Introduction
Cerebrovascular disease is a common and
frequently-occurring disease in clinical practice. It has a high
mortality rate, and its morbidity, mortality and probability of
disability caused by cerebrovascular disease have increased year on
year (1). Global cerebrovascular
disease had a national mortality rate and a prevalence ratio of 28
and 142 per 100,000 persons, respectively in 2015 (2). Ischemic cerebrovascular diseases
account for the vast majority of cerebrovascular diseases, among
which, cerebral ischemia-reperfusion injury (CIRI) is the most
serious type. CIRI refers to the phenomenon in which metabolic
disorders and structural damage of some cells are aggravated,
following the reduction in the amount of blood perfusion, and then
the ischemic damaged tissues resume blood reperfusion (3). Thus, there is an urgent requirement to
treat CIRI.
Traditional Chinese medicine (TCM) has been
gradually used in the clinical treatment of cerebrovascular
diseases, as there are fewer side effects, and improved efficacy
and safety (4). Dihydromyricetin
(DHM) is the key element of the Chinese traditional herb, ginkgo.
Previous studies have confirmed that DHM has a variety of
functions, including antitumor and anti-oxidative effects, and
lowering blood sugar and lipid levels (5–7).
Moreover, compared with western medicine for the treatment of
cerebrovascular disease, DHM has fewer side effects (8). The results from in vitro
studies has revealed that DHM decreased not only the myocardial
infarction area and improved heart function, but also alleviated
oxidative stress in hypoxic/reoxygenation (H/R)-induced primary
cardiomyocytes (9). DHM is a novel
hepatoprotective small molecule, which stimulates the expression of
autophagy-associated genes and inhibits hepatic
ischemia-reperfusion (I/R)-induced apoptosis by increasing the
expression levels of FOXO3a and nuclear translocation (10). In addition, DHM prevents Alzheimer's
disease by inhibiting the activation of NLRP3 inflammasomes in
APP/PS1 transgenic mice to inhibit neuroinflammation (11). However, the role of DHM in cerebral
ischemia-reperfusion cell model has not been investigated.
NF-E2-related factor 2 (Nrf2) is one of the key
regulators of endogenous antioxidant defense and promotes the
transcription of a variety of antioxidant genes, including heme
oxygenase (HO-1) and NAD (P) H:quinone oxidoreductase 1 (NQO1)
(12). The activation of Nrf2/HO-1
signaling pathway by targeting Brg1 improved H/R-induced HT22
neuron cell apoptosis and oxidative stress (13). The results indicated that Nrf2/HO-1
plays an important role in the pathological process of CIRI. In
addition, a variety of drugs targeting the Nrf2/HO-1 signaling
pathway to improve neuronal injury caused by CIRI have been
developed. Inactivated Pseudomonas aeruginosa protects
against myocardial I/R injury through Nrf2 and HO-1 (14). Rutaecarpine improved neuronal injury
and inhibited apoptosis, inflammation and oxidative stress by
regulating the expression of the Nrf2/HO-1 signaling pathway in
rats with CIRI (15). Moreover, DHM
protects umbilical vein endothelial cells from injury through the
Nrf2/HO-1 signaling pathway mediated by ERK and AKT (16). DHM was also found to decrease
lipopolysaccharide (LPS)-induced oxidative stress and promoted the
activity of the antioxidant system by activating superoxide
dismutase (SOD) and the Nrf2/HO-1 signaling pathway (17). Therefore, the present study
investigated whether DHM could influence the oxidative stress and
apoptosis of cells in CIRI by regulating the Nrf2/HO-1 signaling
pathway.
In the present study, HT22 hippocampal neurons were
induced to create an oxygen and glucose deprivation/reoxygenation
(OGD/R) model and the effect of DHM on oxidative stress and
apoptosis of HT22-induced cells was investigated, as well as the
mechanism.
Materials and methods
Cell culture
Mouse HT-22 hippocampal were obtained from The Cell
Bank of Type Culture Collection of the Chinese Academy of Sciences.
The cells were cultured in Dulbecco's modified Eagle's medium
(DMEM) with 10% fetal bovine serum (FBS) (both Gibco; Thermo Fisher
Scientific, Inc.) and 1% antibiotics, at 37°C in a humidified
incubator with 5% CO2. Brusatol (BR, Sigma-Aldrich;
Merck KGaA) at 0.3 µg/ml was used as an Nrf2/HO-1 pathway
inhibitor.
OGD/R model
The OGD/R model is widely recognized as a model for
studying cerebral ischemia at present. By injecting N2,
the concentration of CO2 and O2 in the
incubator can be precisely controlled. The glucose in the culture
medium of neurons can be deprived, thus creating the hypoxic and
glucose-deficient environment for culturing neurons and simulating
cerebral ischemia in vivo. The treatment of HT-22 cells by
ODG/R was conducted as previously described (9). The mouse hippocampal HT22 cells were
cultured in high-glucose Dulbecco's Modified Eagle Medium (DMEM)
containing 10% fetal bovine serum (FBS) in a normoxic 5%
CO2 cell culture incubator at 37°C. OGD/R HT22 cells
were used as an in vitro model of CIRI. Briefly, HT22 cells
were cultured in glucose-free DMEM and then placed in hypoxic
conditions (1% O2, 94% N2, 5% CO2)
at 37°C for 2 h. Thereafter, the medium was discarded, normal DMEM
with glucose was added and the culture was continued for 24 h of
reoxygenation under normoxic condition (95% air, 5% CO2)
to produce OGD/R. HT22 cells cultured in growth culture medium
under normoxic condition served as a control.
Cell Counting Kit-8 (CCK-8)
The CCK-8 kit (Dojindo Molecular Technologies, Inc.)
was used to detect cell proliferation. Briefly, HT-22 cells were
seeded in 96-well plates (2×104 cells/ml) and after the
corresponding treatment, CCK-8 solution (10 µl) was added to each
well. Subsequently, the plates were incubated for 2 h at 37°C and
the absorbance of each well was detected at 450 nm using a
microplate reader (BioTek Instruments; Agilent Technologies,
Inc.).
ELISA
The amount of malondialdehyde (MDA, cat. no.
BMS222), superoxide dismutase (SOD, cat. no. BMS222) and
glutathione (GSH, cat. no. PA5-18651) was evaluated using ELISA
kits (BioSource International; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instruction. The cell supernatant
was centrifuged for 5–10 min at 4°C, at 5,000 × g.
Western blot analysis
The cells were lysed with RIPA lysis buffer
(Beyotime Institute of Biotechnology) and incubated for 30 min on
ice. Total protein was quantified using a BCA protein assay kit.
Following extraction of the total protein from the cells, 10%
SDS-PAGE was used to separate the proteins (30 µg) and transferred
to PVDF membranes, which were blocked with 10% skimmed milk for 1 h
at room temperature. Following overnight incubation at 4°C with
specific primary antibodies, the membranes were washed three times
and incubated with horseradish peroxidase-conjugated secondary
antibodies (1:5,000; cat no. AA24142, Cell Signaling Technology,
Inc.) for 2 h at room temperature. The protein levels were
visualized using the Super signal West Pico Chemiluminescent
substrate (Pierce; Thermo Fisher Scientific, Inc.). Protein
expression levels were semi-quantified using ImageJ software
(version 146; National Institutes of Health) with GAPDH as the
loading control. The following primary antibodies from Cell
Signaling Technology, Inc. were used: Anti-Bax (1:1,000; cat. no.
14796S), anti-caspase3 (1:1,000; cat no. 700128), anti-Bcl-2
(1:1,000; cat. no. 14-1028-82), anti-Nrf2 (1:1,000; cat. no.
12721T), anti-HO-1 (1:1,000; cat. no. 86806S), anti-cleaved
caspase3 (1:1,000; cat. no. PA5-38438), anti-NOX2 (1:1,000; cat.
no. MA5-18052), anti-NOX4 (1:1,000; cat. no. PA5-53304) and
anti-GAPDH (1:1,000; cat. no. MA5-15738).
TUNEL
After fixing 4% paraformaldehyde at room temperature
for 20 min, the cells were washed twice with PBS. Then, 0.2% Triton
X-100 was added to the cells and treated at room temperature for 5
min. The cells were collected and washed with PBS three times, and
treated with 50 µl TUNEL assay solution (Roche Diagnostics GmbH) at
37°C in dark for 60 min and added with stop solution, followed by
incubation with DAB solution and staining with hematoxylin and
eosin for 5 min at room temperature. The cells were randomly
selected for field observation under a light microscope (Carl Zeiss
AG, magnification, ×200). The experiment was repeated three
times.
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). Statistical analyses were performed using SPSS
v22.0 statistical software (IBM Corp.) and one-way ANOVA with
Tukey's multiple comparison post hoc test was employed for
comparison among groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
DHM promotes the viability of
OGD/R-induced HT22 cells
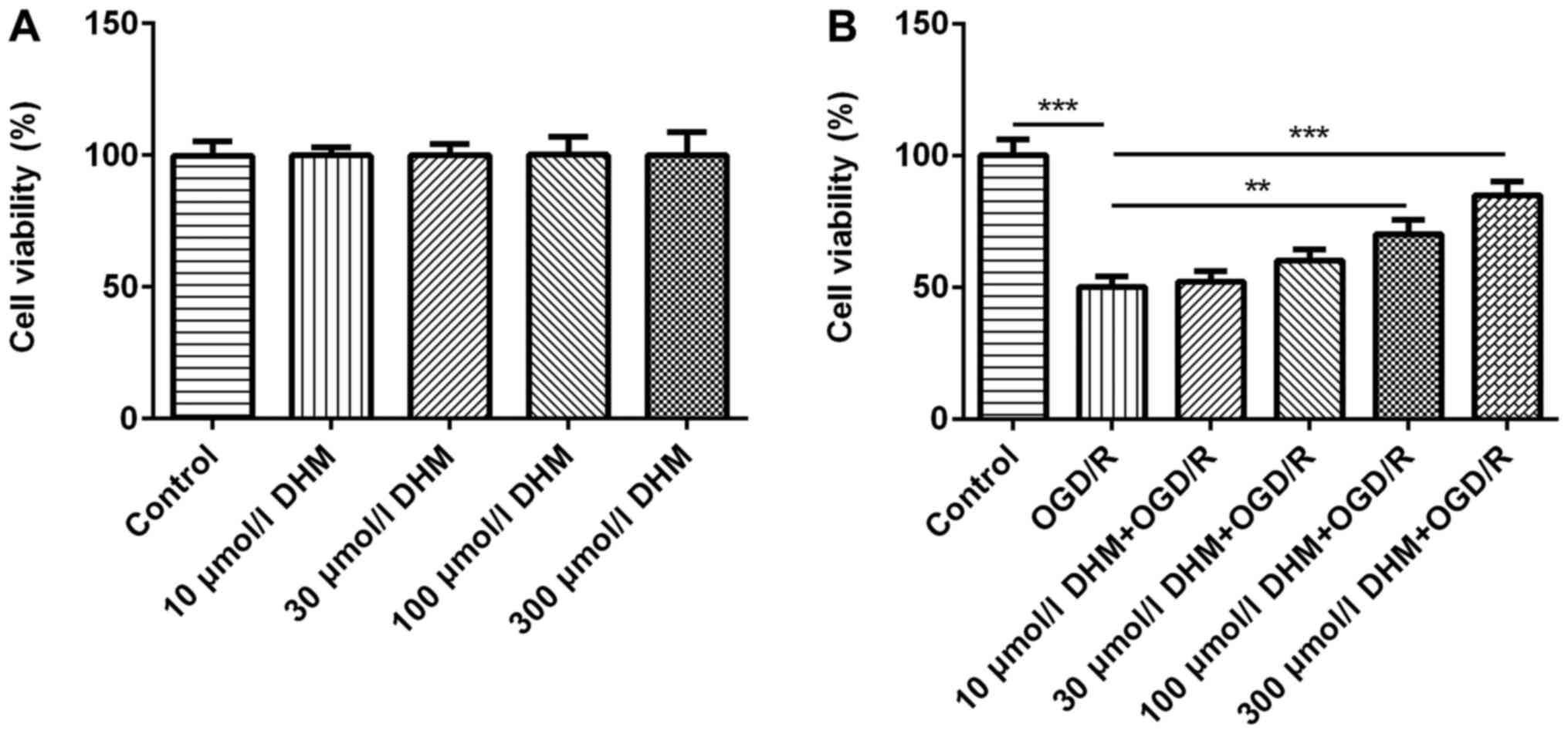
CCK-8 was used to detect the effects of different
concentrations of DHM (0, 10, 30, 100 and 300 µmol/l) on cell
viability and no differences were found (18,19)
(Fig. 1A). Subsequently, the
different aforementioned concentrations of DHM were used in
OGD/R-induced HT22 cells and the CCK-8 results revealed that the
survival rate of OGD/R-induced HT22 cells was significantly
decreased compared with that of the control group. Furthermore, the
cell survival rate of the DHM+OGD/R group was increased in a
dose-dependent manner, compared with the OGD/R group (Fig. 1B). It was found that 300 µmol/l DHM
could significantly promote cell proliferation of OGD/R-induced
HT22 cells, and DHM had no cytotoxic effect on cells at this
concentration. Therefore, 300 µmol/l DHM was used for the
subsequent experimentation.
DHM inhibits the oxidative stress of
OGD/R-induced HT22 cells
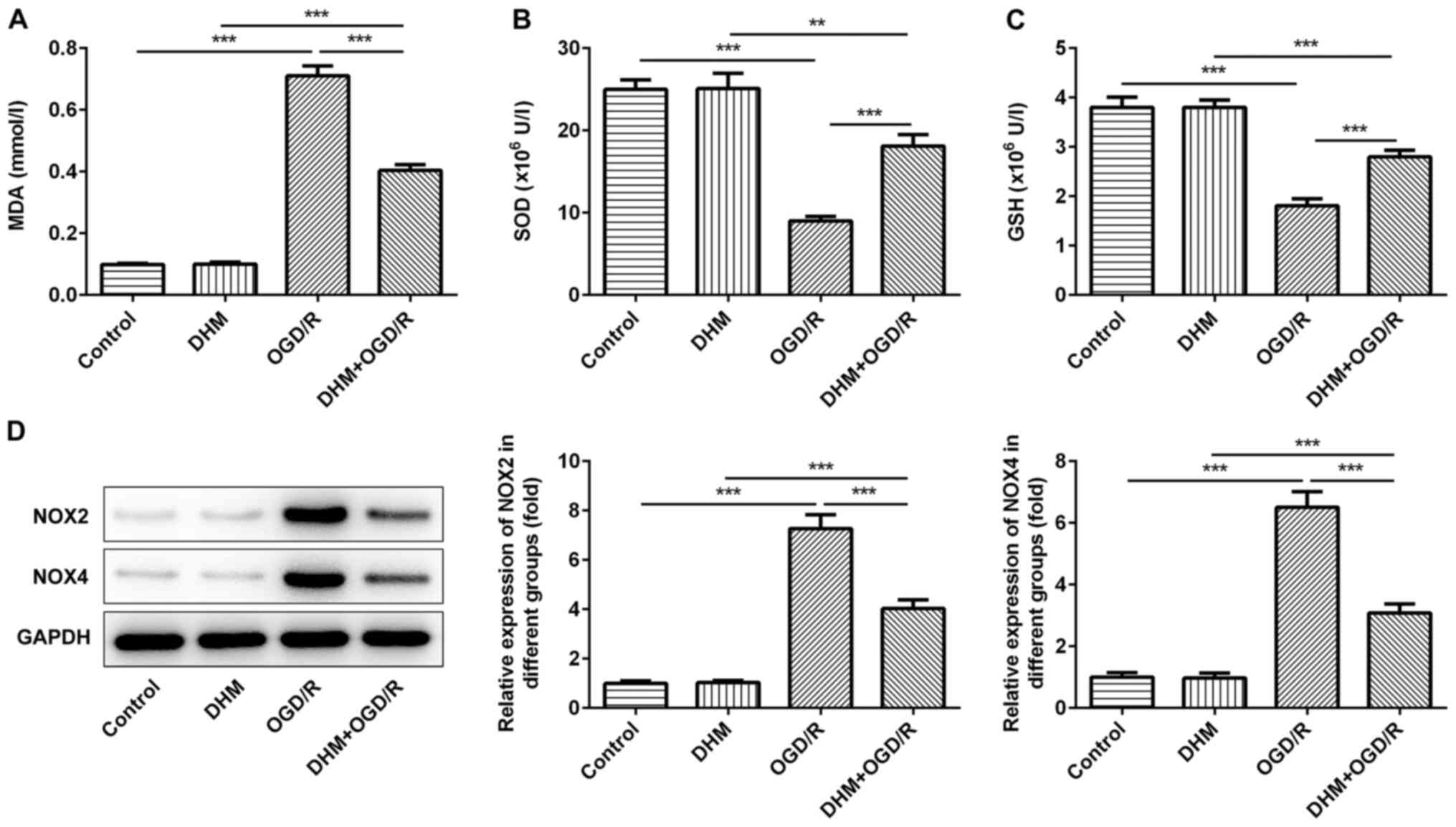
The level of cellular oxidative stress was then
investigated. The cells were grouped into either control, DHM,
OGD/R, DHM+OGD/R groups, and compared with the control group. No
differences were observed in the amount of MDA (Fig. 2A), SOD (Fig. 2B), GSH (Fig. 2C) and the expression levels of the
oxidative stress proteins, NOX2 and NOX4 (Fig. 2D) in cells treated with DHM alone,
while there was a significant increase in the OGD/R group. This
indicated that OGD/R successfully induced oxidative stress in HT22
cells. Compared with that in the OGD/R group, the levels of MDA,
and the expression levels of the oxidative stress proteins, NOX2
and NOX4 in the DHM+OGD/R group were significantly decreased and
the expression of SOD and GSH were increased, indicating that DHM
could inhibit the oxidative stress of OGD/R-induced HT22 cells.
DHM inhibits apoptosis in
OGD/R-induced HT22 cells
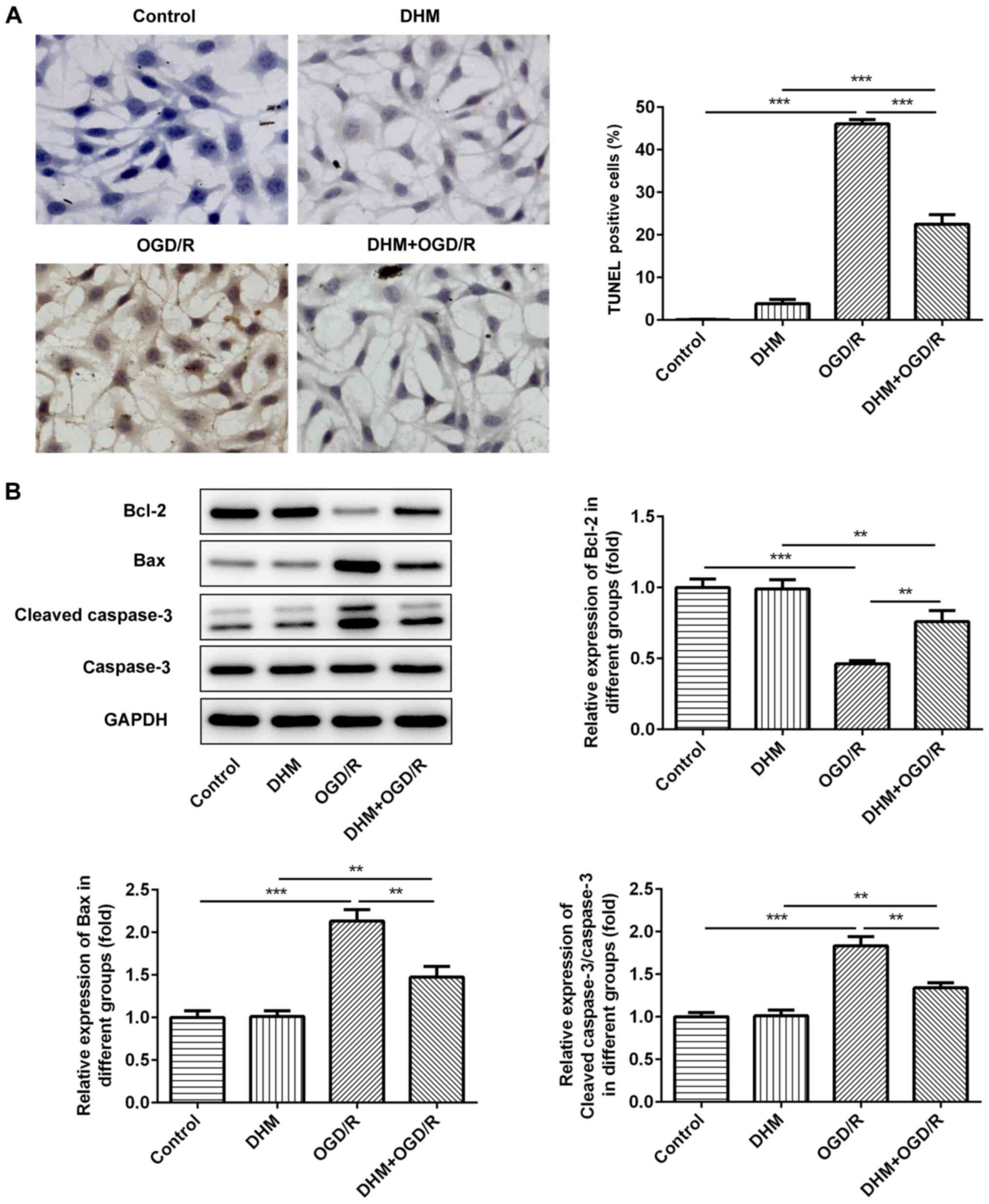
TUNEL staining was used to detect the rate of
apoptosis in cells. Compared with that in the control group, there
was no change in the rate of apoptosis following DHM treatment in
HT22 cells alone, while there was a significant increase in that of
the OGD/R group (Fig. 3A), an
increase in the expression levels of the pro-apoptotic proteins,
Bax and cleaved caspase-3, and a decrease in the anti-apoptotic
protein, Bcl-2 (Fig. 3B). This
indicates that OGD/R successfully induced apoptosis of HT22 cells.
Compared with that in the OGD/R group, cell apoptosis in the
DHM+OGD/R group was decreased, accompanied by the decrease in the
expression levels of Bax and cleaved caspase-3, and the increase in
Bcl-2; indicating that DHM inhibited the apoptosis of OGD/R-induced
HT22 cells.
DHM inhibits oxidative stress and
apoptosis in OGD/R-induced HT22 cells by activating Nrf2/HO-1
signaling pathway
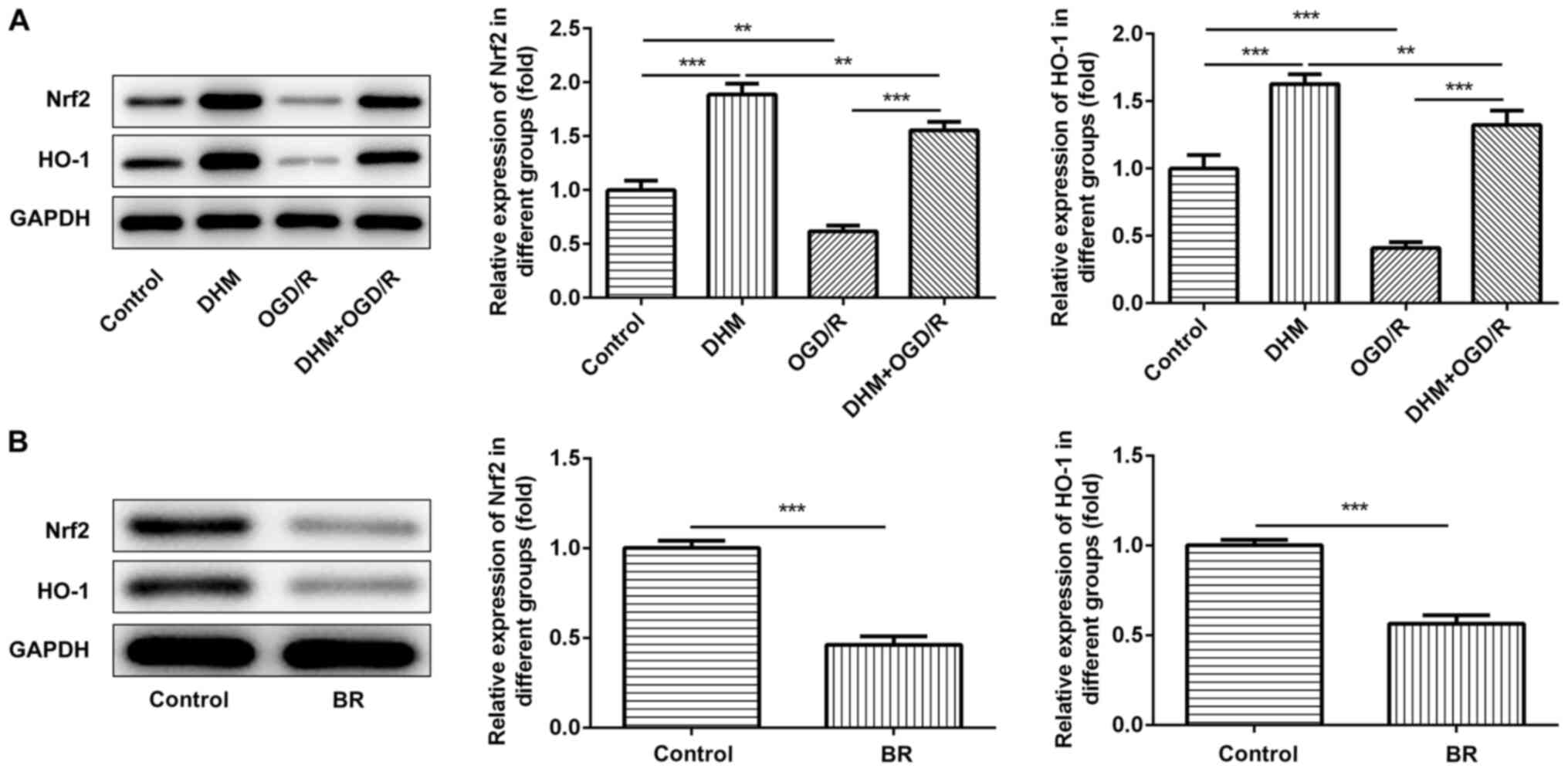
The expression of Nrf2 and HO-1 in the OGD/R group
was significantly decreased compared with the control group, while
the expression of Nrf2 and Ho-1 in the DHM+OGD/R group was reversed
compared with the OGD/R group. In addition, compared with the
control group, the expression of Nrf2 and Ho-1 in the DHM group was
significantly increased. It showed that Nrf2/HO-1 signaling pathway
was inhibited after OGD/R induction, and that DHM could reverse the
inhibitory effect of OGD/R on signaling pathway (Fig. 4A), indicating that the Nrf2/HO-1
signaling pathway was activated following DHM treatment.
Subsequently, the Nrf2/HO-1 signaling pathway inhibitor, BR was
added to the cells, and the protein expression levels of Nrf2 and
HO-1 were detected using western blot analysis (Fig. 4B). Subsequently, the cells were
divided into control, DHM, OGD/R, DHM+OGD/R, and BR+DHM+OGD/R
groups. Compared with that in the DHM+OGD/R group, the levels of
MDA (Fig. 5A), and the protein
expression levels of NOX2 and NOX4 (Fig. 5D) in the BR+DHM+OGD/R group were
significantly increased, the expression of SOD (Fig. 5B) and GSH (Fig. 5C) were decreased, indicating that BR
could reverse the effect of DHM on OGD/R-induced oxidative stress
in HT22 cells. In addition, compared with that in the DHM+OGD/R
group, the rate of apoptosis in the BR+DHM+OGD/R was also increased
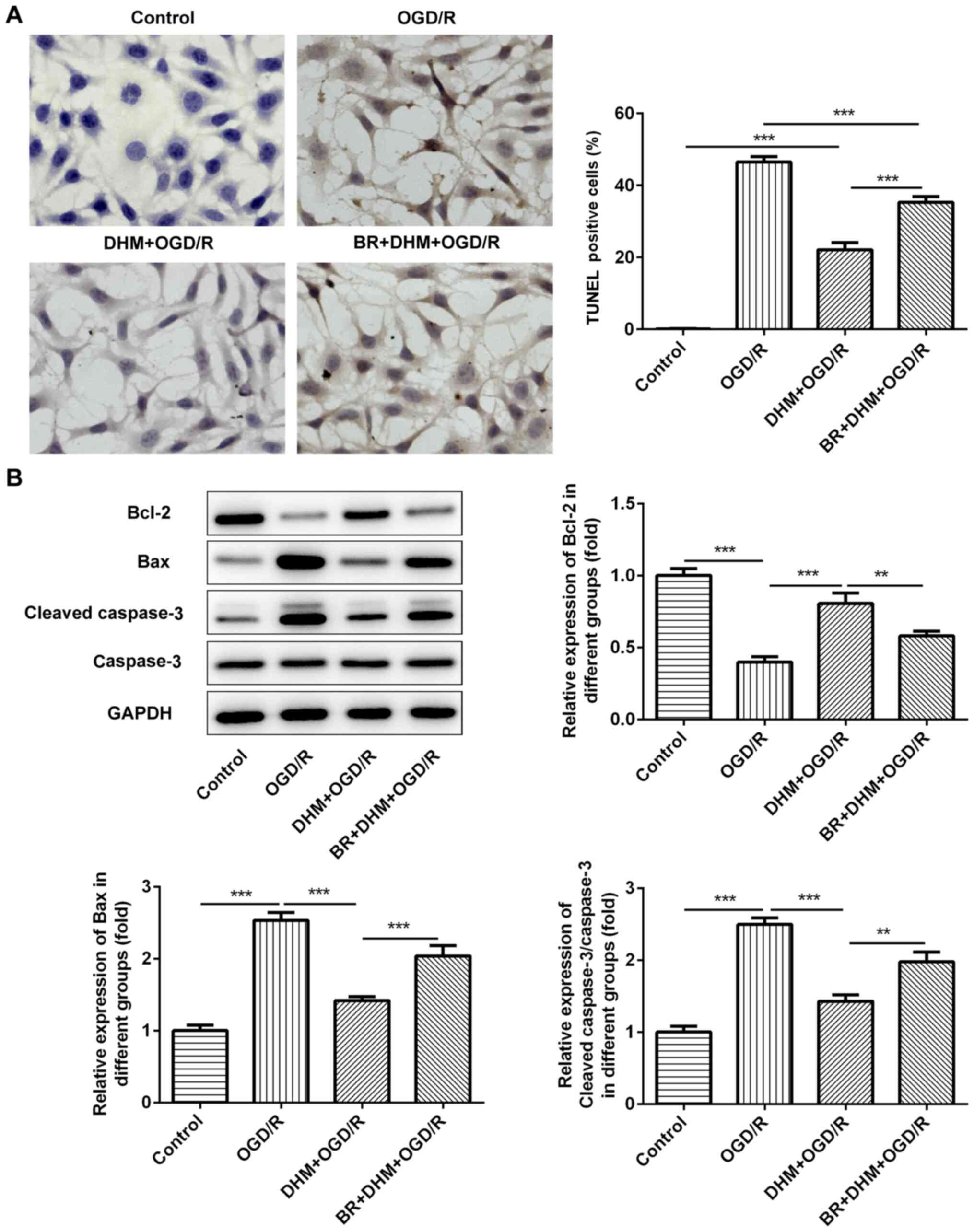
(Fig. 6A), which was accompanied by
an increase in the protein expression levels of Bax and cleaved
caspase-3 (Fig. 6B and Table I), and the decrease in Bcl-2
(Fig. 6B); indicating that BR could
also reverse the effect of DHM on OGD/R-induced apoptosis of HT22
cells. The results indicate that DHM inhibited oxidative stress and
apoptosis of OGD/R-induced HT22 cells by activating the Nrf2/HO-1
signaling pathway.
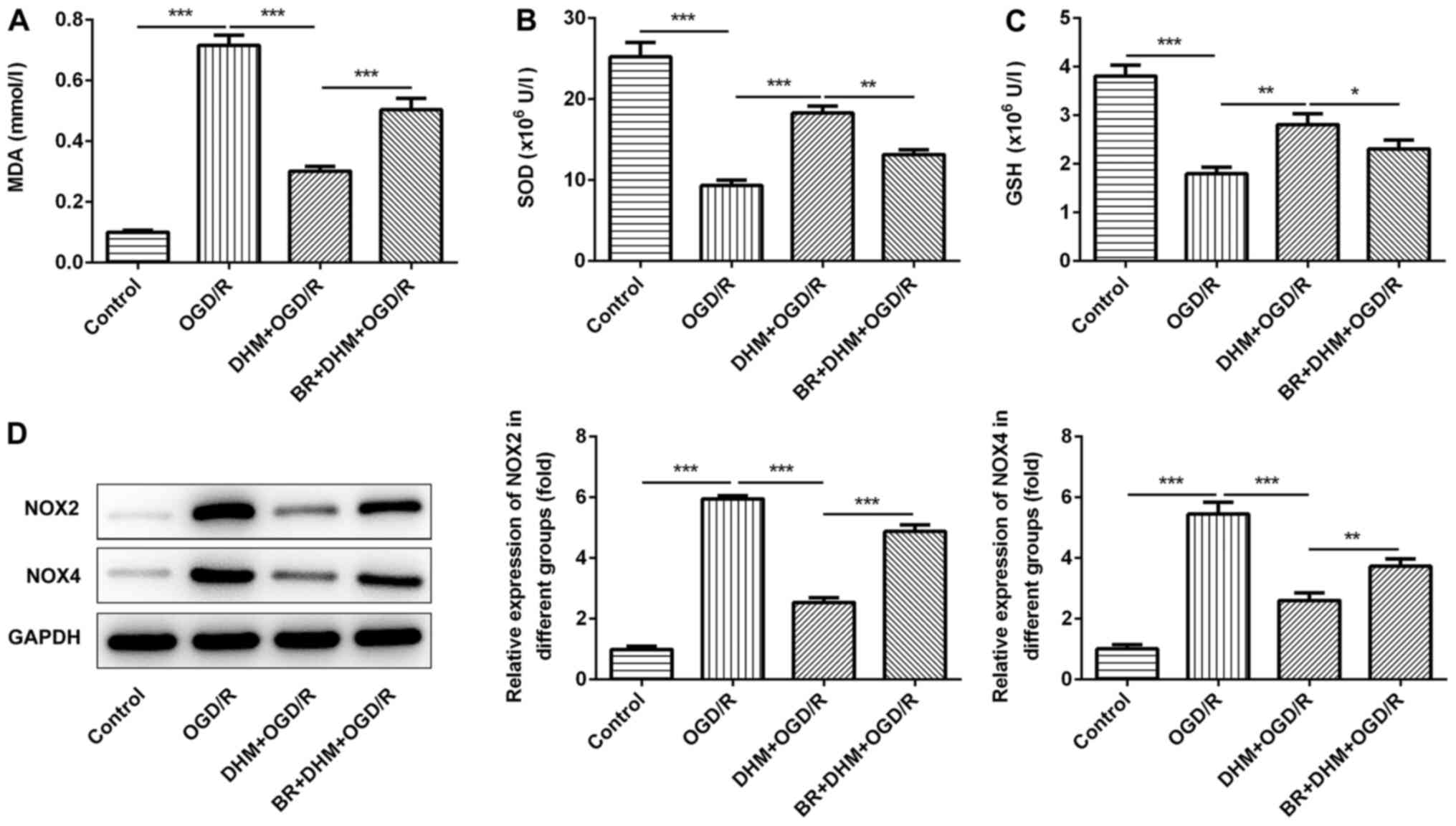 | Figure 5.DHM inhibits oxidative stress of
OGD/R-induced HT22 cells by activating the Nrf2/HO-1 signaling
pathway. MDA (A) SOD (B) and GSH (C) were detected by ELISA assay.
(D) Western blotting was used to detect the expressions of NOX2 and
NOX4. *P<0.05, **P<0.01, ***P<0.001. DHM,
dihydromyricetin; OGD/R, oxygen and glucose
deprivation/reoxygenation; Nrf2, NF-E2-related factor 2; HO-1, heme
oxygenase; MDA, malondialdehyde; SOD, superoxide dismutase; GSH,
glutathione; NOX, NADPH oxidase. |
 | Table I.Values of relative protein expression
of cleaved caspase-3 and caspase-3 in HT22 cells (mean ± standard
deviation). |
Table I.
Values of relative protein expression
of cleaved caspase-3 and caspase-3 in HT22 cells (mean ± standard
deviation).
| Group | Cleaved
caspase-3/caspase-3 | Caspase-3 | Cleaved
caspase-3 |
|---|
| Control | 1.00±0.02 | 1.00±0.07 | 1.00±0.07 |
| OGD/R | 2.50±0.14 | 1.00±0.07 | 2.50±0.07 |
| DHM+OGD/R | 1.42±0.08 | 1.00±0.08 | 1.43±0.07 |
| BR+DHM+OGD/R | 1.94±0.02 | 1.02±0.06 | 1.98±0.11 |
Discussion
At present, the clinical treatment of ischemic
cerebrovascular disease is still limited, and there is a lack of
effective and safe treatment measures. Therefore, there is an
urgent requirement to develop stable and safe drugs to treat
CIRI.
In the present study, mouse hippocampal neuron HT22
cells were induced using OGD/R to create an in vitro model
of CIRI. It was found that following OGR/D induction, the cell
survival rate decreased, oxidative stress was activated, and
apoptosis was also significantly increased, indicating the cell
model was successfully established.
DHM, which is a type of active hydrogenated
flavonol, has been investigated widely in pharmacology in recent
years, and has been found to be effective in treating a variety of
different diseases. DHM has been found to increase endothelial
nitric oxide production and inhibited atherosclerosis through
microRNA-21 in apolipoprotein E-deficient mice (18). DHM also ameliorated chronic social
defeat stress-induced cognitive and affective disorder in mice
(20). In addition, modulation of
SIRT1-mediated signaling cascades in the liver contributed to the
amelioration of non-alcoholic steatohepatitis in middle-aged LDL
receptor knockout mice, that were fed a high-fat diet by DHM
(21). Therefore, DHM has been
shown to have a variety of beneficial functions in the current
study and has become a potential source of treatment of CIRI. A
previous study investigating ischemia reperfusion injury found that
DHM enhances protection during ischemia, decreases myocardial
dysfunction by enhancing anti-inflammatory activities, attenuates
myocardial oxidative injury and prevents apoptosis during
ischemia/reperfusion (22). In a
study investigating cerebral ischemia, DHM was found to effectively
prevent cerebral edema caused by whole brain I/R injury in rats
caused by the ligation of the bilateral common carotid artery
(23). DHM has also been found to
have a neuronal protective effect on PC12 cells induced by
H2O2 (24).
The effect of DHM in CIRI is currently unclear, therefore, in the
present study, DHM was used to treat OGD/R-induced HT22 cells, and
it was found that DHM promoted the proliferation of OGD/R-induced
HT22 cells in a dose-dependent manner. Moreover, DHM inhibited the
oxidative stress response and inhibited cell apoptosis in
OGD/R-induced HT22 cells. Thus, DHM has a protective effect on
neurons, which is consistent with the therapeutic effect of DHM on
I/R injury.
It was found that the Nrf2/HO-1 signaling pathway
was inhibited following OGD/R induction and the Nrf2/HO-1 signaling
pathway plays an important role in I/R injury (25,26).
Different drugs can inhibit cell damage and apoptosis caused by
ischemia and hypoxia by activating the Nrf2/HO-1 signaling pathway
(27,28). Therefore, the present study
investigated whether DHM plays a protective role on OGD/R-induced
HT22 cells through the Nrf2/HO-1 signaling pathway. It was found
that the Nrf2/HO-1 signaling pathway in HT22 cells was activated
following treatment with DHM in HT22 cells alone or in
OGD/R-induced HT22 cells. Furthermore, the Nrf2/HO-1 signaling
pathway inhibitor, BR, was used and it was found that following
inhibition of the Nrf2/HO-1 signaling pathway, the inhibitory
effect of DHM on OGD/R-induced oxidative stress and apoptosis was
reduced. Thus, the findings suggest that DHM inhibited oxidative
stress and apoptosis in OGD/R-induced HT22 cells by activating the
Nrf2/HO-1 signaling pathway.
In conclusion, the present study demonstrated that
DHM inhibited oxidative stress and apoptosis of OGD/R-induced HT22
cells by activating the Nrf2/HO-1 signaling pathway, which
indicates that DHM has the potential to be developed as a stable
and safe treatment option for CIRI.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Science
Foundation of Guangzhou First People's Hospital (grant no.
M2019022).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ and TZ wrote the manuscript and analyzed the
data. JW and HZ carried out the experiments and TZ supervised the
present study. QZ searched the literature and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fisher M: Introducing focused updates in
cerebrovascular disease. Stroke. 48:26532017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yanez N, Useche JN, Bayona H, Porras A and
Carrasquilla G: Analyses of mortality and prevalence of
cerebrovascular disease in colombia, south america (2014–2016): A
Cross-sectional and ecological study. J Stroke Cerebrovasc Dis.
29:1046992020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soares ROS, Losada DM, Jordani MC, Évora P
and Castro-E-Silva O: Ischemia/reperfusion injury revisited: An
overview of the latest pharmacological strategies. Int J Mol Sci.
20:50342019. View Article : Google Scholar
|
|
4
|
Wang M, Liu JX, Yao MJ and Ren JG:
Advances in research on pharmacological and neuroprotective effects
of traditional Chinese medicine after cerebral ischemia. Zhongguo
Zhong Yao Za Zhi. 45:513–517. 2020.(In Chinese). PubMed/NCBI
|
|
5
|
Xu Y, Wang S, Chan HF, Lu H, Lin Z, He C
and Chen M: Dihydromyricetin induces apoptosis and reverses drug
resistance in ovarian cancer cells by p53-mediated downregulation
of survivin. Sci Rep. 7:460602017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Le L, Jiang B, Wan W, Zhai W, Xu L, Hu K
and Xiao P: Metabolomics reveals the protective of Dihydromyricetin
on glucose homeostasis by enhancing insulin sensitivity. Sci Rep.
6:361842016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Wang K, Huang C, Lin D, Zhou Y, Wu
Y, Tian N, Fan P, Pan X, Xu D, et al: SIRT3 activation by
dihydromyricetin suppresses chondrocytes degeneration via
maintaining mitochondrial homeostasis. Int J Biol Sci.
14:1873–1882. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren W, Liao J, Chen J, Li Z and Huang L:
The effect of Chinese herbal medicine combined with western
medicine on vascular endothelial function for patients with
hypertension: Protocol for a systematic review and Meta-analysis.
Medicine (Baltimore). 98:e181342019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei L, Sun X, Qi X, Zhang Y, Li Y and Xu
Y: dihydromyricetin ameliorates cardiac Ischemia/reperfusion injury
through Sirt3 activation. Biomed Res Int. 2019:68039432019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Lv L, Pi H, Qin W, Chen J, Guo D,
Lin J, Chi X, Jiang Z, Yang H and Jiang Y: Dihydromyricetin
protects against liver ischemia/reperfusion induced apoptosis via
activation of FOXO3a-mediated autophagy. Oncotarget. 7:76508–76522.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng J, Wang JX, Du YH, Liu Y, Zhang W,
Chen JF, Liu YJ, Zheng M, Wang KJ and He GQ: Dihydromyricetin
inhibits microglial activation and neuroinflammation by suppressing
NLRP3 inflammasome activation in APP/PS1 transgenic mice. CNS
Neurosci Ther. 24:1207–1218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang R, Xu M, Wang Y, Xie F, Zhang G and
Qin X: Nrf2-a promising therapeutic target for defensing against
oxidative stress in stroke. Mol Neurobiol. 54:6006–6017. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li F, Liang J, Tong H, Zhu S and Tang D:
Inhibition of microRNA-199a-5p ameliorates oxygen-glucose
Deprivation/Reoxygenation-induced apoptosis and oxidative stress in
HT22 neurons by targeting Brg1 to activate Nrf2/HO-1 signalling.
Clin Exp Pharmacol Physiol. 47:1020–1029. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Z, Tang Z, Zhang W, Liu J, Li B and
Ding S: Inactivated Pseudomonas aeruginosa protects against
myocardial ischemia reperfusion injury via Nrf2 and HO-1. Exp Ther
Med. 19:3362–3368. 2020.PubMed/NCBI
|
|
15
|
Han M, Hu L and Chen Y: Rutaecarpine may
improve neuronal injury, inhibits apoptosis, inflammation and
oxidative stress by regulating the expression of ERK1/2 and
Nrf2/HO-1 pathway in rats with cerebral ischemia-reperfusion
injury. Drug Des Devel Ther. 13:2923–2931. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo Y, Lu S, Dong X, Xu L, Sun G and Sun
X: Dihydromyricetin protects human umbilical vein endothelial cells
from injury through ERK and Akt mediated Nrf2/HO-1 signaling
pathway. Apoptosis. 22:1013–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Li X, Fang J, Hou X, Fang H, Guo
F, Li F, Chen A and Huang S: (2R,3R)Dihydromyricetin inhibits
osteoclastogenesis and bone loss through scavenging LPS-induced
oxidative stress and NF-κB and MAPKs pathways activating. J Cell
Biochem. 119:8981–8995. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Q, Zhang T, Yi L, Zhou X and Mi M:
Dihydromyricetin inhibits NLRP3 Inflammasome-dependent pyroptosis
by activating the Nrf2 signaling pathway in vascular endothelial
cells. Biofactors. 44:123–136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Wang L, Peng L, Tian X, Qiu X,
Cao H, Yang Q, Liao R and Yan F: Dihydromyricetin protects HUVECs
of oxidative damage induced by sodium nitroprusside through
activating PI3K/Akt/FoxO3a signalling pathway. J Cell Mol Med.
23:4829–4838. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Li BR, Xiao ZY, Zhao JL and Yu XD:
Dihydromyricetin ameliorates chronic social defeat stress induced
cognitive and affective disorder in mice. Zhongguo Ying Yong Sheng
Li Xue Za Zhi. 35:496–500. 2019.(In Chinese). PubMed/NCBI
|
|
21
|
Zeng Y, Hua YQ, Wang W, Zhang H and Xu XL:
Modulation of SIRT1-mediated signaling cascades in the liver
contributes to the amelioration of nonalcoholic steatohepatitis in
high fat fed Middle-aged LDL receptor knockout mice by
dihydromyricetin. Biochem Pharmacol. 175:1139272020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang D, Zhang X, Qu D, Han J, Meng F, Xu M
and Zheng Q: Astragalin and dihydromyricetin as adjuncts to
histidine-tryptophan-ketoglutarate cardioplegia enhances protection
during cardioplegic arrest. Mol Med Rep. 18:2929–2936.
2018.PubMed/NCBI
|
|
23
|
Dirnagl U, Iadecola C and Moskowitz MA:
Pathobiology of ischaemic stroke: An integrated view. Trends
Neurosci. 22:391–397. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kou X, Shen K, An Y, Qi S, Dai WX and Yin
Z: Ampelopsin inhibits H2O2-induced apoptosis
by ERK and Akt signaling pathways and up-regulation of heme
oxygenase-1. Phytother Res. 26:988–994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li F, Liang J and Tang D: Brahma-related
gene 1 ameliorates the neuronal apoptosis and oxidative stress
induced by oxygen-glucose deprivation/reoxygenation through
activation of Nrf2/HO-1 signaling. Biomed Pharmacother.
108:1216–1224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji Q, Gao J, Zheng Y, Liu X, Zhou Q, Shi
C, Yao M and Chen X: Inhibition of microRNA-153 protects neurons
against ischemia/reperfusion injury in an Oxygen-glucose
deprivation and reoxygenation cellular model by regulating
Nrf2/HO-1 signaling. J Biochem Mol Toxicol. Jul 31–2017.(Epub ahead
of print). doi: 10.1002/jbt.21905. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu L, Zhao Z, Yin Q and Zhang X: TTB
protects astrocytes against oxygen-glucose
deprivation/reoxygenation-induced injury via activation of
Nrf2/HO-1 signaling pathway. Front Pharmacol. 10:7922019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He F, Zhang Y, Chen S, Ye B, Chen J and Li
C: Effect of EGCG on oxidative stress and Nrf2/HO-1 pathway in
neurons exposed to oxygen-glucose deprivation/reperfusion. Zhong
Nan Da Xue Xue Bao Yi Xue Ban. 43:1041–1047. 2018.(In Chinese).
PubMed/NCBI
|