Introduction
Cervical cancer has a considerably high mortality
rate and is the fourth most common cancer in women worldwide
(1). In total, >130,500
individuals are diagnosed with cervical cancer in China every year,
accounting for 30% of the overall cancer-affected population
worldwide (2). Cervical cancer is
caused by the infection of the human papillomavirus (HPV) to the
uterine epithelia. Although cervical cancer can affect other parts
of the body, it progresses slowly and can be treated effectively if
diagnosed at an early stage. However, current treatments, including
chemotherapy and radiotherapy are not ideal due to the side effects
caused. Therefore, it is important to discover novel methods to
effectively treat cervical cancer.
Paeonol (Pae) is a natural product derived from the
root of Cynanchum paniculatum (Bunge) K. Schum and the root
of Paeonia suffruticosa Andr. (Ranunculaceae). It has
received extensive attention due to its multiple biological
activities, such as anti-oxidative, anti-inflammatory and
anti-cancer effects (3–6). Pae relieved the induction of oxidative
stress and inflammation in rats with testicular
ischaemia-reperfusion injury (4). A
previous study demonstrated that by downregulating the expression
of Erb-B2 receptor tyrosine kinase 2 and inhibiting the nuclear
factor-κB signaling pathway, Pae induced the apoptosis of gastric
cancer cells (7). Another study
demonstrated the significant role of Pae in inhibiting the growth
of breast cancer cells, possibly by its ability to induce cell
apoptosis (8). However, whether Pae
can exert significant effects on the proliferation and apoptosis of
cervical cancer cells has not been fully elucidated, thus,
requiring an in-depth study to be conducted on its role. Through
STITCH, it was found that paeonol could regulate
prostaglandin-endoperoxide synthase (PTGS2), and the interaction
between PTGS2 and 5-lipoxygenase (ALOX5; 5-LO) can be found on the
String website.
5-LO is considered the major enzyme involved in the
biosynthesis of a class of bioactive lipids signaling molecules
known as eicosanoids (9). The roles
of lipoxygenase in the pathogenesis of cancer have been recently
identified and novel lipoxygenase inhibitors have been developed
with promising anti-cancer activity (10). Monga et al (11) revealed that pharmacological and
genetic targeting of 5-LO induced prostate cancer cell apoptosis.
Increased expression of 5-LO was detected in clinical samples from
patients with breast cancer (12).
The inhibition of 5-LO impeded the invasion of breast cancer cells
by regulating the production of interleukin-8 and matrix
metlloprotease-9 (MMP-9) (13).
However, the specific role of 5-LO in cervical cancer has not been
fully investigated.
In the present study, the role of Pae on the
proliferation, migration and invasion of cervical cancer cells was
investigated and the potential mechanisms underlying these
processes were explored in association with 5-LO expression.
Materials and methods
Cell culture and treatment
The HeLa cell line was obtained from the Shanghai
Cell Bank of Chinese Academy of Sciences. The human immortalized
cervical epithelial cell line H8 was obtained from Bnbio.
Subsequently, the cells were cultured in RPMI-1640 medium (Thermo
Fisher Scientific, Inc.), containing 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) in an incubator with 5%
CO2 at 37°C. Pae (purity >98%) was purchased from
Dalian Meilun Biotechnology Co., Ltd. (cat. no. MB1762-S). Pae was
dissolved in DMSO and preserved for further experiments. The cells
were exposed to Pae for 24 h at the concentrations of 0.1, 0.2, 0.4
and 0.6 mg/ml. The culture medium was replaced every 2–3 days.
Cell transfection
The overexpression plasmids pcDNA 3.1–5-LO and
control pcDNA 3.1 were generated by Shanghai GenePharma Co., Ltd.
HeLa cells were respectively transfected with 2.5 µg pcDNA 3.1–5-LO
or vector using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) for 48 h according to the manufacturer's
instructions. The transfection efficiency of the cells was
determined by reverse transcription-quantitative PCR (RT-qPCR).
Cell Counting Kit-8 (CCK-8) assay
Following transfection, the cells were seeded into
96-well plates (1×105 cells per well) and re-suspended
in RPMI-1640 containing 10% FBS. Then, 10 µl CCK-8 reagent (Thermo
Fisher Scientific, Inc.) was added to cells treated for 24, 48 and
72 h at 37°C. Subsequently, the absorbance at 450 nm was measured
with a microplate reader (Thermo Fisher Scientific, Inc.) at each
time point according to the manufacturer's instructions.
Colony-formation assay
Treated cells were detached by 0.25% trypsin and
re-suspended in medium. The cells were seeded into culture dishes
at a density of 2,000 cells/well. Following 2 weeks of incubation,
the colonies were visible to the naked eye. The cells were fixed
with 4% paraformaldehyde for 20 min at room temperature and stained
with 0.2% crystal violet for 10 min at room temperature. The number
of colonies was counted using ImageJ software (v.1.52s; National
Institutes of Health).
Wound healing assay
The cells were cultured in 6-well plates
(5×105 cells/well) with RPMI-1640 containing 10% FBS. A
scratch was created on the cell surface with a 200-µl pipette tip.
Following washing to remove the detached cells, the medium was
replaced with serum-free RPMI-1640 medium and cultured at 37°C for
24 h. The images were obtained after 24 h by a light microscope
(Olympus Corporation; magnification ×100).
Transwell assay
Following transfection, the cells were plated in a
serum-free medium at a density of 1×104 cells/ml in the
upper chamber, which was coated with Matrigel (Corning Inc.).
Medium containing 20% FBS was added into the lower chamber.
Following 24-h incubation, 0.05% crystal violet was used to stain
the cells for 30 min at room temperature in the lower chamber. The
cells were counted under a light microscope at a magnification of
×100.
Flow cytometry
Cell apoptosis was measured using the Annexin-FITC
Apoptosis Detection kit (Beyotime Institute of Biotechnology).
Briefly, the cells were collected and washed with PBS twice, gently
resuspended in Annexin V binding buffer and incubated with Annexin
V-FITC/PI at room temperature in dark. The number of apoptotic
cells was analyzed using a flow cytometer (Becton, Dickinson and
Company).
Western blotting
The cells were collected and the total proteins were
extracted using RIPA lysis buffer (Beyotime Institute of
Biotechnology). BCA assay was used to determine the protein
concentration. Briefly, protein samples (20 µg) were loaded at the
same concentration on each lane of the 12% SDS-polyacrylamide gel.
The proteins were transferred to PVDF membranes (EMD Millipore).
Then, 5% skimmed milk was used for blocking the membranes at room
temperature for 1 h. Primary antibodies such as MMP-2 (cat. no.
ab92536; dilution, 1:1,000; Abcam), MMP-9 (cat. no. ab76003;
dilution, 1:1,000; Abcam), Bcl-2 (cat. no. ab182858; dilution,
1:2,000; Abcam), Bax (cat. no. ab32503; dilution, 1:1,000; Abcam),
cleaved-caspase-3 (cat. no. ab32042; dilution, 1:500; Abcam),
cleaved-caspase-9 (cat. no. 20750; dilution, 1:1,000; Cell
Signaling Technology, Inc.), caspase-3 (cat. no. ab32351; dilution,
1:5,000; Abcam), caspase-9 (cat. no. ab32539; dilution, 1:500;
Abcam), ALOX5 (cat. no. ab169755; dilution, 1:1,000; Abcam) and
GAPDH (cat. no. ab8245; dilution, 1:1,000; Abcam) were incubated
with the membranes overnight at 4°C. Subsequently, the membranes
were incubated with horseradish peroxidase-conjugated secondary
antibody (cat. no. 7074; dilution, 1:2,000; Cell Signaling
Technology, Inc.) at room temperature for 2 h prior to ECL
detection. Image J. v.1.52s (National Institutes of Health) was
used to analyze the density of the immunoblots. GAPDH was used as
an internal control.
RT-qPCR
Total RNA was extracted from HeLa cells transfected
with pcDNA 3.1–5-LO and control vector using a Takara MiniBEST RNA
Extraction kit (Takara Bio, Inc). Total RNA was reverse-transcribed
into cDNA using SuperScript IV First-Strand Synthesis system
(Thermo Fisher Scientific, Inc.) at the following thermocycling
conditions: 42°C for 60 min, 70°C for 5 min, preserved at 4°C.
RT-qPCR was detected using a TaqMan gene expression assay kit
(Thermo Fisher Scientific, Inc.). PCR was performed as follows:
Pretreatment at 95°C for 10 min, followed by 35 cycles at 94°C for
15 sec, 60°C for 1 min, 60°C for 1 min and preserved at 4°C. The
2−ΔΔCq method was used to analyze the relative gene
expression (14) and GAPDH was used
for normalization. The primer sequences were as follows: 5-LO
forward: 5′-TGGAATGACTTCGCCGACTTTGAG-3′ and reverse:
5′-TAGCCAAACATCAGGTCTTCCTGC-3′; and GAPDH forward:
5′-ACCACAGTCCATGCCATCAC-3′ and reverse:
5′-TCCACCACCCTGTTGCTGTA-3′.
Statistical analysis
All experimental data are expressed as mean ±
standard deviation, and were statistically analyzed with SPSS 17.0
software (SPSS, Inc.). The Student' s t-test was used to analyze
the comparison between the two groups and the one-way analysis of
variance test followed by the Tukey's post hoc test was performed
to analyze significant differences among multiple groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Pae inhibits the migration and
invasion of HeLa cells
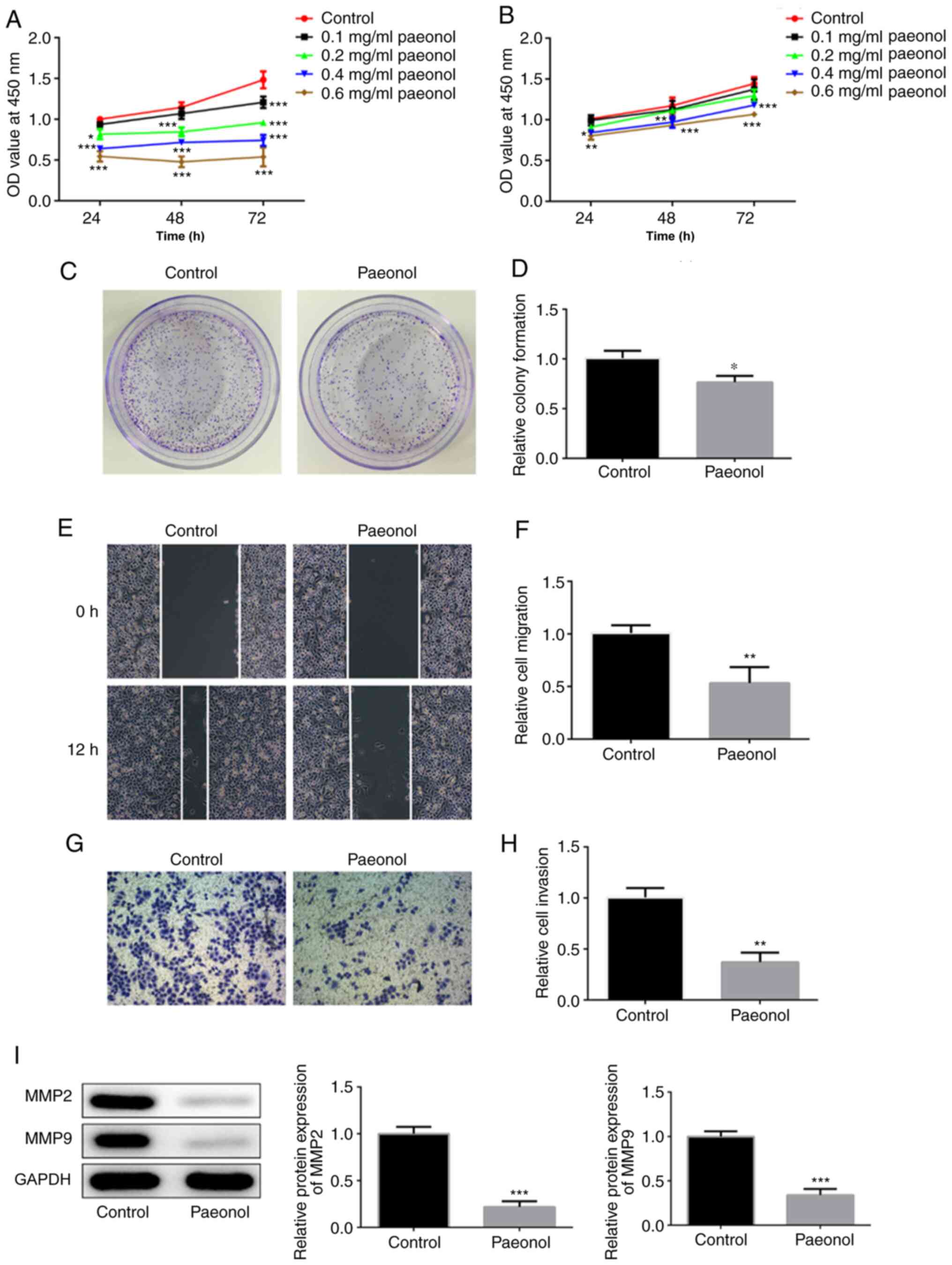
The viability of HeLa cells was decreased with the
increasing doses of Pae (Fig. 1A).
H8 cells treated with low concentrations of Pae demonstrated no
significant changes on cell viability compared with control H8
cells. However, cell viability was decreased with time when the
dose of Pae increased to 0.4 mg/ml (Fig. 1B). Therefore, 0.2 mg/ml Pae was
selected for further experiments. Untreated HeLa cells were used as
the control group, whereas cells treated with 0.2 mg/ml Pae were
used as the Pae group. The colony-formation ability of HeLa cells
treated with Pae was decreased compared with that of the control
group (Fig. 1C and D). Wound
healing and Transwell assays were conducted to assess the invasion
and migration of HeLa cells. The data indicated that the invasive
and migratory activities of HeLa cells were markedly inhibited
following their exposure to Pae (Fig.
1E-H). Western blot analysis was conducted to detect changes in
the expression levels of the invasion- and migration-associated
proteins MMP-2 and MMP-9. The results indicated considerably lower
expression levels in the Pae group compared with those noted in the
control group (Fig. 1I). These
results suggested that Pae inhibited the proliferation, migration
and invasion of HeLa cells.
Pae promotes the induction of HeLa
cell apoptosis
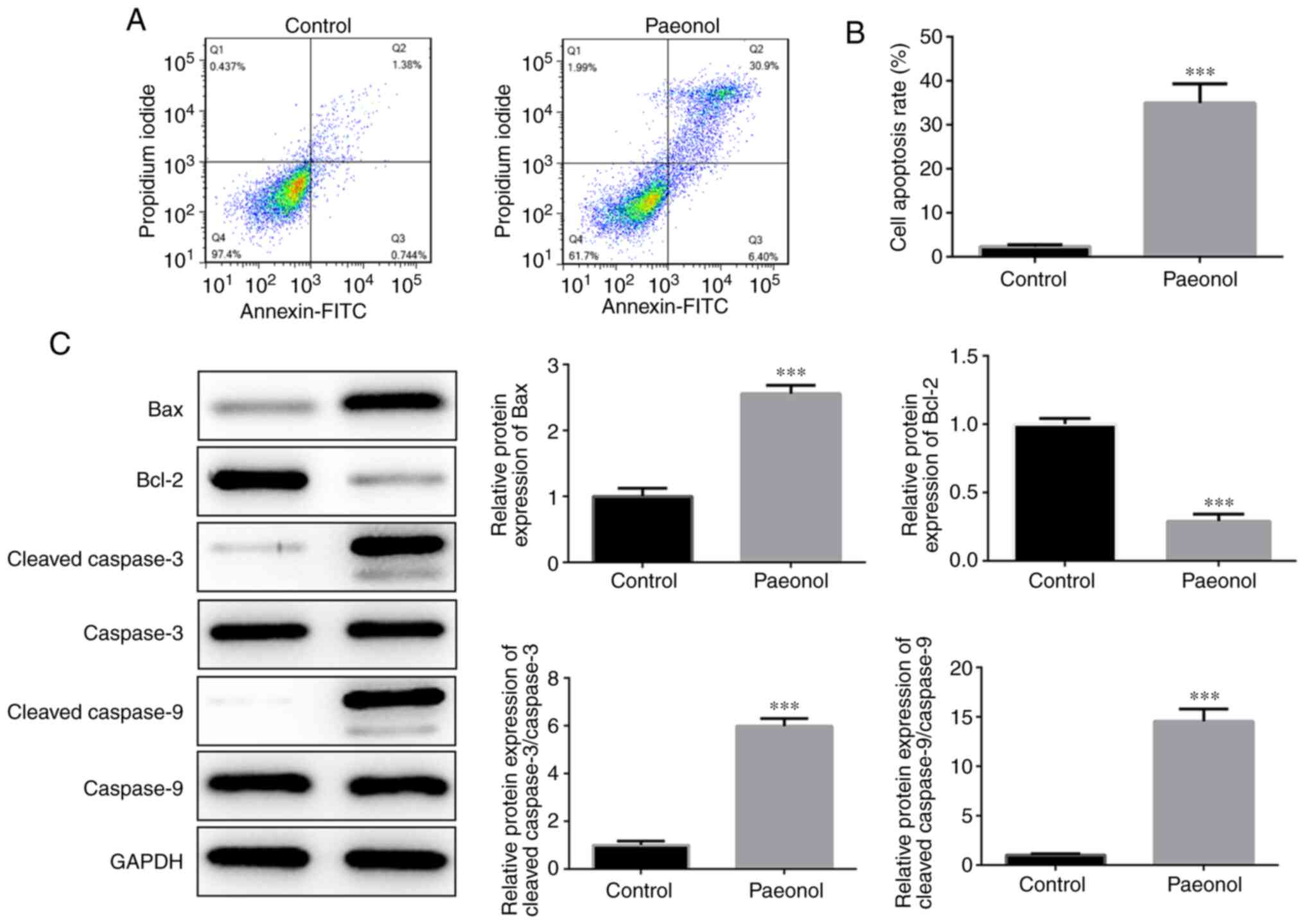
Flow cytometry was utilized to detect the induction
of apoptosis in HeLa cells. The results indicated that the
percentage of apoptotic cells in the Pae group was significantly
increased compared with that of the control cells (Fig. 2A and B). Western blot analysis
indicated that the expression levels of Bcl-2 were markedly
decreased, while those of the pro-apoptotic proteins Bax, cleaved
caspase-3 and cleaved caspase-9 were significantly increased
(Fig. 2C). Therefore, these results
indicated that Pae promoted the induction of apoptosis in HeLa
cells.
Pae inhibits the expression of 5-LO in
HeLa cells
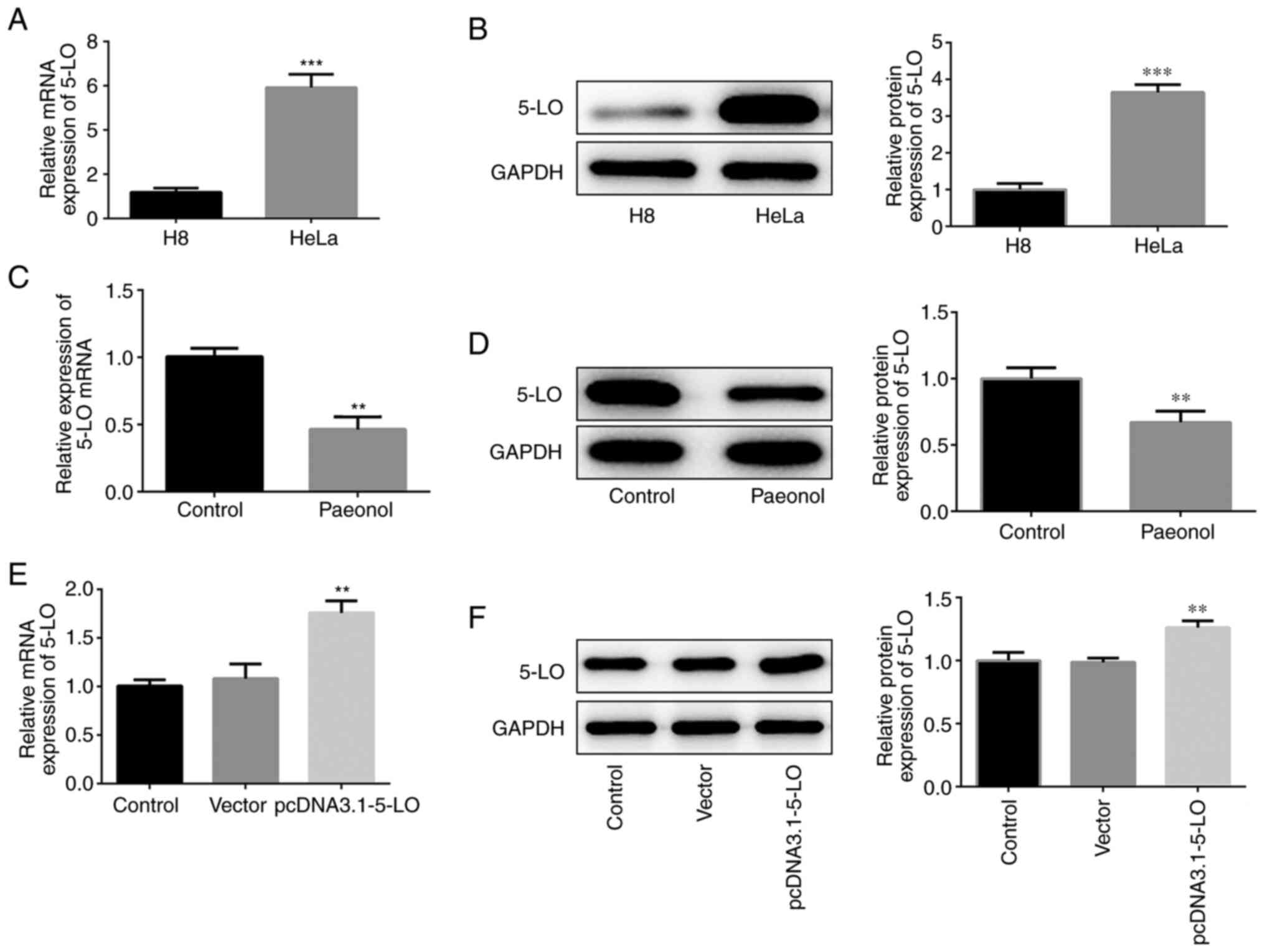
In order to detect the expression of 5-LO in HeLa
cells, western blotting and RT-qPCR analysis were performed. 5-LO
mRNA and protein levels were elevated in HeLa cells (Fig. 3A and B). Pae-treated HeLa cells
exhibited downregulated expression of 5-LO compared with that of
the control cells (Fig. 3C and D).
The transcription and protein levels of 5-LO were increased
following construction and transfection of the overexpression
plasmid of 5-LO into HeLa cells (Fig.
3E and F), indicating that the overexpression plasmid was
effective. Collectively, these results confirmed that 5-LO was
highly expressed in HeLa cells and that Pae inhibited the
expression of 5-LO.
5-LO inhibits Pae-mediated
anti-migratory and anti-invasive effects
The cells were classified into four groups, namely
control, Pae, Pae+vector and Pae+pcDNA 3.1–5-LO groups. Cell
viability and clone-formation ability of the Pae or the Pae+vector
groups were decreased to a relatively low level compared with those
of the control group, while the addition of the 5-LO overexpression
plasmid into HeLa cells disrupted this effect (Fig. 4A-C). As shown in Fig. 4D-G, the Pae or Pae+vector groups
demonstrated decreased invasive and migratory activities compared
with those of the control group, while these effects were recovered
partially in the Pae+pcDNA 3.1–5-LO group as determined by the
expression levels of the invasion- and migration-associated
proteins MMP-2 and MMP-9 (Fig. 4H).
The latter were significantly decreased following treatment of HeLa
with Pae. However, when HeLa cells were transfected with pcDNA
3.1–5-LO the effects of Pae were weakened and the levels of MMP-2
and MMP-9 were increased. Taken together, the results demonstrated
that the anti-migratory and anti-invasive effects of Pae were
5-LO-dependent.
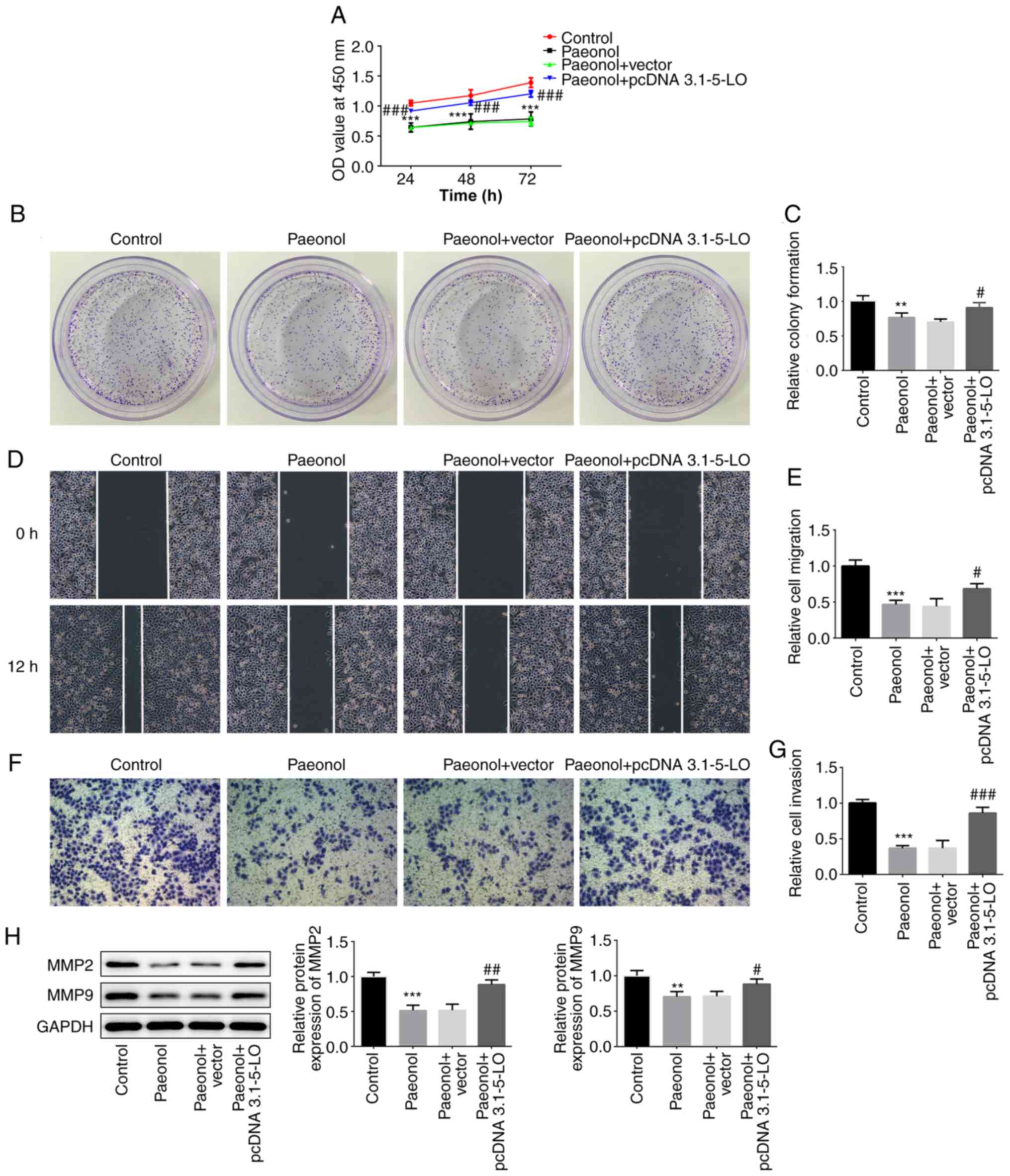 | Figure 4.5-LO inhibits Pae from exerting the
anti-migratory and anti-invasive effects. (A) The cell viability at
24, 48 and 72 h was estimated via Cell Counting Kit-8 assay in HeLa
cells divided into control, Pae, Pae+vector and Pae+pcDNA 3.1–5-LO
groups. (B and C) Colony-formation assay was performed to determine
the colony-forming capacity of HeLa cells in different groups. (D
and E) Wound-healing assay was conducted to evaluate the migratory
capacity of HeLa cells (magnification, ×100). (F and G) Transwell
assay was performed to assess the invasive capacity of HeLa cells
(magnification, ×100). (H) The protein levels of MMP-2, MMP-9 in
HeLa cells were detected by western blotting. **P<0.01,
***P<0.01 vs. control group. #P<0.05,
##P<0.01, ###P<0.001 vs. Pae+vector
group. 5-LO, 5-lipoxygease; MMP, matrix metalloprotease. |
5-LO is required for the pro-apoptotic
effect of Pae
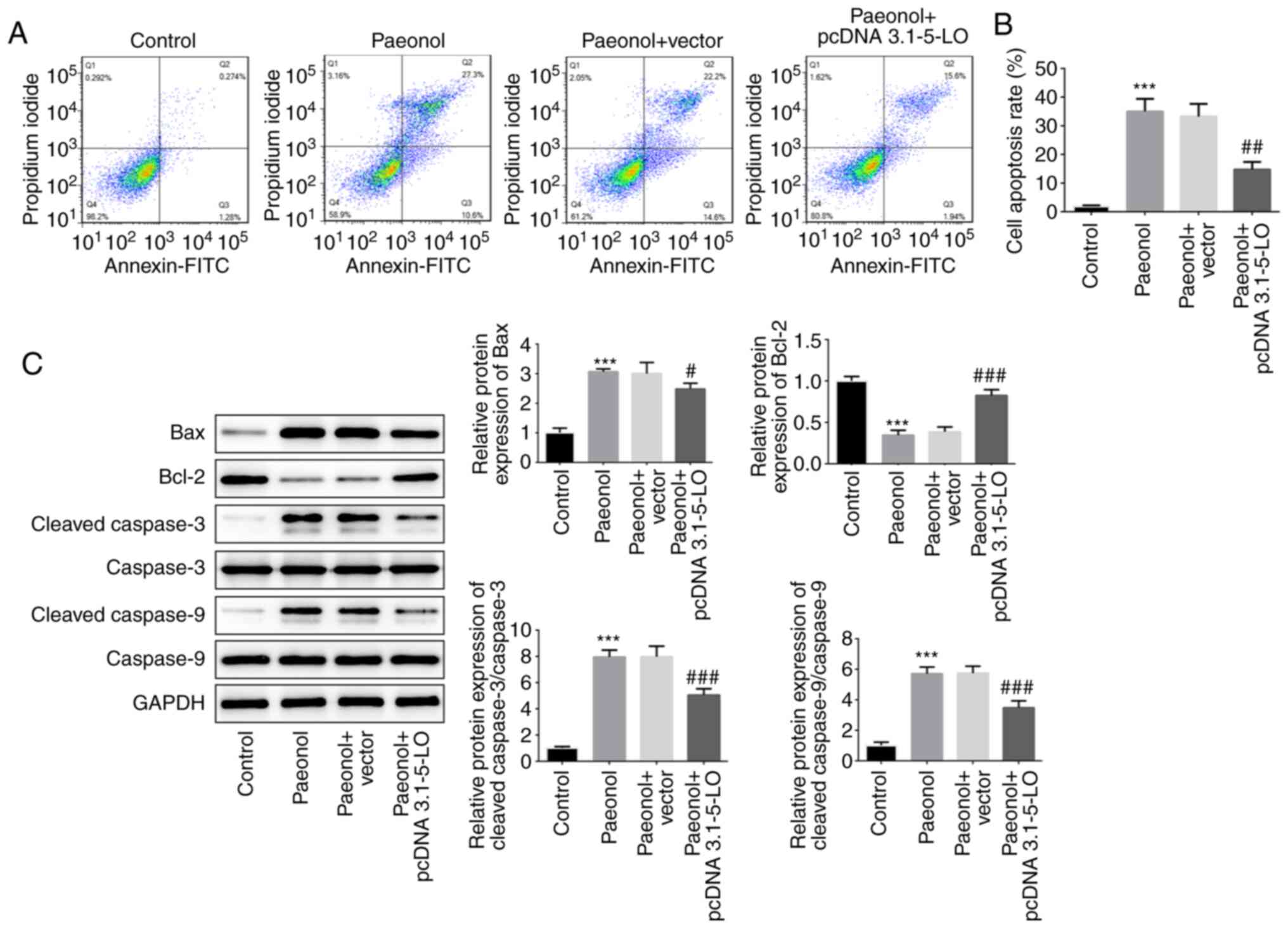
The pro-apoptotic effects of Pae on HeLa cells were
investigated following transfection of the cells with pcDNA
3.1–5-LO. The Pae group indicated enhanced apoptotic rate compared
with that of the control group, whereas the apoptotic rate of the
Pae+pcDNA 3.1–5-LO group was alleviated compared with that of the
Pae+vector group, indicating that overexpression of 5-LO
drastically decreased the pro-apoptotic effects of Pae (Fig. 5A and B). Subsequently, the protein
levels of Bcl-2, Bax, cleaved caspase-3 and cleaved caspase-9 were
estimated. The results indicated that the levels of Bax, cleaved
caspase-3 and cleaved caspase-9 were increased following treatment
of the cells with Pae, while overexpression of 5-LO reversed the
effects of Pae (Fig. 5C). These
results suggested that Pae promoted the induction of cell apoptosis
by regulating 5-LO.
Discussion
As a common malignant tumor of the female
reproductive system (15), cervical
cancer is the third most diagnosed cancer type with a considerably
high frequency worldwide (16). Due
to the advanced healthcare systems, the incidence of cervical
cancer has decreased over the past decade in developed countries
(17). However, it is still high in
developing countries (17). Despite
the unclear pathogenesis of cervical cancer, it has been widely
accepted that HPV infection, due to sexual intercourse, is closely
associated with this disease. Recently a high mortality has been
noted among women under the age of 30 due to cervical cancer
(18). Therefore, the
identification of the mechanism underlying the occurrence and
development of cervical cancer is of considerable importance.
Pae is a phenolic compound isolated from Paeonia
suffruticosa that exerts numerous pharmacological effects
(19), including anti-oxidation and
anti-inflammation (20), which may
benefit the recovery from diseases such as gastric ulcer (21), myocardial infarction (22) and cancer (23). A previous study demonstrated that
Pae plays a protective role against acute lung injury in an
endotoxic rat model by downregulating the expression levels of the
pro-inflammatory cytokine HMGB1 (24), a highly conserved non-histone
DNA-binding protein in the nucleus (25). However, studies that have
investigated the anti-inflammatory effects of Pae and its
interaction with a certain disease are limited. Moreover, a limited
number of studies exist on the effects of traditional Chinese
medicine Pae on the progression of cervical cancer. Therefore, a
series of experiments were conducted to dissect the mechanism
underlying the anticancer effects of Pae with regard to the
inhibition of cell proliferation, migration and invasion of HeLa
cells.
It has been previously shown that Pae regulates the
expression levels of proliferation-associated proteins in order to
exert its anti-metastasis activities (26). The present study demonstrated that
Pae exerted potent anticancer effects by regulating the
proliferation, migration and invasion of HeLa cells. Previous
studies have reported that 5-LO is implicated in the pathogenesis
of certain cancer types. Bai et al (27) reported that 5-LO expression was
associated with poor prognosis in esophageal squamous cell
carcinoma (ESCC), whereas its inhibition decreased the viability
and migration of ESCC cells. Moreover, 5-LO promoted the invasion
of papillary thyroid carcinoma cells by inducing the expression of
MMP-9 (28). It was found that 5-LO
was highly expressed in HeLa cells and that Pae could significantly
decrease its levels. Given the important role of 5-LO in cell
viability, migration and invasion, it was hypothesized that Pae
could hinder the proliferation and migration of cervical cancer
cells and activate the apoptotic cascade by downregulating the
expression levels of 5-LO. In the present study, the elevated
expression levels of 5-LO in HeLa cells were decreased following
treatment of the cells with Pae. Additional investigations
indicated that overexpression of 5-LO recovered the effects noted
on proliferation, invasion and migration of Pae-treated HeLa cells
to a certain extent.
In summary, the data demonstrated that Pae played an
inhibitory role on the proliferation, invasion and migration of
HeLa cells, while inducing apoptosis by regulating the expression
of 5-LO. The current study may offer new insight and provide novel
targets for the treatment of cervical cancer.
Acknowledgements
Not applicable.
Funding
The study was supported by Medical Health Science
and Technology Project of Zhejiang Province (grant no. 2019KY477)
and Key project of Traditional Medical Science and Technology of
Zhejiang Province (grant no. 2018ZZ013).
Availability of data and materials
All data generated or analyzed during this study are
included in the present manuscript.
Authors' contributions
SQS and LYY acquired the data and confirmed the
authenticity of all the raw data. XWZ and HYP contributed to
analysis and interpretation of data. SQS, FYH and JLL contributed
to the design of the study and drafted the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wei J and Wang Y, Shi K and Wang Y:
Identification of core prognosis-related candidate genes in
cervical cancer via integrated bioinformatical analysis. Biomed Res
Int. 2020:89592102020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei H, Wang XW, Chen KM, Ling SR and Yi
CJ: Analysis of gene mutation associated with tyrosine kinase
inhibitor sensitivity of epidermal growth factor receptor in
cervical cancer patients. Eur Rev Med Pharmacol Sci. 22:6280–6287.
2018.PubMed/NCBI
|
|
3
|
Cheng CS, Chen JX, Tang J, Geng YW, Zheng
L, Lv LL, Chen LY and Chen Z: Paeonol inhibits pancreatic cancer
cell migration and invasion through the inhibition of TGF-β1/smad
signaling and epithelial-mesenchymal-transition. Cancer Manag Res.
12:641–651. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mohamed MZ, Morsy MA, Mohamed HH and Hafez
HM: Paeonol protects against testicular ischaemia-reperfusion
injury in rats through inhibition of oxidative stress and
inflammation. Andrologia. 52:e135992020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao L, Wang Z, Lu D, Huang J, Liu J and
Hong L: Paeonol induces cytoprotective autophagy via blocking the
Akt/mTOR pathway in ovarian cancer cells. Cell Death Dis.
10:6092019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou J, Liu Q, Qian R, Liu S, Hu W and Liu
Z: Paeonol antagonizes oncogenesis of osteosarcoma by inhibiting
the function of TLR4/MAPK/NF-κB pathway. Acta histochemica.
122:1514552020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu J, Yu L, Luo J, Huo R and Zhu B:
Paeonol induces the apoptosis of the SGC7901 gastric cancer cell
line by downregulating ERBB2 and inhibiting the NF-κB signaling
pathway. Int J Mol Med. 42:1473–1483. 2018.PubMed/NCBI
|
|
8
|
Saahene RO, Wang J, Wang ML, Agbo E and
Pang D: The antitumor mechanism of paeonol on CXCL4/CXCR3-B signals
in breast cancer through induction of tumor cell apoptosis. Cancer
Biother Radiopharm. 33:233–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moore GY and Pidgeon GP: Cross-talk
between cancer cells and the tumour microenvironment: The role of
the 5-Lipoxygenase pathway. Int J Mol Sci. 18:2362017. View Article : Google Scholar
|
|
10
|
Mahboubi-Rabbani M and Zarghi A:
Lipoxygenase inhibitors as cancer chemopreventives: Discovery,
recent developments, and future perspectives. Curr Med Chem. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Monga J, Subramani D, Bharathan A and
Ghosh J: Pharmacological and genetic targeting of 5-lipoxygenase
interrupts c-Myc oncogenic signaling and kills
enzalutamide-resistant prostate cancer cells via apoptosis. Sci
Rep. 10:66492020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Costa H, Touma J, Davoudi B, Benard M,
Sauer T, Geisler J, Vetvik K, Rahbar A and Soderberg-Naucler C:
Human cytomegalovirus infection is correlated with enhanced
cyclooxygenase-2 and 5-lipoxygenase protein expression in breast
cancer. J Cancer Res Clin Oncol. 145:2083–2095. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Go JH, Wei JD, Park JI, Ahn KS and Kim JH:
Wogonin suppresses the LPSenhanced invasiveness of MDAMB231 breast
cancer cells by inhibiting the 5LO/BLT2 cascade. Int J Mol Med.
42:1899–1908, 20180. PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee YY, Choi CH, Sung CO, Do IG, Huh S,
Song T, Kim MK, Kim HJ, Kim TJ, Lee JW, et al: Prognostic value of
pre-treatment circulating monocyte count in patients with cervical
cancer: comparison with SCC-Ag level. Gynecol Oncol. 124:92–97.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aishanjiang A, Rouzi N, Jiao Z, Wang L,
Wusainahong K, Wumanjiang N, Musha M and Niyazi M: MicroRNA-9
enhances invasion and migration of cervical carcinomas by directly
targeting FOXO1. Eur Rev Med Pharmacol Sci. 22:2253–2260.
2018.PubMed/NCBI
|
|
17
|
Banno K, Iida M, Yanokura M, Kisu I, Iwata
T, Tominaga E, Tanaka K and Aoki D: MicroRNA in cervical cancer:
OncomiRs and tumor suppressor miRs in diagnosis and treatment.
ScientificWorldJournal. 2014:1780752014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meng X, Chu Y, Pan Y, Han L, Meng Z and
Wang X: Preoperative neoadjuvant chemotherapy combined with radical
surgery in cervical cancer. J BUON. 25:125–131. 2020.PubMed/NCBI
|
|
19
|
Al-Taher AY, Morsy MA, Rifaai RA, Zenhom
NM and Abdel-Gaber SA: Paeonol attenuates methotrexate-induced
cardiac toxicity in rats by inhibiting oxidative stress and
suppressing TLR4-Induced NF-κB inflammatory pathway. Mediators
Inflamm. 2020:86410262020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin X, Wang J, Xia ZM, Shang CH, Chao QL,
Liu YR, Fan HY, Chen DQ, Qiu F and Zhao F: Anti-inflammatory and
anti-oxidative activities of paeonol and its metabolites through
blocking MAPK/ERK/p38 signaling pathway. Inflammation. 39:434–446.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hafez HM, Morsy MA, Mohamed MZ and Zenhom
NM: Mechanisms underlying gastroprotective effect of paeonol
against indomethacin-induced ulcer in rats. Hum Exp Toxicol.
38:510–518. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Song F, Duan LR, Sheng JJ, Xie YH,
Yang Q, Chen Y, Dong QQ, Zhang BL and Wang SW: Paeonol and
danshensu combination attenuates apoptosis in myocardial infarcted
rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and
PI3K/Akt pathway. Sci Rep. 6:236932016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng CS, Chen JX, Tang J, Geng YW, Zheng
L, Lv LL, Chen LY and Chen Z: Paeonol inhibits pancreatic cancer
cell migration and invasion through the inhibition of
TGF-beta1/smad signaling and epithelial-mesenchymal-transition.
Cancer Manag Res. 12:641–651. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Xu Q, Mei L, Lei H, Wen Q, Miao J,
Huang H, Chen D, Du S, Zhang S, et al: Paeonol attenuates acute
lung injury by inhibiting HMGB1 in lipopolysaccharide-induced shock
rats. Int Immunopharmacol. 61:169–177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mei L, He M, Zhang C, Miao J, Wen Q, Liu
X, Xu Q, Ye S, Ye P, Huang H, et al: Paeonol attenuates
inflammation by targeting HMGB1 through upregulating miR-339-5p.
Sci Rep. 9:193702019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lyu ZK, Li CL, Jin Y, Liu YZ, Zhang X,
Zhang F, Ning LN, Liang ES, Ma M, Gao W, et al: Paeonol exerts
potential activities to inhibit the growth, migration and invasion
of human gastric cancer BGC823 cells via downregulating MMP2 and
MMP9. Mol Med Rep. 16:7513–7519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bai CY, Zhang JY, Shi TW, Bai YQ, Wu BL,
Du ZP, Wu ZY, Xu XE, Wang SH, Wu JY, et al: Association between
5-lipoxygenase expression, and malignant behaviors and poor
prognosis in esophageal squamous cell carcinoma. Oncol Lett.
15:9353–9360. 2018.PubMed/NCBI
|
|
28
|
Kummer NT, Nowicki TS, Azzi JP, Reyes I,
Iacob C, Xie S, Swati I, Darzynkiewicz Z, Gotlinger KH, Suslina N,
et al: Arachidonate 5 lipoxygenase expression in papillary thyroid
carcinoma promotes invasion via MMP-9 induction. J Cell Biochem.
113:1998–2008. 2012. View Article : Google Scholar : PubMed/NCBI
|