Introduction
Heart failure (HF) remains a globally epidemic
cardiac disease (1,2). HF is preceded by left ventricular (LV)
remodeling, such as enlarged myocardial size or increased LV mass
due to pressure-overload (3).
Myocardial infarction (MI), the coronary artery disease, can
increase the risk of HF and is even life-threatening (4).
LV remodeling is characterized by increased cardiac
interstitial fibrosis resulting from the accumulation of collagen
type I, collagen type III, matrix metalloproteinase (MMP) 2, MMP9,
TGF-β and α-smooth muscle actin (α-SMA) (5–7).
Cardiac fibrosis is common in many cardiovascular diseases, such as
myocardial infarction (8),
hypertension (9) and cardiomyopathy
(10) and is critical to the
evolution of structural LV remodeling and the development of HF.
Currently, the mechanisms of cardiac fibrosis in HF remains to be
elucidated.
Tanshinone IIA (TIIA) is a natural compound
extracted from the roots of Salvia miltiorrhiza Bunge and has been
used in traditional Chinese medicine to protect against organ
injuries (11). Studies have
demonstrated that TIIA exhibits antioxidative, anti-cancer and
anti-inflammatory effects (12–14).
TIIA is also been used to treat cardiovascular diseases (15) because it can increase coronary blood
flow and ameliorates myocardial metabolic disorder induced by
hypoxia (16). Furthermore, TIIA
treatment can reduce the infarction and increase myocardial
regeneration and contractility (16). However, the effects of TIIA on HF
remain to be elucidated.
Oxidative stress serves important roles in
pathological cardiac remodeling and cardiac failure (17,18).
Coronary ligation can result in dilatation of left ventricle with
compensatory hypertrophy of the remaining LV myocardium and the
right ventricle (19). This
remodeling is associated in LV non-infarcted myocardium with
increased oxidative stress and collagen infiltration (19). TIIA can inhibit hydrogen
peroxide-induced cardiomyocyte apoptosis (20). However, whether TIIA administration
can reverse cardiac fibrosis in MI-induced HF rats via inhibiting
oxidative stress is not known. In addition, it is not clear whether
NADPH oxidase (Nox) 4 is involved in the oxidative stress in the
fibrosis of cardiac fibroblasts (CFs) induced by Ang II. These
questions were addressed by the present study.
Materials and methods
Animals
Experiments were carried out on male Sprague-Dawley
(SD) rats (age, 5–6 weeks; weight, 180–200 g; Vital River
Biological Co., Ltd, Beijing, China). A total of 80 rats were used
in the present study. All procedures were approved by the
Experimental Animal Care and Use Committee of Nanjing University of
Chinese Medicine (approval no. 17064512), and were conducted in
accordance with the Guide for the Care and Use of Laboratory
Animals (National Institutes of Health publication no. 85–23,
revised in 1996; pubmed.ncbi.nlm.nih.gov/25121211/). The rats were
kept in a temperature (22±1°C) and humidity (30–60%)-controlled
room on a 12-h light/dark cycle with free access to standard chow
and tap water. The rats were sacrificed using an overdose of sodium
pentobarbital (100 mg/kg intravenously) for sample collection.
Death was confirmed by the absence of heartbeat, corneal reflexes
and paw withdrawal response to a noxious pinch.
Myocardial infarction model
The myocardial infarction (MI) in rats was induced
by coronary artery ligation with sterile techniques as previously
reported (21). Briefly, the rats
were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and
randomly subjected to the ligation of the left anterior descending
(LAD) coronary artery or sham-operation. The heart was exposed
through a left intercostal thoracotomy and the left coronary artery
was looped by a single nylon suture (7–0). The LAD was ligated under 1–1.5 mm
from the left atrial appendage in rats. The heart was then quickly
repositioned into the chest. The sham rats were treated in the same
way as the coronary ligation rats except that their coronary
arteries were not ligated. After 24 h, the rats were randomly
assigned to four groups: i) Sham + saline group, ii) sham + TIIA
group, iii) MI + saline group and iv) MI + TIIA group (n=8 for each
group). TIIA (1.5 mg/kg/d/500 µl, Sigma-Aldrich; Merck KGaA)
(22) was orally gavaged to the
rats in sham + TIIA and MI + TIIA groups for 28 days, while the
rats in the other two groups received the same volume of saline.
The rats were euthanized using an overdose of sodium pentobarbital
(100 mg/kg intravenously) if malaise or surgical site putrescence
presented.
Echocardiography
Transthoracic echocardiography was performed using
an ultrasound system (VisualSonics Inc.) with a 21-MHz probe under
isoflurane anesthesia (2.5%) to measure the LV end-systolic
diameter (LVESD), LV end-diastolic diameter (LVEDD), LV volumes in
diastole (LVVd) and LV volumes in systole (LVVs). The LV ejection
fraction (EF) and fractional shortening (FS) were calculated as EF
= (LVVd-LVVd)/LVVd ×100 and FS = (LVEDD-LVESD)/LVEDD ×100. The
measurements over three consecutive cardiac cycles were
averaged.
Hemodynamic monitoring
Rats were anesthetized with sodium pentobarbital (50
mg/kg, i.p.). A conductance micromanometer catheter (1.4F, Millar
Instruments, Inc.) was inserted into the LV chamber via the left
carotid artery across the aortic valve. The left ventricular
systolic pressure (LVSP) and LV end-diastolic pressure (LVEDP),
maximum of the first differentiation of LV pressure (LV + dp/dt)
and decline (LV - dp/dt) were obtained with a PowerLab data
acquisition system (ADInstruments Pty Ltd.).
Masson trichrome staining
The rats were sacrificed with an overdose of sodium
pentobarbital (100 mg/kg, i.p.) and the hearts were harvested.
Serial dehydration was performed using an ethanol concentration
gradient, followed by paraffin embedding. Sections of the heart (5
µm) were examined by Masson's trichrome staining according to the
manufacturer's instructions (Wuhan Servicebio Technology Co., Ltd.)
to determine the extent of fibrosis. Three to five random fields of
view were selected in three sections from each animal for
observation under a light microscope (Zeiss AG). Captured images
were analyzed using Image-Pro Plus software 6.0 (Media Cybernetics,
Inc.).
Culture of CFs
Rat CFs were isolated from 1–3 day-old SD rats
(Beijing Vital River Laboratory Animal Technology Co., Ltd.). A
total of 60 cubs were used in the present study. The hearts were
collected following the pups anesthesia under isoflurane (3.5%).
Briefly, CFs were separated from cardiomyocytes by gravity
separation and grown to confluence on 10 cm cell culture dishes
with growth media [DMEM (Gibco; Thermo Fisher Scientific, Inc.]
including 10% FBS, 1% penicillin and 1% streptomycin) at 37°C in
humid air with 5% CO2 and 95% O2. The CFs
from the second passage were incubated with Ang II (10-6 M,
Sigma-Aldrich; Merck KGaA) for 24 h to induce the fibrotic
phenotype. The CFs were then assigned to four groups: i) PBS group,
ii) TIIA (10 µM) group, iii) Ang II group and iv) Ang II + TIIA (10
µM) group.
Reverse transcription-quantitative
PCR
The total RNA in LV or CFs (1×106 cells/ml) was
extracted with TRIzol® (Thermo Fisher Scientific, Inc.).
cDNA was extracted from RNA with reverse transcription using 10 µl
random primers according to the instructions of the PrimeScript™ RT
Master Mix (Takara Biotechnology Co., Ltd.) and stored at −70°C
before use. Collagen I, collagen III, α-smooth muscle actin (SMA),
TGF-β, matrix metalloproteinase (MMP) 2 and MMP9 mRNA levels were
determined with Synergy Brands Green I fluorescence (Applied
Biosystems) in accordance with the manufacturer's protocols. All
samples were amplified in triplicates for 45 cycles in a 384-well
plate (95°C for 15 sec, then 60°C for 1 min). The relative gene
expression was determined by calculating the values of ΔCq as a
relative quantity to the endogenous control (23). These experiments were replicated
three times. The primers sequences are listed in Table I.
 | Table I.List primers used for reverse
transcription-quantitative PCR. |
Table I.
List primers used for reverse
transcription-quantitative PCR.
| Gene | Species | Forward primer,
5′-3′ | Reverse primer,
5′-3′ |
|---|
| Collagen I | Rat |
TCAAGATGGTGGCCGTTAC |
CTGCGGATGTTCTCAATCTG |
| Collagen III | Rat |
CGAGATTAAAGCAAGAGGAA |
GAGGCTTCTTTACATACCAC |
| TGF-β | Rat |
CAGGGAGTAAGGGACACGA |
ACAGCAGTTAGGAACCCAGAT |
| α-SMA | Rat |
GTCCCAGACATCAGGGAGTAA |
TCGGATACTTCAGCGTCAGGA |
| MMP2 | Rat |
CCCCATGTGTCTTCCCCTTC |
AGCTCCTGGATCCCCTTGAT |
| MMP9 | Rat |
AGGGCCCCTTTCTTATTGCC |
CGAGTAACGCTCTGGGGATC |
| GAPDH | Rat |
GGCACAGTCAAGGCTGAGAATG |
ATGGTGGTGAAGACGCCAGTA |
Western blotting
CF samples were lysed in modified RIPA buffer
(BioChannel Biological Technology Co., Ltd.). The protein
concentration was determined using a BCA assay (Beyotime Institute
of Biotechnology). A total of 30–50 µg of protein was separated
using SDS-PAGE on 8% gels, then transferred to a PVDF membrane. The
membrane was blocked with 5% skimmed milk powder at room
temperature for 1 h and probed overnight at 4°C with primary
antibodies against collagen I (1:1,000; cat. no. ab254113; Abcam),
collagen III (1:5,000; cat. no. ab7778; Abcam), TGF-β (1:1,000;
cat. no. ab215715; Abcam), α-SMA(1:10,000; cat. no. ab124964;
Abcam), MMP2 (1:2,000; cat. no. ab92536; Abcam) and MMP9 (1:1,000;
cat. no. ab228402; Abcam) primary antibodies (Abcam), followed by
incubation with a HRP-conjugated goat anti-rabbit secondary
antibody (1:10,000, cat. no. ab7090; Abcam) at 37°C for 1 h. The
bands were visualized using the enhanced chemiluminescence (ECL)
substrate (BioChannel Biological Technology Co., Ltd.). The total
AT1R protein level was normalized to the protein level of GAPDH
(Bioworld Technology Inc.). Images were analyzed using Image-Pro
Plus software (version 6.0; XRayScan; CAD/CAM Services, Inc.).
Nox4 overexpression
Recombinant adenoviral vectors harboring green
fluorescent protein (Ad-GFP) and Nox4 (Ad-Nox4) were constructed
and packaged by Shanghai GeneChem Co., Ltd. In the in vivo
experiment, adenovirus (200 µl/rat, 1×1012 plaque-forming units/ml)
was injected into the rats via the tail vein. In the in
vitro experiment, 20 µl original solution was diluted in 2 ml
Enhance Infection Solution (Shanghai GeneChem Co., Ltd.). The CFs
were transfected with serum-free medium containing Ad-Nox4 or
Ad-GFP at 37°C for 24 h.
Superoxide dismutase (SOD) activity
level
The rats were sacrificed with an overdose of
pentobarbital (100 mg/kg, i.p.). The LV samples were collected and
homogenized in lysis buffer (Thermo Fisher Scientific, Inc.). SOD
was measured using a commercial kit (Nanjing Jiancheng
Bioengineering Institute; cat. no. A001-3-2) according to the
manufacturer's instructions using a microplate reader (BioTek
Instruments, Inc.).
Malondialdehyde level in the
heart
After homogenizing the LV samples, malondialdehyde
(MDA) levels in the LV were determined using an ELISA kit (Wuhan
USCN Business Co., Ltd.; cat. no. CEA597Ge) following the
manufacturer's instructions.
Measurement of Nox activity
The Nox activity in the heart was measured by
enhanced lucigenin chemiluminescence. Briefly, NADPH (100 µM) was
added to the media as a substrate to react with Nox and generate
superoxide anions. The light emission produced by the reaction of
lucigenin (5 µM) with superoxide anions was measured with a
microplate reader (BioTek Instruments, Inc.) once every minute for
10 min. The values representing Nox activity were expressed as the
mean light units (MLU) per min per mg of protein.
Measurement of superoxide anions
The level of superoxide anions in the heart was
determined by lucigenin-derived chemiluminescence. Briefly, the
reaction with superoxide anions was started by adding dark-adapted
lucigenin (5 µM) to each sample to cause photon emission, which was
measured with a microplate reader (BioTek Instruments, Inc.) once
every minute for 10 min. The values representing the superoxide
anions level were expressed as the MLU per min per mg of
protein.
Statistical analysis
Data are presented as the mean ± standard error of
the mean and were analyzed using GraphPad Prism 7.0 (GraphPad
software Inc.). Statistics were completed using one-way ANOVA,
followed by Bonferroni test for post hoc analysis when multiple
comparisons were made. P<0.05 was considered to indicate a
statistically significant difference.
Results
TIIA improves cardiac function of rats
with MI-induced HF
EF, FS, LVSP and LV ±dp/dtmax were
reduced in rats with MI-induced HF and this reduction was reversed
by TIIA treatment. LVVs, LVVd, LVESD, LVEDD and LVEDP were
increased in MI-induced HF rats and this increase was attenuated by
TIIA treatment (Fig. 1).
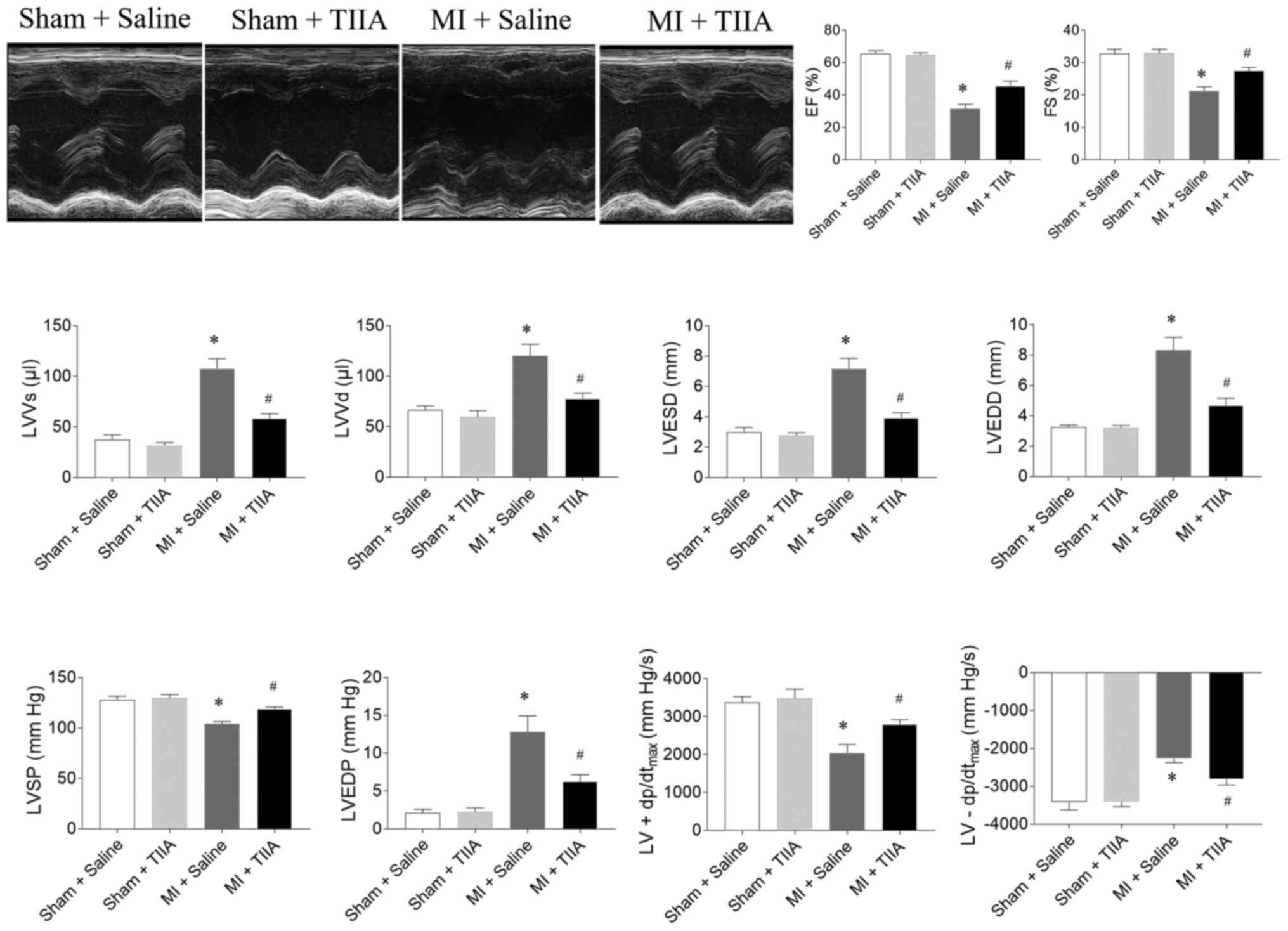 | Figure 1.TIIA improves cardiac dysfunction in
rats with MI-induced heart failure. TIIA treatment improved the
decreases of LV EF, FS, LVSP and the LV ± dp/dtmax and
the increases of LVVS, LVVD, LVESD and LVEDD in MI rats. The
results are expressed as mean ± standard error of the mean. n=8.
*P<0.05 vs. the Sham + Saline group; #P<0.05 vs. the MI +
Saline group. TIIA, tanshinone IIA; MI, myocardial infarction; LV,
left ventricular; EF, ejection fraction; FS, fractional shortening;
LVSP, LV systolic pressure; LV ± dp/dtmax, maximum of
the first differentiation of LV pressure; LVVS, LV volume in
systole; LVVD, LV volume in diastole; LVESD, LV end-systolic
diameter; LVEDD, LV end-diastolic diameter. |
TIIA attenuates LV fibrosis in rats
with MI-induced HF
The mRNA expression levels of collagen I, collagen
III, TGF-β, α-SMA, MMP2 and MMP9 in LV of MI rats were increased
compared with sham rats. The increased levels of collagen I,
collagen III, TGF-β, α-SMA, MMP2 and MMP9 in the LVs of MI rats
were inhibited after TIIA administration (Fig. 2).
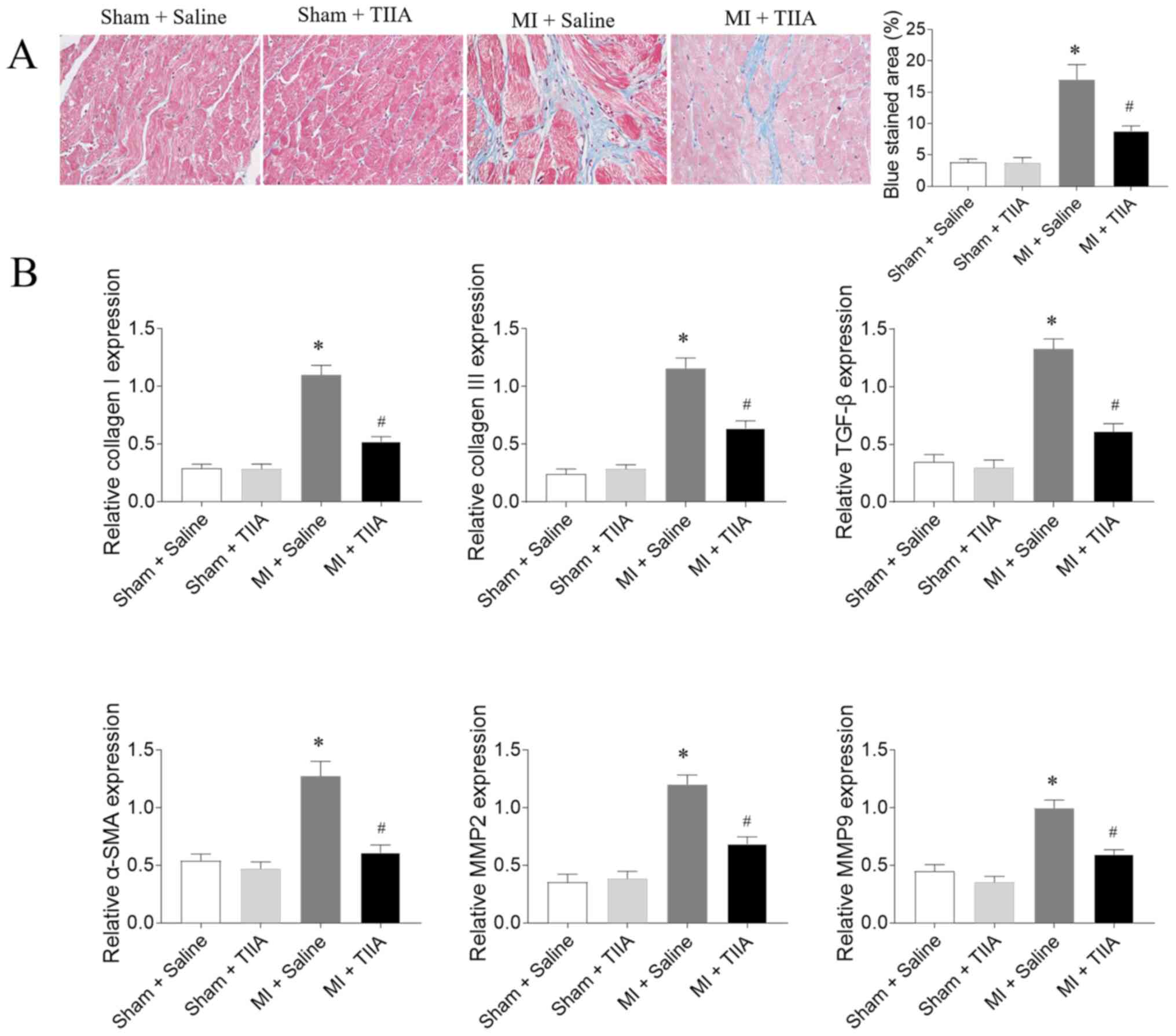 | Figure 2.TIIA attenuates LV fibrosis in rats
with MI-induced heart failure. (A) TIIA treatment attenuated LV
fibrosis in MI rats, the blue area represents the fibrosis.
Magnification, ×400. (B) TIIA treatment inhibited the increases of
collagen I, collagen III, SMA, TGF-β, MMP2 and MMP9 in MI rats. The
results are expressed as mean ± standard error of the mean. n=8.
*P<0.05 vs. the Sham + Saline group; #P<0.05 vs. the MI +
Saline group. TIIA, tanshinone IIA; LV, left ventricular; MI,
myocardial infarction; SMA, α-smooth muscle actin; MMP, matrix
metalloproteinase. |
TIIA attenuates Ang II-induced
fibrosis of CFs
The mRNA expression levels of collagen I, collagen
III, TGF-β, α-SMA, MMP2 and MMP9 increased in Ang II-treated CFs
and this increase was reversed after TIIA treatment (Fig. 3A). The Ang II-induced increases in
protein expression levels of collagen I, collagen III, TGF-β,
α-SMA, MMP2 and MMP9 in CFs were inhibited after TIIA treatment
(Fig. 3B).
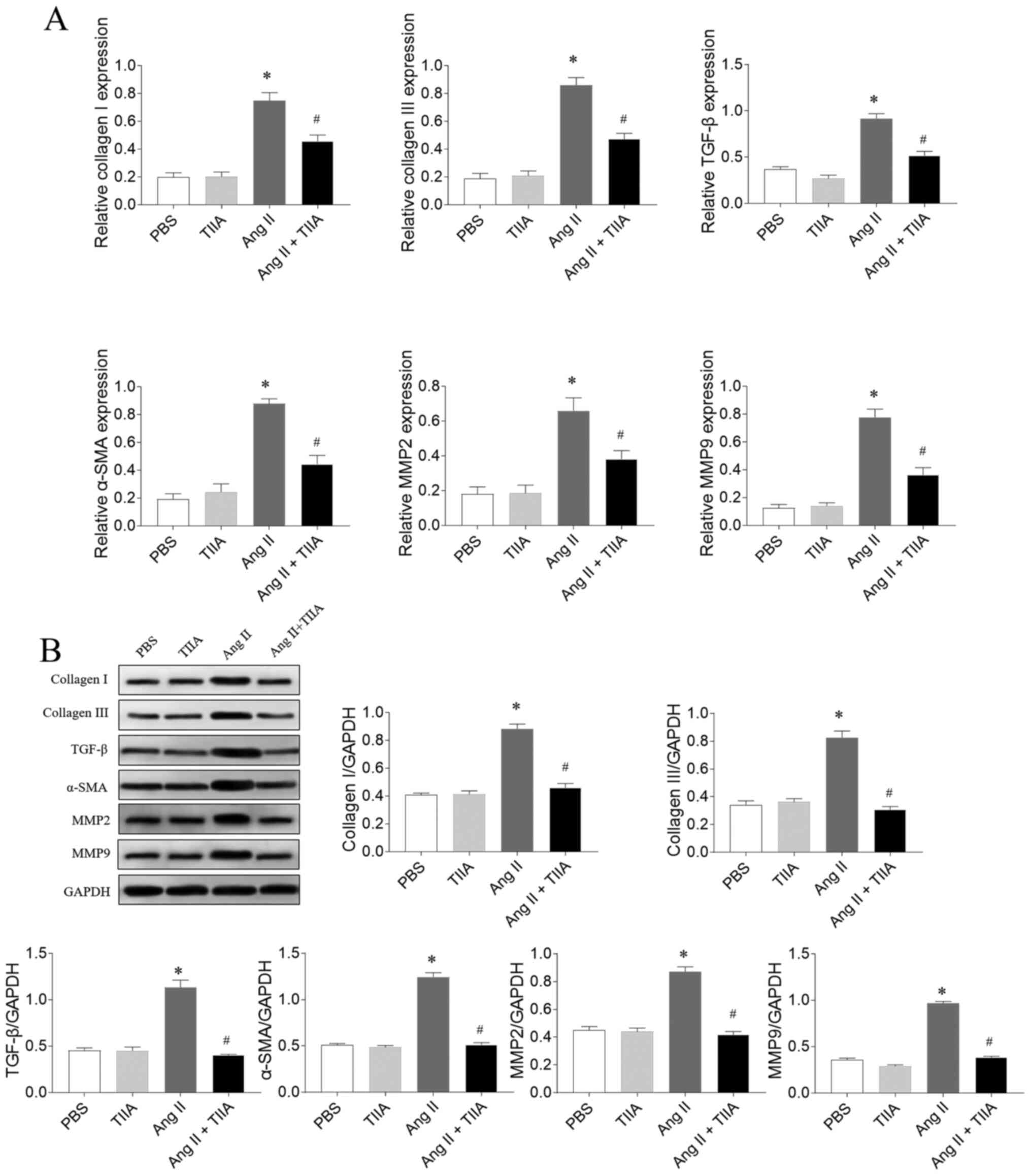 | Figure 3.TIIA attenuates CFs fibrosis induced
by Ang II. (A) TIIA treatment inhibited the increased mRNA
expression levels of collagen I, collagen III, SMA, TGF-β, MMP2 and
MMP9 in Ang II-treated CFs. (B) TIIA treatment inhibited the
increased protein expression of collagen I, collagen III, α-SMA,
TGF-β, MMP2 and MMP9 in Ang II-treated CFs. The results are
expressed as mean ± SE. *P<0.05 vs. the PBS group; #P<0.05
vs. the Ang II group. TIIA, tanshinone IIA; CFs, cardiac
fibroblasts; Ang II, angiotensin II; SMA, α-smooth muscle actin;
MMP, matrix metalloproteinase. |
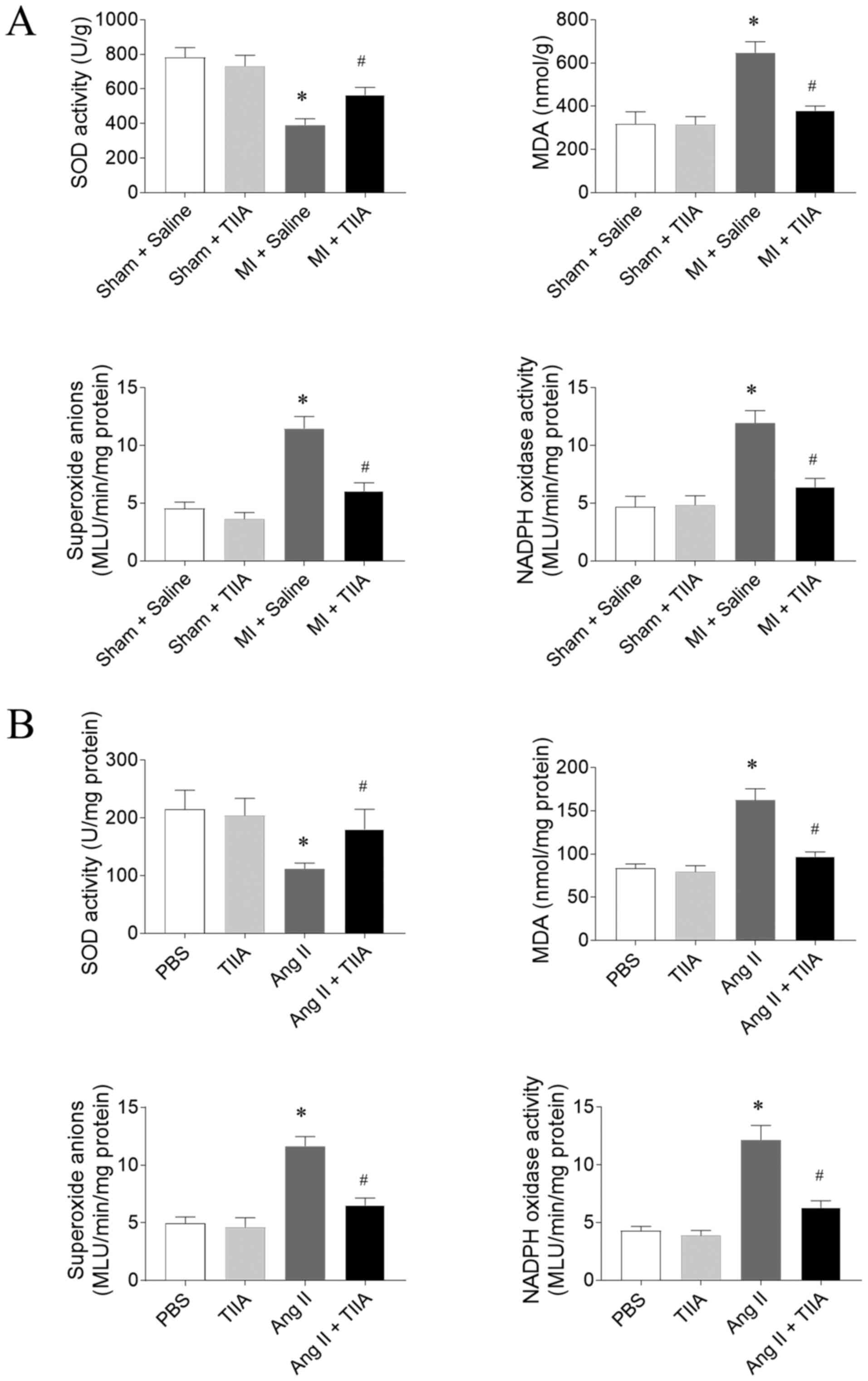
TIIA attenuates oxidative stress in
the LVs of HF rats and Ang II-treated CFs
SOD activity level was reduced in the LVs of
MI-induced HF rats and this reduction was reversed by TIIA. MDA,
superoxide anions and Nox activity levels were significantly
increased in LV of MI-induced HF rats and this increase was
inhibited after TIIA treatment (Fig.
4A). The decrease of SOD activity and the increases of MDA,
superoxide anions and Nox activity levels in Ang II-treated CFs
were reversed by TIIA administration (Fig. 4B).
Nox4 overexpression reverses the
improving effects of TIIA on cardiac function in HF rats
The expression level of Nox was increased in the
heart rats treated with Ad-Nox4 (Fig.
5A). The effects of TIIA in improving LV ± dp/dtmax,
EF, FS and LVSP in MI-induced HF rats were reversed by Nox4
overexpression. Moreover, Nox4 overexpression also reversed the
effects of TIIA in decreasing LVVs, LVVd, LVESD, LVEDD and LVEDP in
MI-induced HF rats (Fig. 5B and
C.).
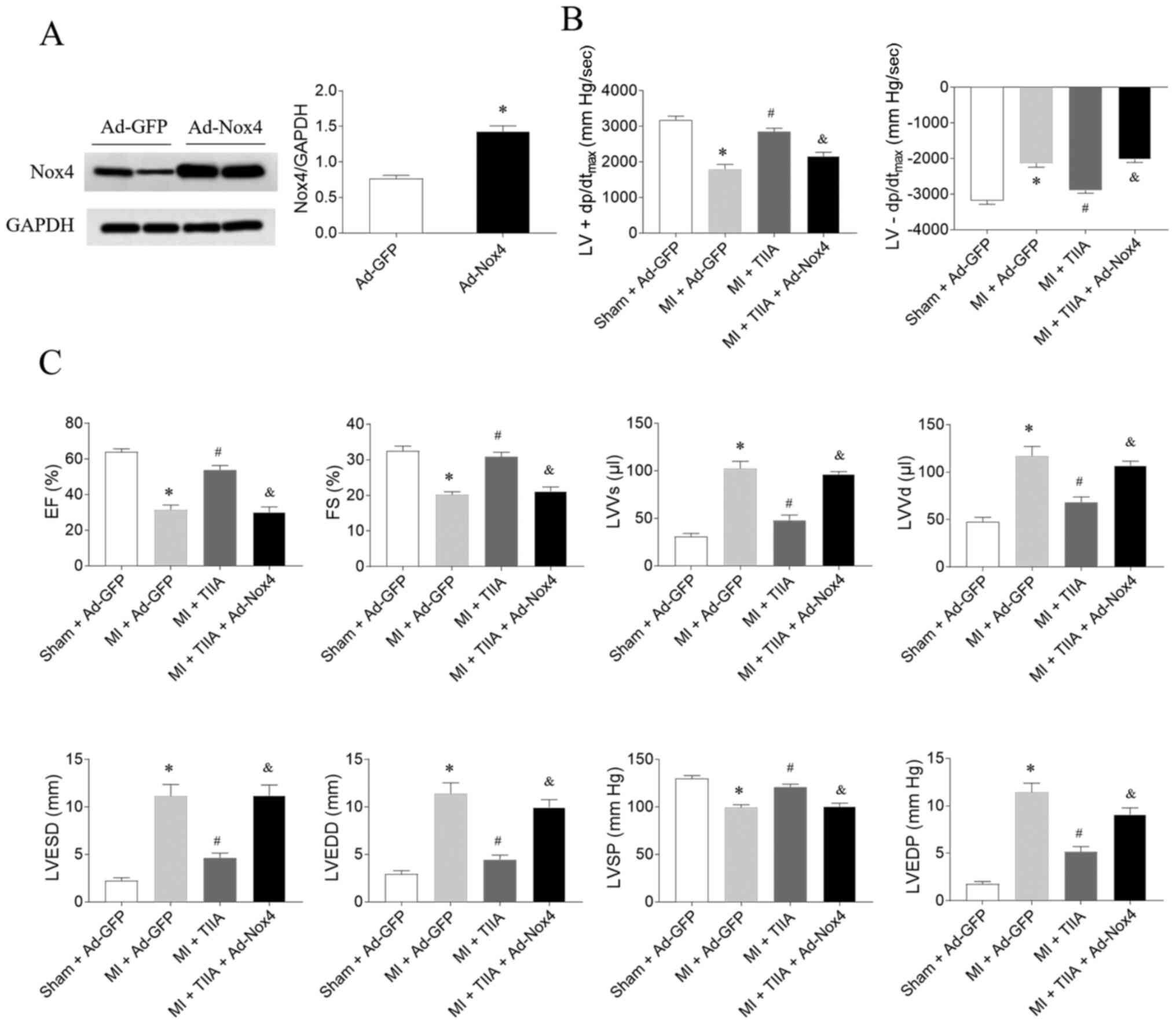 | Figure 5.Nox4 overexpression reverses the
effects of TIIA in improving cardiac dysfunction on rats with
MI-induced heart failure. (A) The expression level of Nox4 was
increased in the heart rats treated with adenoviral (Ad)-Nox4. (B)
Nox4 overexpression reversed the improving effects of TIIA on the
decreases of LV ± dp/dtmax in MI rats. (C) Nox4
overexpression reversed the improving effects of TIIA on the
decreases of LV EF, FS and LVSP and the increases of LVVS, LVVD,
LVESD and LVEDD in MI rats. The results are expressed as mean ±
standard error of the mean. n=8. *P<0.05 vs. the (A) Ad-GFP or
(B and C) Sham + Ad-GFP group; #P<0.05 vs. the MI + Ad-GFP
group; &P<0.05 vs. the MI + TIIA group. Nox, NADPH oxidase;
TIIA, tanshinone IIA; MI, myocardial infarction; LV ±
dp/dtmax, maximum of the first differentiation of LV
pressure; LV, left ventricular; EF, ejection fraction; FS,
fractional shortening; LVSP, LV systolic pressure; LVVS, LV volume
in systole; LVVD, LV volume in diastole; LVESD, LV end-systolic
diameter; LVEDD, LV end-diastolic diameter. |
Nox4 overexpression reverses the
inhibitory effects of TIIA on LV fibrosis in HF rats
Nox4 overexpression reversed the inhibiting effects
of TIIA on the increases in the mRNA expression levels of collagen
I, collagen III, TGF-β, α-SMA, MMP2 and MMP9 in the LVs of rats
with MI-induced HF (Fig. 6).
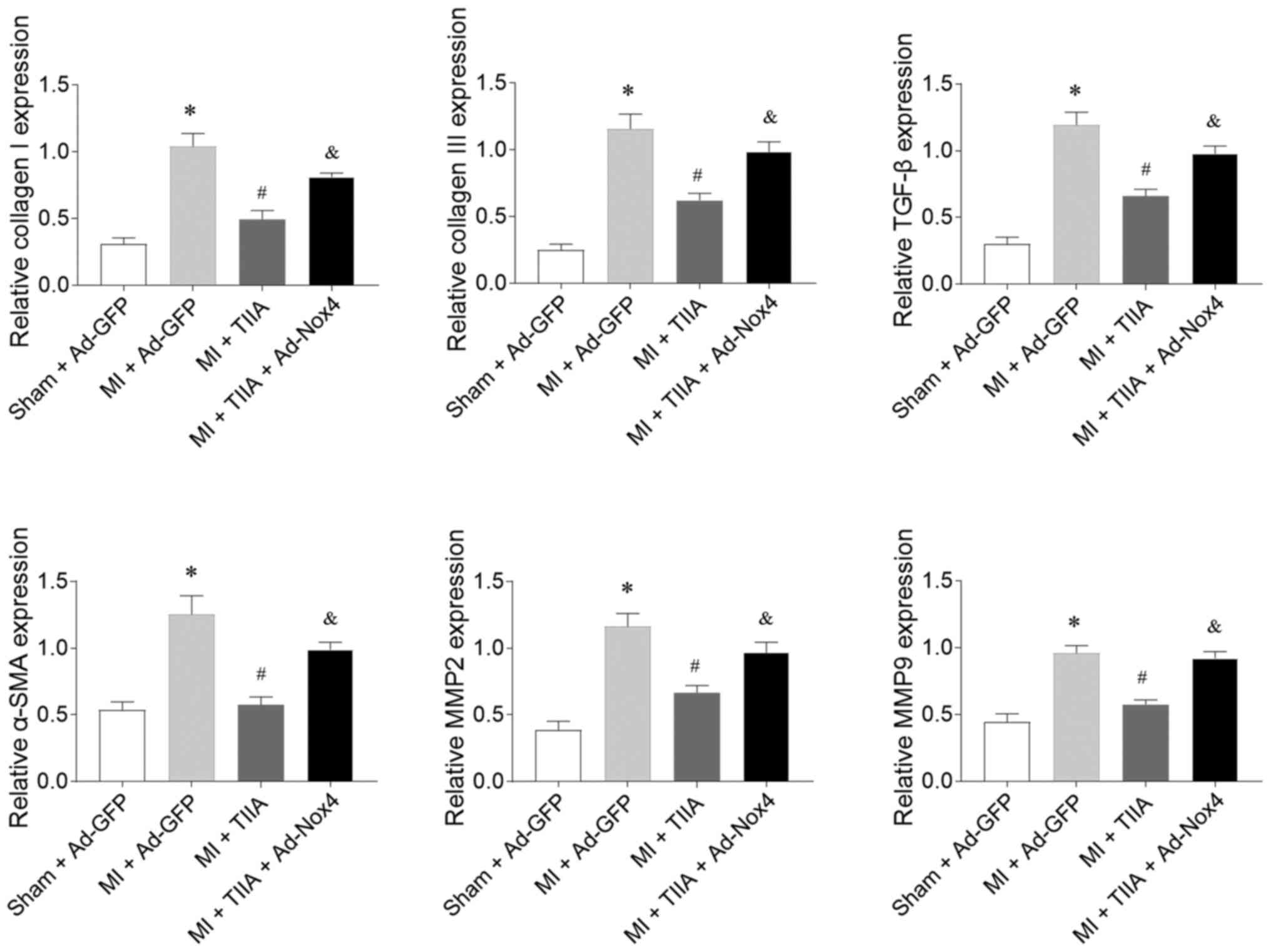 | Figure 6.Nox4 overexpression reverses the
effects of TIIA in inhibiting LV fibrosis in rats with MI-induced
heart failure. Nox4 overexpression reversed the effects of TIIA in
attenuating the increases of collagen I, collagen III, SMA, TGF-β,
MMP2 and MMP9 in the LV of MI rats. The results are expressed as
mean ± standard error of the mean. n=8. *P<0.05 vs. the Sham +
Ad-GFP group; #P<0.05 vs. the MI + Ad-GFP group; &P<0.05
vs. the MI + TIIA group. Nox, NADPH oxidase; TIIA, tanshinone IIA;
LV, left ventricular; MI, myocardial infarction; SMA, α-smooth
muscle actin; MMP, matrix metalloproteinase. |
Nox4 overexpression reverses the
inhibitory effects of TIIA on Ang II-induced CF fibrosis
Nox4 overexpression reversed the inhibitory effects
of TIIA on Ang II-induced increases in the mRNA expression levels
of collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9 in CFs
(Fig. 7).
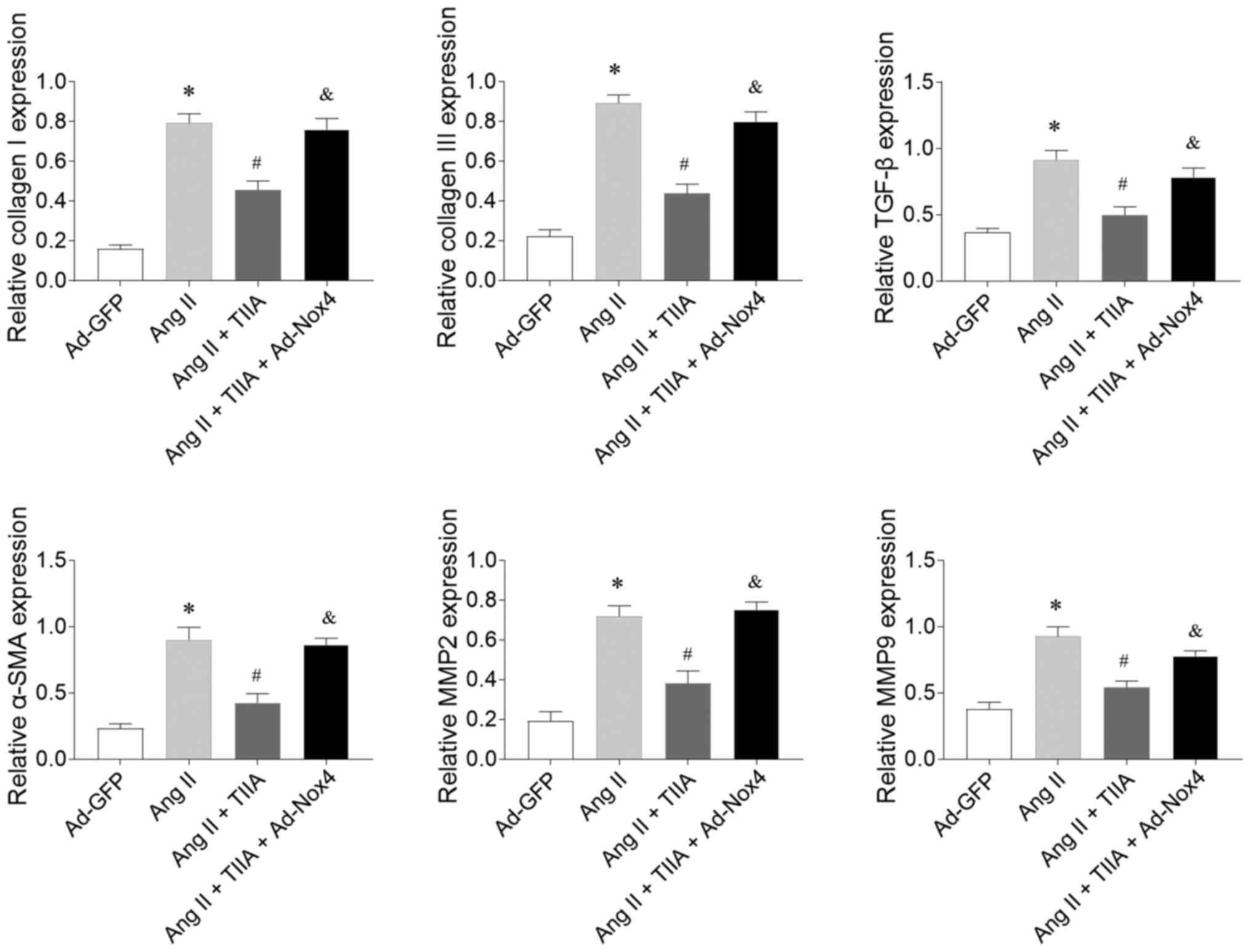 | Figure 7.Nox4 overexpression reversed the
inhibiting effects of TIIA on Ang II-induced fibrosis of CFs. Nox4
overexpression reversed the effects of TIIA in attenuating the
increases of collagen I, collagen III, SMA, TGF-β, MMP2 and MMP9 in
the Ang II-treated CFs. The results are expressed as mean ±
standard error of the mean. *P<0.05 vs. the Ad-GFP group;
#P<0.05 vs. the Ang II group; &P<0.05 vs. the Ang II +
TIIA group. Nox, NADPH oxidase; TIIA, tanshinone IIA; Ang II,
angiotensin II; CFs, cardiac fibroblasts; SMA, α-smooth muscle
actin; MMP, matrix metalloproteinase. |
Discussion
The present study found that TIIA improved cardiac
dysfunction in the rats with MI-induced HF. The fibrosis of LV in
HF rats and Ang II-treated CFs was ameliorated following TIIA
administration. Furthermore, oxidative stress was enhanced in LV of
HF rats and in Ang II-treated CFs, as indicated by the decrease of
SOD activity and the increases of MDA, superoxide anions and Nox
activity levels, all reversed by TIIA treatment. Nox4
overexpression inhibited the effects of TIIA in improving cardiac
dysfunction in HF rats and the fibrosis of LV in HF rats and Ang
II-treated CFs.
The results of the present study demonstrated that
TIIA treatment ameliorated the decreases of EF, FS, LVSP and LV ±
dp/dtmax and the increases of LVVs, LVVd, LVESD, LVEDD
and LVEDP in MI-induced HF rats, indicating that TIIA improved
cardiac dysfunction in HF. This finding is consistent with a
previous finding that TIIA could significantly improve heart
function in left anterior descending (LAD) ligation-induced HF
(22).
As a complex pathological process involving
myocardial fibrosis, cardiac hypertrophy and cardiomyocyte
apoptosis, LV remodeling is often caused by cardiovascular diseases
(CVDs) such as HF, hypertension, or myocardial infarction (24,25).
Cardiac fibrosis is a significant global health problem caused by
pathological stimuli to the heart. Previous studies demonstrated
that TIIA could inhibit the proliferation of mouse cardiac
fibroblasts in cultures by MTT assay (26) and attenuate high glucose-mediated
collagen synthesis through inhibiting the TGF-β1/Smad signaling
pathway in cardiac fibroblasts (27). The present study found that the
increases of collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9
in LV of MI-induced HF rats were abolished following TIIA
administration. In addition, TIIA treatment inhibited the increases
of collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9 in Ang
II-treated CFs. These results suggested that TIIA attenuated the
fibrosis of LV in HF.
As important molecules in the living organisms,
reactive oxygen species (ROS) are involved in many signaling
pathways (28). However, the
overproduction of ROS serves a significant role in the development
of CVDs (29,30). Oxidative stress, a key contributor
to organ damage, is associated with various diseases (31), including cardiac fibrosis (32). Sodium TIIA sulfonate inhibits the
increased production of ROS and expression of TGF-β1 stimulated by
Ang II in human atrial fibroblasts (33). TIIA significantly inhibits
H2O2-induced collagen synthesis via
attenuating Nox activity and ROS generation (34). Due to its antioxidative property,
TIIA protects two-kidney, two-clip hypertensive rats from cardiac
dysfunction and fibrosis, partially via reducing Nox activity, but
without changing blood pressure (35). In the present study, the results
demonstrated that SOD activity was significantly reduced and that
the MDA, superoxide anions and Nox activity levels were increased
in LV of HF rats, then decreased by TIIA treatment. Furthermore,
the decrease of SOD activity and the increases of MDA, superoxide
anions and Nox activity in Ang II-treated CFs were reversed by TIIA
administration. These results indicated that in the heart of
MI-induced HF rats, the oxidants and antioxidants were imbalanced,
which could be reversed by TIIA; TIIA could curb cardiac fibrosis
in HF via inhibiting oxidative stress.
TIIA might be a potent agent against the fibrosis in
the hearts of lipopolysaccharide-treated mice partially via
inhibiting Nox2 (36). The present
study found that Nox4 overexpression reversed the effects of TIIA
in ameliorating cardiac dysfunction in HF rats. The effects of TIIA
in reducing collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9
on LV of HF rats were inhibited following Nox4 overexpression.
These results demonstrated that Nox4 could regulate the inhibitory
effects of TIIA on HF and cardiac fibrosis.
In addition to the oxidative stress explored in the
present study, TIIA is involved in various other signal pathways
related to cardiac diseases. TIIA protects cardiomyocytes and
improves cardiac function by activating the AMP-activated protein
kinase-mammalian target of rapamycin signaling pathway to inhibit
apoptosis and induce autophagy (22). TIIA prevents LV remodeling of MI
rats, mainly through repressing the Toll-like receptor 4/Myeloid
differentiation primary response 88/NF-κB signaling pathway
(37). A previous study
demonstrated that TIIA can activate the phosphatidylinositol
3-kinase/protein kinase B/mTOR signaling pathway to relieve
myocardial ischemia reperfusion injury in rats (12).
In the present study, it was not clear how TIIA
improved cardiac fibrosis of MI rats via inhibiting oxidative
stress. Since only MI model-induced cardiac fibrosis was
investigated in the present study, whether cardiac fibrosis in
other models could also be attenuated by TIIA remains unknown.
In conclusion, oxidative stress was enhanced in the
hearts of HF rats, then attenuated by TIIA. TIIA restored the
imbalance between oxidant and antioxidant levels. TIIA, functioning
as an antioxidant, could improve cardiac dysfunction and attenuate
fibrosis in HF rats and Ang II-induced fibrosis in CFs. Nox4
regulated the inhibitory effects of TIIA in HF and cardiac
fibrosis. It is hoped these results could provide evidence for the
clinical application of TIIA in treating HF-related cardiac
fibrosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key
Discipline Construction Project of Jiangsu Province (grant no.
js1303).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author upon request.
Authors' contributions
RC and WC made substantial contributions to
conception and design of the study, and were involved in drafting
the manuscript or revising it critically for important intellectual
content. XH acquired analysed and interpreted the data. QR made
substantial contributions to conception and design of the study,
and was involved in drafting and revising the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Experimental
Animal Care and Use Committee of Nanjing University of Chinese
Medicine (approval no. 2018031811).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Bui AL, Horwich TB and Fonarow GC:
Epidemiology and risk profile of heart failure. Nat Rev Cardiol.
8:30–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vos T, Flaxman AD, Naghavi M, Lozano R,
Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V,
et al: Years lived with disability (YLDs) for 1160 sequelae of 289
diseases and injuries 1990–2010: A systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2163–2196. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raso A, Dirkx E, Philippen LE,
Fernandez-Celis A, De Majo F, Sampaio-Pinto V, Sansonetti M, Juni
R, El Azzouzi H, Calore M, et al: Therapeutic Delivery of miR-148a
Suppresses Ventricular Dilation in Heart Failure. Mol Ther.
27:584–599. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Timmis A, Townsend N, Gale C, Grobbee R,
Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, et al
ESC Scientific Document Group, : European Society of Cardiology:
Cardiovascular Disease Statistics 2017. Eur Heart J. 39:508–579.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu C, Yang CX, Chen XR, Liu BX, Li Y,
Wang XZ, Sun W, Li P and Kong XQ: Alamandine attenuates
hypertension and cardiac hypertrophy in hypertensive rats. Amino
Acids. 50:1071–1081. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Liu C, Chen X and Li P: Alamandine
attenuates long term hypertension induced cardiac fibrosis
independent of blood pressure. Mol Med Rep. 19:4553–4560.
2019.PubMed/NCBI
|
|
7
|
Yue Y, Meng K, Pu Y and Zhang X:
Transforming growth factor beta (TGF-β) mediates cardiac fibrosis
and induces diabetic cardiomyopathy. Diabetes Res Clin Pract.
133:124–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan X, Pan J, Wen L, Gong B, Li J, Gao H,
Tan W, Liang S, Zhang H and Wang X: miR-144-3p Enhances Cardiac
Fibrosis After Myocardial Infarction by Targeting PTEN. Front Cell
Dev Biol. 7:2492019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rai R, Sun T, Ramirez V, Lux E, Eren M,
Vaughan DE and Ghosh AK: Acetyltransferase p300 inhibitor reverses
hypertension-induced cardiac fibrosis. J Cell Mol Med.
23:3026–3031. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan CY, Wong JX, Chan PS, Tan H, Liao D,
Chen W, Tan LW, Ackers-Johnson M, Wakimoto H, Seidman JG, et al:
Yin Yang 1 Suppresses Dilated Cardiomyopathy and Cardiac Fibrosis
Through Regulation of Bmp7 and Ctgf. Circ Res. 125:834–846. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu QQ, Xu YJ, Yang C, Tang Y, Li L, Cai
HB, Hou BN, Chen HF, Wang Q, Shi XG, et al: Sodium Tanshinone IIA
Sulfonate Attenuates Scopolamine-Induced Cognitive Dysfunctions via
Improving Cholinergic System. BioMed Res Int. 2016:98525362016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Q, Shen L, Wang Z, Jiang HP and Liu LX:
Tanshinone IIA protects against myocardial ischemia reperfusion
injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed
Pharmacother. 84:106–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Wei Y, Yuan S, Liu G, Lu Y, Zhang
J and Wang W: Potential anticancer activity of tanshinone IIA
against human breast cancer. Int J Cancer. 116:799–807. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C, Han X, Zhang H, Wu J and Li B: The
interplay between autophagy and apoptosis induced by tanshinone IIA
in prostate cancer cells. Tumour Biol. 37:7667–7674. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao S, Liu Z, Li H, Little PJ, Liu P and
Xu S: Cardiovascular actions and therapeutic potential of
tanshinone IIA. Atherosclerosis. 220:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen FY, Guo R and Zhang BK: Advances in
cardiovascular effects of tanshinone II(A). Zhongguo Zhong Yao Za
Zhi. 40:1649–1653. 2015.(In Chinese). PubMed/NCBI
|
|
17
|
Sag CM, Santos CX and Shah AM: Redox
regulation of cardiac hypertrophy. J Mol Cell Cardiol. 73:103–111.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madamanchi NR and Runge MS: Redox
signaling in cardiovascular health and disease. Free Radic Biol
Med. 61:473–501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bonnefont-Rousselot D, Mahmoudi A,
Mougenot N, Varoquaux O, Le Nahour G, Fouret P and Lechat P:
Catecholamine effects on cardiac remodelling, oxidative stress and
fibrosis in experimental heart failure. Redox Rep. 7:145–151. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan SH, Zhao NW, Geng ZR, Shen JY, Liu FM,
Yan D, Zhou J, Nie C, Huang CC and Fang ZY: Modulations of
Keap1-Nrf2 signaling axis by TIIA ameliorated the oxidative
stress-induced myocardial apoptosis. Free Radic Biol Med.
115:191–201. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gan XB, Duan YC, Xiong XQ, Li P, Cui BP,
Gao XY and Zhu GQ: Inhibition of cardiac sympathetic afferent
reflex and sympathetic activity by baroreceptor and vagal afferent
inputs in chronic heart failure. PLoS One. 6:e257842011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Wang Q, Wang X, Chen X, Shao M,
Zhang Q, Guo D, Wu Y, Li C, Wang W, et al: Tanshinone IIA protects
against heart failure post-myocardial infarction via
AMPKs/mTOR-dependent autophagy pathway. Biomed Pharmacother.
112:1085992019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koshman YE, Patel N, Chu M, Iyengar R, Kim
T, Ersahin C, Lewis W, Heroux A and Samarel AM: Regulation of
connective tissue growth factor gene expression and fibrosis in
human heart failure. J Card Fail. 19:283–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hou L, Guo J, Xu F, Weng X, Yue W and Ge
J: Cardiomyocyte dimethylarginine dimethylaminohydrolase1
attenuates left-ventricular remodeling after acute myocardial
infarction: Involvement in oxidative stress and apoptosis. Basic
Res Cardiol. 113:282018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Zhang S and Chen X: Tanshinone
IIA protects against cardiac fibrosis through inhibition of
β-tubulin expression. J Biol Regul Homeost Agents. 32:1451–1455.
2018.PubMed/NCBI
|
|
27
|
Tsai YT, Loh SH, Lee CY, Lee SP, Chen YL,
Cheng TH and Tsai CS: Tanshinone IIA Inhibits High Glucose-Induced
Collagen Synthesis via Nuclear Factor Erythroid 2-Related Factor 2
in Cardiac Fibroblasts. Cell Physiol Biochem. 51:2250–2261. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kura B, Szeiffova Bacova B, Kalocayova B,
Sykora M and Slezak J: Oxidative Stress-Responsive MicroRNAs in
Heart Injury. Int J Mol Sci. 21:3582020. View Article : Google Scholar
|
|
29
|
Kim H, Yun J and Kwon SM: Therapeutic
Strategies for Oxidative Stress-Related Cardiovascular Diseases:
Removal of Excess Reactive Oxygen Species in Adult Stem Cells. Oxid
Med Cell Longev. 2016:24831632016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Snezhkina AV, Kudryavtseva AV, Kardymon
OL, Savvateeva MV, Melnikova NV, Krasnov GS and Dmitriev AA: ROS
Generation and Antioxidant Defense Systems in Normal and Malignant
Cells. Oxid Med Cell Longev. 2019:61758042019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Honda T, Hirakawa Y and Nangaku M: The
role of oxidative stress and hypoxia in renal disease. Kidney Res
Clin Pract. 38:414–426. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kura B, Szeiffova Bacova B, Kalocayova B,
Sykora M and Slezak J: Oxidative Stress-Responsive MicroRNAs in
Heart Injury. Int J Mol Sci. 21:3582020. View Article : Google Scholar
|
|
33
|
Chen T, Li M, Fan X, Cheng J and Wang L:
Sodium Tanshinone IIA Sulfonate Prevents Angiotensin II–Induced
Differentiation of Human Atrial Fibroblasts into Myofibroblasts.
Oxid Med Cell Longev. 2018:67125852018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang P, Zhou S, Xu L, Lu Y, Yuan X, Zhang
H, Li R, Fang J and Liu P: Hydrogen peroxide-mediated oxidative
stress and collagen synthesis in cardiac fibroblasts: Blockade by
tanshinone IIA. J Ethnopharmacol. 145:152–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang P, Wu X, Bao Y, Fang J, Zhou S, Gao
J, Pi R, Mou YG and Liu P: Tanshinone IIA prevents cardiac
remodeling through attenuating NAD (P)H oxidase-derived reactive
oxygen species production in hypertensive rats. Pharmazie.
66:517–524. 2011.PubMed/NCBI
|
|
36
|
Huang L, Zhu J, Zheng M, Zou R, Zhou Y and
Zhu M: Tanshinone IIA protects against subclinical
lipopolysaccharide induced cardiac fibrosis in mice through
inhibition of NADPH oxidase. Int Immunopharmacol. 60:59–63. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu DM, Wang YJ, Han XR, Wen X, Li L, Xu L,
Lu J and Zheng YL: Tanshinone IIA prevents left ventricular
remodelling via the TLR4/MyD88/NF-κB signalling pathway in rats
with myocardial infarction. J Cell Mol Med. 22:3058–3072. 2018.
View Article : Google Scholar : PubMed/NCBI
|