Introduction
Cardiovascular diseases are the primary cause of
mortality worldwide, accounting for 31% of all deaths globally in
2016 and it was estimated that 17.9 million people died due to
cardiovascular causes (1).
Atherosclerosis is regarded as the pathological process that
underlies cardiovascular diseases (2). Epidemiological and experimental
studies have shown that age-related endothelial dysfunction is a
major risk factor for atherosclerosis (3). The vascular endothelium is a thin
layer of cells that lines the innermost surface of blood vessels
and functions as a semi-selective barrier, which prevents lipid
infiltration into the vessel wall (4). Accumulating evidence has suggested the
involvement of endothelial cell senescence in endothelial
inflammation, which promotes the recruitment of circulating
monocytes and contributes to the pathogenesis of atherosclerosis
(5,6). Thus, there is a critical need to
identify approaches to protect against endothelial senescence.
MicroRNAs (miRNAs/miRs) are endogenous small
single-strand non-coding RNAs of 18–22 nucleotides that serve a
major role in the development of atherosclerosis (7,8). Our
previous study revealed that miR-216a induced endothelial cell
senescence and inflammation via the Smad3/IκBα signaling pathway
(9). Moreover, the plasma miR-216a
level was elevated in elderly patients with coronary artery disease
(9). These findings suggest that
miR-216 may be a novel biomarker and therapeutic target of
endothelial senescence and atherosclerosis. By using drug-RNA
interaction predictor software, the present study examined the
potential interaction between chemical molecules and miR-216a on
the basis of its sequence and structural features. Here, the
present study tested the hypothesis that ginsenoside Rb2 (Rb2), a
small chemical molecule, suppresses endothelial senescence mediated
by miR-216a.
Rb2, a 20(S)-protopanaxadiol glycoside, is the main
bioactive component extracted from the plant Pannax ginseng,
and is a widely used traditional Chinese medicine (10,11).
The molecular formula of Rb2 is
C53H90O22 and the chemical
structure is shown in Fig. S1. The
Rb2 has been reported to exert anti-inflammatory effects by
upregulating the expression level of G protein-coupled receptor
protein 120, a ω-3 fatty acid receptor in monocytes (12). Rb2 also promotes glucose metabolism
and attenuates fat accumulation via AKT-dependent signaling
mechanisms (13). To date, the
effects of Rb2 on endothelial senescence and inflammation remain
unknown.
In the present study, considering that cellular
senescence and the related inflammatory process are induced by
numerous stressors, such as mitochondrial deterioration, oxidative
stress, expression of certain oncogenes, DNA damage and chromatin
disruption (14), a
miR-216a-induced endothelial senescence model was established by
transfecting cells with Lv-miR-216a to assess the effect of Rb2 on
endothelial senescence and inflammation by acting as a specific
inhibitor of the miR-216a/Smad3 pathway, thus aiming to investigate
the therapeutic potential of Rb2 in endothelial senescence and
atherosclerosis.
Materials and methods
Microscale thermophoresis (MST)
assay
A drug-RNA interaction predictor software
(rnanut.net/drip) was used to examine the
potential interaction between Rb2 and miR-216a on the basis of its
sequence and structural features. The MST assay is a biophysical
technology for testing interactions between protein and protein or
nucleic acid, as well as interactions between nucleic acid and
small molecules (15). In the
current study, the MST experiment was applied to assess the
interaction between Rb2 and miR-216a. The wild-type or mutant-type
probe of miR-216a was labeled with 5-carboxyfluorescein (5′FAM),
and the sequences were 5′-UAAUCUCAGCUGGCAACUGUGA-3′ for wild-type
probe, and 5′-UAUAGAGUGCUGGCAACUGUGA-3′ for mutant-type probe. Rb2
(purity 98.26%; MedChemExpress) was dissolved in RNase-free water,
and then serial doubling dilutions of Rb2 were created from 1 mM to
30 nM for the MST experiment. The 5′FAM-labeled probe of miR-216a
(500 nM) and Rb2 dilutions were mixed in a 1:1 ratio and incubated
for 5 min at room temperature in the dark. The mixtures were then
filled into the capillaries, and the florescence was detected at
400 nm of excitation light wavelength using the Monolith NT.115 MTS
instrument (NanoTemper Technologies GmbH). The binding ability
between miR-216a and Rb2 was assessed using the dissociation
constant (Kd).
Cell culture
The primary human umbilical vein endothelial cells
(HUVECs) were isolated from the human umbilical cord veins, as
described previously (9,16,17).
The umbilical cord samples were donated by 3 healthy pregnant women
(aged, 25–30 years) at Beijing Haidian Material & Child Health
Hospital (Beijing, China) and collected from January to February,
2020. Informed consent was obtained from the donors. The study was
approved by the ethics committee of Fuwai Hospital. The HUVECs were
cultured using the endothelial cell medium (ScienCell Research
Laboratories, Inc.) supplemented with 1% endothelial cell growth
supplement (ScienCell Research Laboratories, Inc.), 5% FBS
(ScienCell Research Laboratories, Inc.) and 1%
penicillin-streptomycin solution in an incubator with 5%
CO2 at 37°C.
A replicative senescent model of endothelial cells
was established by passaging, and the number of population-doubling
level (PDL) was calculated using the equation: PD=log2
(Ch/Cs), in which Ch is the number of viable cells at harvest and
Cs is the number of cells seeded (6). The PDLs vs. time growth curves were
obtained to assess the cellular replication potentiality over time.
PDL8 was identified as young HUVECs at a culture of 5–8 days, and
PDL44 as senescent cells at a culture of ~75 days. In this study,
given the potential difference in the differentiate rate of HUVECs,
in order to calculate the PDLs accurately when establishing the
replicative senescence model, the HUVECs derived from different
donors were not mixed. However, the main experiments were
replicated in HUVECs from three independent donors to assess the
findings.
Human aortic endothelial cells (HAECs) from a pool
of three individuals (lot nos. 11,035, 3,535 and 11,385; cat. no.
6100; ScienCell Research Laboratories, Inc.) were cultured to
evaluate the effects of Rb2. HAECs were cultured in the same
condition as HUVECs.
Rb2 treatment and miR-216a expression
analysis
The PDL8 and PDL44 HUVECs were cultured and grown to
90% confluency, and were then treated with Rb2 at various
concentrations of 0.1, 1, 10 and 100 µM for 24 h at 37°C, in order
to investigate the effect of Rb2 on the expression of endogenous
miR-216a.
miRNA expression analysis was performed via reverse
transcription-quantitative (RT-q)PCR. In brief, the total RNA was
extracted from cultured cells using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.), and cDNA was reverse transcribed
with All-in-One™ miRNA First-Strand cDNA Synthesis kit
(GeneCopoeia, Inc) at 37°C for 15 min and 85°C for 5 min. Next, the
expression of miR-216a was measured by All-in-One™ miRNA qPCR kit
(GeneCopoeia, Inc) using an ABI 7500 system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The reaction conditions were as
follows: initial denaturation at 94°C for 10 min, followed by 40
cycles of denaturation at 94°C for 15 sec, annealing at 55°C for 1
min and extension at 72°C for 10 min. U6 was used as internal
reference and the relative gene expression of miR-216a was analyzed
using the relative quantification (2−ΔΔCq) method
(18). The primer sequences of
miR-216a and U6 (GeneCopoeia, Inc.) were as follows: miR-216a-5p
5′-TCTCAGCTGGCAACTGTGAAA-3′ and U6 5′-GGTCGGGCAGGAAAGAGGGC-3′.
miRNA lentivirus infection
Lentiviruses expressing miR-216a and the negative
control (NC) vector were constructed by Shanghai Genechem Co., Ltd.
According to the manufacturer's instructions, the coding sequence
of pre-miR-216a was inserted into GV369 vector
(Ubi-EGFP-MCS-IRES-puromycin) and the construct was verified via
DNA sequencing. Next, to collect the lentiviral particles, 293T
cells were transfected with 20 µg lentiviral vectors mixed with 1
ml transfection reagent (Genechem Co., Ltd) at 37°C for 48 h, and
cell culture supernatants were harvested 48 h after transfection,
centrifuged at 4,000 × g at 4°C for 10 min and passed through the
0.45 µM filter to remove cellular debris. Finally, lentiviral
particles were resolved in PBS and aliquoted for storage at −80°C
as viral stocks.
To establish the stable cell line of miR-216a,
HUVECs were infected with pre-miR-216a recombinant lentiviruses
(Lv-miR-216a) or NC vectors (Lv-NC) (Shanghai Genechem Co., Ltd.).
PDL4 HUVECs were seeded in 24-well cell culture plate (Corning,
Inc.) at a density of 5×104 cells/well and added with 5
µl lentiviral vector diluent of 1×108 transducing unit
(TU/ml) in each well for a 24-h infection at 37°C, and then the
medium was replaced with fresh growth medium. The multiplicity of
infection was 10. After 3 days, cells were treated with 400 ng/ml
puromycin (Sigma-Aldrich; Merck KGaA) for selection, and the
lentivirus infection efficiency (>95%) was evaluated via the
enhanced green fluorescent protein florescence analysis. Then,
infected HUVECs were passaged every 3 days and treated with
puromycin for selection. PDLs were calculated during the passage.
PDL25 cells with stable expression of miR-216a (at ~40 days
following Lv-miR-216a infection) were assessed as the senescent
cell line and used for subsequent experiments.
miRNA and small interfering (si)RNA
transfection
To examine whether Rb2 could affect the target gene
expression of miR-216a, PDL8 HUVECs were transfected with miR-216a
mimics (Shanghai GenePharma Co., Ltd.) at a final concentration of
50 nM using Lipofectamine® 3000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). After incubation for 6 h at 37°C,
cells were treated with Rb2 (10 µM) at 37°C for 48 h and harvested
for expression analysis.
To evaluate the effect of Rb2 on the Smad3/IκBα
pathway, PDL8 HUVECs were transfected with Smad3 siRNA (si-Smad3;
Shanghai GenePharma Co., Ltd.) at a final concentration of 40 nM
using Lipofectamine 3000 reagent. After incubation for 6 h at 37°C,
cells were treated with Rb2 (10 µM) at 37°C for 48 h and then were
harvested for expression analysis. The sequences were as follows:
miR-216a mimics sense, 5′-UAAUCUCAGCUGGCAACUGUGA-3′ and anti-sense,
5′-ACAGUUGCCAGCUGAGAUUAUU-3′; and Smad3 siRNA sense,
5′-AGGACGAGGUCUGCGUGAAUCCCUA-3′ and anti-sense,
5′-UAGGGAUUCACGCAGACCUCGUCCU-3′.
Assay for senescence-associated
β-galactosidase (SA-β-gal) activity
Endothelial senescence was assessed via in
situ staining for SA-β-gal positive cells using Senescence
β-Galactosidase Staining kit (Beyotime Institute of Biotechnology).
β-galactosidase catalyzes the hydrolysis of X-gal, producing a blue
color that can be easily visualized under a microscope (19). According to the manufacturer's
protocol, cells were treated with fresh β-galactosidase staining
solution (pH 6.0) for 16 h at 37°C without CO2. The
SA-β-gal-positive cells were identified as blue stained cells and
images were analyzed using Leica DMI4000 B inverted light
microscopes (magnification, ×10; Leica Microsystems GmbH). The
percentage of SA-β-gal positive-staining cells was calculated by
counting five random microscopic fields per sample. The experiment
was repeated five times for each group.
Assays for endothelial cell
proliferation and adhesion activity
The endothelial proliferative and adhesive abilities
were assessed in the senescent PDL25 HUVECs (at ~40 days after
transfection of Lv-miR-216a). The cell proliferative ability was
assessed using the Cell Counting Kit-8 (CCK-8) method (Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions. Briefly, HUVECs were seeded into 96-well plate at a
density of 5×103 cells/ml and cultured in a 5%
CO2 incubator at 37°C for 24 h, and were then treated
with Rb2 (10 µM) for 24 h at 37°C. Next, the cells were incubated
with 10 µl CCK-8 solution for an additional 1, 2 and 3 h at 37°C.
The absorbance was detected at a wavelength of 450 nm on a LB960
microplate reader (Titertek-Berthold).
The human acute monocytic leukemia cell line (THP-1;
China Infrastructure of Cell Line Resources, Beijing, China) was
used to assess the endothelial adhesive ability to monocytes. THP-1
cells were labeled with CellTracker™ CM-Dil (red fluorescence;
Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min at 37°C,
washed twice with PBS and then were re-suspended in RPMI-1640
medium (Sigma-Aldrich; Merck KGaA) at a density of 1×106
cells/ml. The CM-Dil labeled THP-1 cells were incubated with PDL25
HUVECs at 37°C for 30 min in an incubator containing 5%
CO2. The non-adherent THP-1 cells were removed by PBS,
and the adherent cells were fixed with 4% paraformaldehyde (Beijing
Leagene Biotechnology Co., Ltd.) at room temperature for 15 min.
The nuclei of cells were stained with DAPI (OriGene Technologies,
Inc.) at room temperature for 20 min, and the number of adhesive
THP-1 cells to endothelial cells was observed and imaged in five
random fields at wavelengths of 359 and 549 nm using a confocal
laser scanning microscope (magnification, ×20) SP8 (Leica
Microsystems GmbH). The experiments were repeated five times.
mRNA expression analysis
The mRNA expression levels of p53, p21, vascular
cell adhesion molecule 1 (VCAM1), intercellular adhesion molecule
(ICAM1) and Smad3 were analyzed via RT-qPCR (primer sequences are
listed in Table SI). Briefly,
total RNA was extracted from cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was reverse
transcribed using PrimeScript™ RT Master Mix (Takara Bio, Inc.)
under the following thermocycling conditions: 37°C for 15 min, 85°C
for 5 sec. RT-qPCR was performed using SYBR Green qPCR mix
(Shanghai Yeasen Biotechnology Co., Ltd.) on an ABI 7500 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The reaction
conditions were conducted as following: Initial denaturation for 10
min at 95°C, followed by 40 cycles of denaturation at 95°C for 15
sec, annealing and elongation at 60°C for 1 min, and final
extension at 72°C for 1 min. GAPDH expression was used as internal
control, and the expression of target gene was calculated using
relative quantification (2−ΔΔCq) method (18).
Western blot analysis
Protein expression levels were determined via
western blot analysis. In brief, HUVECs were harvested and proteins
extracts were isolated with ice-cold RIPA lysis buffer (Beyotime
Institute of Biotechnology) containing protease inhibitor (Roche
Diagnostics GmbH). The total concentration of protein was
determined using a Pierce BCA Protein Assay kit (Invitrogen; Thermo
Fisher Scientific, Inc.). Each sample protein was loaded at 20 µg
and separated by 10% SDS-PAGE, and then transferred to
nitrocellulose membranes (EMD Millipore). Subsequently, the
membrane was blocked with 10% non-fat milk at room temperature for
2 h and then incubated with primary antibodies overnight at 4°C.
The primary antibodies were purchased from Cell Signaling
Technology, Inc., including the anti-p53 (1:1,000; cat. no. 2527T),
anti-p21Waf1/Cip1 (1:1,000; cat. no. 2947T), anti-ICAM1
(1:1,000; cat. no. 67836T), anti-VCAM1 (1:1,000; cat. no. 39036S),
anti-Smad3 (1:1,000; cat. no. 9523T) and anti-IκBα (1:1,000; cat.
no. 4812S). The mouse monoclonal anti-GAPDH antibody (1:2,000; cat.
no. TA309157; OriGene Technologies, Inc.) was applied as an
internal control. Then, membranes were incubated with
HRP-conjugated anti-rabbit IgG secondary antibody (1:5,000; cat.
no. 7074; Cell Signaling Technology, Inc.) or anti-mouse IgG
secondary antibody (1:2,000; ZB2305; OriGene Technologies, Inc.)
for 1.5 h at room temperature. Bands were visualized with FluorChem
R, M and E Systems (ProteinSimple) and analyzed using ImageJ
software (v1.5.1; National Institutes of Health).
Statistical analysis
Data are presented as the mean ± SD. For the
comparison between two groups, group differences were analyzed
compared using an unpaired Student t-test. For the comparisons ≥3
groups, group differences were analyzed using one-way ANOVA with
Tukey's post hoc test. Each experiment was repeated ≥3 times
independently. Data were analyzed using SPSS 19.0 software (IBM
Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Binding ability between Rb2 and
miR-216a
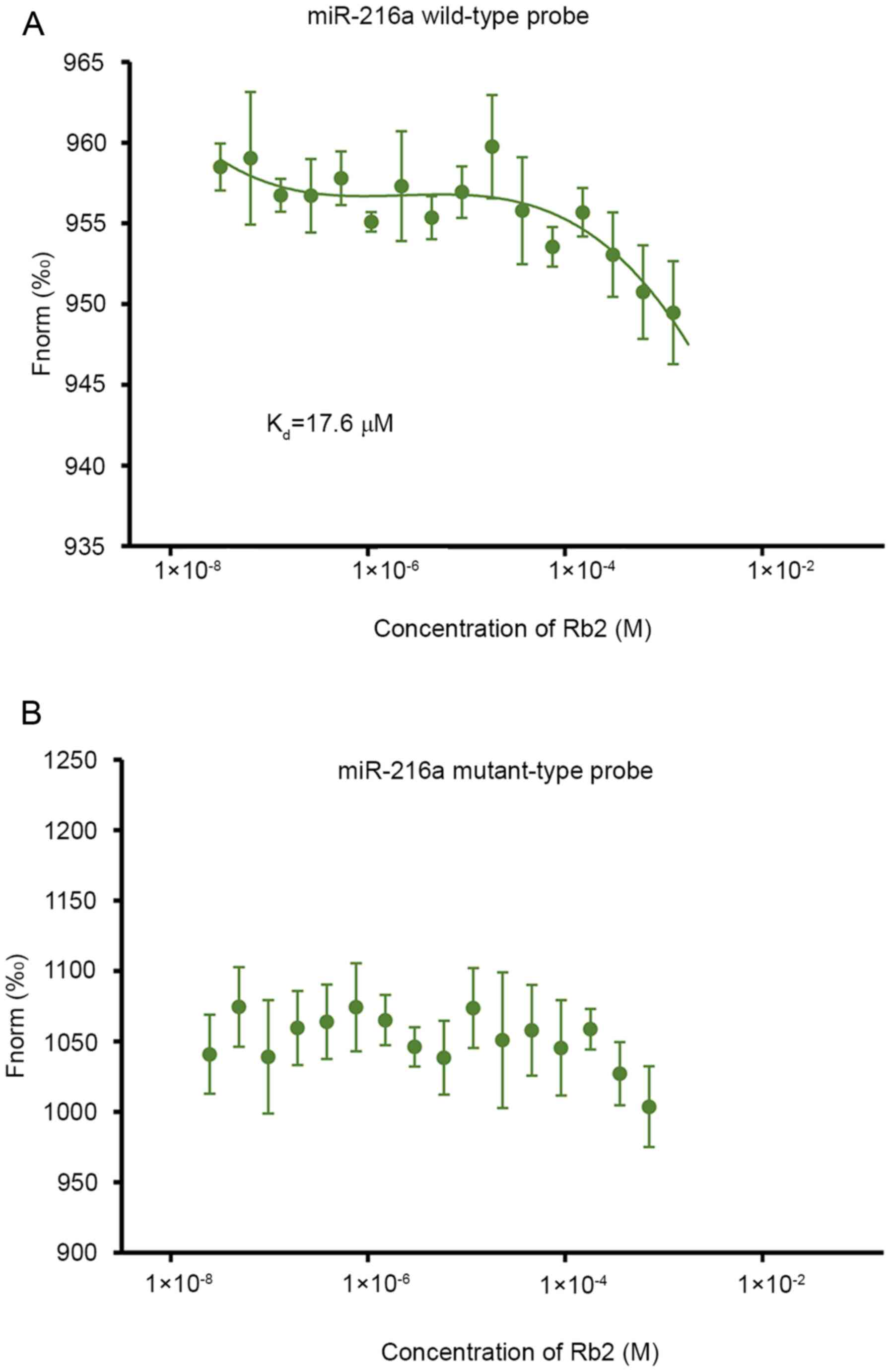
The bioinformatics analysis with drug-RNA
interaction predictor software (rnanut.net/drip) suggested that Rb2 may have a high
affinity with miR-216a. In the subsequent MST experiment for
assessing the interaction between Rb2 and miR-216a, the results
demonstrated that Rb2 bound to the wild-type probe of miR-216a and
the Kd value was 17.6 µM (Fig. 1A). By contrast, if the seed sequence
of miR-216a was mutated, Rb2 was not detected to bind the
mutant-type probe (Fig. 1B),
indicating that Rb2 can bind to miR-216a specifically.
Establishment of the senescent HUVECs
model
A premature senescent model of endothelial cells
induced by miR-216a was established. The PDLs-vs.-time growth
curves indicated that, in the PDL25 line at ~40 days after
transfection with Lv-miR-216a, the cells showed senescent features,
characterized by enlarged size and flattened morphology (Fig. 2A), as well as accumulation of
SA-β-gal within cytoplasm (blue staining; Fig. 2B), which was similar as the
senescent status of naturally passaged PDL44 (at a culture of ~75
days). The percentage of SA-β-gal-positive cells was markedly
increased by 130% in the PDL25 line transfected with Lv-miR-216a
compared with the NC (P=0.01; Fig.
2C). Moreover, the mRNA expression levels of the
senescent-related cell cycle inhibitors p21 and p53 were
upregulated by 34% (P=0.05) and 38% (P=0.02; Fig. 2D), respectively; further protein
expression analysis also showed that p21 (P=0.05) and p53
(P<0.01) were significantly upregulated (Fig. 2E).
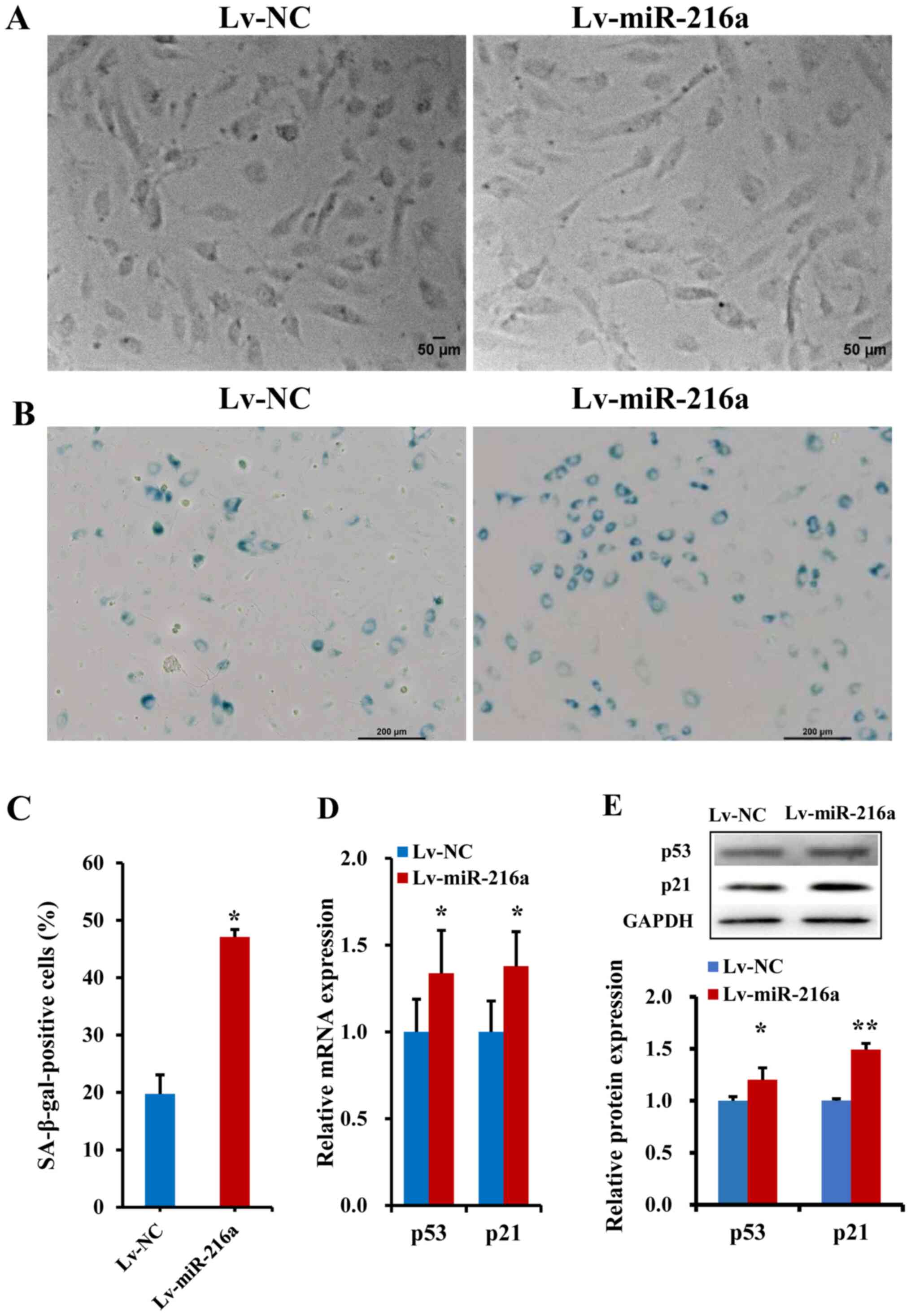 | Figure 2.Establishment of a premature
senescence phenotype in endothelial cells induced by miR-216a. (A)
Images of senescent phenotype, characterized by enlarged size and
flattened morphology, in the PDL25 endothelial cell line
transfected with Lv-miR-216a compared with cells transfected with
Lv-NC vector (n=5). Scale bar, 50 µm. (B) Micrographs of senescent
cells, characterized by accumulation of SA-β-gal within cytoplasm
(blue staining) in PDL25 cells transfected with Lv-miR-216a
compared with the Lv-NC (n=5). Scale bar, 200 µm. (C) Comparisons
of the percentage of SA-β-gal-positive cells (of the total cell
number) between PDL25 cells transfected with the Lv-miR-216a and
the Lv-NC (n=5). (D) mRNA expression levels of senescent-related
genes p53 and p21 (n=5). (E) Protein expression levels of
senescent-related genes p53 and p21 (n=5). The data are presented
as the mean ± SD. *P<0.05, **P<0.01, vs. Lv-NC. miR-216a,
microRNA-216a; Lv-miR-216a, pre-miR-216a recombinant lentiviruses;
NC, negative control; SA-β-gal, senescence-associated
β-galactosidase. |
To verify whether pre-miR-216a recombinant
lentiviruses were successfully transfected in HUVECs, miR-216a
expression was examined using RT-qPCR. Compared with NC (Lv-NC),
the expression level of miR-216a in the Lv-miR-216a cell line was
increased by 6900% (P<0.01; Fig.
S2).
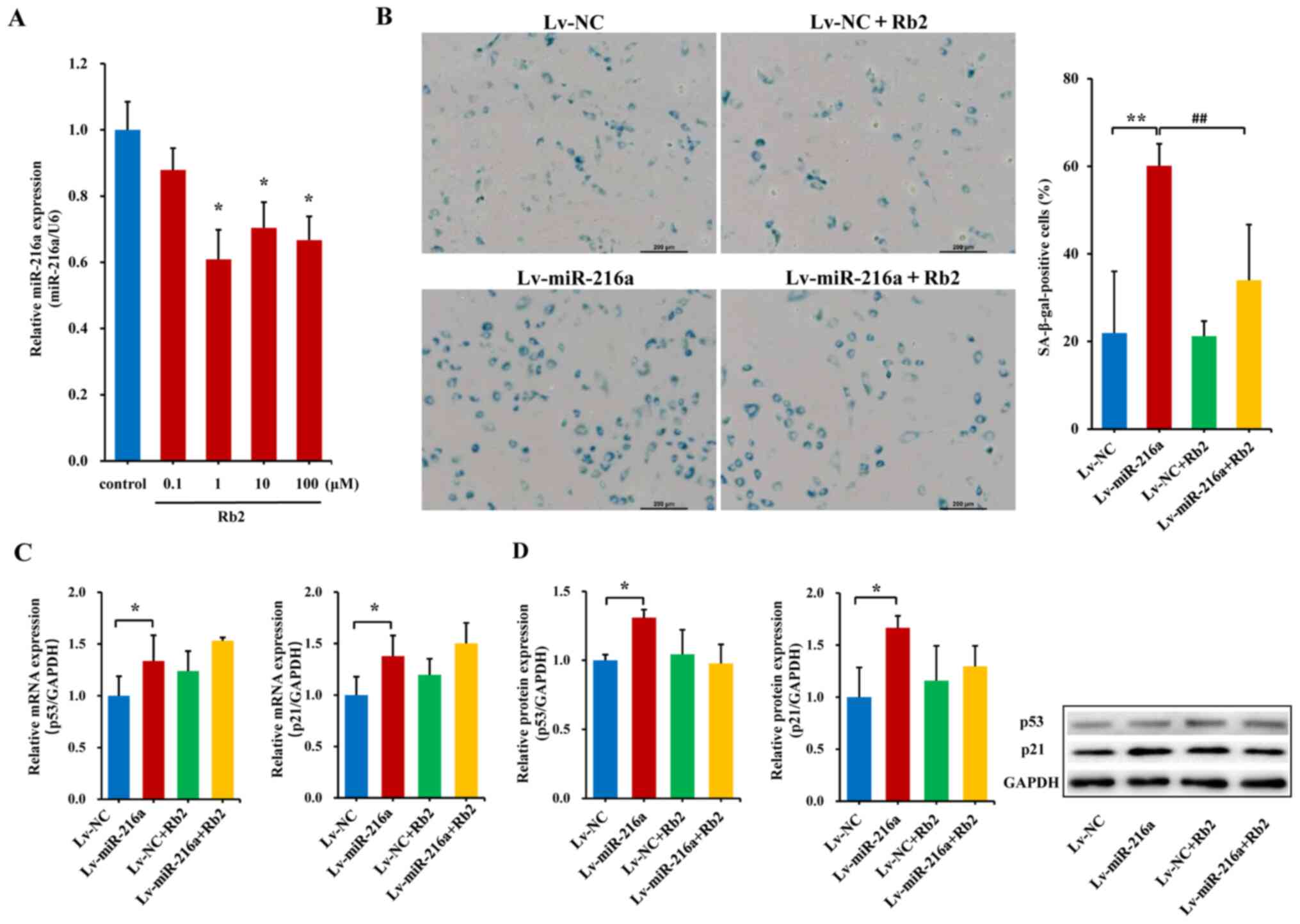
Rb2 attenuates endothelial senescence
induced by miR-216a
Serial concentrations of Rb2 were applied to examine
the effects of Rb2 on endogenous miR-216a expression. In young PDL8
HUVECs, Rb2 treatment with concentrations of 1, 10 or 100 µM
significantly decreased miR-216a expression by 40% (P=0.01), 30%
(P=0.02) and 36% (P=0.02), respectively (Fig. 3A). Moreover, in senescent PDL44
HUVECs, Rb2 did not appear to affect miR-216a expression at low
doses, whereas a relative high dose (100 µM) of Rb2 exerted a
significant inhibitory effect on miR-216a expression by 44%
(P=0.01; Fig. S3), supporting the
previous finding that endogenous miR-216a was highly expressed in
senescent endothelial cells (9).
However, the concentration of 100 µM Rb2 has previously been shown
to induce cytotoxic effects on endothelial cells (20). In HAECs, Rb2 (10 µM) also decreased
the expression level of endogenous miR-216a by 34% (P=0.02;
Fig. S4). Given that the
Kd value was 17.6 µM in the MST experiment assessing the
special binding ability between Rb2 and miR-216a, the concentration
of 10 µM of Rb2 was selected as the optimal dose and used in
further experiments.
Next, the potential role of Rb2 in endothelial
senescence and dysfunctions induced by miR-216a was assessed. In
the premature senescent model of PDL25 HUVECs with Lv-miR-216a
transfection, the percentage of SA-β-gal-positive cells were
increased by 174% (P<0.01; Fig.
3B) compared with the Lv-NC; whereas the senescent features and
accumulation of SA-β-gal within cytoplasm induced by miR-216a were
reversed following Rb2 treatment, and the percentage of
SA-β-gal-positive cells decreased by 76% in the Lv-miR-216a+Rb2
group compared with the Lv-miR-216a group (P<0.01; Fig. 3B). The elevated mRNA and protein
expression levels of p53 (P=0.05) and p21 (P=0.04) induced by
miR-216a were not significantly affected by Rb2 treatment (Fig. 3C and D).
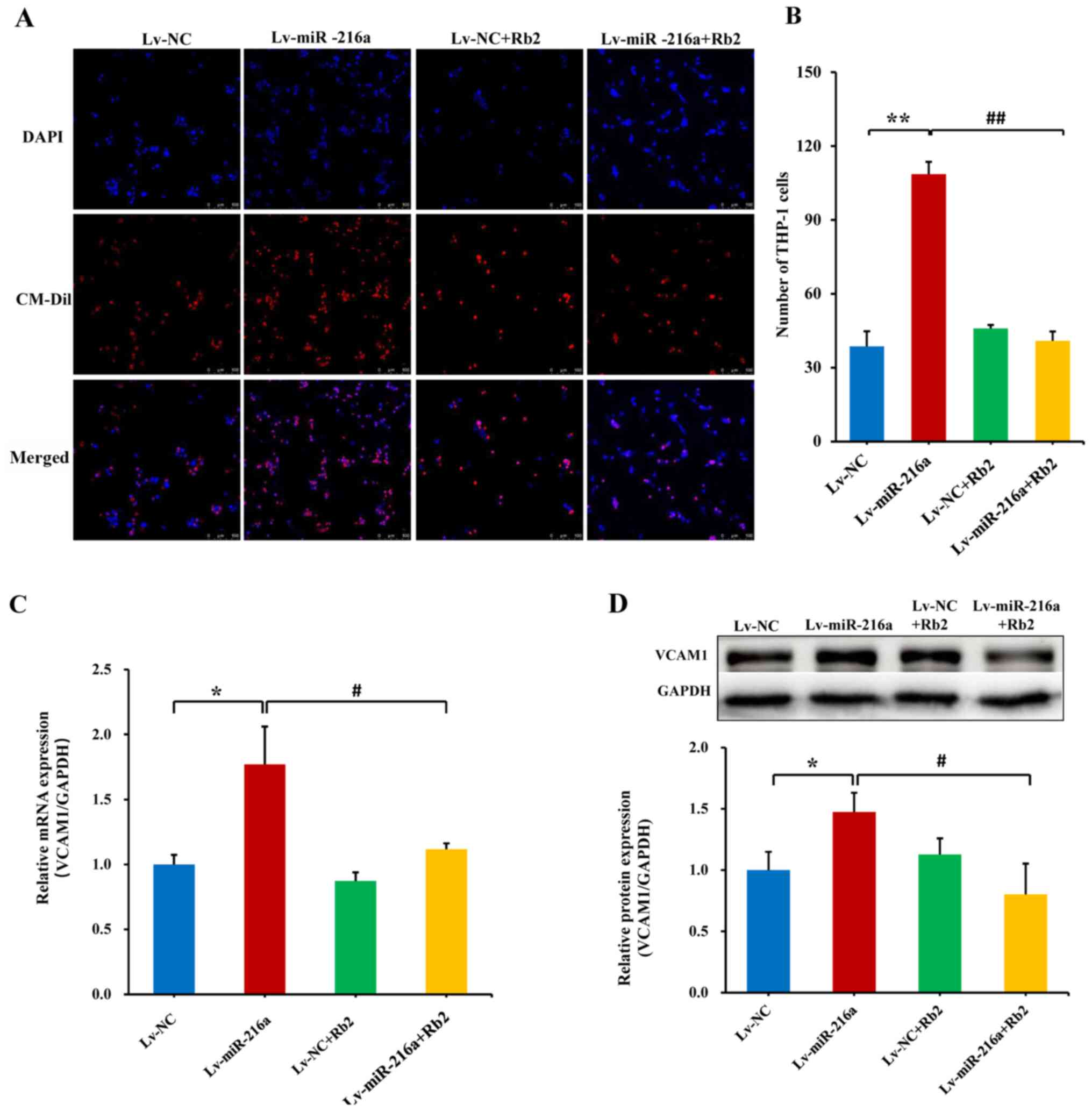
Rb2 inhibits the adhesiveness of
endothelial cells to monocytes induced by miR-216a
In senescent PDL25 HUVECs with overexpression of
miR-216a, the adhesive ability of endothelial cells (DAPI-staining)
to THP-1 cells (CM-Dil-staining) was increased by 180% compared
with the NC (P<0.01), whereas this promoting effect of miR-216a
was significantly inhibited by Rb2 treatment (P<0.01; Fig. 4A and B). Consistently, the mRNA
expression level of adhesion cytokine VCAM1 was upregulated by 78%
by Lv-miR-216a (P=0.02), while this effect was inhibited by Rb2
(P=0.04; Fig. 4C), and these
effects were also observed via further protein expression analysis
(both P<0.05; Fig. 4D).
Additionally, the proliferative ability of endothelial cells was
examined in senescent PDL25 cells, and the inhibitory effect of
miR-216a was not affected by Rb2 (Fig.
S5).
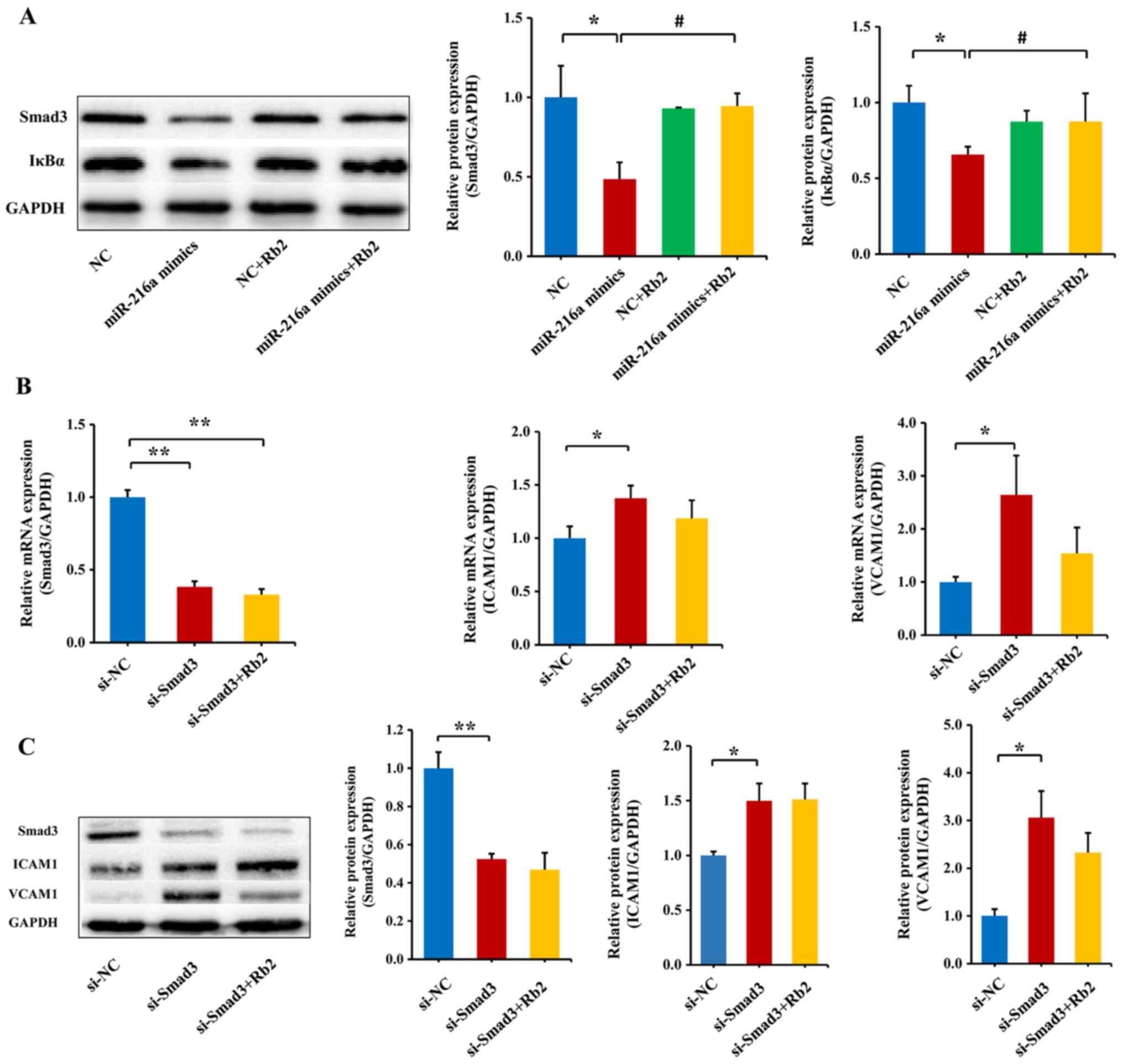
Rb2 reverses the inhibitory role of
miR-216a on the expression of the Smad3/IκBa pathway
Our previous study has shown that miR-216a can
directly inhibit the Smad3/IκBα signaling pathway, and therefore,
promote the endothelial inflammatory process (6). Thus, the current study transfected
miR-216a mimics in PDL8 HUVECs to observe the effect of miR-216a on
the protein expression levels of Smad3 and IκBα. To verify whether
miR-216a mimics was successfully transfected, miR-216a expression
was examined using RT-qPCR. Compared with the NC group, the
expression level of miR-216a was increased by 422-fold (P<0.01;
(Fig. S6). The results
consistently demonstrated that miR-216a decreased the protein
expression levels of Smad3 and IκBα (P<0.01) in PDL8 HUVECs
transfected with miR-216a mimics, and the role of miR-216a was
significantly reversed by Rb2 treatment (P<0.01; Fig. 5A).
To further assess whether Rb2 reversed the role of
endogenous miR-216a in the Smad3/IκBa pathway, Smad3 siRNA was
transfected to PDL8 HUVECs. As expected, the mRNA and protein
expression levels of Smad3 were greatly decreased by silencing
Smad3 (P<0.01; Fig. 5B), and
consequently the mRNA and protein expression levels of downstream
adhesion molecules ICAM1 and VCAM1 were upregulated (both
P<0.05; Fig. 5C). By contrast,
when exposed to Rb2, the expression levels of ICAM1 and VCAM1 were
reversed, although the findings were not significant (Fig. 5C). These results suggested that Rb2
attenuated the inflammatory process by inhibiting the expression
level of Smad3 targeted by miR-216a.
Discussion
To the best of our knowledge, the present study
demonstrated the first time that Rb2 had a specific affinity for
miR-216a, and further experiments identified that Rb2 attenuated
endothelial senescence and the adhesiveness of monocytes to
senescent HUVECs induced by miR-216a. Moreover, miR-216a promoted
the endothelial inflammatory process by inhibiting the Smad3 and
IκBα signaling pathway, and the role of miR-216a was suppressed
after Rb2 treatment. These findings indicated that Rb2 may serve as
a potential therapeutic drug for endothelial cell senescence and
inflammation by targeting miR-216a.
Atherosclerosis, an aging-related, chronic
inflammatory process, is accelerated by endothelial senescence and
dysfunction (21,22). In our previous study, it was found
that miR-216a promoted endothelial senescence and inflammation by
inhibiting the Smad3/IκBα pathway, and notably, elderly patients
with coronary artery disease had an elevated concentrations of
plasma miR-216a, all of which indicate that miR-216a may be a
therapeutic target (9).
miRNAs are emerging as an appealing target for drug
discovery, the these traditionally include oligonucleotides that
are complementary to a miRNA and block its activity, as well as
duplex or chemically-modified single-stranded RNAs that mimic a
miRNA and trigger enhanced activity (23,24).
For example, 2′-F- and 2′-MOE-modified anti-miR-33 has been shown
to reduce atherosclerosis in non-human primates (25). Moreover, the mimics of miR-34
represses oncogene expression and blocks tumor growth (26), while single-stranded
oligonucleotides complementary to miR-122 have been developed to
treat hepatitis C virus (27).
However, there are some drawbacks, such as unwanted exogenous RNA
immunogenicity, poor stability, weak cell permeability and high
costs (28). Preclinical and
clinical studies have also showed that delivery of miRNAs-based
chemistries has side effects and may lead to off-target effects
(29,30).
Recently, targeting miRNAs using chemical small
molecules has become a promising strategy for disease treatment
(31). In the present study,
bioinformatics analysis and validating experiments identified that
Rb2 bound to miR-216a specifically and inhibited the expression
level of endogenous miR-216a in endothelial cells. However, the
inhibitory effect of Rb2 on miR-216a appeared not to occur in a
dose-dependent manner. There are some explanations for these
results as follows: First, the mode of Rb2 binding to miR-216a
remains to be fully elucidated. Drug-RNA interaction predictor
software was used to predict that Rb2 had a high binding score for
miR-216a by identifying cleft and pocket-containing motifs in the
primary sequence and secondary structural elements of miR-216a. The
binding model of RNA motif-Rb2 pairs may cause a conformational
change in miR-216a and influence its RNA binding activity. Of note,
miRNAs can fold into structural composites including base-paired
and non-canonically paired regions with three dimensional
structures. Thus, the direct interactions between miRNAs and small
molecules are complex, including cross-linking and cleavage to
alter the miRNA sequence and recruit nucleases (32,33).
However, approaches remain to be established to identify compound
modules or fragments that selectively bind miRNA motifs, and to
define factors that influence the bioactivities of small chemical
molecules and miRNAs.
It is reasonable to suggest that the intracellular
microenvironment may affect the inhibitory manner of ginsenosides
on miRNA expression in various cell types. Previous studies have
reported that several types of ginsenosides show a dose-dependent
effect on expression levels of targeted miRNAs, whereas others have
not (34–36). For example, notoginsenoside R1
markedly suppresses miR-301a expression in murine chondroprogenitor
cells at a relatively high concentration, but does not influence
miR-301a expression at a low dose (34). It has also been shown that
ginsenoside Rg1 reduced miR-148-5p expression in rat bone marrow
mesenchymal stem cells and that ginsenoside Rg3 that downregulated
miR-221 expression in human oral squamous carcinoma cells; however,
the inhibitory effects of ginsenosides were not in a dose-dependent
manner (35,36). In the current study, various
concentrations (1, 10 or 100 µM) of Rb2 significantly decreased the
expression level of endogenous miR-216a in young PDL8 HUVECs,
whereas only a relative high dose (100 µM) of Rb2 had an inhibitory
effect on miR-216a in PDL44 cells, which supports our previous
finding that endogenous miR-216a was highly expressed in senescent
endothelial cells compared with younger cells (9).
The present study also evaluated the inhibitory
effect of Rb2 on miR-216a in arterial endothelial cells, and found
that Rb2 significantly decreased expression of endogenous miR-216a
in HAECs. However, transfection of miR-216a into HAECs induced the
phenotype of endothelial-mesenchymal transition (EnMT) in an early
cell passage. There are several explanations for this finding. For
instance, HAECs isolated from human adult arteries are highly
differentiated cells, and may have a different mechanism during
endothelial aging implicated in atherosclerosis (37,38).
Moreover, there may be differences in characteristics and functions
between arteries and venous endothelial cells (39,40).
Fleenor et al (41) also
observed that a model of primary human cell aging induced EnMT in
an early passage of HAECs, which was similar to the phenotype
observed in late passage cells. HAECs and HUVECs may react
differently to aging and inflammation induced by miR-216a, and
therefore, more comprehensive experiments are required to further
evaluate the complex effects of Rb2 and miR-216a in vascular
biology, considering the biological difference in vascular
endothelial cells.
Rb2 is one of the most highly abundant components in
ginseng, and has been reported to possess various bioactivities
including anti-inflammatory, anti-oxidative and anti-tumor effects
(12,13). The present study first investigated
the inhibitory effect of Rb2 on endothelial senescence and the
inflammatory process induced by miR-216a. Smad3 is a direct target
gene of miR-216a (9). The present
study found that miR-216a inhibited Smad3 protein expression,
promoted downstream IκBα degradation, and thus, activated NF-κB
responsive genes, such as VCAM1, which promoted the adhesiveness of
endothelial cells to monocytes. The inhibitory effect of miR-216a
on Smad3 and IκBα expression was reversed by Rb2 treatment, which
indicated that Rb2 may exert an anti-inflammation effect, thereby
acting as a potential therapeutic drug for endothelial cell
senescence and inflammation by targeting miR-216a.
Of note, the present study identified that miR-216a
inhibited the proliferative ability of endothelial cells, but the
inhibitory effect of miR-216a was not attenuated by Rb2.
Additionally, expression levels of p53 and p21 genes were not
significantly affected by Rb2. These results are consistent with
the characteristics of senescent cells, such as a flattened and
enlarged cell morphology and growth arrest, which may lead to
irreversible impairment of proliferative ability of endothelial
cells (42). Aging-related genes
p53 and p21 serve an important role in cell-cycle control, DNA
repair and cellular stress responses, and their expression
gradually increases during the process of replicative senescence
(43). Thus, it was suggested that
Rb2 attenuated the endothelial senescence mainly via the
inflammatory process induced by miR-216a, although the underlying
mechanism should be clarified further.
In conclusion, the present study demonstrated that
Rb2 had a specific binding affinity for miR-216a, and further
attenuated the senescent status and inflammatory process induced by
miR-216a via the Smad3/IκBα signaling pathway. These data indicated
that Rb2 may exert an anti-inflammatory effect on the process of
endothelial cell senescence, acting as a potential therapeutic drug
by targeting miR-216a.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the grants from National
Natural Science Foundation of China (grant nos. 81873492 and
81670338) and from State Key Laboratory of Cardiovascular Disease
at Fuwai Hospital (grant no. 2019kf-03).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors contributed to the study conception and
design. Material preparation, data collection and analysis were
performed by YTC, SW, SY, RL and YY. The authenticity of raw data
was assessed and confirmed by YTC, SW, YC and WZ. The draft of the
manuscript was written by YTC, YC and WZ. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Ethics Committee
of Fuwai Hospital, and informed consent was obtained from the
healthy donor.
Patient consent for publication
Not applicable.
Competing interests
The author declare that they have no competing
interests.
References
|
1
|
Evans MA, Sano S and Walsh K:
Cardiovascular disease, aging, and clonal hematopoiesis. Annu Rev
Pathol. 15:419–438. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schaftenaar F, Frodermann V, Kuiper J and
Lutgens E: Atherosclerosis: The interplay between lipids and immune
cells. Curr Opin Lipidol. 27:209–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Libby P, Ridker PM and Hansson GK:
Progress and challenges in translating the biology of
atherosclerosis. Nature. 473:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghosh A, Gao L, Thakur A, Siu PM and Lai
CWK: Role of free fatty acids in endothelial dysfunction. J Biomed
Sci. 24:502017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Menghini R, Stöhr R and Federici M:
MicroRNAs in vascular aging and atherosclerosis. Ageing Res Rev.
17:68–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Childs BG, Gluscevic M, Baker DJ, Laberge
RM, Marquess D, Dananberg J and van Deursen JM: Senescent cells: An
emerging target for diseases of aging. Nat Rev Drug Discov.
16:718–735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feinberg MW and Moore KJ: MicroRNA
regulation of atherosclerosis. Circ Res. 118:703–720. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu Y, Thavarajah T, Gu W, Cai J and Xu Q:
Impact of miRNA of atherosclerosis. Arterioscler Thromb Vasc Biol.
38:e159–e170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang S, Mi X, Chen Y, Feng C, Hou Z, Hui R
and Zhang W: MicroRNA-216a induces endothelial senescence and
inflammation via Smad3/IκBα pathway. J Cell Mole Med. 22:2739–2749.
2018. View Article : Google Scholar
|
|
10
|
Hasegawa H: Proof of the mysterious
efficacy of ginseng: Basic and clinical trials: Metabolic
activation of ginsenoside: Deglycosylation by intestinal bacteria
and esterification with fatty acid. J Pharmacol Sci. 95:153–157.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Han LF, Sakah KJ, Wu ZZ, Liu LL,
Agyemang K, Gao XM and Wang T: Bioactive protopanaxatriol type
saponins isolated from the roots of Panax notoginseng (Burk.) F. H.
Chen. Molecules. 18:10352–10366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Q, Wang T and Wang HY: Ginsenoside
Rb2 enhances the anti-inflammatory effect of ω-3 fatty acid in
LPS-stimulated RAW264.7 macrophages by upregulating GPR120
Expression. Acta Pharmacol Sin. 38:192–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai S, Hong Y, Xu J, Lin Y, Si Q and Gu X:
Ginsenoside Rb2 promotes glucose metabolism and attenuates fat
accumulation via AKT-dependent mechanisms. Biomed Pharmacother.
100:93–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Childs BG, Durik M, Baker DJ and van
Deursen JM: Cellular senescence in aging and age-related disease:
From mechanisms to therapy. Nat Med. 21:1424–1435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wienken CJ, Baaske P, Rothbauer U, Braun D
and Duhr S: Protein-binding assays in biological liquids using
microscale thermophoresis. Nat Commun. 19:1002010. View Article : Google Scholar
|
|
16
|
Jaffe EA, Nachman RL, Becker CG and Minick
CR: Culture of human endothelial cells derived from umbilical
veins. Identification by morphologic and immunologic criteria. J
Clin Invest. 52:2745–2756. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma L, Li G, Cao G, Zhu Y, Du MR, Zhao Y,
Wang H, Liu Y, Yang Y, Li YX, et al: dNK cells facilitate the
interaction between trophoblastic and endothelial cells via VEGF-C
and HGF. Immunol Cell Biol. 95:695–704. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dimri GP, Lee X, Basile G, Acosta M, Scott
G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O,
et al: A biomarker that identifies senescent human cells in culture
and in aging skin in vivo. Proc Natl Acad Sci USA. 92:9363–9367.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu JW, Wei DZ, Du CB and Zhong JJ:
Enhancement of fibrinolytic activity of bovine aortic endothelial
cells by ginsenoside Rb2. Acta Pharmacol Sin. 24:102–108.
2003.PubMed/NCBI
|
|
21
|
Minamino T, Miyauchi H, Yoshida T, Ishida
Y, Yoshida H and Komuro I: Endothelial cell senescence in human
atherosclerosis: Role of telomere in endothelial dysfunction.
Circulation. 105:1541–1544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ling H, Fabbri M and Calin GA: MicroRNAs
and other non-coding RNAs as targets for anticancer drug
development. Nat Rev Drug Discov. 12:847–865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dangwal S and Thum T: MicroRNA
therapeutics in cardiovascular disease models. Annu Rev Pharmacol
Toxicol. 54:185–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rayner KJ, Esau CC, Hussain FN, McDaniel
AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X,
et al: Inhibition of miR-33a/b in non-human primates raises plasma
HDL and lowers VLDL triglycerides. Nature. 478:404–407. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Agostini M and Knight RA: miR-34: From
bench to bedside. Oncotarget. 5:872–881. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thakral S and Ghoshal K: miR-122 is a
unique molecule with great potential in the diagnosis, prognosis of
liver disease and therapy both as miRNA mimic and antimir. Curr
Gene Ther. 15:142–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsui M and Corey DR: Non-coding RNAs as
drug targets. Nat Rev Drug Discov. 16:167–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Rooij E and Olson EN: MicroRNA
therapeutics for cardiovascular disease: Opportunities and
obstacles. Nat Rev Drug Discov. 11:860–872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McClorey G and Wood MJ: An overview of the
clinical application of antisense oligonucleotides for
RNA-targeting therapies. Curr Opin in Pharmacol. 24:52–58. 2015.
View Article : Google Scholar
|
|
31
|
Fan R, Xiao C, Wan X, Cha W, Miao Y, Zhou
Y, Qin C, Cui T, Su F and Shan X: Small molecules with big roles in
microRNA chemical biology and microRNA-targeted therapeutics. RNA
Biol. 16:707–718. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Warner KD, Hajdin CE and Weeks KM:
Principles for targeting RNA with drug-like small molecules. Nat
Rev Drug Discov. 17:547–558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Disney MD: Targeting RNA with small
molecules to capture opportunities at the intersection of
chemistry, biology, and medicine. J Am Chem Soc. 141:6776–6790.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong Y, Yan X, Yang X, Yu C, Deng Y, Song
X and Zhang L: Notoginsenoside R1 suppresses miR-301a via NF-κB
pathway in lipopolysaccharide-treated ATDC5 cells. Exp Mol Pathol.
112:1043552020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng HZ, Fu XK, Shang JL, Lu RX, Ou YF
and Chen CL: Ginsenoside Rg1 protects rat bone marrow mesenchymal
stem cells against ischemia induced apoptosis through miR-494-3p
and ROCK-1. Eur J Pharmacol. 822:154–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng Z and Xing D: Ginsenoside Rg3
inhibits growth and epithelial-mesenchymal transition of human oral
squamous carcinoma cells by down-regulating miR-221. Eur J
Pharmacol. 853:353–363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rippe C, Blimline M, Magerko KA, Lawson
BR, LaRocca TJ, Donato AJ and Seals DR: MicroRNA changes in human
arterial endothelial cells with senescence: Relation to apoptosis,
eNOS and inflammation. Exp Gerontol. 47:45–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiong Y, Yepuri G, Forbiteh M, Yu Y,
Montani JP, Yang Z and Ming XF: ARG2 impairs endothelial autophagy
through regulation of MTOR and PRKAA/AMPK signaling in advanced
atherosclerosis. Autophagy. 10:2223–2238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dela Paz NG and D'Amore PA: Arterial vs.
venous endothelial cells. Cell Tissue Res. 335:5–16. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hirashima M and Suda T: Differentiation of
arterial and venous endothelial cells and vascular morphogenesis.
Endothelium. 13:137–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fleenor BS, Marshall KD, Rippe C and Seals
DR: Replicative aging induces endothelial to mesenchymal transition
in human aortic endothelial cells: Potential role of inflammation.
J Vasc Res. 49:59–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaur J and Farr JN: Cellular senescence in
age-related disorders. Transl Res. 226:96–104. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rufini A, Tucci P, Celardo I and Melino G:
Senescence and aging: The critical roles of p53. Oncogene.
32:5129–5143. 2013. View Article : Google Scholar : PubMed/NCBI
|