Introduction
Acute kidney injury (AKI) refers to an acute
decrease or even loss of kidney function within hours that is
caused by a variety of factors, including sepsis, ischaemia, and
nephrotoxicity (1). According to
the location and aetiology of the lesion, AKI is classified as
prerenal AKI, renal parenchymal or renal vascular disease-related
AKI, and postrenal AKI (2). Sepsis
often leads to septic shock and multiple organ dysfunction, and AKI
is one of the common lethal complications of sepsis (3,4). Acute
peritoneal dialysis and continuous renal replacement therapy are
the most common clinical treatment methods for AKI (5,6).
Although numerous existing studies have focused on the treatment of
AKI, a novel treatment protocol remains to be established.
Autophagy degrades and recycles damaged
macromolecules and organelles to maintain the stability of the
intracellular environment. Autophagy is activated under stress
conditions, including endoplasmic reticulum stress, oxidant injury,
cell starvation, hypoxia, and nutrient deprivation (7,8). Most
of the stress conditions for activating autophagy are involved in
the pathogenesis of AKI (7,8). The reactive oxygen species (ROS) and
oxidative stress involved in AKI trigger autophagy in animal cells
and tissues, resulting in aggravated kidney injury (9). Autophagy can antagonise apoptosis, and
the inhibition of autophagy aggravates sepsis-induced AKI (10,11).
Autophagy in the stress response is mediated by diverse signalling
pathways, such as the phosphoinositide 3-kinase (PI3K)/protein
kinase B (AKT), ROS/c-Jun N-terminal kinase (JNK), and
AMP-activated protein kinase (AMPK)/mammalian target of rapamycin
(mTOR) pathways (12–15). The AMPK/mTOR pathway plays a major
role in AKI autophagy. Previous findings have indicated that
recombinant human erythropoietin suppresses lipopolysaccharide
(LPS)-induced cell apoptosis in AKI rats via AMPK-mediated
autophagy (16). Furthermore,
NAD-dependent deacetylase sirtuin-3 alleviates AKI by inducing
autophagy via regulation of the AMPK/mTOR pathway (17).
Zinc-finger E-box-binding homeobox 1 (ZEB1), a
member of the ZEB family, plays a critical role in
epithelial-mesenchymal transition (EMT) (18–20).
In addition, numerous studies have demonstrated that ZEB1 plays a
major role in a variety of diseases, such as invasive
endometriosis, cerebral arterial thrombosis, brain ischaemia, and
ischaemic stroke (21–24). A previous study reported that ZEB1
overexpression plays a protective role in brain ischaemia (23). ZEB1 overexpression has been
confirmed to ameliorate brain damage after acute ischaemic stroke
by reducing central nervous system inflammation (24). Additionally, ZEB1 protects skeletal
muscle from damage and accelerates skeletal muscle regeneration
(25). However, studies of ZEB1
effects on AKI are limited.
In the present study, an AKI model in rats was
established using the cecal ligation and puncture (CLP) method. The
autophagy, inflammation, apoptosis, and injury of kidney tissues
were evaluated in CLP-induced AKI rats. In addition, the regulatory
mechanism of ZEB1 involving the AMPK/mTOR pathway was analysed in
CLP-induced AKI rats. The present study findings may provide a new
theoretical foundation for the treatment of AKI.
Materials and methods
Establishment of the adenovirus (Ad)
vector
The ZEB1 fragment was amplified and inserted into
the overexpression vector pcDNA 3.1 (promoter CMV; Invitrogen;
Thermo Fisher Scientific, Inc.) to construct pcDNA-ZEB1.
Thereafter, the fragment was sub-cloned into the Ad vector
pAdTrack-CMV (promoter CMV; Agilent Technologies, Inc.) to
construct Ad-ZEB1. The empty pAdTrack-CMV vector was used as a
negative control (Ad-NC).
Rat model of CLP-induced AKI
A total of 70 male Sprague-Dawley rats (age, ~8
weeks; weight, 250±10 g) were obtained from Laboratory Animal
Center of Southern Medical University (Guangzhou, China). All rats
in experiments were provided free access to water and normal rat
chow and were acclimatized for 7 days at 24°C, 60±10% humidity and
12-h light/dark cycles. Rats were randomly divided into seven
groups (n=10 in each group): Blank, sham, CLP, CLP + Ad-NC, CLP +
Ad-ZEB1, CLP + Ad-NC + dorsomorphin (DM; Sigma-Aldrich: Merck
KGaA), and CLP + Ad-ZEB1 + DM. CLP-induced AKI was constructed in
the rats as previously described (26). Briefly, all rats fasted for 12 h
before CLP surgery. The rats underwent 1 cm midline laparotomy
under anaesthesia (intraperitoneal injection of 50 mg/kg
pentobarbital sodium). The caecum was ligated with a 4-0 silk
suture at 1 cm from the end. A 22-gauge needle was used to puncture
the caecum with two holes close to the ligation site. A small
amount of faeces was squeezed out through the two holes to induce
sepsis. Sham rats only underwent laparotomy and bowel manipulation.
Ad-ZEB1 and Ad-NC (2×107 TU/ml) were intravenously
injected into the tail of rats in the CLP + Ad-NC and CLP + Ad-ZEB1
groups, respectively, 2 days before CLP. In the CLP + Ad-NC + DM
group, rats were intravenously injected with Ad-NC caudally and
intraperitoneally injected with DM (20 mg/kg) before CLP. In the
CLP + Ad-ZEB1 + DM group, rats were intravenously injected with
Ad-ZEB1 caudally and intraperitoneally injected with DM (20 mg/kg)
before CLP. The rats were anaesthetised by intraperitoneal
injection of pentobarbital sodium (50 mg/kg) and sacrificed by
cervical dislocation. All experimental protocols were approved by
the Ethics Committee of the First Affiliated Hospital of Shandong
First Medical University. All procedures were performed in
accordance with ethical standards and laboratory care and use
guidelines of the First Affiliated Hospital of Shandong First
Medical University.
Assessment of renal function
Blood samples were collected from the inferior vena
cava of the rats. The blood urea nitrogen (BUN) and serum
creatinine (SCr) levels were measured using an automated
biochemical analyser (TBA-40FR; Toshiba). The 24-h urine volume was
measured gravimetrically. Urinary sodium was measured with an
ion-selective electrode (Nova Biomedical), and urine osmolality was
measured using a freezing-point osmometer (3D3; Advanced
Instruments, Inc.). The mean arterial pressure (MAP) was measured
using a non-invasive blood pressure monitoring system (ALC-NIBP;
Alcott Biotech Co., Ltd.). The renal blood flow (RBF) was recorded
using PowerLab (model 8S) and LabChart software (version 5.1.1,
ADInstruments PTY, Inc.) and calculated as the average flow during
the first 10-sec interval of each minute over a 10-min period after
stabilised flow.
Sepsis score
After treatment for 24 h, the sepsis in rats was
evaluated according to Shrum's scoring system (27). The scores included appearance,
consciousness, activity, response to stimulus, eyes, and
respiration rate and quality (0–4 points). A higher total score
indicates greater sepsis severity.
Hematoxylin and eosin (H&E)
staining
The kidney tissues were collected and fixed in 4%
formaldehyde solution for 24 h at room temperature.
Paraffin-embedded kidney tissues were sectioned into 5-µm-thick
sections. The sections were deparaffinised with xylene, dehydrated
with graded ethanol, and stained with H&E for 15 min at room
temperature. Histopathological changes of the kidney tissues were
observed under a light microscope at a magnification of ×200. Five
fields were selected randomly for each animal. The degree of kidney
damage was assessed according to the following criteria (28): 0, normal; 1, <25%; 2, 25–50%; 3,
50–75%; and 4, 75–100%.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
Cell apoptosis was detected using a TUNEL kit
(TransGen Biotech Co., Ltd.). In brief, the samples were fixed with
4% paraformaldehyde at 4°C for 4 h. Following this, samples were
treated with 3% hydrogen peroxidase and incubated in a labeling
reaction mixture comprised of terminal deoxynucleotidyl transferase
and deoxynucleotides overnight at 4°C. Sections were then subjected
to further incubation with horseradish peroxidase (1:500; Shanghai
Macklin Biochemical Co., Ltd.) for 30 min and treatment with
3,3′-diaminobenzidine for 15 min at 37°C in the dark. Reactions
were stopped with running water and counterstaining was performed
with hematoxylin at 37°C for 10 min. Following dehydration with a
graded ethyl alcohol series and xylene treatment, tissue samples
were mounted on coverslips with neutral gum. Apoptotic nuclei
appeared as dark brown dots. TUNEL-positive cells were counted in
five randomly selected regions (magnification, ×200), and the
percentage of TUNEL-positive cells was calculated using Image-Pro
Plus software (Media Cybernetics, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from kidney tissues was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. For cDNA synthesis, 2
µg of total RNA was reverse transcribed using the PrimeScript™ RT
Reagent Kit with gDNA Eraser (Perfect Real Time; Takara Bio, Inc.).
qPCR was carried out using SYBR-Green PCR Master Mix (Takara Bio,
Inc.) on a Bio-Rad Real-Time PCR instrument (Bio-Rad Laboratories,
Inc.). The following thermocycling conditions were used for qPCR:
Initial denaturation at 95°C for 3 min; followed by 40 cycles at
95°C for 15 sec, annealing at 60°C for 30 sec and elongation at
72°C for 1 min; and final extension at 72°C for 5 min. The
expression levels of ZEB1, interleukin (IL)-6, IL-1β, and tumour
necrosis factor (TNF)-α were normalised to glyceraldehyde
3-phosphate dehydrogenase and calculated with the 2−ΔΔCq
method (29). The primer sequences
used were as follows: ZEB1 forward, 5′-AGCAGTGAAAGAGAAGGGAATGC-3′
and reverse, 5′-GGTCCTCTTCAGGTGCCTCAG-3′; IL-6 forward,
5′-TCCAGTTGCCTTCTTGGGAC-3′ and reverse, 5′-GTACTCCAGAAGACCAGAGG-3′;
IL-1β forward, 5′-CCAGCTTCAAATCTCACAGCAG-3′ and reverse,
5′-CTTCTTTGGGTATTGCTTGGGATC-3′; TNF-α forward,
5′-CACAGAAAGCATGATCCGCGA-3′ and reverse,
5′-CGGCAGAGAGGAGGTTGACTTTCT-3′.
Western blotting
The kidney tissues were lysed with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology). The protein concentration was detected via BCA
Protein Assay kit and 50 µg protein/lane was separated via 10%
sodium dodecyl sulphate polyacrylamide gel electrophoresis and
transferred onto nitrocellulose membranes. The membranes were
blocked with 5% skim milk for 2 h at 25°C and then incubated with
the following primary antibodies: anti-ZEB1 (1:1,000; product code
ab124512; Abcam), anti-AMPKα1 (1:1,000; product code ab32047),
anti-phosphorylated (p)-AMPKα1 (1:1,000; product code ab92701),
anti-mTOR (1:1,000; product code ab32028), anti-p-mTOR (1:1,000;
product code ab109268), anti-caspase-9 (1:1,000; product code
ab184786), anti-caspase-3 (1:1,000; product code ab13847),
anti-microtubule-associated protein 1 light chain-3B (LC3B;
1:1,000; product code ab48394), anti-LC3A/B (1:1,000; product code
ab62721), and anti-Beclin-1 (1:1,000; product code ab210498; all
from Abcam) overnight at 4°C. The membranes were subsequently
incubated with the horseradish peroxidase-conjugated anti-mouse IgG
secondary antibody (1:5,000; product code ab6728; Abcam) for 2 h at
25°C. The protein blots were developed with an enhanced
chemiluminescence kit (Invitrogen; Thermo Fisher Scientific, Inc.).
Quantity One 1-D Analysis Software (Bio-Rad Laboratories, Inc.) was
used to measure the density of the western blot bands.
Statistical analysis
All data were analysed using SPSS Statistics 23.0
software (IBM Corp.). Data were expressed as the mean ± standard
deviation. One-way ANOVA followed by Tukey's post hoc test was used
to evaluate the differences among multiple groups, whereas acute
kidney damage score and sepsis score were analysed using the
Kruskal-Wallis test and Dunn's multiple comparison test,
respectively. P-values <0.05 were considered to indicate
statistically significant differences. All experiments were
performed in triplicate.
Results
ZEB1 expression is downregulated in
CLP-induced AKI
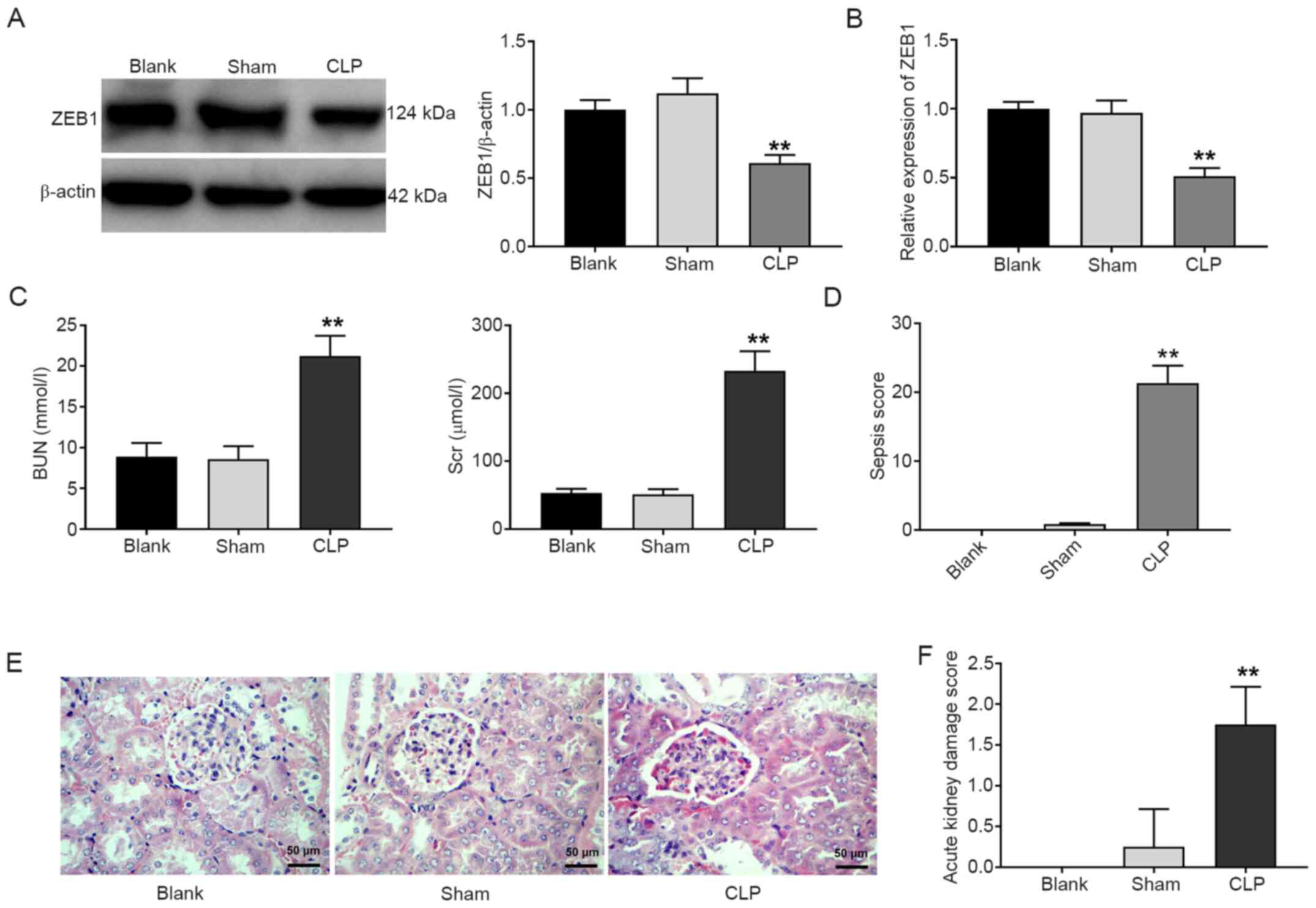
As observed from the results of the western blotting
assay, the expression of the ZEB1 protein in the CLP group was
significantly decreased compared with that in the blank group
(P<0.01; Fig. 1A). In addition,
the RT-qPCR results presented similar findings (P<0.01; Fig. 1B). The BUN and SCr levels in the CLP
group were significantly enhanced compared with those in the blank
group (P<0.01; Fig. 1C).
Compared with the blank group, the sepsis score in the CLP group
was also increased (P<0.01; Fig.
1D). Furthermore, pathological changes of the kidney tissues
were observed under a light microscope. The kidney tubules and
glomerulus structures were normal in the sham group. Telangiectasia
and severe congestion were observed in the kidney tissues of the
CLP group (Fig. 1E). The acute
kidney damage score of the CLP group was higher than that of the
blank group (P<0.01; Fig. 1F).
In addition, several other kidney injury-related parameters were
measured. As presented in Table I,
urine volume and urine sodium were significantly increased, whereas
urine osmolality, MAP, and RBF were decreased in the CLP group
compared with the blank group (P<0.01).
 | Table I.Kidney function-associated parameters
in rats. |
Table I.
Kidney function-associated parameters
in rats.
| Parameters | Blank | Sham | CLP |
|---|
| Urine volume (ml/24
h) | 14.78±2.23 | 15.13±1.12 |
34.61±2.34a |
| Urine osmolality
(mOsm/kg) | 818.29±62.46 | 856.45±57.28 |
349.59±39.62a |
| Urine sodium
(%) | 0.11±0.02 | 0.13±0.01 |
1.59±0.48a |
| MAP (mmHg) | 112.48±11.77 | 106.62±10.33 |
87.46±7.37a |
| RBF (ml/min/g) | 4.28±0.46 | 4.34±0.28 |
2.46±0.13a |
Autophagy and the AMPK/mTOR pathway
are blocked in CLP-induced AKI
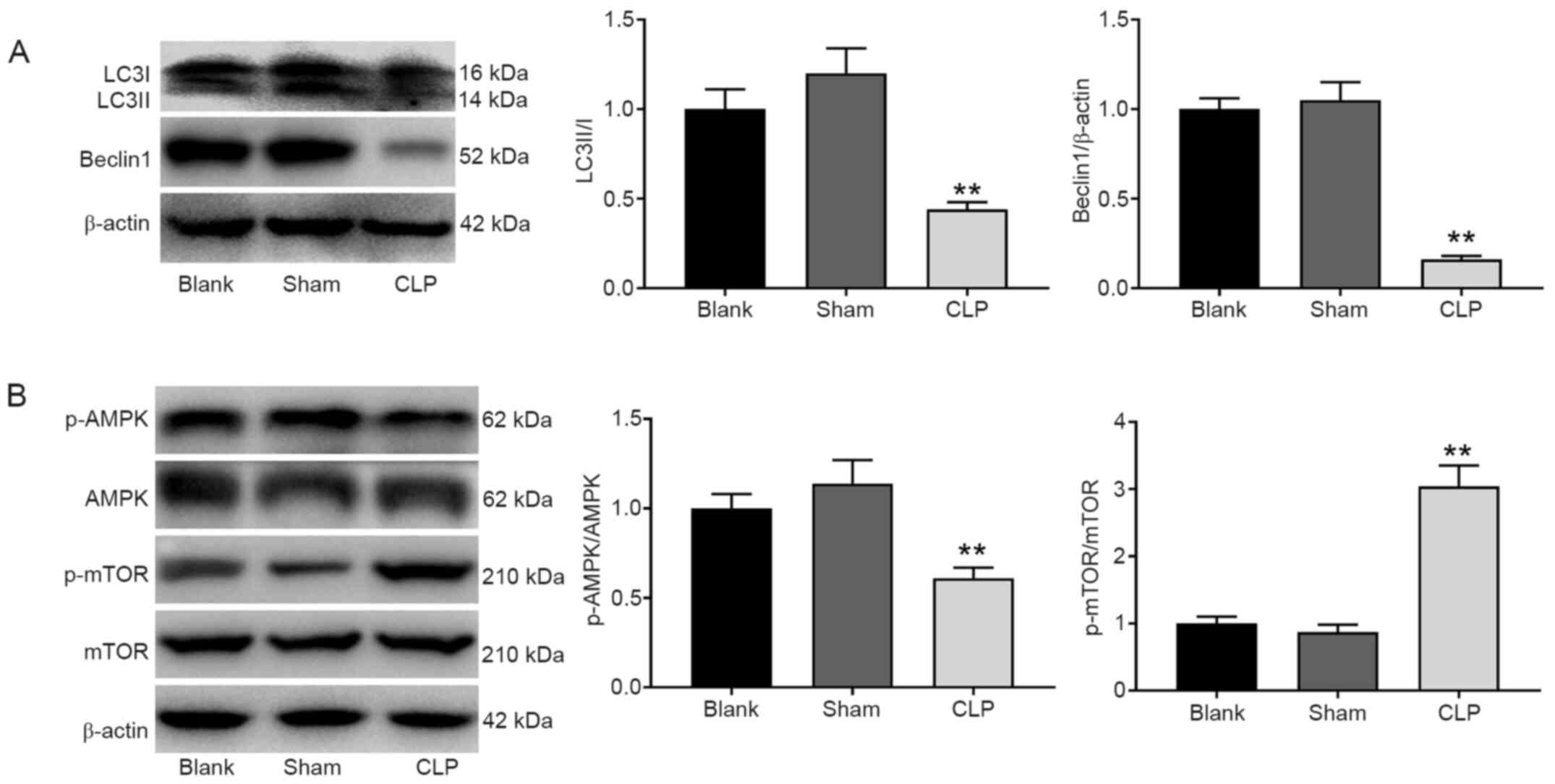
Western blot analysis revealed that the expression
levels of the autophagy marker proteins Beclin-1 and LC3A/B were
significantly decreased in the CLP group compared with the blank
group (P<0.01; Fig. 2A). In
addition, the protein expression of p-AMPK/AMPK was decreased while
the protein expression of p-mTOR/mTOR was increased in the CLP
group compared with the blank group (P<0.01; Fig. 2B).
Inflammation and apoptosis are
enhanced in CLP-induced AKI
As revealed in Fig.
3A, the expression levels of IL-1β, IL-6 and TNF-α in the CLP
group were significantly increased compared with those in the blank
group (P<0.01). An increased number of TUNEL-positive cells was
observed in the CLP group compared with the blank group (P<0.01;
Fig. 3B). The protein expression
levels of caspase-9 and caspase-3 were also detected via western
blotting. Compared with the blank group, the protein expression
levels of caspase-9 and caspase-3 were significantly increased in
the CLP group (P<0.01; Fig.
3C).
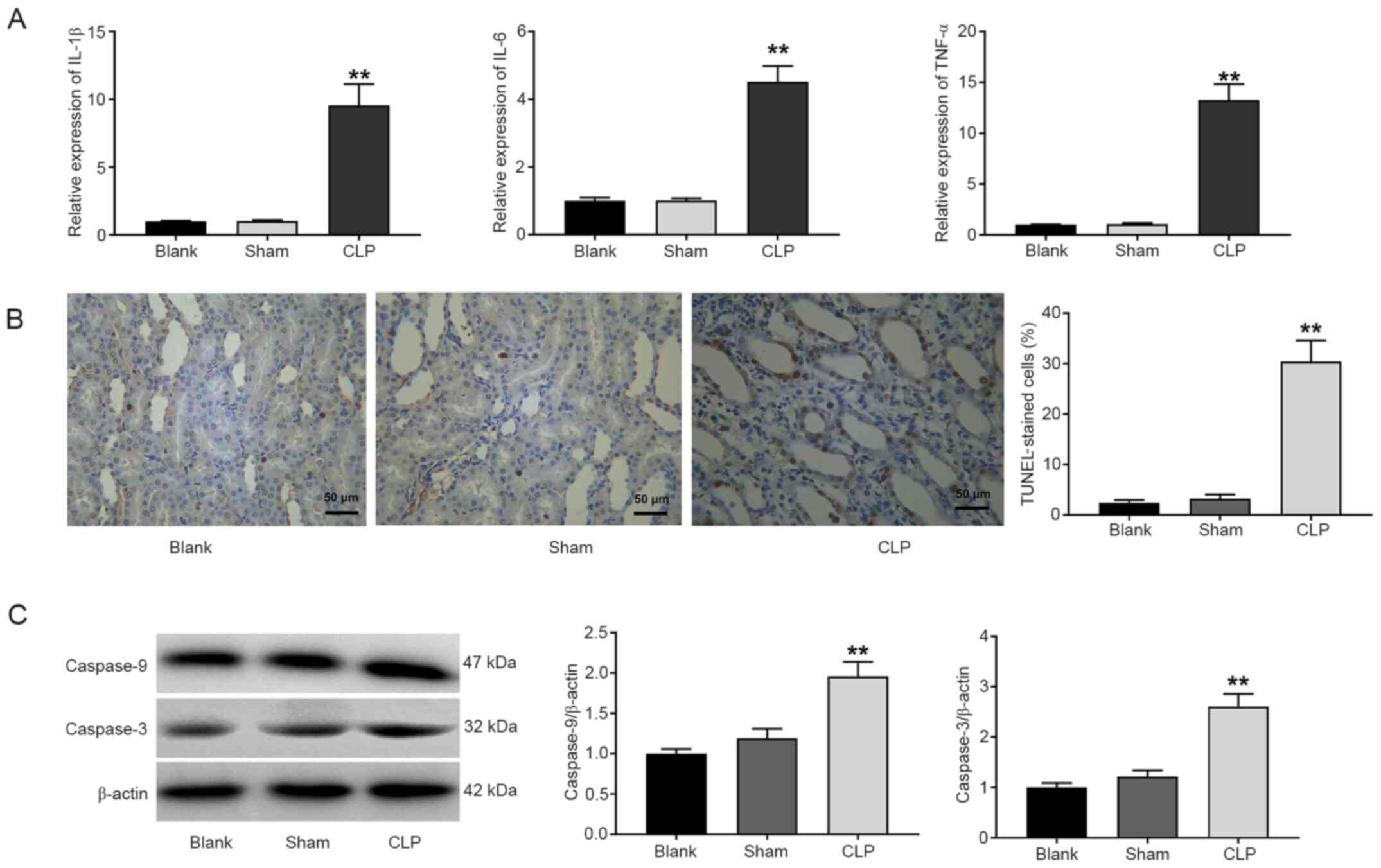 | Figure 3.Inflammation and apoptosis are
enhanced in CLP-induced AKI. (A) Reverse transcription-quantitative
polymerase chain reaction analysis of the levels of IL-6, IL-1β,
and TNF-α. (B) TUNEL staining of apoptosis in kidney tissues (scale
bar, 50 µm). (C) Western blot analysis of caspase-9 and caspase-3
expression in kidney tissues. Blank, normal rats; Sham, rats
underwent laparotomy and bowel manipulation without CLP; CLP, rats
underwent CLP. **P<0.01 compared with the blank group. The data
are presented as the mean ± SD of 3 independent experiments. CLP,
cecal ligation and puncture; AKI, acute kidney injury; IL,
interleukin; TNF-α, tumour necrosis factor-α; TUNEL, terminal
deoxynucleotidyl transferase dUTP nick end labeling. |
Overexpression of ZEB1 activates the
AMPK/mTOR pathway in CLP-induced AKI
To validate whether ZEB1 participated in AKI by
regulating the AMPK/mTOR pathway, the protein expression levels of
ZEB1, p-AMPK/AMPK, and p-mTOR/mTOR were detected after injection of
Ad-ZEB1 and DM into CLP-induced AKI rats. The protein expression of
ZEB1 was significantly increased in the CLP + Ad-ZEB1 group
compared with the CLP group (P<0.01; Fig. 4A). Similarly, through RT-qPCR
analysis, increased ZEB1 expression was detected in the CLP +
Ad-ZEB1 group compared with the CLP group (P<0.01; Fig. 4B). As revealed in Fig. 4C, the expression of p-AMPK/AMPK was
significantly increased while that of p-mTOR/mTOR was significantly
decreased in the CLP + Ad-ZEB1 group compared with the CLP + Ad-NC
group (P<0.01). The effects of ZEB1 overexpression on the
expression levels of p-AMPK/AMPK and p-mTOR/mTOR were significantly
reversed by DM.
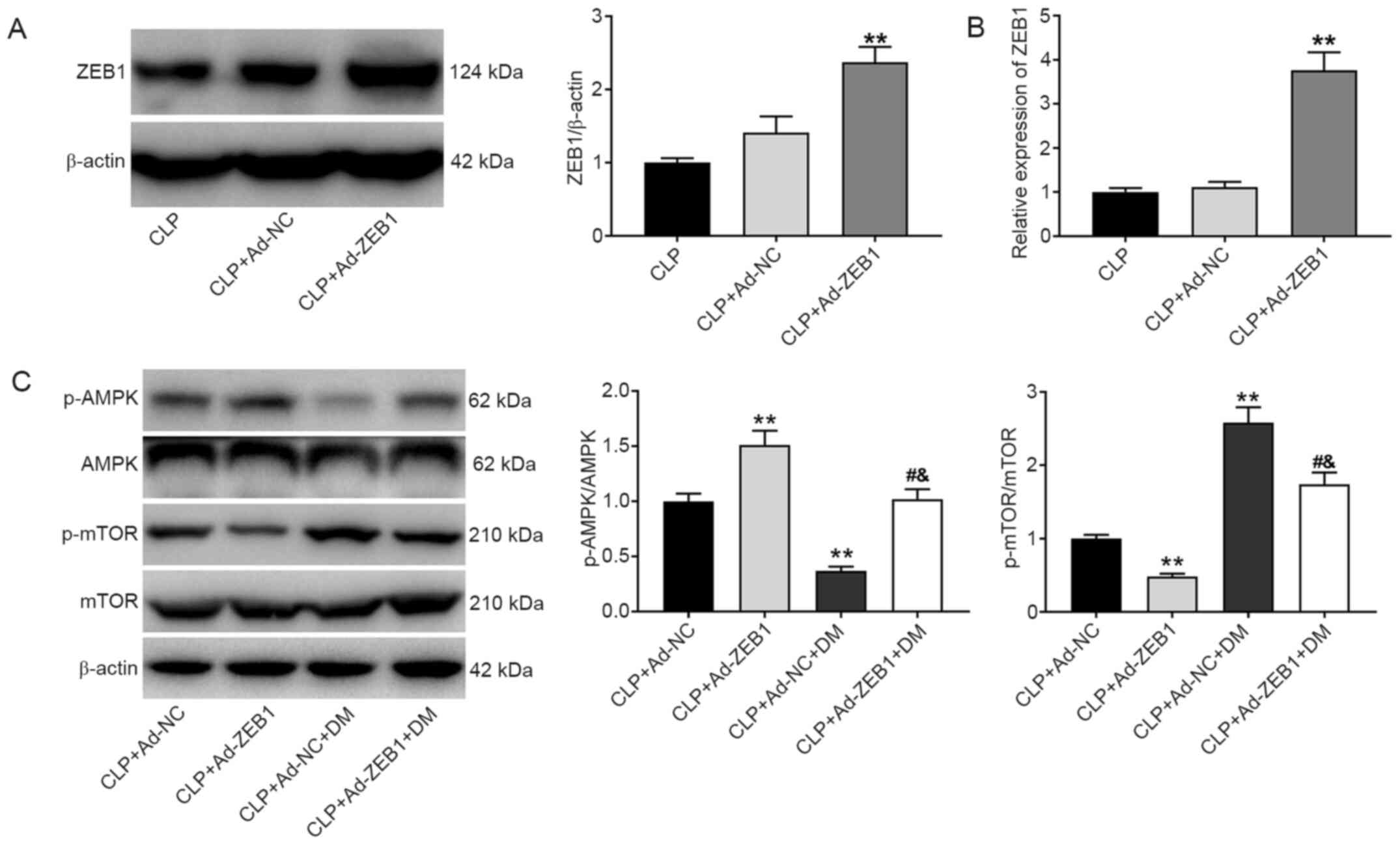 | Figure 4.ZEB1 overexpression activates the
AMPK/mTOR signaling pathway in CLP-induced AKI. (A) Western blot
analysis of ZEB1 expression in kidney tissues. (B) Reverse
transcription-quantitative polymerase chain reaction analysis of
ZEB1 expression in kidney tissues. (C) Western blot analysis of
p-AMPK/AMPK and p-mTOR/mTOR expression in kidney tissues. CLP +
Ad-NC, rats were caudally injected with Ad-NC before CLP. CLP +
Ad-ZEB1, rats were caudally injected with Ad-ZEB1 before CLP. CLP +
Ad-NC + DM, rats were intravenously injected with Ad-NC caudally
and intraperitoneally injected with AMPK inhibitor DM before CLP.
CLP + Ad-ZEB1 + DM, rats were intravenously injected with Ad-ZEB1
caudally and intraperitoneally injected with DM before CLP.
**P<0.01 vs. CLP or CLP + Ad-NC group; #P<0.05
compared with CLP + Ad-ZEB1 group; &P<0.05 vs.
CLP + Ad-NC + DM group. The data are presented as the mean ± SD of
3 independent experiments. ZEB1, zinc-finger E-box-binding homeobox
1; AMPK, AMP-activated protein kinase; mTOR, mammalian target of
rapamycin; CLP, cecal ligation and puncture; AKI, acute kidney
injury; p-, phosphorylated; DM, dorsomorphin. |
Overexpression of ZEB1 alleviates AKI
and enhances autophagy in CLP-induced AKI
The role of ZEB1 in CLP-induced AKI and autophagy
was further evaluated. As presented in Fig. 5A-C, the BUN and SCr levels, acute
kidney damage score, and sepsis score were significantly decreased
by ZEB1 overexpression in CLP-induced AKI (P<0.01). The
inhibitory effect of ZEB1 overexpression on AKI was eliminated by
DM (P<0.01). In addition, according to the histopathological
changes presented in Fig. 5B, it
was observed that overexpression of ZEB1 obviously attenuated the
damage (telangiectasia and severe congestion) of CLP on the kidney
tubules and glomerulus. However, treatment with DM reversed this
condition. The expression levels of the autophagy-related proteins
Beclin-1 and LC3A/B were measured via western blotting.
Overexpression of ZEB1 significantly increased the expression
levels of LC3A/B and Beclin-1 in CLP-induced AKI (P<0.01). DM
reversed the effect of ZEB1 overexpression on the autophagy of
CLP-induced AKI (P<0.01; Fig.
5D).
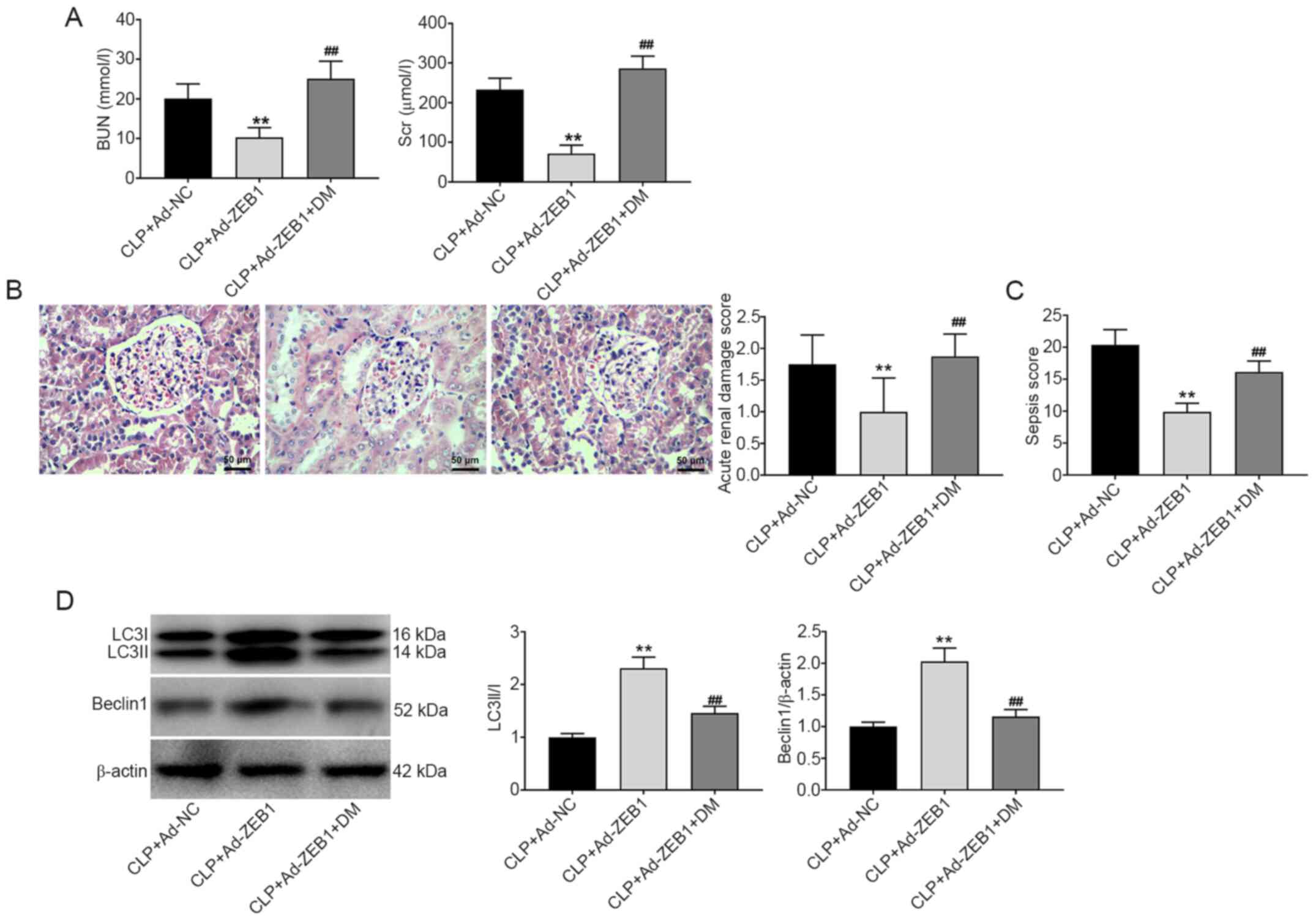 | Figure 5.ZEB1 overexpression alleviates AKI
and enhances autophagy in CLP-induced AKI. (A) The levels of BUN
and SCr in rats. (B) Pathological changes of renal tissues observed
by hematoxylin and eosin staining (scale bar, 50 µm) and the acute
kidney damage score of rats. (C) The sepsis score in rats. (D)
Western blotting analysis of Beclin1 and LC3II/I expression in
kidney tissues. CLP + Ad-NC, rats were intravenously injected with
Ad-NC caudally before CLP. CLP + Ad-ZEB1, rats were intravenously
injected with Ad-ZEB1 caudally before CLP. CLP + Ad-ZEB1 + DM, rats
were intravenously injected with Ad-ZEB1 caudally and
intraperitoneally injected with DM before CLP. **P<0.01 compared
with the CLP + Ad-NC group; ##P<0.01 compared with
the CLP + Ad-ZEB1 group. The data are presented as the mean ± SD of
3 independent experiments. ZEB1, zinc-finger E-box-binding homeobox
1; AKI, acute kidney injury; CLP, cecal ligation and puncture; BUN,
blood urea nitrogen; SCr, serum creatinine; DM, dorsomorphin. |
Overexpression of ZEB1 inhibits
inflammation and apoptosis in CLP-induced AKI
The effects of ZEB1 on inflammation and cell
apoptosis in CLP-induced rats were measured. Compared with the CLP
+ Ad-NC group, the expression levels of IL-1β, IL-6 and TNF-α were
significantly reduced in the CLP + Ad-ZEB1 group (P<0.01;
Fig. 6A). Conversely, DM recovered
the expression levels of IL-6, IL-1β, and TNF-α that were reduced
by ZEB1 overexpression (P<0.01). The cell apoptosis and protein
expression levels of caspase-9 and caspase-3 were detected via
TUNEL and western blotting. The number of TUNEL-positive cells and
the expression levels of caspase-9 and caspase-3 were decreased in
the CLP + Ad-ZEB1 group compared with the CLP + Ad-NC group
(P<0.01). The inhibitory effect of ZEB1 overexpression on cell
apoptosis was reversed by intraperitoneal injection of DM
(P<0.01; Fig. 6B and C).
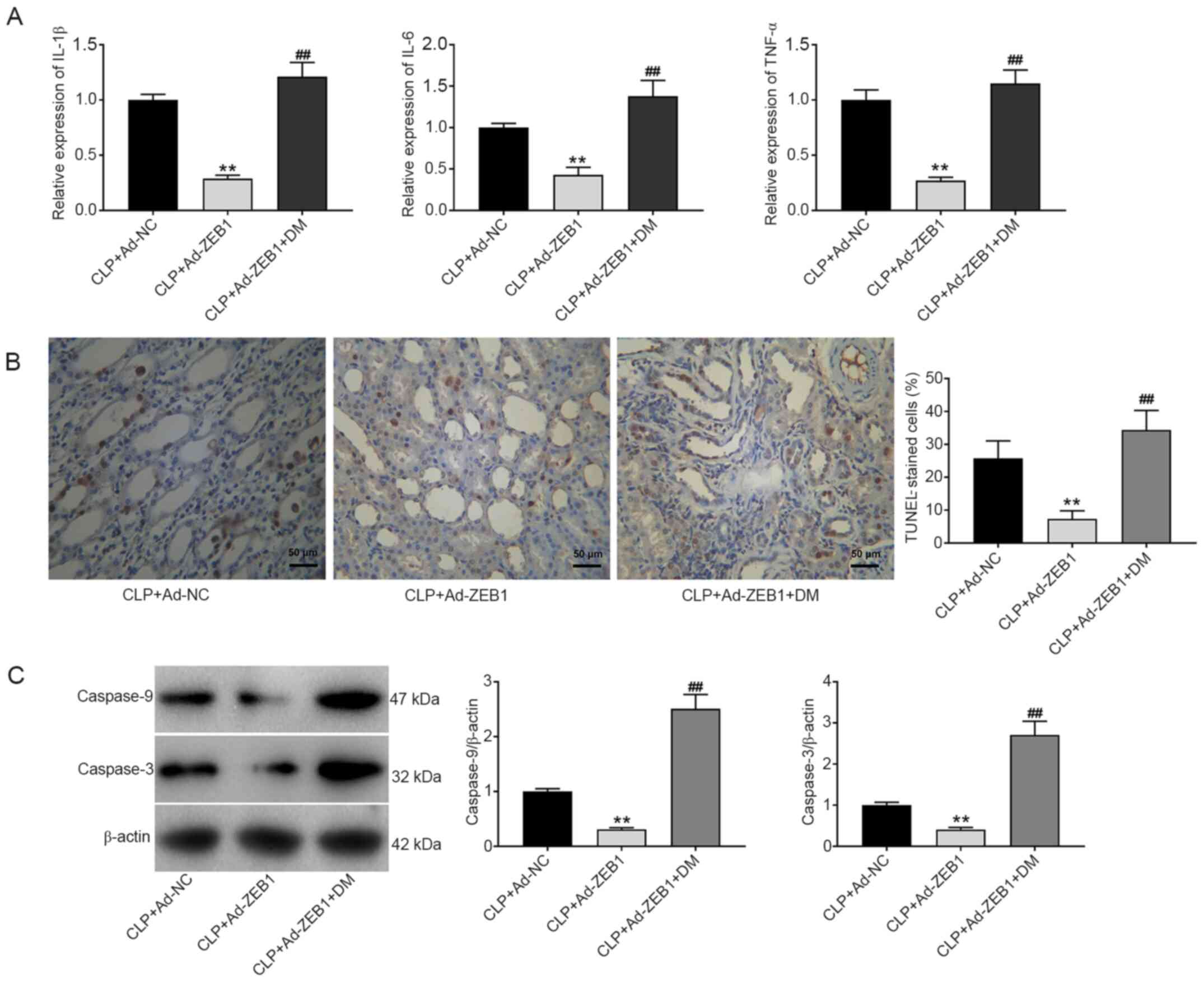 | Figure 6.ZEB1 overexpression inhibits the
inflammation and apoptosis in CLP-induced AKI. (A) Reverse
transcription-quantitative polymerase chain reaction analysis of
the levels of IL-6, IL-1β and TNF-α in kidney tissues. (B) TUNEL
staining of apoptosis in kidney tissues (scale bar, 50 µm). (C)
Western blot analysis of caspase-9 and caspase-3 expression in
kidney tissues. CLP + Ad-NC, rats were intravenously injected with
Ad-NC caudally before CLP. CLP + Ad-ZEB1, rats were intravenously
injected with Ad-ZEB1 caudally before CLP. CLP + Ad-ZEB1 + DM, rats
were intravenously injected with Ad-ZEB1 caudally and
intraperitoneally injected with DM before CLP. **P<0.01 compared
with the CLP + Ad-NC group; ##P<0.01 compared with
the CLP + Ad-ZEB1 group. The data were presented as the mean ± SD
of 3 independent experiments. ZEB1, zinc-finger E-box-binding
homeobox 1; CLP, cecal ligation and puncture; AKI, acute kidney
injury; IL, interleukin; TNF-α, tumour necrosis factor-α; DM,
dorsomorphin; TUNEL, terminal deoxynucleotidyl transferase dUTP
nick end labeling. |
Discussion
Sepsis is the most common cause of AKI in critical
patients and is recognised as the foremost precipitant of AKI
(30,31). At present, in vivo
experiments are the primary means of basic research on sepsis.
Animal models can effectively simulate the pathological process of
sepsis and can be conducive to elucidating the pathogenesis of AKI
in humans (32). In the present
study, the AKI model was evaluated by analysing BUN, SCr, acute
kidney damage score, urine volume, urine osmolality, urine sodium,
MAP, and RBF. The data revealed that the acute kidney damage score,
BUN level, SCr level, urine volume, and urine sodium were higher
while urine osmolality, MAP, and RBF were lower in the CLP group
than those in the blank group. These results indicated that CLP
induced severe kidney injury and that the rat AKI model was
successfully established.
Autophagy is an essential pathway for the
maintenance of cellular homeostasis and the response to stress, and
dysregulation of autophagy is involved in the pathogenesis of
numerous diseases (33–38). Growing evidence has revealed that
autophagy protects the kidneys from various kidney inflammatory
insults (39,40). Eva-1 homolog A-mediated autophagy
was revealed to weaken liver injury in acute liver failure mice by
attenuating inflammatory responses and apoptosis (41). The inhibition of autophagy was
revealed to aggravate apoptosis, inflammation, and oxidative stress
in the lung tissues of traumatic brain injury rats (42). Autophagy activation attenuated the
levels of TNF-α and IL-6 in ischaemia reperfusion injury of the
kidneys (43). In the present
study, the results revealed that the expression levels of Beclin-1
and LC3A/B were significantly decreased in CLP rats. These data
indicated that autophagy was blocked in the progression of AKI. It
was speculated that autophagy activation may relieve AKI by
inhibiting cell apoptosis and inflammation.
A previous study revealed that ZEB1 expression was
suppressed in hypoxia-induced cell injury (44). A similar result was observed in
CLP-induced AKI. In the present study, the expression of ZEB1 was
reduced in CLP-induced rats. In addition, it was revealed that ZEB1
overexpression decreased inflammation in CLP-induced AKI rats. This
result is consistent with a previous study reporting that ZEB1
overexpression reduced the inflammation and brain damage caused by
acute ischaemic stroke (24).
Additionally, ZEB1 was overexpressed in AKI rats by injection of
Ad-ZEB1 and it was revealed that overexpression of ZEB1 increased
the expression levels of Beclin-1 and LC3A/B as well as inhibited
inflammation and apoptosis in the kidney tissues of AKI rats. These
results indicated that ZEB1 may relieve AKI by activating autophagy
and inhibiting inflammation and apoptosis. Furthermore, in addition
to the role of ZEB1 in autophagy, inflammation, and apoptosis, ZEB1
has been reported to be correlated with EMT progression (18,20,45).
Xiong et al (20) revealed
that ZEB1 was a transcriptional repressor of E-cadherin, which
regulated the EMT of tubular epithelial cells. Because EMT plays a
pivotal role in the process of renal fibrosis, it was hypothesised
that ZEB1 may also influence AKI by regulating EMT. However, there
is also a potential limitation in this study. The time-course
expression profiles of ZEB1 were not measured in kidney tissues of
AKI rats after injection of Ad-ZEB1. The optimal action time of
Ad-ZEB1 still needs to be determined.
The AMPK/mTOR pathway is widely involved in the
development of numerous diseases, including inflammation, cardiac
dysfunction, and AKI (17,46–48).
The present study revealed that the AMPK/mTOR pathway was blocked
in CLP-induced AKI rats. Overexpression of ZEB1 activated the
AMPK/mTOR pathway in CLP-induced AKI rats. In addition, the
injection of the AMPK inhibitor DM reversed the protective effect
of ZEB1 on CLP-induced AKI. These results indicated that ZEB1
overexpression relieved CLP-induced AKI by activating the AMPK/mTOR
pathway. A previous study revealed that preconditioning mice with
an AMPK activator ameliorated ischaemic AKI in vivo
(47). The protective effect of
curcumin on LPS-induced acute lung injury was exerted via AMPK
activation (49). Thus, it was
speculated that ZEB1-mediated activation of the AMPK/mTOR pathway
may contribute to AKI remediation.
In conclusion, ZEB1 alleviated CLP-induced AKI in
rats by activating autophagy as well as inhibiting inflammation and
apoptosis. The protective effect of ZEB1 overexpression on AKI was
achieved via activation of the AMPK/mTOR pathway. The present
findings indicated that ZEB1 may be a potential therapeutic target
for AKI. However, further research is required to identify the role
of autophagy and the underlying mechanisms in AKI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors contributed to the study conception and
design. Material preparation, data collection and analysis were
performed by XL, LZ and BZ. The first draft of the manuscript was
written by DS. DS and BZ authenticated all the raw data. All
authors were involved in writing and revising, as well as reading
and approving the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Ethics Committee of The First Affiliated Hospital of Shandong First
Medical University. All procedures were performed in accordance
with ethical standards and laboratory care and use guidelines of
the First Affiliated Hospital of Shandong First Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Makris K and Spanou L: Acute kidney
injury: Definition, pathophysiology and clinical phenotypes. Clin
Biochem Rev. 37:85–98. 2016.PubMed/NCBI
|
|
2
|
Chen H and Busse LW: Novel therapies for
acute kidney injury. Kidney Int Rep. 2:785–799. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wafaisade A, Lefering R, Bouillon B, Sakka
SG, Thamm OC, Paffrath T, Neugebauer E and Maegele M; Trauma
Registry of the German Society for Trauma Surgery, : Epidemiology
and risk factors of sepsis after multiple trauma: An analysis of
29,829 patients from the Trauma Registry of the German Society for
Trauma Surgery. Crit Care Med. 39:621–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arulkumaran N, Sixma ML, Jentho E,
Ceravola E, Bass PS, Kellum JA, Unwin RJ, Tam FW and Singer M:
Sequential analysis of a panel of biomarkers and pathologic
findings in a resuscitated rat model of sepsis and recovery. Crit
Care Med. 45:e821–e830. 2017. View Article : Google Scholar
|
|
5
|
Cho S, Lee YJ and Kim SR: Acute peritoneal
dialysis in patients with acute kidney injury. Perit Dial Int.
37:529–534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ueno T: The roles of continuous renal
replacement therapy in septic acute kidney injury. Artif Organs.
41:667–672. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaushal GP and Shah SV: Autophagy in acute
kidney injury. Kidney Int. 89:779–791. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kundu M and Thompson CB: Autophagy: Basic
principles and relevance to disease. Annu Rev Pathol. 3:427–455.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Srivastava RK, Traylor AM, Li C, Feng W,
Guo L, Antony VB, Schoeb TR, Agarwal A and Athar M: Cutaneous
exposure to lewisite causes acute kidney injury by invoking DNA
damage and autophagic response. Am J Physiol Renal Physiol.
314:F1166–F1176. 2018. View Article : Google Scholar
|
|
10
|
Mei S, Livingston M, Hao J, Li L, Mei C
and Dong Z: Autophagy is activated to protect against endotoxic
acute kidney injury. Sci Rep. 6:221712016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leventhal JS, Ni J, Osmond M, Lee K,
Gusella GL, Salem F and Ross MJ: Autophagy limits endotoxemic acute
kidney injury and alters renal tubular epithelial cell cytokine
expression. PLoS One. 11:e01500012016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
He J, Deng L, Liu H, Chen T, Chen S, Xia S
and Liu Y: BCL2L10/BECN1 modulates hepatoma cells autophagy by
regulating PI3K/AKT signaling pathway. Aging (Albany NY).
11:350–370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Ren Z, Li X, Zhong J, Bi Y, Li R,
Zhao Q and Yu X: Pristimerin induces autophagy-nediated cell death
in K562 cells through the ROS/JNK signaling pathway. Chem
Biodivers. 16:e19003252019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang K, Chu D, Wu J, Zhao M, Zhang M, Li
B, Du W, Du J and Guo R: WITHDRAWN: Cinobufagin induced cell
apoptosis and protective autophagy through the ROS/MAPK signaling
pathway. Life Sci. Jul 11–2019.(Epub ahead of print). doi:
10.1016/j.lfs.2019.116642. View Article : Google Scholar
|
|
16
|
Li K, Liu T-X, Li J-F, Ma YR, Liu ML, Wang
YQ, Wu R, Li B, Shi LZ and Chen C: rhEPO inhibited cell apoptosis
to alleviate acute kidney injury in sepsis by AMPK/SIRT1 activated
autophagy. Biochem Biophys Res Commun. 517:557–565. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao W, Zhang L, Chen R, Lu H, Sui M, Zhu
Y and Zeng L: SIRT3 protects against acute kidney injury via
AMPK/mTOR-regulated autophagy. Front Physiol. 9:15262018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Comijn J, Berx G, Vermassen P, Verschueren
K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D and van Roy
F: The two-handed E box binding zinc finger protein SIP1
downregulates E-cadherin and induces invasion. Mol Cell.
7:1267–1278. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiong M, Jiang L, Zhou Y, Qiu W, Fang L,
Tan R, Wen P and Yang J: The miR-200 family regulates
TGF-β1-induced renal tubular epithelial to mesenchymal transition
through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J
Physiol Renal Physiol. 302:F369–F379. 2012. View Article : Google Scholar
|
|
21
|
Furuya M, Masuda H, Hara K, Uchida H, Sato
K, Sato S, Asada H, Maruyama T, Yoshimura Y, Katabuchi H, et al:
ZEB1 expression is a potential indicator of invasive endometriosis.
Acta Obstet Gynecol Scand. 96:1128–1135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sultan and Aneesa: Functional
characterization of the transcription factor ZEB1 in epithelial to
mesenchymal transition and cancer progression. (Unpublished PhD
thesis). University of Vienna; 2010
|
|
23
|
Bui T, Sequeira J, Wen TC, Sola A, Higashi
Y, Kondoh H and Genetta T: ZEB1 links p63 and p73 in a novel
neuronal survival pathway rapidly induced in response to cortical
ischemia. PLoS One. 4:e43732009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li D, Lang W, Zhou C, Wu C, Zhang F, Liu
Q, Yang S and Hao J: Upregulation of microglial ZEB1 ameliorates
brain damage after acute ischemic stroke. Cell Rep. 22:3574–3586.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siles L, Ninfali C, Cortés M, Darling DS
and Postigo A: ZEB1 protects skeletal muscle from damage and is
required for its regeneration. Nat Commun. 10:13642019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shrum B, Anantha RV, Xu SX, Donnelly M,
Haeryfar SM, McCormick JK and Mele T: A robust scoring system to
evaluate sepsis severity in an animal model. BMC Res Notes.
7:2332014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leelahavanichkul A, Yasuda H, Doi K, Hu X,
Zhou H, Yuen PS and Star RA: Methyl-2-acetamidoacrylate, an ethyl
pyruvate analog, decreases sepsis-induced acute kidney injury in
mice. Am J Physiol Renal Physiol. 295:F1825–F1835. 2008. View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoste EA, Bagshaw SM, Bellomo R, Cely CM,
Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, et
al: Epidemiology of acute kidney injury in critically ill patients:
the multinational AKI-EPI study. Intensive Care Med. 41:1411–1423.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gomez H and Kellum JA: Sepsis-induced
acute kidney injury. Curr Opin Crit Care. 22:546–553. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huan JN: Recognizing prevention and
treatment of burn sepsis with the concept of holistic integrative
medicine. Zhonghua Shao Shang Za Zhi. 33:196–199. 2017.(In
Chinese). PubMed/NCBI
|
|
33
|
Croce KR and Yamamoto A: A role for
autophagy in Huntington's disease. Neurobiol Dis. 122:16–22. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galluzzi L, Bravo-San Pedro JM, Blomgren K
and Kroemer G: Autophagy in acute brain injury. Nat Rev Neurosci.
17:467–484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng L, Liao X, Zhang Y and Wang F:
Protective effects on age-related macular degeneration by activated
autophagy induced by amyloid-β in retinal pigment epithelial cells.
Discov Med. 27:153–160. 2019.PubMed/NCBI
|
|
36
|
Jia G, Cheng G and Agrawal DK: Autophagy
of vascular smooth muscle cells in atherosclerotic lesions.
Autophagy. 3:63–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lipinski MM, Zheng B, Lu T, Yan Z, Py BF,
Ng A, Xavier RJ, Li C, Yankner BA, Scherzer CR, et al: Genome-wide
analysis reveals mechanisms modulating autophagy in normal brain
aging and in Alzheimer's disease. Proc Natl Acad Sci USA.
107:14164–14169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh R, Xiang Y, Wang Y, Baikati K,
Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ and Czaja MJ:
Autophagy regulates adipose mass and differentiation in mice. J
Clin Invest. 119:3329–3339. 2009.PubMed/NCBI
|
|
39
|
Lenoir O, Tharaux PL and Huber TB:
Autophagy in kidney disease and aging: Lessons from rodent models.
Kidney Int. 90:950–964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kimura T, Isaka Y and Yoshimori T:
Autophagy and kidney inflammation. Autophagy. 13:997–1003. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin X, Cui M, Xu D, Hong D, Xia Y, Xu C,
Li R, Zhang X, Lou Y, He Q, et al: Liver-specific deletion of
Eva1a/Tmem166 aggravates acute liver injury by impairing autophagy.
Cell Death Dis. 9:7682018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu X, Zhi T, Chao H, Jiang K, Liu Y, Bao
Z, Fan L, Wang D, Li Z, Liu N, et al: ERK1/2/mTOR/Stat3
pathway-mediated autophagy alleviates traumatic brain
injury-induced acute lung injury. Biochim Biophys Acta Mol Basis
Dis. 1864:1663–1674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ling H, Chen H, Wei M, Meng X, Yu Y and
Xie K: The effect of autophagy on inflammation cytokines in renal
ischemia/reperfusion injury. Inflammation. 39:347–356. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shi K, Sun H, Zhang H, Xie D and Yu B:
miR-34a-5p aggravates hypoxia-induced apoptosis by targeting ZEB1
in cardiomyocytes. Biol Chem. 400:227–236. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chua HL, Bhat-Nakshatri P, Clare SE,
Morimiya A, Badve S and Nakshatri H: NF-kappaB represses E-cadherin
expression and enhances epithelial to mesenchymal transition of
mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2.
Oncogene. 26:711–724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang J, Zhao P, Quan N, Wang L, Chen X,
Cates C, Rousselle T and Li J: The endotoxemia cardiac dysfunction
is attenuated by AMPK/mTOR signaling pathway regulating autophagy.
Biochem Biophys Res Commun. 492:520–527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lieberthal W, Tang M, Lusco M, Abate M and
Levine JS: Preconditioning mice with activators of AMPK ameliorates
ischemic acute kidney injury in vivo. Am J Physiol Renal Physiol.
311:F731–F739. 2016. View Article : Google Scholar
|
|
48
|
Jeon SM: Regulation and function of AMPK
in physiology and diseases. Exp Mol Med. 48:e2452016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim J, Jeong SW, Quan H, Jeong CW, Choi JI
and Bae HB: Effect of curcumin (Curcuma longa extract) on
LPS-induced acute lung injury is mediated by the activation of
AMPK. J Anesth. 30:100–108. 2016. View Article : Google Scholar : PubMed/NCBI
|