Introduction
Ovarian cancer is the seventh commonest type of
cancer in women and the eighth most common cause of cancer death
worldwide, with a 5-year survival rate <45%. The incidence has
increased with increased life expectancy, especially in developing
countries. As the symptoms of early-stage ovarian cancer are mild
and non-specific, most cases are diagnosed at an advanced stage and
require aggressive cytoreductive surgery, followed by platinum and
taxane-based chemotherapy (1). At
present, ~85% of patients with advanced ovarian cancer who have
achieved full remission following surgery and chemotherapy develop
recurrent disease. The median survival time of patients with
advanced ovarian cancer ranges only 12–24 months (2,3).
The kynurenine pathway is an important pathway for
tryptophan metabolism; >95% of the tryptophan in the body is
metabolized through the kynurenine pathway (4,5). In
this pathway, tryptophan is first metabolized into the intermediate
N-formyl kynurenine by one of three first-step enzymes, namely,
indoleamine 2,3-dioxygenase 1 (IDO1), indoleamine 2,3-dioxygenase 2
(IDO2) and tryptophan 2,3-dioxygenase (TDO2) and then converted
into kynurenine by arylformamidase (6). Kynurenine subsequently undergoes a
series of enzymatic reactions and is converted into
3-hydroxykynurenine, 3-hydroxyanthranilic acid,
2-amino-3-carboxymuconatesemialdehyde and quinolinic acid, after
which it is finally converted to nicotinamide adenine dinucleotide
for metabolic use. The metabolites of the kynurenine pathway are
involved in multiple physiological activities, mainly in the
nervous and the immune systems (4,5). The
first-step enzymes, namely IDO1, IDO2 and TDO2, are the most
important rate-limiting enzymes in the kynurenine pathway (7). At the amino acid level, IDO1 and IDO2
have ~43% sequence similarity, whereas TDO2 has little similarity
with IDO1 and IDO2 (8,9). Studies have shown that one or more of
these enzymes are upregulated to different degrees in tumor tissues
(10–16). Upregulation of these enzymes leads
to tryptophan depletion and kynurenine accumulation, thereby
impairing the anticancer immune response (17–23).
The expression and roles of IDO1 in ovarian cancer have been
extensively investigated (24–29).
Inaba et al (30) reported
that higher expression of IDO1 in ovarian cancer tissues is
associated with shorter survival time. Furthermore, overexpression
of IDO1 in the human ovarian carcinoma cell line SKOV3 promotes
xenograft tumor growth (30),
whereas knockdown of IDO1 in SKOV3 cells suppresses xenograft tumor
growth (31). The tumor-promoting
effect of IDO2 in SK-IDO-xenografted mice is blocked by oral
administration of the IDO inhibitor 1-methyl-tryptophan (30). The present study aimed to
investigate the expression and roles of TDO2 in ovarian cancer.
Materials and methods
Plasmid and reagents
The TDO2 overexpression plasmid was designed by Sino
Biological Inc. Briefly, the coding sequence of pCMV3-TDO2 was
amplified using 5′-TAATACGACTCACTATAGGG-3′ and
5′-TAGAAGGCACAGTCGAGG−3′ as primers and inserted into the
KpnI/XbaI site of pCMV3-untagged vector (Sino
Biological Inc.). LM10, which is an effective TDO inhibitor, was
purchased from Selleck Chemicals. The cells were treated with LM10
at a final concentration of 500 µM (32).
Cell culture
The human ovarian epithelial cell lines T29 and T29H
were provided by Dr Jinsong Liu (School & Hospital of
Stomatology, Wenzhou Medical University, China) (33,34).
Briefly, isolated human surface ovarian epithelium cells were
infected sequentially by retroviruses containing SV40 T/t antigens
and hTERT genes to generate T29 cells. The immortalized, but
non-oncogenic T29 cells were further transformed by introducing an
oncogenic H-RASV12 in a pLNCX retroviral vector to form
the T29H cell. The SKOV3 cell line was provided by Dr Xueqiong Zhu
(The Second Affiliated Hospital of Wenzhou Medical University,
China). The OVSAHO (JCRB1046) cells were from JCRB Cell Bank. T29,
T29H and OVSAHO cells were maintained in high-glucose Dulbecco's
modified Eagle's medium (DMEM; HyClone; Cytiva) supplemented with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin-streptomycin (Gibco; Thermo Fisher Scientific,
Inc.). Human ovarian cell line SKOV3 cells were maintained in
Roswell Park Memorial Institute-1640 medium (RPMI-1640; HyClone;
Cytiva) supplemented with 10% FBS and 1% penicillin-streptomycin.
Cells were cultured in an incubator in a 5% CO2
humidified atmosphere at 37°C. All cell lines were mycoplasma free
and cells passaged at the Digestive Cancer Center, The First
Affiliated Hospital of Wenzhou Medical University for >6 months
following receipt were authenticated by genetic profiling using
polymorphic short-tandem repeat loci (35).
Small interfering (si)RNA and
transfection
siRNAs were purchased from Gema Gene. The siRNA
sequences were: siTDO2, 5′-CGUUAAUCGCGUAUAAUACGCGUATT-3′ (sense),
5′-UACGCGUAUUAUACGCGAUUAACGTT-3′ (anti-sense); siNC (negative
control siRNA), 5′-AAUUCUCCGAACGUGUCACGUTT-3′ (sense) and
5′-ACGUGACACGUUCGGAGAAUUTT-3′ (anti-sense).
Cells were seeded into 6-well plates at
3×105/well. The plasmids were transfected 2.5 µg/well
and the siRNAs were transfected at a final concentration of 50 nM
using Lipofectamine® 2000 Reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), following the manufacturer's
instructions. The cells were then collected 48 h after transfection
for subsequent analysis, including western blotting and reverse
transcription-quantitative (RT-q) PCR.
Western blotting
Total protein was extracted from T29, T29H, OVSAHO
and SKOV3 cells using RIPA lysis buffer (Beyotime Institute of
Biotechnology). After mixing with SDS lysis buffer and boiling, the
protein content in cell lysates were measured using BCA Protein
Assay kit (Beyotime Institute of Biotechnology). Equal amounts of
protein (30 µg) were loaded onto 10% SDS-PAGE and separated via
electrophoresis, then separated proteins were transferred to PVDF
membranes. After blocking in 5% skimmed milk at room temperature
for 1 h, membranes were incubated overnight at 4°C with primary
antibodies against TDO2 (cat. no. H00006999-A01; Abnova; 1:1,000)
and anti-GAPDH (cat. no. 2118S; Cell Signaling Technology, Inc;
1:1,000). The membranes were washed with TBST, followed by
incubation with goat anti-mouse IgG (cat. no. ab6789; Abcam;
1:10,000) or goat anti-rabbit IgG (cat. no. ab6721; Abcam;
1:10,000) antibodies at room temperature for 1 h. Subsequently, the
bands were detected with ECL plus reagents (GE Healthcare).
Densitometric analysis was performed using ImageJ software (version
1.8.0; National Institutes of Health). All experiments were
repeated at least three times.
RT-qPCR
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), incubated
with RNase-free DNase I (Promega Corporation) for 30 min and
reverse transcribed using the M-MLV reverse transcription kit
(Promega Corporation) according to the manufacturer's protocol.
qPCR was subsequently performed using a SYBR Green PCR Master mix
(Vazyme Biotech Co., Ltd.) on an ABI PRISM 7300 Sequence Detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used for qPCR: 95°C for 30
sec followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec.
The following primer sequences were used: TDO2:
5′-TCCTCAGGCTATCACTACCTGC(forward) and 5′-ATCTTCGGTATCCAGTGTCGG−3′
(reverse) (36); GAPDH:
5′-GCAAATTCCATGGCACCGTC-3′ (forward) and 5′-CCTGGAAGATGGTGATGGGA-3′
(reverse). The ΔΔCt method was used to measure the relative
expression levels of the subject genes. ΔCq was obtained by
subtracting the Cq (threshold cycle) value of GAPDH from that of
the subject gene and ΔΔCq was calculated by subtracting the ΔCq of
the control sample from that of the subject sample. The fold change
was calculated as 2−ΔΔCq and the relative expression
level of the control sample was defined as 1 (37). All experiments were performed
independently and repeated three times.
Cell proliferation assay
Following cell transfection for 24 h, the T29H,
OVSAHO or SKOV3 cells were seeded in 96-well plates with
2×103 cells at each time point, CellTiter 96 Aqueous One
Solution Cell Proliferation Assay (MTS) kit (Promega Corporation)
was added to the corresponding plates and incubated for 3 h at 37°C
according to the manufacturer's instructions. MTS is bioreduced by
cells into a formazan product that is soluble in tissue culture
medium. The absorbance of the formazan at 490 nm can be measured
directly from the 96-well assay plates using a Multiskan Spectrum
microplate reader (Thermo Fisher Scientific, Inc.) (38).
For cell number counting assays, cells were seeded
into 96-well plates with 1×104/well. Cells were counted
at 12, 24, 48 and 72 h after transfection or drug treatment using a
Countess 3 Automated Cell Counter (Thermo Fisher Scientific,
Inc.).
Colony formation assay
Following cell transfection for 24 h, the cells were
digested with trypsin and resuspended with the cell medium and
inoculated into a six-well plate with 200 cells/well and 2 ml of
the complete medium was added. The six-well plate was placed in the
incubator for further cultivation. After 18 days, the cells were
fixed with absolute methanol for 20 min, stained with 0.5% crystal
violet at room temperature for 20 min and the sample was rinsed and
images captured for counting.
Cell migration and invasion assay
Transwell inserts of 8 µm-pore plain (to assess
migration) or Matrigel-coated (to assess invasion; Costar; Corning,
Inc.) were placed in the wells of 24-well culture plates and 500 µl
of DMEM or RPMI-1640 containing 10% FBS was added to the lower
chamber. The T29H, OVSAHO, or SKOV3 cells were washed once with
Hanks' Balanced Salt Solution (Invitrogen; Thermo Fisher
Scientific, Inc.) 12 h after transfection, resuspended in 100 µl
serum-free medium (8×104 cells) and added to the upper
chamber. After 12 h of incubation at 37°C with 5% CO2,
the cells on the top side of the filter were manually removed with
a cotton swab. The cells adherent to the bottom surface of the
insert were fixed in cold absolute methanol for 10 min and then
stained with 0.01% crystal violet in 20% ethanol at room
temperature. After 10 min, the filters were washed thoroughly in
water and images were captured under a DMI3000 M inverted manual
microscope (Leica Microsystems GmbH). The number of migratory cells
was recorded using an optical microscope at ×100 magnification. The
average number of migrated cells was assessed by counting five
randomly selected microscopic fields. The experiment was performed
in triplicate.
Measurement of kynurenine
Supernatants from the control group and knockdown
TDO2 group cells were collected at 500 × g for 15 min at 4°C.
Levels of kynurenine in the supernatant were determined using the
human kynurenine ELISA kit (Cusabio Biotech Co., Ltd.; cat. no.
CSB-E13659h).
Activities of caspase-3/7
The activities of caspase-3/7 were measured using a
caspase-3/7 activity apoptosis assay kit (Sangon Biotech Co.,
Ltd.), according to the manufacturer's instructions.
The Cancer Genome Atlas (TCGA) and The
Genotype-Tissue Expression (GTEx) dataset analysis
RNA sequencing data of ovarian serous
cystadenocarcinoma were obtained from TCGA database (https://tcga-data.nci.nih.gov/tcga/). RNA
sequencing data of normal ovarian tissues and fallopian tube
tissues were obtained from GTEx project (http://gtexportal.org). The expression data were log2
(TPM+1) transformed. Bindea et al (39) examined the spatio-temporal dynamics
of 24 different tumor-infiltrating immune cells. The relative
quantities of these 24 immune cell types in ovarian cancer were
evaluated by using the R software (www.R-project.org; version 3.6.2) GSVA (version
1.34.0) package (40). Correlation
between the expression of TDO2 and the relative quantity of immune
cells was calculated by the signature gene sets of the immune
cells.
Statistical analysis
Data from three independent experiments are
presented as the mean ± standard error of the mean. Differences
were analyzed using a two-tailed unpaired Student's t-test.
Univariate hazard ratios with 95% confidence intervals were
calculated using the Cox proportional hazards regression and
significance was calculated using Wald's test. Statistical analysis
was performed using SPSS software (version 20.0; IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Upregulation of tryptophan
2,3-dioxygenase in ovarian cancer
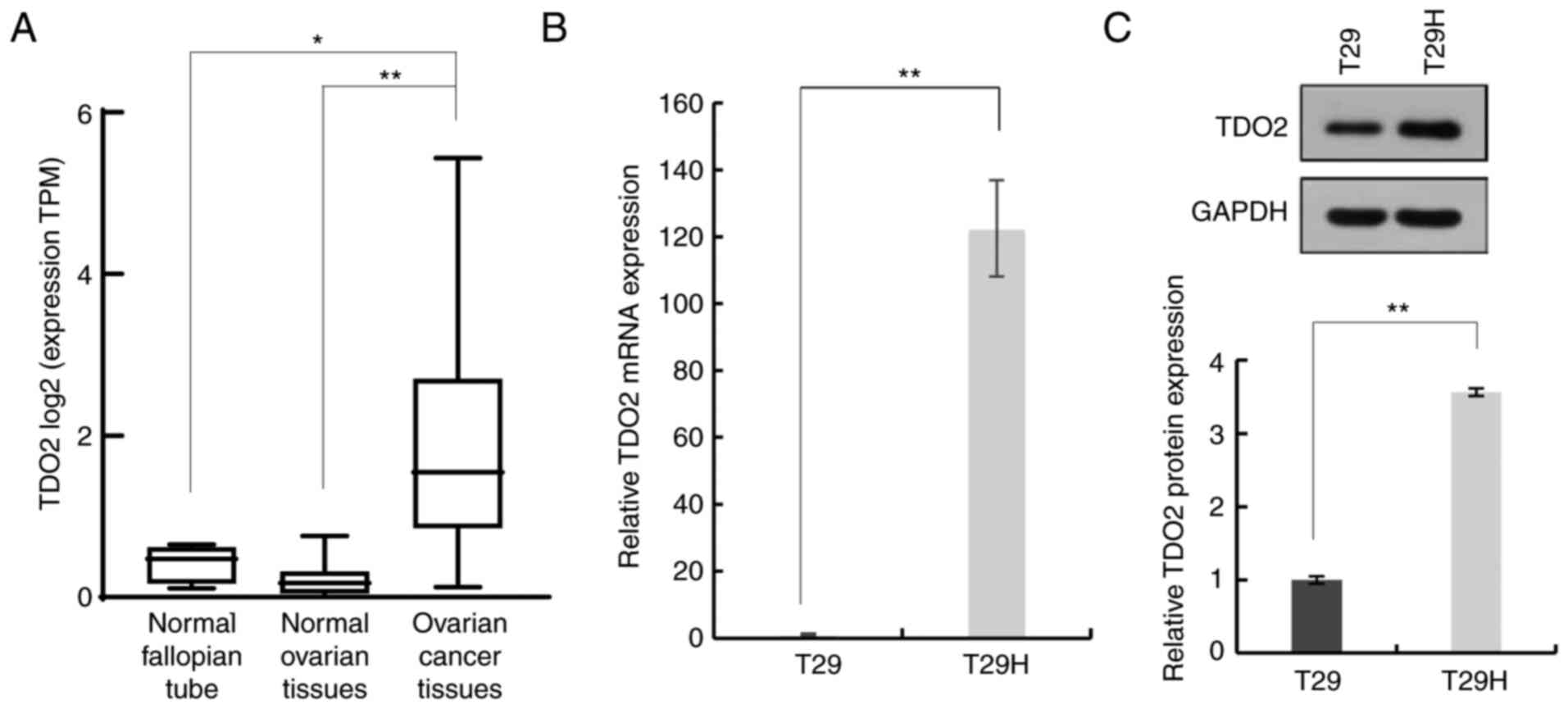
To investigate whether TDO2 serves a role in ovarian
cancer development, the expression of TDO2 in ovarian cancer
tissues, normal ovarian tissues and normal fallopian tube tissues
was compared. As shown in Fig. 1A,
the TDO2 mRNA level was significantly higher in ovarian
cancer tissues compared with normal ovarian tissues and fallopian
tube tissues. It was then evaluated whether TDO2 was upregulated in
a genetically defined model of human ovarian cancer. The T29 cells
were derived from primary human ovarian surface epithelial cells by
stable transfection with the SV40 T/t antigens and hTERT. The
immortalized but non-oncogenic T29 cells were further transformed
by introducing oncogenic HRasV12 to generate the T29H cell line,
which resembles natural ovarian cancer in several aspects. It was
found that the mRNA level of TDO2 was >100-fold higher in
T29H cells compared with T29 cells (Fig. 1B). Western blotting revealed that
the TDO2 protein was ~3-fold higher in T29H cells compared with T29
cells (Fig. 1C). Taken together,
these data indicated that TDO2 is upregulated in ovarian cancer
cells.
Regulation of proliferation, migration
and invasion in ovarian cancer cells by tryptophan
2,3-dioxygenase
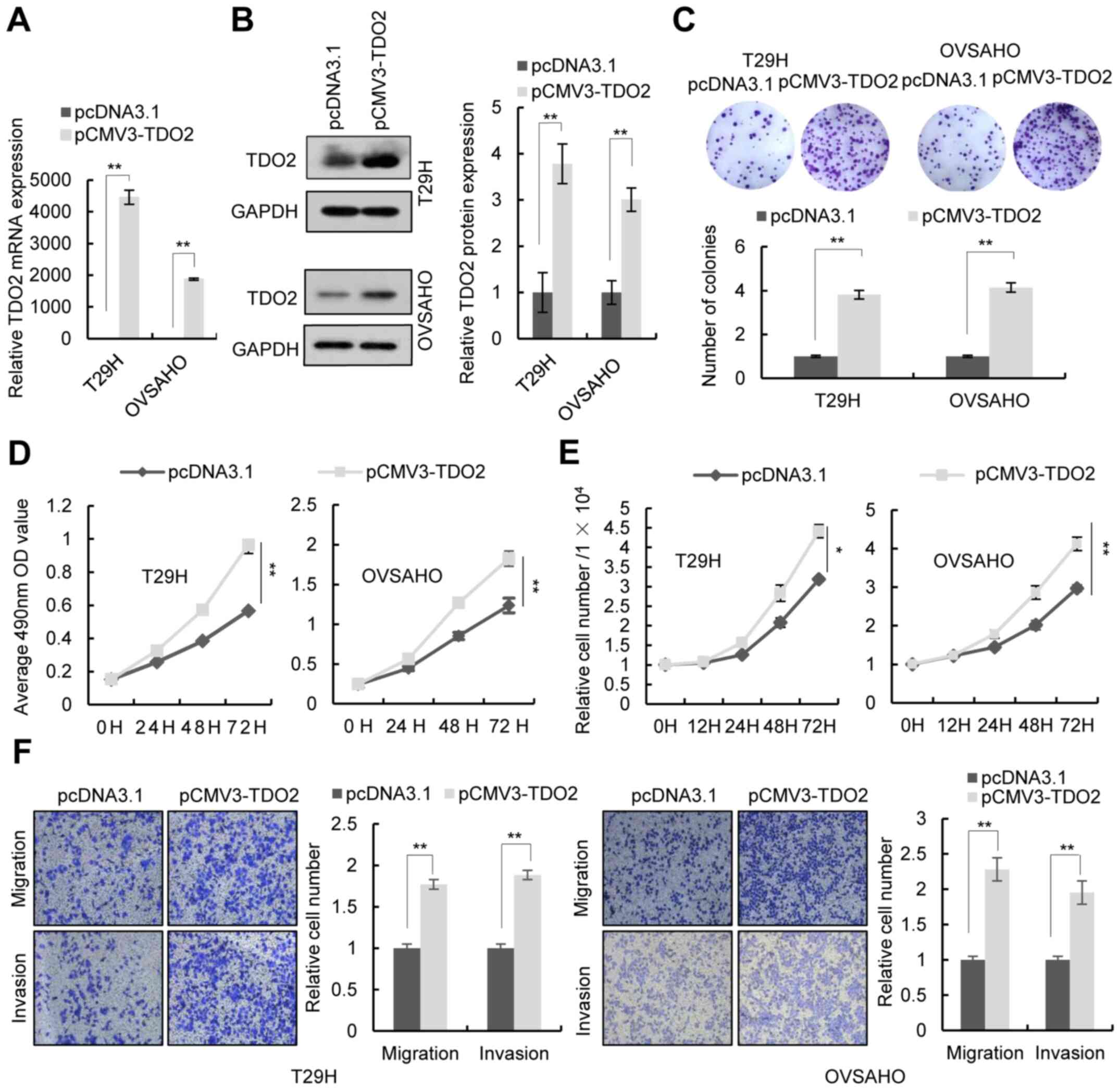
To investigate the function of TDO2 in ovarian
cancer cells, the TDO2 overexpression plasmid was
transfected into the human ovarian cancer cell lines T29H, OVSAHO
and SKOV3. RT-qPCR and western blotting confirmed the
overexpression of TDO2 in these cell lines (Figs. 2A and B and S1A and B). Colony formation assays, MTS
assays and cell number counts revealed that TDO2 overexpression in
T29H, OVSAHO and SKOV3 promoted cell proliferation (Figs. 2C-E and S1C-E). Transwell assays indicated that
TDO2 overexpression promoted the migration and invasion of ovarian
cells (Figs. 2F and S1F). It was then investigated whether
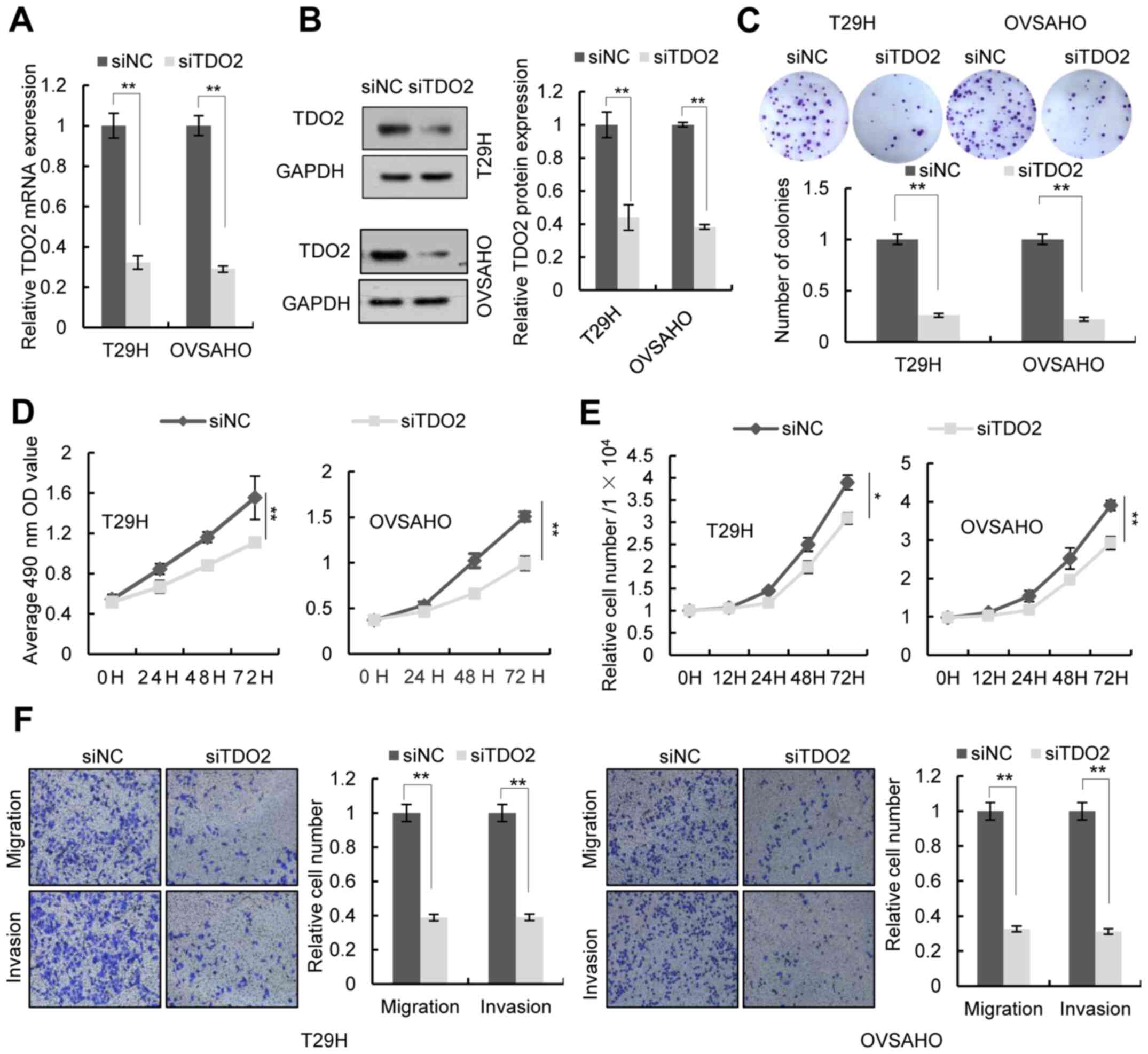
TDO2 knockdown affected the proliferation, migration and invasion
of ovarian cancer cells. Transfection of a TDO2-specific
siRNA led to decreased expression of TDO2 in T29H, OVSAHO and SKOV3
cells (Figs. 3A and B and S1A and B). Colony formation assays, MTS
assays, and cell number counts showed that knockdown of TDO2
decreased cell proliferation (Figs.
3C-E and S1C-E). Transwell
assays indicated that TDO2 knockdown reduced cell migration and
invasion (Figs. 3F and S1F). TDO2 knockdown did not increase the
activity of caspase-3/7, indicating that the decrease of MTS signal
and cell numbers in TDO2 knockdown cells was not a result of cell
apoptosis (Fig. S2).
Inhibition of proliferation, migration
and invasion of ovarian cancer cells by the tryptophan
2,3-dioxygenase inhibitor, LM10
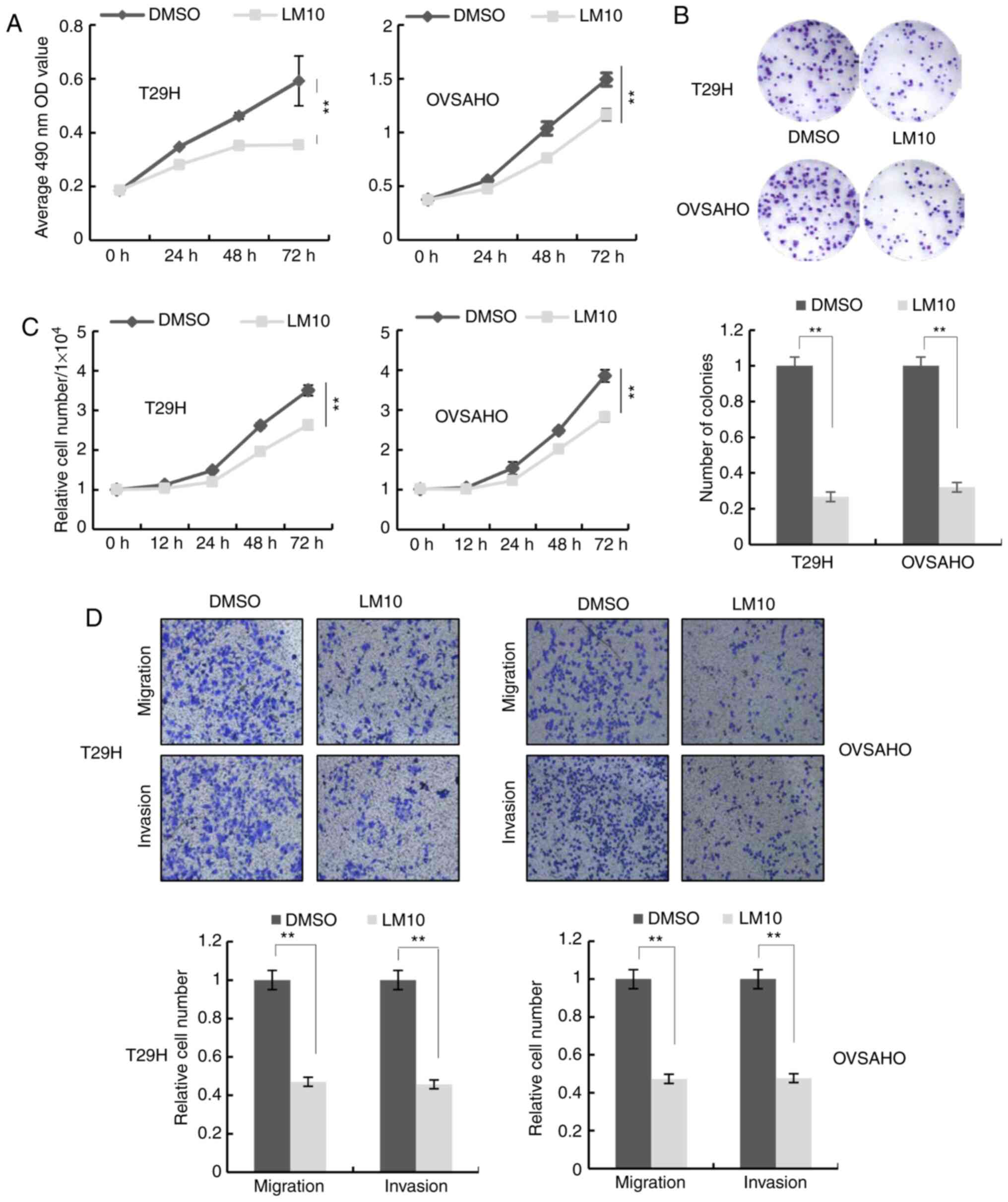
LM10, a TDO2 inhibitor, reportedly exhibits
anti-cancer activity by reversing the tumoral immune resistance
caused by TDO2 overexpression (15). It was therefore examined whether
LM10 could inhibit the proliferation, migration and invasion of
ovarian cancer cells. MTS assays, colony formation assays and cell
number counting revealed that treatment with LM10 inhibited cell
proliferation (Figs. 4A-C and
S1C-E). Transwell assays indicated
that LM10 significantly repressed cell migration and invasion
(Figs. 4D and S1F).
Association of tryptophan
2,3-dioxygenase expression with pathological characteristics in
patients with ovarian cancer
TDO2 is capable of inhibiting anti-CD3-driven T-cell
proliferation and inducing CD8+ T-cell death (41,42).
It was therefore assessed whether the expression of TDO2 was
inversely associated with immune cell infiltration in ovarian
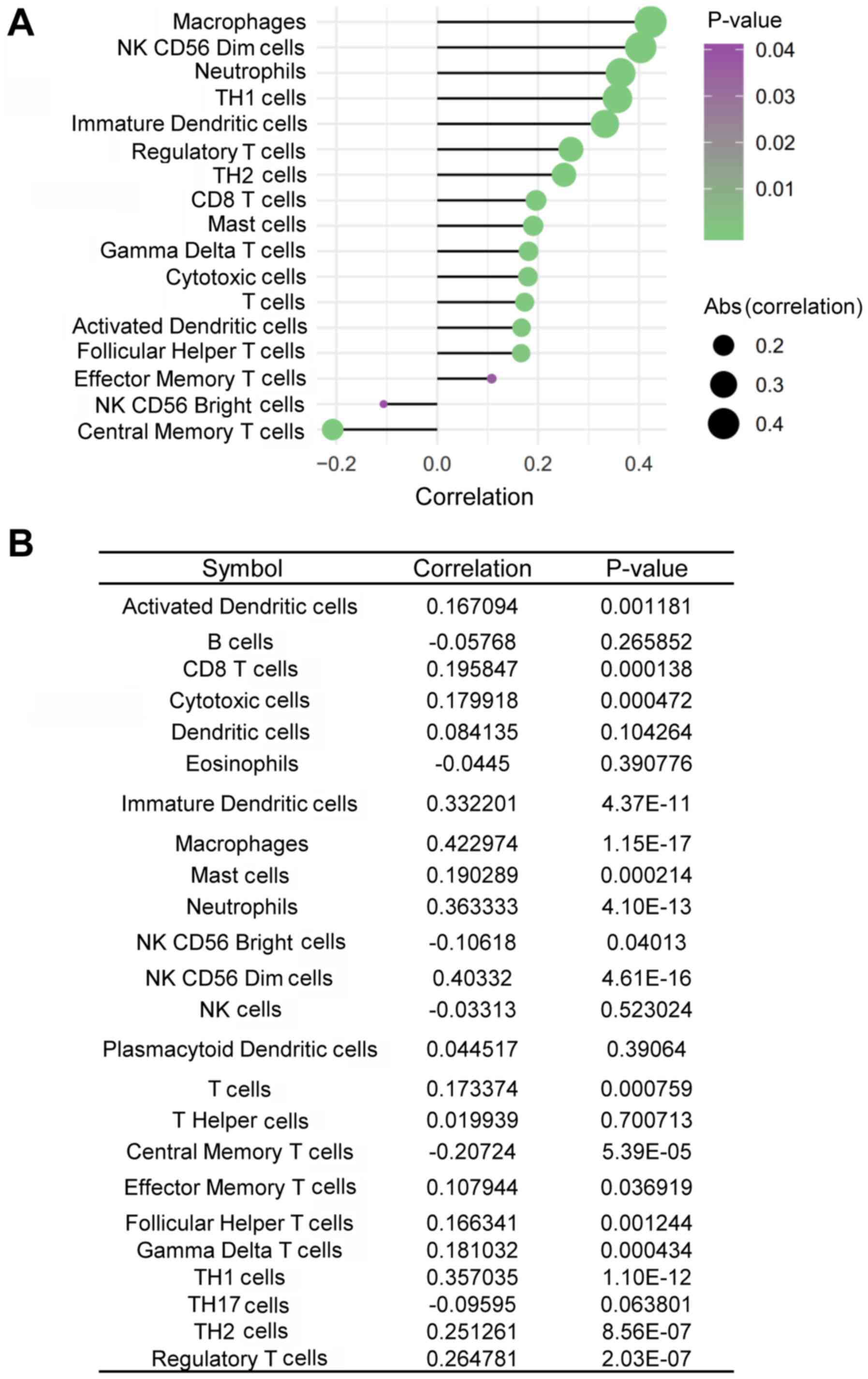
cancer tissues. TDO2 expression was positively, rather than
negatively, associated with the infiltration of CD8+ T
cells in ovarian cancer tissues (Fig.
5A and B). In addition, TDO2 expression was also positively
associated with the infiltration of other immune cells, including
macrophages, CD56dim natural killer (NK) cells, neutrophils,
T-helper type 1 (Th1) cells, immature dendritic cells (iDCs),
regulatory T cells (Tregs), T-helper type 2 (Th2) cells, mast
cells, gamma delta T (γδT) cells, cytotoxic T cells, activated
dendritic cells (aDCs), follicular helper T (TFH) cells and
effector helper T cells in ovarian cancer tissues, whereas it was
negatively associated with the infiltration of CD56bright NK cells
and central memory T (TCM) cells (Fig.
5A and B).
Discussion
TDO2 has been found to be upregulated in different
types of cancers including Merkel cell carcinoma, breast carcinoma,
bladder carcinoma, colorectal carcinoma, hepatocarcinoma and
melanoma (15,43,44).
However, its expression and role in ovarian cancer remain to be
elucidated. The present study found that TDO2 was upregulated in
ovarian cancer tissues compared with that in the normal ovarian
tissues. Overexpression of TDO2 promoted the proliferation,
migration and invasion of ovarian cancer cells, whereas knockdown
of TDO2 suppressed these phenotypes. In addition, inhibiting the
activity of TDO2 with LM10 suppressed the proliferation of ovarian
cancer cells. Kynurenine is reported to promote proliferation,
migration and invasion of cancer cells (6,13). The
results of the present study showed that knockdown of TDO2
by ~70% did not significantly reduce the levels of kynurenine
(Fig. S3), suggesting that TDO2
may promote cancer cell proliferation, migration and invasion in a
kynurenine- independent mechanism. Taken together, these data
suggested that TDO2 functions as an oncogene in ovarian
cancer and that LM10 may be a candidate drug for ovarian cancer
treatment.
TDO2 has been shown to inhibit the proliferation and
activation of T cells in the tumor microenvironment, thereby
allowing tumors to escape immune surveillance (9,45).
Pilotte et al (15) reported
that cancer cells with increased expression of TDO2 exhibited
increased immune tolerance in mice and grew rapidly and the oral
TDO2 inhibitor LM10 reversed this immune tolerance and inhibited
tumor growth by activating anti-tumoral immunity. Consistent with
these studies, the present study found that TDO2 expression was
positively associated with Treg infiltration in ovarian cancer
tissues. By contrast, the expression levels of TDO2 were also
positively associated with CD8+ T-cell infiltration.
These conflicting observations suggested that further studies are
needed to determine whether TDO2 can negatively regulate the
functions of CD8+ T cells. In addition, TDO2 expression
was also positively associated with the infiltration of other
immune cells, including macrophages, CD56dim NK cells, neutrophils,
Th1 cells, iDCs, Th2 cells, mast cells, γδT cells, aDCs and TFH
cells but negatively associated with the infiltration of CD56bright
NK and TCM cells in ovarian cancer tissues. Future efforts should
be directed to address the effects of TDO2 on these immune
cells.
In summary, the findings of the present study
indicated that TDO2 can promote proliferation, migration and
invasion of ovarian cancer cells and that TDO2 expression is
associated with immune cell infiltration in ovarian cancer tissues.
Future studies should construct different animal models to
investigate the in vivo role of TDO2 in ovarian cancer and
to elucidate the underlying molecular mechanisms.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81572780, 81773011 and
81972648) and the Zhejiang Provincial Natural Sciences Foundation
(grant nos. LZ16H160004, LY16C050004 and LY18H030008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KFT conceived and devised the study. KFT, YX, RO,
WC, XW and FT designed the experiments and performed the analysis.
YZ drafted the manuscript and revised the manuscript critically for
important content. FT drafted the original manuscript. YZ, FT, JJ,
LC, JD, XC, QH and SZ performed the experiments and analyzed the
data. KFT, YX, RO, WC and XW contributed reagents and materials.
KFT, YX and YZ confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
Glossary
Abbreviations
Abbreviations:
|
TDO2
|
tryptophan 2,3-dioxygenase
|
|
IDO1
|
indoleamine 2,3-dioxygenase 1
|
|
IDO2
|
indoleamine 2, 3-dioxygenase 2
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
NK
|
natural killer cells
|
|
Th1
|
T-helper type 1 cells
|
|
iDCs
|
immature dendritic cells
|
|
Tregs
|
regulatory T cells
|
|
Th2
|
T-helper type 2 cells
|
|
γδT
|
gamma delta T cells
|
|
aDCs
|
activated dendritic cells
|
|
TFH
|
follicular helper T cells
|
|
TCM
|
central memory T cell
|
References
|
1
|
Orr B and Edwards RP: Diagnosis and
treatment of ovarian cancer. Hematol Oncol Clin North Am.
32:943–964. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gunderson CC and Moore KN: Olaparib: An
oral PARP-1 and PARP-2 inhibitor with promising activity in ovarian
cancer. Future Oncol. 11:747–757. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Corrado G, Salutari V, Palluzzi E,
Distefano MG, Scambia G and Ferrandina G: Optimizing treatment in
recurrent epithelial ovarian cancer. Expert Rev Anticancer Ther.
17:1147–1158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Platten M, Nollen EAA, Röhrig UF,
Fallarino F and Opitz CA: Tryptophan metabolism as a common
therapeutic target in cancer, neurodegeneration and beyond. Nat Rev
Drug Discov. 18:379–401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Savitz J: The kynurenine pathway: A finger
in every pie. Mol Psychiatry. 25:131–147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Venkateswaran N, Lafita-Navarro MC, Hao
YH, Kilgore JA, Perez-Castro L, Braverman J, Borenstein-Auerbach N,
Kim M, Lesner NP, Mishra P, et al: MYC promotes tryptophan uptake
and metabolism by the kynurenine pathway in colon cancer. Genes
Dev. 33:1236–1251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Puccetti P, Fallarino F, Italiano A,
Soubeyran I, MacGrogan G, Debled M, Velasco V, Bodet D, Eimer S,
Veldhoen M, et al: Accumulation of an endogenous tryptophan-
derived metabolite in colorectal and breast cancers. PLoS One.
10:e01220462015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ball HJ, Yuasa HJ, Austin CJ, Weiser S and
Hunt NH: Indoleamine 2,3-dioxygenase-2; a new enzyme in the
kynurenine pathway. Int J Biochem Cell Biol. 41:467–471. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheong JE and Sun L: Targeting the
IDO1/TDO2-KYN-AhR pathway for cancer immunotherapy-challenges and
opportunities. Trends Pharmacol Sci. 39:307–325. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pham QT, Oue N, Sekino Y, Yamamoto Y,
Shigematsu Y, Sakamoto N, Sentani K, Uraoka N and Yasui W: TDO2
overexpression is associated with cancer stem cells and poor
prognosis in esophageal squamous cell carcinoma. Oncology.
95:297–308. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang
S and Li Y: Targeting the IDO1 pathway in cancer: From bench to
bedside. J Hematol Oncol. 11:1002018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D'Amato NC, Rogers TJ, Gordon MA, Greene
LI, Cochrane DR, Spoelstra NS, Nemkov TG, D'Alessandro A, Hansen KC
and Richer JK: A TDO2-AhR signaling axis facilitates anoikis
resistance and metastasis in triple-negative breast cancer. Cancer
Res. 75:4651–4664. 2015. View Article : Google Scholar
|
|
13
|
Bishnupuri KS, Alvarado DM, Khouri AN,
Shabsovich M, Chen B, Dieckgraefe BK and Ciorba MA: IDO1 and
kynurenine pathway metabolites activate PI3K-Akt signaling in the
neoplastic colon epithelium to promote cancer cell proliferation
and inhibit apoptosis. Cancer Res. 79:1138–1150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith C, Chang MY, Parker KH, Beury DW,
DuHadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP,
Laury-Kleintop LD, et al: IDO is a nodal pathogenic driver of lung
cancer and metastasis development. Cancer Discov. 2:722–735. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pilotte L, Larrieu P, Stroobant V, Colau
D, Dolusic E, Frédérick R, De Plaen E, Uyttenhove C, Wouters J,
Masereel B and Van den Eynde BJ: Reversal of tumoral immune
resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl
Acad Sci USA. 109:2497–2502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fatokun AA, Hunt NH and Ball HJ:
Indoleamine 2,3- dioxygenase 2 (IDO2) and the kynurenine pathway:
Characteristics and potential roles in health and disease. Amino
Acids. 45:1319–1329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brenk M, Scheler M, Koch S, Neumann J,
Takikawa O, Häcker G, Bieber T and von Bubnoff D: Tryptophan
deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic
cells favoring the induction of human
CD4+CD25+ Foxp3+ T regulatory
cells. J Immuno. 183:145–154. 2009. View Article : Google Scholar
|
|
18
|
Chen W, Liang X, Peterson AJ, Munn DH and
Blazar BR: The indoleamine 2,3-dioxygenase pathway is essential for
human plasmacytoid dendritic cell-induced adaptive T regulatory
cell generation. J Immunol. 181:5396–5404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung DJ, Rossi M, Romano E, Ghith J, Yuan
J, Munn DH and Young JW: Indoleamine 2,3-dioxygenase-expressing
mature human monocyte-derived dendritic cells expand potent
autologous regulatory T cells. Blood. 114:555–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Curti A, Pandolfi S, Valzasina B, Aluigi
M, Isidori A, Ferri E, Salvestrini V, Bonanno G, Rutella S, Durelli
I, et al: Modulation of tryptophan catabolism by human leukemic
cells results in the conversion of CD25- into CD25+ T
regulatory cells. Blood. 109:2871–2877. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fallarino F, Grohmann U, You S, McGrath
BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML and
Volpi C: The combined effects of tryptophan starvation and
tryptophan catabolites down-regulate T cell receptor zeta-chain and
induce a regulatory phenotype in naive T cells. J Immunol.
176:6752–6761. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hippen KL, O'Connor RS, Lemire AM, Saha A,
Hanse EA, Tennis NC, Merkel SC, Kelekar A, Riley JL, Levine BL, et
al: In vitro induction of human regulatory T cells using conditions
of low tryptophan plus kynurenines. Am J Transplant. 17:3098–3113.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma MD, Baban B, Chandler P, Hou DY,
Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL and Munn DH:
Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes
directly activate mature Tregs via indoleamine 2,3-dioxygenase. J
Clin Invest. 117:2570–2582. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kristeleit R, Davidenko I, Shirinkin V,
El-Khouly F, Bondarenko I, Goodheart MJ, Gorbunova V, Penning CA,
Shi JG, Liu X, et al: A randomised, open-label, phase 2 study of
the IDO1 inhibitor epacadostat (INCB024360) versus tamoxifen as
therapy for biochemically recurrent (CA-125 relapse)-only
epithelial ovarian cancer, primary peritoneal carcinoma, or
fallopian tube cancer. Gynecol Oncol. 146:484–490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang GN, Zhu Y and Huang JM:
Understanding of targeting MyD88, IDO1 and AHR at the heart of
immunosuppressive signaling pathway for immunotherapy of epithelial
ovarian cancer. Zhonghua fu chan ke za zhi. 53:448–451. 2018.(In
Chinese). PubMed/NCBI
|
|
26
|
Awuah SG, Zheng YR, Bruno PM, Hemann MT
and Lippard SJ: A Pt(IV) pro-drug preferentially targets
indoleamine-2,3-dioxygenase, providing enhanced ovarian cancer
immuno-chemotherapy. J Am Chem Soc. 137:14854–14857. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qian F, Villella J, Wallace PK,
Mhawech-Fauceglia P, Tario JD Jr, Andrews C, Matsuzaki J, Valmori
D, Ayyoub M, Frederick PJ, et al: Efficacy of levo-1-methyl
tryptophan and dextro-1-methyl tryptophan in reversing
indoleamine-2, 3-dioxygenase-mediated arrest of T-cell
proliferation in human epithelial ovarian cancer. Cancer Res.
69:5498–5504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanizaki Y, Kobayashi A, Toujima S, Shiro
M, Mizoguchi M, Mabuchi Y, Yagi S, Minami S, Takikawa O and Ino K:
Indoleamine 2,3-dioxygenase promotes peritoneal metastasis of
ovarian cancer by inducing an immunosuppressive environment. Cancer
Sci. 105:966–973. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uyttenhove C, Pilotte L, Theate I,
Stroobant V, Colau D, Parmentier N, Boon T and Van den Eynde BJ:
Evidence for a tumoral immune resistance mechanism based on
tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med.
9:1269–1274. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Inaba T, Ino K, Kajiyama H, Yamamoto E,
Shibata K, Nawa A, Nagasaka T, Akimoto H, Takikawa O and Kikkawa F:
Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in
the progression of ovarian carcinoma. Gynecol Oncol. 115:185–192.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang D, Saga Y, Mizukami H, Sato N, Nonaka
H, Fujiwara H, Takei Y, Machida S, Takikawa O, Ozawa K and Suzuki
M: Indoleamine-2,3-dioxygenase, an immunosuppressive enzyme that
inhibits natural killer cell function, as a useful target for
ovarian cancer therapy. Int J Oncol. 40:929–934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dolusić E, Larrieu P, Moineaux L,
Stroobant V, Pilotte L, Colau D, Pochet L, Van den Eynde B,
Masereel B, Wouters J and Frédérick R: Tryptophan 2,3-dioxygenase
(TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential
anticancer immunomodulators. J Med Chem. 54:5320–5334. 2011.
View Article : Google Scholar
|
|
33
|
Young T, Mei F, Liu J, Bast RC Jr, Kurosky
A and Cheng X: Proteomics analysis of H-RAS-mediated oncogenic
transformation in a genetically defined human ovarian cancer model.
Oncogene. 24:6174–6184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Yang G, Thompson-Lanza JA, Glassman
A, Hayes K, Patterson A, Marquez RT, Auersperg N, Yu Y, Hahn WC, et
al: A genetically defined model for human ovarian cancer. Cancer
Res. 64:1655–1663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Metzgar D, Liu L, Hansen C, Dybvig K and
Wills C: Domain-level differences in microsatellite distribution
and content result from different relative rates of insertion and
deletion mutations. Genome Res. 12:408–413. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li R, Quan Y and Xia W: SIRT3 inhibits
prostate cancer metastasis through regulation of FOXO3A by
suppressing Wnt/β-catenin pathway. Exp Cell Res. 364:143–151. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cory AH, Owen TC, Barltrop JA and Cory JG:
Use of an aqueous soluble tetrazolium/formazan assay for cell
growth assays in culture. Cancer Commun. 3:207–212. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bindea G, Mlecnik B, Tosolini M,
Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T,
Lafontaine L, Berger A, et al: Spatiotemporal dynamics of
intratumoral immune cells reveal the immune landscape in human
cancer. Immunity. 39:782–795. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li L, Feng Q and Wang X: PreMSIm: An R
package for predicting microsatellite instability from the
expression profiling of a gene panel in cancer. Comput Struct
Biotechnol J. 18:668–675. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schmidt SK, Muller A, Heseler K, Woite C,
Spekker K, MacKenzie CR and Däubener W: Antimicrobial and
immunoregulatory properties of human tryptophan 2,3-dioxygenase.
Eur J Immunol. 39:2755–2764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Greene LI, Bruno TC, Christenson JL,
D'Alessandro A, Culp-Hill R, Torkko K, Borges VF, Slansky JE and
Richer JK: A role for tryptophan-2,3-dioxygenase in CD8 T-cell
suppression and evidence of tryptophan catabolism in breast cancer
patient plasma. Mol Cancer Re. 17:131–139. 2019. View Article : Google Scholar
|
|
43
|
Wardhani LO, Matsushita M, Iwasaki T,
Kuwamoto S, Nonaka D, Nagata K, Kato M, Kitamura Y and Hayashi K:
Expression of the IDO1/TDO2-AhR pathway in tumor cells or the tumor
microenvironment is associated with Merkel cell polyomavirus status
and prognosis in Merkel cell carcinoma. Human Pathol. 84:52–61.
2019. View Article : Google Scholar
|
|
44
|
Rogers TJ, Christenson JL, Greene LI,
O'Neill KI, Williams MM, Gordon MA, Nemkov T, D'Alessandro A,
Degala GD, Shin J, et al: Reversal of triple-negative breast cancer
EMT by miR-200c decreases tryptophan catabolism and a program of
immunosuppression. Mol Cancer Res. 17:30–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Badawy AA: Targeting tryptophan
availability to tumors: The answer to immune escape? Immunol Cell
Biol. 96:1026–1034. 2018. View Article : Google Scholar : PubMed/NCBI
|