Introduction
Triple negative breast cancer (TNBC) is
characterized by the absence of estrogen and progesterone receptors
and Erb-b2 receptor tyrosine kinase 2, and this type of cancer has
been the second leading cause of cancer-associated mortalities
among women (1–3). Although significant progression has
been achieved in the therapy of breast cancer, chemotherapy remains
the primary treatment option for patients with TNBC (4). Therefore, the clinical outcome of
patients with TNBC is relatively worse compared with that of other
types of breast cancer due to the inefficiency of endocrine therapy
and targeted therapy (4). As the
metastatic risk of TNBC is high, it is important to elucidate the
molecular mechanism underlying the progression of TNBC and identify
available therapeutic targets.
Long non-coding RNAs (lncRNAs) are a class of
non-coding transcripts >200 nucleotides in length, which have a
wide range of regulatory functions in both physiological and
pathological processes (5,6). As another set of non-coding RNAs,
microRNAs (miRNAs/miRs) are defined as single-stranded, small RNAs
involved in post-transcriptional regulation of gene expression via
interacting with the 3′-untranslated region (UTR) of target mRNAs
(7–10). Interestingly, accumulating evidence
has suggested that lncRNAs and miRNAs participate in the
tumorigenesis of TNBC by modulating the expression of oncogenes or
tumor suppressors (11–15). One functional mechanism consists of
lncRNAs serving as competing endogenous RNAs (ceRNAs) for miRNAs to
regulate miRNA and miRNA-targeted mRNAs (15). For example, lncRNA antisense RNA in
the INK4 locus acted as a ceRNA of miR-199a and promoted the
progression of TNBC (16).
Moreover, a recent study revealed that lncRNA LINC00173 enhanced
the development of TNBC by sponging miR-490-3p (17). These findings indicate that
targeting lncRNAs may be promising to improve the positive outcomes
of TNBC.
Second chromosome locus associated with prostate-1
(SChLAP1), also named LINC00913, is an upregulated lncRNA that is
proven to participate in oncogenesis in prostate cancer (18–20).
SChLAP1 accelerates the proliferation and metastasis of prostate
cancer via sponging miR-198, and subsequently modulates the MAPK1
signaling pathway (20). Highly
expressed SChLAP1 is a possible tissue-based biomarker for
identifying patients with prostate cancer at higher risk of lethal
progression (18). Additionally,
silencing SChLAP1 suppresses the malignant phenotype of bladder
cancer cells (21). This evidence
indicates that abnormal expression of SChLAP1 occurs in cancer, and
interrupting SChLAP1 may be a therapeutic solution for patients
with cancer. However, the expression level and functional mechanism
of SChLAP1 in TNBC have not been fully elucidated.
The aim of the present study was to investigate the
clinical significance and biological function of SChLAP1, as well
as elucidate the associated miRNAs that mediate the regulatory role
of SChLAP1 in the malignancy of TNBC.
Materials and methods
Patients and tissues samples
A total of 50 paired TNBC tissues and matched
adjacent normal tissues (~2 cm from the margin of the TNBC tissues)
were obtained from the Shanxi Provincial Cancer Hospital between
January 2012 and September 2014. Tissues were immediately frozen in
liquid nitrogen and stored at −80°C. None of the patients received
radiotherapy or chemotherapy before the surgery. This study was
performed in accordance with the Declaration of Helsinki and was
approved by the Ethics Committee of Shanxi Provincial Cancer
Hospital (approval no. 0425245-1). Written informed consent
statements were provided by all the enrolled patients. The clinical
data of the patients enrolled in this study are presented in
Table SI.
Cell culture and transfection
Human TNBC cell lines, including MDA-MB-231, SKBR3,
BT-549 and HCC-1937, and the normal breast epithelial cell line
MCF10A were purchased from the American Type Culture Collection.
Cells were cultured in RPMI-1640 medium (Sigma-Aldrich; Merck
KGaA), supplemented with 10% FBS (Invitrogen; Thermo Fisher
Scientific, Inc.) in a humidified atmosphere with 5% CO2
at 37°C.
The small interference RNA (siRNA) pool (two
guaranteed siRNA mixtures, cat. no. 4392420; Thermo Fisher
Scientific, Inc.) targeting SChLAP1, the miR-524-5p mimic
(5′-CUACAAAGGGAAGCACUUUCUC-3′) and the relative controls
(5′-GGUUCGUACGUACACUGUUCA-3′) were obtained from Shanghai
GenePharma Co., Ltd. Flag-tagged High Mobility Group AT-Hook 2
(HMGA2) plasmid (100 ng; cat. no. 25409) was purchased from
Addgene, Inc. A total of 50 nM siRNA or miRNA was transfected into
the TNBC cells using Lipofectamine® 2000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 15 min. Cells were cultured for 48 h before further
analysis.
RNA extraction and reverse
transcription quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from frozen tissues or
cultured cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. For the detection of miRNAs, RT (37°C for 10 min,
85°C for 5 sec and 4°C for 15 min) was performed with the TaqMan
miRNA RT kit (Takara Biotechnology Co., Ltd.). The relative
expression level of miR-524-5p was compared with that of U6 RNA
using the TaqMan Universal PCR Master mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. For lncRNA or mRNA analysis, cDNA was synthesized with
the TaqMan High-Capacity Complementary DNA RT kit (Tiangen Biotech
Co., Ltd.). Gene expression was detected using the TaqMan Fast PCR
Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The expression level
of GAPDH was also examined as the internal control. The
thermocycling conditions were as follows: Initial denaturation at
95°C for 5 min, followed by 40 cycles of denaturation at 95°C for
15 sec, annealing at 60°C for 15 sec and extension at 72°C for 15
sec. The primers used in this study were as follows: SChLAP1
forward, 5′-GGGAAGAAGTGCCAGATGCT-3′ and reverse,
5′-CAGCTTCTTCAGGGAGGTGG-3′; miR-524-5p forward,
5′-GCTGTGACCCTACAAAGGGA-3′ and reverse, 5′-AGCATCAACTTCAACGCTGC-3′;
U6 RNA forward, 5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′; GADPH forward,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′; and HMGA2 forward,
5′-TTCAGCCCAGGGACAACC-3′ and reverse, 5′-GCTGCTTTAGAGGGACTCTTGT-3′.
The relative expression of targets was quantified with the
2−ΔΔCq method (22). The
experiments were performed in triplicate.
Cell Counting Kit-8 (CCK-8) assay
The viability of TNBC cells transfected with the
indicated expressing vectors was determined using CCK-8 (Beyotime
Institute of Biotechnology) following the manufacturer's
instructions. Cells were seeded in a 96-well plate with 1,000 cells
per well. After culturing overnight, 10 µl CCK-8 reagent was added
to the medium at the indicated time interval (1, 2, 3, 4 and 5
day), and the plates were incubated for additional 4 h at 37°C. The
absorbance of each well was measured at the wavelength of 450 nm
with a microplate reader (EnSpire 2300; PerkinElmer, Inc.).
Bioinformatics analysis
The mRNA expression profiles of TNBC (Homo
sapiens) were downloaded from the Gene Expression Omnibus (GEO;
http://www.ncbi.nlm.nih.gov/geo/)
database. GSE58135 (expression profiling via high throughput
sequencing) was used to identify hub lncRNAs associated with TNBC.
The dataset provided gene expression profiles of 16 TNBC primary
tumors and 16 breast tissues adjacent to TNBC primary tumors
(female patients, 39–64 years old). High throughput sequencing raw
data (Fastq files) for GSE58135 were evaluated using FastQC
(version 0.11.8, http://www.bioinformatics.babraham.ac.uk/projects/fastqc/).
Prefiltering of the quality of reads was performed using
trim_galore (version 0.6.4_dev, http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/).
Filtered reads were aligned using STAR for the 65,217 annotated
genes obtained from the GRCh38 (v79) build of the human genome
(23). The read counts per gene
were quantified using the featureCounts (version 1.6.4) using
annotations from the GRCh38 (v79) build of the human genome
(24). The Bioconductor R package
DESeq2 (version 1.26.0; http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html)
was used to identify the differentially expressed genes between
patients with TNBC and controls in the raw expression counts, which
were adjusted for library size. Genes were considered to be
differentially expressed when the Benjamini-Hochberg adjusted
P-value [false discovery rate (FDR)] was <0.05 and the log2
fold-change (FC) ≥4 between TNBC tissues and control groups.
Target prediction
The targeted miRNAs of SChLAP1 were predicted using
the online software program miRDB (version 5.0; http://mirdb.org/) (25). The highest rank among all the
candidates was assigned to miR-524-5p. The potential targets of
miR-524-5p were identified using the TargetScan database (version
7.2; http://www.targetscan.org/). The 3′-UTR
of HMGA2 was found to contain complementary binding sites of
miR-524-5p.
Luciferase reporter assay
The wild-type (WT) or mutated (Mut) fragment of
SChLAP1 containing the predicted binding sites of miR-524-5p was
inserted into the pmirGLO reporter vector (Promega Corporation).
TNBC cells were co-transfected with 50 nM miR-524-5p mimic and 200
ng WT or Mut luciferase plasmid of SChLAP1 using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The luciferase activity was measured after
transfection for 48 h using the Dual-Luciferase reporter analysis
kit (Promega Corporation) following the manufacturer's protocol.
The activity of Renilla luciferase was detected to normalize
the activity of firefly. The experiments were performed in three
replicates.
Western blot analysis
TNBC cells were lysed with RIPA buffer (Beijing
Solarbio Science & Technology Co., Ltd.) containing protease
inhibitor and subjected to BCA protein assay to evaluate the
protein concentration. Next, 20 µg protein lysates were loaded onto
15% SDS-PAGE gels for electrophoresis, and subsequently separated
proteins were transferred onto PVDF membranes. The membranes were
blocked with 5% non-fat milk for 1 h at room temperature and
subsequently incubated with primary antibody against HMGA2
(1:1,000; cat. no. ab97276; Abcam) or GAPDH (1:3,000; cat. no.
ab181602; Abcam) overnight at 4°C. After washing three times with
PBS with 0.1% Tween-20, the membrane was incubated with Goat
Anti-Rabbit IgG H&L (HRP) secondary antibody (1:5,000; cat. no.
ab205718; Abcam) for 1 h at room temperature. The expression of
GAPDH was detected as the loading control. Protein bands were
visualized with an ECL kit (Pierce; Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. The semi-quantification
of the western blotting bands was performed using the ImageJ
software (v1.8.0; National Institutes of Health).
Colony formation assay
TNBC cells were transfected with siRNA-Control or
siRNA-SChLAP1 and seeded into 6-well plates with 500 cells per
well. Cells were cultured for 2 weeks with RPMI-1640 medium
containing 10% FBS. Colonies were washed with cold PBS and fixed
with 75% methanol (Beyotime Institute of Biotechnology) at room
temperature for 10 min. Then, colonies were stained with 0.1%
crystal violet for 15 min at room temperature and counted with a
light microscope (magnification, ×40).
Cell apoptosis
Cells were transfected with siRNA-Control or
siRNA-SChLAP1 for 48 h. The apoptosis of cells (early and late
apoptotic cells) was analyzed via flow cytometry (FACScalibur; BD
Biosciences) using the Annexin V-FITC/PI kit (BD Biosciences).
Cells were resuspended with the binding buffer and stained with the
Annexin V-FITC at room temperature for 30 min followed by staining
with PI in the dark for 10 min at room temperature. The cell
apoptosis profile was analyzed using the CellQuest Software version
0.9.3.1 (BD Biosciences).
Statistical analysis
The data are presented as the mean ± SD from three
independent experiments. Statistical significance was determined
with paired Student's t-test for data in Fig. 1B and C, or one-way ANOVA followed by
Tukey's test using GraphPad software (version 7.0; GraphPad
Software, Inc.). Differences between two groups were analyzed using
an unpaired t-test. The correlation between miR-524-5p and SChLAP1
was assessed via the Pearson's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
SChLAP1 expression is increased in
TNBC tissues and cell lines
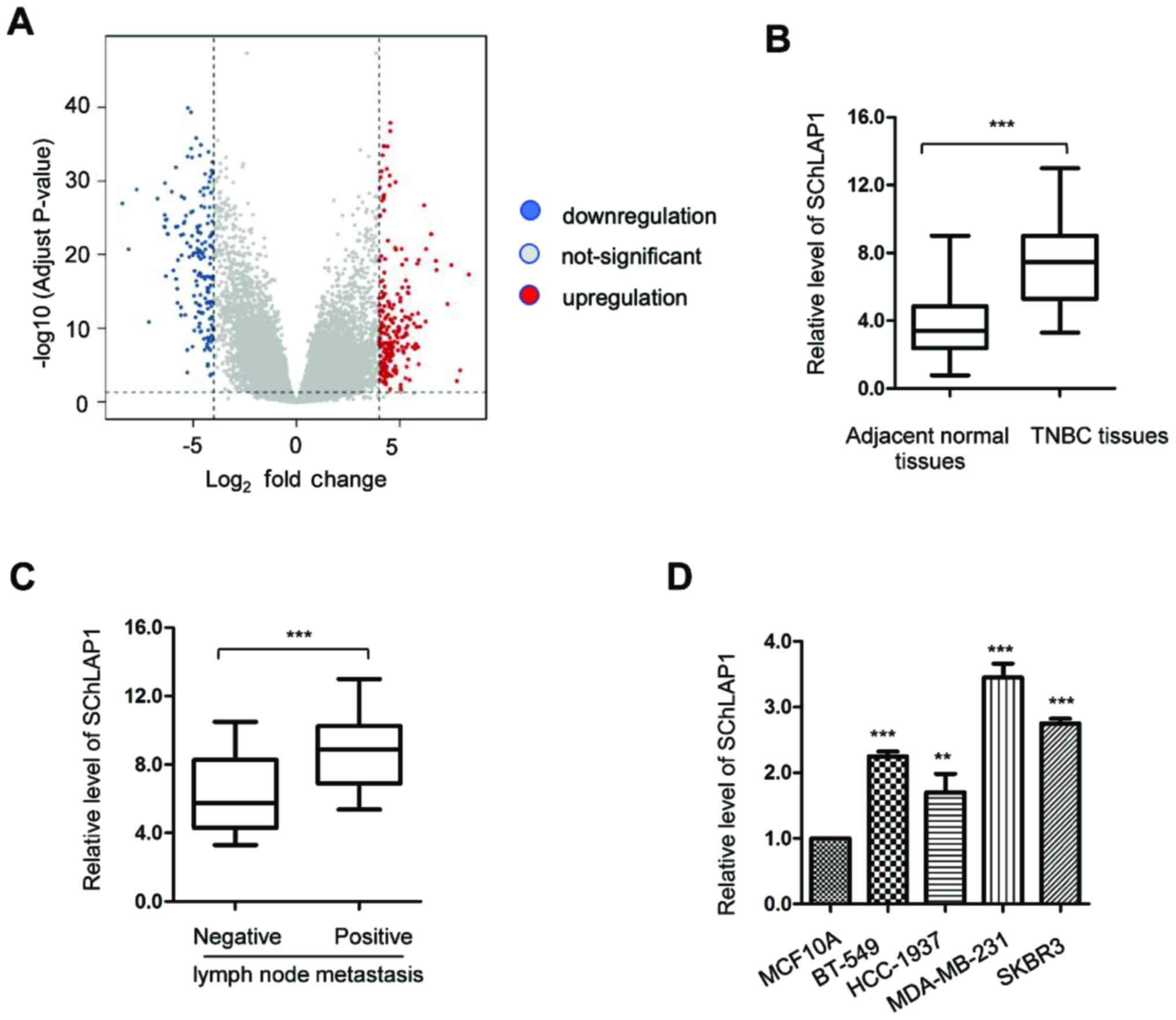
To determine which lncRNAs were aberrantly expressed
in TNBC, bioinformatics analysis was conducted using the GEO
(http://www.ncbi.nlm.nih.gov/geo/)
database that covered the gene expression profiles obtained from 16
TNBC tissues and 16 adjacent non-cancerous tissues. It was found
that lncRNA SChLAP1 was among the 10 most upregulated lncRNAs in
TNBC tissues (Figs. 1A and S1).
To verify the increased expression of SChLAP1 in
TNBC, the expression levels of SChLAP1 in a larger sample size
(n=50) of TNBC tissues and matched non-cancerous tissues were
determined via RT-qPCR analysis. The results demonstrated that the
expression of SChLAP1 was significantly upregulated in TNBC tissues
compared with that of the adjacent normal tissues (Fig. 1B). In addition, patients with
distant lymph node metastasis (n=16) had a relative higher
expression level of SChLAP1 compared with patients without
metastasis (n=34) (Fig. 1C). To
further confirm the increased expression of SChLAP1 in TNBC, the
expression level of SChLAP1 in TNBC cell lines was also determined.
Significantly increased expression levels of SChLAP1 were found in
TNBC cell lines compared with those of normal MCF10A cells
(Fig. 1D). These findings indicated
that the SChLAP1 was upregulated in TNBC.
Knockdown of SChLAP1 suppresses the
viability and colony formation of TNBC cells, and also triggers
apoptosis of TNBC cells
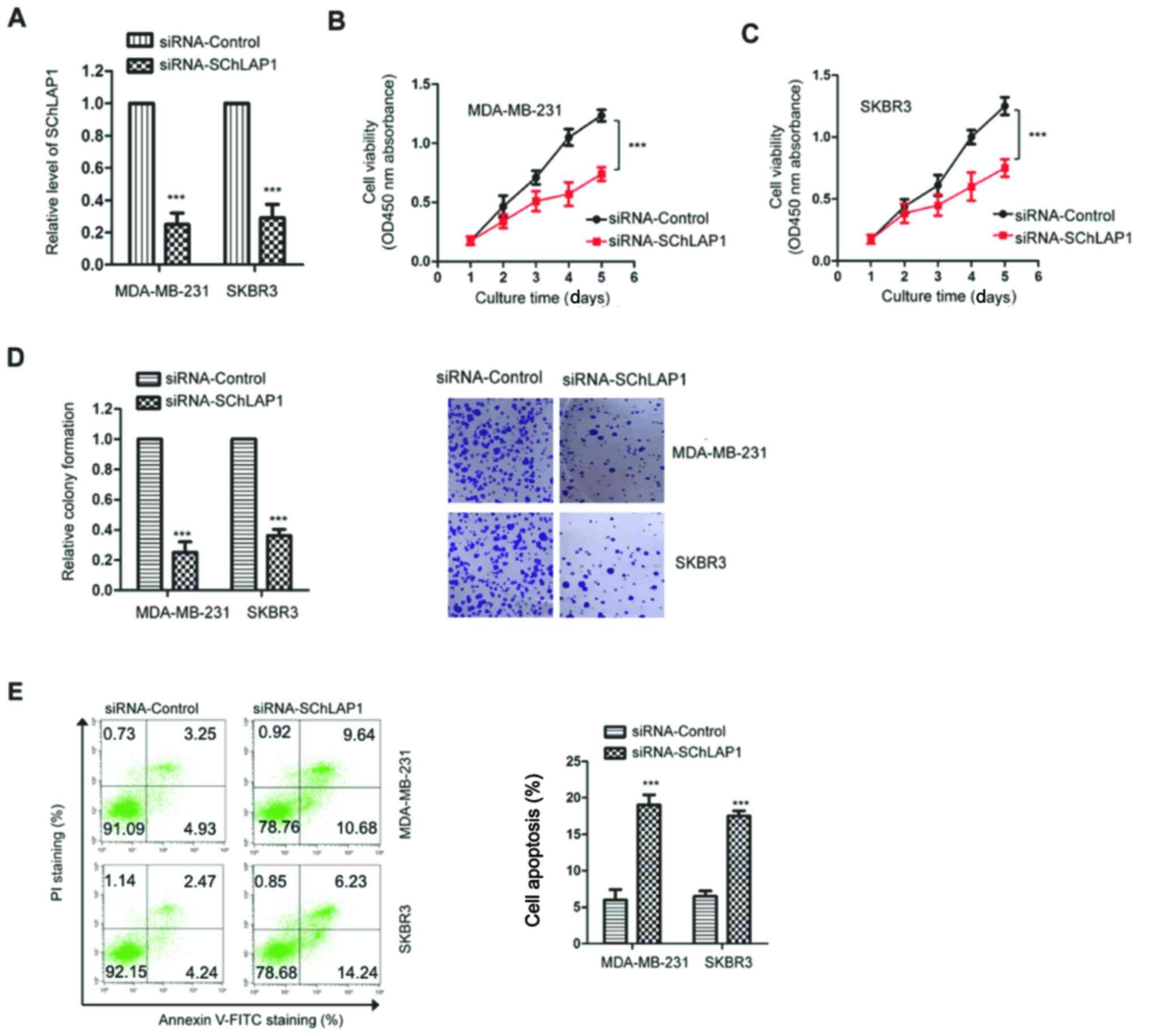
Given the upregulation of SChLAP1 in TNBC, the
effects of SChLAP1 on the malignant behaviors of TNBC cells were
examined via knockdown of the expression of SChLAP1. MDA-MB-231 and
SKBR3 cells were transfected with siRNA-SChLAP1 or non-targeting
control (siRNA-control), and the expression of SChLAP1 was
validated via RT-qPCR. The results demonstrated that the expression
of SChLAP1 was significantly decreased after the transfection of
siRNA-SChLAP1 in TNBC cells (Fig.
2A).
The influence of SChLAP1 on the viability of TNBC
cells was determined via the CCK-8 assay. As presented in Fig. 2B and C, compared with cells
transfected with siRNA-control, knockdown of SChLAP1 significantly
suppressed the proliferative capacity of both MDA-MB-231 and SKBR3
cells. Consistently, the colony formation analysis also indicated
that knockdown of SChLAP1 resulted in the suppressed viability of
TNBC cells (Fig. 2D).
To further validate the inhibitory effects on the
malignant behaviors of TNBC cells induced by knockdown of SChLAP1,
the apoptosis of both MDA-MB-231 and SKBR3 cells was evaluated
using flow cytometry analysis. The results indicated that knockdown
of SChLAP1 significantly increased the apoptotic percentage of TNBC
cells compared with the control group (Fig. 2E). Collectively, these findings
suggested that SChLAP1 may be essential in regulating the malignant
phenotypes of TNBC cells.
SChLAP1 serves as a sponge of
miR-524-5p
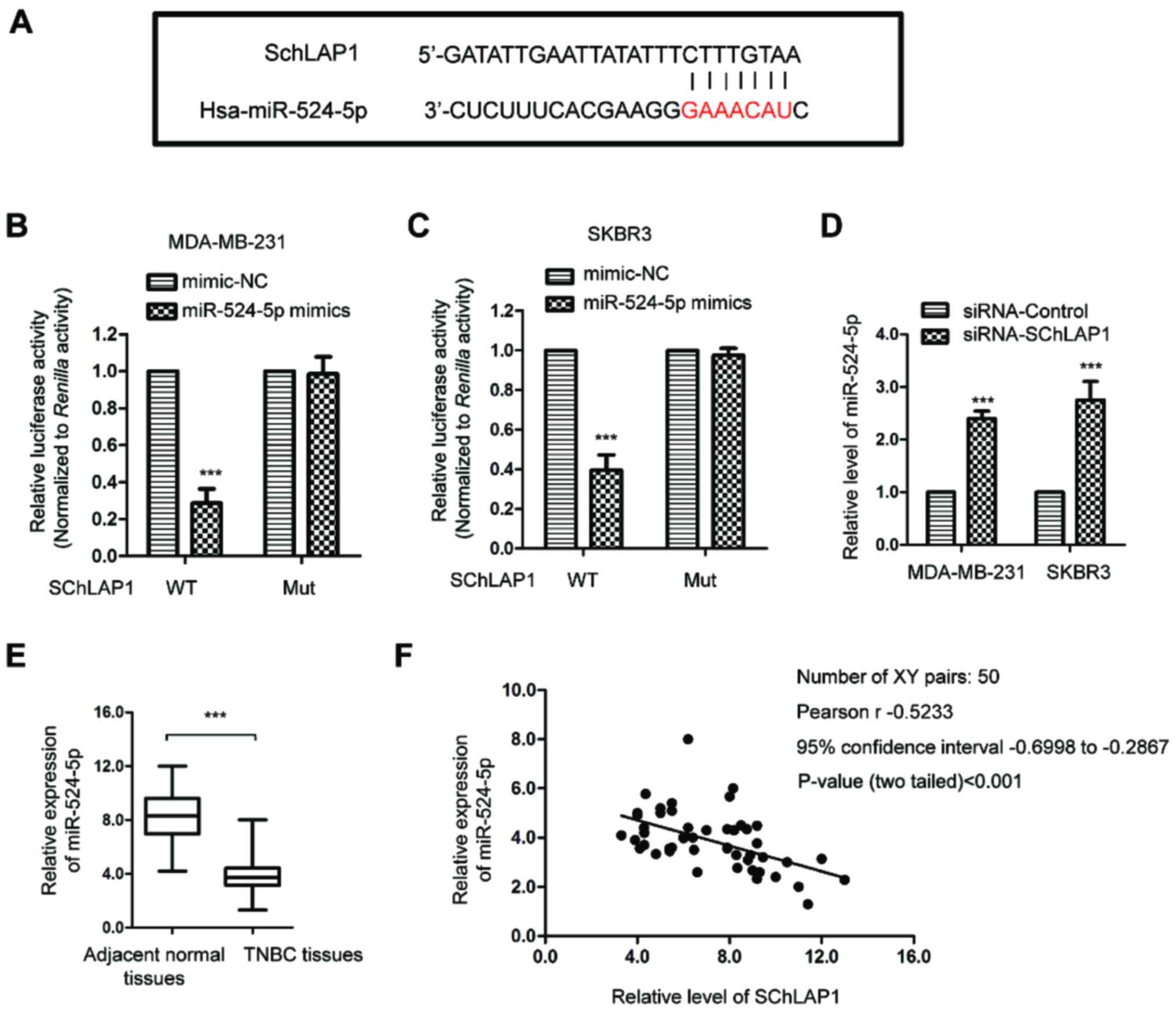
To examine the possible underlying mechanism via
which SChLAP1 affected the activities of TNBC cells, the targeted
miRNAs of SChLAP1 were predicted using the online tool (http://mirdb.org/). The bioinformatics analysis
identified that miR-524-5p harbored the possible binding sites of
SChLAP1 (Fig. 3A). To confirm the
binding between SChLAP1 and miR-524-5p, a luciferase reporter assay
was performed by co-transfecting luciferase vector carrying WT or
Mut SChLAP1 and miR-524-5p mimic. As indicated in Fig. 3B and C, overexpression of miR-524-5p
significantly decreased the luciferase activity of TNBC cells
expressing WT but not Mut SChLAP1, which suggested that there was
specific binding between SChLAP1 and miR-524-5p.
To investigate whether the binding affected the
stability of miR-524-5p, the expression level of miR-524-5p in TNBC
cells transfected with siRNA-SChLAP1 or siRNA-control was
determined via RT-qPCR. The data demonstrated that knockdown of
SChLAP1 significantly upregulated the expression level of
miR-524-5p in both MDA-MB-231 and SKBR3 cells (Fig. 3D), which suggested that there was
negative regulation of SChLAP1 upon the expression of miR-524-5p.
To support this conclusion, the expression of miR-524-5p in TNBC
tissues and matched non-cancerous tissues was detected, and it was
determined that miR-524-5p was significantly downregulated in TNBC
tissues compared with that of the adjacent normal tissues (Fig. 3E). Moreover, Pearson's correlation
analysis indicated that the expression level of miR-524-5p in TNBC
tissues was moderately, negatively correlated with SChLAP1
expression (Fig. 3F). These results
demonstrated that SChLAP1 sponged miR-524-5p and negatively
regulated the expression of miR-524-5p in TNBC cells.
HMGA2 is a target of miR-524-5p in
TNBC
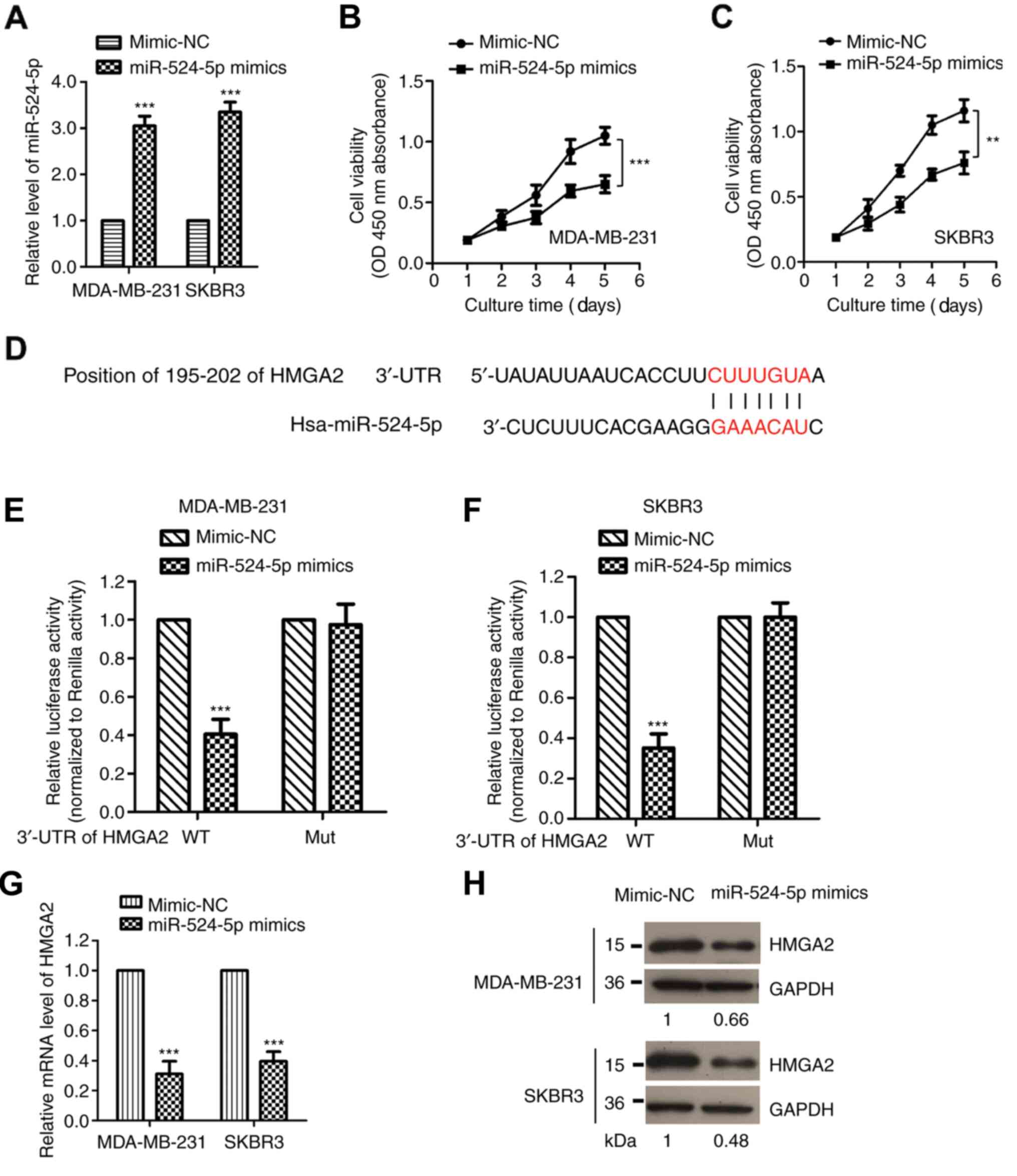
As miR-524-5p was identified as a target of SChLAP1,
to further understand the importance of miR-524-5p in mediating the
function of SChLAP1, the effects of miR-524-5p on the viability of
TNBC cells were determined by transfecting miR-524-5p mimic into
both MDA-MB-231 and SKBR3 cells. The overexpression of miR-524-5p
was confirmed via RT-qPCR (Fig.
4A). The CCK-8 assay demonstrated that overexpression of
miR-524-5p significantly inhibited the viability of both MDA-MB-231
and SKBR3 cells (Fig. 4B and C),
which suggested that miR-524-5p had suppressive effects, and was
consistent with the downregulated expression of miR-524-5p in
TNBC.
To investigate the functional mechanism of
miR-524-5p in TNBC, the downstream targets of miR-524-5p were also
predicted using the online TargetScan database. It was determined
that HMGA2 was a functional target of miR-524-5p, as the 3′-UTR of
HMGA2 harbored the complementary binding sites of miR-524-5p
(Fig. 4D). To validate this
hypothesis, the WT or corresponding Mut 3′-UTR of HMGA2 was
inserted into the luciferase vector. Both MDA-MB-231 and SKBR3
cells were co-transfected with miR-524-5p mimic and luciferase
plasmid expressing the WT or Mut 3′-UTR of HMGA2. The results
demonstrated that the luciferase intensity was significantly
suppressed by miR-524-5p in WT group, but not by the Mut 3′-UTR of
HMGA2 (Fig. 4E and F).
To further confirm that HMGA2 was a target of
miR-524-5p, the mRNA and protein expression levels of HMGA2 were
detected via RT-qPCR and western blotting, respectively. The data
identified that HMGA2 expression was significantly decreased after
the transfection of miR-524-5p (Fig. 4G
and H). These results demonstrated HMGA2 was a functional
target of miR-524-5p in TNBC.
A SChLAP1/miR-524-5p/HMGA2 regulatory
pathway is critical for the proliferation of TNBC cells
To investigate the regulation of SChLAP1 on the
expression of HMGA2, the mRNA and protein expression levels of
HMGA2 in MDA-MB-231 and SKBR3 cells were detected after the
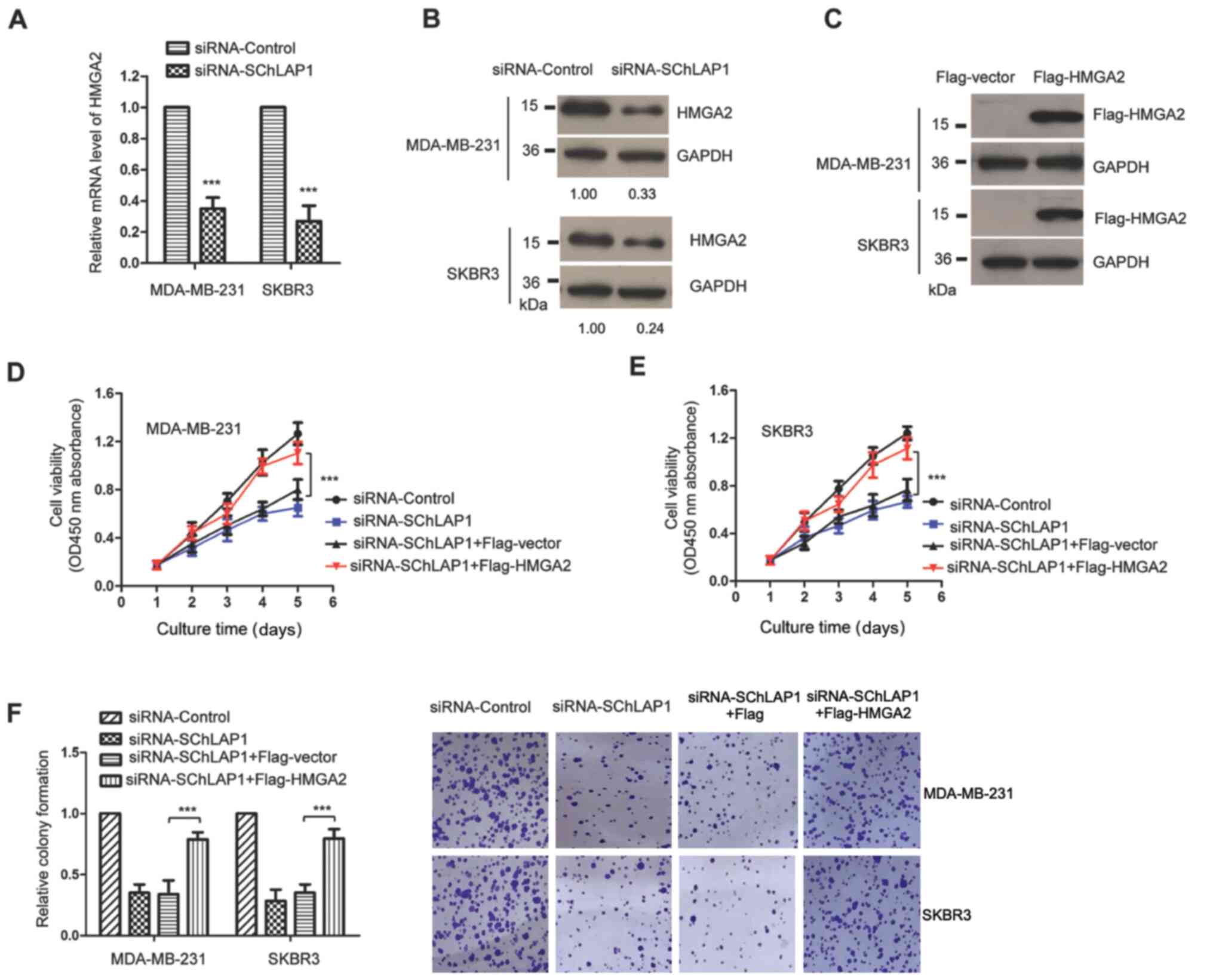
knockdown of SChLAP1. As presented in Fig. 5A and B, knockdown of SChLAP1
significantly decreased both the mRNA and protein expression levels
of HMGA2 in TNBC cells.
To determine whether SChLAP1 exerted its role via
the miR-524-5p/HMGA2 pathway, rescue experiments were performed by
transfecting HMGA2. The expression of transfected Flag-tagged HMGA2
was validated via western blotting using anti-Flag antibody
(Fig. 5C). The CCK-8 assay
demonstrated that knockdown of SChLAP1 suppressed the viability of
TNBC cells, while overexpression of HMGA2 significantly rescued the
viability of SChLAP1-knockdown TNBC cells (Fig. 5D and E). Consistently,
overexpression of HMGA2 also attenuated the suppressed colony
formation capacity of TNBC cells that was induced by SChLAP1
knockdown (Fig. 5F). These findings
demonstrated the critical function of the miR-524-5p/HMGA2 pathway
in mediating the role of SChLAP1 in the malignancy of TNBC.
Discussion
Multiple lncRNAs have been identified as oncogenes
or tumor suppressors that can regulate the progression of different
human cancer types, including TNBC (12,13).
Based on the sequencing data, the expression of SChLAP1 was
significantly decreased in TNBC tissues compared with that of the
non-cancerous tissues, ranking top 10 among all the genes. Aberrant
expression of SChLAP1 has been reported to serve an oncogenic role
in the development of glioblastoma (26) and prostate cancer (19,20).
Increased expression levels of SChLAP1 in prostate cancer tissues
may serve as a biomarker for the prognosis of patients with cancer
(18). Overexpressed SChLAP1
promotes the proliferation and metastasis of cancer cells, and
predicts poor outcomes of patients with cancer (19). These findings suggested that
targeting SChLAP1 may be a potential strategy to disrupt cancer
progression; however, the role of SChLAP1 in TNBC remains poorly
understood.
The present results indicated that SChLAP1 was
upregulated and associated with lymph node metastasis of patients
with TNBC. The functional analysis revealed that knockdown of
SChLAP1 significantly inhibited the viability and colony formation,
as well as induced the apoptosis of TNBC cells. These findings
demonstrated the essential role of SChLAP1 in the progression of
TNBC. However, further study will be necessary to evaluate the
correlation of SChLAP1 with the clinical parameters of patients
with TNBC to determine whether aberrant expression of SChLAP1 can
be used as an independent biomarker for the prognosis of TNBC. In
addition to SChLAP1, those genes that are differentially expressed
in TNBC also warrant further investigation to identify their role
in the development of TNBC.
Increasing evidence has revealed that lncRNAs exert
their functions in cancer by acting as ceRNAs to sponge miRNA and
regulate the expression of miRNAs (27–29). A
recent study reported that SChLAP1 accelerated the malignant
phenotype of prostate cancer by sponging miR-198 and activating
MAPK1 signaling (20). In the
current study, the potential miRNAs capable of binding to SChLAP1
were predicted using the online tool. Both the prediction and the
luciferase reporter assay validated the binding between SChLAP1 and
miR-524-5p. Interestingly, miR-524-5p exhibits a low expression
level and acts as a tumor suppressor in multiple cancer types,
including gastric cancer, glioma and papillary thyroid carcinoma
(30–34).
In the present study, knockdown of SChLAP1 increased
the expression of miR-524-5p in TNBC cells. Consistent with the
upregulation of SChLAP1 in TNBC, miR-524-5p expression was
downregulated and negatively correlated with that of SChLAP1 in
TNBC tissues. Additionally, overexpression of miR-524-5p suppressed
the viability of TNBC cells, suggesting that miR-524-5p exerts a
tumor-suppressive role in TNBC. However, it is necessary to
investigate the clinical significance of abnormally expressed
miR-524-5p and validate the inhibitory function of miR-524-5p in
TNBC using in vivo experiments.
HMGA2 is a member of the high motility group protein
family and can bind the DNA minor groove at sequences rich with A
and T nucleotides to activate gene transcription (35). Accumulating evidence has indicated
that there was increased expression of HMGA2 in different human
cancer types, including pancreatic cancer and gastric cancer, and
that it was involved in the epithelial-to-mesenchymal transition of
multiple epithelial carcinomas (36). Overexpression of HMGA2 is strongly
associated with the poor prognosis of patients with colorectal
cancer (37). HMGA2 has also been
identified as a target of miRNAs that modulates the development of
cancer (38–42). For example, miR-9 exerted
anti-cancer effects in hepatocellular carcinoma by targeting HMGA2
(43), while miR-204-3p decreased
HMGA2 and repressed the malignant phenotype of colon cancer
(44).
In the current study, it was found that miR-524-5p
bound to the 3′-UTR of HMGA2 and inhibited the expression of HMGA2
in TNBC cells. Consistent with the increased expression levels of
miR-524-5p, knockdown of SchLAP1 suppressed both the mRNA and
protein expression levels of HMGA2 in TNBC cells. The rescue
experiments demonstrated that overexpression of HMGA2 attenuated
the reduced growth of TNBC cells by downregulating SChLAP1. These
findings indicate the critical function of the miR-524-5p/HMGA2
axis in the oncogenic role of SChLAP1 in TNBC.
In conclusion, the present study demonstrated that
SChLAP1 was upregulated and positively associated with the advanced
progression of TNBC. The results suggested that SChLAP1 regulates
the malignant behaviors of TNBC cells by sponging miR-524-5p. This
evidence provides a novel perspective on the molecular mechanism of
SChLAP1 in the progression of TNBC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XB, HZ and JX designed the study. XB performed the
experiments. SZ and JQ collected the tissue samples and analyzed
gene expression via reverse transcription-quantitative PCR. XX and
WL performed the dual-luciferase reporter assay and western
blotting. XB, HZ and JX wrote the manuscript. HZ and JX confirmed
the authenticity of all raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This work was approved by the Ethics Committee of
Shanxi Provincial Cancer Hospital. All patients provided written
informed consent prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Medina MA, Oza G, Sharma A, Arriaga LG,
Hernandez Hernandez JM, Rotello VM and Ramirez JT: Triple-negative
breast cancer: A review of conventional and advanced therapeutic
strategies. Int J Environ Res Public Health. 17:20782020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
da Silva JL, Cardoso Nunes NC, Izetti P,
de Mesquita GG and de Melo AC: Triple negative breast cancer: A
thorough review of biomarkers. Crit Rev Oncol Hematol.
145:1028552020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pandy JGP, Balolong-Garcia JC,
Cruz-Ordinario MVB and Que FVF: Triple negative breast cancer and
platinum-based systemic treatment: A meta-analysis and systematic
review. BMC Cancer. 19:10652019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Laurentiis M, Cianniello D, Caputo R,
Stanzione B, Arpino G, Cinieri S, Lorusso V and De Placido S:
Treatment of triple negative breast cancer (TNBC): Current options
and future perspectives. Cancer Treat Rev. 36 (Suppl 3):S80–S86.
2010. View Article : Google Scholar
|
|
5
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodriguez Bautista R, Ortega Gómez A,
Hidalgo Miranda A, Zentella Dehesa A, Villarreal-Garza C,
Ávila-Moreno F and Arrieta O: Long non-coding RNAs: Implications in
targeted diagnoses, prognosis, and improved therapeutic strategies
in human non- and triple-negative breast cancer. Clin Epigenetics.
10:882018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Q, Gao S, Li H, Lv M and Lu C: Long
noncoding RNAs (lncRNAs) in triple negative breast cancer. J Cell
Physiol. 232:3226–3233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kong X, Liu W and Kong Y: Roles and
expression profiles of long non-coding RNAs in triple-negative
breast cancers. J Cell Mol Med. 22:390–394. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Piasecka D, Braun M, Kordek R, Sadej R and
Romanska H: MicroRNAs in regulation of triple-negative breast
cancer progression. J Cancer Res Clin Oncol. 144:1401–1411. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naorem LD, Prakash VS, Muthaiyan M and
Venkatesan A: Comprehensive analysis of dysregulated lncRNAs and
their competing endogenous RNA network in triple-negative breast
cancer. Int J Biol Macromol. 145:429–436. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu ST, Xu JH, Zheng ZR, Zhao QQ, Zeng XS,
Cheng SX, Liang YH and Hu QF: Long non-coding RNA ANRIL promotes
carcinogenesis via sponging miR-199a in triple-negative breast
cancer. Biomed Pharmacother. 96:14–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan H, Yuan J and Li X, Ma Y, Wang X, Xu B
and Li X: LncRNA LINC00173 enhances triple-negative breast cancer
progression by suppressing miR-490-3p expression. Biomed
Pharmacother. 125:1099872020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mehra R, Udager AM, Ahearn TU, Cao X, Feng
FY, Loda M, Petimar JS, Kantoff P, Mucci LA and Chinnaiyan AM:
Overexpression of the Long Non-coding RNA SChLAP1 independently
predicts lethal prostate cancer. Eur Urol. 70:549–552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prensner JR, Iyer MK, Sahu A, Asangani IA,
Cao Q, Patel L, Vergara IA, Davicioni E, Erho N, Ghadessi M, et al:
The long noncoding RNA SChLAP1 promotes aggressive prostate cancer
and antagonizes the SWI/SNF complex. Nat Genet. 45:1392–1398. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Luo H, Xiao N, Duan J, Wang Z and
Wang S: Long noncoding RNA SChLAP1 accelerates the proliferation
and metastasis of prostate cancer via targeting miR-198 and
promoting the MAPK1 pathway. Oncol Res. 26:131–143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Shi Z, Nan Y and Li M: Inhibiting
malignant phenotypes of the bladder cancer cells by silencing long
noncoding RNA SChLAP1. Int Urol Nephrol. 48:711–716. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liao Y, Smyth GK and Shi W: featureCounts:
An efficient general purpose program for assigning sequence reads
to genomic features. Bioinformatics. 30:923–930. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y and Wang X: miRDB: An online
database for prediction of functional microRNA targets. Nucleic
Acids Res. 48:D127–D131. 2020. View Article : Google Scholar
|
|
26
|
Ji J, Xu R, Ding K, Bao G, Zhang X, Huang
B, Wang X, Martinez A, Wang X, Li G, et al: Long Noncoding RNA
SChLAP1 forms a growth-promoting complex with HNRNPL in human
glioblastoma through stabilization of ACTN4 and activation of NF-κB
signaling. Clin Cancer Res. 25:6868–6881. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomson DW and Dinger ME: Endogenous
microRNA sponges: Evidence and controversy. Nat Rev Genet.
17:272–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan JJ and Tay Y: Noncoding RNA: RNA
regulatory networks in cancer. Int J Mol Sci. 19:3102018.
View Article : Google Scholar
|
|
29
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu H, Chen X, Lin T, Chen X, Yan J and
Jiang S: MicroRNA-524-5p suppresses the progression of papillary
thyroid carcinoma cells via targeting on FOXE1 and ITGA3 in cell
autophagy and cycling pathways. J Cell Physiol. 234:18382–18391.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu GH, Liu YH, Yang Z, Zhu AL and Zhao
CL: MicroRNA-524-5p suppresses the growth and invasive abilities of
gastric cancer cells. Oncol Lett. 11:1926–1932. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu CY, Meng FQ and Liu J: MicroRNA-524-5p
suppresses cell proliferation and promotes cell apoptosis in
gastric cancer by regulating CASP3. Eur Rev Med Pharmacol Sci.
23:7968–7977. 2019.PubMed/NCBI
|
|
33
|
Zhen W, Qiu D, Zhiyong C, Xin W, Mengyao
J, Dimin Z, Chonghui H, Haijun W and Yonghong Z: MicroRNA-524-5p
Functions as a tumor suppressor in a human pituitary tumor-derived
cell line. Horm Metab Res. 49:550–557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao K, Wang Q, Wang Y, Huang K, Yang C,
Li Y, Yi K and Kang C: EGFR/c-myc axis regulates TGFβ/Hippo/Notch
pathway via epigenetic silencing miR-524 in gliomas. Cancer Lett.
406:12–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang S, Mo Q and Wang X: Oncological role
of HMGA2 (Review). Int J Oncol. 55:775–788. 2019.PubMed/NCBI
|
|
36
|
De Martino M, Fusco A and Esposito F: HMGA
and Cancer: A review on patent literatures. Recent Pat Anticancer
Drug Discov. 14:258–267. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang YM, Cheng CH, Pan SL, Yang PM, Lin
DY and Lee KH: Gene expression signature-based approach identifies
antifungal drug ciclopirox as a novel inhibitor of HMGA2 in
colorectal cancer. Biomolecules. 9:6882019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang NN, Ge SL, Zhang RQ, Huang YL, Liu SD
and Wu KM: MiR-490-3p inhibited the proliferation and metastasis of
esophageal squamous cell carcinoma by targeting HMGA2. Eur Rev Med
Pharmacol Sci. 22:8298–8305. 2018.PubMed/NCBI
|
|
39
|
Xing F, Song Z and He Y: MiR-219-5p
inhibits growth and metastasis of ovarian cancer cells by targeting
HMGA2. Biol Res. 51:502018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun J, Qiao Y, Song T and Wang H: MiR495
suppresses cell proliferation by directly targeting HMGA2 in lung
cancer. Mol Med Rep. 19:1463–1470. 2019.PubMed/NCBI
|
|
41
|
Wang MJ, Zhang H, Li J and Zhao HD:
microRNA-98 inhibits the proliferation, invasion, migration and
promotes apoptosis of breast cancer cells by binding to HMGA2.
Biosci Rep. 38:BSR201805712018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J, Liang H, Ge H, Guo X, Gu D and
Yuan Y: MicroRNA3633p inhibits hepatocarcinogenesis by targeting
HMGA2 and is associated with liver cancer stage. Mol Med Rep.
19:935–942. 2019.PubMed/NCBI
|
|
43
|
Xu X, Zou H, Luo L, Wang X and Wang G:
MicroRNA-9 exerts antitumor effects on hepatocellular carcinoma
progression by targeting HMGA2. FEBS Open Bio. 9:1784–1797. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xi X, Teng M, Zhang L, Xia L, Chen J and
Cui Z: MicroRNA-204-3p represses colon cancer cells proliferation,
migration, and invasion by targeting HMGA2. J Cell Physiol.
235:1330–1338. 2020. View Article : Google Scholar : PubMed/NCBI
|