Introduction
Oral squamous cell carcinoma (OSCC) is one of the
most frequent malignant tumors with a poor prognosis (1,2), and
the incidence of OSCC is ~300,000 new cases per year worldwide
(3). Recent reports revealed that
betel quid chewing, smoking and human papillomavirus infections are
the three biggest risk factors that contribute to the tumorigenesis
of OSCC (4,5). In addition, OSCC is known to exhibit a
high propensity for metastasis (6).
Currently, the primary treatment options for OSCC are surgery,
chemotherapy and radiotherapy (7);
however, these therapeutic strategies have limited effects,
particularly for the patients with advanced stage OSCC (8). Therefore, it is necessary to
investigate novel effective strategies for the treatment of
OSCC.
Previous studies have reported that non-coding RNAs
(ncRNAs) play important roles in multiple diseases (9,10).
Among these ncRNAs are long ncRNAs (lncRNAs), which are >200
nucleotides in length (11). In
addition, lncRNAs are involved in OSCC. For example, Ghapanchi
et al (12) revealed that
lncRNA H19 could increase the proliferation of OSCC cells. Wu et
al (13) revealed that lncRNA
RC3H2 was an oncogene via regulating microRNA (miRNA/miR)-101-3p in
OSCC.
Meanwhile, the lncRNA deleted in lymphocytic
leukemia 1 (DLEU1) has been confirmed to regulate the progression
of multiple types of cancer (12,13),
and elevated DLEU1 expression contributes to the development of
OSCC, suggesting that DLEU1 may play an important role in OSCC
(14); however, the detailed
function of DLEU1 in OSCC remains unclear.
miRNAs are endogenic non-coding small RNAs that are
found in abundance in the human body (15). In addition, dysregulation of miRNAs
are known to be associated with the progression of OSCC (16). For example, it was previously
reported that miR-770 could promote the migration and invasion of
OSCC cells by regulating the NAD-dependent protein deacetylase
sirtuin-7 (Sirt7)/Smad4 pathway (17). Moreover, miR-128 and miR-142 could
regulate the tumorigenesis and epithelial-mesenchymal transition in
OSCC through mediation of homeobox protein Hox-A10 (18). Meanwhile, Luo et al (19) found that miR-149-5p could regulate
cisplatin chemosensitivity, cell growth, and metastasis of OSCC
cells by targeting TGFβ2. However, the association between DLEU1
and miR-149-5p in OSCC is unclear.
The present study aimed to investigate the
biological function of DLEU1 in OSCC, in order to identify novel
potential therapeutic strategies for treating OSCC.
Materials and methods
Cell culture and cell
transfection
OSCC cell lines (Cal-27 and SCC-9; American Type
Culture Collection) were maintained in DMEM (Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) and antibiotics (100 U/ml
penicillin and 100 µg/ml streptomycin) in an incubator at 37°C with
5% CO2.
Small interfering (si)RNAs targeted against DLEU1
(DLEU1 siRNA1, DLEU1 siRNA2 and DLEU1 siRNA3; 10 nM) and a negative
control (NC) siRNA (siRNA-NC) were purchased from Guangzhou RiboBio
Co., Ltd., and were transfected into OSCC cells (5×103)
using Lipofectamine® 2000 (Thermo Fisher Scientific,
Inc.). Then, cells were incubated at 37°C for 6 h and the
transfection efficiency was determined via reverse
transcription-quantitative PCR (RT-qPCR). After 24 h of incubation,
transfected cells were used for subsequent experiments. The siRNA
sequences were as follows: siRNA-NC, 5′-UUCUCCGAACGUGUCACGUTT-3′;
DLEU1 siRNA1, 5′-GGAAUGAAGCAACUGAGAUUU-3′; DLEU1 siRNA2,
5′-GGGTTACGATTGCCCAGAT-3′; and DLEU1 siRNA3,
5′-CGTTAAGGTTCCGGACGAC-3′.
The NC, miR-149-5p agomir and miR-149-5p antagomir
were synthesized and obtained from Shanghai GenePharma Co., Ltd.
Cells (5×103) were transfected with 10 nM control agomir
(NC), miR-149-5p agomir or miR-149-5p antagomir for 24 h with
Lipofectamine 2000 at 37°C. After 24 h of transfection, transfected
cells were used for subsequent experiments. Meanwhile, the
concentration of miR-149-5p agomir/antagomir (50 nM) was selected
according to the instructions of the manufacturer. The sequences
were as follows: miR-149-5p agomir, 5′-UCUGGCUCCGUGUCUUCACUCCC-3′;
miR-149-5p antagomir, 5′-GGGAGUGAAGACACGGAGCCAGA-3′; and NC,
5′-UUCUCCGAACGUGUCACGUTT-3′.
Tissue collection
In total, 10 pairs of OSCC samples and adjacent
normal tissues (~2 cm from tumor) were collected from the
Affiliated Stomatological Hospital of Guizhou Medical University
(Guiyang, China) between April 2018 and April 2019. The patients
were informed of the purpose of the experiments and provided
written informed consent. The clinical information of the patients
is listed in Table I. Patients were
diagnosed with OSCC following the American College of Rheumatology
classification criteria for the diagnosis of OSCC (20). Meanwhile, the samples were used for
investigation of DLEU1 and CDK6 levels. The present study was
approved by the Institutional Ethical Committee of Affiliated
Stomatological Hospital of Guizhou Medical University (approval no.
ASHGMU20190523).
 | Table I.Clinical characteristics of patients
with OSCC in the present study. |
Table I.
Clinical characteristics of patients
with OSCC in the present study.
| Clinical
characteristics | Number |
|---|
| Age, years |
|
≥60 | 4 |
|
<60 | 6 |
| Sex |
|
Male | 7 |
|
Female | 3 |
| Tumor size, cm |
| ≥3 | 6 |
|
<3 | 4 |
| TNM stage |
|
I/II | 6 |
|
III/IV | 4 |
RT-qPCR
Total RNA was extracted from OSCC cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using the
PrimeScript RT reagent kit (Takara Bio, Inc.) according to the
manufacturer's instructions. Then, RT-qPCR was performed using a
SYBR® Premix Ex Taq™ II kit (Takara Bio, Inc.) on a
7900HT system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the following conditions: 60°C for 1 min, 90°C for 15
min, followed by 40 cycles of application at 90°C for 15 sec and
55°C for 60 sec. The primers used were as follows: U6 forward,
5′-CGCTTCGGCAGCACATATAC-3′ and reverse, 5′-AAATATGGAACGCTTCACGA-3′;
DLEU1 forward, 5′-GGTCCACGGCACGTTAACA-3′ and reverse,
5′-CCAATTGAAGGCCTTAAGG-3′; miR-149-5p forward,
5′-TGCGCTAGCAGCGGGAACAGTTC-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGTATT-3′; and β-actin forward,
5′-GTCCACCGCAAATGCTTCTA-3′ and reverse, 5′-TGCTGTCACCTTCACCGTTC-3′.
The relative expression level was quantified by normalizing to
β-actin or U6 using the 2−ΔΔCq method (21).
Bioinformatics analysis
The survival curve was calculated based on the data
from The Cancer Genome Atlas (TCGA). The result shown here is in
part based upon data generated by the TCGA Research Network
(https://www.cancer.gov/tcga). In
addition, the data from TCGA were analyzed using Gene Expression
Profiling Interactive Analysis (GEPIA) as previously described
(22).
Bioinformatics prediction
The potential downstream miRNA of DLEU1 was
predicted using StarBase (http://starbase.sysu.edu.cn/index.php). In addition,
the target mRNA of miR-149-5p was predicted using TargetScan
(http://www.targetscan.org/vert_72/),
miRDB (http://www.mirdb.org/) and miRWalk
(http://mirwalk.umm.uni-heidelberg.de/).
Cell Counting Kit-8 (CCK-8) assay
SCC-9 or Cal-27 cells (5.0×103 cells/well) were
transfected with siRNA-NC, DLEU1 siRNA2 or DLEU1 siRNA3 for 0, 24,
48 or 72 h. Then, 10 µl CCK-8 reagent (Beyotime Institute of
Biotechnology) was added into each well, and the plate was
incubated for 2 h at 37°C. The absorbance was detected at 450 nm
using a microplate reader (Bio-Rad Laboratories, Inc.).
Immunofluorescence staining
OSCC cells were plated onto a 96-well plate at a
density of 5.0×103 cells/well. Following incubation,
cells were transfected with siRNA-NC or DLEU1 siRNA2 for 72 h.
After that, cells were fixed with 4% paraformaldehyde for 20 min at
room temperature and blocked with 2% bovine serum albumin (Beyotime
Institute of Biotechnology) at room temperature for 30 min.
Subsequently, cells were incubated with rabbit monoclonal antibody
anti-Ki67 (1:100; cat. no. ab92742; Abcam) at 4°C overnight. Then,
cells were incubated with anti-rabbit IgG secondary antibody
(1:1,000; cat. no. ab150077; Abcam) for 1 h at room temperature.
The results were observed under a fluorescence microscope (Olympus
BX53; Olympus Corporation).
Transwell assay
For the cell migration assay, 2×105 OSCC
cells were seeded into the upper chambers of the 24-well plates in
200 µl serum-free DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 0.2% bovine serum albumin (Beyotime Institute of
Biotechnology). The lower chambers contained RPMI-1640 medium
supplemented with 1% FBS. After incubation for 24 h at 37°C, the
non-migrating cells were gently removed from the upper side of each
chamber with a cotton swab, while the cells that had migrated were
fixed with 95% alcohol for 10 min at room temperature and stained
with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA) for 5 min at
room temperature. Finally, cells were counted under an inverted
light microscope (Olympus Corporation) at ×400 magnification.
For the cell invasion assay, the upper chamber was
pre-treated with 100 µl Matrigel (BD Biosciences) for 4 h at 37°C.
Subsequently, transfected OSCC cells (2×105) were seeded
into the upper chambers with serum-free DMEM, while the media in
the lower chambers was supplemented with 1% FBS. After incubation
for 24 h, the Transwell chambers were fixed with 95% alcohol for 10
min at room temperature and stained with 0.1% crystal violet for 5
min at room temperature. The Transwell chamber was observed and the
invaded cells were counted under a microscope.
Flow cytometry assay
Cells were trypsinized and resuspended in binding
buffer. Then, cells were stained with 5 µl Annexin V-FITC (BD
Biosciences) and propidium iodide (PI; BD Biosciences) in the dark
at 37°C for 30 min. Flow cytometry (FACScan™; BD Biosciences) was
applied to analyze the apoptosis rate using CellQuest™ software
(version 5.1; BD Biosciences).
Western blotting
OSCC cells were lysed in RIPA lysis buffer (Nanjing
KeyGen Biotech Co., Ltd.), and the protein concentration was
determined using a BCA Assay kit (Beijing Solarbio Science &
Technology Co., Ltd.). Proteins (30 µg per lane) were separated via
SDS-PAGE (10% gel), and separated proteins were then transferred
onto PVDF membranes (Thermo Fisher Scientific, Inc.). The membranes
were blocked in 5% non-fat dried milk in TBS with Tween-20 (10%)
for 1 h at room temperature. Then, the PVDF membranes were
incubated at 4°C overnight with the following primary antibodies:
Anti-Bax (cat. no. ab182733; 1:1,000), anti-cleaved caspase-3 (cat.
no. ab49822; 1:1,000), anti-Bcl-2 (cat. no. ab196495; 1:1,000),
anti-CDK6 (cat. no. ab131469; 1:1,000), anti-CDK4 (cat. no.
ab68266; 1:1,000), anti-cyclin-dependent kinase inhibitor p27 (p27
Kip1; cat. no. ab32034; 1:1,000) and anti-β-actin (cat. no. ab8226;
1:1,000). Then, the membrane was incubated with horseradish
peroxidase-labeled goat anti-rabbit secondary antibody (cat. no.
ab7090; 1:5,000) at room temperature for 1 h. All antibodies were
obtained from Abcam. Enhanced chemiluminescence reagent (Thermo
Fisher Scientific, Inc.) was used to visualize the protein bands.
ImageJ software (version 2.0; National Institutes of Health) was
used to quantify the intensity of the bands.
Dual-luciferase reporter assay
CDK6 3′-untranslated region (3′UTR) containing the
putative binding site of miR-149-5p (CDK6 WT 3′UTR) and the CDK6
3′UTR with the mutation binding site (CDK6 MT 3′UTR) were
synthesized by Shanghai GenePharma Co., Ltd. The mutation was
generated using a site directed mutagenesis kit (Promega
Corporation). The partial sequences of DLEU1 were synthesized by
Shanghai GenePharma Co., Ltd. These were then cloned into the
vectors (pmirGLO; Promega Corporation). Subsequently, DLEU1 (WT/MT)
and CDK6 (WT/MT) were transfected into OSCC cells using
Lipofectamine 2000. After 48 h of transfection, the relative
luciferase activity was detected using the Dual-Luciferase Reporter
kit (Promega Corporation). The data were normalized to
Renilla luciferase activity.
RNA pull-down assay
Probe-control or probe-DLEU1 was transcribed and
labeled with a Biotin RNA Labeling Mix (Roche Diagnostics). Cells
were lysed with Poly-lysis buffer (ELK bioscience), washed with PBS
and centrifuged at 1000 × g for 5 min at 4°C. Secondary structure
formation in the biotin-labeled RNAs was induced with RNA structure
buffer (Thermo Fisher Scientific, Inc.). Streptavidin beads (75 µl;
Thermo Fisher Scientific, Inc.) were washed and incubated
overnight. After that, streptavidin bead-RNA complexes were
obtained by separating the mixture. Then, cell lysates
(5×107 cells) were added to the complexes and incubated
for 1 h. Following incubation, the mixture was separated again, and
the supernatant of cell lysates was utilized to detect the
enrichment of miR-149-5p. RT-qPCR was used to detect the enrichment
of miR-149-5p.
Cell cycle detection
Cell cycle distribution was assessed as previously
described (23). Briefly, OSCC
cells (5×105) were harvested, fixed with 75% ethanol on
ice for 20 min, permeabilized with 0.25% Triton X-100 and stained
with Pharmingen PI/RNase (BD Biosciences). After incubation for 15
min at 4°C, cells were analyzed using a flow cytometer (BD FACSAria
III; BD Biosciences) and ModFit (version 3.0; Verity Software
House, Inc.). The data were quantified using FlowJo software
(version 3.0; FlowJo, LLC).
Statistical analysis
Data are presented as the mean ± SD. The CCK-8 assay
was performed five times. Immunofluorescence staining, RT-qPCR,
western blotting, flow cytometry and Transwell invasion assays were
repeated in triplicate. In addition, all other experiments were
repeated three times. One-way analysis of variance and Tukey's post
hoc tests were used for comparisons between ≥3 groups. A paired
Student's t-test was used for comparisons between tumor tissues and
adjacent normal tissues of the same patients, while an unpaired
Student's t-test was used for comparisons between unpaired groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
DLEU1 expression is negatively
associated with the survival rate of patients with OSCC
To detect the role of DLEU1 in OSCC, TCGA data were
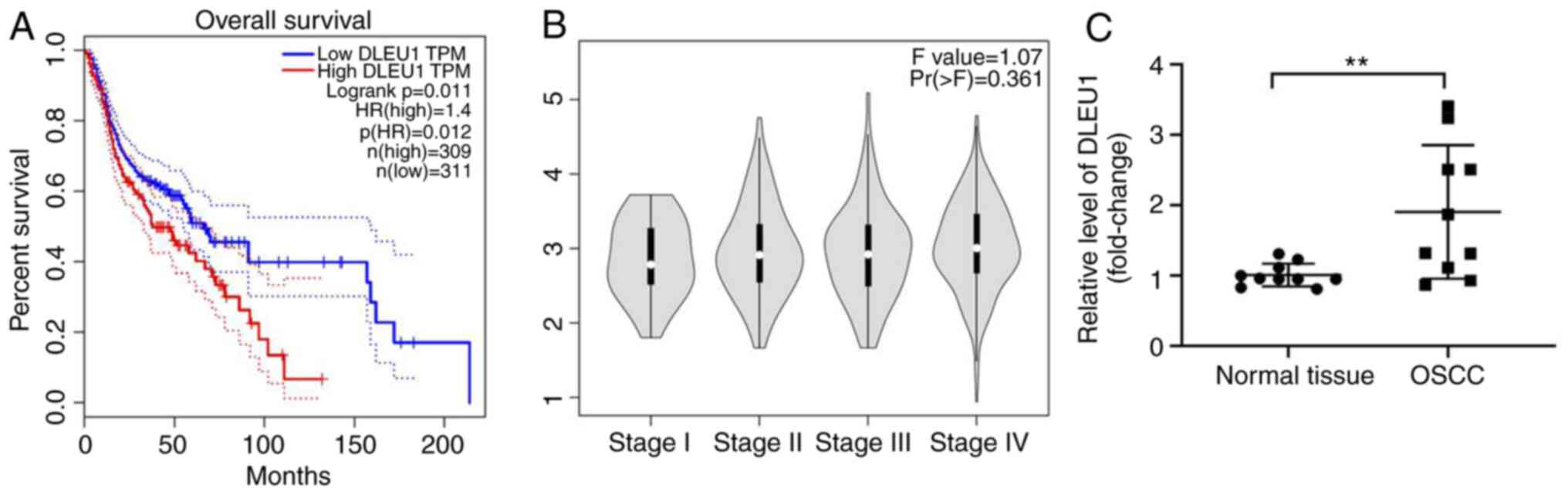
analyzed. As presented in Fig. 1A,
DLEU1 level was negatively associated with the survival rate of
patients with OSCC. In addition, DLEU1 was closely associated with
patients with advanced stage OSCC (Fig.
1B). Furthermore, the expression of DLEU1 was significantly
higher in OSCC tissues (Fig. 1C).
Taken together, the expression of DLEU1 was upregulated in
OSCC.
Silencing of DLEU1 significantly
inhibits the proliferation of OSCC cells
In order to evaluate the efficacy of cell
transfection, RT-qPCR was performed. The data indicated that the
expression of DLEU1 was significantly downregulated in OSCC cells
when transfected with DLEU1 siRNAs (Fig. 2A and B). In addition, knockdown of
DLEU1 significantly suppressed the cell viability of OSCC cells
(Fig. 2C and D). DLEU1 siRNA2 had
the most significant inhibitory effects on the expression of DLEU1,
thus DLEU1 siRNA2 was used in subsequent investigations.
Furthermore, the results of the Ki67 staining revealed that DLEU1
siRNA2 significantly inhibited the proliferation of OSCC cells
(Fig. 2E and F), and it was
revealed that Cal-27 cells were more susceptible to DLEU1 siRNA2
treatment. Thus, Cal-27 cells were selected for use in the
subsequent analyses. Overall, silencing of DLEU1 significantly
inhibited the proliferation of OSCC cells.
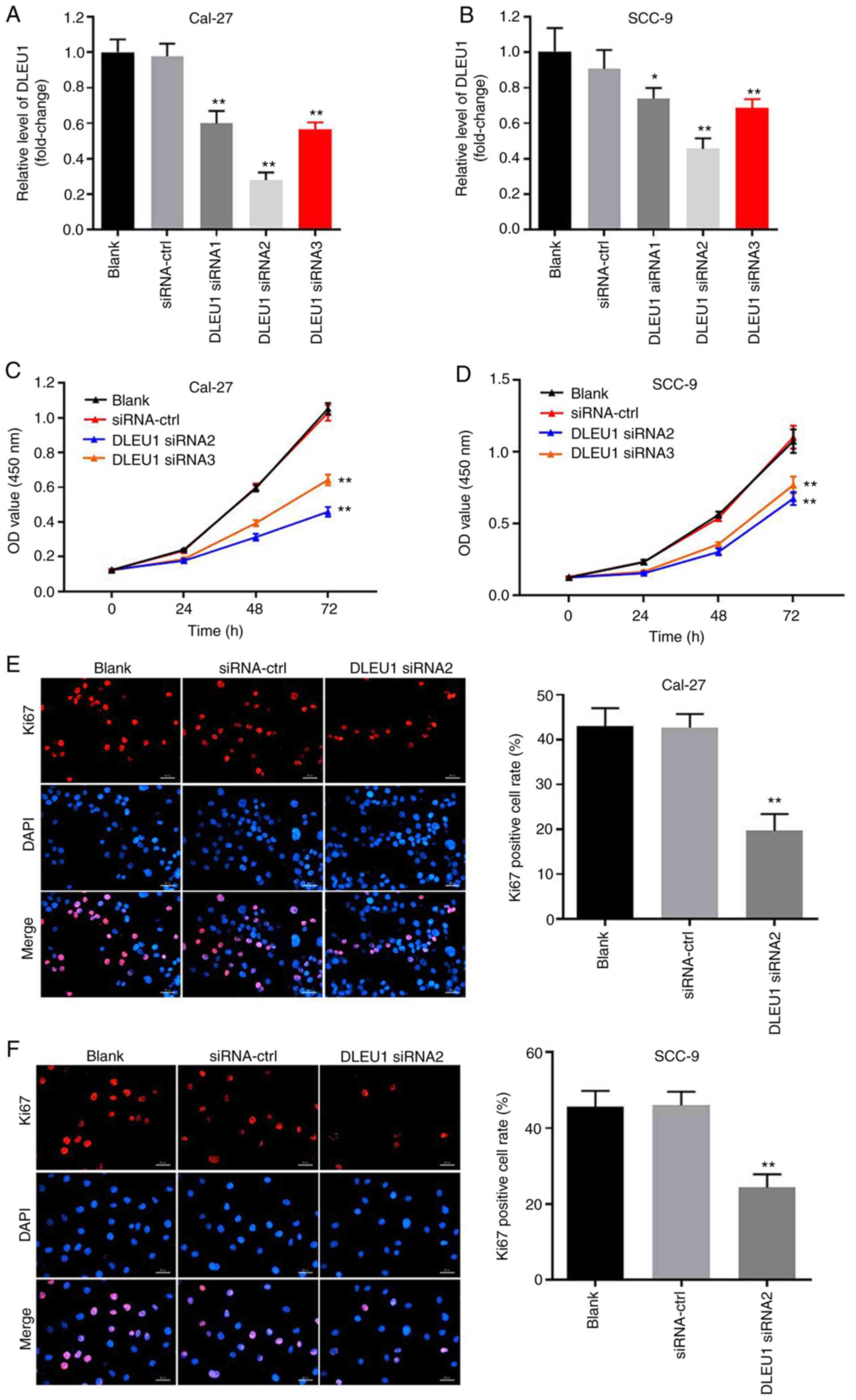 | Figure 2.Silencing of DLEU1 significantly
inhibits the proliferation of OSCC cells. (A) Cal-27 or (B) SCC-9
cells were transfected with NC, DLEU1 siRNA1, DLEU1 siRNA2 or DLEU1
siRNA3 for 24 h. Then, the efficiency of DLEU1 siRNA transfection
was detected via reverse transcription-quantitative PCR. (C) Cal-27
or (D) SCC-9 cells were treated with NC, DLEU1 siRNA2 or DLEU1
siRNA3 for 0, 24, 48 or 72 h. Then, OD values of OSCC cells were
determined via a Cell Counting Kit-8 assay. (E and F) The
proliferation of OSCC cells was measured by Ki67 staining. Red
fluorescence shows Ki67 expression. Blue fluorescence shows DAPI
staining. Scale bar, 100-µm. *P<0.05, **P<0.01 vs. control.
DLEU1, deleted in lymphocytic leukemia 1; OSCC, oral squamous cell
carcinoma; NC, negative control; siRNA, small interfering RNA. |
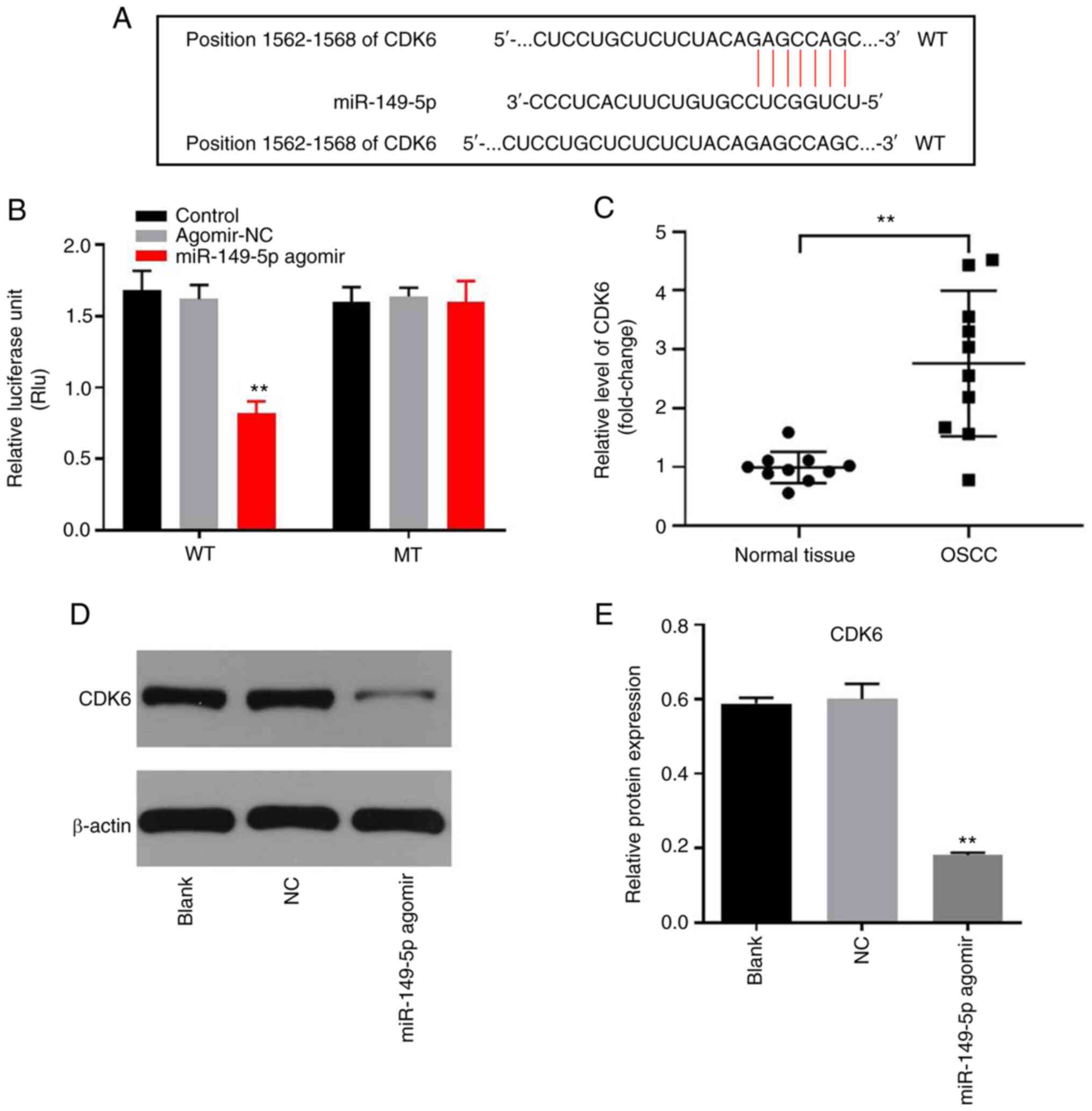
DLEU1 sponges miR-149-5p in OSCC
In order to investigate the underlying mechanism by
which DLEU1 mediated the proliferation of OSCC cells, StarBase was
used. As indicated in Fig. 3A,
DLEU1 had a putative miR-149-5p targeting site. In addition,
miR-149-5p is known to regulate the tumorigenesis of OSCC (19). Thus, miR-149-5p was selected for
further analysis. The level of miR-149-5p in OSCC cells was
upregulated by miR-149-5p agomir, but was downregulated by
miR-149-5p antagomir (Fig. 3B).
This result suggested that miR-149-5p agomir or antagomir was
stably transfected into OSCC cells. Co-transfection of the WT-DLEU1
vector with miR-149-5p agomir resulted in a significant decrease in
the luciferase activity when compared with the MT-DLEU1 vector
(Fig. 3C). Furthermore, the
expression of miR-149-5p in OSCC cells was significantly increased
by the knockdown of DLEU1, while this was completely reversed by
miR-149-5p antagomir (Fig. 3D). In
addition, the results of the pull-down assay suggested that DLEU1
bound to miR-149-5p directly in OSCC cells (Fig. 3E). Overall, DLEU1 could sponge
miR-149-5p in OSCC.
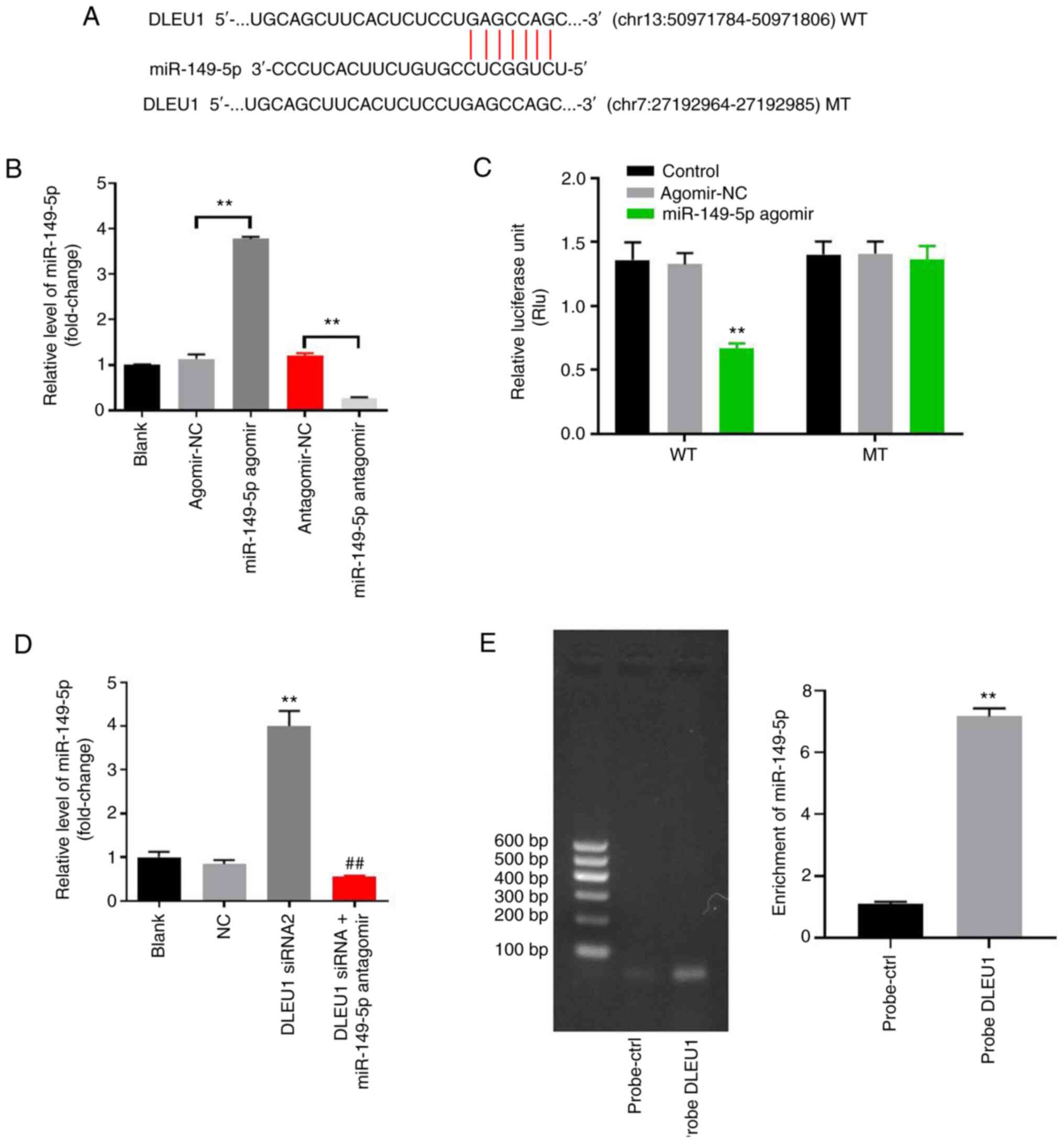 | Figure 3.DLEU1 can sponge miR-149-5p in OSCC.
(A) Gene structure of DLEU1 indicated the binding site of
miR-149-5p in its 3′UTR. (B) OSCC cells were transfected with
antagomir/agomir NC, miR-149-5p agomir/antagomir for 24 h. Then,
the expression of miR-149-5p in OSCC cells was detected via
RT-qPCR. (C) The relative luciferase activity in Cal-27 cells after
co-transfection with miR-149-5p and WT/MT DLEU1 3′UTR was measured
using a dual-luciferase reporter assay. (D) Cal-27 cells were
treated with NC, DLEU1 siRNA2 or DLEU1 siRNA2 + miR-149-5p. Then,
the expression of miR-149-5p in Cal-27 cells was detected via
RT-qPCR. (E) The association between DLEU1 and miR-149-5p was
explored using an RNA pull-down assay. **P<0.01 vs. control;
##P<0.01 vs. DLEU1 siRNA2. DLEU1, deleted in
lymphocytic leukemia 1; OSCC, oral squamous cell carcinoma; NC,
negative control; siRNA, small interfering RNA; miR, microRNA; UTR,
untranslated region; WT, wild-type; MT, mutant; RT-qPCR, reverse
transcription-quantitative PCR. |
Knockdown of DLEU1 inhibits the
tumorigenesis of OSCC via regulation of miR-149-5p
In order to further investigate the underlying
mechanism by which DLEU1 regulated the progression of OSCC, flow
cytometry was used. As indicated in Fig. 4A, DLEU1 siRNA2 significantly induced
cell apoptosis, which was partially reversed by miR-149-5p
antagomir (Fig. 4A). In addition,
the migration and invasion of OSCC cells was significantly
inhibited following knockdown of DLEU1, and this was also reversed
by the miR-149-5p antagomir (Fig.
4B). Meanwhile, the expression levels of Bax and cleaved
caspase-3 in OSCC cells were significantly increased by DLEU1
siRNA2 (Fig. 4C-E). By contrast,
DLEU1 siRNA2 significantly inhibited the expression of Bcl-2 in
OSCC cells (Fig. 4C and F).
However, the effects of DLEU1 knockdown on these three proteins
were significantly reversed by miR-149-5p antagomir (Fig. 4C-F). Knockdown of DLEU1 inhibited
the tumorigenesis of OSCC via mediation of miR-149-5p.
miR-149-5p directly targets CDK6 in
OSCC cells
In order to identify the downstream target of
miR-149-5p, TargetScan, miRDB and miRWalk were used in the present
study. As presented in Fig. 5A,
CDK6 was demonstrated to be the potential target of miR-149-5p
using these three online tools. The co-transfection of the WT-CDK6
vector with miR-149-5p agomir significantly decreased the
luciferase activity when compared with the MT-CDK6 vector (Fig. 5B). In addition, the level of CDK6
was significantly higher in OSCC tissues, compared with adjacent
normal tissues (Fig. 5C).
Furthermore, the protein expression of CDK6 in OSCC cells was
significantly inhibited by miR-149-5p agomir (Fig. 5D and E). In summary, miR-149-5p
directly targeted CDK6 in OSCC cells.
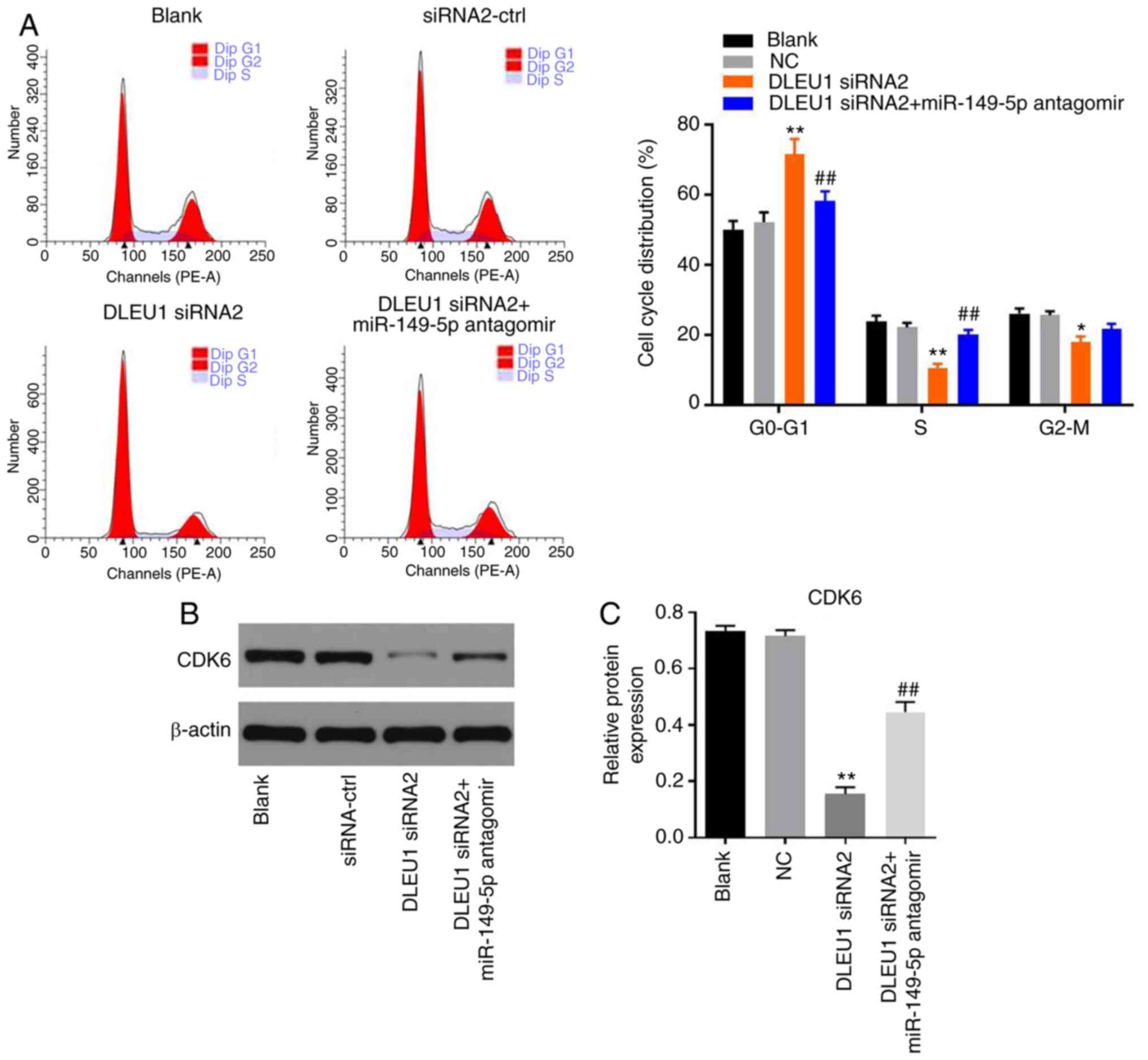
Knockdown of DLEU1 induces G1 arrest
in OSCC cells via mediation of CDK6
In order to determine cell cycle distribution, flow
cytometry was performed in the present study. The data revealed
that DLEU1 knockdown significantly induced G1 cell cycle arrest in
OSCC cells, which was partially rescued by miR-149-5p antagomir
(Fig. 6A). In addition, the
expression of CDK6 in OSCC cells was significantly inhibited by
DLEU1 siRNA2, while this phenomenon was partially reversed by
miR-149-5p antagomir (Fig. 6B and
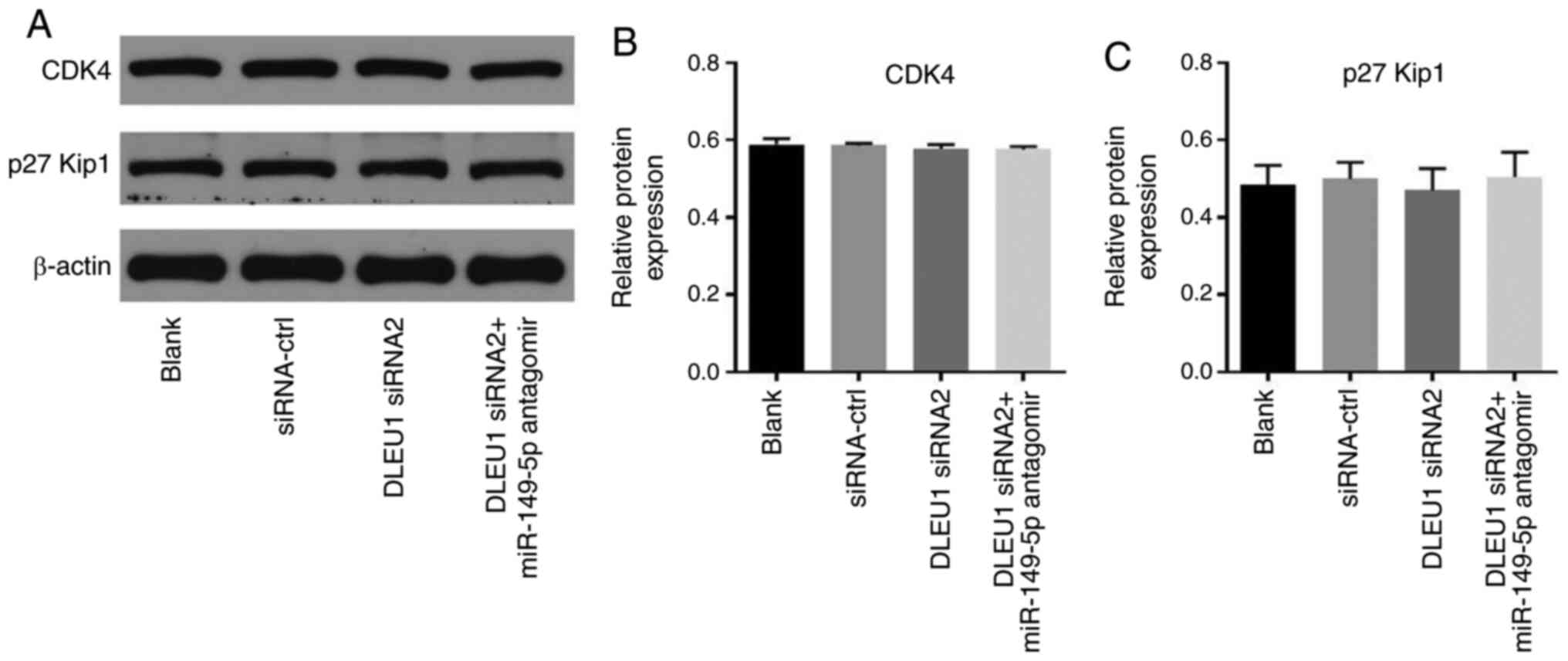
C). Meanwhile, the expression levels of CDK4 and p27 Kip1 in
OSCC cells were not affected by the knockdown of DLEU1 or the
addition of miR-149-5p antagomir (Fig.
7A-C). In summary, knockdown of DLEU1 induced G1 cell cycle
arrest in OSCC cells via mediation of CDK6.
Discussion
Several lncRNAs have been found to be up- or
downregulated in OSCC, and dysregulated lncRNAs have been
implicated to play an important role during the tumorigenesis of
OSCC (24–26). A previous study indicated that the
expression of DLEU1 was upregulated in OSCC tissues (14). The present study further
investigated the function of DLEU1 in OSCC, confirming that DLEU1
could act as a key biomarker in OSCC. In addition, Lin et al
(27) reported that upregulation of
DLEU1 could promote the tumorigenesis of esophageal squamous cell
carcinoma. Miao et al (24)
revealed that DLEU1 promoted the progression of clear cell renal
cell carcinoma. Based on these data, it can be suggested that DLEU1
could function as an oncogene in certain tumor types. According to
Liu et al (28), Sp1
transcription factor (SP1) could bind to the promoter region of
DLEU1 to activate DLEU1 transcription. In addition, SP1 could
promote the tumorigenesis of OSCC (29). Thus, DLEU1 may be upregulated by SP1
in OSCC.
It was previously reported that miR-149-5p could act
as a suppressor in certain types of malignancies (30,31).
Luo et al (19) revealed
that miR-149-5p could regulate cell proliferation and tumor
metastasis in OSCC by targeting TGFβ2. Consistently, the results of
the present study indicated that miR-149-5p antagomir could inhibit
the effect of DLEU1 knockdown on the proliferation of OSCC cells,
suggesting that DLEU1 could sponge miR-149-5p in OSCC. However,
Chen et al (32) indicated
that knockdown of DLEU1 inhibited the proliferation of osteosarcoma
cells via sponging miR-671-5p. This difference may be due to the
different tumor type.
miRNAs can mediate cancer tumorigenesis via the
repression of target gene expression (33). In the present study, it was revealed
that miR-149-5p could directly target CDK6 in OSCC cells. CDK6 is a
regulator of the cell cycle that can prevent G0/G1 arrest in
non-small cell lung, liver and gastric cancer cell lines (34–36).
In addition, CDK6 has a specific oncogenic role in a variety of
tumors (32). Li et al
(33) revealed that Tanshinone IIA
promoted cardiac differentiation and enhanced cell motility via
mediation of CDK6; Liu et al (34) confirmed that CDK6 was directly
targeted by miR-149-5p in lung cancer. Consistently, the results of
the present study suggested that DLEU1 siRNA inhibited the
tumorigenesis of OSCC via indirectly targeting CDK6. Meanwhile, a
previous report revealed that knockdown of DLEU1 could induce G1
phase cell cycle arrest in gastric cancer cells, and DLEU1 could
mediate the expression of KLF2 (37). The present study revealed that
silencing of DLEU1 could increase G1 phase distribution in OSCC
cells. KLF2 is known to act as a key regulator in G1 phase
distribution (38,39). This similar function between CDK6
and KLF2 may contribute to the consistent data between the present
study and the previous report. On the other hand, knockdown of
DLEU1 could inhibit the invasion of OSCC cells, while miR-149-5p
antagomir reversed this phenomenon. In addition, miR-149-5p
directly targeted CDK6. Thus, DLEU1 knockdown may inhibit the
invasion of OSCC cells via indirectly targeting CDK6. According to
Chen et al (40),
overexpression of CDK6 relieved the inhibitory action of miR-770-5p
upregulation on cancer cell invasion. Thus, the present study was
consistent with this previous research.
There are some limitations in this research, which
are as follows: i) The underlying mechanism by which DLEU1 is
upregulated in OSCC is unknown; ii) the detailed effect of CDK6 on
cell invasion of OSCC is not clear; iii) the association between
DLEU1 and three risk factors (betel quid chewing, smoking and human
papillomavirus infections) for OSCC needs to be further
investigated; iv) more tissue samples need to be collected in
further study; and v) DLEU1 overexpression experiments need to be
supplemented. Thus, in future studies, we will improve these
limitations. In addition, more underlying mechanisms by which DLEU1
mediates the tumorigenesis of OSCC will be investigated.
In conclusion, silencing of DLEU1 suppressed the
tumorigenesis of OSCC via mediation of the miR-149-5p/CDK6 axis in
the present study. Therefore, DLEU1 may serve as a target for the
treatment of OSCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TL, HL and WH conceived and supervised the study. TL
and WH confirm the authenticity of all the raw data. TL and HL
designed the study. TL, HL and YW performed the experiments and
analyzed the data. All authors reviewed the results, and read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethical Committee of Affiliated Stomatological Hospital of Guizhou
Medical University (Guiyang, China). Written informed consent was
obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Andisheh-Tadbir A, Goharian AS and Ranjbar
MA: Glypican-3 expression in patients with oral squamous cell
carcinoma. J Dent (Shiraz). 21:141–146. 2020.PubMed/NCBI
|
|
2
|
Ueda S, Kanda M, Sato Y, Baba H, Nakamura
S, Sawaki K, Shimizu D, Motoyama S, Fujii T, Kodera Y, et al:
Chromobox 2 expression predicts prognosis after curative resection
of oesophageal squamous cell carcinoma. Cancer Genomics Proteomics.
17:391–400. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsao YC, Chang YJ, Wang CH and Chen L:
Discovery of isoplumbagin as a novel NQO1 substrate and anti-cancer
quinone. Int J Mol Sci. 21:212020. View Article : Google Scholar
|
|
4
|
Yang Y, Ci HS, Mao YL, Li JW and Zuo JH:
CircRNA_002178 promotes the proliferation and migration of oral
squamous cell carcinoma cells by activating the Akt/mTOR pathway.
Eur Rev Med Pharmacol Sci. 24:6122–6130. 2020.PubMed/NCBI
|
|
5
|
Zhu L, Yan D, Chen Y, Chen S, Chen N and
Han J: The identification of autophagy-related genes in the
prognosis of oral squamous cell carcinoma. Oral Dis. 26:1659–1667.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng W, Fu J, Wang T, Chen JX, Fu LB and
Peng W: Hsa_circRNA_101036 acts as tumor-suppressor in oral
squamous cell carcinoma cells via inducing endoplasmic reticulum
stress. Eur Rev Med Pharmacol Sci. 24:6111–6121. 2020.PubMed/NCBI
|
|
7
|
Zhou YM, Yao YL, Liu W, Shen XM, Shi LJ
and Wu L: MicroRNA-134 inhibits tumor stem cell migration and
invasion in oral squamous cell carcinomas via downregulation of
PI3K-Akt signaling pathway by inhibiting LAMC2 expression. Cancer
Biomark. 29:51–67. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gissi DB, Gabusi A, Tarsitano A, Asioli S,
Rossi R, Marchetti C, Montebugnoli L, Foschini MP and Morandi L:
Application of a non-invasive oral brushing procedure based on
bisulfite sequencing of a 13-gene panel to study high-risk OSCC
patients. Cancer Biomark. 28:499–510. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma YS, Chu KJ, Ling CC, Wu TM, Zhu XC, Liu
JB, Yu F, Li ZZ, Wang JH, Gao QX, et al: Long Noncoding RNA
OIP5-AS1 promotes the progression of liver hepatocellular carcinoma
via regulating the hsa-miR-26a-3p/EPHA2 axis. Mol Ther Nucleic
Acids. 21:229–241. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li A, Mallik S, Luo H, Jia P, Lee DF and
Zhao Z: H19, a Long Non-coding RNA, mediates transcription factors
and target genes through interference of MicroRNAs in pan-cancer.
Mol Ther Nucleic Acids. 21:180–191. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan C, Zhang Z, Bao S, Hou P, Zhou M, Xu C
and Sun J: Computational methods and applications for identifying
disease-associated lncRNAs as potential biomarkers and therapeutic
targets. Mol Ther Nucleic Acids. 21:156–171. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ghapanchi J, Ranjbar Z, Mokhtari MJ,
Koohpeima F, Derakhshan M, Khademi B, Ghaderi H, Sheikhbahaei S and
Aliabadi E: The LncRNA H19 rs217727 polymorphism is associated with
oral squamous cell carcinoma susceptibility in iranian population.
BioMed Res Int. 2020:16342522020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu K, Jiang Y, Zhou W, Zhang B, Li Y, Xie
F, Zhang J, Wang X, Yan M, Xu Q, et al: Long noncoding RNA RC3H2
facilitates cell proliferation and invasion by targeting
MicroRNA-101-3p/EZH2 axis in OSCC. Mol Ther Nucleic Acids.
20:97–110. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishiyama K, Maruyama R, Niinuma T, Kai M,
Kitajima H, Toyota M, Hatanaka Y, Igarashi T, Kobayashi JI, Ogi K,
et al: Screening for long noncoding RNAs associated with oral
squamous cell carcinoma reveals the potentially oncogenic actions
of DLEU1. Cell Death Dis. 9:8262018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saikishore R, Velmurugan P, Ranjithkumar
D, Latha R, Sathiamoorthi T, Arun A, Ravi AV and Sivakumar S: The
circular RNA-miRNA Axis: A special RNA signature regulatory
transcriptome as a potential biomarker for OSCC. Mol Ther Nucleic
Acids. 22:352–361. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Padmavathi G and Ramkumar KM: MicroRNA
mediated regulation of the major redox homeostasis switch, Nrf2,
and its impact on oxidative stress-induced ischemic/reperfusion
injury. Arch Biochem Biophys. 698:1087252021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia B, Zhang S, Wu S, Zhu Q and Li W:
MiR-770 promotes oral squamous cell carcinoma migration and
invasion by regulating the Sirt7/Smad4 pathway. IUBMB Life.
73:264–272. 2021. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao Y, Xu Q, Yan L, Jiao Y, Su Q, Li X,
Liu C and Zhao F: MiRNA-128 and MiRNA-142 regulate tumorigenesis
and EMT in oral squamous cell carcinoma through HOXA10. Cancer
Manag Res. 12:9987–9997. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo K, He J, Yu D and Açil Y: MiR-149-5p
regulates cisplatin chemosensitivity, cell growth, and metastasis
of oral squamous cell carcinoma cells by targeting TGFβ2. Int J
Clin Exp Pathol. 12:3728–3739. 2019.PubMed/NCBI
|
|
20
|
Jain A, Kotimoole CN, Ghoshal S, Bakshi J,
Chatterjee A, Prasad TS and Pal A: Identification of potential
salivary biomarker panels for oral squamous cell carcinoma. Sci
Rep. 11:33652021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar
|
|
23
|
Roudnicky F, Poyet C, Buser L, Saba K,
Wild P, Otto VI and Detmar M: Characterization of tumor blood
vasculature expression of human invasive baldder cancer by laser
capture microdissection and transcriptional profiling. Am J Pathol.
190:1960–1970. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miao T, Si Q, Wei Y, Fan R, Wang J and An
X: Identification and validation of seven prognostic long
non-coding RNAs in oral squamous cell carcinoma. Oncol Lett.
20:939–946. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghafouri-Fard S, Dashti S and Taheri M:
The role of long non-coding RNA CASC2 in the carcinogenesis
process. Biomed Pharmacother. 127:1102022020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin Y, Tan Y, Yao Y, Lu N and Zhang F:
SNHG12/miR-326/E2F1 feedback loop facilitates the progression of
oral squamous cell carcinoma. Oral Dis. 26:1631–1639. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin P, Li Q, Lv X, Qu J, Wang D, Li A and
Jiang G: HMGA1 promotes the development of esophageal squamous cell
carcinoma by mediating miR-671-5p/lncRNA DLEU1. Panminerva Med. Jan
30–2020.(Epub ahead of print). doi:
10.23736/S0031-0808.19.03843-6.
|
|
28
|
Liu X, Chen R and Liu L:
SP1-DLEU1-miR-4429 feedback loop promotes cell proliferative and
anti-apoptotic abilities in human glioblastoma. Biosci Rep.
39:392019. View Article : Google Scholar
|
|
29
|
Liu XB, Wang J, Li K and Fan XN: Sp1
promotes cell migration and invasion in oral squamous cell
carcinoma by upregulating Annexin A2 transcription. Mol Cell
Probes. 46:1014172019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou C, Wang P, Tu M, Huang Y, Xiong F and
Wu Y: Long non-coding RNA PART1 promotes proliferation, migration
and invasion of hepatocellular carcinoma cells via
miR-149-5p/MAP2K1 axis. Cancer Manag Res. 12:3771–3782. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tian D, Tian M, Ma ZM, Zhang LL, Cui YF
and Li JL: Anesthetic propofol epigenetically regulates breast
cancer trastuzumab resistance through IL-6/miR-149-5p axis. Sci
Rep. 10:88582020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Zhang C and Wang X: Long noncoding
RNA DLEU1 aggravates osteosarcoma carcinogenesis via regulating the
miR-671-5p/DDX5 axis. Artif Cells Nanomed Biotechnol. 47:3322–3328.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Shi B, Dong F, Zhu X, Liu B and Liu
Y: Long bon-coding RNA DLEU1 promotes cell proliferation, invasion,
and confers cisplatin resistance in bladder cancer by regulating
the miR-99b/HS3ST3B1 axis. Front Genet. 10:2802019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu L, Chen Y, Li Q and Duan P: lncRNA
HNF1A-AS1 modulates non-small cell lung cancer progression by
targeting miR-149-5p/Cdk6. J Cell Biochem. 120:18736–18750. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li K, Wang X, Fan C, Wu C, Li S and Liu H:
Tanshinone IIA promotes cardiac differentiation and improves cell
motility by modulating the Wnt/β-catenin signaling pathway. Mol Med
Rep. 22:1839–1846. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang D, Sun Y, Li W, Ye F, Zhang Y, Guo Y,
Zhang DY and Suo J: Antiproliferative effects of the CDK6 inhibitor
PD0332991 and its effect on signaling networks in gastric cancer
cells. Int J Mol Med. 41:2473–2484. 2018.PubMed/NCBI
|
|
37
|
Li X, Li Z, Liu Z, Xiao J, Yu S and Song
Y: Long non-coding RNA DLEU1 predicts poor prognosis of gastric
cancer and contributes to cell proliferation by epigenetically
suppressing KLF2. Cancer Gene Ther. 25:58–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng S, Qian Z, Jiang F, Ge D, Tang J,
Chen H, Yang J, Yao Y, Yan J, Zhao L, et al: CircRNA LRP6 promotes
the development of osteosarcoma via negatively regulating KLF2 and
APC levels. Am J Transl Res. 11:4126–4138. 2019.PubMed/NCBI
|
|
39
|
Hu CC, Liang YW, Hu JL, Liu LF, Liang JW
and Wang R: LncRNA RUSC1-AS1 promotes the proliferation of breast
cancer cells by epigenetic silence of KLF2 and CDKN1A. Eur Rev Med
Pharmacol Sci. 23:6602–6611. 2019.PubMed/NCBI
|
|
40
|
Chen B, Ji F, Wen X and Jin Z: Circular
RNA circ_ASAP2 promotes cell viability, migration, and invasion of
gastric cancer cells by regulating the miR-770-5p/CDK6 axis. Int J
Clin Exp Pathol. 13:2806–2819. 2020.PubMed/NCBI
|