Introduction
Gastric cancer (GC) is the fourth most frequent
malignant tumor and the second leading cause of cancer-related
mortality globally (1). Over
950,000 patients are diagnosed with GC annually and ~750,000 cases
succumb to this malignancy (2).
Helicobacter pylori (H. pylori) infection is a major
risk factor for GC (3). Following
H. pylori infection, chronic inflammation is induced in the
stomach, which is accompanied by abnormal cell proliferation,
apoptosis and certain genetic or epigenetic changes, eventually
leading to carcinogenesis (4).
Following diagnosis, the preferred treatment for patients with GC
is surgery. However, the majority of these patients relapse, and
other treatment strategies, including endoscopic therapy,
radiotherapy and systemic therapy, may be beneficial for certain
patients (5). Unfortunately, the
5-year overall survival of GC is poor and the mortality rate is
very high (1). The development of
novel drugs may improve the efficacy of therapeutic options for
patients with GC.
Traditional Chinese medicine (TCM) is widely used in
China for the treatment of various illnesses, such as cancer and
depression (6–8). Musk is an essential material used in
the perfume industry (9). Musk
ketones are the major components of musk and are included in the
TCM concoctions. Recently, musk ketone has attracted the attention
of researchers from different scientific fields (10,11).
Increasing evidence has demonstrated that musk ketone may be
helpful for the prevention of certain diseases. For example, musk
ketone was found to significantly repress the growth and induce the
apoptosis of lung cancer cells, whereas the expression levels of
IL-24 and DNA damage-inducible transcript 3 protein were
upregulated following musk ketone treatment (11). In addition, musk ketone was shown to
induce the growth and differentiation of neural stem cells in
cerebral ischemia by activating the PI3K/AKT signaling pathway
(10). However, the effects and
underlying mechanisms of musk ketone in GC remain unclear.
The present study aimed to explore the antitumor
effects of musk ketone in GC. The IC50 value of musk
ketones was assessed in AGS and HGC-27 cells. Based on the results,
a specific concentration was used for GC cell treatment. Cell
proliferation and colony growth were examined following musk ketone
treatment, as were cell cycle progression and apoptosis. It was
also investigated whether this compound induced dysregulation of
numerous genes at the molecular level, including sorbin and SH3
domain containing 2 (SORBS2). The present study sought to determine
whether musk ketone can suppress GC cell growth by regulating
SORBS2 expression.
Materials and methods
Cell lines and cell culture
The human GC cells AGS and HGC-27 were purchased
from the American Type Culture Collection. AGS and HGC-27 cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.), which was supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin and streptomycin solution (Gibco; Thermo Fisher
Scientific, Inc.). All the cells were maintained at 37°C with 5%
CO2.
Measurement of the IC50 of
musk ketone in AGS and HGC-27 cells
Musk ketone (purity ≥98%) was purchased from
Sigma-Aldrich (Merck KGaA). The IC50 of musk ketone was
detected in AGS and HGC-27 cells. Briefly, a total of 2,000 AGS and
HGC-27 cells were seeded in triplicate in 96-well plates. Different
dosages (0, 0.0031, 0.031, 0.31, 3.1, 31 µM) of musk ketone were
added into each well. After 48 h, the culture medium was removed
and each well was added with 100 µl culture medium and 10 µl Cell
Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology). After
incubation for 3 h, cell viability was analyzed by measuring the
OD450.
SORBS2 interference
Small interfering (si)RNAs against siCtrl
(5′-UUCUCCGAACGUGUCACGU-3′), siSORBS2-1
(5′-AUACCCCACAGCUAUUCUAGU-3′) and siSORBS2-2
(5′-GGGCAUCUUCCCGAUCUCAUA-3′) were synthesized from Huzhou Hippo
Biotechnology Co., Ltd. A total of 3×105 cells were
seeded in 6-well plates and transfected with siRNAs (50 nM/well)
using RNAiMAX (4 µl/well; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols. After 48 h, knockdown
efficacy was determined and the cells were subjected to cell
function experiments.
SORBS2 overexpression
pCDNA3.1 plasmids (Tianyi Huiyuan Biotech Co., Ltd.)
were used to overexpress SORBS2 in GC cells. AGS and HGC27 cells
were seeded in 6-well plates at a density of 5×105
cells/well. Cells were transfected with pCDNA3.1-empty and
pCDNA3.1-SORBS2 plasmids (5 µg/well) using VigoFect for 6 h at room
temperature (2 µg/well; Vigorous Biotechnology Beijing Co., Ltd.).
After 48 h, cells were subjected to immunoblotting and apoptosis
analysis.
Cell viability analysis
To determine the anticancer effects of musk ketone
in GC cells, equal numbers of AGS and HGC-27 cells were seeded in
96-well plates, which contained 100 µl DMEM. Musk ketone was added
and the cells were maintained for 1–3 and 4 days. Finally, 10 µl
CCK-8 solution was added to each well and the wells were incubated
at 37°C for 3 h. Cell viability was analyzed by measuring the
OD450.
Colony formation assay
A total of 1,000 AGS and HGC-27 cells were seeded in
triplicate in 6-well plates to observe colony formation. The cells
were incubated with vehicle or musk ketone. Following 7 days of
culture, the colonies were washed with PBS three times.
Subsequently, the colonies were fixed with 100% methanol at room
temperature for 15 min and stained with 0.2% crystal violet
solution at room temperature for 30 min. Images were captured using
a camera (Nikon Corporation). Cell colonies (>50 cells) were
counted manually.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA extracted from indicated cells were subjected to
reverse transcription and cDNA quantification, according to the
methods as described previously (12). qPCR was performed using the SYBR
Premix Ex Taq™ II kit (Takara Biotechnology Co., Ltd.). The
following thermocycling conditions were used for qPCR: 94°C for 5
min, 40 cycles of 94°C for 5 sec and 60°C for 1 min. Relative
expression levels were calculated using the 2−ΔΔCq
method (13). The primer sequences
were as follows: SORBS2 forward, 5′-AAAGACCCATGAGTTCTGCAAG-3′ and
reverse, 5′-GCTCGCACTTTGATCTCCCA-3; β-actin forward,
5′-GAGCTGCGTGTGGCTCCC-3′ and reverse, 5′-CCAGAGGCGTACAGGGATAGCA-3′;
and GAPDH forward, 5′-GACTCATGACCACAGTCCATGC-3′ and reverse,
5′-AGAGGCAGGGATGATGTTCTG-3′.
Western blotting
Total proteins were extracted from GC cells treated
with vehicle or musk ketone using lysis buffer (Beyotime Institute
of Biotechnology). The concentration of total proteins was detected
by the BCA Protein Assay Kit. A total of 30 µg protein per lane
were separated on 12% SDS-PAGE, and were subsequently transferred
onto PVDF membranes. Following blocking with 5% skimmed milk at
room temperature for 2 h, the membranes were incubated with the
indicated primary antibodies at 4°C overnight, followed by
incubation with HRP-conjugated secondary antibodies at room
temperature for 2 h. Protein expression was detected by
SuperSignal™ West Pico PLUS Chemiluminescent Substrate (Thermo
Fisher Scientific, Inc.) on a Tanon 4600 system. Image-Pro Plus
software v6.0 (Media Cybernetics, Inc.) was used to analyze the
protein signals. The antibody against SORBS2 (1:1,000; cat. no.
24643-1-AP) was obtained from ProteinTech Group, Inc. Antibodies
against cleaved (Cle)-caspase 3 (1:500; cat. no. ab32042) and
caspase 3 (1:500; cat. no. ab184787) were from Abcam. The GAPDH
primary antibody (1:4,000; cat. no. sc-47724) and horseradish
peroxidase-conjugated secondary antibodies (both 1:10,000; normal
mouse IgG, cat. no. sc-2748; normal rat IgG, cat. no. sc-2750) were
purchased from Santa Cruz Biotechnology, Inc.
Cell cycle analysis
The cell cycle was examined using propidium iodide
(PI) staining (Cell Cycle and Apoptosis Analysis kit; Shanghai
Yeasen Biotech Co., Ltd.). Briefly, a total of 2×106 GC
cells were seeded in 6-well plates and treated with vehicle or musk
ketone. Following 48 h of incubation, the cells were fixed with 70%
alcohol overnight on ice. Subsequently, the cells were stained with
staining buffer at 37°C for 30 min and the cell cycle was analyzed
by flow cytometry (cytoFlex, Beckman Coulter, Inc.). The data were
analyzed by Cytexpert (version 2.4.0.28; Beckman Coulter,
Inc.).
Apoptosis assay
The induction of apoptosis was detected using
PI/Annexin V staining (Annexin V-FITC/PI Apoptosis Detection kit,
Shanghai Yeasen Biotech Co., Ltd.), according to the manufacturer's
protocols. The cells were washed with PBS and stained with PI and
Annexin V in room temperature for 15 min in the dark. The level of
apoptosis was analyzed by flow cytometry (cytoFlex, Beckman
Coulter, Inc.). The data were analyzed by Cytexpert (Version
2.4.0.28; Beckman Coulter, Inc.). The
FITC+/PI− cells represent early apoptosis,
while FITC+/PI+ cells represent late
apoptosis.
Transwell assay
A total of 8×104 HGC27 cells in 200 µl
FBS-free DMEM were seeded in upper surface of 8.0-µm filter
migration chambers (Corning, Inc.). A total of 500 µl complete DMEM
with 10% FBS (both Gibco; Thermo Fisher Scientific, Inc.) was added
in lower compartment of 24-well plates. The plates were maintained
in the cell incubator. After 24 h, the cells attached on the upper
surface were removed and the cells attached on the lower surface
were fixed with 100% methanol and were stained with 0.2% crystal
violet solution at room temperature for 30 min.
Microarray analysis
Total RNA was extracted from AGS cells treated with
vehicle or musk ketone using TRIzol® reagent (Thermo
Fisher Scientific, Inc.), according to the manufacturer's
protocols. Microarray analysis was performed by Shanghai OE Biotech
Co., Ltd. The dysregulated genes were identified based on
statistical significance at P<0.05 and fold-change >1.5.
Statistical analysis
All the quantification results and statistical
significance were analyzed using GraphPad Prism software 6.0
(GraphPad Software, Inc.). The enrichment analysis of signaling
pathways were assessed by Gene Set Enrichment Analysis (GSEA;
version 4.0.3) (14). The results
are shown as the mean ± standard error of the mean for three
independent experiments. Unpaired Student's t-test was applied to
analyze the differences between the two groups. One-way ANOVA
followed by a Tukey's post hoc test was applied to analyze the
differences among groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Musk ketone significantly represses
the proliferation of GC cells
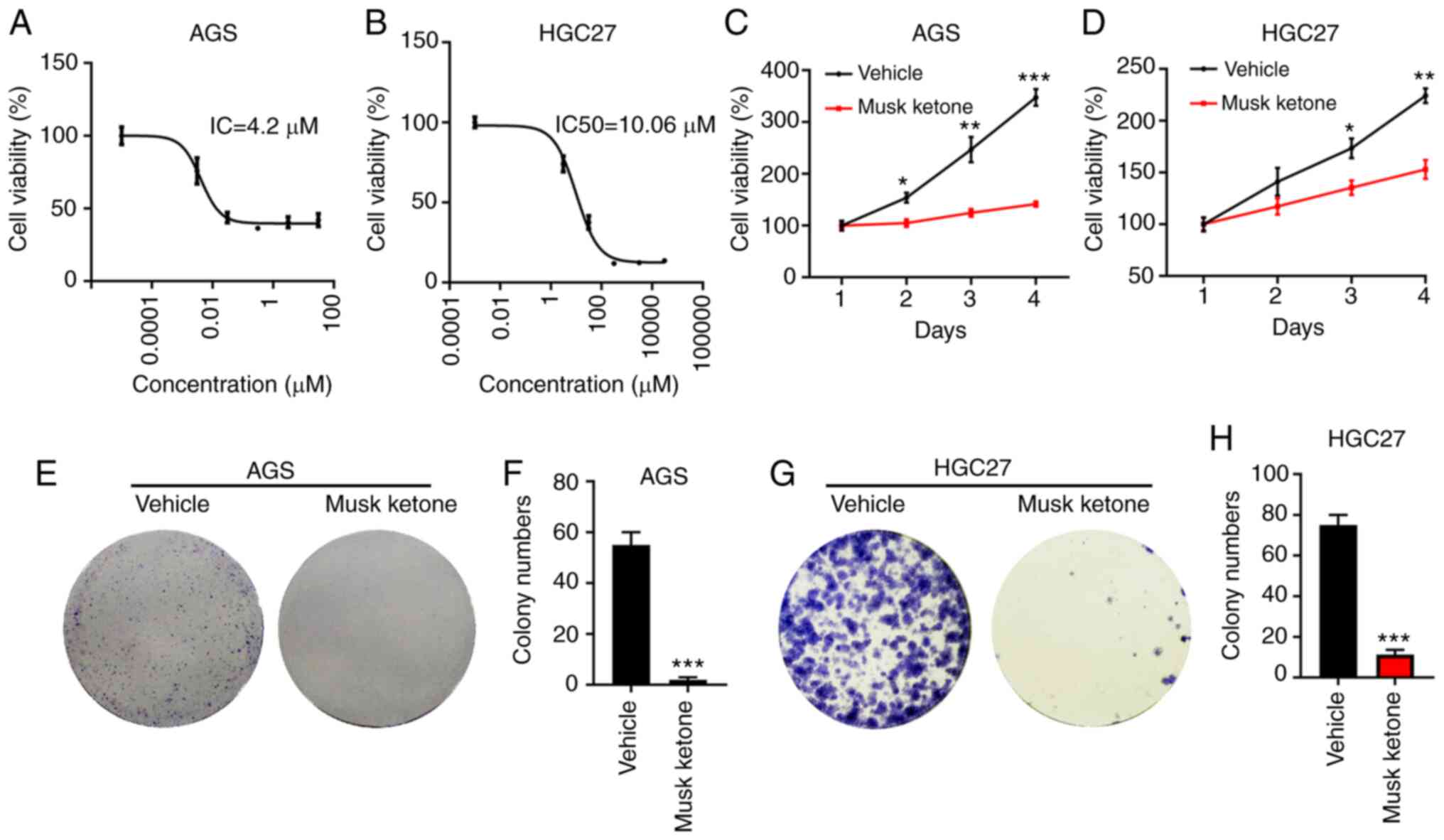
To explore the inhibitory effects of musk ketone on
GC cells, the IC50 of this compound was evaluated in AGS
and HGC-27 cells. These cell lines were seeded in 96-well plates
and were treated with different concentrations of musk ketone. Cell
viability was detected CCK-8 48 h following musk ketone incubation.
The results indicated that the IC50 of musk ketone was
4.2 µM in AGS cells and 10.06 µM in HGC-27 cells (Fig. 1A and B). The results indicated that
the IC50 values of musk ketone in AGS and HGC-27 cells
were 4.2 and 10.06 µM, respectively. Subsequently, the
time-dependent inhibitory effects of musk ketone on GC cells were
examined. Briefly, equal numbers of AGS and HGC-27 cells were
seeded in 96-well plates and the cells were incubated with vehicle
or musk ketone. Cell proliferation was assessed by CCK-8 on days
1–3 and 4. The data indicated that musk ketone inhibited the
proliferation of AGS and HGC-27 cells on day 1 following treatment.
This inhibitory effect was more prominent when the cells were
treated for longer time periods (Fig.
1C and D). To confirm this hypothesis, the colony formation
assay was performed in AGS and HGC-27 cells treated with or without
musk ketone. The data indicated that AGS and HGC-27 cells incubated
with vehicle formed a significantly higher number of colonies
compared with those incubated with musk ketone (Fig. 1E-H). These results suggested that
musk ketone exerted a suppressive effect on GC cells.
Musk ketone promotes cell cycle arrest
and apoptosis
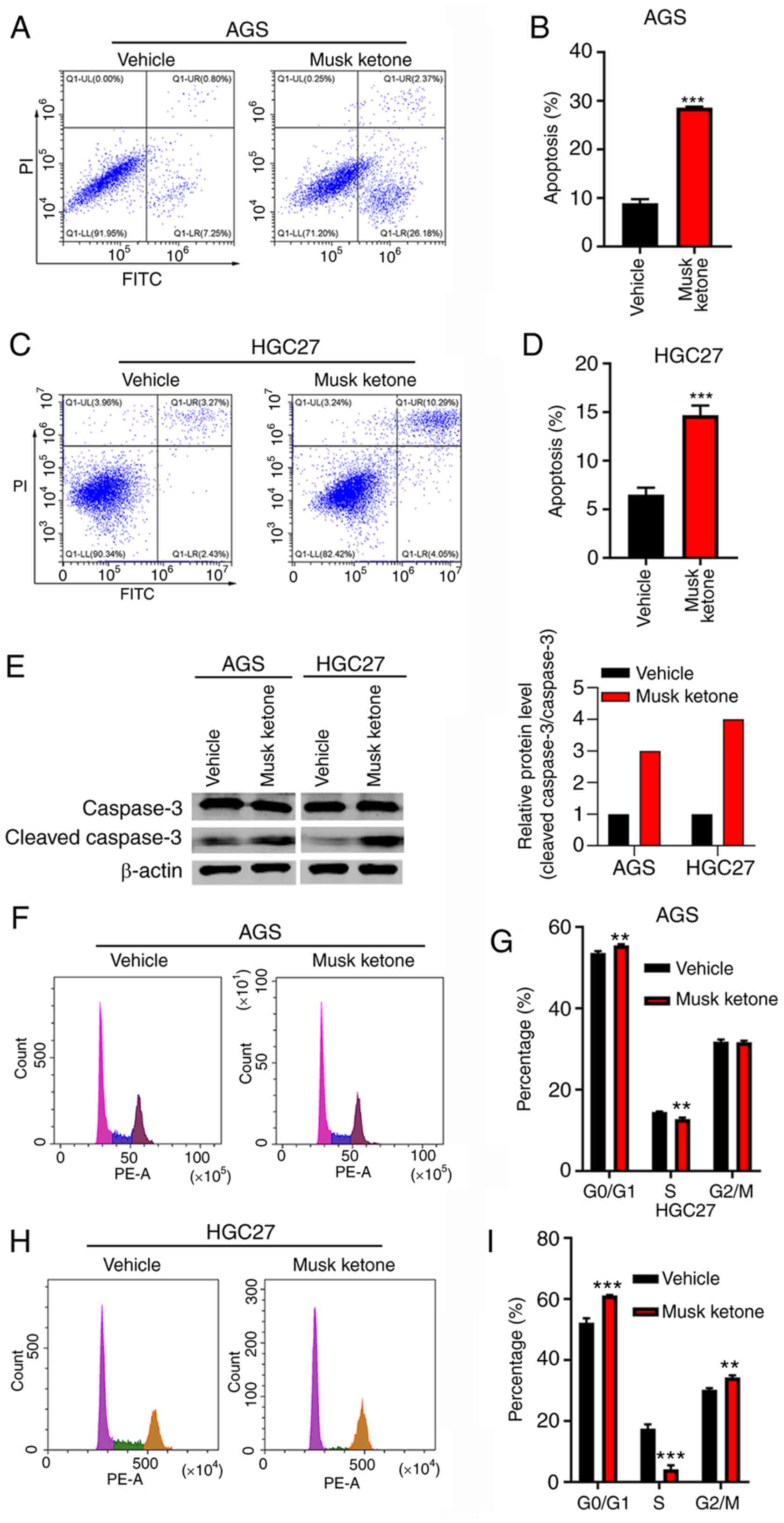
Cell cycle and apoptosis deregulation are hallmarks
of cancer. It was herein examined whether musk ketone regulated
cell cycle arrest and apoptosis. AGS and HGC-27 cells were treated
with vehicle and musk ketone for 48 h. The cells were harvested for
cell cycle and apoptosis analyses. It was demonstrated that musk
ketone treatment enhanced apoptosis of AGS and HGC-27 cells
(Fig. 2A-D). Moreover, musk ketone
treatment resulted in increased ratio of Cle-caspase 3 to caspase 3
in both cells (Fig. 2E).
Furthermore, musk ketone treatment increased the percentage of
cells at the G0/G1 phase and decreased the
percentage of cells at the S phase in both cell lines (Fig. 2F-I). These results indicated that
musk ketone treatment led to cell cycle arrest and increased
apoptosis of GC cells.
Gene expression profiling following
musk ketone treatment
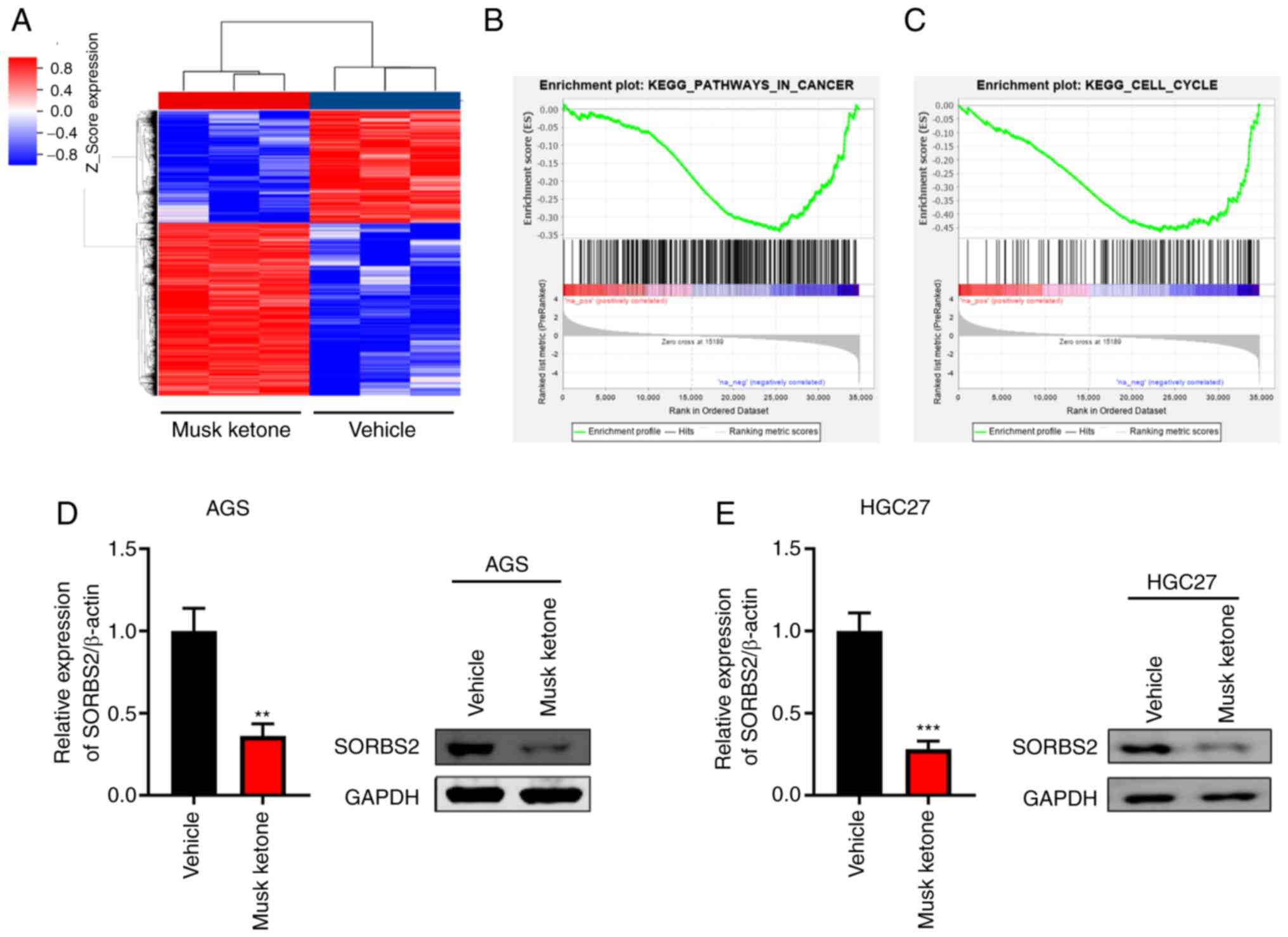
To profile the downstream effectors of musk ketone,
AGS cells were treated with vehicle or musk ketone and subjected to
microarray analysis. Thousands of genes were regulated by musk
ketone, including 2,657 upregulated and 1,728 downregulated genes
(Fig. 3A and Table SI). GSEA indicated that ‘Pathways
In Cancer’ and ‘Cell Cycle’ were negatively regulated by musk
ketone (Fig. 3B and C). In
addition, microarray analysis indicated downregulation of SORBS2
(Fig. 3A). Furthermore, western
blotting and RT-qPCR analyses confirmed that musk ketone repressed
the expression of SORBS2 (Fig. 3D and
E).
Knockdown of SORBS2 suppresses the
proliferation and growth of GC cells
To investigate the role of SORBS2 downregulation in
GC, the SORBS2 gene was knocked down in HGC-27 cells. Western
blotting and RT-qPCR analyses indicated that SORBS2 was efficiently
silenced by siRNA treatment (Fig. 4A
and B). The cells were subsequently analyzed by CCK-8 and
colony formation assays. The data indicated that SORBS2 knockdown
significantly suppressed the proliferation and colony formation of
HGC-27 cells (Fig. 4C-E).
Furthermore, apoptosis was induced by SORBS2 knockdown (Fig. 4F and G). By contrast, SORBS2
overexpression, which was verified via western blotting (Fig. S1A), suppressed the apoptosis of GC
cells (Fig. S1B and C). Transwell
results indicated that SORBS2 knockdown suppressed the migration of
HGC-27 cells (Fig. 4H and I). These
results suggested that SORBS2 functioned as a potential oncogene in
GC.
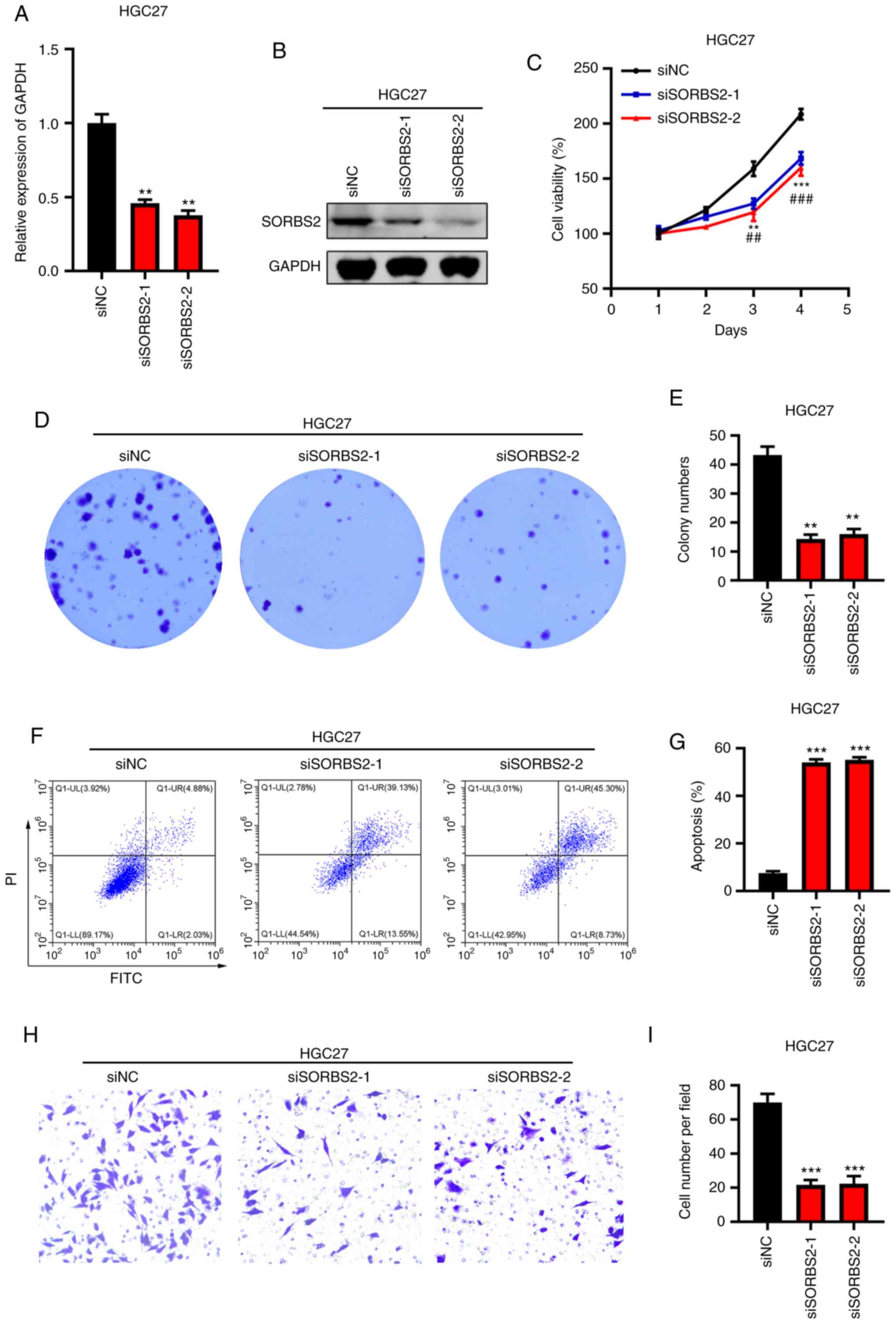 | Figure 4.Knockdown of SORBS2 inhibits the
proliferation of gastric cancer cells. (A) Reverse
transcription-quantitative PCR analysis of SORBS2 in siCtrl-,
siSORBS2-1- and siSORBS2-2-transfected HGC-27 cells. **P<0.01
vs. siNC group. (B) Immunoblotting analysis of SORBS2 in siCtrl-,
siSORBS2-1- and siSORBS2-2-transfected HGC-27 cells. (C) Cell
proliferation was detected by the Cell Counting Kit-8 assay in
siCtrl-, siSORBS2-1- and siSORBS2-2-transfected HGC-27 cells.
**P<0.01, ***P<0.001 siSORBS2-1 vs. siCtrl;
##P<0.01, ###P<0.001 siSORBS2-2 vs. si
Ctrl. (D and E) Colony formation was analyzed in siCtrl-,
siSORBS2-1- and siSORBS2-2-transfected HGC-27 cells. Left, images
of colonies. Right, quantification results. (F and G) Apoptosis was
detected in siCtrl-, siSORBS2-1- and siSORBS2-2-transfected HGC-27
cells. Left, images of apoptosis. Right, quantification results. (H
and I) The migratory activity of siCtrl-, siSORBS2-1- and
siSORBS2-2-transfected HGC-27 cells was assessed by Transwell
assay. Left, images of migration. Right, quantification results.
**P<0.01, ***P<0.001 vs. siNC group. SORBS2, sorbin and SH3
domain containing 2; si, small interfering RNA; NC, negative
control; Ctrl, control. |
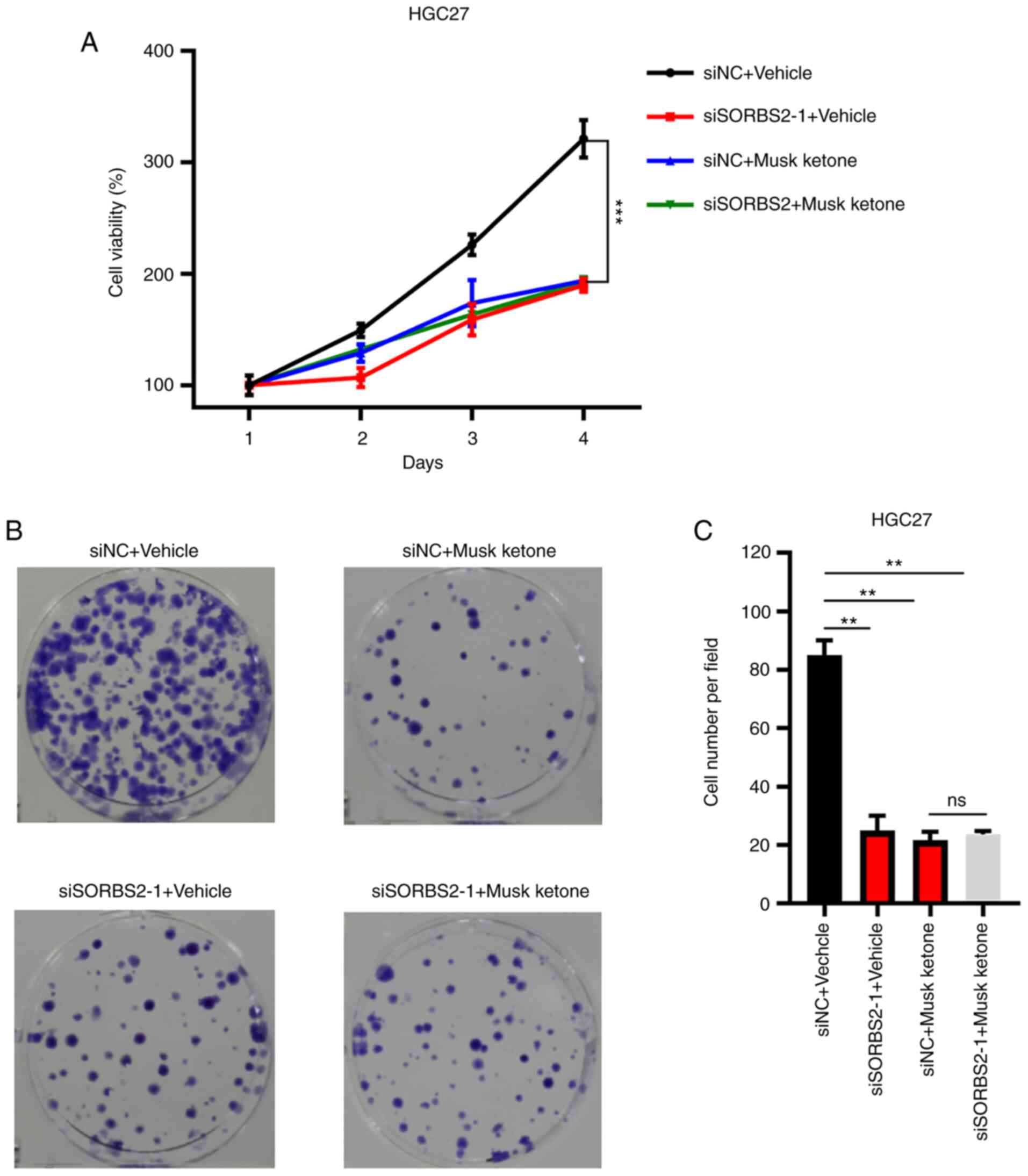
Knockdown of SORBS2 reduces the
anticancer effects of musk ketone
To validate whether the suppression of GC cell
proliferation was dependent on the expression of SORBS2, siNC and
siSORBS2-transfected HGC-27 cells were treated with vehicle or musk
ketone. It was observed that musk ketone significantly suppressed
the proliferation and colony formation of siNC HGC-27 cells,
whereas it exerted no obvious effects on siSORBS2 HGC-27 cells
(Fig. 5A-C). These results
suggested that the anticancer effects of musk ketone may be
mediated via regulating SORBS2 expression.
Discussion
TCM has long been used in China to treat several
diseases, including depression, gastric precancerous lesions and
postoperative abdominal adhesions (7,15,16).
The most well-known TCM drug is artemisinin (qinghaosu), which has
potent therapeutic effects against malaria (17–19).
Recently, increasing evidence has demonstrated that TCM is a
promising approach in the treatment of malignancies. In the present
study, musk ketone, which is a TCM compound, markedly suppressed
the proliferation of GC cells. Musk ketone treatment resulted in
cell cycle arrest and apoptosis in AGS and HCG-27 cells. Transcript
analysis of musk ketone-treated GC cells indicated that numerous
genes were dysregulated following musk ketone treatment.
The initial evidence indicating that musk ketone may
be used for cancer treatment dates back to the 1990s (20). Musk is the major ingredient of this
compound. Two years later, Zheng et al (20) demonstrated that the musk residue,
which contained musk ketone, could be used as a chemopreventive
agent. A toxicity study based on an in vivo mouse lymphoma
model and on in vitro unscheduled DNA synthesis and
cytogenetics assays revealed that musk ketone did not possess
genotoxic potential (21).
Recently, Xu and Cao (11)
demonstrated that musk and musk ketone exerted suppressive effects
on the proliferation and growth of lung cancer cells. However, the
role of musk ketone in GC remains poorly understood. Therefore, the
present study attempted to determine the IC50 of musk
ketone in GC cells. The IC50 values were estimated to be
4.2 and 10.06 µM in AGS and HGC-27 cells, respectively. One dose of
musk ketone could significantly repress the proliferation and
colony formation of both cell types. Furthermore, musk ketone
treatment resulted in cell cycle arrest and enhanced apoptosis in
AGS and HGC-27 cells. These results suggested that musk ketone
exerted potent anticancer effects on GC.
SORBS2, also referred to as ArgBP2, is located on
chromosome 4. Physiologically, SORBS2 regulates actin dynamics,
cytoskeleton establishment and signal transduction (22,23).
Dysregulation of SORBS2 participates in cancer development. For
example, the RNA-binding protein SORBS2 functions as a tumor
suppressor in hepatocellular carcinoma (HCC) by regulating nuclear
receptor ROR-a mRNA transcription (24). Furthermore, SORBS2 suppresses the
metastasis of HCC by inhibiting the ERK signaling pathway (25). In addition, SORBS2 suppresses
ovarian cancer metastasis by modulating tumor-suppressive
immunomodulatory transcripts (26).
However, SORBS2 can promote cell growth and inhibit cell apoptosis
in human renal glomerular endothelial cells and human glomerular
mesangial cells (27). These
studies suggest that SORBS2 may play a distinct role on cell
proliferation and apoptosis in a context-dependent manner. In GC,
SORBS2 is downregulated by heat shock factor protein 1, which
promotes the proliferation and invasion of GC cells (28). However, the association between musk
ketone and SORBS2, as well as the precise function of SORBS2 in GC,
are largely unknown. Based on the microarray data, the present
study indicated that musk ketone significantly reduced the
expression levels of SORBS2 in GC cells. Since SORBS2 is important
for actin dynamics, maintaining the cell cytoskeleton and signal
transduction (22,23), it was predicted that musk ketone
suppresses the growth of GC cell at least partly through regulating
the expression of SORBS2. Based on loss-of-function and
gain-of-function experiments, the present study demonstrated that
SORBS2 expression was essential to maintain the proliferation of GC
cells. Of note, SORBS2 silencing decreased the sensitivity of GC
cells to musk ketone treatment, indicating that SORBS2 may act as
an oncogene in GC, and that the expression levels of SORBS2 in GC
cells may have a role in the efficacy of musk ketone treatment.
There were certain limitations in the present study.
The effect of musk ketone on the tumor growth of GC cells in nude
mice was not investigated and the molecular mechanisms by which
musk ketone regulates SORBS2 are still unknown. Further studies are
required to address these points.
In summary, the present study provided initial
evidence that musk ketone is a promising TCM compound for GC
treatment. The IC50 of musk ketone was determined in
different GC cells. Musk ketone significantly suppressed the
proliferation, colony formation and cell cycle progression of GC
cells and enhanced apoptosis. At the molecular level, musk ketone
downregulated SORBS2 expression in GC cells. Finally, silencing of
SORBS2 reduced GC cell proliferation and the sensitivity of GC
cells to musk ketone treatment.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Applied Basic Research of Qinghai (grant no. 2018-ZJ-744), the CAS
(Light of the West China) Program (grant no. 2019-33), the National
Natural Science Foundation of China (grant no. 81460429), the Open
Project of State Key Laboratory of Plateau Ecology and Agriculture,
Qinghai University (grant no. 2019-ZZ-07) and the Chunhui Plan of
Ministry of Education of China (grant no. Z2017037).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JA and HYW designed the study. JA, HYW, XMM, BWH,
YFY, YPY and ZHS performed the experiments and analyzed the data.
JA and HYW wrote the manuscript draft. ZHS revised the manuscript.
All authors have read and approved the final version of the
manuscript. JA and HYW confirmed the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sexton R, Al Hallak M, Diab M and Azmi A:
Gastric cancer: A comprehensive review of current and future
treatment strategies. Cancer Metastasis Rev. 39:1179–1203. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schulz C, Schütte K, Mayerle J and
Malfertheiner P: The role of the gastric bacterial microbiome in
gastric cancer: Helicobacter pylori and beyond. Therap Adv
Gastroenterol. 12:17562848198940622019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ajani JA, D'Amico TA, Almhanna K, Bentrem
DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, et
al: Gastric cancer, version 3.2016, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 14:1286–1312. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu T, Luo S, Libby P and Shi GP:
Cathepsin L-selective inhibitors: A potentially promising treatment
for COVID-19 patients. Pharmacol Ther. 213:1075872020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li C, Huang J, Cheng YC and Zhang YW:
Traditional Chinese medicine in depression treatment: From
molecules to systems. Front Pharmacol. 11:5862020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang J, Zhu X, Yuan P, Liu J, Wang B and
Wang G: Efficacy of traditional Chinese medicine combined with
chemotherapy in patients with non-small cell lung cancer (NSCLC): A
meta-analysis of randomized clinical trials. Support Care Cancer.
28:3571–3579. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y and Ha CY: Research progress on
musk and artificial propagation technique of forest musk deer.
Zhongguo Zhong Yao Za Zhi. 43:3806–3810. 2018.(In Chinese).
PubMed/NCBI
|
|
10
|
Zhou Z, Dun L, Wei B, Gan Y, Liao Z, Lin
X, Lu J, Liu G, Xu H, Lu C and An H: Musk ketone induces neural
stem cell proliferation and differentiation in cerebral ischemia
via activation of the PI3K/Akt signaling pathway. Neuroscience.
435:1–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu L and Cao Y: Native musk and synthetic
musk ketone strongly induced the growth repression and the
apoptosis of cancer cells. BMC Complement Altern Med. 16:5112016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Gao M, An J, Wang X, Jia Y, Xu J,
Zhu J, Cui J, Li W, Xing R, et al: Dysregulation of
MiR-30a-3p/gastrin enhances tumor growth and invasion through
STAT3/MMP11 pathway in gastric cancer. Onco Targets Ther.
13:8475–8493. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang L, Li J, Hu Z, Fan X, Cai T, Zhou H
and Pan H: A systematic review of the mechanisms underlying
treatment of gastric precancerous lesions by traditional Chinese
medicine. Evid Based Complement Alternat Med.
2020:91547382020.PubMed/NCBI
|
|
16
|
Wu F, Liu W, Feng H, Long L, Hou L and Hou
C: Application of traditional chinese medicines in postoperative
abdominal adhesion. Evid Based Complement Alternat Med.
2020:80734672020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tu Y: The discovery of artemisinin
(qinghaosu) and gifts from Chinese medicine. Nat Med. 17:1217–1220.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Xu C, Wong YK, Liao FL, Jiang T
and Tu Y: Malaria eradication. Lancet. 395:e692020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tu T: Artemisinin-A gift from traditional
Chinese medicine to the world (nobel lecture). Angew Chem Int Ed
Engl. 55:10210–10226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng GQ, Kenney PM and Lam LK: Isolation
and biological evaluation of potential cancer chemopreventive
agents from ambrette musk residue. J Pharm Sci. 81:950–953. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Api AM, Pfitzer EA and San RH: An
evaluation of genotoxicity tests with Musk ketone. Food Chem
Toxicol. 34:633–638. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sanger JM, Wang J, Gleason LM, Chowrashi
P, Dube DK, Mittal B, Zhukareva V and Sanger JW: Arg/Abl-binding
protein, a Z-body and Z-band protein, binds sarcomeric, costameric,
and signaling molecules. Cytoskeleton (Hoboken). 67:808–823. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kioka N, Ueda K and Amachi T: Vinexin,
CAP/ponsin, ArgBP2: A novel adaptor protein family regulating
cytoskeletal organization and signal transduction. Cell Struct
Funct. 27:1–7. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han L, Huang C and Zhang S: The
RNA-binding protein SORBS2 suppresses hepatocellular carcinoma
tumourigenesis and metastasis by stabilizing RORA mRNA. Liver Int.
39:2190–2203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan B, Peng Z and Xing C: SORBS2, mediated
by MEF2D, suppresses the metastasis of human hepatocellular
carcinoma by inhibitiing the c-Abl-ERK signaling pathway. Am J
Cancer Res. 9:2706–2718. 2019.PubMed/NCBI
|
|
26
|
Zhao L, Wang W, Huang S, Yang Z, Xu L,
Yang Q, Zhou X, Wang J, Shen Q, Wang C, et al: The RNA binding
protein SORBS2 suppresses metastatic colonization of ovarian cancer
by stabilizing tumor-suppressive immunomodulatory transcripts.
Genome Biol. 19:352018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jie R, Zhu P, Zhong J, Zhang Y and Wu H:
LncRNA KCNQ1OT1 affects cell proliferation, apoptosis and fibrosis
through regulating miR-18b-5p/SORBS2 axis and NF-ĸB pathway in
diabetic nephropathy. Diabetol Metab Syndr. 12:772020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tong Y, Li Y, Gu H, Wang C, Liu F, Shao Y
and Li F: HSF1, in association with MORC2, downregulates ArgBP2 via
the PRC2 family in gastric cancer cells. Biochim Biophys Acta Mol
Basis Dis. 1864:1104–1114. 2018. View Article : Google Scholar : PubMed/NCBI
|