Introduction
Lung cancer is the most common malignant tumor
worldwide. With increased industrialization, air quality around the
world has been reduced to varying degrees (1). The incidence of lung cancer remains
high, with a 5-year survival rate of 16–18% worldwide (2). Lung cancer can be divided into small
cell lung cancer and non-small cell lung cancer (NSCLC), which
accounts for 85% of all lung cancer cases, according to
pathological morphology and the degree of differentiation (3). Therefore, investigating the
pathogenesis of NSCLC and identifying novel therapeutic drugs and
targets for the disease is important.
Germacrone (GM), a natural product isolated from the
traditional Chinese medicine zedoary, displays a wide range of
pharmacological functions, including antitumor (4), antioxidation (5) and anti-inflammation activities
(6). In addition, it has been
reported that GM displays antitumor activity in vitro
(7). GM induces prostate cancer
cell apoptosis and autophagy by inhibiting the Akt/mTOR signaling
pathway (8). GM also serves an
anticancer role by inducing G2/M phase cell cycle arrest
and promoting apoptosis in gastric cancer cells (4). Furthermore, GM inhibits breast cancer
cell line proliferation by inducing cell cycle arrest at the
G0/G1 and G2/M phases, and
promoting cell apoptosis (9). An
et al (10) demonstrated
that GM displays a significant protective effect against
lipopolysaccharide-induced acute lung injury in neonatal rats.
However, to the best of our knowledge, the effect of GM on lung
cancer has not yet been reported.
A previous study revealed that zedoary inhibits
tumor cell proliferation, invasion and migration by inhibiting the
Akt signaling pathway, suggesting a potential antitumor mechanism
(11). The Akt/MDM2 proto-oncogene
(MDM2)/p53 signaling pathway serves an important role in the
regulation of apoptosis and proliferation (12,13).
The Akt signaling pathway is a classic intracellular signaling
pathway that causes tumorigenesis via a range of mechanisms
(14,15). The malignancy of numerous different
types of cancer, including lung cancer, is associated with
increased abnormal activity of the Akt signaling pathway (16–18).
Akt phosphorylates the p53 suppressor MDM2 and promotes MDM2 to
translocate to the nucleus, thereby inhibiting the function of p53
(19).
Therefore, the present study investigated the role
of GM in lung cancer cells and its underlying molecular mechanism,
with the aim of providing a theoretical basis for the treatment of
lung cancer.
Materials and methods
Cell culture
The human bronchial epithelial cell line (BEAS-2B)
and lung cancer cell line (A549) were purchased from The Cell Bank
of Type Culture Collection of The Chinese Academy of Sciences.
Cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C with 5% CO2. Cells were cultured with different
concentrations (6, 12, 25, 50, 100, 200 and 400 µM) of GM for 48 h
at 37°C (purity >98%; Shanghai YuanYe Biotechnology Co., Ltd.).
Cells in the control group were treated with equal concentrations
of DMEM.
Cell Counting Kit-8 (CCK-8) assay
A549 cell viability was assessed by performing the
CCK-8 assay (Beyotime Institute of Biotechnology). Following
treatment, 10 µl CCK-8 solution was added to each well and
incubated for 4 h at 37°C. Absorbance was measured at a wavelength
of 450 nm using a microplate reader.
Colony formation assay
Cells were seeded (5×102 cells/well) into
6-well plates. Following incubation for 15 days at 37°C, visible
colonies were fixed with 4% (w/v) paraformaldehyde for 15 min at
room temperature and stained with crystal violet for 5 min at room
temperature. Stained colonies (≥50 cells) were visualized using a
light microscope (magnification, ×100, Olympus Corporation)
equipped with a ColorView II Soft Imaging System digital camera
(Olympus Corporation).
TUNEL assay
Cell apoptosis was assessed by performing the
Click-iT® TUNEL Alexa Fluor® Imaging assay
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells were fixed with
4% paraformaldehyde for 30 min at room temperature and then washed
with PBS. Subsequently, 0.3% Triton X-100 in PBS was added and
incubated for 5 min at room temperature. Cells were stained with
DAPI at room temperature for 10 min (Thermo Fisher Scientific,
Inc.). A total of 50 µl TUNEL reaction mixture was added for 1 h at
37°C. Cells were sealed with anti-fluorescence quenched sealing
solution and FITC-labeled TUNEL-positive cells were visualized in
three randomly selected fields of view using an IX70 confocal
microscope (Olympus Corporation) at ×20 magnification. To calculate
the proportion of apoptotic cells, the number of apoptotic cells
and the number of total cells were counted.
Western blotting
The effect of GM treatment on the expression levels
of apoptosis-related proteins was determined via western blotting.
Briefly, total protein was extracted from cells using RIPA buffer
(Nantong Chem-Base Co., Ltd.). Following centrifugation at 300 × g
at 4°C for 5 min, protein concentrations were determined using a
BCA kit (Nantong Chem-Base Co., Ltd.). Proteins (40 µg/lane) were
separated via 10% SDS-PAGE and transferred to PVDF membranes
(Bio-Rad Laboratories, Inc.). Following blocking with 5% skimmed
milk for 2 h at 37°C, the membranes were incubated overnight at 4°C
with the following primary antibodies (all Abcam): Anti-Bcl-2
(1:1,000; cat. no. ab32124), anti-Bax (1:1,000; cat. no. ab182733),
anti-cleaved (C)-caspase 3 (1:1,000; cat. no. ab32042),
anti-caspase 3 (1:1,000; cat. no. ab13847), anti-C-poly(ADP-ribose)
polymerase (PARP; 1:1,000; cat. no. ab32064), anti-PARP (1:1,000;
cat. no. ab191217), anti-Cyclin D1 (1:1,000; cat. no. ab16663),
anti-CDK4 (1:1,000; cat. no. ab108357), anti-CDK6 (1:1,000; cat.
no. ab124821), anti-p-Akt (1:1,000; cat. no. ab38449), anti-Akt
(1:1,000; cat. no. ab8805), anti-p-MDM2 (1:1,000; cat. no.
ab22710), anti-MDM2 (1:1,000; cat. no. ab16895), anti-p53 (1:1,000;
cat. no. ab26) and anti-GAPDH (1:1,000; cat. no. ab9485).
Subsequently, the membranes were incubated with the corresponding
secondary antibody (1:5,000; cat. no. ab150077; Abcam) for 2 h at
37°C. Subsequently, the membranes were incubated with goat
anti-rabbit horseradish peroxidase-conjugated IgG secondary
antibodies (1:5,000; cat. no. ab150077; Abcam). Protein bands were
visualized using the HRP-ECL system (Nantong Chem-Base Co., Ltd.).
ImageJ software (version 146; National Institutes of Health) was
used to analyze the fold change in protein levels.
Flow cytometry
Cell cycle was detected by EzCell™ Cell Cycle
Analysis kit (cat. no. K920-100; BioVision, Inc.). A549 cells
(5×102 cells/well) were synchronized in serum-free
medium for 24 h. Subsequently, cells were treated with 8 µg/ml SC79
(cat. no. ab146428; Abcam) or GM (50, 100 or 200 µM) at 37°C for 24
h. Cells in the control group were treated with equal
concentrations of DMEM. Following washing twice with PBS, cells
were trypsinized and collected by centrifugation at 300 × g at 4°C
for 5 min, followed by fixation with 70% ethanol at 4°C for 24 h.
After washing with PBS, cells were stained with PI in staining
solution supplemented with RNase A for 30 min at room temperature.
The cell cycle was assessed using the FACScan flow cytometer (BD
Biosciences) and analyzed using CellQuest software (version 7.6.1;
Flow Jo LLC.).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 13.0; SPPS, Inc.). Data are presented as the mean
± standard deviation. Comparisons among multiple groups were
analyzed using one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
GM inhibits lung cancer cell
proliferation
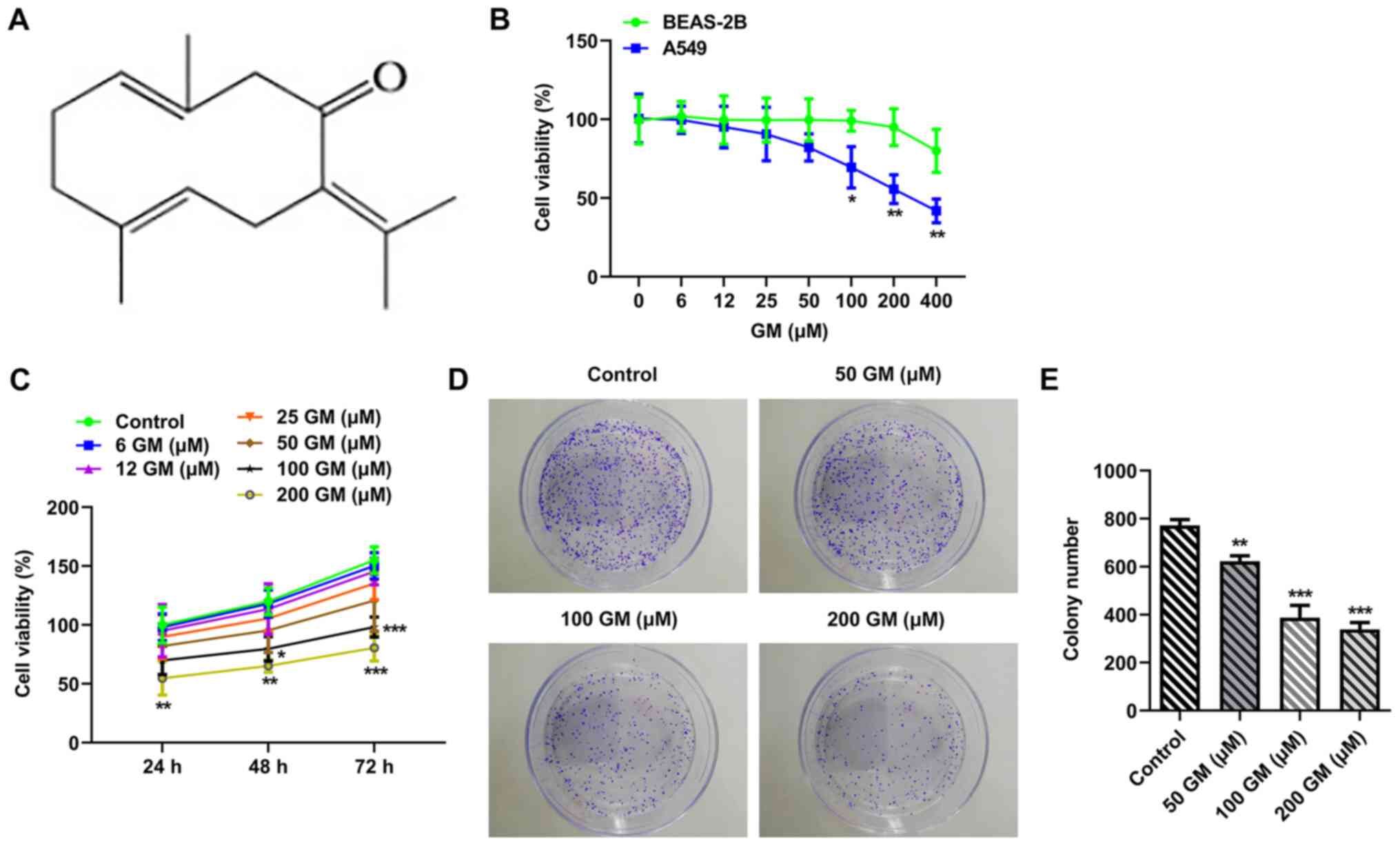
The chemical formula of GM is presented in Fig. 1A. The CCK-8 assay was performed to
evaluate the effect of different concentrations of GM (0, 6, 12,
25, 50, 100, 200 and 400 µM) on cell viability (20,21).
Cell viability was markedly decreased by GM treatment in a
concentration-dependent manner, with the effects of GM on A549 cell
viability being more obvious compared with BEAS-2B cell viability
(Fig. 1B). Subsequently, A549 cells
were treated with different concentrations of GM for 24, 48 or 72 h
(Fig. 1C). The results demonstrated
that A549 cell proliferation was significantly decreased by GM
treatment (200 µM) at 24 h compared with the control group.
Therefore, cells were treated with 50, 100 or 200 µM GM for 24 h
for subsequent experiments. The colony formation assay results
demonstrated that cell proliferation was significantly decreased by
GM treatment in concentration-dependent manner compared with the
control group (Fig. 1D and E).
GM induces lung cancer cell apoptosis
and G1/S cycle arrest
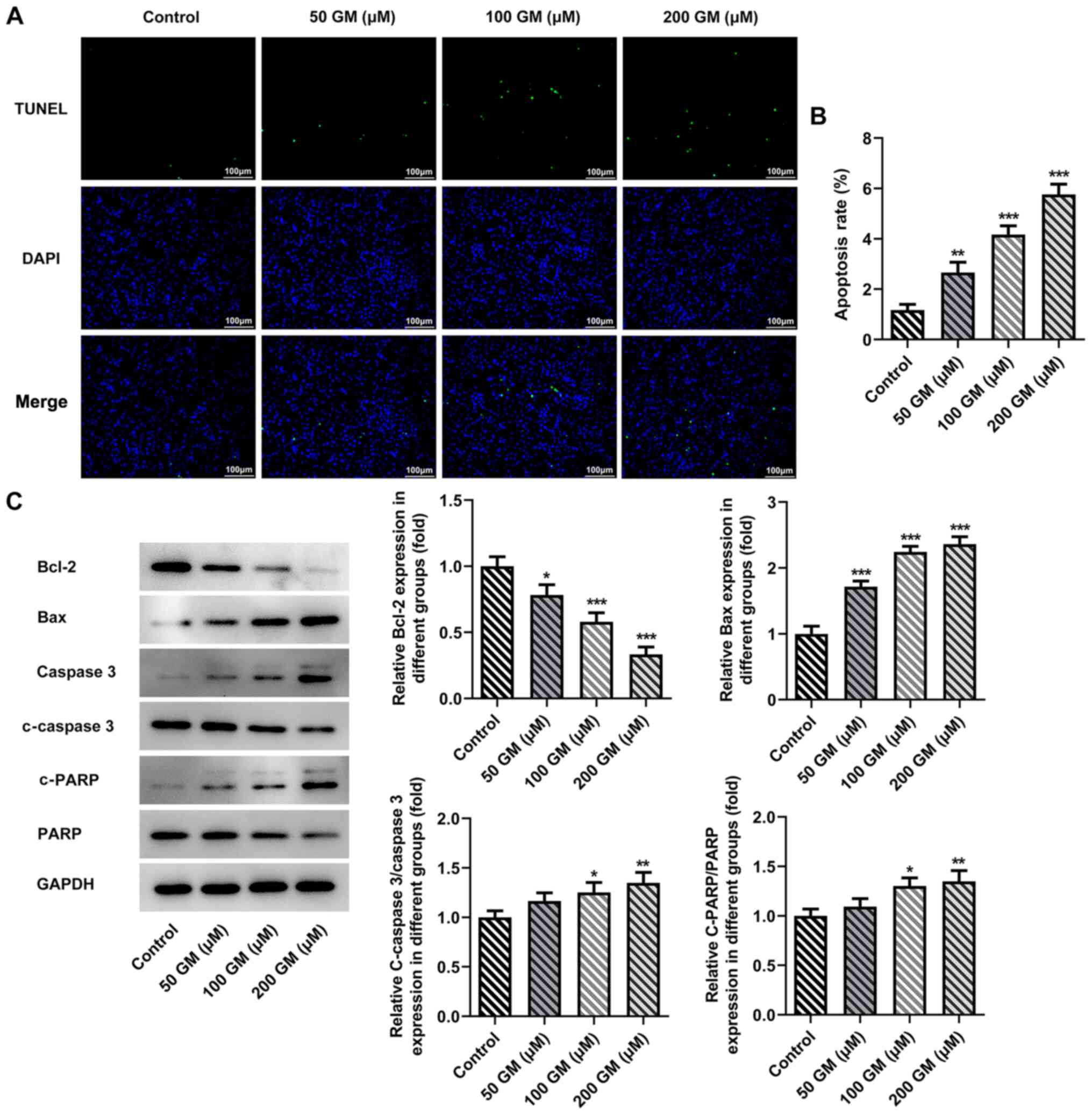
Cell apoptosis was detected by performing TUNEL
assays. The results demonstrated that cell apoptosis was
significantly increased by GM treatment in a
concentration-dependent manner compared with the control group
(Fig. 2A and B). Moreover,
GM-treated cells (100 and 200 µM) displayed significantly increased
expression levels of apoptosis-related proteins, including Bax,
c-caspase 3 and c-PARP, and significantly decreased expression
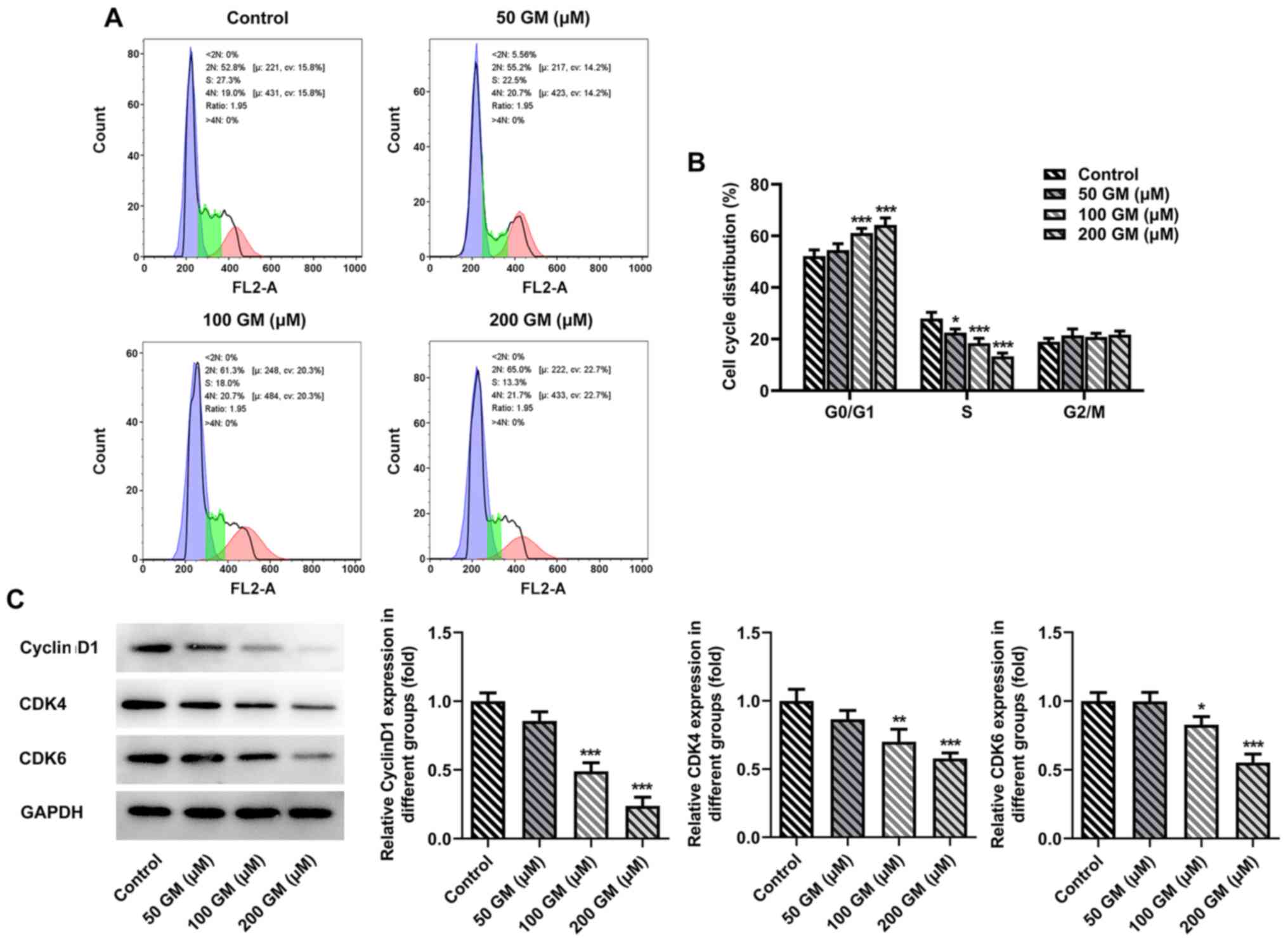
levels of Bcl-2 compared with the control group (Fig. 2C). The cell cycle was detected via
flow cytometry. The results indicated that the proportion of
GM-induced lung cancer cells (50, 100 and 200 µM) arrested in the
G1/S cell cycle phase was significantly increased
compared with the control group (Fig.
3A and B). Subsequently, western blotting was performed to
detect the expression levels of cell cycle-related proteins,
including cyclin D1, CDK4 and CDK6. Compared with the control
group, the expression levels of cyclin D1, CDK4 and CDK6 following
treatment with 100 or 200 µM GM were significantly downregulated in
a concentration-dependent manner (Fig.
3C). Collectively, the aforementioned results indicated that GM
induced lung cancer cell apoptosis and G1/S phase cell
cycle arrest.
GM alters the Akt/MDM2/p53 signaling
pathway
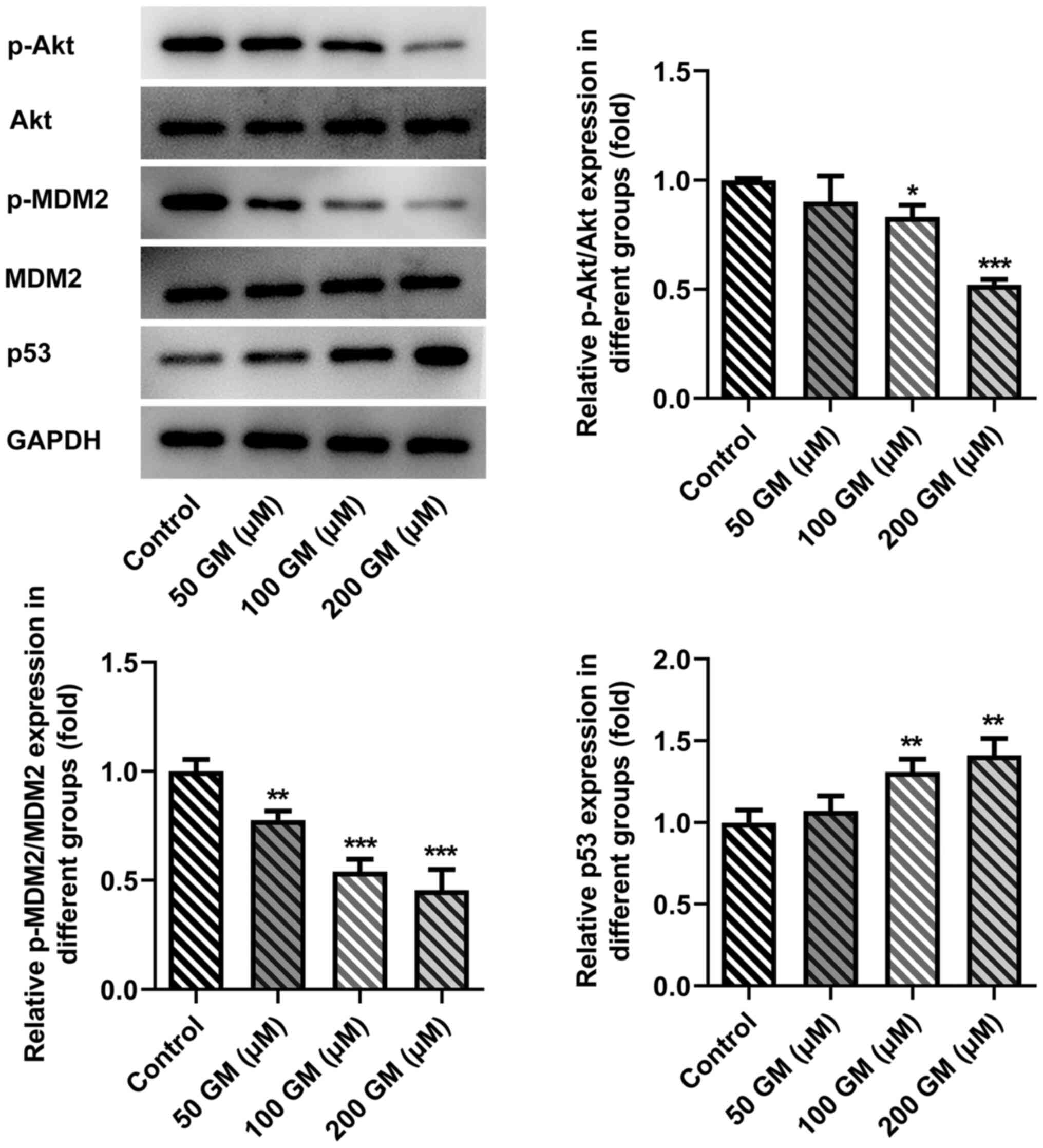
Compared with the control group, the expression
levels of phosphorylated (p)-Akt and p-MDM2 were significantly
decreased, and the expression levels of p53 were significantly
increased following 100 and 200 µM GM administration (Fig. 4). Therefore, the results indicated
that GM altered the Akt/MDM2/p53 signaling pathway. The most
notable effects on protein expression were observed following
treatment with 200 µM GM, so this concentration was selected for
subsequent experiments.
SC79 reverses the antiproliferative,
proapoptotic and pro-cell cycle arrest effects of GM on lung cancer
cells
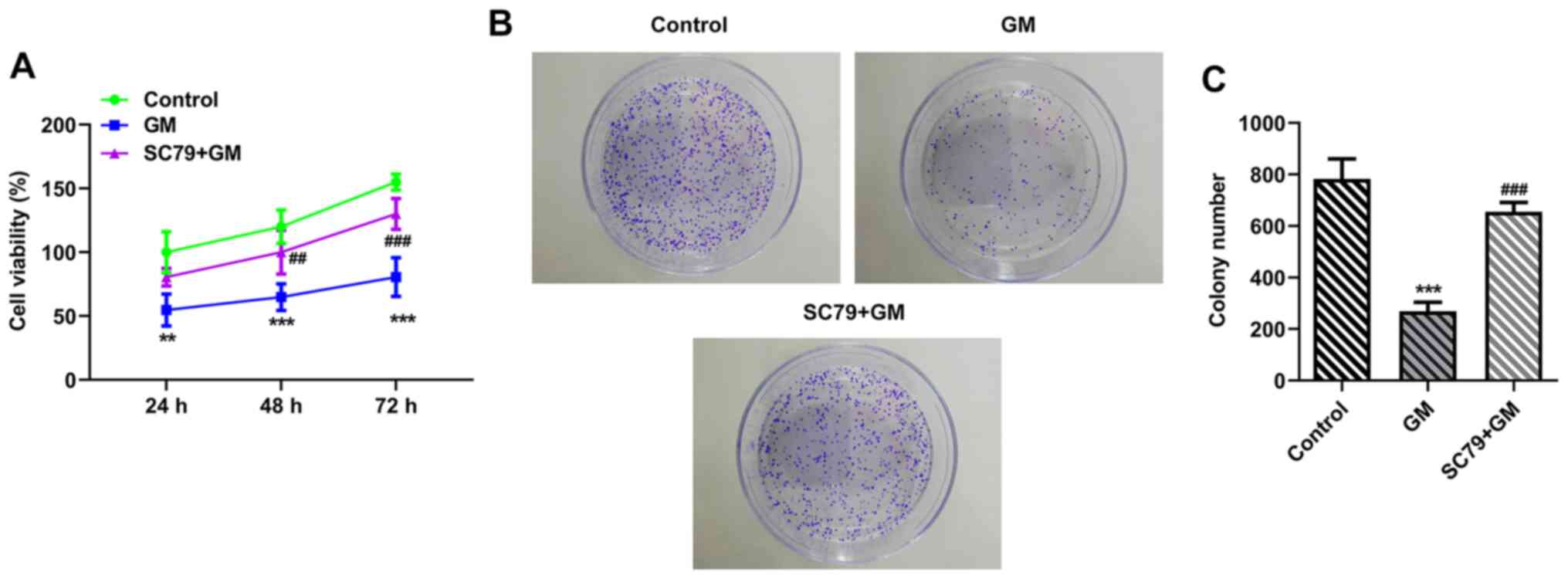
To further verify the aforementioned results, cells
were treated with Akt activator SC79. Cells were divided into the
following two groups: i) GM; and ii) SC79+GM. The CCK-8 (Fig. 5A) and colony formation (Fig. 5B and C) assay results demonstrated
that cell viability (at 48 and 72 h) and proliferation were
significantly increased in the SC79+GM group compared with the GM
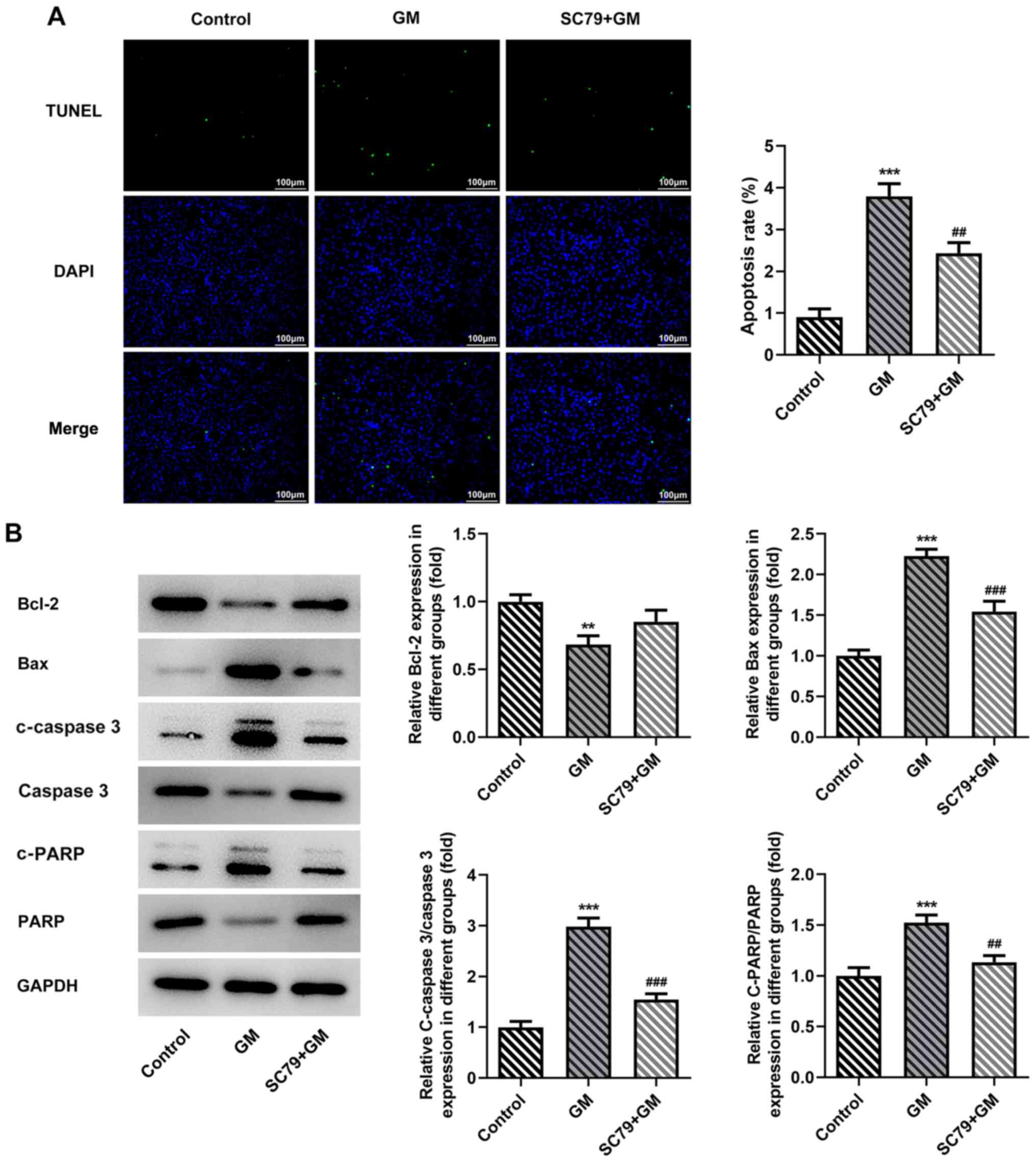
group, respectively. Cell apoptosis was detected by performing
TUNEL assays. Compared with the GM group, the apoptosis rate of the
SC79+GM group was significantly decreased (Fig. 6A). Furthermore, compared with the GM
group, the SC79+GM group displayed significantly decreased
expression levels of Bax, C-Caspase 3 and c-PARP, and slightly
increased expression levels of Bcl-2 (Fig. 6B). The flow cytometry results
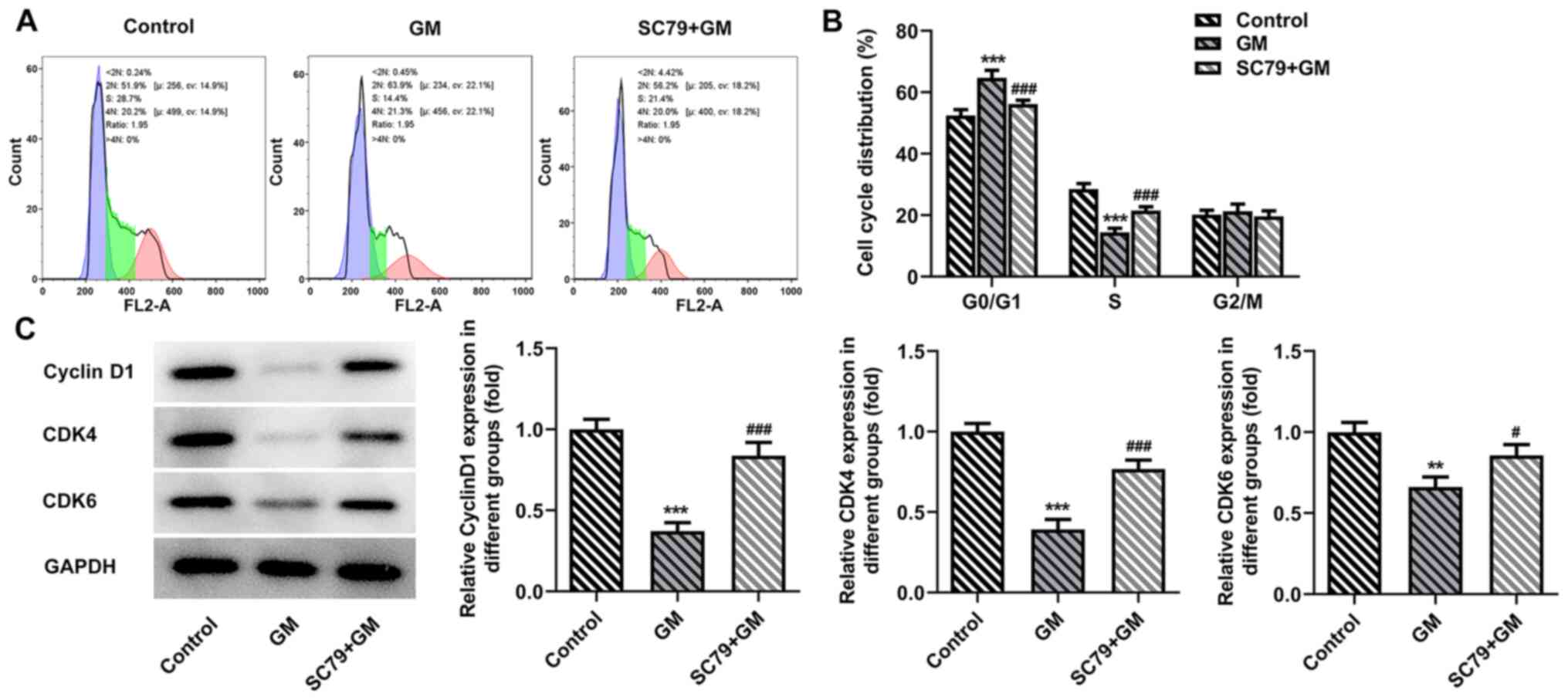
demonstrated that SC79 significantly reversed GM-induced
G1/S phase cell cycle arrest (Fig. 7A and B). Subsequently, western
blotting was performed to detect the expression levels of cyclin
D1, CDK4 and CDK6. Compared with the GM group, the expression
levels of cyclin D1, CDK4 and CDK6 in the SC79+GM group were
significantly increased (Fig. 7C).
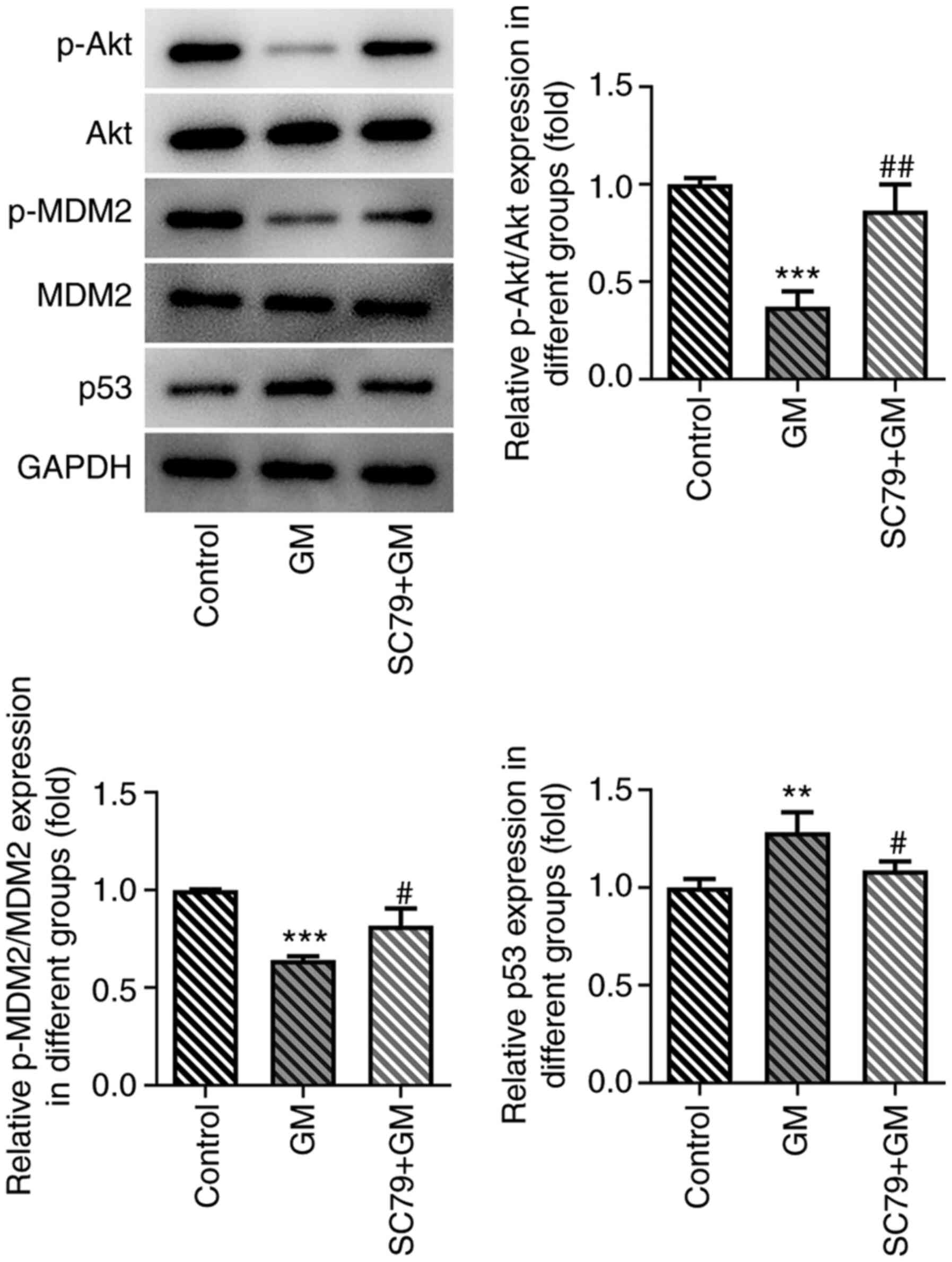
In addition, the expression levels of Akt/MDM2/p53 signaling
pathway-related proteins were detected. The results demonstrated
that p-Akt and p-MDM2 expression levels were significantly
upregulated, and p53 expression levels were significantly
downregulated in the SC79+GM group compared with the GM group
(Fig. 8). Collectively, the results
demonstrated that SC79 reversed the antiproliferative, proapoptotic
and pro-cell cycle arrest effects of GM on lung cancer cells.
Discussion
GM displays antiviral, antioxidant,
anti-inflammatory and immunomodulatory pharmacological effects
(20,22). Pharmacological studies have
demonstrated that GM also displays antitumor effects on gastric
cancer (4), glioma (21) and breast cancer (9) by inducing cell cycle arrest and
promoting cell apoptosis. Cai et al (23) reported that curcumol extracted from
zedoary enhanced celecoxib-induced growth inhibition and apoptosis
in NSCLC. However, to the best of our knowledge, the effects of GM
isolated from zedoary in lung cancer have not been previously
reported. Therefore, the present study investigated the effects of
GM on lung cancer cells. The results of the present study
demonstrated that GM induced lung cancer cell apoptosis and cell
cycle arrest, thereby inhibiting the development of lung
cancer.
Following further investigation into the effects of
GM on lung cancer cell apoptosis and cell cycle arrest and its
molecular mechanism, the present study demonstrated that the
expression levels of p-Akt and p-MDM2 were significantly
downregulated, whereas the expression levels of p53 were
significantly upregulated by different concentrations of GM (100
and 200 µM) compared with the control group. Malignancy in various
different types of cancer cells (such as breast, ovarian
epithelial, prostate and gastric cancer) is associated with
increased abnormal Akt signaling (24). Akt phosphorylates the p53 suppressor
MDM2, promoting nuclear translocation of MDM2, thereby inhibiting
p53 (25). As a classical tumor
suppressor gene, p53 mutations occur in >50% of all malignant
tumors (26). The protein encoded
by p53 is a transcription factor that controls the initiation of
the cell cycle, and regulates cell proliferation and apoptosis via
a complex molecular network (27).
It has been previously reported that the expression levels of p-Akt
and p-MDM2 are upregulated in prostate cancer cells, which
downregulates p53 expression and induces cell cycle arrest
(28). Similarly, genistein
inhibited esophageal cancer cell proliferation by inhibiting
activation of the Akt/MDM2/p53 signaling pathway (13).
Furthermore, GM induces prostate cancer cell
apoptosis and autophagy by inhibiting the Akt/mTOR signaling
pathway (8). GM can serve a role in
breast cancer cells by targeting the estrogen receptor, MAPK, AKT
and other signaling pathways (29,30).
The aforementioned studies suggested that GM could target the Akt
signaling pathway. Following administration of SC79, the
antiproliferative, proapoptotic and pro-cell cycle arrest effects
of GM on lung cancer cells were significantly reversed.
Collectively, the results of the present study demonstrated that GM
induced lung cancer cell apoptosis and cell cycle arrest via the
Akt/MDM2/p53 signaling pathway. However, it has been previously
reported that GM can decrease the expression levels of p-Akt in
cerebral ischemia-reperfusion injury model rats, which was
inconsistent with the results of the present study; therefore,
future studies should investigate this inconsistency further
(31).
A key limitation of the present study was that the
therapeutic effect of GM on lung cancer was not studied in a mouse
tumor model. Therefore, future studies should investigate the
therapeutic effect of GM on lung cancer in vivo.
In conclusion, the present study demonstrated that
GM promoted lung cancer cell apoptosis and cell cycle arrest by
inhibiting the Akt/MDM2/p53 signaling pathway. The results of the
present study provided a theoretical basis for identifying
potential drugs and targets for the treatment of lung cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and JC contributed to the conception and design
of the present study, analyzed and interpreted the data, and
critically revised the manuscript for important intellectual
content. KS, HL and JD contributed to designing the study, analyzed
the data, and drafted and revised the manuscript. DH, ZL and WW
substantially contributed to the conception and design of the
study, acquired, analyzed and interpreted the data, and drafted and
critically revised the manuscript for important intellectual
content. YZ and JC confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rivera GA and Wakelee H: Lung Cancer in
never smokers. Adv Exp Med Biol. 893:43–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garon EB, Hellmann MD, Rizvi NA, Carcereny
E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L,
et al: Five-year overall survival for patients with advanced
non-small-cell lung cancer treated with pembrolizumab: Results from
the phase I KEYNOTE-001 study. J Clin Oncol. 37:2518–2527. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiang AC and Herbst RS: Frontline
immunotherapy for NSCLC-the tale of the tail. Nat Rev Clin Oncol.
17:73–74. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu L, Wang L, Tian X, Zhang J and Feng H:
Germacrone exerts anti-cancer effects on gastric cancer through
induction of cell cycle arrest and promotion of apoptosis. BMC
Complement Med Ther. 20:212020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen QF, Wang G, Tang LQ, Yu XW, Li ZF and
Yang XF: Effect of germacrone in alleviating HUVECs damaged by
H2O2-induced oxidative stress. Zhongguo Zhong Yao Za Zhi.
42:3564–3571. 2017.(In Chinese). PubMed/NCBI
|
|
6
|
Wang Z, Zhuo F, Chu P, Yang X and Zhao G:
Germacrone alleviates collagen-induced arthritis via regulating
Th1/Th2 balance and NF-κB activation. Biochem Biophys Res Commun.
518:560–564. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nair A, Amalraj A, Jacob J, Kunnumakkara
AB and Gopi S: Non-curcuminoids from turmeric and their potential
in cancer therapy and anticancer drug delivery formulations.
Biomolecules. 9:132019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu Z, Xu J, Shao M and Zou J: Germacrone
induces apoptosis as well as protective autophagy in human prostate
cancer cells. Cancer Manag Res. 12:4009–4016. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong Z, Chen X, Tan W, Xu Z, Zhou K, Wu
T, Cui L and Wang Y: Germacrone inhibits the proliferation of
breast cancer cell lines by inducing cell cycle arrest and
promoting apoptosis. Eur J Pharmacol. 667:50–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
An JF, Sun Y, Zhang QL, Zhang FL and Zhang
JL: The effects of germacrone on lipopolysaccharide-induced acute
lung injury in neonatal rats. Cell Mol Biol (Noisy-le-Grand).
60:8–12. 2014.PubMed/NCBI
|
|
11
|
Hashem S, Nisar S, Sageena G, Macha MA,
Yadav SK, Krishnankutty R, Uddin S, Haris M and Bhat AA:
Therapeutic effects of curcumol in several diseases; An overview.
Nutr Cancer. 73:181–195. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu W, Gao L, Li T, Zheng J, Shao A and
Zhang J: Mesencephalic astrocyte-derived Neurotrophic factor (MANF)
protects against neuronal apoptosis via activation of Akt/MDM2/p53
signaling pathway in a rat model of intracerebral hemorrhage. Front
Mol Neurosci. 11:1762018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao J, Xia R, Chen J, Gao J, Luo X, Ke C,
Ren C, Li J and Mi Y: Inhibition of esophageal-carcinoma cell
proliferation by genistein via suppression of JAK1/2-STAT3 and
AKT/MDM2/p53 signaling pathways. Aging (Albany NY). 12:6240–6259.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J
and Wen X: The PI3K/AKT pathway in the pathogenesis of prostate
cancer. Front Biosci (Landmark Ed). 21:1084–1091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iida M, Harari PM, Wheeler DL and Toulany
M: Targeting AKT/PKB to improve treatment outcomes for solid
tumors. Mutat Res. 819-820:1116902020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song M, Bode AM, Dong Z and Lee MH: AKT as
a therapeutic target for cancer. Cancer Res. 79:1019–1031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reyes-Farias M and Carrasco-Pozo C: The
Anti-Cancer Effect of Quercetin: Molecular implications in cancer
metabolism. Int J Mol Sci. 20:31772019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen G, Park D, Magis AT, Behera M,
Ramalingam SS, Owonikoko TK, Sica GL, Ye K, Zhang C, Chen Z, et al:
Mcl-1 Interacts with Akt to promote lung cancer progression. Cancer
Res. 79:6126–6138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mayo LD and Donner DB: A
phosphatidylinositol 3-kinase/Akt pathway promotes translocation of
Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA.
98:11598–11603. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie XH, Zhao H, Hu YY and Gu XD:
Germacrone reverses Adriamycin resistance through cell apoptosis in
multidrug-resistant breast cancer cells. Exp Ther Med. 8:1611–1615.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu B, Gao YQ, Wang XM, Wang YC and Fu LQ:
Germacrone inhibits the proliferation of glioma cells by promoting
apoptosis and inducing cell cycle arrest. Mol Med Rep.
10:1046–1050. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He W, Zhai X, Su J, Ye R, Zheng Y and Su
S: Antiviral Activity of Germacrone against Pseudorabies Virus in
vitro. Pathogens. 8:2582019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai F, Chen M, Zha D, Zhang P, Zhang X,
Cao N, Wang J, He Y, Fan X, Zhang W, et al: Curcumol potentiates
celecoxib-induced growth inhibition and apoptosis in human
non-small cell lung cancer. Oncotarget. 8:115526–115545. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Revathidevi S and Munirajan AK: Akt in
cancer: Mediator and more. Semin Cancer Biol. 59:80–91. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abraham AG and O'Neill E:
PI3K/Akt-mediated regulation of p53 in cancer. Biochem Soc Trans.
42:798–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Oijen MG and Slootweg PJ:
Gain-of-function mutations in the tumor suppressor gene p53. Clin
Cancer Res. 6:2138–2145. 2000.PubMed/NCBI
|
|
27
|
Flemming A: Cancer: Mutant p53 rescued by
aggregation inhibitor. Nat Rev Drug Discov. 15:852016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han CT, Schoene NW and Lei KY: Influence
of zinc deficiency on Akt-Mdm2-p53 and Akt-p21 signaling axes in
normal and malignant human prostate cells. Am J Physiol Cell
Physiol. 297:C1188–C1199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lim MS, Choung SY and Jeong KW: Germacrone
inhibits estrogen receptor α-mediated transcription in MCF-7 breast
cancer cells. Phytother Res. 30:2036–2043. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kong Q, Ma Y, Yu J and Chen X: Predicted
molecular targets and pathways for germacrone, curdione, and
furanodiene in the treatment of breast cancer using a
bioinformatics approach. Sci Rep. 7:155432017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu T, Yin F, Kong H and Peng J: Germacrone
attenuates cerebral ischemia/reperfusion injury in rats via
antioxidative and antiapoptotic mechanisms. J Cell Biochem.
120:18901–18909. 2019. View Article : Google Scholar : PubMed/NCBI
|