Introduction
Depression is the fourth most prevalent disease in
the world, with about 322 million individuals suffering from it
worldwide, accounting for about 4.4% of the global population
(1). Depression (and suicide) has
begun to appear in those of increasingly younger age (in university
students and even in primary and secondary school students). Common
symptoms include inattention, negative self-evaluation, a sense of
self-guilt and worthlessness (even in mild episodes), self-injuring
or suicidal ideas or behaviors and sleep disorders (2). Depression does not belong to the class
of neurodegenerative diseases, but its occurrence is closely
related to microglial activation (3,4).
Microglial activation occurs as a response to
neuroinflammation and is a potential target for treating
depression. Ionized calcium binding adapter molecule 1 (Iba-1) is a
specific marker of microglial activation, which has been shown by
previous studies to be significantly increased in the hippocampus
of stressed rats (5). Activation of
microglia can be classified into either M1 or M2 microglial
activation (6). When M1 microglia
are activated, expression levels of proinflammatory cytokines are
raised, such as inducible nitric oxide synthase (iNOS), TNF-α and
IL-1β (7). It is known that the
abundant expression of Toll-like receptor (TLR) 4 induces
microglial activation, triggering the association of recombinant
interleukin 1 receptor-associated kinase 1 (IRAK1) and TNF
receptor-associated factor 6 (TRAF6) (8). Thus, blocking microglial activation is
of great importance in treating neuroinflammation-associated
diseases, including depression.
miR-146 contains two evolutionarily conserved genes:
miR-146a and miR-146b. In individuals, these loci are located at
chromosomes 5 and 10 respectively and they differ by only 2
nucleotides at the 3′terminus (9).
In 2006, Taganov et al (10)
first reported that miR-146a can suppress the immune response via
the NF-κB signaling pathway. It also negatively regulates the
expression of TLR4 pathway-related molecules and downstream
inflammatory factors (11,12). Studies have found that increasing
miR-146a expression reduces microglia-mediated neuroinflammatory
responses, while silencing miR-146a expression induces the opposite
effect (13,14).
The present study investigated the effect of
miR-146a on depression model mice and further investigated
microglia-mediated neuroinflammatory responses in hippocampal and
BV-2 cells, including iNOS, TRAF6, IRAK1 and NF-κB.
Materials and methods
Animals
A total of 144 healthy male C57BL/6J mice, weighing
25–30 g at 6–8 weeks of age, were purchased from Jinan Pengyue
Experimental Animal Breeding Co., Ltd. [animal production license
no. SCXK (lu) 2014-0007]. All experiments followed the requirements
for animal ethics specified by the Institutional Animal Care and
Use Committee (IACUC) of Binzhou People's Hospital (approval no.
201910072). Mice were raised in a clean environment with a
temperature of 20±2°C, relative humidity of 50–70%, 12-h light/dark
cycle and food and water ad libitum. Following adaptive
feeding for one week, animals underwent experimental grouping and
model preparation.
Lipopolysaccharide (LPS)-induced
depression model
Following Ren et al (15), animals were treated with LPS using
intraperitoneal injection (ip) at 0.83 mg/kg for five consecutive
days to establish the depression model.
Animal groups in LPS induced
depression
In order to test the effect of miR-146a on LPS
induced depression mice, 72 mice were divided into 6 groups, 12
mice in each group: i) Control group (Control; 0.9% saline,
intraperitoneal injection); ii) LPS group (LPS; 0.83 mg/kg,
intraperitoneal injection); iii) miR-146a mimic (brain injection of
miR-146a mimic and i.p. LPS); iv) mimic-negative control (NC; brain
injection of miR-146a mimic NC and i.p. LPS); v) miR-146a short
interfering (si)RNA (brain injection of miR-146a siRNA and i.p.
LPS); and vi) siRNA-NC (brain injection of miR-146a siRNA NC and
i.p. LPS). miR-146a mimic (5′-UGAGAACUGAAUUCCAUGGGUU-3′), miR-146a
mimic negative control (5′-UUGUACUACACAAAAGUACUG-3′), miR-146a
siRNA (5′-AACCCAUGGAAUUCAGUUCUCA-3′) and miR-146a siRNA negative
control (5′-CAGUACUUUUGUGUAGUACAA-3′) were all purchased from
Guangzhou RiboBio Co., Ltd. One week before LPS injection, 1 µl
miR-146a mimic (0.5 nmol/µl), 5 µl miR-146a siRNA (0.5 nmol/µl) and
NC (0.5 nmol/µl) were injected into the hippocampus (0.2 µl/min) at
2.06 mm at antero-posterior, approximately 1.5 mm medio-lateral and
2 mm below the skull surface using a 33-gauge beveled NanoFil
needle, a NanoFil syringe and a Microsyringe Pump Controller
(16). The miR-146a mimic and siRNA
were injected once every two days. After 1 week, animals were
co-treated with LPS or normal saline using intraperitoneal
injection for five consecutive days.
Chronic unpredictable mild stress
(CUMS) depression model
Studies have shown that microglial cells are closely
involved in the occurrence of depression (3,17).
Therefore, to further study the effect of miR-146a on depressed
mice, a CUMS depression model was established. According to
previous reports, the present study employed stimulation with
different sources of stress, including fasting, water prohibition,
isolation, cage tilting, cage emptying, diurnal inversion,
restraint, environmental dampness, stroboscopic lighting and
diurnal disturbance (18,19). Every day, two or three stress
sources were randomly selected and the model was repeated every
Monday for five consecutive weeks.
Animal groups in CUMS depression
A total of 72 mice were divided into 6 groups, 12
mice in each group: i) Control group (Control), in which the mice
were not treated; ii) depression group (CUMS), in which the CUMS
model was induced in mice; iii) miR-146a-overexpressing control +
depression group (mimic-NC+CUMS), in which one week before CUMS,
mice were hippocampally injected with a blank vector containing
miR-146a mimic; iv) miR-146a-overexpressing + depression group
(miR-146a mimic+CUMS), in which one week before CUMS, mice were
hippocampally injected with miR-146a mimic; v) miR-146a-silenced
control + depression group (si-NC+CUMS), in which one week before
CUMS, a blank vector containing miR-146a siRNA was hippocampally
injected; and vi) miR-146a-silenced + depression group
(si-miR-146a+CUMS), in which one week before CUMS, the miR-146a
siRNA was hippocampally injected.
Behavioral testing
Sucrose preference test (SPT)
The test was divided into two parts, the training
period and the testing period. During the 48-h training period,
mice were given 2 bottles of sucrose water (1% concentration)
during the first 24 h and 1 bottle of sucrose water (1%
concentration) and 1 bottle of pure water during the second 24 h.
In the 8 h before the test, the mice did not eat, nor did they
drink any water. During the test period of 2 h, each animal was
given 1 bottle of sucrose water (1% concentration) and 1 bottle of
pure water. To avoid biases in the results caused by positional
preferences, the positions of the two bottles were changed after 1
h of testing. Following the test, the sucrose water preference
index (sucrose water preference index=sucrose water
consumption/total liquid consumption ×100%) was calculated
(20).
Forced swimming test (FST)
Mice were put in a transparent cylinder with a
height of ~22 cm, a diameter of ~14 cm and a water depth of ~15 cm.
The water temperature was ~20–25°C. The forced swimming lasted for
6 min (21).
Tail suspension test (TST)
The inverted tail of each mouse being tested was
fixed to the tail suspension device, with the head of the mouse ~5
cm away from the table, so that there was no place the mouse could
grasp. There were baffles between the mice to block the line of
sight, in order to prevent mutual interference. The percentage of
time during the last 4 min that each mouse spent immobile within
the complete 6 min testing time was recorded (22).
Locomotor activity
Locomotor activity was measured according to the
protocol previously described (23). Briefly, each mouse was monitored in
a 30×30×30 cm open-field cage. The total distance travelled by each
mouse was measured over 30 min using open-field activity software
(Med Associates). To avoid affecting the behavior of the next
experimental animal, the cage was cleaned in between trials using
20% alcohol.
Reverse transcription-quantitative
(RT-q) PCR
RT-qPCR was performed according to the
manufacturer's instructions. Total RNA was extracted from both
sides of the hippocampus in one single mouse (100 mg) using a Total
RNA Isolation kit (cat. no. A27828; MagMAX MiRVana Total RNA
Isolation kit; Thermo Fisher Scientific, Inc.). RNA (10 µl), Oligo
(dT)18 Primer (0.5 µl) and Random Hexamer Primer (0.5 µl) were
mixed, then made up to 15 µl using ribonuclease free deionized
water. The mixture were reacted at 65°C for 5 min then cooled to
room temperature. Subsequently, 4 µl 5X Reaction Buffer and 1 µl
Servicebio RT Enzyme Mix (Wuhan Servicebio Technology Co., Ltd.)
were added. The reaction conditions were: 42°C 60 min, 70°C 5 min.
qPCR was subsequently performed using SYBR Green qPCR Master Mix
(MedChemExpress) and 2 µl cDNA as a template. The reaction
conditions were: 10 min at 95°C, 15 sec at 95°C and 1 min at 60°C.
The RNA was amplified for a total of 40 cycles. The relative
expression of miR-146a mRNA was calculated with U6 as the internal
parameter using the 2−ΔΔCq method (24). RT-qPCR was performed in triplicate.
The primer sequences were: miR-146a, F:
5′-CCTGAGAAGTGAATTCCATGGG-3′ and R:
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAACCCATG-3′; U6, F:
5′-GGAGATTACTGCCCTGGCTCCTA-3′ and R:
5′-GACTCATCGTACTCCTGCTTGCTG-3′.
ELISA
The concentrations of TNF-α (cat. no. BMS607-3FIVE)
and IL-1β (cat. no. BMS6002) in the hippocampal tissue (100 mg) of
mice or BV-2 cells (100 mg) were analyzed using an ELISA kit
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions.
Immunofluorescence
Following the behavioral tests, mice were
anesthetized using 0.3% pentobarbital sodium (45 mg/kg) and
sacrificed by decapitation. The hippocampus was dissected from the
brain tissues and a portion was preserved at −80°C, while another
portion was fixed in 4% paraformaldehyde at room temperature
overnight, dehydrated and embedded in paraffin. Then the sections
were cut into 4-µm thick sections. The sections were dewaxed,
treated with citrate buffer (pH 6.0) antigen for 10 min,
inactivated with 3% H2O2 for 20 min and
sealed with 5% BSA (cat. no. ml064298; Shanghai Enzyme-linked
Biotechnology Co., Ltd.) for 20 min. Rabbit anti-Iba-1 antibody
(1:200; cat. no. ab48004, Abcam) was incubated and reacted
overnight at 4°C. Following rewarming, donkey polyclonal Secondary
Antibody to Goat IgG-H&L (Alexa Fluor® 488; 1:200;
cat. no. ab150129, Abcam) was incubated with secondary antibodies
for 2 h at room temperature in dark. Following washing with PBS,
DAPI (cat. no. I029-1-1, Nanjing Jiancheng Bioengineering
Institute) was added and cultured for 3 min at room temperature.
Following washing with PBS, anti-fluorescence quenching sealing
agent (cat. no. IH0252; Beijing Leagene Biotech Co., Ltd.) was used
to seal. A total of five fields were randomly selected at ×400
magnification using a MiRax Scan (Zeiss GmbH, Jena) and the results
were analyzed using ImageJ 1.49p (National Institutes of
Health).
Western blotting
The expression levels of iNOS, IL-1β, TRAF6, IRAK1
and NF-κB p65 in the hippocampus of each group or in BV-2 cells
were detected by western blotting. Total protein in the tissue (100
mg) or BV-2 cells was extracted using RIPA buffer containing
protein inhibitors (cat. no. W062-1-1; Nanjing Jiancheng
Bioengineering Institute) and the concentration of the protein
sample was measured with a quantitative BCA Protein Assay kit (cat.
no. 23225; Thermo Fisher Scientific, Inc.). Samples (40 µg) from
each group were collected, separated by 10% SDS-PAGE using a
Mini-PROTEAN 3 (Bio-Rad Laboratories, Inc.) and transferred to a
PVDF membrane (EMD Millipore). Blocking was performed using 5%
skimmed milk for 1 h at 37°C and 5% BSA was used to dilute the
following rabbit anti-mouse primary antibodies: iNOS (1:250; cat.
no. ab15323; Abcam); IL-1β (1:2,000; cat. no. ab9722; Abcam); TNF-α
(1:1,000, cat. no. ab183218; Abcam); TRAF6 (1:500; cat. no.
ab62488; Abcam); IRAK1 (1:2,000; cat. no. ab238; Abcam); NF-κB p65
(1:1,000; cat. no. ab16502; Abcam); and β-actin (1:1,000; cat. no.
ab8227; Abcam). Following incubation at 4°C overnight, the
membranes were washed 3 times with TBST (TBS, 1 ml/l Tween-20) for
10 min each time. Then they were incubated in goat anti-rabbit HRP
IgG (1:2,000; cat. no. ab6721; Abcam) for 2 h at room temperature
and the membrane was washed 3 times, for 10 min each time.
Detection was performed using the enhanced chemiluminescence kit
(cat. no. SW2010; Beijing Solarbio Science & Technology Co.,
Ltd.). ImageJ software 1.49p (National Institutes of Health) was
used for grayscale image analysis.
Cell culture and treatment
The mouse microglial BV-2 cells were purchased from
the Shanghai Institute of Biochemistry and Cell Biology. The cells
were cultured in DMEM/F12 medium supplemented with 10% fetal bovine
serum and 1% penicillin streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) in 5% CO2 at 37°C.
BV-2 cells were randomly divided into 4 groups with
3 replicates: i) Control cells (Control); ii) LPS-treated (cells
were stimulated with 1 µg/ml LPS) (16); iii) LPS + miR-146a mimic
(miR-146a+LPS; cells were cultured with 1 µg/ml LPS for 24 h, then
transfected with 50 nM miR-146a mimic); and iv) LPS + miR-146a
negative control (miR-146a-NC+LPS). miR-146a mimic
(5′-UGAGAACUGAAUUCCAUGGGUU-3′) and negative control (Guangzhou
RiboBio Co., Ltd.) were transfected using Lipofectamine®
3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C
and then added to the BV-2 cells. Following transfection for 48 h,
miR-146a expression was assessed by RT-PCR.
Statistical analysis
Data analysis was performed using SPSS 19.0 software
(IBM Corp.). All results are presented as the mean ± standard
deviation. Multiple data sets were compared using the one-way
analysis of variance and Tukey's test was used for subsequent
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
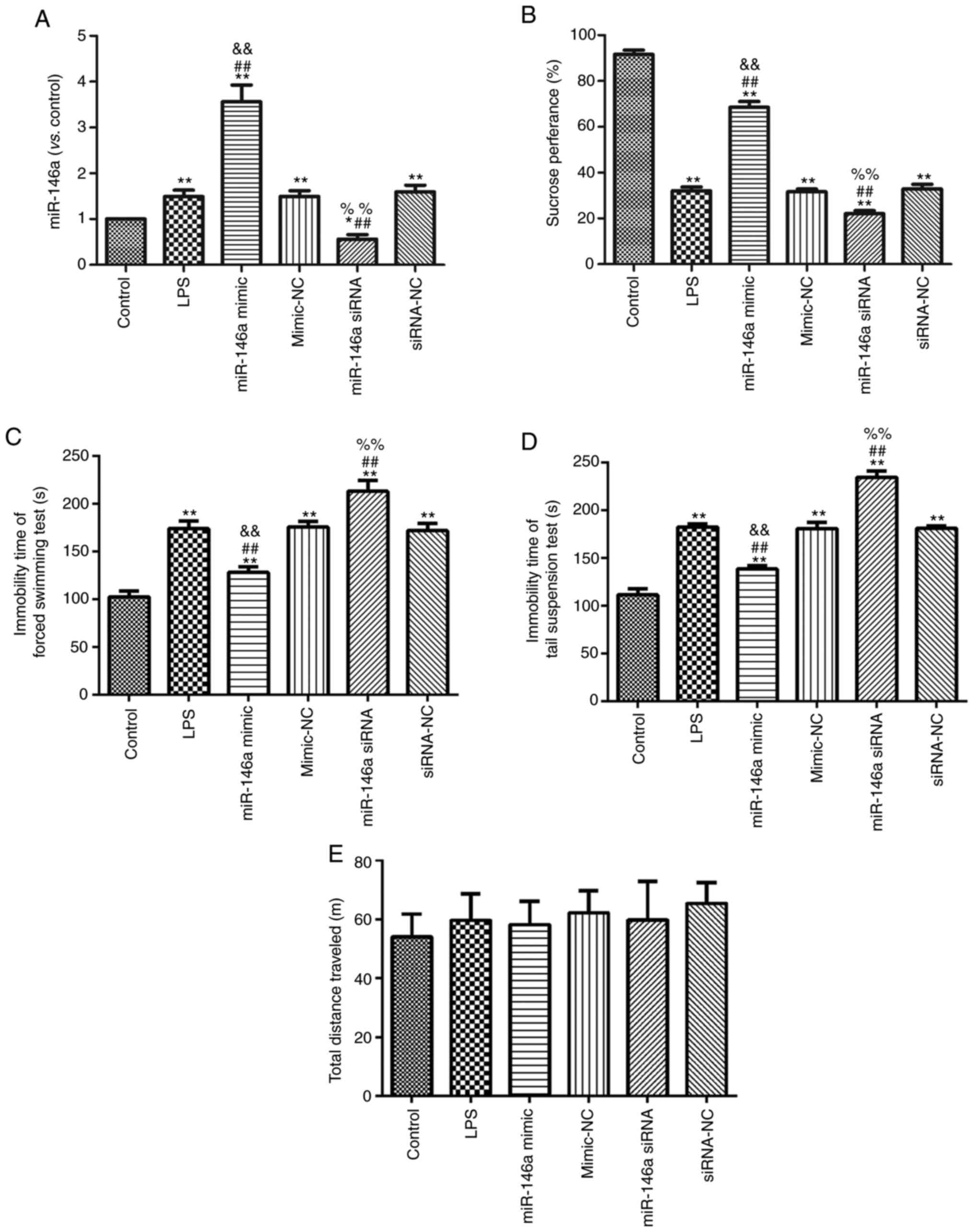
Overexpression of miR-146a suppresses
LPS-induced depression in mice
One week before LPS injection, miR-146a mimic and
siRNA were injected into mice once every two days. After 1 week,
mice were co-treated with LPS. Hippocampal miR-146a expression was
measured in each experimental group using RT-PCR, as shown in
Fig. 1A. Expression of miR-146a was
significantly enhanced in the miR-146a mimic group, while miR-146a
silencing effectively decreased miR-146a expression in the miR-146a
siRNA group (P<0.05). The behavior of the mice in each group was
tested and the results are shown in Fig. 1B-E. Compared with the control group,
LPS induction significantly decreased sucrose preference (Fig. 1B) and significantly increased the
scores on the FST (Fig. 1C) and the
TST (Fig. 1D; all P<0.05).
Overexpression of miR-146a significantly enhanced sucrose
preference and reduced the scores on the FST and the TST in the
LPS-induced mice. miR-146a silencing significantly decreased
sucrose preference and increased scores on the FST and the TST in
LPS-induced mice (all P<0.05). However, there was no significant
difference in the total distance traveled among groups (P>0.05;
Fig. 1E).
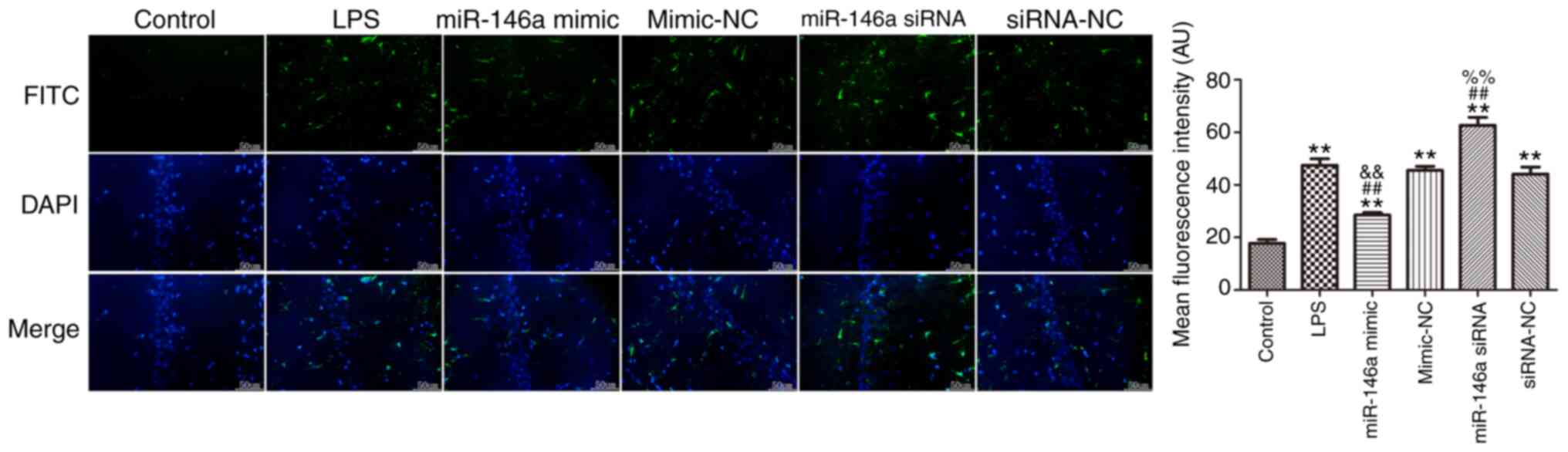
Overexpression of miR-146a suppresses
microglial cell activation in the hippocampus of LPS-induced
mice
Iba-1 was used as a specific marker of microglia
cells and its expression was measured using immunofluorescence, as
shown in Fig. 2. LPS induced a
significant increase in Iba-1 expression in the hippocampus
compared with the control group (P<0.01). Overexpression of
miR-146a significantly attenuated the level of Iba-1 in the
hippocampus of LPS-induced mice, while miR-146a silencing markedly
increased the expression of Iba-1 (P<0.05).
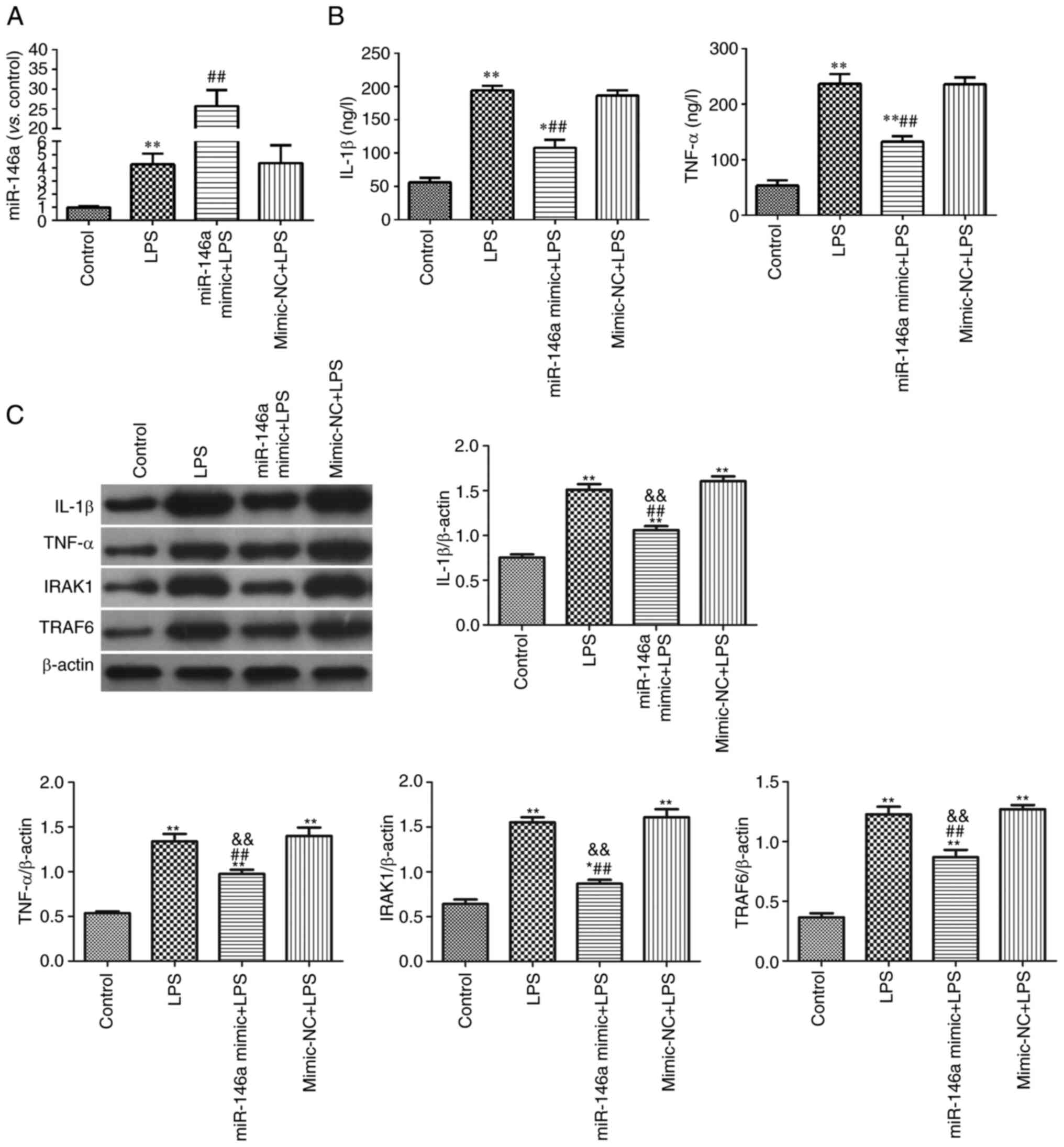
Overexpression of miR-146a inhibits
the expression of microglia-mediated neuroinflammatory proteins in
the hippocampus of LPS-induced mice
Expression levels of microglia-mediated
neuroinflammatory proteins were measured in the hippocampus of the
experimental mice (Fig. 3).
Compared with the control group, the expression levels of iNOS,
IL-1β, TNF-α, IRAK1, TRAF6 and p-NF-κB p65 in the LPS-induced group
were significantly increased (P<0.05). Overexpression of
miR-146a effectively inhibited the expression levels of these
neuroinflammatory proteins, while miR-146a silencing significantly
upregulated their expression compared with LPS group
(P<0.01).
 | Figure 3.Overexpression of miR-146a inhibited
neuroinflammatory response in hippocampus of LPS-induced mice. (A)
The relative expression of iNOS, IL-1β, TNF-α; (B) The relative
expression of IRAK1, TRAF6, p-NF-κB p65/ NF-κB p65. *P<0.05 and
**P<0.01 vs. control group; #P<0.05 and
##P<0.01 vs. LPS group;
&&P<0.01 vs. mimic-NC group;
%%P<0.01 vs. siRNA-NC group. miR, microRNA; LPS,
lipopolysaccharide; NC, negative control; si, short interfering;
iNOS, inducible nitric oxide synthase; IRAK1, recombinant
interleukin 1 receptor-associated kinase 1; TRAF6, TNF
receptor-associated factor 6; p-, phosphorylated. |
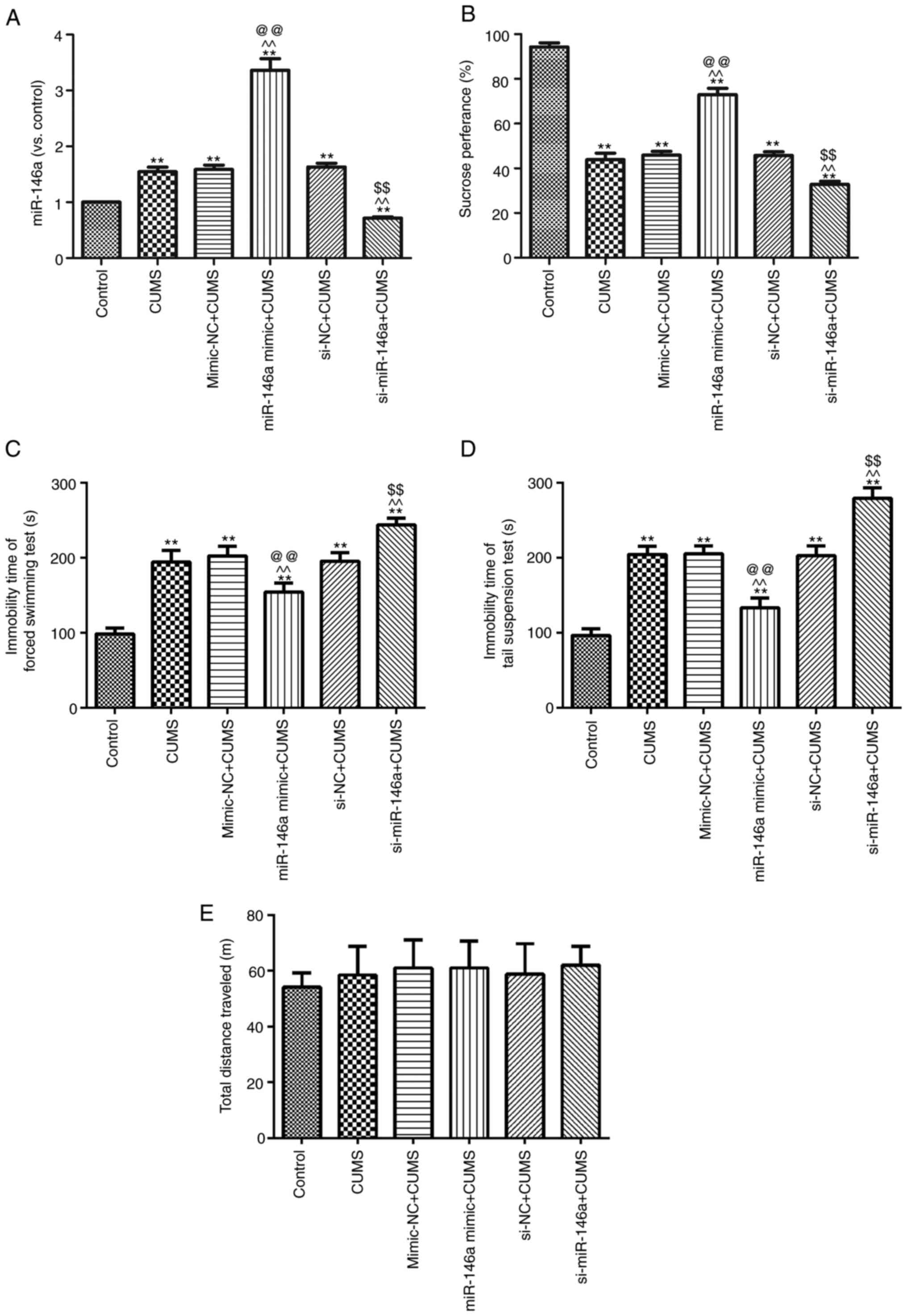
Overexpression of miR-146a reduces
depressive behaviors in CUMS mice
In order to study the effect of miR-146a on
depressed mice, miR-146a mimic and siRNA were used to treat CUMS
mice (Fig. 4A). Compared with the
untreated CUMS group, miR-146a expression in the hippocampal region
of CUMS mice was effectively increased following treatment with
miR-146a agomir, but significantly decreased following treatment
with miR-146a antagomir (both P<0.05). The behaviors of CUMS
mice were also tested (Fig. 4B-D).
In contrast to the Control group, sucrose preference (Fig. 4B) was notably decreased in the CUMS
group, but scores on the FST (Fig.
4C) and the TST (Fig. 4D) were
significantly increased (all P<0.05). Following overexpression
of miR-146a, sucrose preference was clearly increased in the CUMS
mice and scores on the FST and the TST were significantly decreased
(all P<0.05). Meanwhile, miR-146a silencing led to more serious
behavioral impairments compared with those in the CUMS group
(P<0.05). There was no difference in locomotor activity among
groups (P>0.05; Fig. 1E).
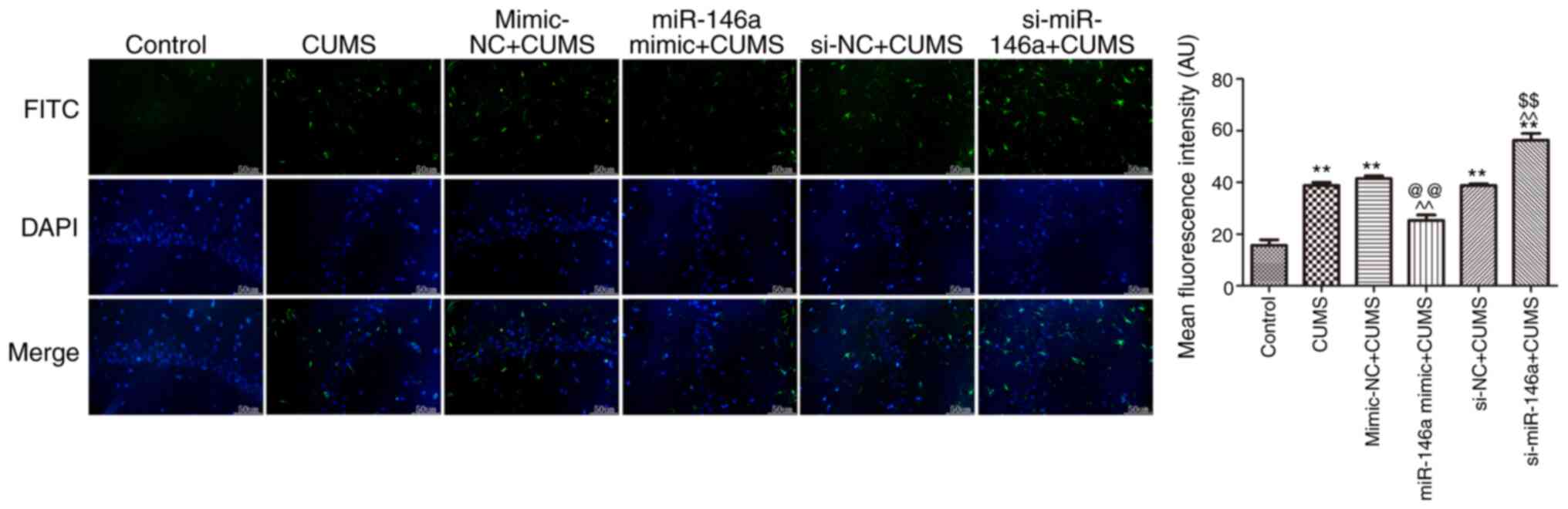
Overexpression of miR-146a suppresses
activation of microglia in the hippocampus of CUMS mice
The expression level of Iba-1 in the hippocampus of
CUMS mice was measured by immunofluorescence, as shown in Fig. 5. Iba-1 expression was effectively
increased in CUMS mice compared with that in control mice
(P<0.05). Overexpression of miR-146a significantly suppressed
Iba-1 expression in the hippocampus of CUMS mice, while miR-146a
silencing significantly promoted Iba-1 expression (both P<0.05).
This indicates that microglia in the hippocampus of CUMS mice
become abnormally activated and that overexpression of miR-146a can
significantly reduce such activation.
Overexpression of miR-146a suppresses
microglia-mediated neuroinflammatory proteins in the hippocampus of
CUMS mice
The expression levels of iNOS, IL-1β, TNF-α, IRAK1,
TRAF6 and p-NF-κB p65 in the hippocampus in CUMS mice were
significantly enhanced compared with those in control mice
(P<0.05; Fig. 6). Overexpression
of miR-146a effectively lowered the expression levels of these
proteins, while miR-146a silencing effectively increased their
expression (P<0.05; Fig. 6).
These data suggest that overexpression of miR-146a markedly
inhibits microglia-mediated neuroinflammatory protein
expression.
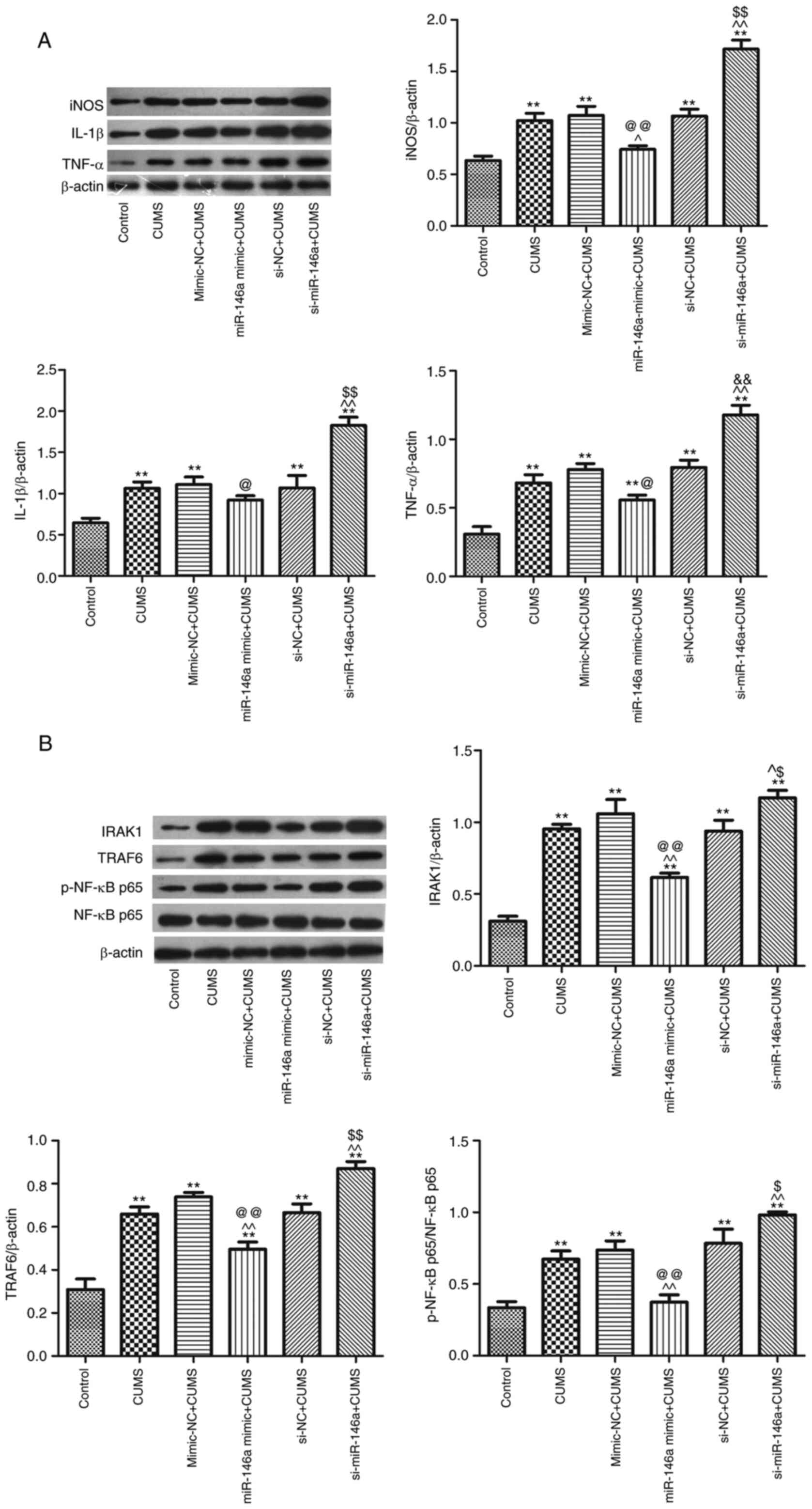 | Figure 6.Overexpression of miR-146a inhibits
neuroinflammatory response in hippocampus of CUMS mice. (A) The
relative expression of iNOS, IL-1β, TNF-α; (B) The relative
expression of IRAK1, TRAF6, p-NF-κB p65/NF-κB p65. **P<0.01 vs.
control group; ^P<0.05, ^^P<0.01 vs.
CUMS group; @P<0.05 and @@P<0.01 vs.
mimic-NC + CUMS group; $P<0.05 and
$$P<0.01 vs. siRNA-NC + CUMS group. miR, microRNA;
CUMS, chronic unpredictable mild stress; iNOS, inducible nitric
oxide synthase; IRAK1, recombinant interleukin 1
receptor-associated kinase 1; TRAF6, TNF receptor-associated factor
6; p-, phosphorylated; NC, negative control; si, short
interfering. |
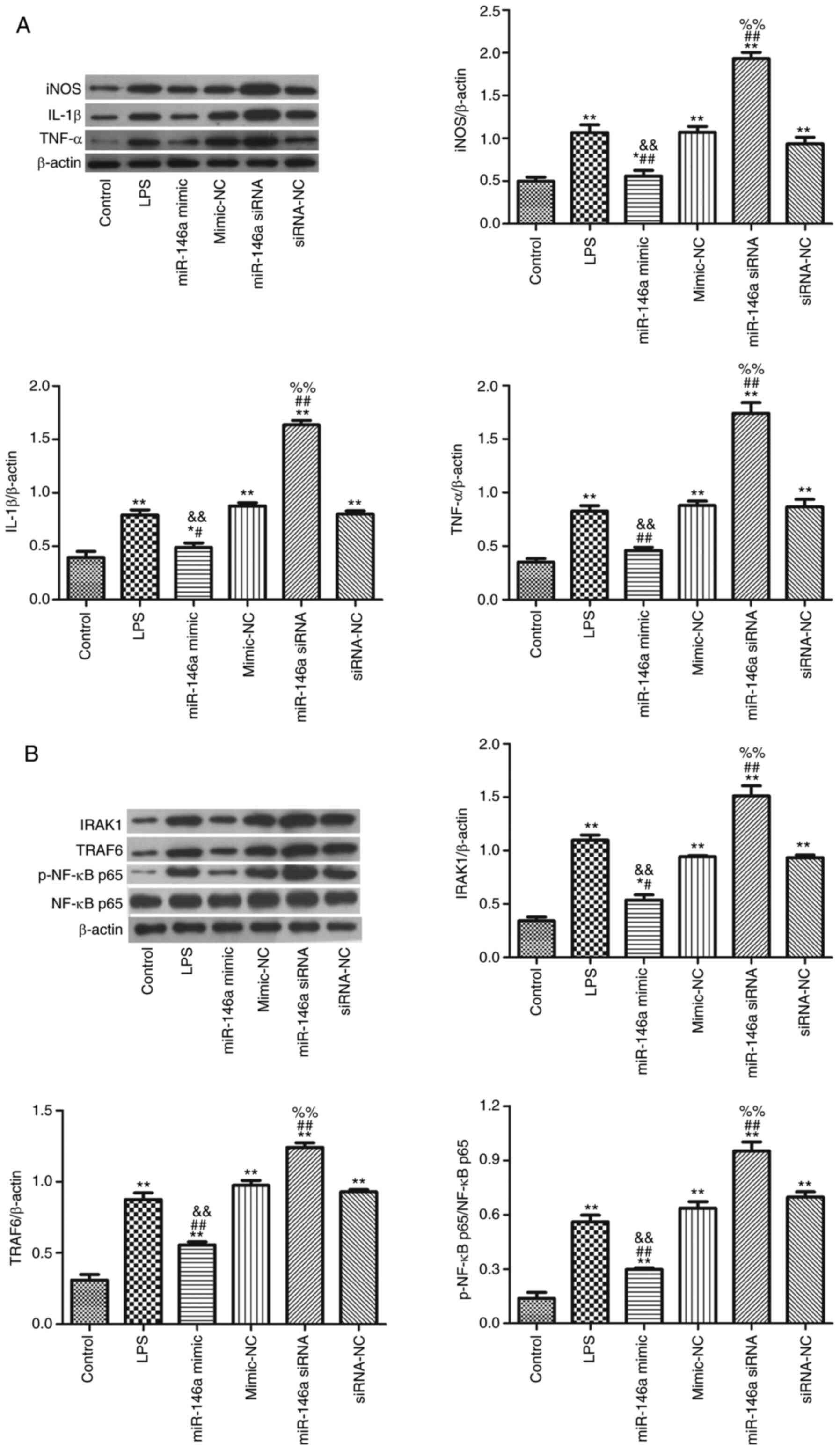
Overexpression of miR-146a suppresses
the neuroinflammatory response in LPS-induced BV-2 cells
The expression of miR-146a was quantified, as shown
in Fig. 7A. Following transfection
with miR-146a mimic, the level of miR-146a was clearly increased
compared with that of the LPS group (P<0.05). Additionally,
transfection with miR-146a mimic notably decreased the levels of
IL-1β and TNF-α in LPS-induced BV-2 cells compared with those of
LPS-treated cells (Fig. 7B;
P<0.05). The western blotting results showed that levels of
IL-1β, TNF-α, IRAK1 and TRAF6 were significantly reduced in the
miR-146a+LPS group compared with those in the miR-146a-NC+LPS group
(Fig. 7C; P<0.05).
Discussion
In the present study, two mouse models of depression
were established, namely the LPS-induced depression and CUMS
depression models. Notably, miR-146a was expressed at a high level
in depressed mice accompanied with increased expression of IRAK1
and TRAF6. Further investigation showed that overexpression of
miR-146a suppressed microglial activation in the hippocampus of
depressed mice and inhibited neuroinflammation-related proteins,
including iNOS, IL-1β, IRAK1, TRAF6 and p-NF-κB p65. From these
contradictory results, it may be that the expression of miR-146a
was too low to suppress the expression of inflammatory proteins in
depression models. However, the mechanisms of miR-146a for
neuroinflammation require further elucidation.
The LPS immune activation model is an important
animal model for studying the cytokine hypothesis of depression. A
number of studies have shown that depression is associated with
immune activation and manifests as an increased expression of
pro-inflammatory cytokines (25–27).
Immune activation results in changes of mood and behavior;
therefore, cytokines can be seen as biomarkers for depression.
Cytokines are divided into pro-inflammatory cytokines and
anti-inflammatory cytokines (28).
The present study found that the pro-inflammatory factors iNOS,
TNF-α and IL-1β were clearly increased in hippocampus of depressed
mice. In a study of surgical trauma-induced cognitive decline in
mice, miR-146a suppresses hippocampal neuroinflammation and improve
cognitive function (16). In an
Alzheimer's disease mouse model, intranasal administration of
miR-146a agomir rescues the pathological process and cognitive
impairment (29). Consistent with
these previous studies (16,29),
the present study found that injection of miR-146a mimic suppressed
TNF-α and IL-1β expression and also ameliorated depression-like
behaviors, as measured by the SPT, FST and TST. The data suggested
that miR-146a served a positive role in depression. The theoretical
basis of the CUMS model is close to that of human depression; that
is, depression is promoted by medium-chronic and low-level
stressors (30). Consistent with
LPS-induced depression, expression levels of iNOS and IL-1β were
clearly increased, while miR-146a inhibited pro-inflammatory factor
expression.
Recent studies have shown that microglial activation
is closely related to depression (3–5).
Postmortem examination of the dorsal anterior cingulate cortex in
patients with major depression has revealed microglial activation
and macrophage aggregation (31).
The immune response of the central nervous system is mainly
regulated by microglia and astrocytes. Similarly, the present study
found that microglial cells were activated in the hippocampus of
depressed mice. Together, these studies suggest that microglial
activation can be considered an important marker of depression.
Therefore, exploring how to inhibit the activation of microglia is
essential to treating patients with depression. miR-146a has been
shown to serve a protective role in surgical trauma-induced
cognitive decline in mice via suppression of the IRAK1/TRAF6/NF-κB
pathway in the hippocampus (16).
Based on these previous reports, it was hypothesized that miR-146a
acted as a positive regulator for depression, suppressing
microglial activation and neuroinflammation in the hippocampus.
The results of the present study supported the
hypothesis that miR-146a effectively inhibited the activation of
microglial cells and suppressed levels of IRAK1, TRAF6 and NF-κB
p65 in the hippocampus of depressed mice. The present study
suggested, for the first time to the best of the authors'
knowledge, that miR-146a acted as an antidepressant by inhibiting
microglial activation. However, more experimental studies are
needed to explore the role that microRNAs serve in the pathogenesis
of depression.
In summary, the present study found that miR-146a
overexpression inhibited microglial activation by reducing levels
of the neuroinflammation-related proteins TRAF6, IRAK1 and NF-κB
p65 in the hippocampus of depressed mice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
CPL and MZ carried out the experimental work and the
data collection and interpretation. CPL, MZ and JXS participated in
the design and coordination of experimental work and acquisition of
data. JH, FXQ and YG participated in the data collection, analysis
of data and preparation of the manuscript. CPL, MZ and JXS carried
out the study design, the analysis and interpretation of data and
drafted the manuscript. CPL and MZ confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study has been approved by the Institutional
Animal Care and Use Committee (IACUC) of Binzhou People's Hospital
(201910072).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Carlessi AS, Borba LA, Zugno AI, Quevedo J
and Reus GZ: Gut-microbiota-brain axis in depression: The role of
neuroinflammation. Eur J Neurosci. 53:222–235. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amidfar M, Reus GZ, Quevedo J and Kim YK:
The role of memantine in the treatment of major depressive
disorder: Clinical efficacy and mechanisms of action. Eur J
Pharmacol. 827:103–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shibata M and Suzuki N: Exploring the role
of microglia in cortical spreading depression in neurological
disease. J Cereb Blood Flow Metab. 37:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yirmiya R, Rimmerman N and Reshef R:
Depression as a microglial disease. Trends Neurosci. 38:637–658.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou S, Chen S, Xie W, Guo X and Zhao J:
Microglia polarization of hippocampus is involved in the mechanism
of Apelin-13 ameliorating chronic water immersion restraint
stress-induced depression-like behavior in rats. Neuropeptides.
81:1020062020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mikita J, Dubourdieu-Cassagno N, Deloire
MS, Vekris A, Biran M, Raffard G, Brochet B, Canron MH, Franconi
JM, Boiziau C and Petry KG: Altered M1/M2 activation patterns of
monocytes in severe relapsing experimental rat model of multiple
sclerosis. Amelioration of clinical status by M2 activated monocyte
administration. Mult Scler. 17:2–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tobon-Velasco JC, Cuevas E and
Torres-Ramos MA: Receptor for AGEs (RAGE) as mediator of NF-κB
pathway activation in neuroinflammation and oxidative stress. CNS
Neurol Disord Drug Targets. 13:1615–1626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hui B, Zhang L, Zhou Q and Hui L:
Pristimerin inhibits LPS-triggered neurotoxicity in BV-2 Microglia
cells through modulating IRAK1/TRAF6/TAK1-Mediated NF-κB and AP-1
signaling pathways in vitro. Neurotox Res. 33:268–283. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Testa U, Pelosi E, Castelli G and Labbaye
C: miR-146 and miR-155: Two key modulators of immune response and
tumor development. Noncoding RNA. 3:222017.PubMed/NCBI
|
|
10
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He X, Zheng Y, Liu S, Shi S, Liu Y, He Y,
Zhang C and Zhou X: MiR-146a protects small intestine against
ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-κB
pathway. J Cell Physiol. 233:2476–2488. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loubaki L, Chabot D, Pare I, Drouin M and
Bazin R: MiR-146a potentially promotes IVIg-mediated inhibition of
TLR4 signaling in LPS-activated human monocytes. Immunol Lett.
185:64–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng M, Du G, Zhao J and Du X: miR-146a
negatively regulates the induction of proinflammatory cytokines in
response to Japanese encephalitis virus infection in microglial
cells. Arch Virol. 162:1495–1505. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma N, Verma R, Kumawat KL, Basu A and
Singh SK: miR-146a suppresses cellular immune response during
Japanese encephalitis virus JaOArS982 strain infection in human
microglial cells. J Neuroinflammation. 12:302015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren Z, Yan P, Zhu L, Yang H, Zhao Y, Kirby
BP, Waddington JL and Zhen X: Dihydromyricetin exerts a rapid
antidepressant-like effect in association with enhancement of BDNF
expression and inhibition of neuroinflammation. Psychopharmacology
(Berl). 235:233–244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Dong R, Lu Y, Zhou Y, Li K, Zhang
Z and Peng M: MicroRNA-146a protects against cognitive decline
induced by surgical trauma by suppressing hippocampal
neuroinflammation in mice. Brain Behav Immun. 78:188–201. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singhal G and Baune BT: Microglia: An
interface between the loss of neuroplasticity and depression. Front
Cell Neurosci. 11:2702017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma K, Guo L, Xu A, Cui S and Wang JH:
Molecular mechanism for stress-induced depression assessed by
sequencing miRNA and mRNA in medial prefrontal cortex. PLoS One.
11:e01590932016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma K, Xu A, Cui S, Sun MR, Xue YC and Wang
JH: Impaired GABA synthesis, uptake and release are associated with
depression-like behaviors induced by chronic mild stress. Transl
Psychiatry. 6:e9102016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Strekalova T, Couch Y, Kholod N, Boyks M,
Malin D, Leprince P and Steinbusch HM: Update in the methodology of
the chronic stress paradigm: Internal control matters. Behav Brain
Funct. 7:92011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rupniak NM, Carlson EJ, Webb JK, Harrison
T, Porsolt RD, Roux S, de Felipe C, Hunt SP, Oates B and Wheeldon
A: Comparison of the phenotype of NK1R-/- mice with pharmacological
blockade of the substance P (NK1) receptor in assays for
antidepressant and anxiolytic drugs. Behav Pharmacol. 12:497–508.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zomkowski AD, Santos AR and Rodrigues AL:
Putrescine produces antidepressant-like effects in the forced
swimming test and in the tail suspension test in mice. Prog
Neuropsychopharmacol Biol Psychiatry. 30:1419–1425. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nishida S, Araki R, Baba A, Asari S,
Tachibana S, Nakajima Y, Iwakumo A and Yabe T: Post-weaning folate
deficiency induces a depression-like state via neuronal immaturity
of the dentate gyrus in mice. J Pharmacol Sci. 143:97–105. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak JK and Schmittgen TD: Analysis of
relative gene expression data using quantitative PCR and the
2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leff-Gelman P, Mancilla-Herrera I,
Flores-Ramos M, Cruz-Fuentes C, Reyes-Grajeda JP, García-Cuétara
Mdel P, Bugnot-Pérez MD and Pulido-Ascencio DE: The immune system
and the role of inflammation in perinatal depression. Neurosci
Bull. 32:398–420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robson MJ, Quinlan MA and Blakely RD:
Immune system activation and depression: Roles of serotonin in the
central nervous system and periphery. ACS Chem Neurosci. 8:932–942.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ronovsky M, Berger S, Zambon A, Reisinger
SN, Horvath O, Pollak A, Lindtner C, Berger A and Pollak DD:
Maternal immune activation transgenerationally modulates maternal
care and offspring depression-like behavior. Brain Behav Immun.
63:127–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim YK, Na KS, Myint AM and Leonard BE:
The role of pro-inflammatory cytokines in neuroinflammation,
neurogenesis and the neuroendocrine system in major depression.
Prog Neuropsychopharmacol Biol Psychiatry. 64:277–284. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mai H, Fan W, Wang Y, Cai Y, Li X, Chen F,
Chen X, Yang J, Tang P, Chen H, et al: Intranasal administration of
miR-146a agomir rescued the pathological process and cognitive
impairment in an AD mouse model. Mol Ther Nucleic Acids.
18:681–695. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li H, Linjuan L and Wang Y: G-CSF improves
CUMS-induced depressive behaviors through downregulating
Ras/ERK/MAPK signaling pathway. Biochem Biophys Res Commun.
479:827–832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bollinger JL and Wohleb ES: The formative
role of microglia in stress-induced synaptic deficits and
associated behavioral consequences. Neurosci Lett. 711:1343692019.
View Article : Google Scholar : PubMed/NCBI
|