Introduction
Bigelow et al (1) first applied therapeutic hypothermia in
the clinic in 1950, and this technique remains one of the most
important measures of myocardial protection during myocardial
ischemia-reperfusion injury. Reperfusion of the ischemic myocardium
itself can conversely worsen myocardial injury (2). Therapeutic hypothermia can reduce
myocardial oxygen consumption and inhibit myocardial metabolism,
thereby enhancing myocardial tolerance to hypoxia (3). However, therapeutic hypothermia can
also cause several adverse side effects. For example, hypothermia
can deactivate the Na+-K+-ATPase and
Ca2+-ATPase of the sarcolemma and sarcoplasmic
reticulum, which may cause cell volume dysregulation and cell
swelling (4,5). Furthermore, therapeutic hypothermia
has been reported to reduce cell membrane potential and substance
transport (6). As such, the optimal
treatment of myocardial ischemic perfusion with therapeutic
hypothermia remains unclear. A greater understanding of the
molecular and cellular mechanisms that are active during
therapeutic hypothermia in the clinic is required; this includes
determining the indications for treatment and to reduce potential
side effects.
Small ubiquitin-related modifier protein (SUMO) is a
member of the ubiquitin family of proteins, all of which possess
similar structures, but different functions. SUMO can covalently
modify numerous proteins to regulate their stability, function and
localization (7). Similar to
ubiquitin modification, SUMO modification (8) (termed SUMOylation) is a dynamic
process catalyzed by a small number of E1-activating enzymes, a
single E2 binding enzyme (ubiquitin carrier protein 9; Ubc9) and
multiple E3 ligases. Furthermore, SUMOylated proteins can be
de-SUMOylated through SUMO-specific proteases (8).
Hypoxia-inducible factor (HIF)-1 is an important
factor that mediates the response of cells to hypoxia (9). HIF-1 is comprised of two subunits
termed α and β (10). The
transcriptional activity and protein stability of HIF-1α are
regulated by intracellular oxygen concentration. Under normoxic
conditions, HIF-1α is rapidly degraded by the ubiquitin-proteasome
pathway. However, under hypoxic conditions, the stability increases
and it enters the nucleus to combine with HIF-1β to form a dimer
that regulates hypoxia-induced gene transcription, including
vascular endothelial growth factor (VEGF), glucose transporter 1
(Glut-l), matrix metalloproteinases and multidrug transporter
(11–14). Notably, HIF-1α can be modified by
SUMO1, which can affect the stability of HIF-1α (15). Nevertheless, the role and regulatory
mechanisms of HIF-1α SUMOylation in normal physiological function
remain unclear (16). Therapeutic
hypothermia can enhance the binding capacity of SUMO2/3 to its
target proteins, and can serve a role in inhibiting apoptosis and
improving neural function in the early stage of ischemia (17,18).
However, it remains unclear whether regulation of HIF-1α protein by
SUMOylation is involved in the protective mechanism underlying the
effects of therapeutic hypothermia on myocardial ischemia.
In the present study, the SUMOylation of HIF-1α was
examined to determine if it participated in the protective
mechanism of therapeutic hypothermia against myocardial ischemia.
The present results indicated that SUMOylation of HIF-1α had an
indispensable role in the protective mechanism of therapeutic
hypothermia on the ischemic myocardium. This new knowledge on the
mechanism of therapeutic hypothermia in myocardial protection may
aid in the rational application of therapeutic hypothermia to treat
myocardial ischemia-reperfusion.
Materials and methods
Cell culture and hypoxia or
hypothermia exposure
Rat cardiomyocyte H9C2 cells were obtained from the
American Type Culture Collection, and were cultured in Dulbecco's
modified Eagle's medium supplemented with 10% fetal bovine serum,
100 µg/ml penicillin and 50 µg/ml streptomycin sulfate (all from
Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. To evaluate the effects of hypoxia on H9C2 cells,
culture medium without glucose, L-aspartic acid, L-glutamic acid or
sodium pyruvate was equilibrated overnight in an anoxic chamber
with 85% N2, 10% H2 and 5% CO2.
The cultures were transferred to an anoxic chamber and washed three
times with anoxic medium. After 24 h of oxygen-glucose deprivation
at 37°C, the cells were transferred back to the incubator at 37°C
with 5% CO2 for an additional 24 h. To simulate
therapeutic hypothermic stress, the culture dishes loaded with H9C2
cells were placed in incubators for 24 h at 34°C with 5%
CO2. Cells cultured in normal conditions (37°C with 5%
CO2) were used as controls.
Blockade of the SUMOylation
pathway
H9C2 cells were seeded in 6-well plates at a density
of 1×105 cells and incubated overnight at 37°C in
normoxic conditions. Spectomycin B1 (20 µM; Nanjing Chemlin
Chemical Co., Ltd.) was added to the culture medium 30 min prior to
reperfusion for 24 h to block the effects of Ubc9, as previously
reported (18).
Western blotting
Total protein was extracted from cells or tissues
using RIPA buffer (Beijing Solarbio Science & Technology Co.,
Ltd.) supplemented with 1 mM phenylmethane sulfonyl fluoride and 20
mM N-ethylmaleimide, as previously reported (19). The protein homogenate (40 µg per
lane; 2 µg/µl) was resolved by SDS-PAGE using 10% gels, blotted
onto Immobilon PVDF membranes (EMD Millipore), blocked with 5%
skimmed milk for 90 min at room temperature, and incubated with the
appropriate primary antibody at 4°C overnight. The β-actin antibody
was re-probed after stripping. Information on all antibodies is
shown in Table I. Primary
antibodies were recovered and the membrane was incubated with the
appropriate secondary antibodies for 1 h at room temperature. The
membranes were visualized with enhanced chemiluminescence reagents
(EMD Millipore). Densitometric semi-quantification analysis of the
western blot bands was performed using image analysis software
(ImageJ v1.48; National Institutes of Health).
 | Table I.Antibody information. |
Table I.
Antibody information.
| Antibody
target | Supplier | Catalogue
number | Dilution |
|---|
| SUMO1 | Abcam | ab11672 | 1:1,000 |
| HIF-1α | Abcam | ab216842 | 1:500 |
| VEGF | Abcam | ab69479 | 1:500 |
| Ubc9 | Abcam | ab75854 | 1:2,000 |
| Caspase 3 | Abcam | ab13847 | 1:500 |
| Cleaved-caspase
3 | Abcam | ab2302 | 1:1,000 |
| Bax | Abcam | ab32503 | 1:5,000 |
| Bcl2 | Abcam | ab692 | 1:500 |
| β-actin | Abcam | ab8227 | 1:1,000 |
| Goat anti-rabbit
IgG | Jackson
ImmunoResearch | 111-035-003 | 1:2,000 |
| Goat anti-mouse
IgG | Jackson
ImmunoResearch | 111-035-003 | 1:2,000 |
Lactate dehydrogenase (LDH)
detection
H9C2 cells were seeded into 96-well plates
8×104 cells/ml and incubated under hypoxic and/or
hypothermic conditions with or without spectomycin B1. The cells
were then collected, sonicated twice at interval setting 0.5 with a
UP-200S sonicator and centrifuged at 13,680 × g at 4°C for 10 min.
The LDH content in the supernatant obtained from centrifugation of
the sonicated cells was measured using an ELISA (cat. no. YM-S0351;
Shanghai Yuanmu Biotechnology Co., Ltd.), in accordance with the
manufacturer's instructions.
Detection of apoptosis using flow
cytometry
H9C2 cells (1×105 cells) were collected
following EDTA-trypsin digestion and washed twice with cold PBS.
Apoptosis was then assessed within 1 h using an Annexin V
fluorescein isothiocyanate apoptosis detection kit (BD Biosciences)
and a FACSCalibur flow cytometer (BD Biosciences), according to the
manufacturer's protocol. The samples cells were analyzed using the
FlowJo software version 9 (FlowJo LLC).
Rats and experimental grouping
Animal experiments were performed according to the
regulations and guidelines approved by the Animal Ethics Committee
of The Fifth Central Hospital of Tianjin (Tianjin, China; approval
no. TJWZX2018044). Animal studies were conducted according to the
Guide for the Care and Use of Laboratory Animals (8th edition,
2011) and the guidelines for Animal Research Reporting In Vivo
Experiments guidelines (https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf).
A total of 48 6-week-old Sprague-Dawley female rats (mean weight,
200 g) were purchased from the Animal Center of Nanjing University
(Nanjing, China. Animals were housed in the Experimental Animal
Center of The Fifth Central Hospital of Tianjin, and maintained
under a controlled temperature (22–24°C), stable humidity (40-60%)
and a 12 h-light/dark cycle with ad libitum access to food and
water. Animals were randomly divided into four groups (n=6 each):
i) Control group, the isolated heart of each rat was perfused at
37°C for 30 min, exposed to ischemia for 30 min and reperfusion for
120 min. The animals in the control group did not receive an
injection of spectomycin B1. ii) Therapeutic hypothermia group, the
isolated heart of each rat was perfused at 37°C for 30 min, then
exposed to ischemia for 30 min and reperfusion at 34°C for 120 min.
Animals in this group did not receive an injection of spectomycin
B1. iii) Spectomycin B1 group, the isolated heart of each rat was
perfused at 37°C for 30 min, then exposed to ischemia for 30 min
and reperfusion for 120 min. At 30 min prior to the start of
surgery, animals received intraperitoneal injection of spectomycin
B1 (2 mg/kg) according to the reagent instructions. iv) Therapeutic
hypothermia + spectomycin B1 group, the isolated heart of each rat
was perfused at 37°C for 30 min, followed by ischemia for 30 min
and reperfusion at 34°C for 120 min. At 30 min prior to the start
of surgery, animals received intraperitoneal injection of
spectomycin B1 (2 mg/kg), according to the reagent
instructions.
Production of the isolated heart
perfusion model
The rats were anesthetized with sodium pentobarbital
(60 mg/kg intraperitoneally; Merial) and fixed to the operating
table. Briefly, after the thorax was opened, the heart was removed
and mounted on a Langendorff apparatus. All hearts were perfused
with Krebs-Henseleit buffer containing 118.5 mM sodium chloride,
4.7 mM potassium chloride, 1.2 mM magnesium sulfate, 24.8 mM sodium
bicarbonate, 1.8 mM calcium chloride, 1.2 mM potassium hydrogen
phosphate and 10 mM glucose, which was heated to 37°C and gassed
with 95% O2/5% CO2 to stop the hearts from
beating. Subsequently, a 4-0 silk suture was placed around the left
coronary artery and the ends of the suture were passed through a
small piece of soft vinyl tubing to form a snare. Regional ischemia
was induced by fixing the snare to the heart using a hemostat.
After 30 min of ischemia, the hearts were reperfused for 120 min by
releasing the hemostat. Rats were sacrificed by exsanguination
during the Langendorff procedure.
Measurement of myocardial infarction
(MI)
After reperfusion was complete, the heart was
removed. All hearts were cut into 1-mm-thick cross-sections and
incubated in 1% triphenyltetrazolium chloride (Sigma-Aldrich; Merck
KGaA) at 37°C for 20 min. The sections at the level of the
papillary muscle were photographed with a ruler. The unstained area
was considered infarcted myocardium. Total myocardial area (TMA),
and infarct area (IA) at the mid-papillary muscle were measured by
planimetry. The percentage of infarct area was calculated as IA/TMA
×100%.
Confocal imaging of mitochondrial
membrane potential
Mitochondrial membrane potential of H9C2 cells was
measured using a commercial assay (JC-10 mitochondrial membrane
potential assay kit; Beijing Solarbio Science & Technology Co.,
Ltd.). Fluorescence changes were detected with a laser scanning
confocal microscope. The maximum excitation wavelength of the JC-10
monomer was 515 nm and the maximum emission wavelength was 529 nm
(green). The maximum excitation wavelength of the JC-10 polymer was
585 nm and the maximum emission wavelength was 590 nm (red).
Statistical analysis
All statistical analyses were performed using SPSS
19.0 for Windows (IBM Corp.). Each experiment was performed at
least three times. Data are presented as the mean ± SD. The
differences between the experimental and control group were tested
using an unpaired t-test. For comparing differences among more than
two experimental groups, the means were compared using one-way
ANOVA followed by a Tukey-Kramer test. P<0.05 was considered to
indicate a statistically significant difference.
Results
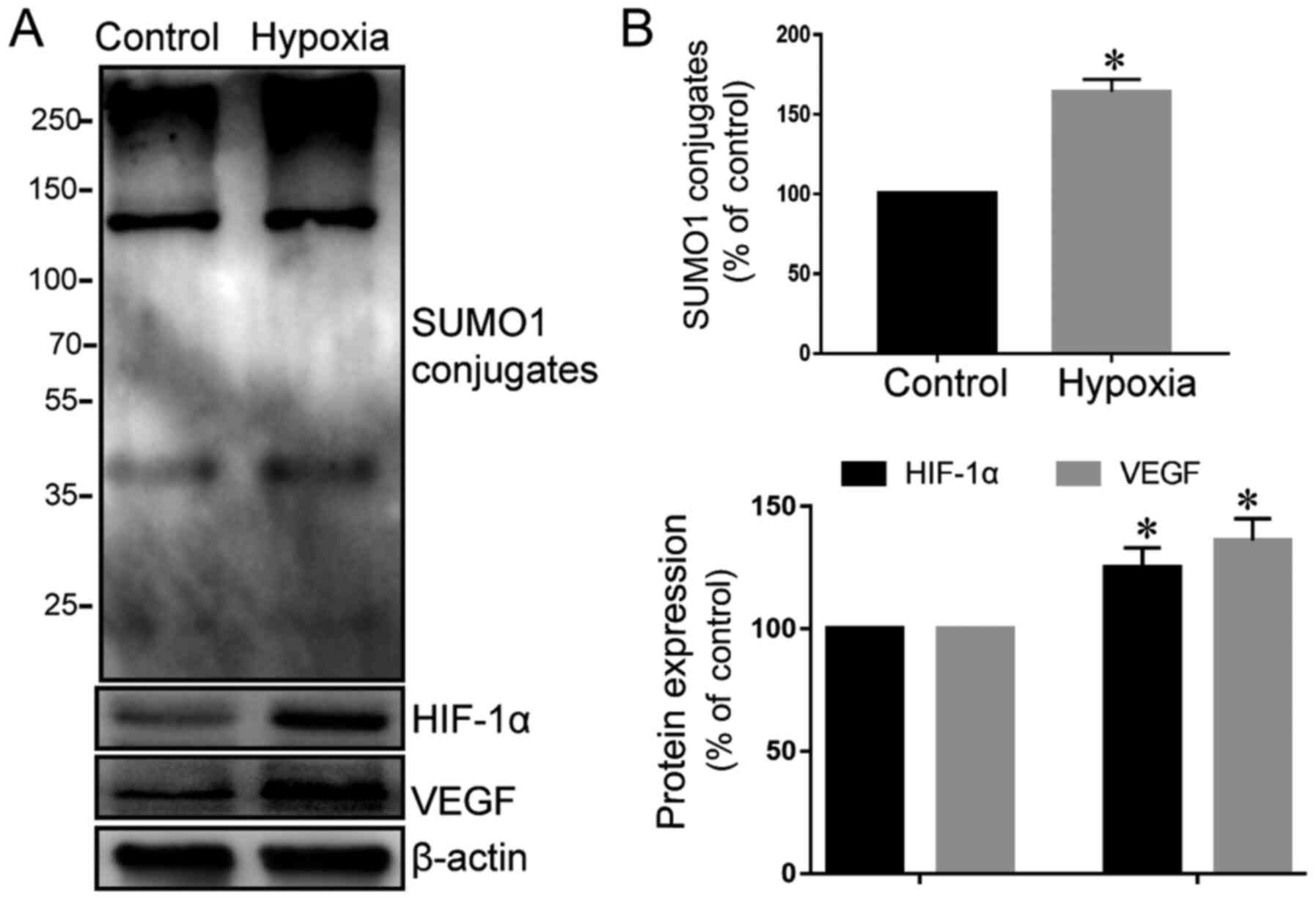
Hypoxia can significantly increase the
expression of SUMO1 in cardiomyocytes and activate the HIF-1α/VEGF
pathway
Because HIF-1α has previously been identified as a
target protein of SUMO1 (15), the
expression of SUMO1 in cardiomyocytes was initially examined under
normal and hypoxic conditions. The present results showed that
cardiomyocytes expressed low levels of SUMO1 under normal oxygen
conditions, which significantly increased following exposure to
hypoxic conditions (Fig. 1A and B).
In addition, HIF-1α and VEGF expression were examined. The protein
expression levels of HIF-1α and VEGF in cardiomyocytes were
significantly increased under hypoxic conditions compared with
normoxia. Bae et al (20)
have demonstrated that the protein level and transcriptional
activity of HIF-1α can be upregulated by SUMO1. These findings
validated the hypothesis that hypoxia may increase the expression
of SUMO1 in cardiomyocytes, increase the protein levels of HIF-1α
and continuously activate its downstream VEGF transcription,
thereby antagonizing the actions of hypoxia (Fig. 1A and B).
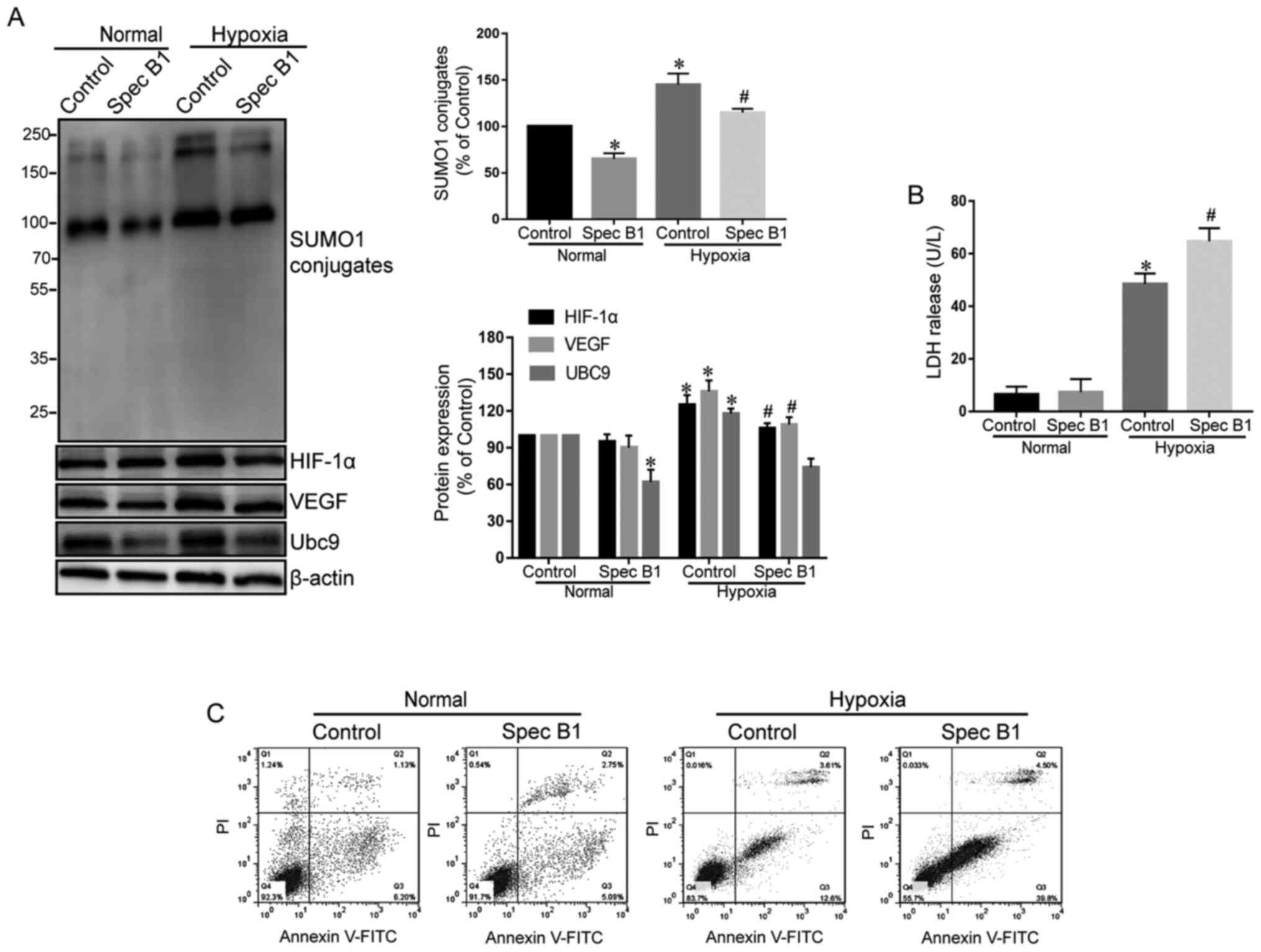
Inhibition of SUMOylation can reduce
signaling of the HIF-1α/VEGF pathway and aggravate cardiomyocyte
injury following hypoxia
To examine whether the hypoxia-activated HIF-1α/VEGF
pathway is SUMOylation-dependent, cardiomyocytes were pretreated
with spectomycin B1, a specific inhibitor of the E2 binding enzyme
Ubc9, in order to inhibit the binding of SUMO1 to target proteins,
prior to induction of hypoxia. The results demonstrated that
spectomycin B1 effectively inhibited the activity of Ubc9 (19), which reduced the amount of SUMO1
conjugation to target proteins and decreased the levels of HIF-1α
and VEGF proteins in myocardial cells under hypoxia (Fig. 2A).
Subsequently, the effect of inhibition of the
SUMOylation pathway on myocardial injury following hypoxia was
tested. Treatment with spectomycin B1 alone did not induce
significant LDH release and cell apoptosis in the cardiomyocytes
(Fig. 2B and C), but caused a
significant reduction in hypoxic tolerance of cardiomyocytes,
characterized by higher levels of LDH released (Fig. 2B) and increased cellular late
apoptotic (Q2) and early apoptotic (Q3) test by flow
cytometry(Fig. 2C).
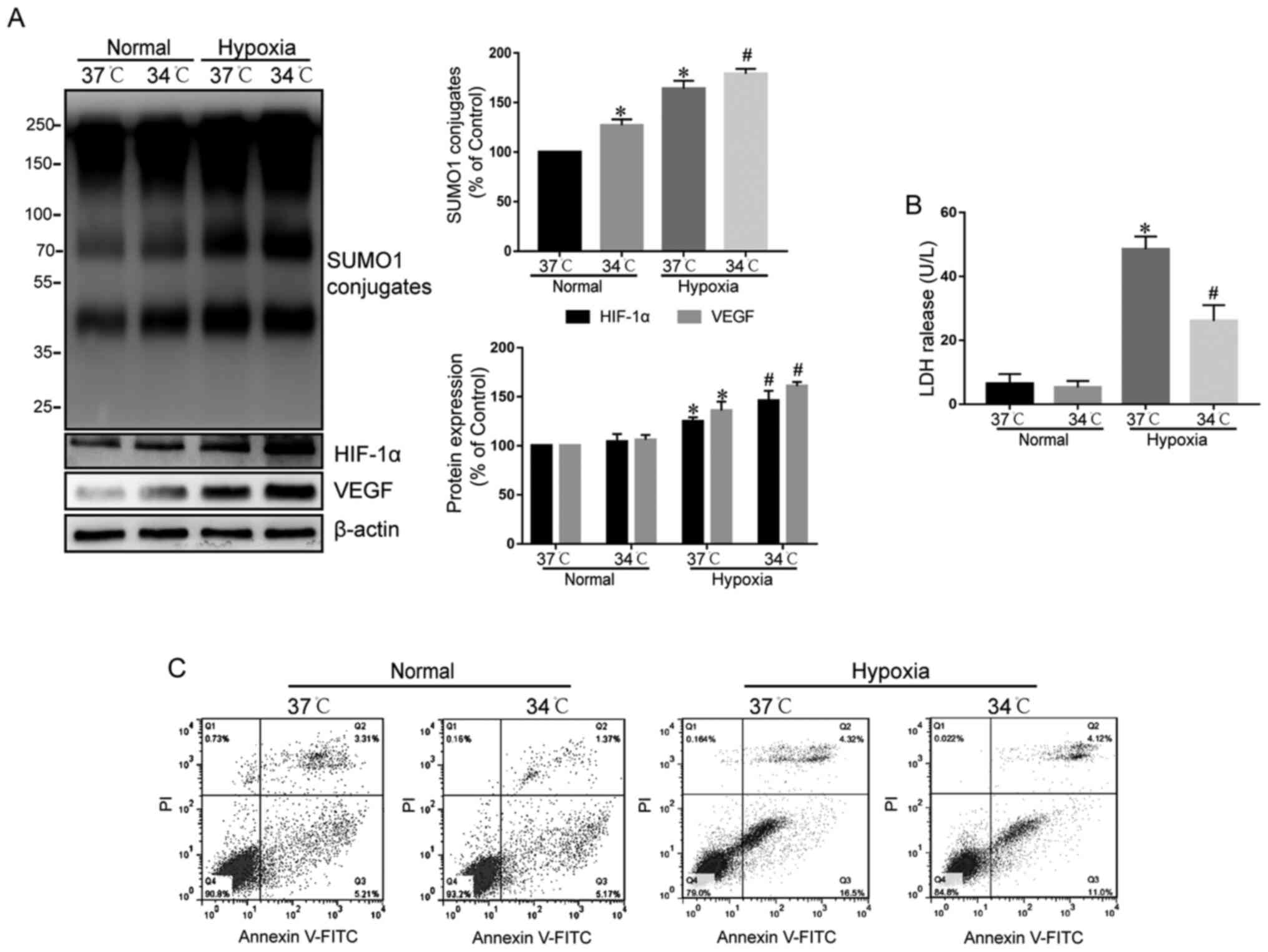
Therapeutic hypothermia further
increases protein SUMOylation, stabilizes the HIF-1α/VEGF pathway,
and enhances hypoxic tolerance of cardiomyocytes
Next, the effects of therapeutic hypothermia were
examined on protein SUMOylation in normoxic and hypoxic
cardiomyocytes (Fig. 3A).
Therapeutic hypothermia significantly increased the frequency of
SUMO1 conjugates in normoxic cardiomyocytes, and also significantly
increased protein SUMOylation in hypoxic cardiomyocytes compared
with the 37°C control (Fig. 3A). By
contrast, therapeutic hypothermia had a minimal effect on HIF-1α
and VEGF protein levels in normoxic cardiomyocytes, but
significantly increased HIF-1α and VEGF protein levels in hypoxic
cardiomyocytes (Fig. 3A).
Next, the effect of therapeutic hypothermia on the
cytotoxicity to cardiomyocytes was examined under normoxic and
hypoxic conditions. Therapeutic hypothermia did not affect LDH
release or cardiomyocyte apoptosis under normoxia (Fig. 3B and C). However, under hypoxic
conditions, therapeutic hypothermia significantly reduced LDH
release and the percentage of apoptotic cardiomyocytes (Fig. 3B and C). These data suggested that
therapeutic hypothermia may increase the protein levels of HIF-1α
by increasing protein SUMOylation, resulting in protein levels of
the downstream VEGF, thereby antagonizing hypoxia-induced
cardiomyocyte injury.
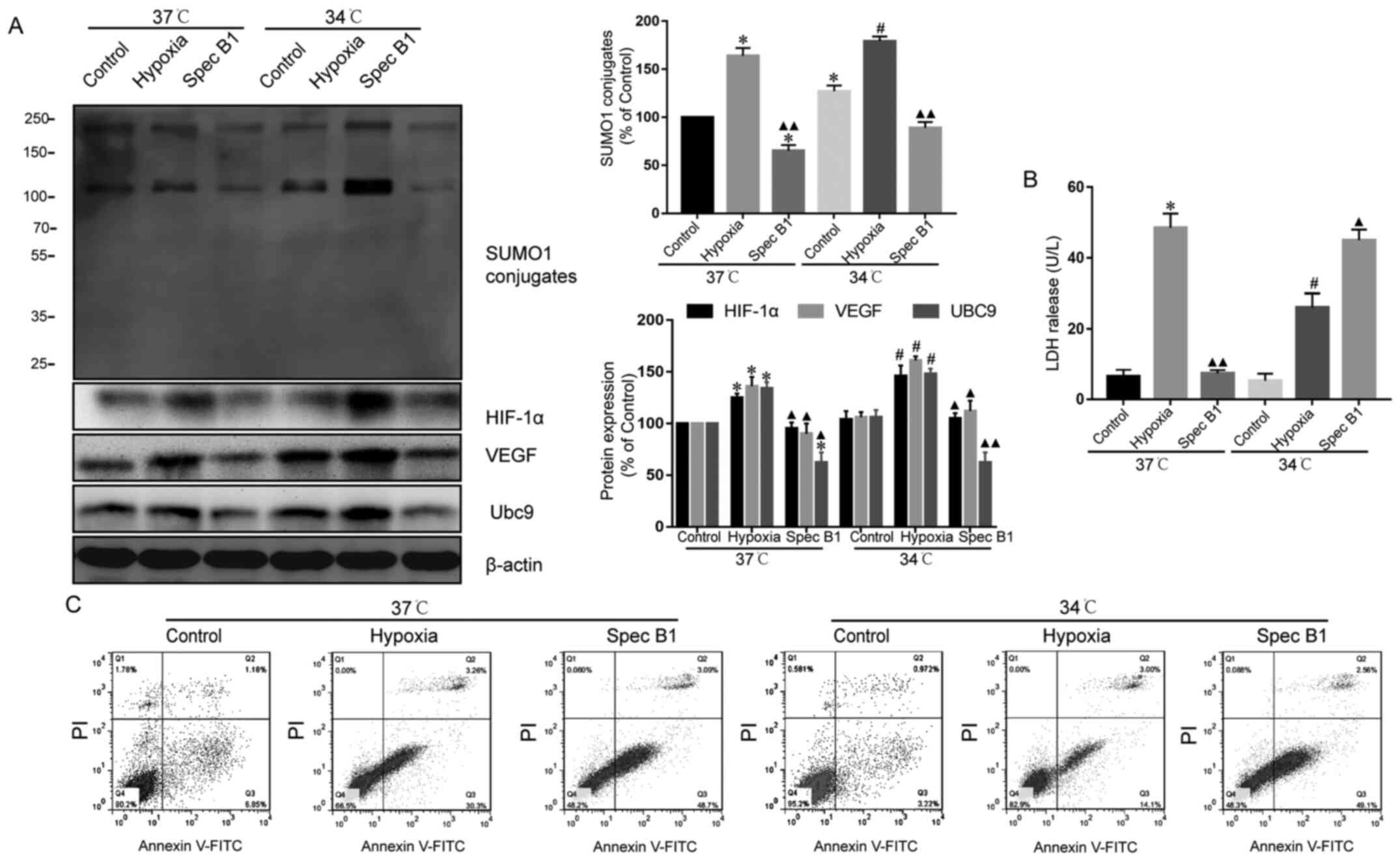
Inhibition of SUMOylation can reduce
the protective effect of therapeutic hypothermia on hypoxic
cardiomyocytes
To assess whether the protective action of
hypothermia on hypoxic cardiomyocytes was SUMOylation-dependent,
cardiomyocytes were treated with spectomycin B1. Spectomycin B1
significantly reduced the protein levels of Ubc9 and conjugated
SUMO1 under both normoxic and hypoxic conditions. By contrast,
therapeutic hypothermia had no effect on the myocardial cells after
spectomycin B1 treatment, compared with untreated cells under the
same conditions (Fig. 4A).
Correspondingly, when cardiomyocytes were treated with spectomycin
B1, therapeutic hypothermia did not increase the protein levels of
HIF-1α and VEGF (Fig. 4A) and
reduce the protective effect of hyothermia (Fig. 4B and C). These data suggested that
the percentage of apoptotic cardiomyocytes under hypoxia was
increased following treatment with the Ubc9 inhibitor.
Nevertheless, as there are an abundant number of SUMO-conjugated
proteins involved in various cellular processes, treatment with a
Ubc9 inhibitor will block all modification by SUMO1 of HIF-1α as
well as other proteins involved in cellular apoptosis, which could
also be modified by SUMO2/3. Consequently, the present findings
(Figs. 2C and 4C) may also involve de-SUMOylation of
other proteins due to the inhibition of Ubc9.
Therapeutic hypothermia can reduce the
area of MI after ischemia-reperfusion, while inhibition of the SUMO
pathway can offset this protective effect
Finally, a rat model of myocardial
ischemia-reperfusion was used to test whether the protective effect
of therapeutic hypothermia on ischemic myocardium was
SUMO-dependent. As expected, therapeutic hypothermia reduced
myocardial infarct size in rats exposed to myocardial ischemia and
reperfusion (Fig. 5A). Furthermore,
treatment with spectomycin B1 to inhibit SUMO conjugation further
increased MI in ischemia-reperfusion rats (Fig. 5A). In addition, when therapeutic
hypothermia was administered to rats undergoing myocardial
ischemia-reperfusion pretreated with spectomycin B1, the protective
effect of therapeutic hypothermia was significantly reduced and was
characterized by a large myocardial infarct area (Fig. 5A), decreased mitochondrial membrane
potential (Fig. 5B), increased
cleaved-caspase 3/caspase 3 ratio and a decreased Bcl2/Bax ratio
(Fig. 5C).
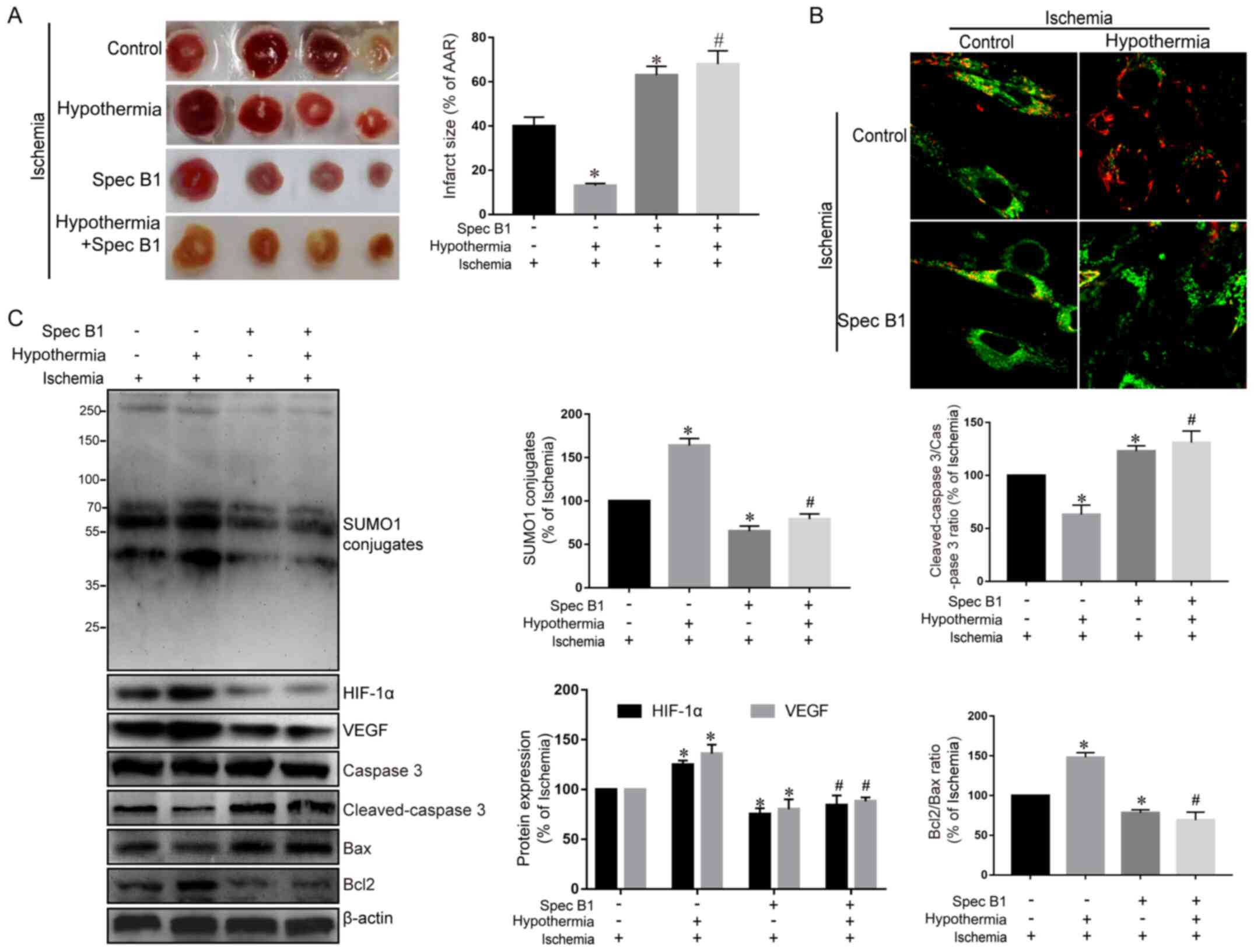 | Figure 5.Therapeutic hypothermia reduces the
area of myocardial infarction after ischemia-reperfusion, whereas
inhibition of the SUMO pathway can offset this protective effect.
(A) Representative images and analysis of myocardial infarction in
rats that underwent myocardial ischemia-reperfusion in the
different treatment groups. (B) Confocal imaging of mitochondrial
membrane potential. Magnification, ×400. (C) SUMO1, HIF-1α, VEGF,
cleaved-caspase 3, caspase 3, Bcl2 and Bax protein expression
levels were examined using western blotting and were
semi-quantified. Data are presented as the mean ± SD (n=3).
*P<0.05 vs. control group. #P<0.05 vs. hypoxia
group. SUMO, small ubiquitin-related modifier; HIF, hypoxia
inducible factor; VEGF, vascular endothelial growth factor; Spec
B1, spectomycin B1. |
Discussion
Ischemia-reperfusion injury is a common
pathophysiological phenomenon following treatment of ischemic heart
disease, including coronary artery bypass grafting after acute MI,
thrombolysis after acute MI and open-heart surgery under
extracorporeal circulation (21–23).
The stress response of cardiomyocytes to hypoxia is a complex and
delicately regulated process, involving a series of biological
response reactions that alter the proteome and genome, activate
angiogenesis, anaerobic metabolism and other signaling pathways.
The stress response is also known to regulate the cell cycle, cell
differentiation, apoptosis and necrosis (24,25).
Thus, it is important to reduce and prevent myocardial
ischemia-reperfusion injury.
Therapeutic hypothermia involves reducing the core
body temperature to within a suitable range. Adult patients with
cardiac arrest outside the hospital can be given low temperature
therapy (32–35°C) for 12–24 h, which has a protective effect
against ischemia-reperfusion injury (26). Therapeutic hypothermia has also been
reported to reduce reperfusion injury in animal models of acute MI
and improve ventricular remodeling after MI (27). However, most randomized controlled
clinical trials have revealed that therapeutic hypothermia does not
benefit patients with acute MI, but can benefit patients with
large-scale MI and rapid temperature targeting before reperfusion
(28). Nevertheless, the exact
molecular and cellular mechanisms of therapeutic hypothermia remain
to be fully elucidated, and are important for its clinical
application.
The balance of post-translational modification of
proteins involving ubiquitin and SUMO is essential for eukaryotic
cells to respond to hypoxic stress (29,30).
Unlike the degradation of target proteins caused by ubiquitination,
SUMO1, which is present in all eukaryotic cells, can regulate
protein-protein interactions, transcriptional activity, enhance
substrate stability and affect the target protein subcellular
localization (31,32). Numerous SUMO1 substrates, including
HIF-1α, IĸBα, poly(ADP-ribose) polymerase 1, p53, Mdm2, c-jun,
Glut-1 and Glut-4, have important roles in the oxygen response
(33). Furthermore, hypothermia has
been shown to significantly increase the expression of SUMO1 in
cells, thereby protecting downstream target proteins from enzymatic
hydrolysis (34).
In the present study, protein SUMO1 modification was
examined to determine if SUMO1 was involved in the protective
action of therapeutic hypothermia on myocardial injury induced by
ischemia-reperfusion. Using an in vitro oxygen glucose
deprivation model and an in vivo model of myocardial
ischemia-reperfusion in rats, it was verified that protein
SUMOylation was essential for hypoxic tolerance and the therapeutic
hypothermia-mediated cytoprotection of cardiomyocytes.
Specifically, blockade of SUMO conjugation by spectomycin B1 was
associated with a reduction in the protective effect of therapeutic
hypothermia on cardiomyocytes. These data confirmed that
therapeutic hypothermia-mediated myocardial protection was
dependent on protein SUMOylation. Thus, the protein SUMOylation
pathway involving SUMO1 should be considered when treating
myocardial ischemia-reperfusion using therapeutic hypothermia
because any drug or clinical intervention that increases or
inhibits protein SUMOylation may affect the protective action of
therapeutic hypothermia on the ischemic myocardium or be associated
with additional complications.
Acknowledgements
Not applicable.
Funding
This study was supported by grant from Tianjin
Natural Science Foundation of China (grant nos. 18JCQNJC12800 and
19JCZDJC35200), Tianjin Special Project of New Generation
Artificial Intelligence Technology (grant no. 18ZXZNSY00260), and
Binhai Health and Family Planning Commission Science and Technology
Projects (grant no. 2019BWKQ030).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JFF and XZL designed the experiments. HQL, XYB, MLX,
XFM, CYZ and JJJ performed the experiments, and collected and
analyzed data. HQL and XYB drafted the manuscript. All authors
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. All authors
read and approved of the final version to be published.
Ethics approval and consent to
participate
Animal experiments were performed according to the
regulations and guidelines approved by the Animal ethics Committee
of The Fifth Central Hospital of Tianjin (Tianjin, China).
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Bigelow WG, Lindsay WK and Greenwood WF:
Hypothermia; its possible role in cardiac surgery: An investigation
of factors governing survival in dogs at low body temperatures. Ann
Surg. 132:849–866. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jennings RB: Historical perspective on the
pathology of myocardial ischemia/reperfusion injury. Circ Res.
113:428–438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Otterspoor LC, van Nunen LX, van't Veer M,
Johnson NP and Pijls NHJ: Intracoronary hypothermia before
reperfusion to reduce reperfusion injury in acute myocardial
infarction: A novel hypothesis and technique. Ther Hypothermia Temp
Manag. 7:199–205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han Y, Rajah GB, Hussain M and Geng X:
Clinical potential of pre-reperfusion hypothermia in ischemic
injury. Neurol Res. 41:697–703. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paquot F, Huart J, Defraigne JO,
Krzesinski JM and Jouret F: Implications of the calcium-sensing
receptor in ischemia/reperfusion. Acta Cardiol. 72:125–131. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Witcher R, Dzierba AL, Kim C, Smithburger
PL and Kane-Gill SL: Adverse drug reactions in therapeutic
hypothermia after cardiac arrest. Ther Adv Drug Saf. 8:101–111.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao X: SUMO-mediated regulation of
nuclear functions and signaling processes. Mol Cell. 71:409–418.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosik J, Szostak B, Machaj F and Pawlik A:
Potential targets of gene therapy in the treatment of heart
failure. Expert Opin Ther Targets. 22:811–816. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Liang X, Liang H and Wang B:
SENP1/HIF-1α feedback loop modulates hypoxia-induced cell
proliferation, invasion, and EMT in human osteosarcoma cells. J
Cell Biochem. 119:1819–1826. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chachami G, Stankovic-Valentin N,
Karagiota A, Basagianni A, Plessmann U, Urlaub H, Melchior F and
Simos G: Hypoxia-induced changes in SUMO conjugation affect
transcriptional regulation under low oxygen. Mol Cell Proteomics.
18:1197–1209. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomasi ML and Ramani K: SUMOylation and
phosphorylation cross-talk in hepatocellular carcinoma. Transl
Gastroenterol Hepatol. 3:202018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou HJ, Xu Z, Wang Z, Zhang H, Zhuang ZW,
Simons M and Min W: SUMOylation of VEGFR2 regulates its
intracellular trafficking and pathological angiogenesis. Nat
Commun. 9:33032018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sallais J, Alahari S, Tagliaferro A,
Bhattacharjee J, Post M and Caniggia I: Factor inhibiting HIF1-A
novel target of SUMOylation in the human placenta. Oncotarget.
8:114002–114018. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ciria M, García NA, Ontoria-Oviedo I,
González-King H, Carrero R, De La Pompa JL, Montero JA and
Sepúlveda P: Mesenchymal stem cell migration and proliferation are
mediated by hypoxia-inducible factor-1α upstream of notch and SUMO
pathways. Stem Cells Dev. 26:973–985. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han X, Wang XL, Li Q, Dong XX, Zhang JS
and Yan QC: HIF-1α SUMOylation affects the stability and
transcriptional activity of HIF-1α in human lens epithelial cells.
Graefes Arch Clin Exp Ophthalmol. 253:1279–1290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui CP, Wong CC, Kai AK, Ho DW, Lau EY,
Tsui YM, Chan LK, Cheung TT, Chok KS, Chan ACY, et al: SENP1
promotes hypoxia-induced cancer stemness by HIF-1α deSUMOylation
and SENP1/HIF-1α positive feedback loop. Gut. 66:2149–2159. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Ren W, Jiang Z, Su Z, Ma X, Li Y,
Jiang R, Zhang J and Yang X: Hypothermia inhibits the proliferation
of bone marrow-derived mesenchymal stem cells and increases
tolerance to hypoxia by enhancing SUMOylation. Int J Mol Med.
40:1631–1638. 2017.PubMed/NCBI
|
|
18
|
Li G, Liu X, Su Z and Zhang D: Hypothermia
exerts early neuroprotective effects involving protein conjugation
of SUMO 2/3 in a rat model of middle cerebral artery occlusion. Mol
Med Rep. 16:3217–3223. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hirohama M, Kumar A, Fukuda I, Matsuoka S,
Igarashi Y, Saitoh H, Takagi M, Shin-ya K, Honda K, Kondoh Y, et
al: Spectomycin B1 as a novel SUMOylation inhibitor that directly
binds to SUMO E2. ACS Chem Biol. 8:2635–2642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bae SH, Jeong JW, Park JA, Kim SH, Bae MK,
Choi SJ and Kim KW: Sumoylation increases HIF-1alpha stability and
its transcriptional activity. Biochem Biophys Res Commun.
324:394–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li R, Wei J, Jiang C, Liu D, Deng L, Zhang
K and Wang P: Akt SUMOylation regulates cell proliferation and
tumorigenesis. Cancer Res. 73:5742–5753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Del Re DP, Amgalan D, Linkermann A, Liu Q
and Kitsis RN: Fundamental mechanisms of regulated cell death and
implications for heart disease. Physiol Rev. 99:1765–1817. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rossello X and Yellon DM:
Cardioprotection: The disconnect between bench and bedside.
Circulation. 134:574–575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heusch G and Gersh BJ: The pathophysiology
of acute myocardial infarction and strategies of protection beyond
reperfusion: A continual challenge. Eur Heart J. 38:774–784.
2017.PubMed/NCBI
|
|
25
|
Aggarwal S and Natarajan G: Biventricular
function on early echocardiograms in neonatal hypoxic-ischaemic
encephalopathy. Acta Paediatr. 106:1085–1090. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spath NB, Mills NL and Cruden NL: Novel
cardioprotective and regenerative therapies in acute myocardial
infarction: A review of recent and ongoing clinical trials. Future
Cardiol. 12:655–672. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nosaka N, Okada A and Tsukahara H: Effects
of therapeutic hypothermia for neuroprotection from the viewpoint
of redox regulation. Acta Med Okayama. 71:1–9. 2017.PubMed/NCBI
|
|
28
|
Kloner RA, Hale SL, Dai W and Shi J:
Cardioprotection: Where to from here? Cardiovasc Drugs Ther.
31:53–61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Wang Y and Lu L: De-SUMOylation of
CCCTC binding factor (CTCF) in hypoxic stress-induced human corneal
epithelial cells. J Biol Chem. 287:12469–12479. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Z, Siraj S, Liu L and Chen Q:
MARCH5-FUNDC1 axis fine-tunes hypoxia-induced-mitophagy. Autophagy.
13:1244–1245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee YJ, Bernstock JD, Nagaraja N, Ko B and
Hallenbeck JM: Global SUMOylation facilitates the multimodal
neuroprotection afforded by quercetin against the deleterious
effects of oxygen/glucose deprivation and the restoration of
oxygen/glucose. J Neurochem. 138:101–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou F, Dai A, Jiang Y, Tan X and Zhang X:
SENP 1 enhances hypoxia induced proliferation of rat pulmonary
artery smooth muscle cells by regulating hypoxia inducible factor
1α. Mol Med Rep. 13:3482–3490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang F, Cai F, Shi R, Wei JN and Wu XT:
Hypoxia regulates sumoylation pathways in intervertebral disc
cells: Implications for hypoxic adaptations. Osteoarthritis
Cartilage. 24:1113–1124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee YJ, Mou Y, Klimanis D, Bernstock JD
and Hallenbeck JM: Global SUMOylation is a molecular mechanism
underlying hypothermia-induced ischemic tolerance. Front Cell
Neurosci. 8:4162014. View Article : Google Scholar : PubMed/NCBI
|