Introduction
The majority of gingival tumors are highly
differentiated squamous cell carcinomas, including gingival
squamous cell carcinoma (GSCC) (1).
GSCC occurs in either the maxilla or the mandible (2,3). GSCC
typically resembles a common periodontal lesion; therefore, the
early presentation of GSCC is occasionally misdiagnosed as a benign
inflammatory condition or oral allergy syndrome (4). In addition, GSCC is associated with a
high risk of metastasis and bone invasion with delayed diagnosis
(5,6). In the early stages, GSCC is able to
infiltrate into mandibular alveoli, leading to bone destruction,
tooth mobility and pain (1).
Chemotherapy is the main therapeutic strategy used to treat
malignancies; for example, cisplatin, a DNA-damaging agent, has
potent antitumor properties and is commonly used to treat various
cancer types, including lung cancer (7), ovarian cancer (8), and head and neck carcinomas,
particularly oral squamous cell carcinoma (9). Although cisplatin inhibits GSCC
growth, it leads to severe adverse effects (10) and can lead to drug resistance
(11). Therefore, more effective
strategies with less toxic effects are required. As an alternative
approach, malignancies may be more sensitive to antitumor agents
obtained from natural products than traditional chemotherapy
(12–15).
It has been >20 years since the identification of
P21 as a P53-regulated cell-cycle inhibitor (16). The tumor suppressor P53 induces the
expression of P21 to mediate G1 arrest and cell
apoptosis in tumors (17). P21
expression has been observed in tumors, and it has been widely
recognized that P21 acts to restrain proliferation and tumor growth
(18), and P21 is uniquely involved
in maintaining G1 cell-cycle arrest. Under unfavorable
conditions, such as radiation, DNA-damaging chemotherapy or
nutrient deprivation, the P53/P21 G1 checkpoint is
triggered and causes growth arrest until the damage is repaired or
nutrients become available (18).
However, it has been reported that P21 might have controversial
roles, and it has been indicated that P21 may act as an
anti-apoptotic agent (19).
Lapiferin is purified from the roots of
Ferulalapidosa and has been identified as a complex ester of
sesquiterpene alcohols. Although the configuration and structure of
lapiferin has been revealed through chemical transformations and
analysis of spectral characteristics (20), its role in gingival tumors remains
to be elucidated.
In view of the antitumor potential of P21, the
present study used P21-based screening of natural compounds to
detect compounds that could promote the expression of P21 and
confer antitumor effects. All compounds from a commercially
available compound library source were examined. The natural
compounds that exhibited the highest capacity to promote P21
transcription were selected and subjected to further evaluation of
antitumor properties. The present study identified lapiferin as a
potent antitumor natural compound that exerted its effects via
positively regulating P21 expression. To the best of our knowledge,
this study is the first to identify an effective natural compound
that targets GSCC, based on broad-spectrum drug screening.
Materials and methods
Cell culture
YD-38 human GSCC cells were purchased from the Cell
Bank of Chinese Academy of Sciences (Shanghai, China), and were
cultured according to the manufacturer's protocol. Cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.). The HGF-1 cell line
(CRL-2014) was purchased from American Type Culture Collection
(ATCC) and was also cultured in DMEM supplemented with 10% FBS. The
culture media were replaced every 2 days, unless otherwise
specified. Cells were incubated at 37°C in a humidified environment
containing 5% CO2.
Plasmid and small interfering (si)RNA
transfection
The P21 luciferase reporter plasmid was constructed,
according to the following procedures. The pGL3-basic vector
(Promega Corporation) was digested using KpnI and
HindIII restriction enzymes. Primers (forward,
5′-GACTCTGTGATCAATTTCTT-3′ and reverse, 5′-TTGTATCTCTGCAGGCG-3′)
were used to clone the P21 promoter from THLE-2 normal liver cells
(ATCC), to which the KpnI and HindIII restriction
enzymes were added (21). After
PCR, double digestion and DNA gel (1% agarose) extraction (22), the fragments were ligated together.
Sequencing was used to ensure that the P21 luciferase reporter
plasmid was successfully constructed. siRNAs were designed and
synthesized by Shanghai GenePharma Co., Ltd. and were transfected
into cells at a concentration of 10 µM. The sequences were as
follows: siNC, sense 5′-ACUUACGAGUGACAGUAGA-3′, antisense
5′-UCUACUGUCACUCGUAAGU-3′; and siP21, sense
5′-CCAACUCAUUCUCCAAGUA-3′ and antisense 5′-UACUUGGAGAAUGAGUUGG-3′.
Briefly, YD-38 cells were seeded into 96-well plates at a density
of 2,000 cells/well and transfection was performed using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. A
total of 48 h post-transfection, subsequent experiments were
conducted.
Luciferase reporter assay
Luciferase reporter assays were performed using the
Dual-Luciferase® Reporter assay system (cat. no. E1910;
Promega Corporation) according to the manufacturer's protocol.
Briefly, YD-38 cells seeded into a 24-well plate at a density of
30,000 cells/well were transfected by Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.) with P21
luciferase plasmid (1 µg/well) and Renilla vector (100
ng/well) and were treated with various natural products from the
compound library at the Chinese Academy of Sciences (working
concentration, 10 mg/ml for each compound) for 24 h at 37°C. Cells
were then lysed with lysis buffer (Beyotime Institute of
Biotechnology) for 20 min at room temperature with agitation (150
rpm). Subsequently, the lysate was incubated with luciferase assay
reagent II and the absorbance of all wavelengths was immediately
analyzed. Finally, the Stop & Glo reagent was added to the
cells and absorbance was measured again (Tecan). Luciferase
activities were determined by obtaining the ratio of the two
readings; luciferase activity was normalized to Renilla
luciferase activity.
Cell proliferation assay
YD-38 cells were seeded into 96-well plates (3,000
cells/well) and allowed to grow overnight. Cells transfected with
siP21 (10 µM) were subsequently treated with lapiferin (10 µg/ml),
followed by incubation in DMEM for a further 72 h. Cell
proliferation was determined for 2 consecutive days using the MTT
assay. Briefly, 2 mg/ml MTT solution was added to each well and the
cells were incubated for 4 h at 37°C. Subsequently, media were
removed and 200 µl dimethyl sulfoxide was added. The plate was
agitated for 5 min and the optical density was subsequently
determined at 490 nm using a spectrophotometer.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cultured GSCC cells
using the RNA kit (Qiagen, Inc.) according to the manufacturer's
protocol. Total RNA was reverse transcribed into cDNA using
QuantiTect Reverse Transcription kit (Takara Bio, Inc.), according
to the manufacturer's protocol. Primer sequences were as follow:
p21, forward 5′-GAAAAGGAGAACACGGGATG-3′, reverse
5′-AAAGTCACTAAGAATCATTTATTG-3′; and β-actin, forward
5′-CGGAGTCAACGGATTTGGTC-3′ and reverse 5′-AGCCTTCTCCATGGTCGTGA-3′.
RT-qPCR was performed using a TaqMan miRNA RT-qPCR assay (Takara
Bio, Inc.) equipped with ABI-Prism 7300 system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). RT-qPCR thermocycling conditions
were performed as follows: Denaturation at 95°C for 30 sec,
followed by 45 cycles at 95°C for 3 sec and 60°C for 30 sec.
Expression was quantified using the 2−ΔΔCq method
(23).
Cell apoptosis detection
The Annexin V/propidium iodide (PI) assay was
performed according to the manufacturer's protocol (Invitrogen;
Thermo Fisher Scientific, Inc.). Briefly, YD-38 cells
(1×105/well) were plated into 6-well plates and treated
with lapiferin (10 µg/ml) in the presence or absence of siP21 for
48 h. Subsequently, cells were washed with pre-chilled PBS,
trypsinized and resuspended in 100 µl binding buffer containing 2.5
µl FITC-conjugated Annexin V and 1 µl PI (100 µg/ml). Cells were
then incubated at room temperature for 15 min in the dark. Finally,
≥10,000 cells were collected and analyzed by flow cytometry
(FACSCanto II; BD Biosciences) with FlowJo VX software (FlowJo,
LLC). Cell apoptotic rates were assessed as follows: (Apoptotic
cells in the control group-apoptotic cells in the lapiferin
group)/apoptotic cells in the control group × 100.
Western blot analysis
Total proteins were extracted from cultured YD-38
cells. Briefly, cells were grown until they reached 95% confluence;
after washing with PBS, cells were lysed using RIPA lysis buffer
(Beyotime Institute of Biotechnology) to obtain the total protein
lysate. Equal amounts of protein (30 µg/lane), the concentration of
which was determined by the BCA method (Thermo Fisher Scientific,
Inc.), were then subjected to 12 or 15% SDS-PAGE. Subsequently, the
proteins were transferred to a PVDF membrane (0.22 µm; EMD
Millipore,) and blocked with 5% milk dissolved in TBS-Tween (0.1%)
for 1 h at room temperature. GAPDH was detected as a loading
control. Immunoreactivity was determined using enhanced
chemiluminescence autoradiography (Thermo Fisher Scientific, Inc.).
The membrane was incubated with primary antibodies overnight at 4°C
and with secondary antibodies at room temperature for 1 h. The
following antibodies were used for western blotting: P21 (cat. no.
ab109520; 1:1,000; Abcam), proliferating cell nuclear antigen
(PCNA; cat. no. 10205-2-AP; 1:1,000; ProteinTech Group, Inc.), Ki67
(cat. no. ab15580; 1:1,000; Abcam), cell division cycle 25C (Cdc
25C; cat. no. ab32444; 1:1,000; Abcam), cyclin B1 (cat. no. ab72;
1:1,000; Abcam), cleaved (cl)-caspase-3 (cat. no. 9664; 1:1,000;
Cell Signaling Technology, Inc.), cl-caspase-9 (cat. no. 20750;
1:1,000; Cell Signaling Technology, Inc.), Bax (cat. no. ab32503;
1:1,000; Abcam), cytochrome c (cyto. C; cat. no. ab13575;
1:1,000; Abcam), cl-poly(ADP-ribose) polymerase (PARP; cat. no.
ab32064; 1:1,000; Abcam), GAPDH (cat. no. sc-47724; 1:2,000; Santa
Cruz Biotechnology, Inc.), and horseradish peroxidase-conjugated
secondary antibodies (cat. nos. sc-2004 and sc-2005; 1:5,000; Santa
Cruz Biotechnology, Inc.). Each experiment was repeated at least
three times.
Statistical analysis
Data are expressed as the mean ± standard deviation.
One-way analysis of variance was used for comparisons among
multiple groups (≥3 groups), followed by a least significant
difference post hoc test. P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
at least three times, unless otherwise stated.
Results
Lapiferin promotes P21 luciferase
activity in a human GSCC cell line
To identify natural products that target P21,
natural compounds from the Chinese Academy of Sciences compound
library, which consists of 480 natural products, were purchased.
YD-38 cells were transfected with P21 luciferase plasmid and
Renilla control plasmid for 24 h in 96-well plates, after
which the natural products were added to each well and mixed
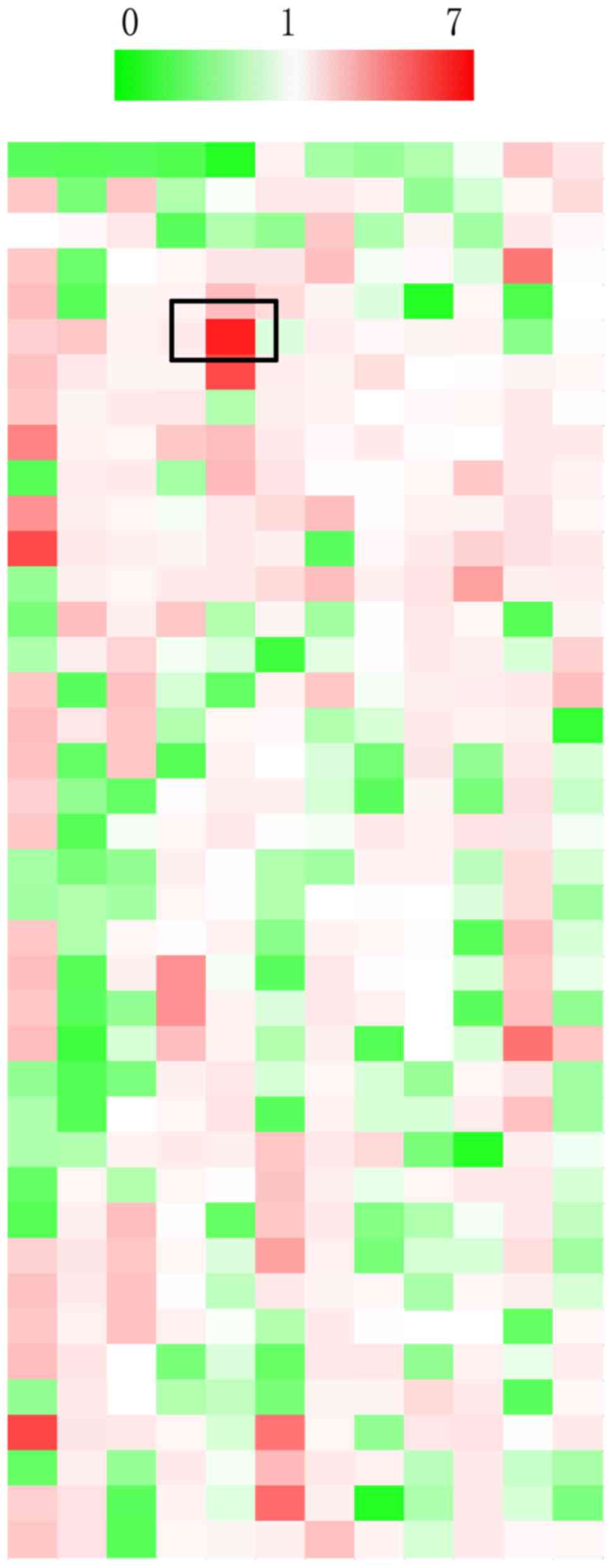
gently. As shown in Fig. 1, ~30% of
the natural products inhibited the luciferase activities of P21
(green) and 53% of the natural products promoted the luciferase
activities of P21in GSCC cells (red); the remaining compounds had
no effect on P21 transcriptional activities. Of all the effective
natural compounds, lapiferin increased P21 activity to the highest
level compared with in untreated cells (6.32-fold). Therefore, this
natural product was selected for subsequent analysis.
Lapiferin decreases cell proliferation
in a dose- and time-dependent manner in GSCC
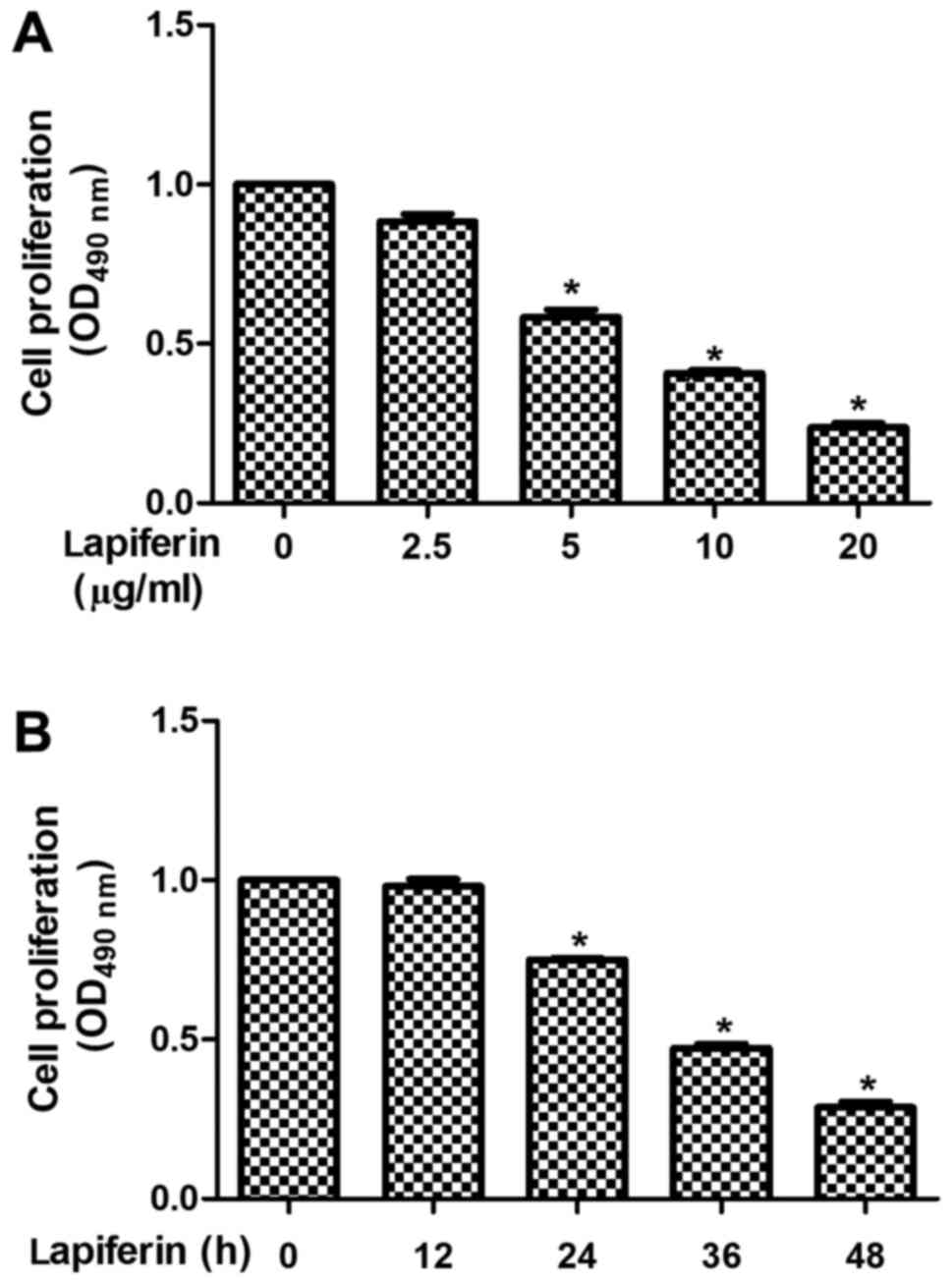
The present study examined the detailed effects of
lapiferin on human GSCC cell proliferation. As shown in Fig. 2A, when YD-38 cells were treated with
lapiferin, the proliferative rates remained stable in response to a
low dose of lapiferin (2.5 µg/ml), but gradually decreased as the
concentration of lapiferin increased (5, 10 and 20 µg/ml). In
addition, cell proliferation was determined at various time points.
Similarly, cell proliferation was stable in response to lapiferin
treatment for 12 h; however, the cell proliferative rates were
decreased to 75% when YD-38 cells were stimulated with lapiferin
for 24 h; the suppressive effects were further enhanced as the
treatment time was extended (Fig.
2B). These findings suggested that lapiferin decreased cell
proliferation in a dose- and time-dependent manner in human GSCC
cells.
Lapiferin increases cell apoptosis in
a dose- and time-dependent manner in GSCC
Increased cell proliferation and decreased cell
apoptosis are the two main manifestations of human malignancies.
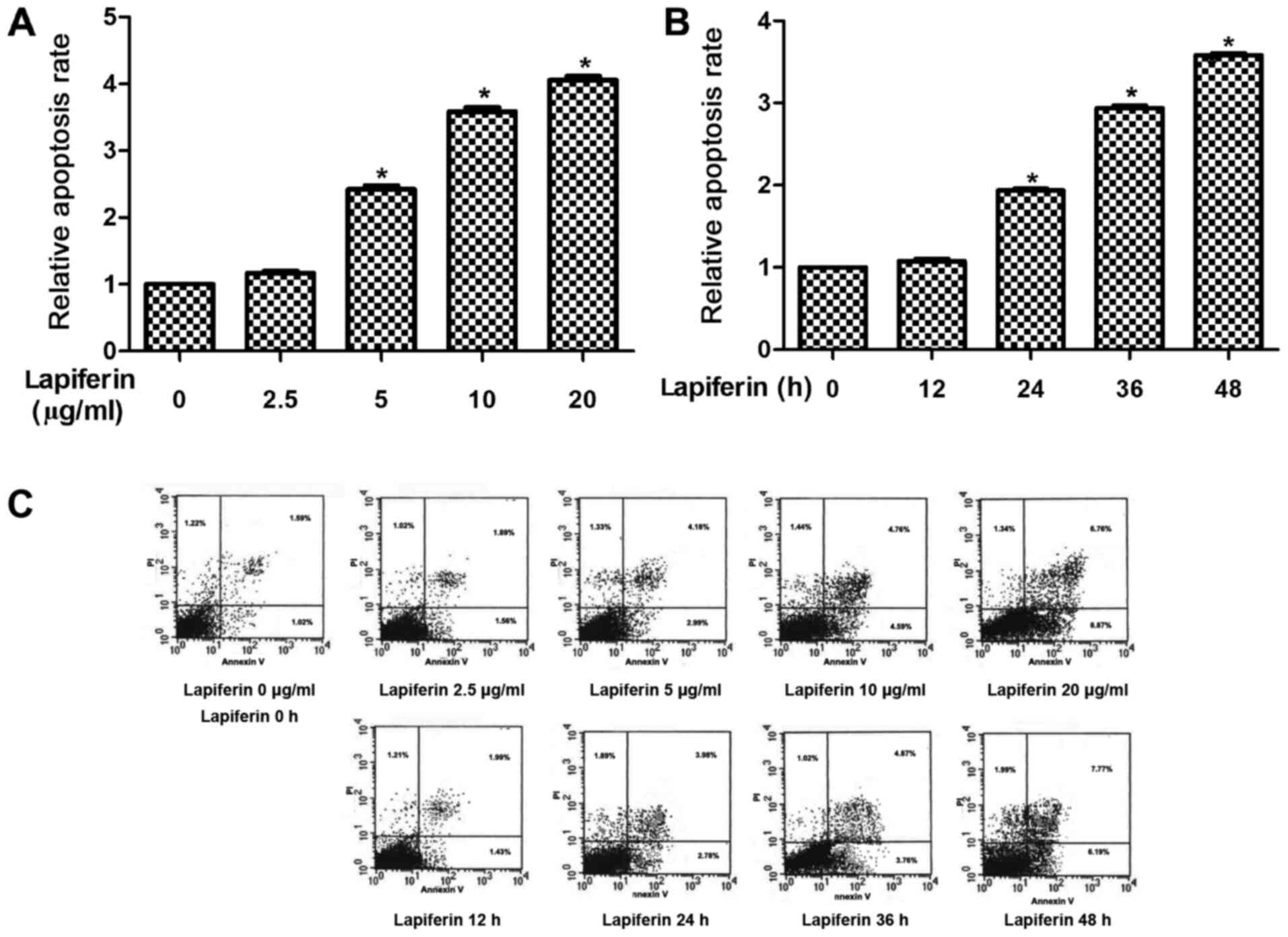
Therefore, the role of lapiferin in human GSCC cell apoptosis was
determined. The results revealed that when cells were treated with
lapiferin at a concentration of 5 µg/ml, the cell apoptotic rates
were significantly increased to 2.2-fold compared with in the
control YD-38 group (Fig. 3A).
Furthermore, cell apoptosis was assessed when cells were treated
with lapiferin for various durations. As shown in Fig. 3B, the cell apoptotic rates remained
stable at 12 h and were markedly increased when YD-38 cells were
treated with lapiferin for increased durations. Cell apoptotic
rates peaked (3.5-fold) when cells were treated with lapiferin for
48 h. These results suggested that lapiferin increased cell
apoptosis in a dose- and time-dependent manner in GSCC.
Lapiferin suppresses the protein
expression of cell proliferation regulators in YD-38 cells
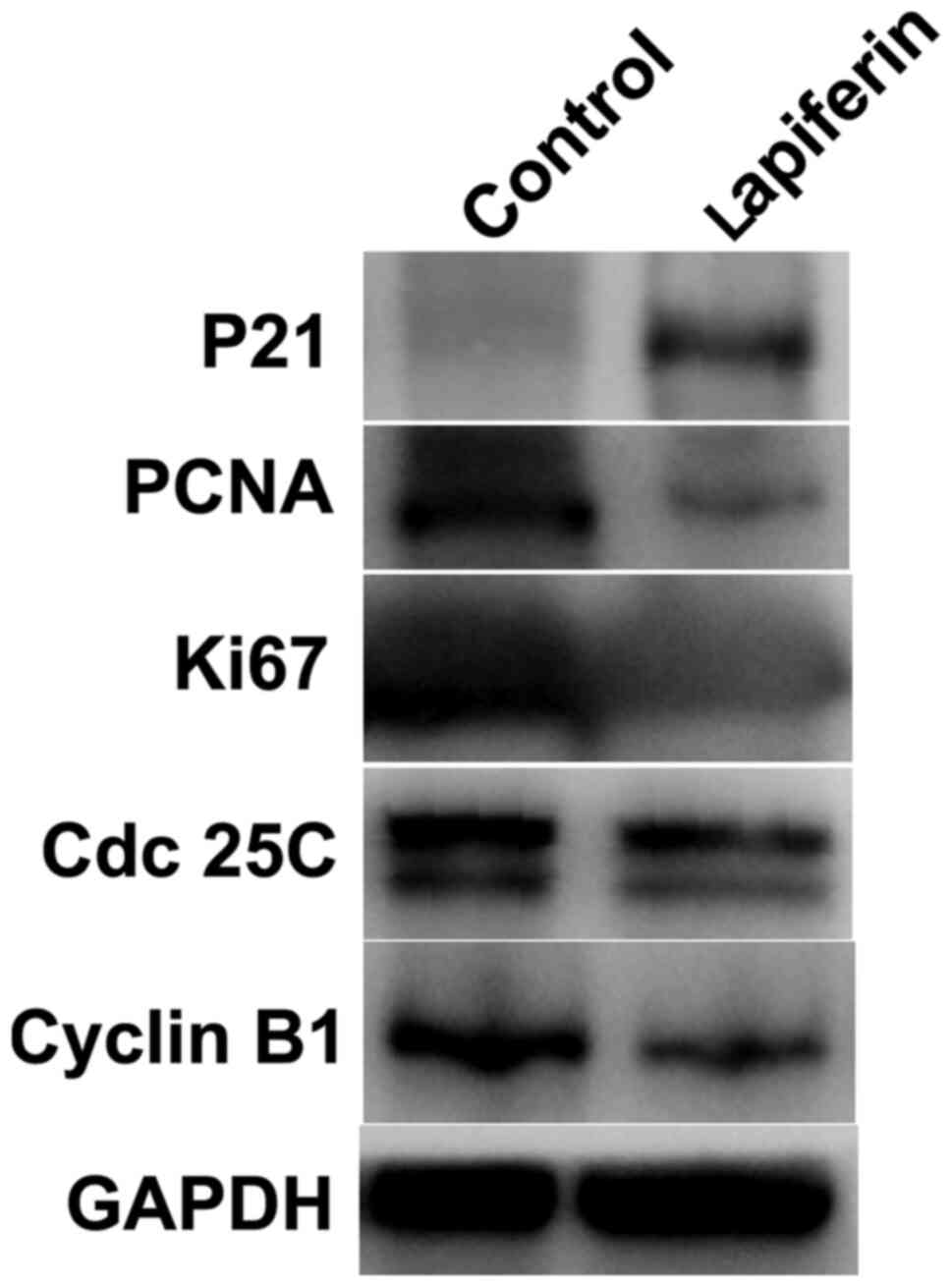
As shown in Fig. 1,
lapiferin increased the luciferase activity of P21. The present
study subsequently examined the effects of lapiferin by western
blotting. GAPDH was included as an internal control. As shown in
Fig. 4, when YD-38 cells were
treated with lapiferin for 24 h, the protein expression levels of
P21 were markedly increased, which was consistent with the findings
presented in Fig. 1. However, PCNA
and Ki67, two cell proliferation markers, and Cdc 25C and Cyclin
B1, key cell cycle regulators, were downregulated by lapiferin;
these findings are concordant with those presented in Fig. 2. These data further suggested that
treatment of cells with lapiferin decreased cell proliferation.
Lapiferin increases the protein
expression levels of pro-apoptotic factors in YD-38 cells
The expression levels of cell apoptosis-related
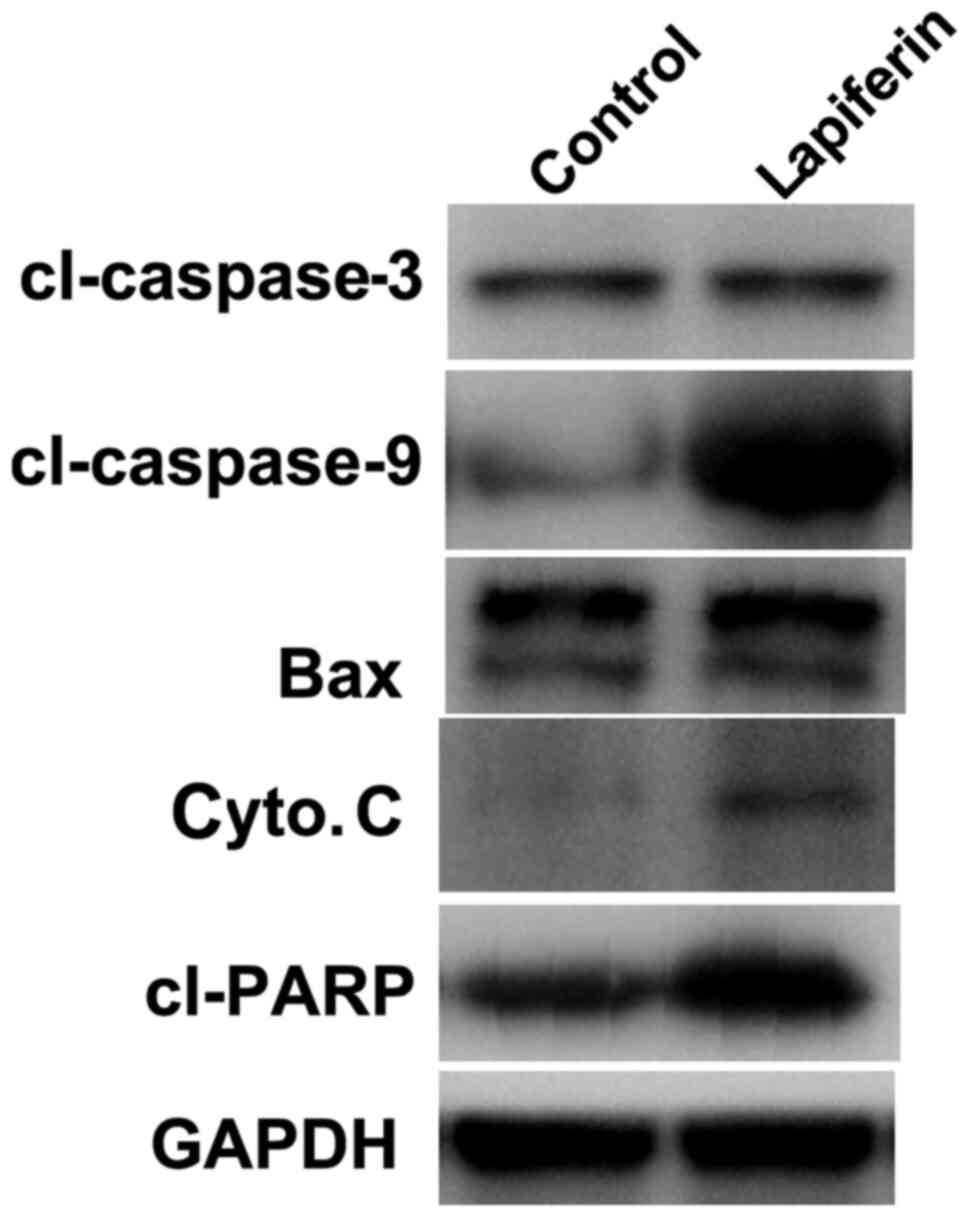
proteins were also examined by western blot analysis. As shown in
Fig. 5, when the human GSCC cell
line YD-38 was stimulated with lapiferin for 24 h at a
concentration of 10 µg/ml, the protein expression levels of
cl-caspase-3, cl-caspase-9, Bax, cyto. C and cl-PARP were markedly
increased; expression of the internal control GAPDH remained
stable. These data indicated that lapiferin increased the
expression of cell apoptosis-associated proteins in human GSCC.
Lapiferin decreases cell proliferation
and increases cell apoptosis through regulating P21 in GSCC
Furthermore, the detailed mechanism underlying the
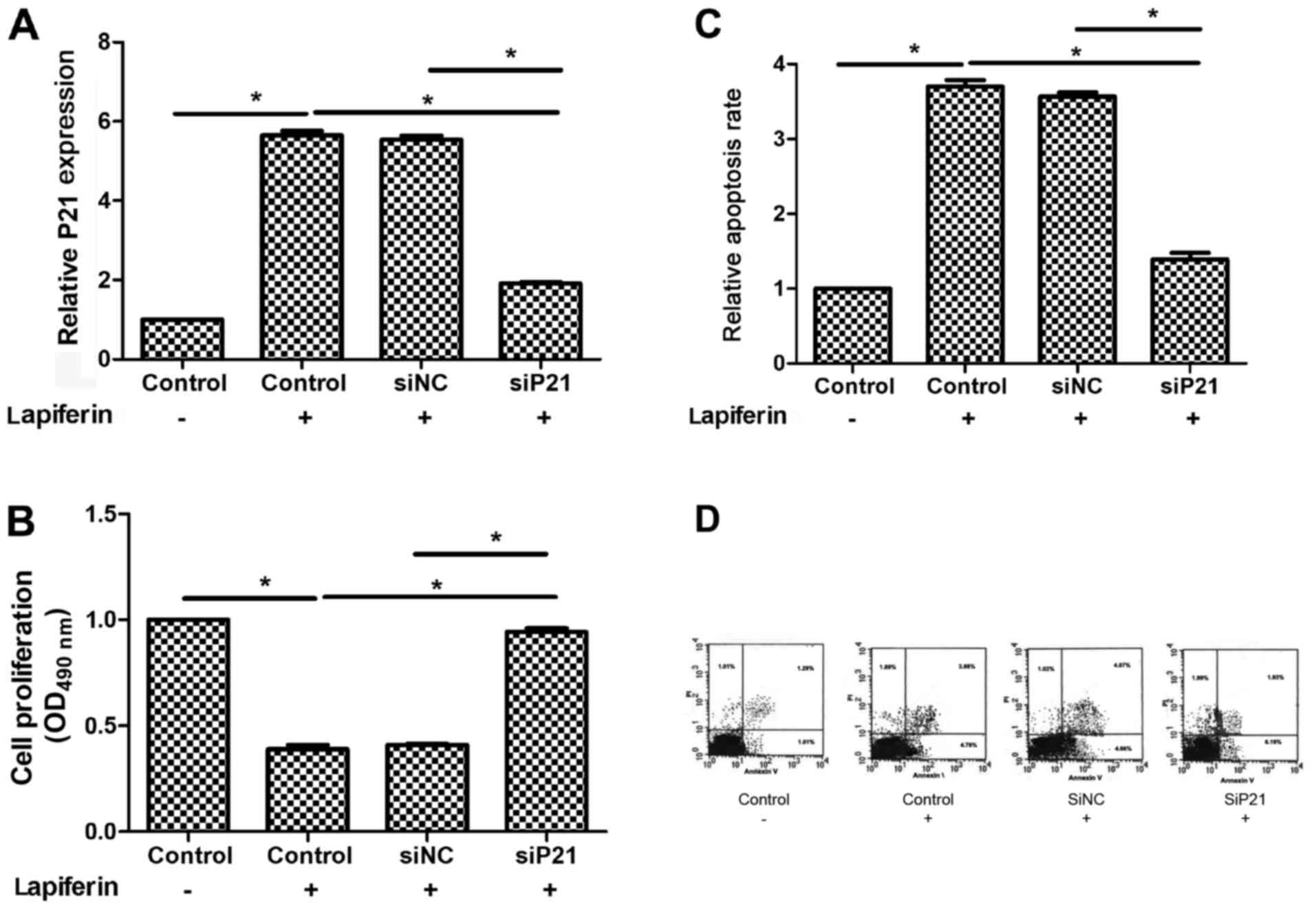
role of lapiferin in human GSCC was explored. Firstly, the
expression of P21 was knocked down in YD-38 cells using a specific
siRNA against P21. As shown in Fig.
6A, the basal expression levels of P21 were low and treatment
with lapiferin markedly increased P21; however, when cells were
transfected with siP21, the mRNA expression levels of P21 returned
to basal levels. Accordingly, cell proliferation was decreased
(Fig. 6B) and cell apoptosis was
increased in response to treatment with lapiferin for 24 h
(Fig. 6C). Notably, cell
proliferation and apoptosis returned to a level comparable to the
control when cells were transfected with siP21 (Fig. 6B and C). Taken together, these
results suggested that lapiferin decreased cell proliferation and
increased cell apoptosis through regulating P21 in human GSCC.
Discussion
Over 90% of cases of malignant neoplasms in the oral
cavity are squamous cell carcinoma, the characteristics of which
include rapid cell growth and progressive invasion and migration
(2,24). Squamous cell carcinoma is most
commonly located in the lower lip, tongue and floor of the mouth,
and GSCC accounts for ~10% of cases of intraoral carcinoma
(25).
Induction of P21 as a consequence of P53 activation
has been widely recognized as exerting a tumor suppressive effect
(18). P21 is currently accepted as
a potent universal cyclin-dependent kinase (CDK) inhibitor
(26), which physically interacts
with, and inhibits, the activity of cyclin-CDK2, -CDK1 and -CDK4/6
complexes, thus functioning as a regulator of cell cycle
progression during the G1 and S phases (27). Unraveling of the cell-cycle
checkpoints, in particular the P53-dependent P21-requiring
cell-cycle checkpoint after DNA damage has provided a rationale for
the development of tumor-specific therapeutic strategies (18).
The present study aimed to identify a potent natural
compound that targetsP21 based on the rationale that natural
compound-activated P21 expression may confer antitumor potential.
Using a compound library, it was demonstrated that lapiferin was
one of the most effective natural compounds that promoted the
expression of P21 at both transcriptional and protein levels.
Lapiferin is a complex ester of sesquiterpene alcohols with
aliphatic acid, which does not possess ionophoric properties
(28). Earlier studies have
purified lapiferin from the roots of F. lapidosa, and
established its structure and configuration based on chemical
transformations and analysis of spectral characteristics (29,30).
In addition, lapiferin extracted from F. communis and F.
arrigoniisardinian has been shown to exert anti-proliferative
activity against human colon cancer via interactions with type II
estrogen-binding sites (20).
Similarly, it has been reported that lapiferin derived from F.
vesceritensis induces the apoptosis pathway in MCF-7 breast
cancer cells (30). With the aid of
compound library-based drug screening, this study revealed that
lapiferin potently promoted P21expression. The proliferation of
YD-38 GSCC cells was inhibited in response to lapiferin in a
dose-dependent manner. Furthermore, the apoptosis of YD-38 GSCC
cells was promoted by lapiferin treatment in a dose-dependent
manner. Conversely, following knockdown of P21, lapiferin-mediated
suppression of proliferation was markedly inhibited, indicating the
P21-dependent mechanism of lapiferin-mediated antitumor activity.
Previous reports (20,30), together with the current study,
indicated that lapiferin may confer potent antitumor effects on
solid tumors.
Understanding the mechanism underlying the effects
of P53/P21 on tumor suppression has important implications for
patients and cancer therapy (18).
This study provided evidence to suggest that lapiferin may serve as
a potential anti-proliferative natural compound against GSCC.
Similar to previous reports (20,30),
this study revealed that lapiferin may regulate tumor cell
apoptosis and proliferation; however, whether lapiferin has further
functional roles in GSCC, including on cell migration and invasion,
remains to be elucidated. Since GSCC has a high propensity to
invade into adjacent tissues (31),
the roles of lapiferin in GSCC cell metastasis warrant further
investigation, and whether lapiferin-mediated protection from cell
metastasis relies on P21 activation remains to be elucidated.
In conclusion, the present study used a multi-drug
screening approach to identify lapiferin as a critical mediator of
P21 activation that may lead to suppression of cell proliferation
and induction of cell apoptosis in GSCC. To the best of our
knowledge, this study is the first to systemically investigate the
roles of lapiferin in GSCC. The findings indicated that lapiferin
may serve as a potent anti-proliferative agent against GSCC and may
have critical clinical implications.
Acknowledgements
The authors would like to thank Dr David White
(Johns Hopkins University) for revising this manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL performed most of the experiments. CP designed
the project and analyzed the data. YR and QZ revised the manuscript
and conducted some experiments. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gong Y, Yang H and Tian X: Elucidating the
mechanism of miRNA-214 in the regulation of gingival carcinoma. Exp
Ther Med. 13:2544–2550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Keshava A, Gugwad S, Baad R and Patel R:
Gingival squamous cell carcinoma mimicking as a desquamative
lesion. J Indian Soc Periodontol. 20:75–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okura M, Yanamoto S, Umeda M, Otsuru M,
Ota Y, Kurita H, Kamata T, Kirita T, Yamakawa N, Yamashita T, et
al: Prognostic and staging implications of mandibular canal
invasion in lower gingival squamous cell carcinoma. Cancer Med.
5:3378–3385. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hinchy NV, Jayaprakash V, Rigual N, Reid
M, Frustino JL, Rossitto R, Groman A and Sullivan MA: Progression
of gingival squamous cell carcinoma from early to late stage after
invasive dental procedure. Gen Dent. 64:38–43. 2016.PubMed/NCBI
|
|
5
|
Fitzpatrick SG, Neuman AN, Cohen DM and
Bhattacharyya I: Papillary variant of squamous cell carcinoma
arising on the gingiva: 61 cases reported from within a larger
series of gingival squamous cell carcinoma. Head Neck Pathol.
7:320–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi EJ, Zhang X, Kim HJ, Nam W and Cha
IH: Prognosis of gingival squamous cell carcinoma diagnosed after
invasive procedures. Asian Pac J Cancer Prev. 12:2649–2652.
2011.PubMed/NCBI
|
|
7
|
Zhang F, Duan S, Tsai Y, Keng PC and Chen
Y, Lee SO and Chen Y: Cisplatin treatment increases stemness
through upregulation of hypoxia-inducible factors by interleukin-6
in non-small cell lung cancer. Cancer Sci. 107:746–754. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao Y, Shan N, Zhao C, Wang Y, Xu F, Li J,
Yu X, Gao L and Yi Z: LY2109761 enhances cisplatin antitumor
activity in ovarian cancer cells. Int J Clin Exp Pathol.
8:4923–4932. 2015.PubMed/NCBI
|
|
9
|
Florea AM and Büsselberg D: Cisplatin as
an anti-tumor drug: Cellular mechanisms of activity, drug
resistance and induced side effects. Cancers (Basel). 3:1351–1371.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang C and Yu Y: Synergistic cytotoxicity
of β-elemene and cisplatin in gingival squamous cell carcinoma by
inhibition of STAT3 signaling pathway. Med Sci Monit. 23:1507–1513.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang X, Tang C, Luo H, Wang H and Zhou X:
Shp2 confers cisplatin resistance in small cell lung cancer via an
AKT-mediated increase in CA916798. Oncotarget. 8:23664–23674. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu X, Jiang H, Li J, Xu J and Fei Z:
Anticancer effects of paris saponins by apoptosis and PI3K/AKT
pathway in gefitinib-resistant non-small cell lung cancer. Med Sci
Monit. 22:1435–1441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao PJ, Song SC, Du LW, Zhou GH, Ma SL,
Li JH, Feng JG, Zhu XH and Jiang H: Paris Saponins enhance
radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell
line by inducing apoptosis and G2/M cell cycle phase arrest. Mol
Med Rep. 13:2878–2884. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song S, Du L, Jiang H, Zhu X, Li J and Xu
J: Paris saponin I sensitizes gastric cancer cell lines to
cisplatin via cell cycle arrest and apoptosis. Med Sci Monit.
22:3798–3803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang H, Zhao PJ, Su D, Feng J and Ma SL:
Paris saponin I induces apoptosis via increasing the Bax/Bcl-2
ratio and caspase-3 expression in gefitinib-resistant non-small
cell lung cancer in vitro and in vivo. Mol Med Rep.
9:2265–2272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
el-Deiry WS, Tokino T, Velculescu VE, Levy
DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and
Vogelstein B: WAF1, a potential mediator of p53 tumor suppression.
Cell. 75:817–825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
el-Deiry WS, Harper JW, O'Connor PM,
Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill
DE, Wang Y, et al: WAF1/CIP1 is induced in p53-mediated G1 arrest
and apoptosis. Cancer Res. 54:1169–1174. 1994.PubMed/NCBI
|
|
18
|
El-Deiry WS: p21(WAF1) mediates cell-cycle
inhibition, relevant to cancer suppression and therapy. Cancer Res.
76:5189–5191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Georgakilas AG, Martin OA and Bonner WM:
p21: A two-faced genome guardian. Trends Mol Med. 23:310–319. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poli F, Appendino G, Sacchetti G, Ballero
M, Maggiano N and Ranelletti FO: Antiproliferative effects of
daucane esters from Ferula communis and F. arrigonii
on human colon cancer cell lines. Phytother Res. 19:152–157. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abbasi-Kenarsari H, Shafaghat F, Baradaran
B, Movassaghpour AA, Shanehbandi D and Kazemi T: Cloning and
expression of CD19, a human B-cell marker in NIH-3T3 cell line.
Avicenna J Med Biotechnol. 7:39–44. 2015.PubMed/NCBI
|
|
22
|
Zhang L, Lin J, Ma Y, Wei D and Sun M:
Construction of a novel shuttle vector for use in Gluconobacter
oxydans. Mol Biotechnol. 46:227–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoffman HT, Karnell LH, Funk GF, Robinson
RA and Menck HR: The national cancer data base report on cancer of
the head and neck. Arch Otolaryngol Head Neck Surg. 124:951–962.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoon TY, Bhattacharyya I, Katz J, Towle HJ
and Islam MN: Squamous cell carcinoma of the gingiva presenting as
localized periodontal disease. Quintessence Int. 38:97–102.
2007.PubMed/NCBI
|
|
26
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gartel AL and Radhakrishnan SK: Lost in
transcription: p21 repression, mechanisms, and consequences. Cancer
Res. 65:3980–3985. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abramov AY, Zamaraeva MV, Hagelgans AI,
Azimov RR and Krasilnikov OV: Influence of plant terpenoids on the
permeability of mitochondria and lipid bilayers. Biochim Biophys
Acta. 1512:98–110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Golovina LA, Saidkhodzhaev AI, Abdullaev
ND, Malikov VM and Yagudaev MR: Structure and stereochemistry of
lapiferin. Chem Nat Compd. 19:281–285. 1983. View Article : Google Scholar
|
|
30
|
Gamal-Eldeen AM and Hegazy ME: A crystal
lapiferin derived from Ferula vesceritensis induces
apoptosis pathway in MCF-7 breast cancer cells. Nat Prod Res.
24:246–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kademani D: Oral cancer. Mayo Clin Proc.
82:878–887. 2007. View
Article : Google Scholar : PubMed/NCBI
|