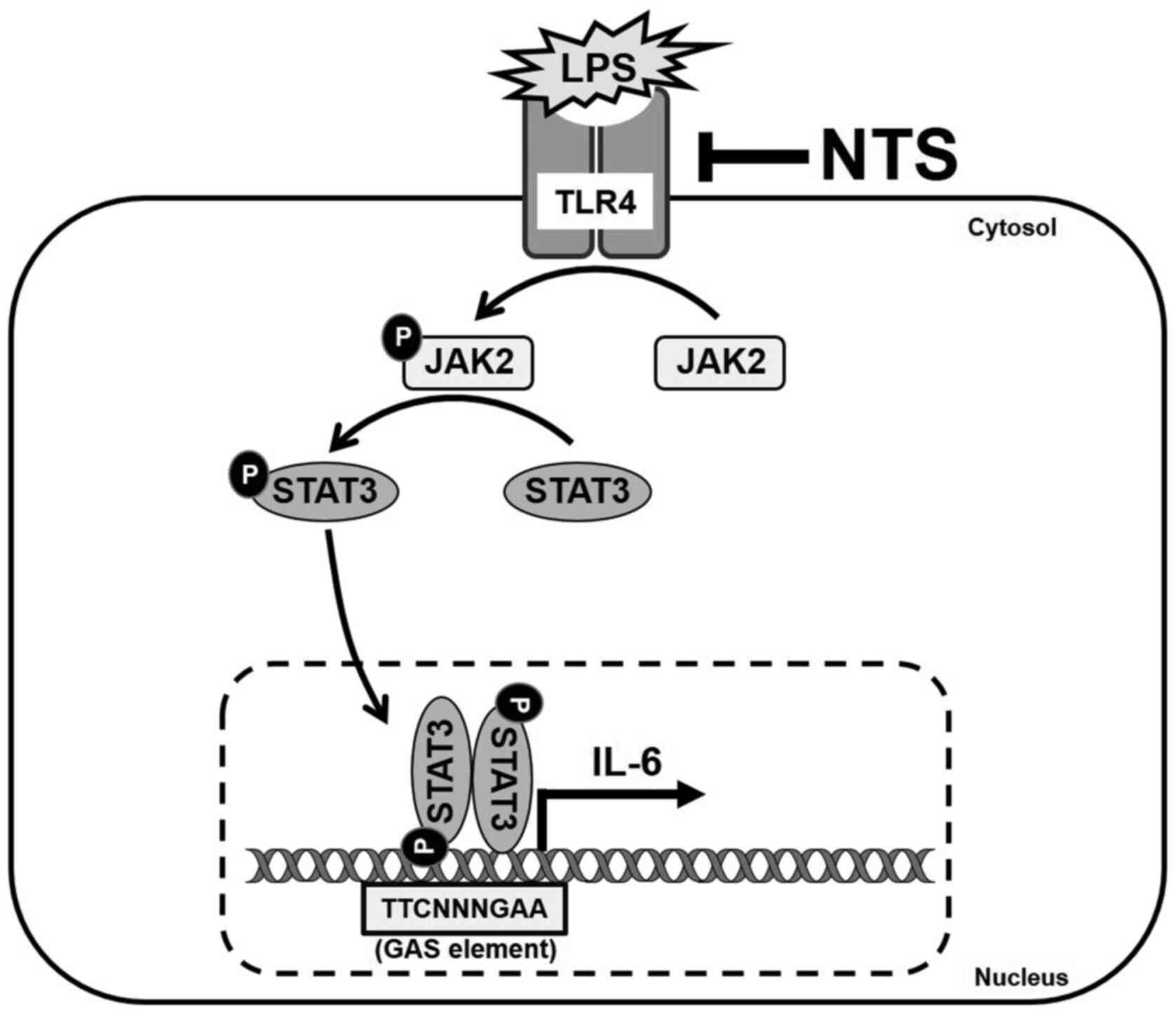
Introduction
Inflammation is considered a localized immune
response against harmful or irritating stimuli that activate the
immune system. Generally, the immune system recognizes stimuli that
cause inflammation and induces an immune response depending on the
type of stimuli (1). In a cell
culture system, lipopolysaccharide (LPS), a component in the outer
membrane of gram-negative bacteria, is used to induce inflammation
(2). It induces inflammatory
responses by regulating Toll-like receptor (TLR) signaling
(3). Among the TLRs, TLR-2 and
TLR-4 are considered to serve key roles in inflammation, which
subsequently activates NF-κB signaling (4,5). In
response to LPS-induced inflammation, NF-κB activates
pro-inflammatory cytokines, such as TNF-α, IL-1β, or IL-6 (6–8).
The Janus kinase (JAK) family of protein tyrosine
kinases takes part in various cellular responses against stimuli,
including cancer (9). JAK2 is a
ligand that binds to the cell membrane receptor to trigger
downstream signaling events from the receptor to the target
molecule. Once a stimulus activates the receptor, tyrosine
phosphorylation of JAK2 occurs, which signals to other downstream
targets. JAK2 is involved in LPS-induced inflammation (10). In particular, LPS-induced
inflammation activates TLR-4, triggering the phosphorylation of
JAK2, which is required for the activation of various signaling
pathways, including Src-kinase, MAPK, PI3K-AKT and STAT (11).
Of the JAK2-mediated signaling pathways, STAT3
serves an important role in inflammation (12). STAT3 is also considered an oncogene
and it can transduce signals from stimuli at the cell surface to
the nucleus (13). When JAK2 is
phosphorylated, the Src-homology 2 domain of STAT3 binds to JAK2,
leading to the phosphorylation of STAT3; this in turn translocates
to the nucleus and binds to gene promoters to initiate
transcription (14). When
inflammation occurs, STAT3 activation leads to its binding to the
promoter of IL-6, which is a pro-inflammatory cytokine and key
mediator in inflammation (15). A
recent study has shown that the JAK2/STAT3 signaling pathway is
required for cell death and inflammation in pancreatic cells
(16). In addition, elevated
expression levels of JAK2/STAT3/IL-6 are involved in inflammation
during tumorigenesis (17) and
increased production of IL-6 has also been identified in cancer
(18). Therefore, focusing on the
JAK2/STAT3/IL-6 pathway for inflammation may provide a target to
develop an anti-inflammatory drug.
A number of sulfur compounds are known to exhibit
anti-inflammatory activities (19).
Methylsulfonylmethane (MSM) acts as an anti-inflammatory drug
against LPS-induced inflammatory responses (20). Sulfur is an essential element that
is indirectly consumed by our body in the form of foods, such as
onion, garlic and duck meat (21).
For the consumption of naturally existing mineral sulfur, different
substances are combined with mineral sulfur to eliminate its toxic
substances. A number of countries use a non-toxic form of dietary
sulfur or natural sulfur for the treatment of different diseases,
including inflammatory diseases (22). Non-toxic sulfur (NTS) has been used
in livestock feed to improve meat quality and immunity. It has been
reported that repeated oral administration of NTS in rats did not
induce cell death (23,24). In addition, it has been demonstrated
that NTS can enhance growth hormone signaling by regulating the
JAK2/STAT5b/IGF-1 axis (25). The
present study hypothesized that NTS exhibits anti-inflammatory
activity against LPS-induced inflammation by regulating
JAK2/STAT3/IL-6 signaling.
Materials and methods
Antibodies and cell culture
reagents
Dulbecco's modified Eagle's medium (DMEM),
penicillin-streptomycin solution, Trypsin-EDTA (0.05%) and fetal
bovine serum (FBS) were purchased from Gibco (Thermo Fisher
Scientific, Inc.). Antibodies specific for β-actin (cat. no.
sc-47778) and TLR-4 (cat. no. sc-293072) and secondary antibodies
[goat anti-mouse (cat. no. sc-516102) and anti-rabbit (cat. no.
sc-2357)] were obtained from Santa Cruz Biotechnology, Inc. The
anti-IL-6 (cat. no. ab6672) and Tata-binding protein (TBP; cat. no.
ab63766) antibodies were purchased from Abcam, and phosphorylated
(p)-Jak2 (cat. no. 3776s), Jak2 (cat. no. 3230s), p-STAT3 (cat. no.
9145) and STAT3 (cat. no. 9139) antibodies were purchased from Cell
Signaling Technology, Inc. NTS was donated by Nara Bio Co., Ltd.
LPS (cat. no. L4391), and the STAT3 inhibitor
2-hydroxy-4-methylphenyl sulfonyl oxy acetyl amino-benzoic acid
(cat. no. S3I-201) and TLR-4 inhibitor (TLR4-C34; cat. no. SML0832)
were purchased from Sigma-Aldrich (Merck KGaA).
Cell culture and treatment
C2C12 myoblasts (cat. no. CRL-1772; American Type
Culture Collection) were cultured in DMEM supplemented with 10% FBS
and 1% penicillin-streptomycin at 37°C in 5% CO2. For
each experiment, cells at 70–80% confluency cells were gently
washed twice with phosphate-buffered saline (PBS). Unless otherwise
specified, cells were treated with 0.2 µg/ml NTS in dimethyl
sulfoxide (DMSO; cat. no. D8418; Sigma-Aldrich; Merck KGaA) for 24
h at 37°C.
Cell viability assay
Cell viability was assessed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
cat. no. M2128; Sigma-Aldrich; Merck KGaA) assay. Briefly, cells
were re-suspended in DMEM 1 day prior to drug treatment at a
density of 1×104 cells/well in 24-well culture plates. The next
day, the culture medium was replaced with fresh medium containing
DMEM (vehicle control) or different concentrations of NTS (0.1–2
µg), followed by incubation for 24 h at 37°C. Following this
treatment, MTT (5 mg/ml) was added and the culture plates were
incubated at 37°C for 4 h. The resulting formazan product was
dissolved in DMSO and absorbances were measured at 560 nm using the
Ultra Multifunctional Microplate Reader (Tecan US, Inc.). Cell
viability was determined from these readings using the formula %
viability = (fluorescence value of MSM/fluorescence value of
non-treated control) ×100. All measurements were performed in
triplicate and experiments were repeated at least three times. Cell
viability was confirmed by WST-1 (Roche Diagnostics GmbH) assay.
Briefly, cells were re-suspended in DMEM 1 day prior to drug
treatment at a density of 4×103 cells/well in 96-well culture
plates. The next day, the culture medium was replaced with fresh
medium containing DMEM (vehicle control) or different
concentrations of NTS (0.1–2 µg) and cells were incubated for 24 h
at 37°C. Following this treatment, 10 µl/well cell proliferation
Reagent WST-1 was added and the culture dishes were incubated at
37°C for 4 h. Absorbances were measured at 460 nm using an Ultra
Multifunctional Microplate Reader (Tecan US, Inc.). Cell viability
was determined from these readings using the formula % viability =
(fluorescence value of MSM/fluorescence value of non-treated
control) ×100. All measurements were performed in triplicate and
experiments were repeated at least three times.
Apoptosis analysis
Fluorescein-conjugated Annexin V (Annexin V-FITC)
was used to measure the apoptosis in C2C12 cells. The NTS or LPS
treated cells were washed with PBS and re-suspended in a binding
buffer at a concentration of 1×106 cells. Then, the cells were
stained with Annexin V-FITC and PI for 10 min in the dark at room
temperature. The percentage of apoptotic cells was measured by flow
cytometry using a FACSCalibur flow cytometer (Bio-Rad Laboratories,
Inc.) and the analysis was performed using FlowJo software v10
(FlowJo LLC). Apoptotic rate was calculated using live cells vs.
dead cells, which included early apoptosis, late apoptosis or
necrosis.
Western blotting
Whole cell lysates were prepared from untreated or
NTS-treated C2C12 myoblasts by incubating the cells on ice with
radioimmunoprecipitation lysis buffer (cat. no. 20-188; EMD
Millipore) containing phosphatase and protease inhibitors. Cells
were lysed by aspirating through a 23-gauge needle and the lysate
was centrifuged at 18,300 × g for 10 min at 4°C to remove cellular
debris. Protein concentrations were measured using the Bradford
method (Thermo Fisher Scientific, Inc.). Equal amounts of protein
(100 µg per lane) were subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. Then, the separated
proteins were transferred onto nitrocellulose membranes. The blots
were blocked for 1 h with 5% skimmed milk (cat. no. 90002-594; BD
Biosciences) in TBS with Tween-20 (TBST) buffer [20 mM Tris-HCl
(cat. no. 10708976001; Sigma-Aldrich; Merck KGaA), pH 7.6, 137 mM
NaCl (Formedium Ltd.) and 0.1X Tween-20 (cat. no. 0777; Scientific
Sales, Inc.)]. Membranes were then probed overnight at 4°C with the
indicated primary antibodies (1:1,000; anti-TLR-4, anti-IL-6,
anti-p-Jak2, anti-Jak2, anti-p-STAT3, anti-STAT3 and anti-β-actin)
diluted in 5% bovine serum albumin (BSA; EMD Millipore) or 5%
skimmed milk (Difco™ skim milk; BD Biosciences). Membranes were
then washed with TBST and incubated for 1–1.5 h at room temperature
with horseradish peroxidase (HRP)-conjugated secondary antibodies
(1:1,000). Detection was performed using the Enhanced
Chemiluminescence Plus detection kit (Cytiva) and LAS-4000 imaging
device (Version 1.0; Fujifilm Corporation). Blots were stripped
with Restore Western Blot Stripping Buffer (Thermo Fisher
Scientific, Inc.). Densitometry was performed using ImageJ software
(version 1.8.0_172; National Institutes of Health).
Reverse transcription
(RT)-semi-quantitative PCR
Total RNA was extracted from the cells using the
RNeasy Mini kit (Qiagen GmbH) according to the manufacturer's
instructions. RNA was spectrophotometrically quantified at 260 nm.
Subsequently, RT-semi-quantitative PCR analyses were performed to
detect TLR-4, IL-6 and GAPDH RNA expression. Briefly, cDNA was
synthesized from total RNA by incubating the samples at 42°C for 1
h and then at 95°C for 5 min using a First-Strand cDNA Synthesis
kit (cat. no. K-2041; Bioneer Corporation) and oligod(T) primers.
The RT-PCR Premix kit (cat. no. K-2016; Bioneer Corporation) was
used to amplify TLR-4, IL-6 and GAPDH with primers synthesized by
Bioneer Corporation. To generate a 359-bp TLR-4 fragment, the
following primers were used: TLR-4 sense,
5′-GCTTTCACCTCTGCCTTCAC-3′ and antisense,
5′-CGAGGCTTTTCCATCCAATA-3′. To generate a 396-bp IL-6 fragment, the
following primers were used: IL-6 sense, 5′-AGCCCTGAGAAAGGAGACAT-3′
and antisense, 5′-CTGCGCAGAATGAGATGAGT-3′. Then, a 320-bp GAPDH
mRNA fragment was generated with the following primers: GAPDH
sense, 5′-AAGGCCATCACCATCTTCCA-3′ and antisense,
5′-ACGATGCCAAAGTGGTCATG-3′. The PCR conditions for TLR-4 were as
follows: 95°C for 5 min, 32 cycles of 95°C for 60 sec, 58°C for 60
sec and 72°C for 60 sec and 72°C for 10 min. The PCR conditions for
IL-6 and GAPDH were as follows: 95°C for 5 min, 32 cycles of 95°C
for 60 sec, 60°C for 60 sec and 72°C for 60 sec and 72°C for 10
min. PCR products were resolved via electrophoresis on a 2% agarose
gel and visualized with ethidium bromide (cat. no. E7637;
Sigma-Aldrich; Merck KGaA) staining.
Flow cytometry analysis
After cultured cells were washed with cold PBS, cell
pellets were isolated by centrifugation at 14,000 × g for 5 min at
4°C and incubated with 10% BSA on ice for 20 min. Then, cells
(3×106) were stained with the following antibodies (1:200) on ice
for 30 min: PE CD282 (TLR4; cat. no. 312806; BioLegend, Inc.).
Stained cells were washed with pre-cold PBS and flow cytometric
analysis was performed using the FACSCalibur flow cytometer (BD
Bioscience) and analysis was performed using FlowJo software v10
(FlowJo LLC).
Chromatin immunoprecipitation (ChIP)
assay
The ChIP assay was performed using the Imprint ChIP
kit (cat. no. CHP1; Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocol. Briefly, C2C12 myoblasts were fixed by
adding 1% formaldehyde to media containing cells immediately and
quenched with 1.25 M glycine at room temperature. Following washing
with PBS, the cells were suspended in nuclei preparation buffer and
shearing buffer (provided in ChIP kit) and sonicated under
optimized conditions (20 sec on and off for 15 min with 25%
amplitude on ice). The sheared DNA was then centrifuged at 16,000 ×
g for 10 min at 4°C and the cleared supernatant was used for
protein/DNA immunoprecipitation. The clarified supernatant was
diluted with buffer (1:1 ratio) and 5-µl aliquots of the diluted
samples were used as internal controls. Then, the diluted
supernatant was incubated with anti-STAT3 antibody (1:1,000) in
pre-coated wells for 90 min at 4°C. The controls were incubated
with normal goat IgG (1:1,000) and then with anti-RNA polymerase II
for 90 min at 4°C. The unbound DNA was washed with
immunoprecipitation wash buffer and the bound DNA was collected by
the cross-link reversal method using a DNA release buffer
containing proteinase K. The released DNA and DNA from the internal
control were purified using the GenElute Binding Column G
(Sigma-Aldrich; Merck KGaA). The DNA was then quantified via
RT-qPCR. The primer sequences were: IL-6 sense,
5′-GACTGAGCCTAAGGGTGCAT-3′ and antisense,
5′-ACCACTAGAGGGCCAAGTCA-3′.
Nuclear protein extraction
Nuclear protein extracts were isolated using a
nuclear extract kit (cat. no. AY2002; Panomics, Inc.). Briefly,
cells were washed with PBS and buffer A containing DTT, Protease
Inhibitor, Phosphates Inhibitor I and Phosphates Inhibitor II were
added and incubated in ice on a rocking platform at 200 rpm for 10
min. The samples were transferred to micro centrifuge tubes and the
supernatant removed by centrifugation at 14,000 × g for 3 min at
4°C. Then buffer B containing DTT, Protease Inhibitor, Phosphates
Inhibitor I and Phosphates Inhibitor II were added and incubated on
ice for 1 h. The nuclear extract was collected by taking the
supernatant by centrifugation at 14,000 × g for 5 min at 4°C and
western blotting was performed as described above for further
analysis.
Enzyme-linked immunosorbent assay
(ELISA)
ELISA was performed for the quantitative detection
of IL-6 using a mouse IL-6 ELISA kit (cat. no. ab100713; Abcam).
C2C12 cells were treated with NTS for 24 h and with 100 ng/ml LPS
for 4 h and the spent media were used for the assay. The samples
were added to anti-mouse IL-6-coated microwells along with sample
diluent and a biotin-conjugate solution. Following incubation,
streptavidin-HRP was added and the plates were further incubated
for 1 h in a shaker at room temperature. Then,
3,3′,5,5′-tetramethylbenzidine solution was added after washing.
Finally, a stop solution was added once the high-concentrated
standard turned a dark blue color. The absorbance was read with a
microplate reader at 450 nm and calculations were performed
according to the assay protocol.
Statistical analyses
All experiments were performed at least three times.
Results are expressed as the mean ± standard error of the mean.
Statistical analyses were conducted using the one-way analysis of
variance (ANOVA) or unpaired Student's t-test. One-way ANOVA was
followed by Tukey's post hoc test. Analyses were performed using
the SAS 9.3 program (SAS Institute, Inc.). P<0.05 was considered
to indicate a statistically significant difference.
Results
Protective effect of NTS on
LPS-induced loss of cell viability
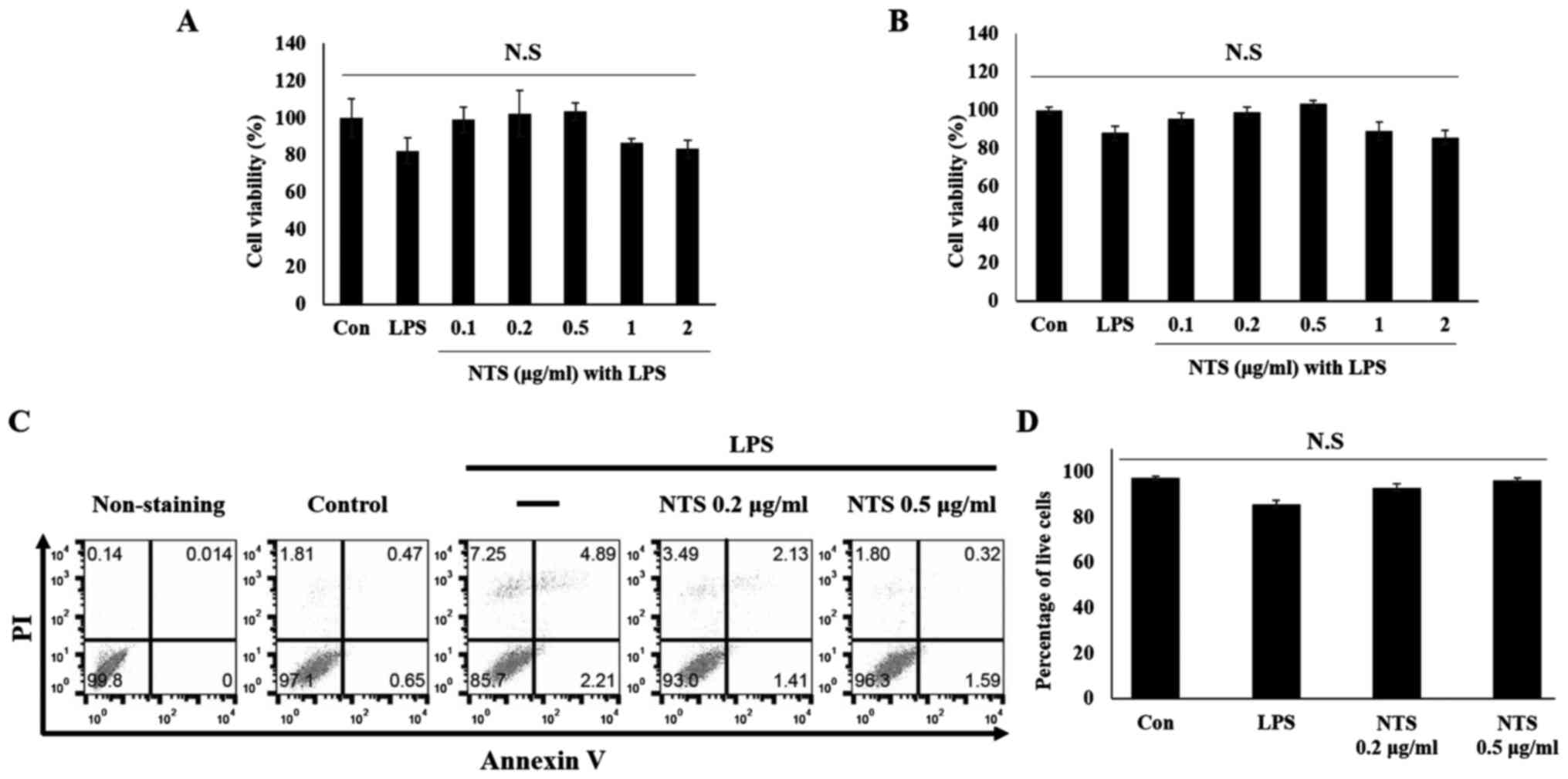
To examine whether NTS exhibits a protective effect
on cell viability, the MTT assay (Fig.
1A) and WST-1 assay (Fig. 1B)
were performed. It was found that LPS induced ~20% of cell death
during a treatment period of 24 h. To determine the optimum
concentration of NTS, cells were treated with 0.1, 0.2, 0.5, 1 and
2 µg/ml NTS in the presence of 100 ng/ml LPS. A non-significant
increase in cell numbers with 0.1, 0.2 and 0.5 µg/ml NTS was
observed, whereas 1 and 2 µg/ml NTS decreased cell viability. In
order to confirm the cyto protective effect of NTS, flow cytometry
analysis was conducted in C2C12 myoblasts (Fig. 1C). The results confirmed an increase
in cell proliferation compared with LPS-treated cells (Fig. 1D). The pro-apoptotic factor BAX
protein was also analyzed and this confirmed the cyto protective
effect of NTS against LPS-induced cell death (Fig. S1). From these results, 0.2 and 0.5
µg/ml NTS were selected for further studies.
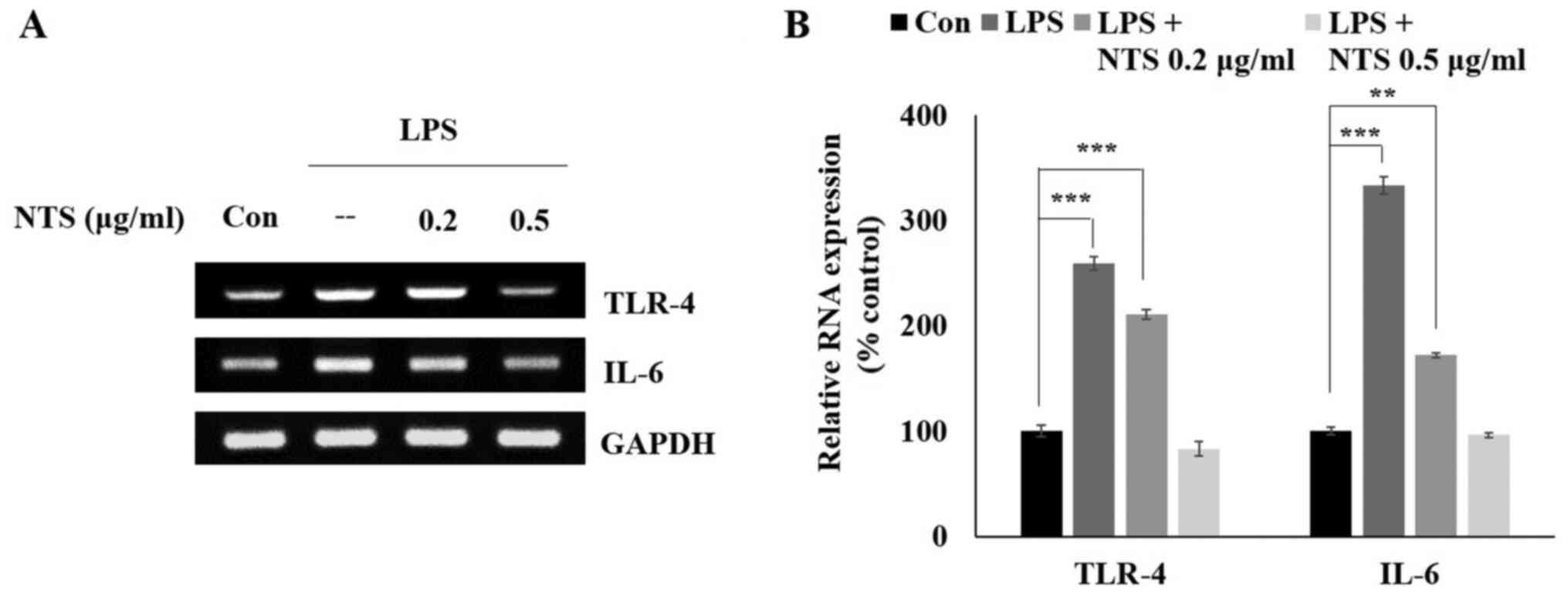
NTS inhibits LPS-induced mRNA
expression of TLR4 and IL-6
From the cell viability assay, the optimal
concentrations of NTS were determined to be 0.2 and 0.5 µg/ml.
Subsequently, it was determined whether NTS can inhibit LPS-induced
expression of the inflammatory receptor TLR-4 and pro-inflammatory
cytokine IL-6. C2C12 cells were treated with LPS or NTS and then
mRNA was isolated from these cells to analyze the expression of
TLR-4 and IL-6 via RT-semi-quantitative PCR (Fig. 2A). The obtained results suggested an
increase in the expression of TLR-4 and IL-6 with LPS treatment,
which was inhibited by NTS treatment (Fig. 2B). These results suggested the
anti-inflammatory effect of NTS.
NTS downregulates LPS-induced
JAK2/STAT3/IL-6 signaling
It was observed that NTS inhibited LPS-induced mRNA
expression of TLR-4 and IL-6 mRNA. Therefore, the protein
expression of TLR-4 and JAK2/STAT3/IL-6 was measured with western
blotting. The obtained results demonstrated an increase in the
expression of TLR-4 and JAK2/STAT3/IL-6 in LPS-treated cells, which
was downregulated by 0.5 µg/ml NTS (Fig. 3A) without affecting the expression
of total JAK2 and STAT3. To determine the role of TLR-4 and STAT3
in the anti-inflammatory effect of NTS, cells were treated with a
TLR-4 inhibitor (TLR4-C34) or STAT3 inhibitor S3I-201 (100 µM). It
was observed that the TLR-4 inhibitor demonstrated a similar
significant pattern to NTS; by contrast, the STAT3 inhibitor
inhibited the expression of p-STAT3 and IL-6. These results
provided evidence of the role of TLR-4 and STAT3 in the
anti-inflammatory activity of NTS (Fig.
3B). The ratio of phosphorylated proteins to their total form
also demonstrated similar result to the phosphorylated proteins
compared with β-actin (Fig. 3C). In
addition, the role of TLR-4 in inflammation was confirmed by
examining LPS-induced expression of TLR-4 and its inhibition by NTS
treatment via flow cytometry analysis (Fig. 3D).
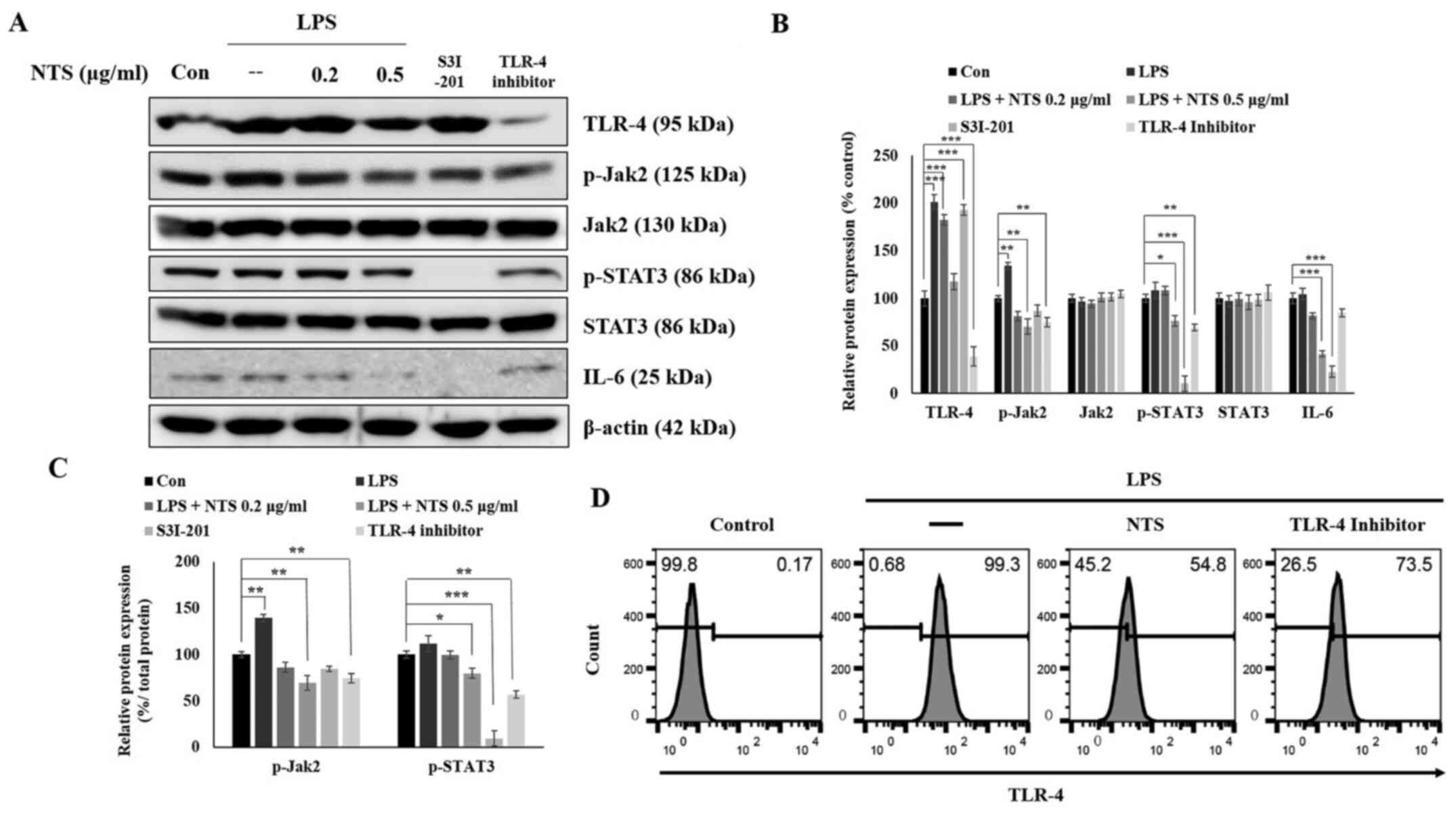 | Figure 3.NTS inhibits TLR-4, JAK2, STAT3 and
IL-6 signaling. (A) Western blotting showing the expression of
TLR-4, JAK2, p-JAK2, STAT3, p-STAT3 and IL-6 proteins following the
treatment of C2C12 cells with 100 ng/ml LPS and 0.2 or 0.5 µg/ml
NTS or 100 µM S3I-201 or 80 µM TLR4-C34 for 24 h. (B) Relative
protein expression levels of TLR-4, JAK2, p-JAK2, STAT3, p-STAT3
and IL-6 were determined via densitometry and were normalized to
β-actin. Data are representative of three independent experiments.
(C) Relative expression levels of p-JAK2 and p-STAT3 were
determined via densitometry and were normalized to total JAK2 and
STAT3. (D) Flow cytometry (fluorescence-activated cell sorting)
analysis of TLR-4 expression in C2C12 cells showing the expression
levels of LPS-induced TLR-4 and its inhibition by 0.5 µg/ml NTS or
80 µM Toll-like receptor 4 inhibitor (TLR4-C34) for 24 h.
*P<0.05, **P<0.01 and ***P<0.001 vs. control. NTS,
non-toxic sulfur; TLR-4, Toll-like receptor 4; JAK2, Janus kinase
2; p-, phosphorylated; LPS, lipopolysaccharide; Con, control. |
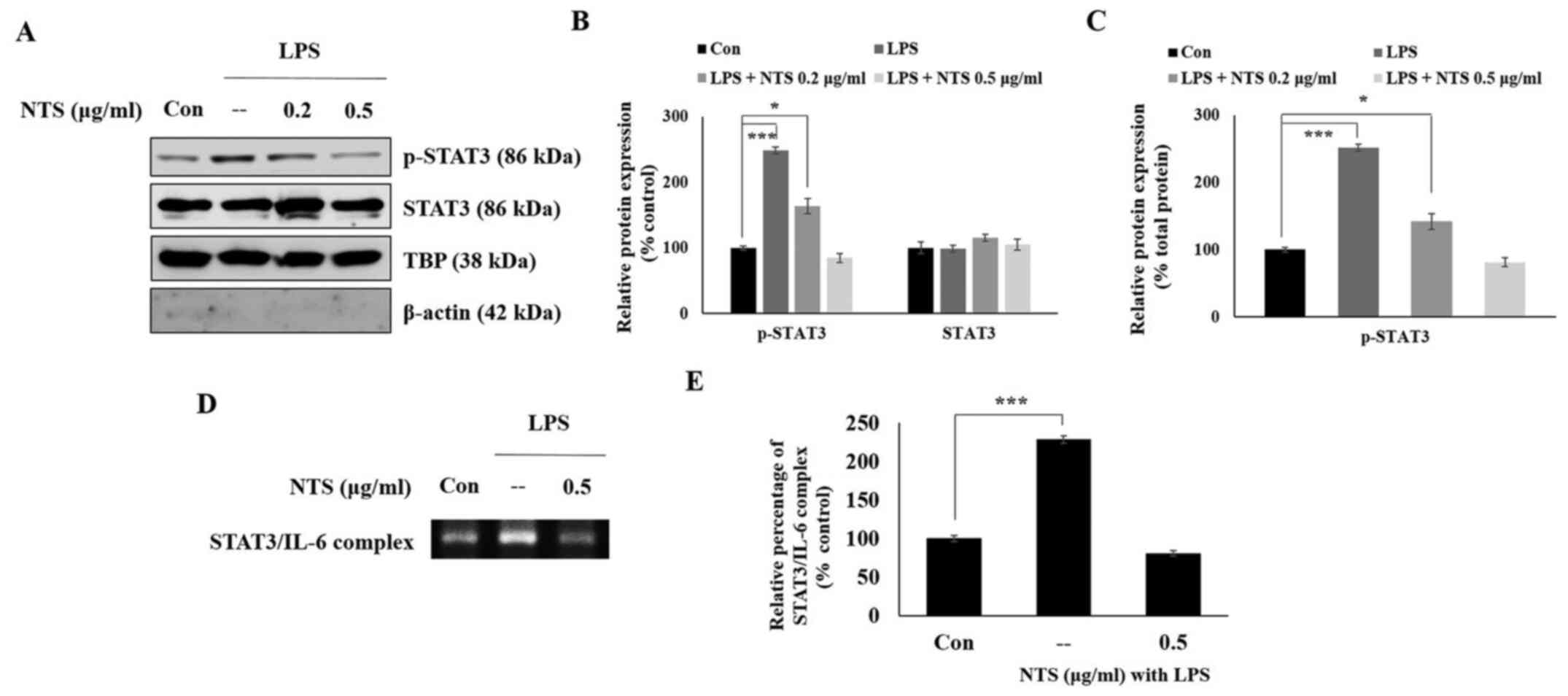
NTS inhibits expression of nuclear
p-STAT3 and its binding to the IL-6 promoter
The present study demonstrated that NTS inhibited
LPS-induced expression of TLR-4, JAK2, STAT3 and IL-6. It was
hypothesized that STAT3 would translocate from the cytoplasm to the
nucleus to bind to the promoters of pro-inflammatory cytokines in
LPS-induced inflammation. Therefore, C2C12 cells were treated with
or without LPS or NTS for 24 h and nuclear proteins were isolated
to analyze the expression of STAT3 via western blotting. The
expression of p-STAT3 increased in LPS-treated cells, whereas total
STAT3 levels remained unchanged in non-treated control cells
(Fig. 4A). NTS inhibited the
expression of p-STAT3 without affecting the expression levels of
STAT3, indicating the possible inhibition of the nuclear
translocation of STAT3 by NTS (Fig.
4B). The ratio of nuclear p-STAT3 protein to total nuclear
STAT3 protein also demonstrated a similar result to the p-STAT3
expression compared with TBP (Fig.
4C). It was hypothesized that nuclear p-STAT3 may bind to the
IL-6 promoter. To confirm this, the expression of the STAT3-IL-6
complex was analyzed by ChIP assay (Fig. 4D). The result demonstrated a
significant increase in LPS-induced expression of the STAT3-IL-6
complex, which was downregulated by 0.5 µg/ml NTS (Fig. 4E).
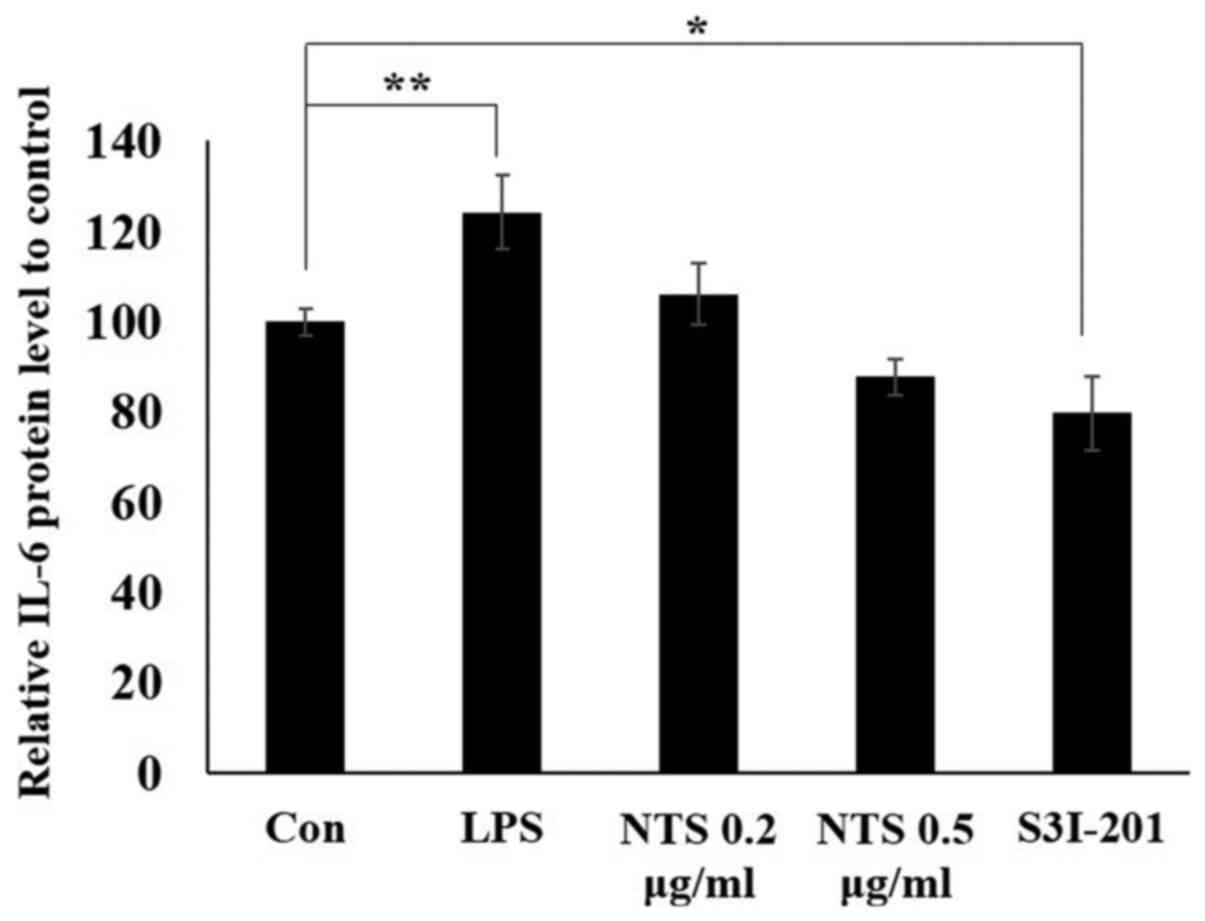
NTS inhibits LPS-induced
STAT3-dependent IL-6 expression
The inhibition of the STAT3-IL-6 complex by NTS was
demonstrated in LPS-induced inflammation. The present study
assessed whether NTS could inhibit IL-6 release into the media.
C2C12 cells were treated with or without LPS or NTS and the media
was collected to conduct a mouse IL-6 ELISA. The result
demonstrated a significant increase in LPS-induced IL-6 expression,
whereas NTS decreased IL-6 expression (Fig. 5). A similar pattern was observed in
the STAT3 inhibitor-treated group. These results demonstrated that
NTS exhibited an anti-inflammatory effect by regulating STAT3.
Taken together, NTS inhibited LPS-induced inflammation by
inhibiting LPS-induced expression of TLR-4, JAK2, STAT3 and IL-6.
It also inhibited the expression of IL-6 mRNA to induce its
anti-inflammatory effects (Fig.
6).
Discussion
The treatment of inflammation with naturally active
compounds is a promising strategy as a number of natural compounds
exhibit anti-inflammation activities with fewer side effects
(26). After calcium and
phosphorus, sulfur is suggested to be the most abundant mineral
element present in the human body that is used for normal
maintenance and it is generally derived from the sources of dietary
protein because of the toxicity associated with direct
administration (27).
Sulfur-containing amino acids are essential for optimal growth and
protein synthesis (28).
Sulfur-containing compounds possess various activities, including
anticancer (29), anti-inflammatory
(30) and antioxidant activity
(31). NTS is considered a good
source as it enables the supplementation of the non-toxic form of
sulfur. If NTS were able to inhibit LPS-induced inflammation, then
it could be considered a candidate drug for the treatment of
inflammation.
If a natural compound protects against LPS-induced
cell death by increasing the cell viability of a normal cell, then
it can be considered that the particular concentration of that
natural compound is not associated with significant adverse
effects. The present study used C2C12 mouse myoblasts for
anti-inflammatory studies as these cells are used for inflammation
studies. Inflammation by LPS can activate the ubiquitin-proteasome
pathway via TLR4 and induces catabolism in cultured C2C12 (32). These C2C12 myoblasts are commonly
used for the regulation of LPS-induced inflammation by candidate
drugs in vitro (33,34). However, the lack of a more relevant
cell model could be considered as a limitation of this study. The
results demonstrated that LPS induced ~20% of cell death in C2C12
cells, whereas concentrations >0.5 µg/ml NTS demonstrated a
protective effect on C2C12 cells by increasing cell viability
compared with LPS treatment. Inhibition in apoptosis by NTS also
gave evidence for our previous study that demonstrated NTS can act
as a growth hormone by inducing growth hormone signaling (25). Therefore, from the results of the
cell viability and apoptosis assay, it was hypothesized that 0.5
µg/ml NTS could inhibit LPS-induced inflammation.
To examine the anti-inflammatory activity of NTS,
the effect of NTS on LPS-induced expression of the inflammation
response receptor TLR-4 and pro-inflammatory cytokine IL-6 was
initially assessed. TLR-4 is considered one of the main receptors
triggering inflammatory responses (35). The addition of LPS leads to the
activation of TLR-4 by binding to cluster of differentiation (CD)14
that is anchored to raft proteins (36). IL-6 is a key factor in inflammation
response pathways and it takes part in both non-classical
inflammation pathways and Hippo pathways (37). It also serves an important role in
maintaining the delicate balance between inflammation and immune
resolution (38). The results of
the present study demonstrated an increase in the expression of
TLR-4 and IL-6 with LPS treatment, whereas NTS decreased their
expression at both transcriptional and translational levels and
their levels in spent media. These results suggested the role of
NTS as an effective anti-inflammatory drug against LPS-induced
inflammation.
The JAK/STAT pathway is one of the key signaling
pathways as a target for autoimmune diseases and inflammation
(39,40). JAK/STAT signals promote inflammation
by regulating the development of innate lymphoid cells in immune
responses (41). It is the major
pathway in inflammation that acts via signaling from TLR-4 in
natural compound-based anti-inflammatory activity (42). The present study demonstrated that
LPS inhibition induced the expression of TLR-4. In addition, it
found that NTS inhibited LPS-induced expression of activated JAK2
and STAT3 without affecting the total forms of these proteins.
Therefore, the results suggested that the anti-inflammatory
activity of NTS was mediated via TLR-4 and JAK2/STAT3 signaling.
STAT3 is a transcription factor for the pro-inflammatory cytokine
IL-6 during inflammation (43). The
results of the present study demonstrated that NTS inhibited the
nuclear levels of activated STAT3 and its binding to the promoter
region of IL-6, indicating that the inflammatory response via
STAT3/IL-6 signaling was downregulated by NTS treatment. In
particular, it blocks the transcription of IL-6, thereby inhibiting
inflammation.
In conclusion, the present study demonstrated that
the natural sulfur-containing compound NTS inhibited LPS-induced
inflammation by modulating TLR-4 and JAK2/STAT3 signaling pathways.
NTS also reduced the nuclear p-STAT3 protein levels and inhibits
its binding to the promoter region of the pro-inflammatory cytokine
IL-6. Taken together, the results suggested that NTS could be a
potential candidate drug for the treatment of inflammation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Nara Bio Co.,
Ltd., Republic of Korea, in 2018 and by the Cooperative Research
Program for Agriculture Science and Technology Development (project
no. PJ01325702) and by the National Research Foundation of Korea
(NRF) grant funded by the Korean government (MSIT; grant no.
2018R1C1B6006146).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YMY and KJJ designed the experiments. DYK, NS and
ESJ performed most of the experiments. AR, HDK, IK, JCP and SWB
helped with experiments and discussions. YMY, KJJ, DYK and NS
analyzed the data. NS and DYK wrote the manuscript. HDK and IHK
from Bio Co., Ltd., provided NTS and took part in project design
and evaluation. DYK and NS confirm the authenticity of all the raw
data. All authors helped to revise the manuscript and approved the
final version for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Hyoung Do Kim is affiliated with Nara Bio Co., Ltd.,
which provided funding for this study and supplied non-toxic
sulfur. The remaining authors declare that they have no competing
interests.
References
|
1
|
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng
J, Li Y, Wang X and Zhao L: Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget.
9:7204–7218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Catorce MN and Gevorkian G: LPS-induced
murine neuroinflammation model: Main features and suitability for
pre-clinical assessment of nutraceuticals. Curr Neuropharmacol.
14:155–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heinbockel L, Weindl G, Martinez-de-Tejada
G, Correa W, Sanchez-Gomez S, Bárcena-Varela S, Goldmann T, Garidel
P, Gutsmann T and Brandenburg K: Inhibition of lipopolysaccharide-
and lipoprotein-induced inflammation by antitoxin peptide
Pep19-2.5. Front Immunol. 9:17042018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawai T and Akira S: Signaling to
NF-kappaB by Toll-like receptors. Trends Mol Med. 13:460–469. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther. 2:22017.
View Article : Google Scholar
|
|
6
|
Yamakawa T, Eguchi S, Matsumoto T,
Yamakawa Y, Numaguchi K, Miyata I, Reynolds CM, Motley ED and
Inagami T: Intracellular signaling in rat cultured vascular smooth
muscle cells: Roles of nuclear factor-kappaB and p38
mitogen-activated protein kinase on tumor necrosis factor-alpha
production. Endocrinology. 140:3562–3572. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olson CM, Hedrick MN, Izadi H, Bates TC,
Olivera ER and Anguita J: p38 mitogen-activated protein kinase
controls NF-kappaB transcriptional activation and tumor necrosis
factor alpha production through RelA phosphorylation mediated by
mitogen- and stress-activated protein kinase 1 in response to
Borrelia burgdorferi antigens. Infect Immun. 75:270–277. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ngkelo A, Meja K, Yeadon M, Adcock I and
Kirkham PA: LPS induced inflammatory responses in human peripheral
blood mononuclear cells is mediated through NOX4 and Giα dependent
PI-3kinase signalling. J Inflamm (Lond). 9:12012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nipin SP, Darvin P, Yoo YB, Joung YH, Kang
DY, Kim DN, Hwang TS, Kim SY, Kim WS, Lee HK, et al: The
combination of methylsulfonylmethane and tamoxifen inhibits the
Jak2/STAT5b pathway and synergistically inhibits tumor growth and
metastasis in ER-positive breast cancer xenografts. BMC Cancer.
15:4742015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okugawa S, Ota Y, Kitazawa T, Nakayama K,
Yanagimoto S, Tsukada K, Kawada M and Kimura S: Janus kinase 2 is
involved in lipopolysaccharide-induced activation of macrophages.
Am J Physiol Cell Physiol. 285:C399–C408. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JJ, Kim DH, Kim DG, Lee HJ, Min W,
Rhee MH, Cho JY, Watarai M and Kim S: Toll-like receptor 4-linked
Janus kinase 2 signaling contributes to internalization of Brucella
abortus by macrophages. Infect Immun. 81:2448–2458. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kasembeli MM, Bharadwaj U, Robinson P and
Tweardy DJ: Contribution of STAT3 to inflammatory and fibrotic
diseases and prospects for its targeting for treatment. Int J Mol
Sci. 19:192018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Darnell JE Jr, Kerr IM and Stark GR:
Jak-STAT pathways and transcriptional activation in response to
IFNs and other extracellular signaling proteins. Science.
264:1415–1421. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moran A, Akcan Arikan A, Mastrangelo MA,
Wu Y, Yu B, Poli V and Tweardy DJ: Prevention of trauma and
hemorrhagic shock-mediated liver apoptosis by activation of
stat3alpha. Int J Clin Exp Med. 1:213–247. 2008.PubMed/NCBI
|
|
15
|
Moran A, Tsimelzon AI, Mastrangelo MA, Wu
Y, Yu B, Hilsenbeck SG, Poli V and Tweardy DJ: Prevention of
trauma/hemorrhagic shock-induced lung apoptosis by IL-6-mediated
activation of Stat3. Clin Transl Sci. 2:41–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen WD, Zhang JL, Wang XY, Hu ZW and Qian
YB: The JAK2/STAT3 signaling pathway is required for inflammation
and cell death induced by cerulein in AR42J cells. Eur Rev Med
Pharmacol Sci. 23:1770–1777. 2019.PubMed/NCBI
|
|
17
|
Wang SW and Sun YM: The IL-6/JAK/STAT3
pathway: Potential therapeutic strategies in treating colorectal
cancer (Review). Int J Oncol. 44:1032–1040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung YC and Chang YF: Serum interleukin-6
levels reflect the disease status of colorectal cancer. J Surg
Oncol. 83:222–226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van der Merwe M and Bloomer RJ: The
influence of methylsulfonylmethane on inflammation-associated
cytokine release before and following strenuous exercise. J Sports
Med (Hindawi Publ Corp). 2016:74983592016.PubMed/NCBI
|
|
20
|
Kim YH, Kim DH, Lim H, Baek DY, Shin HK
and Kim JK: The anti-inflammatory effects of methylsulfonylmethane
on lipopolysaccharide-induced inflammatory responses in murine
macrophages. Biol Pharm Bull. 32:651–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koh E and Surh J: Influence of sulfur
fertilization on the antioxidant activities of onion juices
prepared by thermal treatment. Prev Nutr Food Sci. 21:160–164.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caron JM, Bannon M, Rosshirt L, Luis J,
Monteagudo L, Caron JM and Sternstein GM: Methyl sulfone induces
loss of metastatic properties and reemergence of normal phenotypes
in a metastatic cloudman S-91 (M3) murine melanoma cell line. PLoS
One. 5:e117882010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim CI, Choe HS, Kang C, Lee BK and Ryu
KS: Effects of dietary organic sulfur on performance, egg quality
and cell-mediated immune response of laying hens. Korean J Poult
Sci. 45:97–107. 2018. View Article : Google Scholar
|
|
24
|
Lee JS, Kwon JK, Han SH and An IJ:
Toxicity study of detoxication sulphur at 3 months post-treatment
in rats. J Fd Hyg Saf. 25:263–268. 2010.
|
|
25
|
Kang DY, Sp N, Jo ES, Kim HD, Kim IH, Bae
SW, Jang KJ and Yang YM: Non toxic sulfur enhances growth hormone
signaling through the JAK2/STAT5b/IGF 1 pathway in C2C12 cells. Int
J Mol Med. 45:931–938. 2020.PubMed/NCBI
|
|
26
|
Kang DY, Darvin P, Yoo YB, Joung YH, Sp N,
Byun HJ and Yang YM: Methylsulfonylmethane inhibits HER2 expression
through STAT5b in breast cancer cells. Int J Oncol. 48:836–842.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nimni ME, Han B and Cordoba F: Are we
getting enough sulfur in our diet? Nutr Metab (Lond). 4:242007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Griffith OW: Mammalian sulfur amino acid
metabolism: An overview. Methods Enzymol. 143:366–376. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lim EJ, Hong DY, Park JH, Joung YH, Darvin
P, Kim SY, Na YM, Hwang TS, Ye SK, Moon ES, et al:
Methylsulfonylmethane suppresses breast cancer growth by
down-regulating STAT3 and STAT5b pathways. PLoS One. 7:e333612012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sousa-Lima I, Park SY, Chung M, Jung HJ,
Kang MC, Gaspar JM, Seo JA, Macedo MP, Park KS, Mantzoros C, et al:
Methylsulfonylmethane (MSM), an organosulfur compound, is effective
against obesity-induced metabolic disorders in mice. Metabolism.
65:1508–1521. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Henrotin Y and Mobasheri A: Natural
products for promoting joint health and managing osteoarthritis.
Curr Rheumatol Rep. 20:722018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Doyle A, Zhang G, Abdel Fattah EA, Eissa
NT and Li YP: Toll-like receptor 4 mediates
lipopolysaccharide-induced muscle catabolism via coordinate
activation of ubiquitin-proteasome and autophagy-lysosome pathways.
FASEB J. 25:99–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu S, Adewole D, Yu L, Sid V, Wang BOK
and Yang C: Rutin attenuates inflammatory responses induced by
lipopolysaccharide in an in vitro mouse muscle cell (C2C12) model.
Poult Sci. 98:2756–2764. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baker LA, Martin NRW, Kimber MC, Pritchard
GJ, Lindley MR and Lewis MP: Resolvin E1 (Rv E1) attenuates LPS
induced inflammation and subsequent atrophy in C2C12 myotubes. J
Cell Biochem. 119:6094–6103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rogero MM and Calder PC: Obesity,
inflammation, Toll-like receptor 4 and fatty acids. Nutrients.
10:102018. View Article : Google Scholar
|
|
36
|
Płóciennikowska A, Hromada-Judycka A,
Borzęcka K and Kwiatkowska K: Co-operation of TLR4 and raft
proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life
Sci. 72:557–581. 2015. View Article : Google Scholar
|
|
37
|
Yeung YT, Aziz F, Guerrero-Castilla A and
Arguelles S: Signaling pathways in inflammation and
anti-inflammatory therapies. Curr Pharm Des. 24:1449–1484. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taams LS: Inflammation and immune
resolution. Clin Exp Immunol. 193:1–2. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Banerjee S, Biehl A, Gadina M, Hasni S and
Schwartz DM: Erratum to: JAK-STAT signaling as a target for
inflammatory and autoimmune diseases: Current and future prospects.
Drugs. 77:12612017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Banerjee S, Biehl A, Gadina M, Hasni S and
Schwartz DM: JAK-STAT signaling as a target for inflammatory and
autoimmune diseases: Current and future prospects. Drugs.
77:521–546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stabile H, Scarno G, Fionda C, Gismondi A,
Santoni A, Gadina M and Sciumè G: JAK/STAT signaling in regulation
of innate lymphoid cells: The gods before the guardians. Immunol
Rev. 286:148–159. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou GY, Yi YX, Jin LX, Lin W, Fang PP,
Lin XZ, Zheng Y and Pan CW: The protective effect of juglanin on
fructose-induced hepatitis by inhibiting inflammation and apoptosis
through TLR4 and JAK2/STAT3 signaling pathways in fructose-fed
rats. Biomed Pharmacother. 81:318–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zimmers TA, Fishel ML and Bonetto A: STAT3
in the systemic inflammation of cancer cachexia. Semin Cell Dev
Biol. 54:28–41. 2016. View Article : Google Scholar : PubMed/NCBI
|