Introduction
A hypertrophic scar (HPS) is a serious dermal
condition resulting from burn or traumatic injuries, which severely
impairs the quality of life by causing cosmetic disturbance and
functional deformities (1).
Currently, no satisfactory therapeutics have been developed for the
treatment of HPS. Therefore, the development of novel
pharmacological agents for preventing and treating HPS is
required.
HPS formation involves an abnormal fibrous wound
healing process characterized by the over-proliferation of local
fibroblasts and the excessive deposition of collagen and other
extracellular matrix (ECM) proteins such as proteoglycans and
fibronectin (2). During the
development of HPS, fibroblasts differentiate into myofibroblasts,
which increases ECM synthesis and tissue contraction (3). Myofibroblasts are a particular type of
fibroblast characterized by the abundant expression of α-smooth
muscle actin (α-SMA) (4). Cellular
communication network factor 2 (CCN2) also serves an important role
in the proliferation of HPS fibroblasts and ECM deposition
(5). Currently, the underlying
mechanisms of HPS are not fully understood. However, it is widely
accepted that the TGF-β1/SMAD3 signaling pathway serves an
essential role in HPS formation (6). SMAD3 is a major intracellular effector
of TGF-β1 that is able to regulate the expression levels of
collagen, α-SMA and CCN2 in the fibrotic response, indicating it is
a potential target for HPS therapy (7). Previous studies have reported that the
downregulation of SMAD3 expression or activation in HPS fibroblasts
can significantly reduce the fibrotic reactivity (8–11).
Therefore, pharmacological agents with inhibitory activity over
SMAD3 may have a significant clinical potential in the treatment of
HPS.
In the present study, the ability of specific
compounds, derived from a library of existing therapeutics, was
analyzed with regard to their inhibitory function towards SMAD3.
Ivermectin was selected from the library, which is a semi-synthetic
macrocyclic lactone derivative of the avermectin family used to
treat parasitic diseases (12).
Recently, ivermectin was discovered to be an inhibitor of cancer
stem-like cells and to exhibit anti-inflammatory properties
(13,14). However, to the best of our
knowledge, the effects of ivermectin on HPS fibroblasts have not
been previously reported. In the present in vitro study, the
inhibitory effect of ivermectin on the phosphorylation of SMAD3 was
examined in scar fibroblasts. Furthermore, the experiments
performed in the current study aimed to determine whether
ivermectin treatment could suppress the proliferation of scar
fibroblasts and the expression levels of type I collagen, α-SMA and
CCN2. The results indicated that ivermectin may be a potential
therapeutic agent for HPS treatment.
Materials and methods
Materials
Ivermectin was purchased from Sigma-Aldrich; Merck
KGaA. DMEM, FBS and trypsin were purchased from Thermo Fisher
Scientific, Inc. Anti-Collagen-I (cat. no. ab260043), anti-α-SMA
(cat. no. ab119952), anti-CCN2 (cat. no. ab209780), anti-SMAD3
(cat. no. ab40854), anti-phosphorylated (p)SMAD3 (cat. no. ab52903)
and anti-fibroblast surface protein (FSP; cat. no. ab11333)
antibodies were purchased from Abcam. The anti-GAPDH antibody (cat.
no. sc-365062) was purchased from Santa Cruz Biotechnology, Inc.
The Cell Counting Kit-8 (CCK-8) was obtained from Beyotime
Institute of Biotechnology. The Annexin V Apoptosis Detection kit I
was purchased from BD Biosciences. TRIzol® reagent was
purchased from Invitrogen; Thermo Fisher Scientific, Inc.
Isolation and culture of HPS
fibroblasts
Human HPS fibroblasts were isolated from HPS tissues
of five male patients who had undergone surgical excision 4–6
months after HPS occurred. The recruitment was started in April
2015 and ended in August 2015. The patients were 20–40 years old.
HPS tissues were excised from shoulder and chest. The experiments
were approved by the Ethics Committee of Changhai Hospital
(approval no. CHEC2014-096). All HPS tissues were obtained with
written informed consent according to the Declaration of Helsinki
principles. The tissues in this study were from patients, and the
informed consent was obtained and signed. All patients agreed to
participate in this study. The nature of HPS was confirmed
histologically using hematoxylin and eosin-stained sections of skin
tissues. The tissues were fixed in 10% formaldehyde for 2 days at
room temperature and sectioned at 1 cm2 area and 5 µm
thickness. The sections were stained in hematoxylin solution for 10
min and eosin solution for 10 sec at room temperature and observed
under a light microscope (magnification, ×40). The specimens were
washed three times in sterile DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with an antibiotic/antimycotic (5%
penicillin and streptomycin) preparation and were subsequently cut
into 5×5 mm sections. The sections were incubated in DMEM with 0.2%
dispase overnight at 4°C. The epidermis was removed and the dermis
was minced and digested in DMEM in the presence of 0.2% collagenase
type I for 3 h at 37°C with intermittent shaking. The digestive
action was quenched with DMEM and the obtained cells were cultured
in DMEM supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 0.1 g/ml streptomycin at 37°C in a
humidified incubator with 5% CO2 (15). Only cells from the third to fifth
passages were used in each experiment.
Ivermectin stimulation
The cells were seeded at a density of
1×105 cells/ml into 6-well plates in DMEM containing 10%
FBS (DMEM/10% FBS) for the analysis of mRNA and protein expression
levels. Upon reaching 80% confluence, the medium was removed and
the cells were incubated at 37°C in serum-free DMEM for 24 h.
Subsequently, different concentrations (0.1, 0.3, 1.0 and 3.0
µmol/l) of ivermectin were added simultaneously in DMEM/10% FBS to
treat the cells at 37°C for 48 h. The control cells were grown in
DMEM/10% FBS without the addition of ivermectin.
Cell proliferation assay
The CCK-8 assay is a colorimetric assay that detects
the metabolic activity of viable cells (16). In the present study, the CCK-8 assay
was used according to the manufacturer's instructions to assess the
proliferative activity of HPS fibroblasts. The cells were seeded at
an initial density of 2,000 cells/well into 96-well plates and
subsequently treated at 37°C with ivermectin at different
concentrations (0.1, 0.3, 1.0 and 3.0 µmol/l) for 1, 3 and 5 days.
CCK-8 solution (10 µl) was added to the wells and incubated with
the cells for 2 h at 37°C. The absorbance of the supernatant was
measured using a Multiskan spectrum microplate reader at 450
nm.
In vitro assessment of cell apoptosis
via flow cytometry
Cell apoptosis was detected using the Annexin V-FITC
Apoptosis Detection kit I. Briefly, HPS fibroblasts were treated at
37°C with ivermectin at different concentrations (0.3 and 3.0
µmol/l) for 48 h. Subsequently, the cells were digested with 0.25%
trypsin, washed twice with cold PBS, resuspended in binding buffer
and adjusted to a final concentration of 1×106 cells/100
µl. The cell suspension was incubated with Annexin V-FITC and PI
for 20 min at room temperature in the dark and the samples were
finally measured via flow cytometry (CytoFLEX; Beckman Coulter).
The data was analyzed by Flowjo 10.0.7 (Tree Star FlowJo).
Reverse transcription-quantitative PCR
(RT-qPCR)
Following treatment with ivermectin at different
concentrations (0.1, 0.3, 1.0 and 3.0 µmol/l) for 48 h at 37°C, the
fibroblasts were lysed and total RNA was extracted using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. First strand cDNA was
synthesized from 1 µg RNA using Superscript™ reverse transcriptase
and oligo (dT) primers and PrimeScript RT Master Mix (Takara Bio,
Inc.) at 37°C for 15 min, 85°C for 5 sec and 4°C 5 for sec
(15). qPCR was subsequently
performed using the SYBR Premix Ex Taq (Takara Bio, Inc.) assay for
human collagen-I, α-SMA, CCN2 and the housekeeping gene GAPDH,
following the manufacturer's instructions. Solutions were
predenatured at 95°C for 15 sec, denatured at 95°C for 5 sec,
annealed at 60°C for 30 sec, extended at 72°C for 30 sec, and
amplified for 40 cycles. The relative quantification of the target
gene levels was assessed using RT-qPCR and the ΔΔCt method
(17). The following primer
sequences were used: GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and
reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′; Collagen-I forward,
5′-GAGGGCCAAGACGAAGACATC-3′ and reverse,
5′-CAGATCACGTCATCGCACAAC-3′; α-SMA forward,
5′-GTGTTGCCCCTGAAGAGCAT-3′ and reverse,
5′-GCTGGGACATTGAAAGTCTCA-3′; and CCN2, forward
5′-ACCGACTGGAAGACACGTTTG-3′ and reverse,
5′-CCAGGTCAGCTTCGCAAGG-3′.
Western blot analysis
Following treatment with ivermectin at different
concentrations (0.1, 0.3, 1.0 and 3.0 µmol/l) for 48 h at 37°C, the
cells were washed with ice-cold PBS and lysed in cell lysis buffer
(cat. no. P0013; Beyotime Institute of Biotechnology). Protein
concentrations were determined using a bicinchoninic acid kit (cat.
no. P0012; Beyotime Institute of Biotechnology). The protein
lysates (2 µg) were separated by SDS-PAGE (10% gel) and transferred
to a PVDF membrane. Following blocking with TBS-Tween-20 (0.1%
Tween-20) containing 5% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.) for 1 h at room temperature, the membranes were
immunoblotted with monoclonal rabbit anti-type I collagen antibody
(1:2,000; cat. no. ab260043; Abcam), monoclonal mouse anti-α-SMA
antibody (1:250; cat. no. ab119952; Abcam), monoclonal rabbit
anti-CCN2 antibody (1:500; cat. no. ab209780; Abcam), monoclonal
rabbit anti-SMAD3 antibody (1:1,000; cat. no. ab40854; Abcam),
monoclonal rabbit anti-pSMAD3 antibody (1:2,000; cat. no. ab52903;
Abcam) and monoclonal mouse anti-GAPDH antibody (1:2,000; cat. no.
sc-365062; Santa Cruz Biotechnology, Inc.) at 4°C overnight.
Subsequently, the bound antibodies were incubated with horseradish
peroxidase-conjugated secondary antibodies at room temperature for
1 h and visualized using an ECL chemiluminescence system (BeyoECL
Star; Beyotime Institute of Biotechnology) (15). ImageJ software (v1.51; National
Institutes of Health) was used for densitometry.
Statistical analysis
All experiments were repeated three times. Data are
presented as the mean ± SD. The western blotting bands were
semi-quantified via densitometry. The mRNA and protein expression
levels of each target gene and protein were normalized according to
the corresponding expression levels of GAPDH. SPSS software (v23;
IBM Corp.) was used for the statistical analysis. A one-way ANOVA
was used to compare the statistical differences between the groups
and the post hoc test used the Tukey's method. P<0.05 was
considered to indicate a statistically significant difference.
Results
Identification of primary HPS
fibroblasts
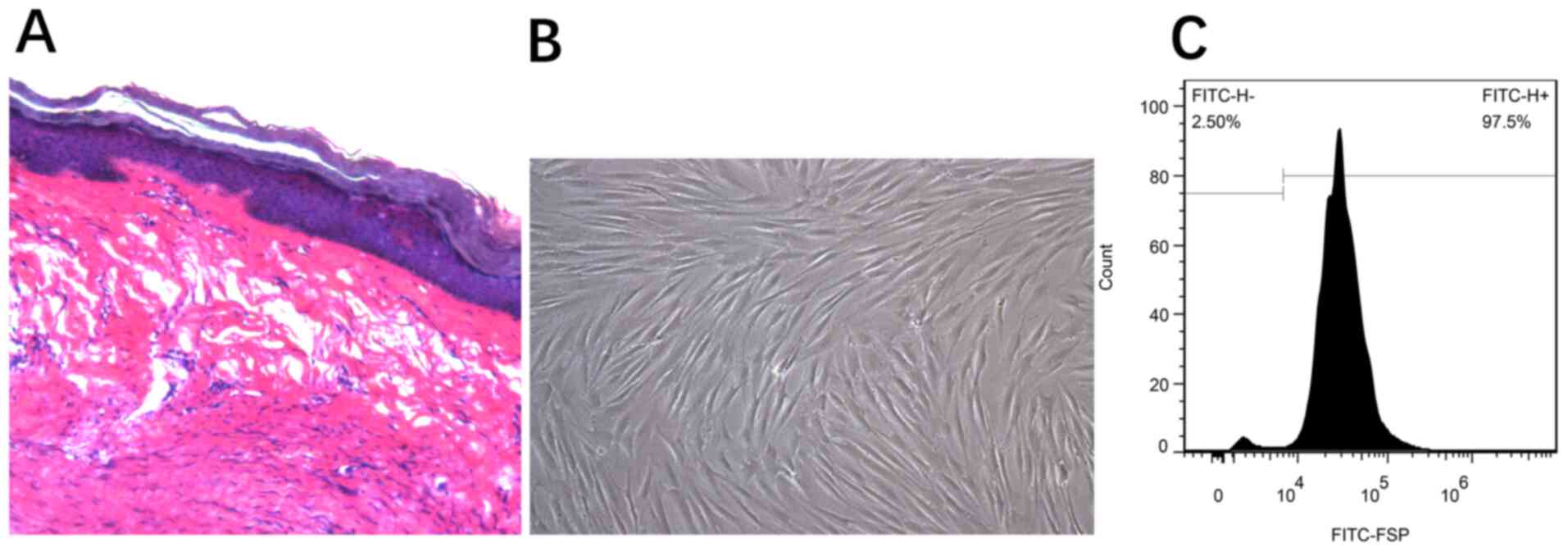
Hematoxylin and eosin-stained sections from the
tissues were histologically examined to confirm the diagnosis of
HPS (Fig. 1A). The adherent
fibroblasts were spindle or star-shaped squamous cells with
multi-protrusions (Fig. 1B). Using
flow cytometry, the cells were detected according to their FSP
expression (anti-FSP antibody); the positive rate was estimated to
be 97.5% (Fig. 1C).
Ivermectin inhibits the expression of
pSMAD3 in HPS fibroblasts without affecting the production of
SMAD3
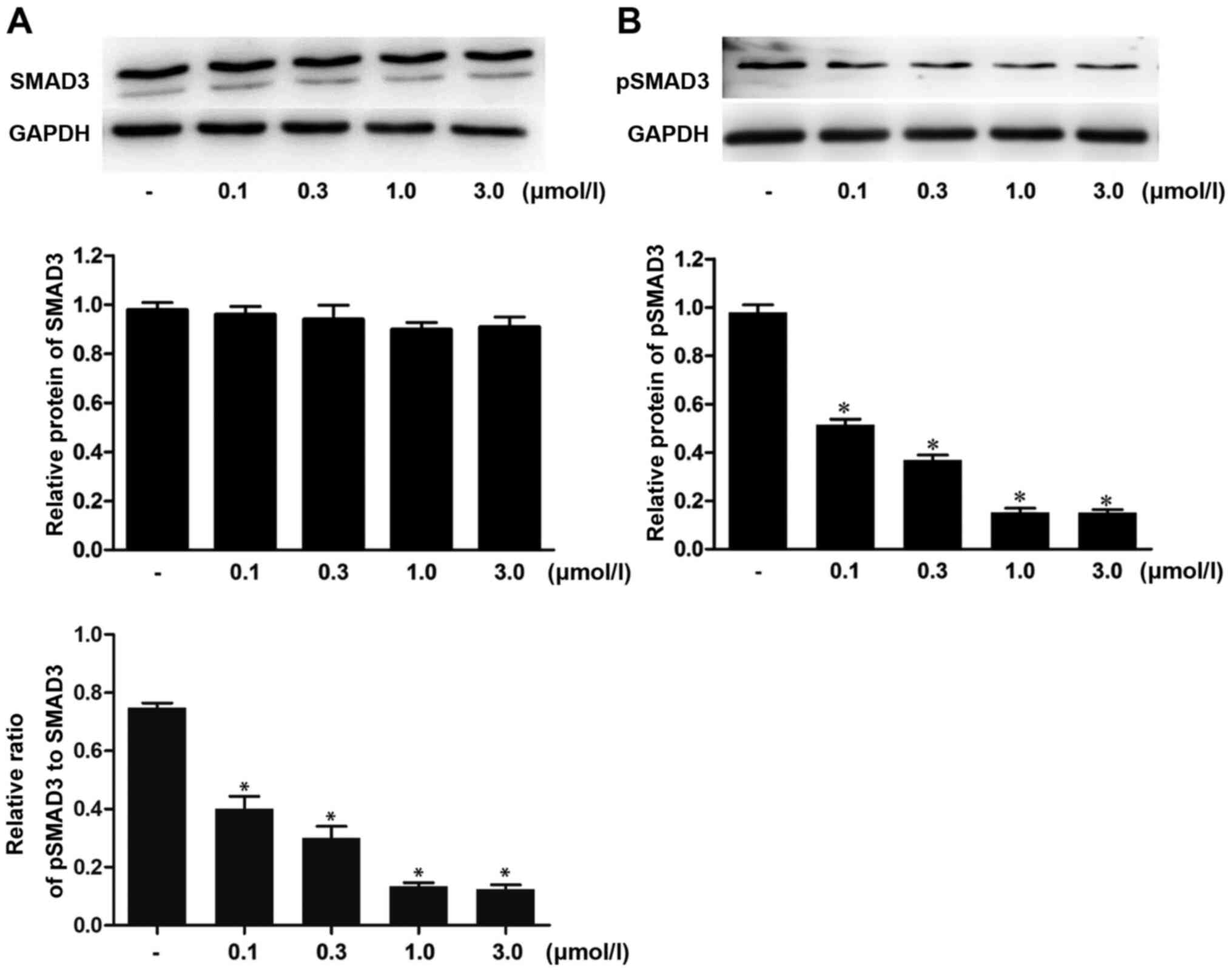
Western blot analysis was performed to examine the
effects of ivermectin on the expression levels of SMAD3 and pSMAD3
in HPS fibroblasts. The data indicated no significant differences
in the expression of SMAD3 between the different groups (Fig. 2A), whereas the expression levels of
pSMAD3 in the ivermectin treatment groups (0.1, 0.3, 1.0 and 3.0
µmol/l) were significantly downregulated compared with those in the
blank control group (Fig. 2B).
These results suggested that ivermectin may be able to inhibit the
expression of pSMAD3 in HPS fibroblasts without affecting the
production of SMAD3.
Ivermectin inhibits HPS fibroblast
proliferation, but does not affect cell apoptosis
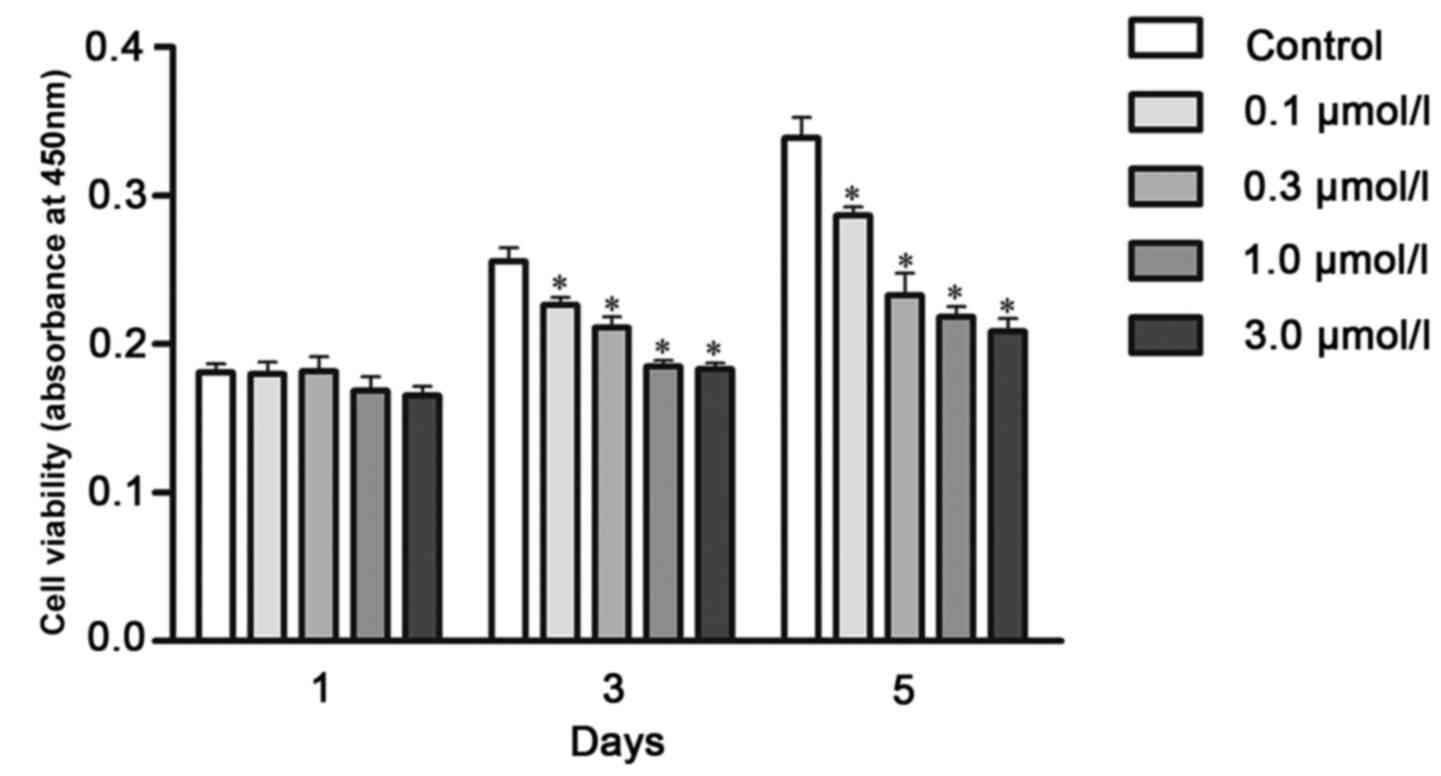
The CCK-8 assay was performed to detect cell
proliferation. On day 1, no significant changes were observed with
different concentrations of ivermectin treatment. On day 3,
ivermectin treatment significantly diminished cell proliferation by
9, 14, 21 and 28%, at doses of 0.1, 0.3, 1.0 and 3.0 µmol/l,
respectively, compared with the control group. On day 5, the
proliferation rate following ivermectin treatment at 0.1, 0.3, 1.0
and 3.0 µmol/l decreased by 15, 31, 35 and 38%, respectively
(Fig. 3).
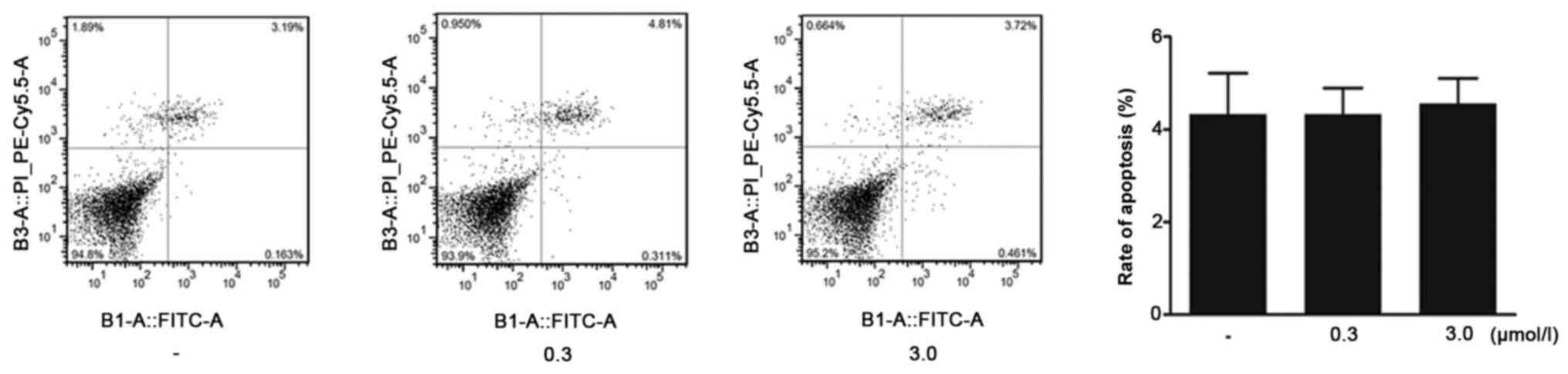
HPS fibroblasts were treated with ivermectin for 48
h and flow cytometry was subsequently performed to assess cell
apoptosis. Flow cytometric analysis indicated that the apoptotic
rates in the groups receiving ivermectin treatment (0.3 and 3.0
µmol/l) were not significantly different compared with the blank
control group (Fig. 4). These
findings suggested that ivermectin could suppress the proliferation
of HPS fibroblasts without affecting cell apoptosis.
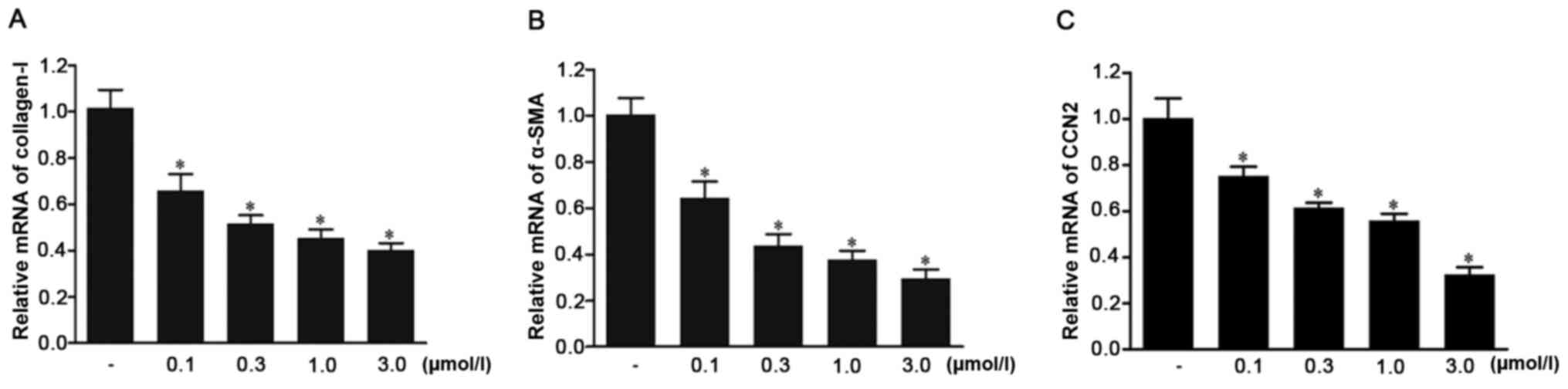
Ivermectin downregulates the mRNA
expression levels of type I collagen, α-SMA and CCN2 in HPS
fibroblasts
The effects of ivermectin on the mRNA expression
levels of type I collagen, α-SMA and CCN2 in HPS fibroblasts were
assessed using RT-qPCR. The results demonstrated that the mRNA
expression levels of all genes were significantly downregulated in
the cell groups treated with ivermectin (0.1, 0.3, 1.0 and 3.0
µmol/l) compared with those in the control group (Fig. 5). The effects became stronger with
the increase of concentration. These findings indicated that
ivermectin may decrease the mRNA expression levels of type I
collagen, α-SMA and CCN2 in HPS fibroblasts.
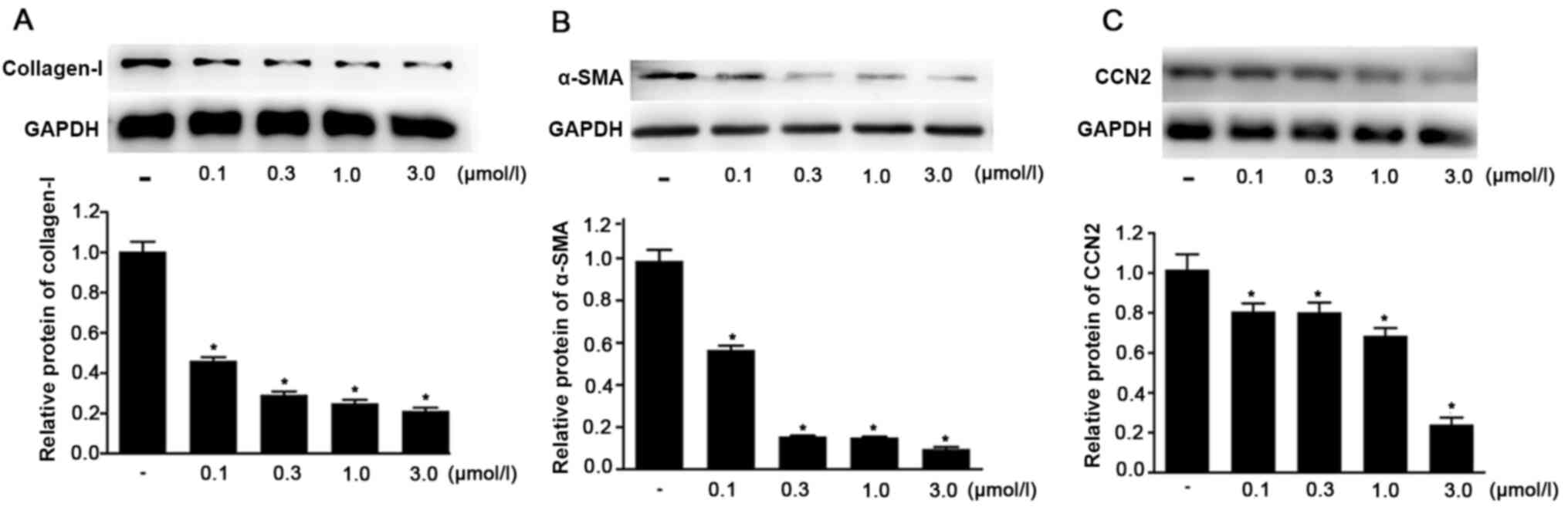
Ivermectin downregulates the protein
expression levels of type I collagen, α-SMA and CCN2 in HPS
fibroblasts
Western blot analysis was performed to assess the
effects of ivermectin on the protein expression of type I collagen,
α-SMA and CCN2 in HPS fibroblasts. It was identified that the
protein expression levels of all these factors were significantly
decreased in the ivermectin treatment groups (0.1, 0.3, 1.0 and 3.0
µmol/l) compared with the control group (Fig. 6). The effects became stronger with
the increase of concentration. These data suggested that ivermectin
may significantly decrease the protein expression levels of type I
collagen, α-SMA and CCN2 in HPS fibroblasts.
Discussion
Ivermectin is a type of macrocyclic lactone
parasiticide that displays anti-inflammatory properties (14). In the present study, the inhibitory
effects of ivermectin on fibrotic reactivity in HPS fibroblasts
were investigated. The main findings of the study demonstrated that
ivermectin could inhibit the phosphorylation of SMAD3 in HPS
fibroblasts. In addition, it was identified that ivermectin could
inhibit cell proliferation and decrease the expression levels of
type I collagen, α-SMA and CCN2. To the best of our knowledge,
these effects of ivermectin on HPS fibroblasts have not been
previously reported.
The accumulation of ECM in fibrotic diseases usually
results from elevated mRNA expression levels due to increased
transcriptional activation (18).
It has been shown that TGF-β1 serves an essential role in
modulating ECM gene expression and that this process is
SMAD3-dependent (19). Following
the binding of the receptor on the cell membrane, TGF-β1 promotes
TGF-β1 receptor kinase to phosphorylate SMAD3 (20). Subsequently, pSMAD3 translocates
into the nucleus and activates gene transcription to mediate
collagen production (21,22). pSMAD3 is the activated version of
SMAD3 and has been reported to be an important modulator of ECM
expression (23). Studies have
revealed that SMAD3 is upregulated and phosphorylated in HPS
fibroblasts (24,25). Thus, the inhibition of the
production or phosphorylation of SMAD3 may be used as a potential
treatment strategy for HPS. Small interfering RNA targeting SMAD3
can decrease ECM deposition and attenuate the process of fibrosis
(26). In the present study, the
results demonstrated that ivermectin treatment significantly
decreased the expression of pSMAD3 in HPS fibroblasts in a
dose-dependent manner. However, the effects on SMAD3 production
were not investigated. Collectively, the current findings indicated
that ivermectin could suppress the phosphorylation of SMAD3 and
that it may be able to inhibit the fibrotic reactivity of HPS
fibroblasts.
The aberrant proliferation of fibroblasts is usually
observed in pathological scars compared with scarless healing
(27). Previous studies have
revealed that the regulation of the proliferation and apoptosis of
fibroblasts are altered in HPS (4,28). HPS
fibroblasts exhibit a higher proliferation rate compared with
normal fibroblasts (29). The
inhibition of the proliferation of HPS fibroblasts may be a
potential treatment for HPS. The present results suggested that
ivermectin could significantly inhibit HPS fibroblast
proliferation, but it did not affect cell apoptosis. The inhibitory
effect was enhanced following an increase in the dose and treatment
duration of ivermectin. It has been previously reported that TGF-β1
was able to promote the proliferation of fibroblasts via the SMAD3
signaling pathway (24). Although,
the precise mechanism remains unknown, it was suggested that it may
be associated with the inhibitory effect caused on SMAD3.
Overproduction and aggregation of ECM is the
principal feature of HPS (4).
Collagen is the key component of ECM and is mainly synthesized by
fibroblasts (30). Fibroblasts can
differentiate into myofibroblasts following stimulation with
TGF-β1; this process also depends on the action of SMAD3 (31). Myofibroblasts exhibit increased
proliferative and secretory properties, as well as serve a major
role in HPS formation by persistently synthesizing collagen
(32). These myofibroblasts are
contractile cells promoting scar contraction; they also overexpress
α-SMA, which is a well-known marker of HPS (33,34).
CCN2 is a type of profibrogenic cytokine that can improve the
proliferation of fibroblasts and ECM deposition; its expression is
also regulated by the TGF-β1/SMAD3 signaling pathway (35). The inhibition of the expression
levels of type I collagen, α-SMA and CCN2 is the main mechanism of
HPS treatment. The present study demonstrated that ivermectin could
significantly decrease the protein and mRNA expression levels of
type I collagen, α-SMA and CCN2 in HPS fibroblasts using RT-qPCR
and western blot analyses. These findings are important in
considering that ivermectin may be able to decrease the deposition
of the ECM and diminish tissue contraction. Ivermectin has been
used as an antiparasitic agent for several years and has been
identified to be safe for human use (14). Since SMAD3 serves an essential role
in HPS formation, the effects of ivermectin on suppressing type I
collagen, α-SMA and CCN2 expression may be associated with the
inhibition of SMAD3 phosphorylation.
In conclusion, the present study demonstrated that
ivermectin was able to inhibit the proliferation of HPS
fibroblasts. Ivermectin could also suppress the phosphorylation of
SMAD3 and decrease the production of type I collagen, α-SMA and
CCN2, which are phenotypic and functional markers of fibrogenesis.
The results suggested that ivermectin may be a promising
therapeutic agent for HPS. However, further studies using animal
models of dermal fibrosis and placebo-controlled clinical studies
are required to conclusively identify the effects of
ivermectin.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81120108015 and 81571897),
the National Basic Research Program of China (973 Program; grant
no. 2012CB518100) and the ‘Twelfth Five-Year’ Scientific Program of
China (grant no. AWS11J008).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZX, RS and JL supervised the study. ZX and ST
conceived and designed the experiments. ST conceived the current
study, analyzed the data and drafted the manuscript. YZ, SX and PL
advised on the design of the experiments and guidance on the
process. YZ, SX and PL also made manuscript revisions and helped
with the experiments. RS and JL selected ivermectin from the
library, designed the current study and supervised the experiments.
All authors reviewed and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Changhai Hospital (approval no. CHEC2014-096). The
tissues in this study were from patients, and the informed consent
was obtained and signed. All patients agreed to participate in this
study.
Patient consent for publication
Consent for publication was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Finnerty CC, Jeschke MG, Branski LK,
Barret JP, Dziewulski P and Herndon DN: Hypertrophic scarring: The
greatest unmet challenge after burn injury. Lancet. 388:1427–1436.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang J, Li Y, Bai X, Li Y, Shi J and Hu
D: Recent advances in hypertrophic scar. Histol Histopathol.
33:27–39. 2018.PubMed/NCBI
|
|
3
|
Lebonvallet N, Laverdet B, Misery L,
Desmouliere A and Girard D: New insights into the roles of
myofibroblasts and innervation during skin healing and innovative
therapies to improve scar innervation. Exp Dermatol. 27:950–958.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu Z, Ding J, Shankowsky HA and Tredget
EE: The molecular mechanism of hypertrophic scar. J Cell Commun
Signal. 7:239–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamanaka O, Saika S, Ikeda K, Miyazaki K,
Kitano A and Ohnishi Y: Connective tissue growth factor modulates
extracellular matrix production in human subconjunctival
fibroblasts and their proliferation and migration in vitro. Jpn J
Ophthalmol. 52:8–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kiritsi D and Nystrom A: The role of TGF
beta in wound healing pathologies. Mech Ageing Devt. 172:51–58.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Flanders KC: Smad3 as a mediator of the
fibrotic response. Int J Exp Pathol. 85:47–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Terada Y, Hanada S, Nakao A, Kuwahara M,
Sasaki S and Marumo F: Gene transfer of Smad7 using electroporation
of adenovirus prevents renal fibrosis in post-obstructed kidney.
Kidney Int. 61 (1 Suppl):S94–S98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu C, Jiang J, Boye A, Jiang Y and Yang Y:
Compound Astragalus and Salvia miltiorrhiza extract
suppresses rabbits' hypertrophic scar by modulating the TGF-β/Smad
signal. Dermatology. 229:363–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mun JH, Kim YM, Kim BS, Kim JH, Kim MB and
Ko HC: Simvastatin inhibits transforming growth factor-β1-induced
expression of type I collagen, CTGF, and α-SMA in keloid
fibroblasts. Wound Repair Regen. 22:125–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang GY, Yi CG, Li X, Ma B, Li ZJ, Chen
XL, Guo SZ and Gao WY: Troglitazone suppresses transforming growth
factor-beta1-induced collagen type I expression in keloid
fibroblasts. Br J Dermatol. 160:762–770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lynagh T, Webb TI, Dixon CL, Cromer BA and
Lynch JW: Molecular determinants of ivermectin sensitivity at the
glycine receptor chloride channel. J Biol Chem. 286:43913–43924.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dominguez-Gomez G, Chavez-Blanco A,
Medina-Franco JL, Saldivar-Gonzalez F, Flores-Torrontegui Y, Juarez
M, Diaz-Chavez J, Gonzalez-Fierro A and Duenas-Gonzalez A:
Ivermectin as an inhibitor of cancer stem-like cells. Mol Med Rep.
17:3397–3403. 2018.PubMed/NCBI
|
|
14
|
Deeks ED: Ivermectin: A review in rosacea.
Am J Clin Dermatol. 16:447–452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang B, Zhu B, Liang Y, Bi L, Hu Z, Chen
B, Zhang K and Zhu J: Asiaticoside suppresses collagen expression
and TGF-β/Smad signaling through inducing Smad7 and inhibiting
TGF-βRI and TGF-βRII in keloid fibroblasts. Arch Dermatol Res.
303:563–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qin T, Lu XT, Li YG, Liu Y, Yan W, Li N
and Sun YY: Effect of Period 2 on the proliferation, apoptosis and
migration of osteosarcoma cells, and the corresponding mechanisms.
Oncol Lett. 16:2668–2674. 2018.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sidgwick GP and Bayat A: Extracellular
matrix molecules implicated in hypertrophic and keloid scarring. J
Eur Acad Dermatol Venereol. 26:141–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verrecchia F and Mauviel A: Transforming
growth factor-beta and fibrosis. World J Gastroenterol.
13:3056–3062. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kajdaniuk D, Marek B, Borgiel-Marek H and
Kos-Kudla B: Transforming growth factor β1 (TGFβ1) in physiology
and pathology. Endokrynol Pol. 64:384–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu R and Cen Y: Transforming growth factor
beta1/Smad3 signal transduction pathway and post-traumatic scar
formation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 26:330–335.
2012.(In Chinese). PubMed/NCBI
|
|
22
|
Leask A and Abraham DJ: TGF-beta signaling
and the fibrotic response. FASEB J. 18:816–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang CJ, Yen YH, Hung LY, Wang SH, Pu CM,
Chien HF, Tsai JS, Lee CW, Yen FL and Chen YL: Thalidomide inhibits
fibronectin production in TGF-β1-treated normal and keloid
fibroblasts via inhibition of the p38/Smad3 pathway. Biochem
Pharmacol. 85:1594–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-beta signaling in fibrosis. Growth Factors.
29:196–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chin GS, Liu W, Peled Z, Lee TY,
Steinbrech DS, Hsu M and Longaker MT: Differential expression of
transforming growth factor-beta receptors I and II and activation
of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 108:423–429.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Gao Z, Shi Y, Sun Y, Lin Z, Jiang
H, Hou T, Wang Q, Yuan X, Zhu X, et al: Inhibition of Smad3
expression decreases collagen synthesis in keloid disease
fibroblasts. J Plast Reconstr Aesthet Surg. 60:1193–1199. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gauglitz GG, Korting HC, Pavicic T,
Ruzicka T and Jeschke MG: Hypertrophic Scarring and Keloids:
Pathomechanisms and current and emerging treatment strategies. Mol
Med. 17:113–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Linge C, Richardson J, Vigor C, Clayton E,
Hardas B and Rolfe K: Hypertrophic scar cells fail to undergo a
form of apoptosis specific to contractile collagen-the role of
tissue transglutaminase. J Invest Dermatol. 125:72–82. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo S, Benathan M, Raffoul W, Panizzon RG
and Egloff DV: Abnormal balance between proliferation and apoptotic
cell death in fibroblasts derived from keloid lesions. Plast
Reconstr Surg. 107:87–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wolfram D, Tzankov A, Pulzl P and
Piza-Katzer H: Hypertrophic scars and keloids-a review of their
pathophysiology, risk factors, and therapeutic management. Dermatol
Surg. 35:171–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu L, Zhu YJ, Yang X, Guo ZJ, Xu WB and
Tian XL: Effect of TGF-beta/Smad signaling pathway on lung
myofibroblast differentiation. Acta Pharmacol Sin. 28:382–391.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Conte E, Gili E, Fagone E, Fruciano M,
Iemmolo M and Vancheri C: Effect of pirfenidone on proliferation,
TGF-β-induced myofibroblast differentiation and fibrogenic activity
of primary human lung fibroblasts. Eur J Pharm Sci. 58:13–19. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Darby IA, Laverdet B, Bonté F and
Desmoulière A: Fibroblasts and myofibroblasts in wound healing.
Clin Cosmet Investig Dermatol. 7:301–311. 2014.PubMed/NCBI
|
|
34
|
Meran S and Steadman R: Fibroblasts and
myofibroblasts in renal fibrosis. Int J Exp Patho. 92:158–167.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Colwell AS, Phan TT, Kong W, Longaker MT
and Lorenz PH: Hypertrophic scar fibroblasts have increased
connective tissue growth factor expression after transforming
growth factor-beta stimulation. Plast Reconstr Surg. 116:1387–1992.
2005. View Article : Google Scholar : PubMed/NCBI
|