Introduction
Gastric cancer is a major global health concern and
is one of the major causes of cancer-related mortality (1). Over the past few years, the incidence
and mortality of gastric cancer in East Asia have markedly
increased (2). Despite advances in
diagnostic technology and optimization of surgical treatment plans
providing novel treatment strategies for patients with gastric
cancer, patient prognosis remains poor (3,4).
Therefore, identifying the molecular mechanism underlying gastric
cancer is important for the development of novel treatment
strategies.
Endoplasmic reticulum stress has been observed
during the development of various tumors, such as breast and lung
cancer (5–7). In several cell types, endoplasmic
reticulum stress can lead to an increase in protein unfolding and
cell apoptosis (8). The Cancer
Genome Atlas database analysis (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga)
demonstrated that the expression of the key gene GPR78 for
endoplasmic reticulum stress in gastric cancer samples was
significantly increased. It has also been reported that endoplasmic
reticulum stress could induce the repeated use of abnormally folded
proteins in cells, which may promote proliferation and metastasis
of tumor tissues (9). Therefore,
the aforementioned studies indicated that endoplasmic reticulum
stress might promote cancer development.
X-box binding protein-1 (XBP-1) serves an important
role in the occurrence and development of endoplasmic reticulum
stress and the ratio of the two splicing mutants (XBP-1S and
XBP-1U) caused by differential mRNA translation in cells often
determines the main physiological functions of the cells (10). A previous study revealed that the
unfolded protein response, which is induced by endoplasmic
reticulum stress, might promote the development of multiple types
of cancer, such as neck squamous carcinoma cells and epidermoid
carcinoma (11). The active form of
XBP-1 (XBP-1S) may promote the development of endoplasmic reticulum
stress, thus inducing the unfolded protein response. By contrast,
the expression of XBP-1U represses the development of endoplasmic
reticulum stress, inhibiting the unfolded protein response
(12).
Furthermore, it has been reported that microRNA
(miRNA/miR)-500a may promote tumor development and progression by
downregulating XBP-1U expression and upregulating XBP-1S expression
(13). Further research using
StarBase database (http://starbase.sysu.edu.cn/) revealed that lncRNA
NEAT1 might target and downregulate the expression of miR-500a-3p
(14). However, whether lncRNA
NEAT1 alters the expression levels of miR-500a-3p and XBP-1 or
influences the development of gastric carcinoma is not completely
understood. Therefore, the present study investigated the roles and
regulatory mechanism underlying lncRNA NEAT1 in gastric carcinoma
cells.
Materials and methods
Cell culture and treatment
Normal epithelial cells of the gastric mucosa (GES1)
and gastric carcinoma cell lines (AGS, HST2 and FU97) were obtained
from American Type Culture Collection. Cells were cultured in
RPMI-1640 (HyClone; Cytiva) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2.
lncRNA NEAT1 overexpression vector (GV369, 1×108 TU/ml)
and its negative control (empty vector), miR-500a-3p mimics (25 µl,
AUGCACCUGGGCAAGGAUUCUG) and its control (mimic NC,
ACAGCAGUGCCAAUUGGUGGUCUGC) were purchased from Shanghai GeneChem
Co., Ltd. Polybrene reagent (Shanghai GeneChem Co., Ltd.) was
applied to promote transfection efficacy of lncRNA NEAT1
overexpression vector in AGS cells (2×104 cells/well).
After transfection for 24 h at 37°C, the cells were used for
further experiment. When the confluence of AGS cells was 30%, the
transfection of mimic was performed using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 24 h at 37°C
and cells were used for further experiment.
Collection of clinical samples
A total of 30 clinical gastric cancer tissue samples
and 30 adjacent healthy tissue samples were obtained from patients
from the Zhongda Hospital Southeast University (Nanjing, China)
between September 2018 and May 2020. Inclusion criteria were that
the gastric cancer had clear pathological diagnosis and no distant
metastasis, the patient had not undergone radiation and
chemotherapy and no other malignant tumors were found. After
gastrectomy, tumor and adjacent healthy tissue (normal gastric
mucosal tissue 5 cm from gastric cancer tissue) samples were
collected. All experimental procedures were approved by the Ethics
Committee of Zhongda Hospital Southeast University (approval number
2018ZDSYLL136291). Written informed consent was obtained from all
patients. The clinical characteristics of the patients are
summarized in Table I.
 | Table I.Characteristics of patients with
gastric cancer. |
Table I.
Characteristics of patients with
gastric cancer.
| Characteristic | Cases (n=30) |
|---|
| Age (year) | 58.2±6.1 |
| Male, n (%) | 17 (56.7) |
| Smoking, n (%) | 10 (33.3) |
| Drinking, n
(%) | 13 (43.3) |
| Family history of
cancer (any type), n (%) | 12 (40.0) |
| Hypertension, n
(%) | 16 (53.3) |
| Diabetes, n
(%) | 17 (56.7) |
Cell Counting Kit-8 (CCK-8)
assays
AGS cells (6×103 cells/well) were seeded
into 96-well plates. Following culture for 24 h, 10 µl CCK-8
solution (Dojindo Molecular Technologies, Inc.) was added to each
well and incubated for 1.5 h. Absorbance was measured at a
wavelength of 450 nm using a spectrophotometer (Thermo Fisher
Scientific, Inc.).
Dual-luciferase reporter assay
The AGS cells were seeded into 12-well plates at 60%
confluence and transfected within 24 h with luciferase reporter
gene plasmids (pGL3-basic, 1 µg) containing the 3′UTR of NEAT1 or
XBP1, and miR-500a-3p mimic (20 µM) or miR-NC. Subsequently, 48 h
after cell transfection (Shanghai GeneChem Co., Ltd.) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) the luciferase activities were detected according
to the instructions of the Dual-Luciferase reporter gene assay kit
(Thermo Fisher Scientific, Inc.). Renilla luciferase
plasmids (0.02 µg) was used as internal reference.
Apoptosis assay
AGS cells (4×105) were plated into 60-mm
culture dishes and their apoptosis levels were evaluated using
Annexin V-FITC/PI kit (Thermo Fisher Scientific, Inc.). Cell
suspensions were prepared and washed with PBS. Subsequently, cells
were incubated with Annexin V (5 µl) at room temperature for 10 min
in the dark and then PI (10 µl) in an ice-bath (Beyotime Institute
of Biotechnology) in the dark for 30 min. Following washing with
PBS, flow cytometry was performed to detect cell apoptosis using
CytoFLEX flow cytometry instrument (Beckman Coulter, Inc.). The
apoptosis levels were analyzed using FlowJo 10.0 software (FlowJo
Software). The upper right quadrant
(FITC+/PI+) were late apoptotic cells and
lower right quadrant (FITC+/PI−) were early
apoptotic cells. The apoptosis levels were evaluated through
calculating the percentage of late apoptotic cells and late
apoptotic cells.
Immunofluorescence
AGS cells were seeded onto a circular glass sheet.
Cells were fixed using 4% paraformaldehyde for 15 min at room
temperature, permeabilized with 0.1% Triton X-100 and blocked with
normal goat serum (Gibco; Thermo Fisher Scientific, Inc.) for 30
min at room temperature. The anti-LC3II/I antibody (cat. no.
ab192890; 1:50, Abcam) was used to incubate overnight at 4°C,
followed by an incubation at room temperature for 1 h with a goat
secondary antibody to Rabbit IgG (Alexa Fluor 488; ab150081;
1:10,000) to detect LC3II/I protein expression using a confocal
microscope (Leica Microsystems GmbH). DAPI was used for labelling
nuclear DNA for incubation for 15 min at room temperature. All
experiments were performed according to the manufacturer's
protocol.
Western blotting
Total protein from cells was extracted using RIPA
buffer (Beyotime Institute of Biotechnology). Protein
concentrations were determined using the BCA assay (Beyotime
Institute of Biotechnology). Subsequently, proteins (50 µg) were
uploaded and separated via 10% SDS-PAGE (Beyotime Institute of
Biotechnology) and transferred to PVDF membranes (EMD Millipore).
Following blocking with 5% skimmed milk powder dissolved into PBST
containing 0.05% Tween-20 for 1 h at 4°C, the membranes were
incubated at 4°C overnight with primary antibodies (all purchased
from Abcam) targeted against: 78-kDa glucose-regulated protein
(GRP78; 1:100; cat. no. ab21685), XBP-1S (1:1,000; cat. no.
ab220783), XBP-1U (1:1,000; cat. no. ab37152), caspase-4 (1:1,000;
cat. no. ab238124), C/EBP homologous protein (CHOP; 1:200; cat. no.
ab11419), Bcl-2 (1:1,000; cat. no. ab32124), Bax (1:1,000; cat. no.
ab32503), cleaved-caspase-3 (1:500; cat. no. ab32042), LC3I/II
(1:50; cat. no. ab232940), beclin-1 (1:1,000; cat. no. ab210498),
autophagy-related gene 5 (1:1,000; Atg5; cat. no. ab109490) and
GAPDH (1:5,000; cat. no. ab8245). Following washing with PBST, the
membranes were incubated with secondary antibodies (goat anti-mouse
IgG; cat. no. ab216772; Goat anti Rabbit IgG; cat. no. ab216777,
1:10,000; Abcam) for 2 h at 4°C. Proteins bands were visualized
using ECL reagents (Pierce; Thermo Fisher Scientific, Inc.). GAPDH
was used as the loading control. The grey value of protein bands
was analyzed using ImageJ software 1.46r (National Institutes of
Health).
Reverse transcription-quantitative PCR
(qPCR)
Total RNA from gastric tissue of patients or cells
was isolated using TRIzol® reagent (Thermo Fisher
Scientific, Inc.). Total RNA was reverse transcribed into cDNA
using the reverse transcription kit (Transcriptor cDNA Synth. Kit
2; cat. no. 4897030001, Roche Diagnostics) for 15 min at 37°C.
Subsequently, qPCR was performed with Applied Biosystems PowerUp
SYBR Green using the ABI 7500 system (Thermo Fisher Scientific,
Inc.). The following primers were used for qPCR: lncRNA NEAT1
forward, 5′-TTGGGACAGTFFACGTGTGG-3′ and reverse,
5′-TCAAGTCCAGCAGAGCA-3′; miR-500a-3p forward,
5′-UAAUCCUUGCUACCUGGGUGAGA-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′; GRP78 forward,
5′-CATCACGCCGTCCTATGTCG-3′ and reverse, 5′-CGTCAAAGACCGTGTTCTCG-3′;
XBP-1S, forward, 5′-TGTGCTGAGTCCGCAGCAG-3′ and reverse,
5′-TGGTTGCTGAAGAG-3′; XBP-1U, forward, 5′-TGGTTGCTGAAGAG-3′ and
reverse, 5′-GAGATGTTCTGGAGGGGTGACAACTG-3′; caspase-4 forward,
5′-CAAGAGAAGCAACGTATGGCA-3′ and reverse,
5′-AGGCAGATGGTCAAACTCTGTA-3′; Bax forward,
5′-CCCGAGAGGTCTTTTTCCGAG-3′ and reverse,
5′-CCAGCCCATGATGGTTCTGAT-3′; Bcl-2 forward,
5′-GGTGGGGTCATGTGTGTGG-3′ and reverse,
5′-CGGTTCAGGTACTCAGTCATCC-3′; Atg5 forward,
5′-AAAGATGTGCTTCGAGATGTGT-3′ and reverse,
5′-CACTTTGTCAGTTACCAACGTCA-3′; GAPDH forward,
5′-TGACGTGCCGCCTGGAGAAC-3′ and reverse,
5′-CCGGCATCGAAGGTGGAAGAG-3′; and U6 forward,
5′-TGCTGGGGCTTTCCGGCAGCGC-3′ and reverse,
5′-CCCAGTGAGGTCCGGAGGT-3′. The PCR reaction conditions were 95°C
pre-denaturation for 2 min, 95°C for 15 sec and 60°C for 30 sec for
40 cycles. miRNA and mRNA expression levels were quantified using
the 2−∆∆Cq method (15)
and normalized to the internal reference genes U6 and GAPDH,
respectively.
Colony formation assay
AGS cells were seeded (4×102 cells/well)
into 12-well plates. Following culture for 2 weeks, cells were
fixed with 4% paraformaldehyde for 15 min at room temperature and
stained with crystal violet solution at room temperature for 10 min
(Thermo Fisher Scientific, Inc.). The images were captured using an
inverted microscope (Olympus Corporation).
Transwell assays
AGS cells (5×105 cells/ml) were cultured
in serum-free medium for 12 h. Matrigel was used to pre-coat upper
chamber at 37°C for 30 min. Subsequently, cells were prepared into
single-cell suspension using serum-free medium and plated into the
upper chamber of Transwell inserts (Corning, Inc.).
Serum-containing medium was added to the lower chamber. Following
culture for 24 h, invading cells were fixed with 4%
paraformaldehyde at room temperature for 15 min and stained with
crystal violet solution at room temperature for 20 min (Thermo
Fisher Scientific, Inc.). Invading cells were visualized using an
inverted phase contrast microscope (Olympus Corporation).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 6.0; GraphPad Software Inc.). Data are
presented as the mean ± SD. All the experiments were repeated three
times. Comparisons among multiple groups were analyzed using
one-way ANOVA followed by Tukey's post hoc test. Comparisons
between two groups were analyzed using a paired t-test. Multiple
linear regression and linear regression were performed to assess
the association between expression levels of different proteins.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Endoplasmic reticulum stress-related
protein expression levels are increased in gastric cancer
tissues
Gastric cancer and adjacent healthy tissues were
collected for the detection of endoplasmic reticulum stress- and
apoptosis-related protein expression levels. A decrease in XBP-1
expression levels may indicate decreased GRP78 expression levels,
leading to ER stress and apoptosis, which alters protein folding
and can lead to protein accumulation (16,17).
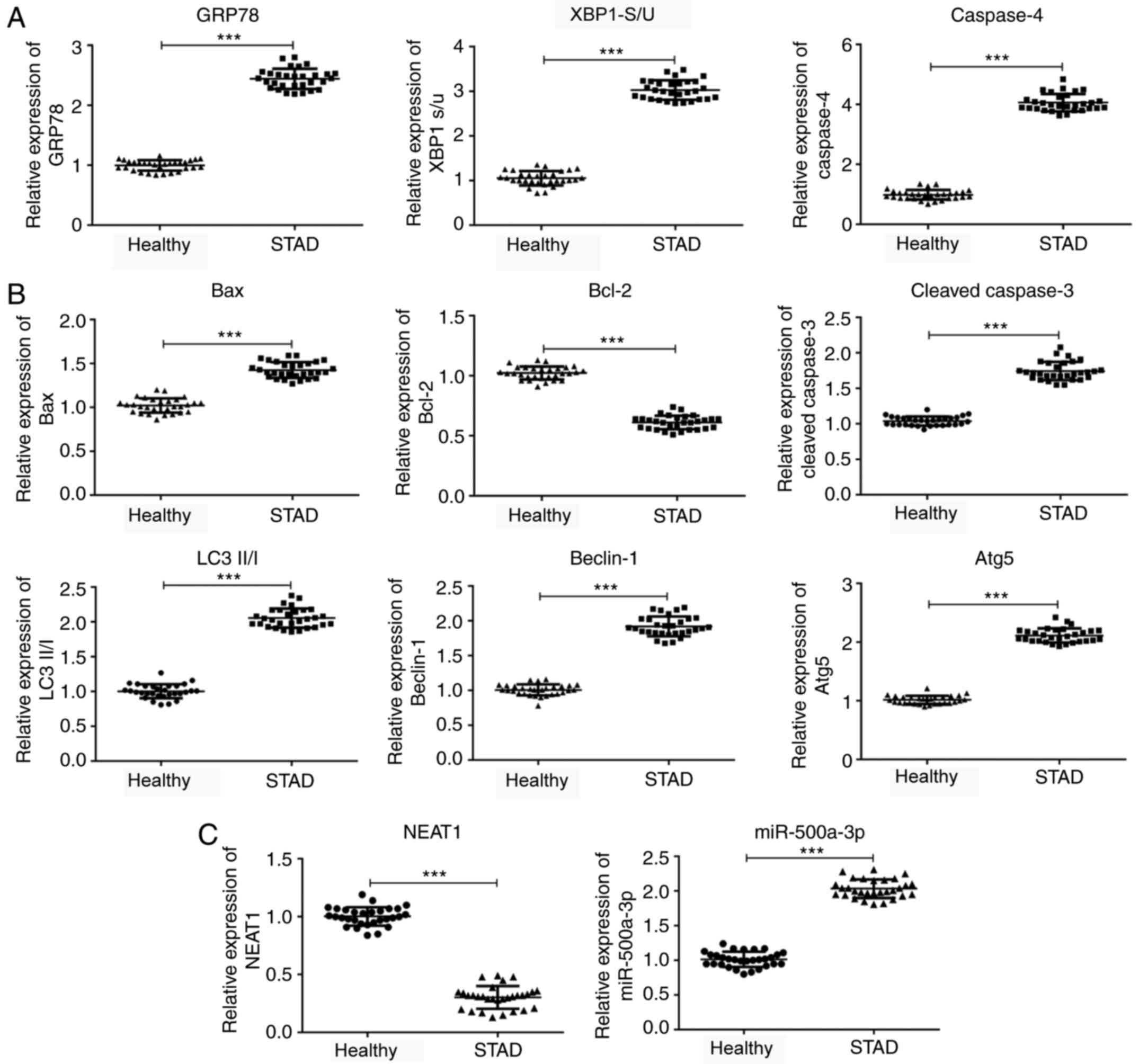
The results of the present study demonstrated that GRP78 and
Caspase4 expression levels were significantly increased in gastric
cancer tissues compared with adjacent healthy tissues (Fig. 1A). Similarly, the ratio of
XBP-1S/XBP-1U was also significantly increased in gastric cancer
tissues compared with adjacent healthy tissues. Bax and
cleaved-caspase-3 expression levels were significantly increased,
whereas Bcl-2 expression levels were significantly decreased in
gastric cancer tissues compared with adjacent healthy tissues
(Fig. 1B). Moreover, the expression
levels of autophagy-related proteins (LC3II/I, Beclin-1 and Atg5)
were significantly increased in gastric cancer tissues compared
with adjacent healthy tissues. Furthermore, lncRNA NEAT1 expression
levels were significantly downregulated, whereas miR-500a-3p
expression levels were significantly upregulated in gastric cancer
tissues compared with adjacent healthy tissues (Fig. 1C).
lncRNA NEAT1 expression is negatively
associated with the occurrence of endoplasmic reticulum stress,
apoptosis and autophagy
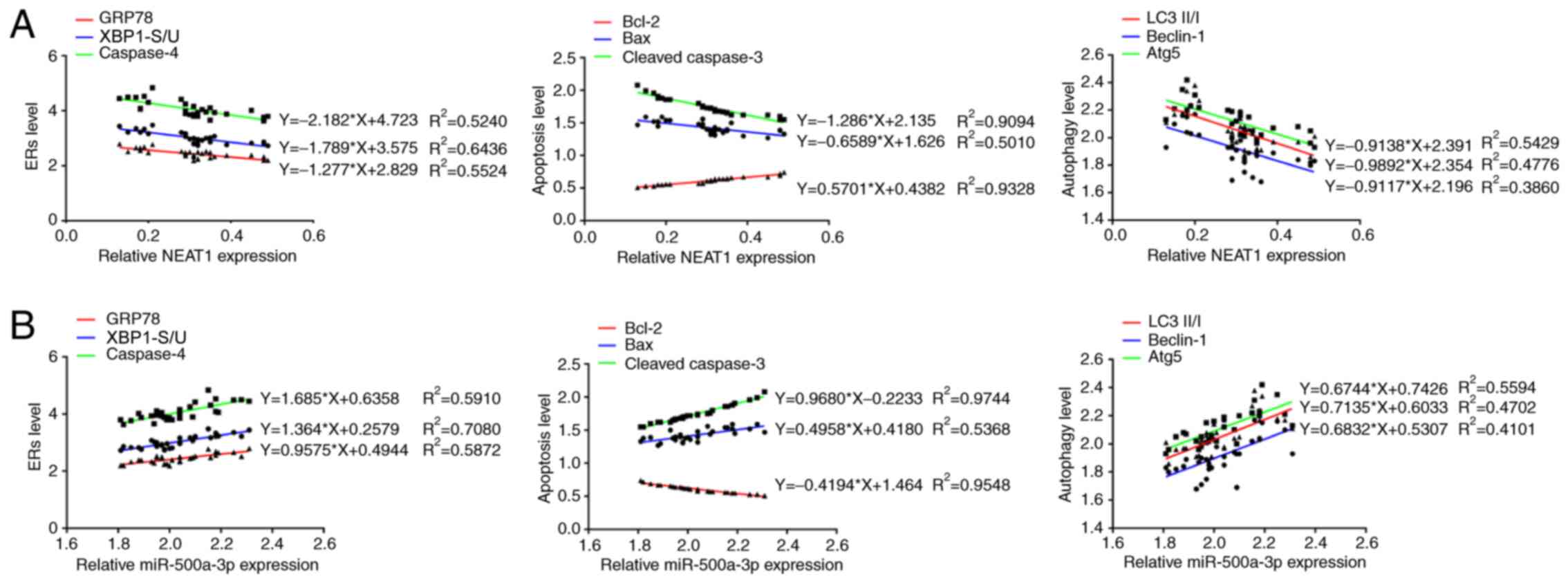
Multiple linear regression was used to analyze the
association between the expression of lncRNA NEAT1 or miR-500a-3p
and the occurrence of endoplasmic reticulum stress, autophagy and
apoptosis. The results demonstrated that lncRNA NEAT1 expression
was negatively associated with the expression of endoplasmic
reticulum stress-related proteins (caspase-4 and GRP78),
apoptosis-related proteins (Bax and cleaved-caspase-3) and
autophagy-related proteins (LC3II/I, Beclin-1 and Atg5) (Fig. 2A). lncRNA NEAT1 expression was also
negatively associated with the XBP-1S/XBP-1U ratio. However,
miR-500a-3p expression was positively associated with the
expression of endoplasmic reticulum stress-related proteins
(caspase-4 and GRP78), apoptosis-related proteins (Bax and
cleaved-caspase-3) and autophagy-related proteins (LC3II/I,
Beclin-1 and Atg5) (Fig. 2B).
Moreover, miR-500a-3p expression was also positively associated
with the XBP-1S/XBP-1U ratio.
Endoplasmic reticulum stress is
increased in gastric cancer cell lines
Normal gastric mucosal epithelial cells (GES1) and
gastric cancer cells (AGS, HST2 and FU97) were selected for
subsequent experiments. Compared with normal gastric mucosal
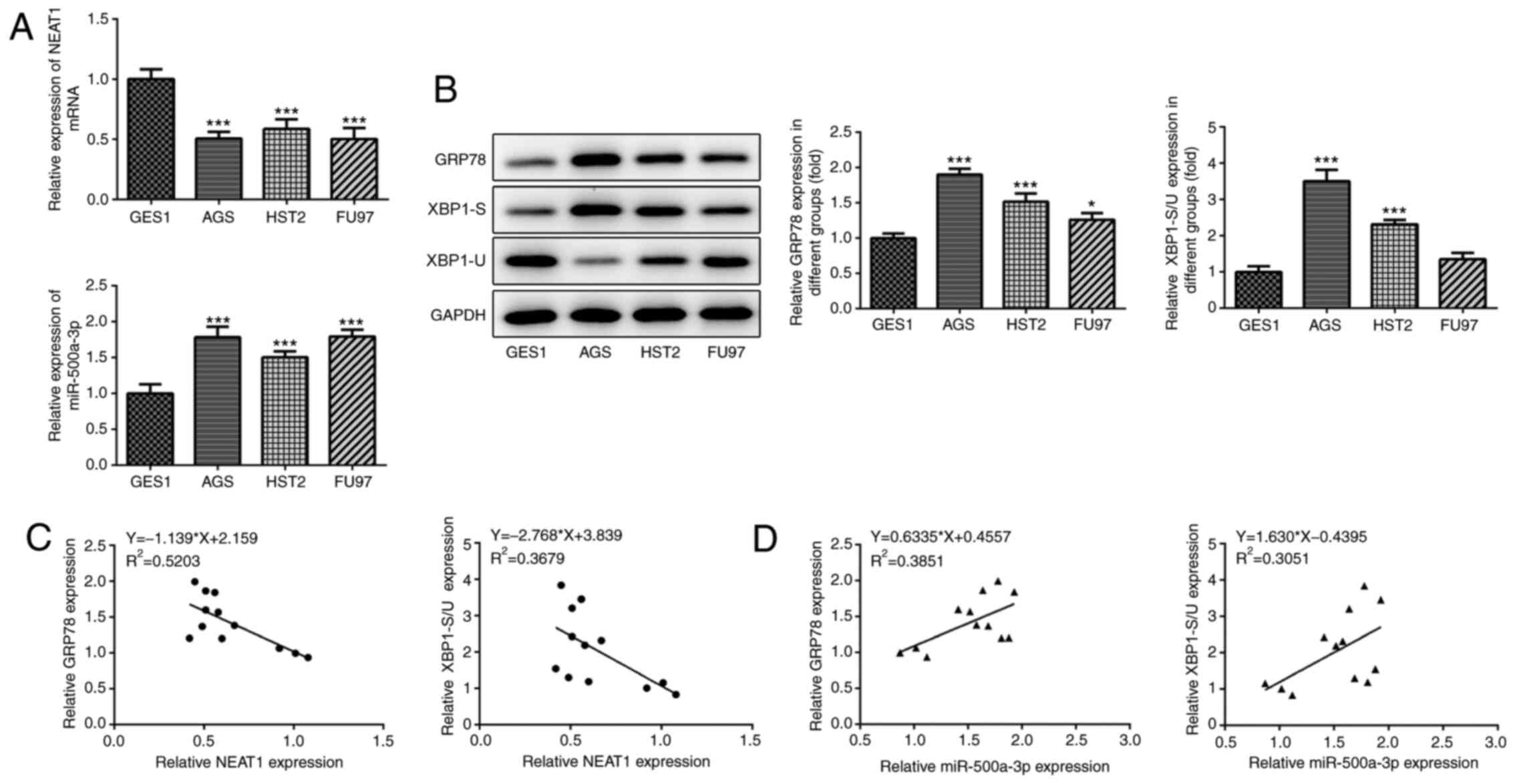
epithelial cells, lncRNA NEAT1 expression levels were significantly
downregulated, whereas miR-500a-3p expression levels were
significantly upregulated in gastric cancer cells (Fig. 3A). Subsequently, the expression
levels of endoplasmic reticulum stress-related proteins were
determined via western blotting. The expression levels of GRP78 and
XBP-1S were markedly increased, whereas the expression levels of
XBP-1U were markedly decreased in gastric cancer cells compared
with normal gastric mucosal epithelial cells (Fig. 3B). The results also demonstrated
that the XBP-1S/XBP-1U ratio was significantly decreased in gastric
cancer cells compared with normal gastric mucosal epithelial cells
(Fig. 3B). The present study
observed decreased levels of NEAT1 and increased levels of
miR-500a-3p, GPR 78 and XBP1 s/u in AGS cells compared with GES1
cells. The increased trend in the expression of GPR78 when compared
with GES1 group appeared to be higher in AGS cells compared with
other cancer cell lines used in the present study (Fig. 3B). Therefore, AGS cells were used
for subsequent experiments. The linear regression analysis results
indicated that lncRNA NEAT1 expression was negatively associated
with the expression of GRP78 and the XBP-1S/XBP-1U ratio (Fig. 3C), whereas miR-500a-3p expression
levels were positively associated with the expression of GRP78 and
the XBP-1S/XBP-1U ratio in gastric cancer cells (Fig. 3D).
lncRNA NEAT1 inhibits gastric cancer
cell proliferation and invasion, but promotes apoptosis
Subsequently, lncRNA NEAT1- and
miR-500a-3p-overexpression gastric cancer cells were established to
investigate the effects of lncRNA NEAT1 and miR-500a-3p on gastric
cancer (Fig. 4A). Luciferase
reporter assays were performed to investigate the association
between miR-500a-3p and lncRNA NEAT1 or XBP-1. The results revealed
that the fluorescein intensity was significantly decreased in the
wild-type lncRNA NEAT1 + miR-500a-3p mimic group compared with the
wild-type NEAT1 + mimic NC group (Fig.
4B). In addition, the fluorescein intensity was also
significantly decreased in the wild-type XBP-1 + miR-500a-3p mimic
group compared with the wild-type XBP-1 + mimic NC group (Fig. 4C). CCK-8 and colony formation assays
were performed to detect the alterations in cell proliferation. AGS
cell proliferation was significantly inhibited by lncRNA NEAT1
overexpression compared with the vector group (Fig. 4D). However, lncRNA NEAT1
overexpression-mediated effects on cell proliferation were
significantly reversed by miR-500a-3p overexpression. Similar
results were obtained from the colony formation assays. Moreover,
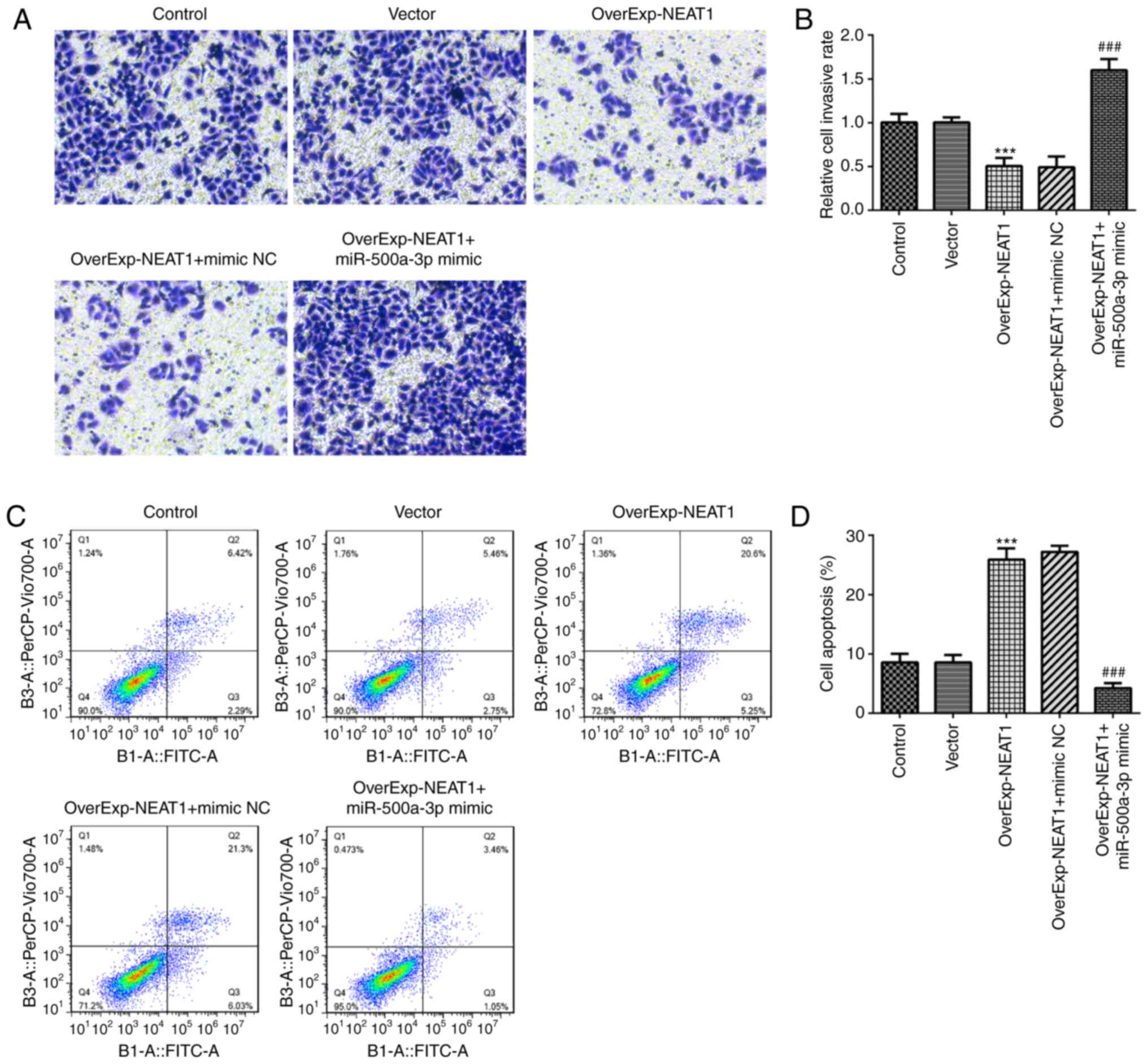
cell invasion was significantly inhibited by lncRNA NEAT1
overexpression compared with the vector group, which was also
significantly reversed by miR-500a-3p overexpression (Fig. 5A and B). In addition, cell apoptosis
was significantly increased by lncRNA NEAT1 overexpression compared
with the vector group, but miR-500a-3p overexpression significantly
reversed lncRNA NEAT1 overexpression-induced cell apoptosis
(Fig. 5C and D). Finally,
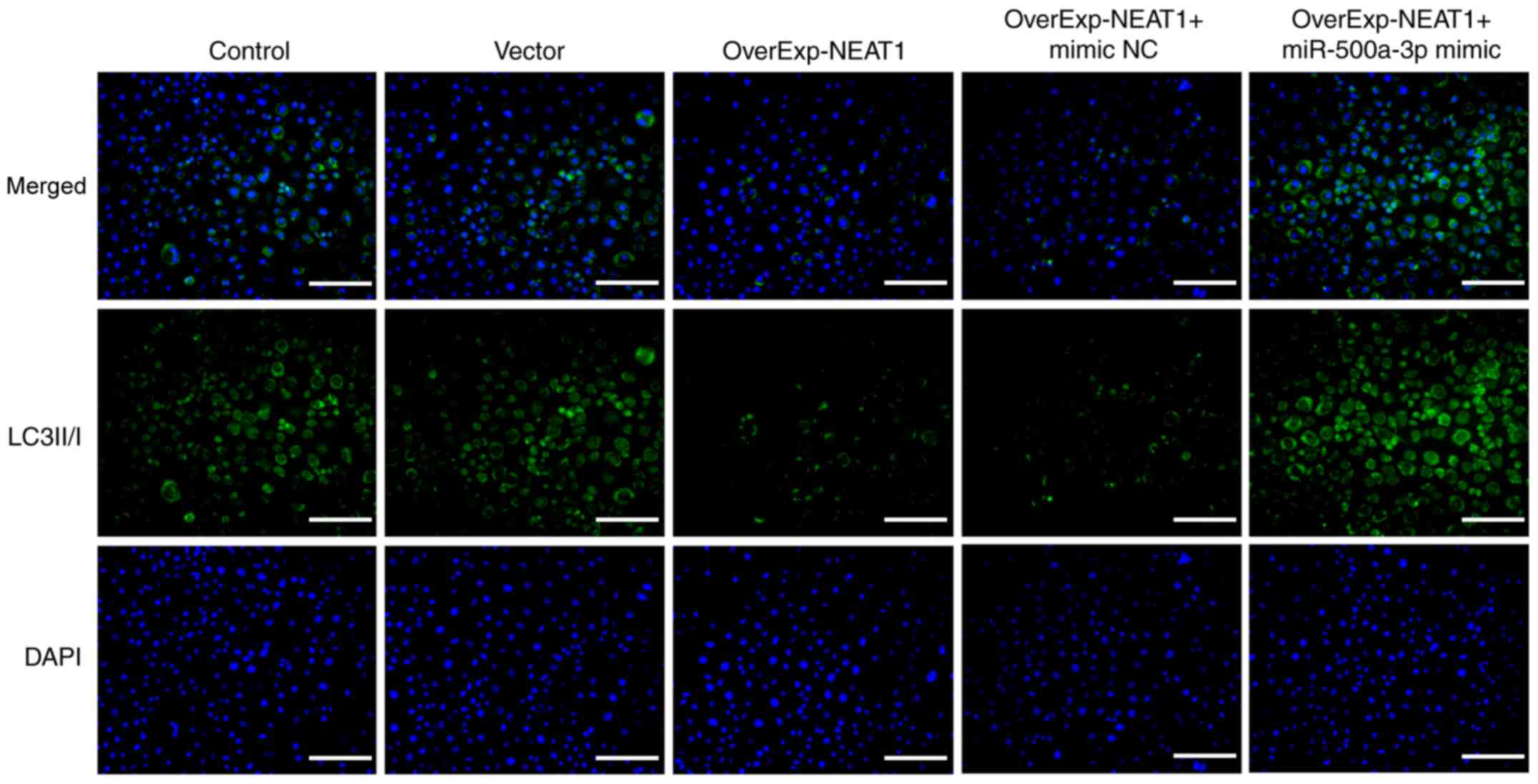
immunofluorescence assays were performed to detect the expression
of autophagy-related protein LC3II/I. The results demonstrated that
the expression of LC3II/I was markedly downregulated by lncRNA
NEAT1 overexpression compared with the vector group, which was
obviously reversed by miR-500a-3p overexpression (Fig. 6).
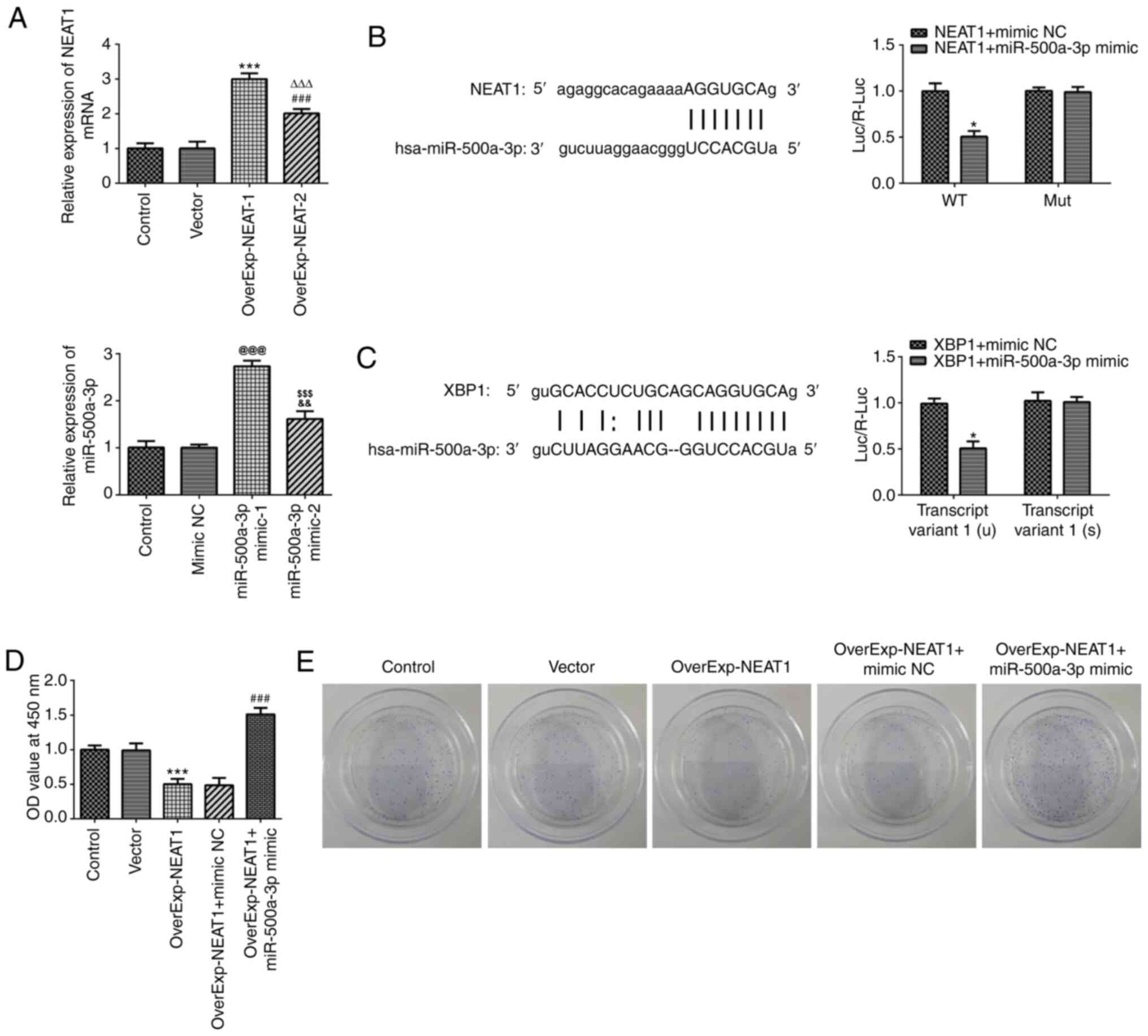 | Figure 4.lncRNA NEAT1 targets miR-500a in
gastric cancer cells. (A) Transfection efficiency of
OverExp-NEAT-1, OverExp-NEAT-2, miR-500a-3p mimic-1 and
miR-500a-3p-2 as determined via reverse transcription-quantitative
PCR. ***P<0.001 vs. vector; ###P<0.001 vs. vector;
∆∆∆P<0.001 vs. OverExp-NEAT-1.
@@@P<0.001 vs. mimic NC;
&&P<0.01 vs. mimic NC;
$$$P<0.001 vs. miR-500a-3p mimic-1. Luciferase
reporter assays were performed to assess the interactions between
miR-500a and (B) lncRNA NEAT1, *P<0.05 vs. NEAT1 + mimic NC or
XBP1 + mimic NC or (C) XBP-1, *P<0.05 vs. XBP1 + mimic NC. (D)
Cell Counting Kit-8 assays were performed to assess the effect of
lncRNA NEAT1 overexpression on gastric cancer cell proliferation.
***P<0.001 vs. vector; ###P<0.001 vs.
OverExp-NEAT1 + mimic NC. (E) Colony formation assays were
performed to assess the effect of lncRNA NEAT1 overexpression on
gastric cancer cell proliferation. lncRNA, long non-coding RNA;
NEAT1, nuclear paraspeckle assembly transcript 1; miR, microRNA;
OverExp, overexpression; NC, negative control; XBP-1, X-box binding
protein-1; OD, optical density; WT, wild-type; Mut, mutant. |
lncRNA NEAT1 overexpression inhibits
endoplasmic reticulum stress and apoptosis in gastric cancer
cells
The expression levels of endoplasmic reticulum
stress-related proteins were detected via western blotting. The
results revealed that GRP78 and Caspase4 expression levels were
significantly downregulated, whereas CHOP expression levels were
significantly upregulated by lncRNA NEAT1 overexpression compared
with the vector group (Fig. 7A). In
addition, lncRNA NEAT1 overexpression significantly decreased the
XBP-1S/XBP-1U ratio compared with the vector group. However,
miR-500a-3p overexpression reversed lncRNA NEAT1
overexpression-mediated effects on endoplasmic reticulum
stress-related protein expression levels. Furthermore, compared
with the vector group, lncRNA NEAT1 overexpression significantly
decreased the expression levels of Bcl-2, LC3II/I, Beclin-1 and
Atg5, but significantly increased the expression levels of Bax and
cleaved-caspase-3. miR-500a-3p overexpression significantly
reversed lncRNA NEAT1-overexpression mediated effects on apoptosis-
and autophagy-related protein expression levels (Fig. 7B). The present study confirmed
increased NEAT1 and decreased miR-500a-3p levels following the
induction of NEAT1 overexpression, while their levels could be
reversed by miR-500a-3p overexpression (Fig. 7C).
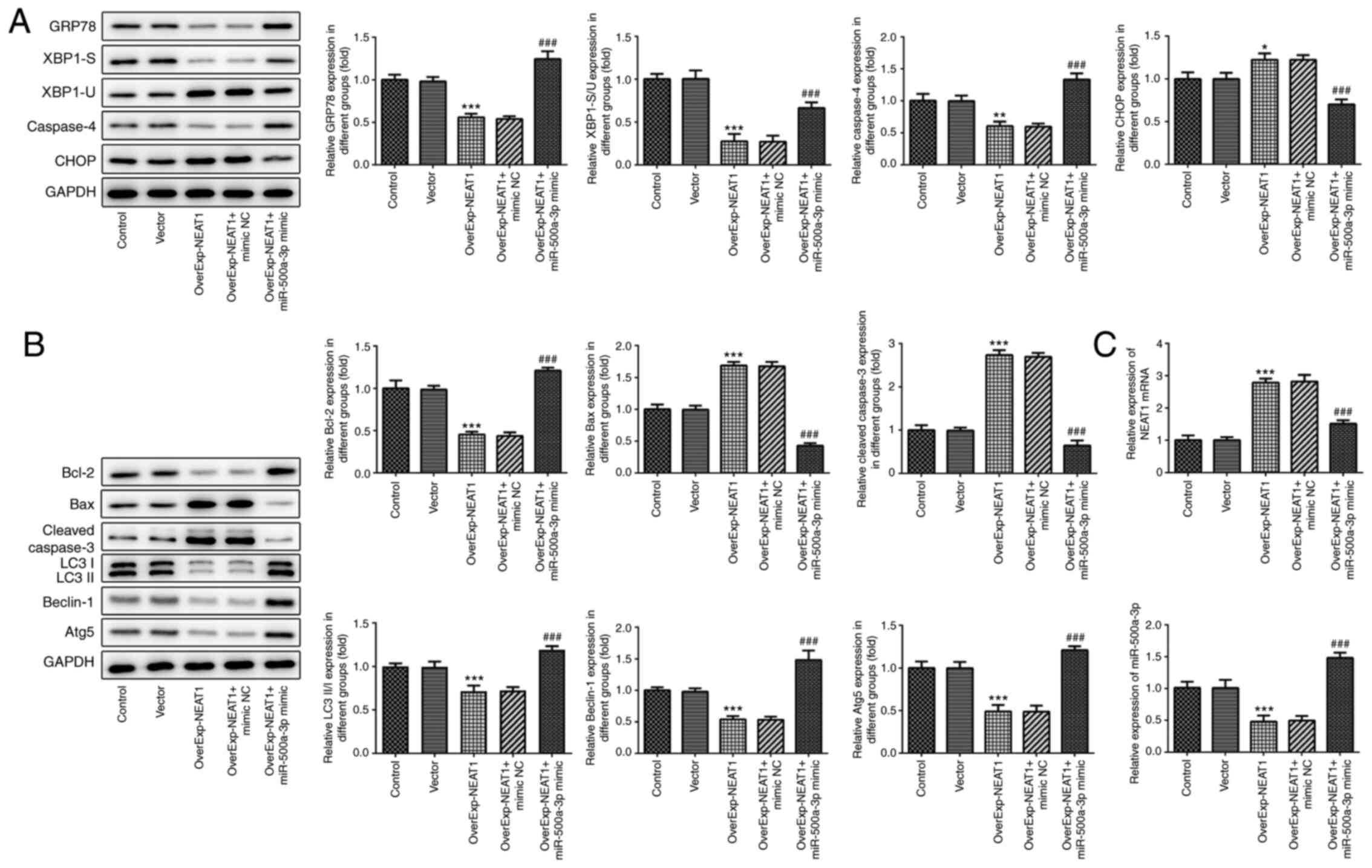 | Figure 7.lncRNA NEAT1 downregulates the
expression levels of endoplasmic reticulum stress-related proteins.
Expression levels of endoplasmic reticulum stress-, (A and B)
apoptosis- and autophagy-related proteins were detected via western
blotting. (C) lncRNA NEAT1 and miR-500a expression levels were
determined via reverse transcription-quantitative PCR. *P<0.05,
**P<0.01 and ***P<0.001 vs. vector; ###P<0.001
vs. OverExp-NEAT1 + mimic NC. lncRNA, long non-coding RNA; NEAT1,
nuclear paraspeckle assembly transcript 1; OverExp, overexpression;
miR, microRNA; NC, negative control; GRP78, 78-kDa
glucose-regulated protein; XBP-1, X-box binding protein-1; CHOP,
C/EBP homologous protein; Atg5, autophagy-related gene 5. |
Discussion
Gastric cancer is the fifth commonest cancer and is
the third commonest cause of cancer-related mortality (18). Despite significant progress in the
elucidation of the pathogenesis and clinical research of gastric
cancer, increasing cases of stomach cancer can be expected in the
future due to an aging population (19). Previous studies have demonstrated
that the development and progression of gastric cancer is
associated with the expression of several genes, such as
miR-17-5p/20a and hosphoinositide 3-kinase/Akt, which affect tumor
cell proliferation, migration and invasion (20,21).
In addition, lncRNAs, a class of genes that do not
encode proteins, serve a key role in cell metabolism, senescence
and apoptosis (22). It has been
reported that lncRNA NEAT1 expression might induce colorectal
cancer progression by interacting with DEAD-box helicase 5
(23). However, the role of lncRNA
NEAT1 in gastric cancer is not completely understood. The present
study demonstrated that lncRNA NEAT1 overexpression significantly
inhibited gastric cancer cell proliferation and invasion, but
significantly promoted cell apoptosis compared with the vector
group. Furthermore, lncRNA NEAT1 expression was significantly
downregulated in gastric cancer tissues compared with adjacent
healthy tissues.
In addition, miR-500a also promotes hepatocarcinoma
cell proliferation and invasion (24). miR-500a enhanced hepatocellular
carcinoma cell migration and invasion by activating the
Wnt/β-catenin signaling pathway (25). In the present study, miR-500a-3p
overexpression significantly promoted gastric cancer cell
proliferation and invasion, but significantly suppressed apoptosis
in lncRNA NEAT1-overexpression gastric cancer cells. In addition,
the results indicated that lncRNA NEAT1 targeted and downregulated
the expression of miR-500a-3p.
Furthermore, previous studies have suggested that
the occurrence of endoplasmic reticulum stress may induce the
development of multiple types of cancer, such as carcinomas of the
breast, stomach, esophagus and liver (26,27).
XBP-1 is a protein that serves a key role during the development of
endoplasmic reticulum stress (28).
Due to differences in splicing, XBP-1 has two subtypes, XBP-1S and
XBP-1U. During the development of multiple tumors, higher
expression levels of XBP-1S induce the unfolded protein response
and enhance tumor proliferation and metastasis. However, the
expression of XBP-1U restricts tumor development by suppressing the
unfolded protein response and endoplasmic reticulum stress
(29). The present study indicated
that miR-500a-3p might affect tumor proliferation and metastasis by
regulating the expression of XBP-1. The results demonstrated that
miR-500a-3p targeted XBP-1S and its overexpression affected the
expression of XBP-1S. Therefore, the results of the present study
indicated that miR-500a-3p mediated gastric cancer cell
proliferation and invasion affected by NEAT1, which possibly was
associated with the inhibition of endoplasmic reticulum stress.
Furthermore, autophagy is a spontaneous
auto-degradation process of cells, and the survival of tumor cells
often depends on autophagy (30).
The present study demonstrated that lncRNA NEAT1 overexpression
significantly downregulated autophagy-related protein (LC3II/I,
Beclin-1 and Atg5) expression levels compared with the vector
group, which was significantly reversed by miR-500a-3p
overexpression. The results indicated that lncRNA NEAT1 inhibited
the autophagy process to restrict the development of gastric
cancer. Cancer cell apoptosis also inhibits cancer development
(31). In the present study,
compared with the vector group, lncRNA NEAT1 overexpression
significantly increased the expression levels of apoptosis-related
proteins (Bax and cleaved-caspase-3), which was significantly
reversed by miR-500a overexpression. The results indicated that the
lncRNA NEAT1 might suppress the development of gastric cancer by
promoting gastric cancer cell apoptosis. Previous studies have
identified miR-500a-3p as a potential prognostic predictor in
certain types of cancer, including hepatocellular carcinoma and
glioblastoma, and have reported an association between miR-500a and
TNM stage in lung cancer and conjunctival malignant melanoma
(32–35). Other studies have reported that
XBP-1 is closely associated with clinical outcome (36,37). A
key limitation of the present study was that the prognostic impact
and cancer staging applications of lncRNA NEAT1, miR-500a-3p and
XBP-1 in gastric cancer were not investigated; therefore, further
investigation is required. To conclude, the present study
demonstrated that the regulatory role of the lncRNA
NEAT1/miR-500a-3p axis in endoplasmic reticulum stress, apoptosis
and autophagy in gastric cancer cells was potentially mediated by
XBP-1. Therefore, the present study identified several potential
diagnostic markers for gastric cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author or first author
on reasonable request.
Authors' contributions
YZ, ZS and HC made substantial contributions to the
conception and design of the study, acquired, analyzed and
interpreted the data, and drafted and revised the manuscript for
important intellectual content. YZ, ZS, YY, SW and HC performed the
experiments and interpreted the data. YZ, ZS and HC confirm the
authenticity of the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Ethics Committee of Zhongda Hospital Southeast University (approval
no. 2018ZDSYLL136291). Written informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li R, Liu B and Gao J: The application of
nanoparticles in diagnosis and theranostics of gastric cancer.
Cancer Lett. 386:123–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rona KA, Schwameis K, Zehetner J, Samakar
K, Green K, Samaan J, Sandhu K, Bildzukewicz N, Katkhouda N and
Lipham JC: Gastric cancer in the young: An advanced disease with
poor prognostic features. J Surg Oncol. 115:371–375. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saito Y, Li L, Coyaud E, Luna A, Sander C,
Raught B, Asara JM, Brown M and Muthuswamy SK: LLGL2 rescues
nutrient stress by promoting leucine uptake in ER+
breast cancer. Nature. 569:275–279. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Travis WD, Brambilla E and Riely GJ: New
pathologic classification of lung cancer: Relevance for clinical
practice and clinical trials. J Clin Oncol. 31:992–1001. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohamed E, Cao Y and Rodriguez PC:
Endoplasmic reticulum stress regulates tumor growth and anti-tumor
immunity: A promising opportunity for cancer immunotherapy. Cancer
Immunol Immunother. 66:1069–1078. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu H, Tian M, Ding C and Yu S: The C/EBP
homologous protein (CHOP) transcription factor functions in
endoplasmic reticulum stress-induced apoptosis and microbial
infection. Front Immunol. 9:30832019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Urra H, Dufey E, Avril T, Chevet E and
Hetz C: Endoplasmic reticulum stress and the hallmarks of cancer.
Trends Cancer. 2:252–262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duplan E, Giaime E, Viotti J, Sévalle J,
Corti O, Brice A, Ariga H, Qi L, Checler F and Alves da Costa C:
ER-stress-associated functional link between Parkin and DJ-1 via a
transcriptional cascade involving the tumor suppressor p53 and the
spliced X-box binding protein XBP-1. J Cell Sci. 126:2124–2133.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu SK, Chiu CC, Dahms HU, Chou CK, Cheng
CM, Chang WT, Cheng KC, Wang HD and Lin IL: Unfolded protein
response (UPR) in survival, dormancy, immunosuppression,
metastasis, and treatments of cancer cells. Int J Mol Sci.
20:25182019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding LH, Ye QN, Lu QJ, Zhu JH, Yan JH,
Wang ZH and Huang CF: Expression of XBP-1 in breast cancer cell
lines and its role in ERalpha signaling. Yi Chuan Xue Bao.
31:380–384. 2004.(In Chinese). PubMed/NCBI
|
|
13
|
Zhang L, Ding Y, Yuan Z, Liu J, Sun J, Lei
F, Wu S, Li S and Zhang D: MicroRNA-500 sustains nuclear factor-κB
activation and induces gastric cancer cell proliferation and
resistance to apoptosis. Oncotarget. 6:2483–2495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42((Database Issue)): D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sepúlveda D, Barrera MJ, Castro I,
Aguilera S, Carvajal P, Lagos C, González S, Albornoz N, Bahamondes
V, Quest AF, et al: Impaired IRE1α/XBP-1 pathway associated to DNA
methylation might contribute to salivary gland dysfunction in
Sjögren's syndrome patients. Rheumatology (Oxford). 57:1021–1032.
2018. View Article : Google Scholar
|
|
17
|
Chern YJ, Wong JCT, Cheng GSW, Yu A, Yin
Y, Schaeffer DF, Kennecke HF, Morin G and Tai IT: The interaction
between SPARC and GRP78 interferes with ER stress signaling and
potentiates apoptosis via PERK/eIF2α and IRE1α/XBP-1 in colorectal
cancer. Cell death Dis. 10:5042019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: A global overview. Int J Cancer. 125:666–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang M, Gu H, Qian H, Zhu W, Zhao C, Zhang
X, Tao Y, Zhang L and Xu W: miR-17-5p/20a are important markers for
gastric cancer and murine double minute 2 participates in their
functional regulation. Eur J Cancer. 49:2010–2021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu HG, Ai YW, Yu LL, Zhou XD, Liu J, Li
JH, Xu XM, Liu S, Chen J, Liu F, et al: Phosphoinositide
3-kinase/Akt pathway plays an important role in chemoresistance of
gastric cancer cells against etoposide and doxorubicin induced cell
death. Int J Cancer. 122:433–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kopp F and Mendell JT: Functional
classification and experimental dissection of long noncoding RNAs.
Cell. 172:393–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang M, Weng W, Zhang Q, Wu Y, Ni S, Tan
C, Xu M, Sun H, Liu C, Wei P and Du X: The lncRNA NEAT1 activates
Wnt/β-catenin signaling and promotes colorectal cancer progression
via interacting with DDX5. J Hematol Oncol. 11:1132018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Y and Wang Y and Wang Y: Up-regulated
miR-500a enhances hepatocarcinoma metastasis by repressing PTEN
expression. Biosci Rep. 37:BSR201708372017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo Y, Chen L, Sun C and Yu C:
MicroRNA-500a promotes migration and invasion in hepatocellular
carcinoma by activating the Wnt/β-catenin signaling pathway. Biomed
Pharmacother. 91:13–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cubillos-Ruiz JR, Bettigole SE and
Glimcher LH: Tumorigenic and immunosuppressive effects of
endoplasmic reticulum stress in cancer. Cell. 168:692–706. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oakes SA and Papa FR: The role of
endoplasmic reticulum stress in human pathology. Annu Rev Pathol.
10:173–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu XM, Yao FH, Yao YM, Dong N, Yu Y and
Sheng ZY: Endoplasmic reticulum stress and its regulator XBP-1
contributes to dendritic cell maturation and activation induced by
high mobility group box-1 protein. Int J Biochem Cell Biol.
44:1097–1105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barez SR, Atar AM and Aghaei M: Mechanism
of inositol-requiring enzyme 1-alpha inhibition in endoplasmic
reticulum stress and apoptosis in ovarian cancer cells. J Cell
Commun Signal. 14:403–415. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mowers EE, Sharifi MN and Macleod KF:
Functions of autophagy in the tumor microenvironment and cancer
metastasis. FEBS J. 285:1751–1766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lopez J and Tait SW: Mitochondrial
apoptosis: Killing cancer using the enemy within. Br J Cancer.
112:957–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bao L, Zhang M, Han S, Zhan Y, Guo W, Teng
F, Liu F, Guo M, Zhang L, Ding G and Wang Q: MicroRNA-500a promotes
the progression of hepatocellular carcinoma by
post-transcriptionally targeting BID. Cell Physiol Biochem.
47:2046–2055. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Su D, Qi X and Ma J: MiR-500a-5p
promotes glioblastoma cell proliferation, migration and invasion by
targeting chromodomain helicase DNA binding protein 5. Mol Med Rep.
18:2689–2696. 2018.PubMed/NCBI
|
|
34
|
Larsen AC: Conjunctival malignant melanoma
in Denmark: Epidemiology, treatment and prognosis with special
emphasis on tumorigenesis and genetic profile. Acta Ophthalmol.
94:1–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liao XH, Xie Z and Guan CN: MiRNA-500a-3p
inhibits cell proliferation and invasion by targeting lymphocyte
antigen 6 complex locus K (LY6K) in human non-small cell lung
cancer. Neoplasma. 65:673–682. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Davies MP, Barraclough DL, Stewart C,
Joyce KA, Eccles RM, Barraclough R, Rudland PS and Sibson DR:
Expression and splicing of the unfolded protein response gene XBP-1
are significantly associated with clinical outcome of
endocrine-treated breast cancer. Int J Cancer. 123:85–88. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hsu HT, Hsing MT, Yeh CM, Chen CJ, Yang JS
and Yeh KT: Decreased cytoplasmic X-box binding protein-1
expression is associated with poor prognosis and overall survival
in patients with oral squamous cell carcinoma. Clin Chim Acta.
479:66–71. 2018. View Article : Google Scholar : PubMed/NCBI
|