Introduction
Gallbladder cancer (GBC) is the most aggressive
cancer type in the biliary tract, accounting for 80–95% of all
biliary tract malignancies (1).
Despite significant efforts in clinical research on GBC, the
prognosis of GBC remains poor, with a 5-year survival rate of ~5%,
due to late diagnosis and easy metastasis (2,3).
Therefore, it is important to elucidate the underlying molecular
mechanisms of GBC that may serve a key role in developing
GBC-targeted therapies.
MicroRNAs (miRNAs/miRs) are a type of small
endogenous RNA that serve an important role in the regulation of
gene expression by reducing the post-transcriptional translation of
target mRNAs (4). Previous studies
have reported that miRNAs are involved in the proliferation,
migration and invasion of cancer cells (5,6).
Moreover, miRNAs can be used as markers of cancer diagnosis and
prognosis. For example, miR-135a-5p is significantly downregulated
in several cancer types, such as gastric carcinoma, and low
expression of miR-135a-5p is associated with a low overall survival
in gastric cancer (7). Furthermore,
miR-135a-5p induces apoptosis in prostate cancer cells by targeting
the regulation of STAT6 expression (8). It has also been shown that miR-135a-5p
inhibits glioma cell proliferation and invasion by targeting and
regulating FOXO1 expression (9).
Other previous studies have also identified miRNAs that affect GBC
patient survival, such as miR-146b-5p, miR-335 and miR-101, amongst
others (10–12).
Our previous studies have observed that miR-135a-5p
was significantly downregulated in GBC tissues, as determined by
analyzing a miRNA microarray (13).
In addition, the relationship between miR-135a-5p and GBC was
further examined through in vivo and in vitro
experiments, and it was found that miR-135a-5p exerted strong
therapeutic effects on GBC (14).
However, few studies have focused on the mechanism of action of the
miR-135a-5p target genes in GBC.
Angiopoietin-2 (ANGPT2) is a ligand for the
tyrosine-protein kinase receptor Tie-2, which functions as a
vascular stabilizing molecule and is an important regulator of
vascular maturation (15,16). ANGPT2 is produced by endothelial
cells and promotes angiogenesis. ANGPT2 is lowly expressed in
normal tissues but tends to be highly upregulated in tumor blood
vessels (17). It has been reported
that elevated circulating ANGPT2 is associated with poor prognosis
and tumor invasion in several cancer types, such as gastric
carcinoma (18,19). However, no relevant studies
reporting the function of ANGPT2 in GBC were identified in a
preliminary search. Thus, the present study aimed to investigate
the regulatory role of miR-135a-5p signaling and the function of
ANGPT2 in GBC.
Materials and methods
Tissues, cell lines and culture
GBC and matched adjacent non-tumorous gallbladder
tissues (≥2 cm away from the tumor tissue) were obtained from 10
patients (age range, 45–63 years, mean age 53.8±8.6 years; seven
female patients and five male patients) who underwent surgery in
The Seventh People's Hospital Affiliated to Shanghai University of
Traditional Chinese Medicine between January 2019 and December
2019. The study was approved by the Ethics Committee of The Seventh
People's Hospital Affiliated to Shanghai University of Traditional
Chinese Medicine. All enrolled patients signed an informed consent
form before surgery. The Cancer Genome Atlas (TCGA) survival
analysis data were evaluated using OncoLnc (oncolnc.org).
A human GBC cell line (GBC-SD) was purchased from
The Chinese Academy of Sciences. Cells were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin/streptomycin (Corning, Inc.) with 5% CO2 at
37°C.
Immunohistochemistry (IHC)
IHC staining was performed according to a method
described in a previous study (20). GBC and matched adjacent non-tumorous
gallbladder tissues (≥2 cm away from the tumor tissue) were fixed
with 4% paraformaldehyde at room temperature for 30 min and then
embedded in paraffin and sliced into 0.5-µm sections, followed by
IHC. Briefly, the paraffin-embedded sections were dewaxed in xylene
and rehydrated in a graded alcohol series (100, 95 and 80%).
Subsequently, the sections were blocked with 5% BSA (cat. no.
ST025; Beyotime Institute of Biotechnology) at 37°C for 1 h, and
heated in a microwave oven in sodium citrate buffer (0.1 mM; pH
6.0) for 5 min for antigen retrieval. The sections were then
incubated overnight at 4°C with primary antibodies against ANGPT2
(cat. no. ab56301; 1:200; Abcam). Following which, the sections
were incubated with a secondary antibody (cat. no. ab6728; 1:1,000;
Abcam) at 37°C for 1 h. The sections were then stained with
diaminobenzidine at room temperature for 3–15 min and
counterstained with hematoxylin at room temperature for 5 min.
Finally, the images were captured using an Olympus light microscope
(Olympus Corporation) under ×200 magnification (21). In total, two independent
investigators performed the scoring of the respective expression
profiles. The number of positive cells was graded as follows: 0
(<5%), 1 (6–20%), 1 (21–40%), 3 (41–60%), 4 (61–80%) or 5
(>80%) (22).
RNA isolation and reverse
transcription-quantitative (RT-q) PCR
Total RNA from gallbladder tissues and GBC-SD cells
was extracted using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA was reverse-transcribed using a
ReverTra Ace™ qPCR RT kit (Toyobo Life Science) according to the
manufacturer's protocol. RT-qPCR was performed using a SYBR
Supermix PCR kit (Kapa Biosystems; Roche Diagnostics) under the
following conditions: 95°C for 3 min, followed by 40 cycles at 95°C
for 10 sec and 60°C for 1 min, and melting curve analysis,
according to the manufacturer's protocol. Primers were obtained
from Sangon Biotech Co., Ltd., and are presented in Table I. GAPDH and U6 were used as
endogenous controls. Data were analyzed using the 2−ΔΔCq
calculation (23).
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| ANGPT2 |
AACTTTCGGAAGAGCATGGAC |
CGAGTCATCGTATTCGAGCGG |
| miR-135a-5p |
CCAGGCTTCCAGTACCATTAGG |
GTTTCCGAGAGAGGCAGGTG |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
| GAPDH |
GAGTCCACTGGCGTCTTCAC |
TGCTGATGATCTTGAGGCTGTT |
Transfection of miRNAs and small
interfering (si)RNAs
Synthesized RNA duplexes of miR-135a-5p mimics,
miRNA mimics negative control (mi-NC), ANGPT2 siRNA and scrambled
siRNA NC (si-NC) were designed and synthesized by Shanghai
GenePharma Co., Ltd.. A total of 1×106 cells/well were
seeded into the 6-well plates and transfected with miR-135a-5p
mimics and inhibitor using HilyMAX reagent (Dojindo Molecular
Technologies, Inc.), according to the manufacturer's instructions.
Transfection was performed using the following reagents: 120 µl
Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.); 80 pmol (4 µl)
mimics or inhibitor; 12 µl HilyMAX reagent, at room temperature for
15 min. GBC-SD cells were harvested for RT-qPCR at 48 h after
transfection. The siRNA sequences and miRNA mimics are shown in
Table II.
 | Table II.ANGPT2 siRNA and miR-135a-5p mimics
sequences. |
Table II.
ANGPT2 siRNA and miR-135a-5p mimics
sequences.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| ANGPT2 siRNA |
GCATAGGAAAGAAGCAATATT |
UAUUGCUUCUUUCCUAUGCTT |
| si-NC |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
| miR-135a-5p
mimics |
UAUGGCUUUUCAUUCCUAUGUGA |
ACAUAGGAAUGAAAAGCCAUAUU |
| mi-NC |
UAUAUCGUGUUAUUAGCGUUCCU |
GAACGCUAAUAACACGAUAUAUU |
Luciferase activity assay
Wild-type (WT) and mutant (MUT) ANGPT2
3′-untranslated regions (UTRs) were cloned into pmiRGLO vectors
(Shanghai GenePharma Co., Ltd.). For the luciferase activity assay,
GBC-SD cells (1×105) were seeded in a 24-well plate.
Cells were transfected with the miR-135a-5p mimics or mimic control
and WT or MUT ANGPT2 3′-UTR, according to the manufacturer's
instructions. Before transfection, the miR-135a-5p mimics or mimic
control and WT or MUT ANGPT2 3′-UTR were incubated separately with
HilyMAX reagent at room temperature for 15 min. Incubation was
performed using the following reagents: 120 µl Opti-MEM; 4 µg WT or
MUT ANGPT2 3′-UTR; 80 pM miR-135a-5p mimics or mimic control; 12 µl
HilyMAX reagent. Then, cells were transfected with the incubated
mixture at 37°C for 4 h. After transfection, cells were cultured in
DMEM (10% FBS) for 48 h. Subsequently, the relative luciferase
activity was determined using a luciferase reporter system (Promega
Corporation). Luciferase activity was normalized to Renilla
luciferase activity.
Cell proliferation assays
Cell proliferation was determined using a Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.),
according to the manufacturer's protocol. GBC-SD cells
(5×103 cells/100 µl) were seeded in a 96-well plate.
After 24, 48 and 72 h of culturing at 37°C, proliferation was
assessed using the CCK-8 solution. The cells in each well were
incubated with 10 µl CCK-8 solution for 1 h on a shaker according
to the manufacturer's instructions. Subsequently, the absorbance
value was measured at 450 nm using a microplate reader (Thermo
Fisher Scientific, Inc.). The experiments were performed in
triplicate.
Wound healing assay
For the wound-healing assay, GBC-SD cells
(1×105) were seeded in 24-well plates and cultured at
37°C overnight. When they had reached 80% confluency, a 10-µl tip
was used to create a 1-mm wide strip at the bottom of wells, and
the floating cells were gently washed twice with DMEM. Cells were
cultured at 37°C in DMEM (1% FBS) for 24 h. The cells migrating
into the wounded areas were captured using a light microscope at
×100 magnification at 0 and 24 h. Wound healing was assessed using
MShot Image Analysis System 1.3.10 (Guangzhou Mingmei Photoelectric
Technology Co., Ltd.).
Transwell assays
For the Transwell assay, Matrigel was diluted with
serum-free medium (dilution concentration was no less than 1:3),
and 50 µl Matrigel was added to the upper chamber (pore size, 8 µm)
of each well in a 24-well plate, and incubated at 37°C for 3–5 h,
Subsequently, 70 µl serum-free medium was added to the upper
chamber of each well and incubated at 37°C for 30 min; residual
liquid in the chamber was removed. GBC-SD cells were seeded into
the upper chamber of Matrigel-plated with DMEM without serum
(5×104 cells/well), and the lower wells contained 500 µl
complete medium (DMEM and 10% FBS), following a routine procedure.
After 48 h at 37°C, cells invading into the lower chamber were
collected, fixed with 5% glutaraldehyde for 10 min at room
temperature. stained with 0.5% crystal violet and then counted
under a light microscope at ×100 magnification.
Western blotting
Western blot analysis was performed as previously
described to determine migration- and invasion-related protein
expression (24). The samples or
cells were lysed in RIPA buffer (cat. no. P0013K; Beyotime
Institute of Biotechnology). The obtained proteins were quantified
using BCA Protein Assay kit (Sangon Biotech Co., Ltd.) and 30 µg
proteins were separated by sodium dodecyl sulfate polyacrylamide
gel electrophoresis on 10% gels for 2 h. Subsequently, the
separated proteins were transferred onto nitrocellulose membranes
(Thermo Fisher Scientific, Inc.). These membranes were then
immersed in 5% skimmed milk (Sangon Biotech Co., Ltd.) diluted with
PBS-0.1% Tween 20 at room temperature for 3 h to prevent
nonspecific protein binding, followed by incubation with primary
antibodies, including the endogenous reference, overnight at 4°C
and with HRP-conjugated anti-rabbit secondary antibodies (cat. no.
ab205718; dilution, 1:5,000; Abcam) for 1 h at room temperature.
The following primary antibodies were used: E-cadherin (E-cad; cat.
no. ab40772; dilution, 1:5,000; Abcam) and Rho associated
coiled-coil containing protein kinase 1 (Rock1; cat. no. ab97592;
dilution, 1:500; Abcam). Anti-GAPDH (cat. no. ab9485; dilution,
1:2,500; Abcam) was used as the endogenous reference. Finally,
bound HRP-conjugated antibodies were detected using Western
Lightning Plus-ECL reagents (PerkinElmer, Inc.). The gray level of
each band was obtained using ImageJ software (version 1.8.0;
National Institutes of Health) (25).
Statistical analysis
The results are presented as the mean ± SD and all
experiments were repeated at least three times. Statistical
analyses were performed using GraphPad Prism (version 7.0; GraphPad
Software, Inc.). The miRNA target gene was determined using
prediction databases [TargetScan Release 7.2 (http://www.targetscan.org/vert_72/) and miRDB
version 6.0 (http://mirdb.org/index.html)]. Survival analysis was
conducted using the Kaplan-Meier method, and the Renyi test was
used to compare the overall survival curves. An unpaired Student's
t-test was used for comparison between two groups in cell assay,
while a paired Student's t-test was used for comparison between two
groups in Fig. 1, with the
exception of IHC data. IHC data are presented as the median and
range, and were analyzed used Wilcoxon test. One-way ANOVA followed
by Tukey's post hoc test was used for comparisons between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
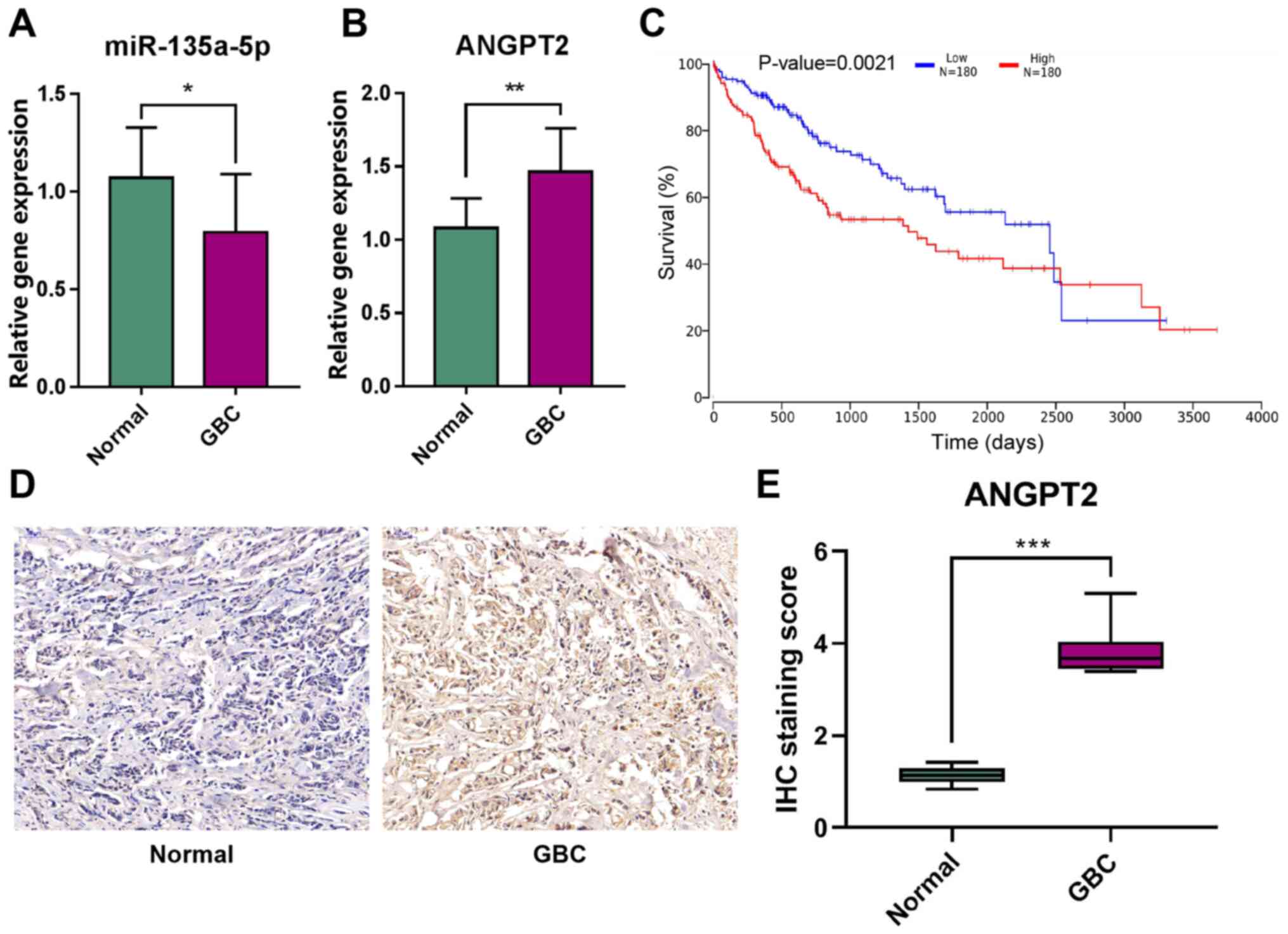
Expression levels of miR-135a-5p and
ANGPT2 in patients with GBC
To determine the expression levels of miR-135a-5p
and ANGPT2 in GBC, RT-qPCR analysis was first performed for 10 GBC
tissues and matched adjacent non-tumorous gallbladder tissues. The
results demonstrated that miR-135a-5p expression was significantly
downregulated in GBC tissues (Fig.
1A). By contrast, the expression level of ANGPT2 in GBC tissues
was significantly higher compared with that in normal tissues
(Fig. 1B). Further TCGA survival
(26) analysis identified that
ANGPT2 expression had a significant impact on the prognosis of the
patients with liver and biliary tumors, as patients with high
ANGPT2 expression had a poorer prognosis (Fig. 1C). The IHC results demonstrated that
the positive rate of ANGPT2 expression in GBC tissues was
significantly higher compared with that in normal tissues (Fig. 1D and E). These results suggested
that miR-135a-5p and ANGPT2 may be key biomarkers of GBC.
Overexpression of miR-135a-5p inhibits
in vitro GBC cell proliferation, migration and invasion
To further investigate the effect of miR-135a-5p on
GBC cell proliferation and migration, miR-135a-5p interference
experiments were performed in vitro. The RT-qPCR results
identified the overexpression of miR-135a-5p by miRNA mimics, which
indicated that the transfection was successful (Fig. 2A). The CCK-8 assays demonstrated
that the overexpression of miR-135a-5p significantly reduced the
proliferation of GBC-SD cells (Fig.
2B). Moreover, the wound healing assay identified that the
overexpression of miR-135a-5p effectively inhibited the migration
of GBC cells. (Fig. 2C and D).
Transwell assays results also revealed that GBC cell invasion was
significantly inhibited after miR-135a-5p overexpression (Fig. 2E and F). Furthermore, the western
blotting results demonstrated that Rock1 expression was decreased,
while E-cad expression was notably increased in miR-135a-5p mimics
group (Fig. 2G). These data
indicated that the overexpression of miR-135a-5p could inhibit the
proliferation, migration and invasion of GBC-SD cells.
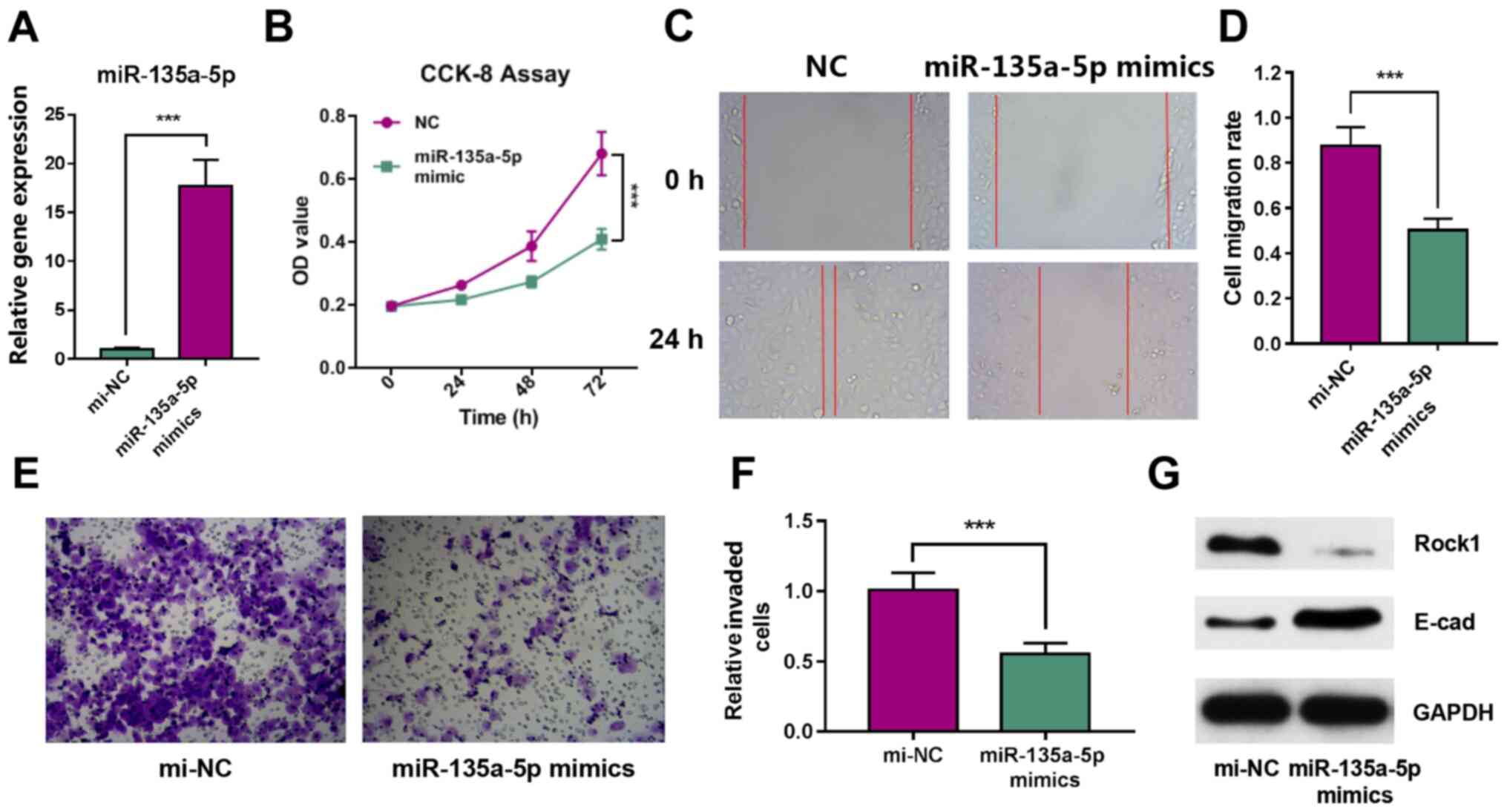 | Figure 2.miR-135a-5p inhibits the
proliferation, migration and invasion of GBC-SD cells. (A)
miR-135a-5p expression in GBC-SD cells after transfection with
miRNA mimics. (B) CCK-8 cell proliferation assay results. (C) Wound
healing assay image and (D) quantification of results.
Magnification, ×200. (E) Cell invasion images and (F)
quantification of results. Magnification, ×200. (G) Expression
levels of Rock1 and E-cad were determined using western blot
analysis. ***P<0.001. miR/mi, microRNA; ANGPT2, angiopoietin-2;
E-cad, E-cadherin; Rock1, Rho associated coiled-coil containing
protein kinase 1; OD, optical density; NC, negative control; CCK,
Cell Counting Kit. |
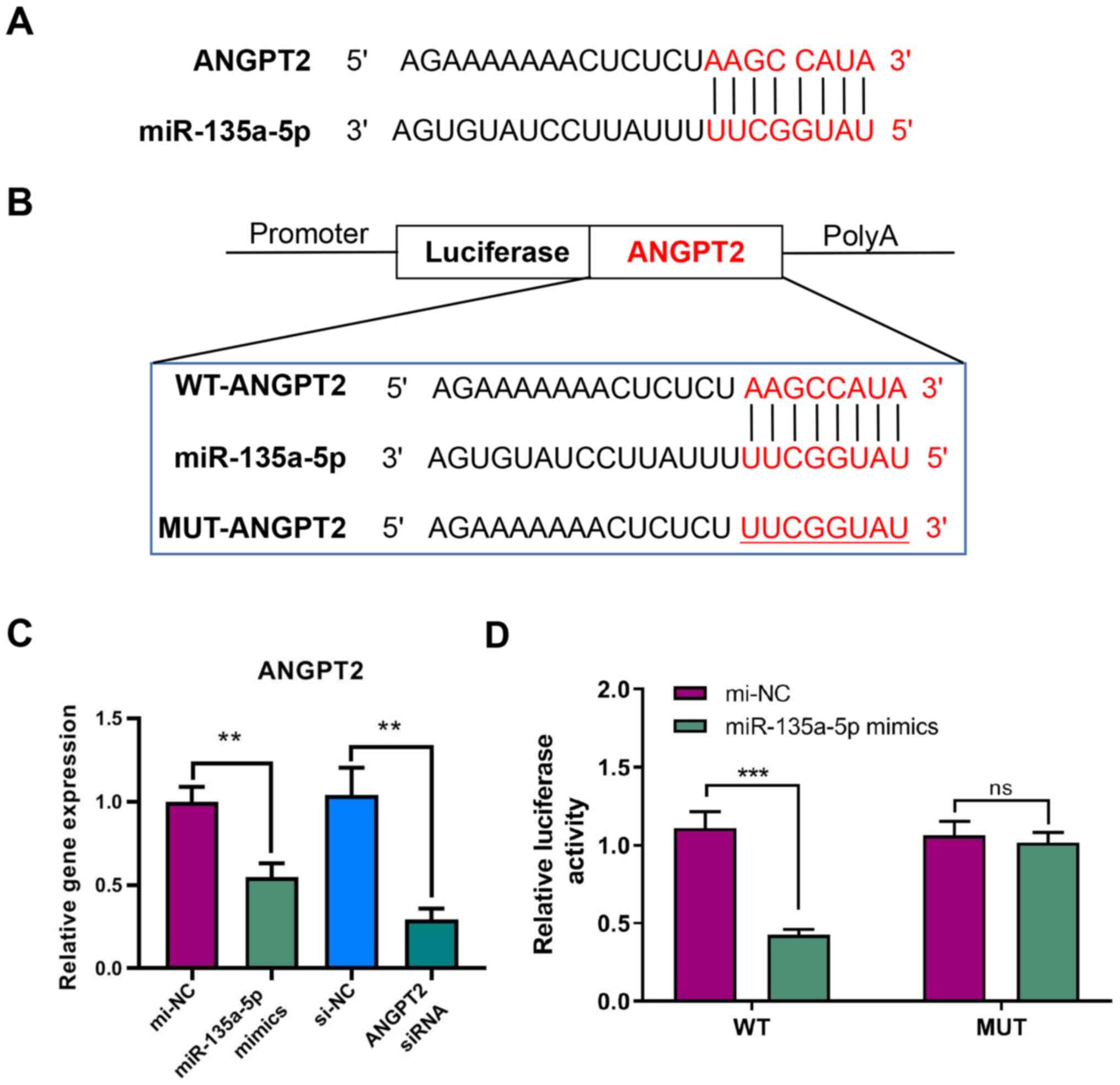
ANGPT2 is the target gene of
miR-135a-5p
Analysis of miRNA target gene prediction databases
(TargetScan Release 7.2 and miRDB version 6.0) indicated that
ANGPT2 may be a target gene of miR-135a-5p based on the putative
target sequence ANGPT2 3′-UTR (Fig.
3A).
RT-qPCR analysis was performed to verify the
regulatory relationship between miR-135a-5p and ANGPT2. The results
demonstrated that ANGPT2 expression was significantly decreased
after transfecting miR-135a-5p mimics in GBC-SD cells. Moreover,
ANGPT2 was significantly inhibited by siRNA, indicating that
transfection was successful (Fig.
3C). The luciferase reporter assay demonstrated that the signal
from WT-ANGPT2 3′-UTR was significantly decreased after
transfecting miR-135a-5p mimics, while the signal from MUT-ANGPT2
3′-UTR showed no significant difference (Fig. 3B and D). These results indicated
that miR-135a-5p could directly target ANGPT2.
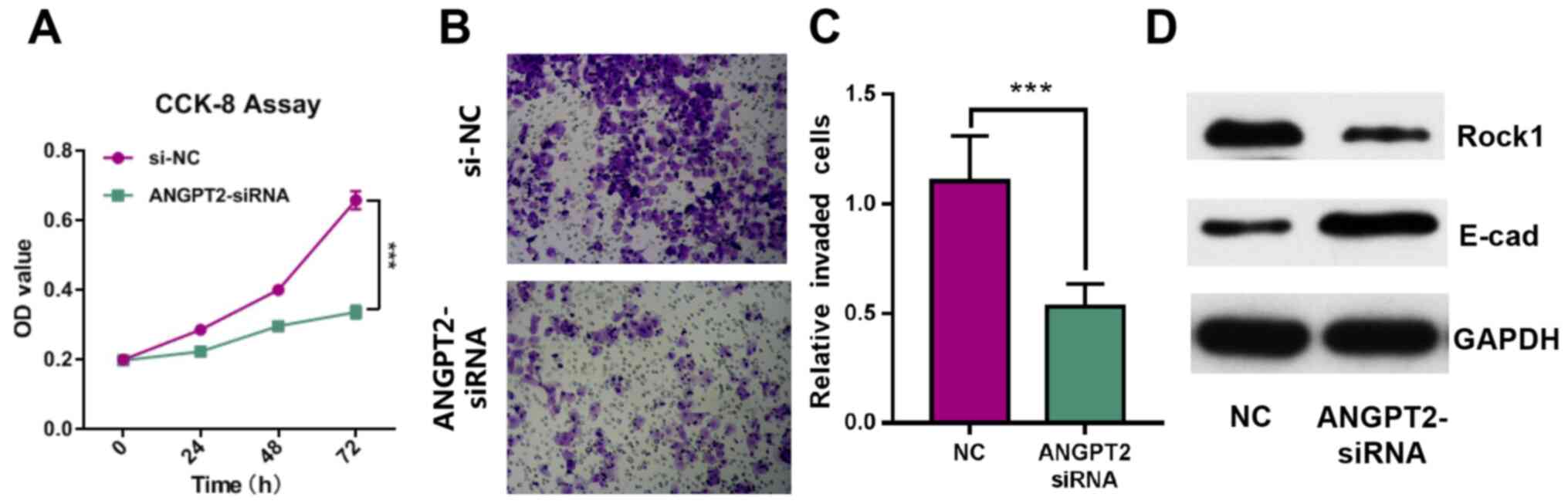
ANGPT2 inhibits the proliferation and
invasion of GBC-SD cells
To investigate the effect of ANGPT2 on GBC cell
proliferation and invasion, GBC-SD cell lines were transfected with
siRNA targeting ANGPT2. The results demonstrated that ANGPT2
expression was significantly decreased after transfection (Fig. 2C). The CCK-8 and Transwell assay
results indicated that the proliferation and invasion of GBC-SD
cells were significantly inhibited by ANGPT2 knockdown (Fig. 4A-C). Western blot analysis results
identified that Rock1 expression was inhibited, and E-cad
expression was notably increased in ANGPT2-siRNA group (Fig. 4D). These data suggested that ANGPT2
inhibited the proliferation and invasion of GBC-SD cells.
Discussion
miRNAs are involved in the occurrence of a variety
of tumors. The regulation of miRNAs on tumor suppressor genes and
oncogenes is a new research approach. Each miRNA has hundreds of
target genes that are involved in the translation or degradation of
mRNAs in a base pairing (27).
Recent studies have shown that miR-135a-5p served important but
contradictory roles in different cancer progression (28,29).
miR-135a-5p acts as a promoter or inhibitor of cancer cell
proliferation and invasion by regulating specific signaling
pathways and target genes. miR-135a-5p also regulates
epithelial-mesenchymal transformation and chemoresistance in cancer
cells (30). Previous studies
reported that miR-135a-5p was upregulated in several cancer types,
such as breast cancer, bladder cancer, melanoma and colorectal
adenocarcinomas (31–34). By contrast, other studies have
revealed that miR-135a-5p was downregulated, such as in lung
cancer, prostate cancer and pancreatic cancer (30,35–37).
The present study demonstrated that miR-135a-5p
expression was downregulated in GBC tissue, which was in line with
previous studies (13,14). Furthermore, the overexpression of
miR-135a-5p significantly inhibited the proliferation and migration
of GBC-SD cells. The expression level of ANGPT2 was suppressed in
the miR-135a-5p mimics group, suggesting that miR-135a-5p can
specifically regulate the expression level of ANGPT2 in GBC-SD
cells.
ANGPT2 is a ligand for the tyrosine-protein kinase
receptor Tie-2, which functions as a vascular stabilizing molecule
and is an important regulator of vascular maturation (16). It has previously been reported that
angiogenesis serves a key role in the development and progression
of various malignant tumors (16).
Vascular formation and dilation are closely associated with VEGFs
(38). It has been shown that VEGFs
regulate ANGPT2, and the upregulation of ANGPT2 has been found
clinically to be one of the mechanisms of acquired resistance
during anti-VEGF treatment (39,40).
Xu et al (41) revealed that
ANGPT2 may be a serum marker for lung cancer prognosis. The present
results suggested that ANGPT2 was a potential target gene of
miR-135a-5p in GBC. Furthermore, knockdown of ANGPT2 expression
significantly inhibited the proliferation and invasion of GBC-SD
cells.
There are several potential limitations to the
present study; one of which was the small number of patient cases
enrolled. Moreover, there were few GBC-related data in the present
study and TCGA dataset; therefore, there may be some sampling
errors in the survival analysis. In future studies, additional
clinical information of patients with GBC will be collected for
relevant verification. Further verification may improve confidence
in the present results, which indicated that the protein expression
levels of ANGPT2 were reduced after miR-135a-5p mimics or siRNA
treatment. Additional validation experiments in future animal model
experiments will help to provide a more detailed understanding of
the relationship between ANGPT2 and miR-135a-5p.
In conclusion, the present study demonstrated that
miR-135a-5p was downregulated and was negatively associated with
malignancies in GBC. It was identified that miR-135a-5p affected
GBC cell proliferation and invasion by targeting ANGPT2. Moreover,
miR-135a-5p may be a potential biomarker for GBC progression and a
potential target for GBC therapeutic intervention.
Acknowledgements
Not applicable.
Funding
This study was funded by the Health Science and
Technology Project of Shanghai Pudong New Area Health Commission
(grant no. PW2020A-33), the Pudong New Area Key Specialty of
Thyroid (grant no. PWZzk2017-21) and the ‘Postgraduate Innovation
Training Special Project’ of Shanghai University of Traditional
Chinese Medicine in 2021 (grant no. Y2021023).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GHY and HYD designed the work. GHY, HYD, XX and BZ
contributed to the experiment, analyzed the data and drafted the
manuscript. GHY and HYD confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Seventh People's Hospital Affiliated to Shanghai
University of Traditional Chinese Medicine. All enrolled patients
signed an informed consent form before surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rakić M, Patrlj L, Kopljar M, Kliček R,
Kolovrat M, Loncar B and Busic Z: Gallbladder cancer. Hepatobiliary
Surg Nutr. 3:221–226. 2014.
|
|
2
|
Sharma A, Sharma KL, Gupta A, Yadav A and
Kumar A: Gallbladder cancer epidemiology, pathogenesis and
molecular genetics: Recent update. World J Gastroenterol.
23:3978–3998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI
|
|
4
|
Farr RJ, Joglekar MV and Hardikar AA:
Circulating microRNAs in diabetes progression: Discovery,
validation, and research translation. Exp Suppl. 106:215–244.
2015.PubMed/NCBI
|
|
5
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Acunzo M and Croce CM: MicroRNA in cancer
and cachexia-A mini-review. J Infect Dis. 212 (Suppl 1):S74–S77.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie Y, Li F, Li Z and Shi Z: miR-135a
suppresses migration of gastric cancer cells by targeting
TRAF5-mediated NF-κB activation. Onco Targets Ther. 12:975–984.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu B, Lu X, Zhao Y, Liu C, Huang X, Chen
S, Zhu W, Zhang L and Chen M: MicroRNA-135a induces prostate cancer
cell apoptosis via inhibition of STAT6. Oncol Lett. 17:1889–1895.
2019.PubMed/NCBI
|
|
9
|
Shi HZ, Wang DN, Xu F, Teng JH and Wang
YL: miR-135a inhibits glioma cell proliferation and invasion by
directly targeting FOXO1. Eur Rev Med Pharmacol Sci. 22:4215–4223.
2018.PubMed/NCBI
|
|
10
|
Lv YP, Shi W, Liu HX, Kong XJ and Dai DL:
Identification of miR-146b-5p in tissues as a novel biomarker for
prognosis of gallbladder carcinoma. Eur Rev Med Pharmacol Sci.
21:518–522. 2017.PubMed/NCBI
|
|
11
|
Peng HH, Zhang YD, Gong LS, Liu WD and
Zhang Y: Increased expression of microRNA-335 predicts a favorable
prognosis in primary gallbladder carcinoma. Onco Targets Ther.
6:1625–1630. 2013.PubMed/NCBI
|
|
12
|
Bao RF, Shu YJ, Hu YP, Wang XA, Zhang F,
Liang HB, Ye YY, Li HF, Xiang SS, Weng H, et al: miR-101 targeting
ZFX suppresses tumor proliferation and metastasis by regulating the
MAPK/Erk and Smad pathways in gallbladder carcinoma. Oncotarget.
7:22339–22354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou H, Guo W, Zhao Y, Wang Y, Zha R, Ding
J, Liang L, Yang G, Chen Z, Ma B and Yin B: MicroRNA-135a acts as a
putative tumor suppressor by directly targeting very low density
lipoprotein receptor in human gallbladder cancer. Cancer Sci.
105:956–965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang G and Yin B: Therapeutic effects of
long-circulating miR-135a-containing cationic immunoliposomes
against gallbladder carcinoma. Sci Rep. 7:59822017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fiedler U and Augustin HG: Angiopoietins:
A link between angiogenesis and inflammation. Trends Immunol.
27:552–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang H, Bhat A, Woodnutt G and Lappe R:
Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer.
10:575–585. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Z, Zhu S, Hong J, Soutto M, Peng D,
Belkhiri A, Xu Z and El-Rifai W: Gastric tumour-derived ANGPT2
regulation by DARPP-32 promotes angiogenesis. Gut. 65:925–934.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jary M, Vernerey D, Lecomte T, Dobi E,
Ghiringhelli F, Monnien F, Godet Y, Kim S, Bouché O, Fratte S, et
al: Prognostic value of angiopoietin-2 for death risk
stratification in patients with metastatic colorectal carcinoma.
Cancer Epidemiol Biomarkers Prev. 24:603–612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dreikhausen L, Blank S, Sisic L, Heger U,
Weichert W, Jäger D, Bruckner T, Giese N, Grenacher L, Falk C, et
al: Association of angiogenic factors with prognosis in esophageal
cancer. BMC Cancer. 15:1212015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Yu Y, Chen X, He Y, Hu Q, Li H,
Han Q, Ren F, Li J, Li C, et al: MiR-139-5p is associated with poor
prognosis and regulates glycolysis by repressing PKM2 in
gallbladder carcinoma. Cell Prolif. 51:e125102018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun S, Wang R, Yi S, Li S, Wang L and Wang
J: Roles of the microRNA3383p/NOVA1 axis in retinoblastoma. Mol Med
Rep. 23:3942021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Y, Ma L, Guo Q and Zhang S: Expression
of bone morphogenetic protein-2 and its receptors in epithelial
ovarian cancer and their influence on the prognosis of ovarian
cancer patients. J Exp Clin Cancer Res. 29:852010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma DJ, Cao Z, Wang BS and Sun YL: Effect
of silencing hepatocyte growth factor receptor c-Met expression on
biological characteristics of colon cancer cells. Zhonghua Zhong
Liu Za Zhi. 42:362–368. 2020.(In Chinese). PubMed/NCBI
|
|
25
|
Liu Y, Fu X, Wang X, Liu Y and Song X:
Long non-coding RNA OIP5-AS1 facilitates the progression of ovarian
cancer via the miR-128-3p/CCNG1 axis. Mol Med Rep. 23:3882021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anaya J: OncoLnc: Linking TCGA survival
data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput Sci. 2:e672016.
View Article : Google Scholar
|
|
27
|
Rebane A and Akdis CA: MicroRNAs in
allergy and asthma. Curr Allergy Asthma Rep. 14:4242014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao Z, Qiu J, Yang G, Liu Y, Luo W, You L,
Zheng L and Zhang T: MiR-135a biogenesis and regulation in
malignancy: A new hope for cancer research and therapy. Cancer Biol
Med. 17:569–582. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang C, Zheng X, Ye K, Sun Y, Lu Y, Fan Q
and Ge H: miR-135a inhibits the invasion and migration of
esophageal cancer stem cells through the hedgehog signaling pathway
by targeting Smo. Mol Ther Nucleic Acids. 19:841–852. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi H, Ji Y, Zhang D, Liu Y and Fang P:
MiR-135a inhibits migration and invasion and regulates EMT-related
marker genes by targeting KLF8 in lung cancer cells. Biochem
Biophys Res Commun. 465:125–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Y, Zhang J, Wang H, Zhao J, Xu C, Du
Y, Luo X, Zheng F, Liu R, Zhang H and Ma D: miRNA-135a promotes
breast cancer cell migration and invasion by targeting HOXA10. BMC
Cancer. 12:1112012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mao XP, Zhang LS, Huang B, Zhou SY, Liao
J, Chen LW, Qiu SP and Chen JX: Mir-135a enhances cellular
proliferation through post-transcriptionally regulating PHLPP2 and
FOXO1 in human bladder cancer. J Transl Med. 13:862015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ren JW, Li ZJ and Tu C: MiR-135
post-transcriptionally regulates FOXO1 expression and promotes cell
proliferation in human malignant melanoma cells. Int J Clin Exp
Pathol. 8:6356–6366. 2015.PubMed/NCBI
|
|
34
|
Nagel R, le Sage C, Diosdado B, van der
Waal M, Oude Vrielink JA, Bolijn A, Meijer GA and Agami R:
Regulation of the adenomatous polyposis coli gene by the miR-135
family in colorectal cancer. Cancer Res. 68:5795–5802. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fukagawa S, Miyata K, Yotsumoto F,
Kiyoshima C, Nam SO, Anan H, Katsuda T, Miyahara D, Murata M, Yagi
H, et al: MicroRNA-135a-3p as a promising biomarker and nucleic
acid therapeutic agent for ovarian cancer. Cancer Sci. 108:886–896.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu B, Tao T, Wang Y, Fang F, Huang Y, Chen
S, Zhu W and Chen M: hsa-miR-135a-1 inhibits prostate cancer cell
growth and migration by targeting EGFR. Tumour Biol.
37:14141–14151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang Y, Cao G, Zhao G, Wang C and Qin Q:
LncRNA differentiation antagonizing non-protein coding RNA promotes
proliferation and invasion through regulating miR-135a/NLRP37 axis
in pancreatic cancer. Invest New Drugs. 38:714–721. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rakoczy PE, Brankov M, Fonceca A, Zaknich
T, Rae BC and Lai CM: Enhanced recombinant adeno-associated
virus-mediated vascular endothelial growth factor expression in the
adult mouse retina: A potential model for diabetic retinopathy.
Diabetes. 52:857–863. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song Z, Wu Y, Yang J, Yang D and Fang X:
Progress in the treatment of advanced gastric cancer. Tumour Biol.
39:10104283177146262017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rigamonti N, Kadioglu E, Keklikoglou I,
Wyser Rmili C, Leow CC and De Palma M: Role of angiopoietin-2 in
adaptive tumor resistance to VEGF signaling blockade. Cell Rep.
8:696–706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu Y, Zhang Y, Wang Z, Chen N, Zhou J and
Liu L: The role of serum angiopoietin-2 levels in progression and
prognosis of lung cancer: A meta-analysis. Medicine (Baltimore).
96:e80632017. View Article : Google Scholar : PubMed/NCBI
|