Introduction
Perioperative neurocognitive disorder (PND) is a
common cognitive complication of surgery, especially among older
patients, which is characterized by progressive cognitive
deterioration in the preoperative or postoperative period. PND has
several clinical manifestations, including attention deficits,
learning disabilities and memory loss (1,2). In
the long term, PND can result in prolonged hospitalization,
decreased quality of life and increased financial burden on the
family and society (3).
Nevertheless, the mechanisms underlying PND are not completely
understood. Netto et al (4)
reported that PND model rats displayed increased levels of
nitrite/nitrate, which are metabolites of nitric oxide (NO). Zhang
et al (5) demonstrated that
anesthesia and surgery increased reactive oxygen species (ROS) and
decreased the level of antioxidant enzymes in the hippocampus of
mice. The superoxide anion (O2•−) is an
important specie of ROS (6).
Inducible NO synthase (iNOS), which is primarily
expressed in pathological conditions, is able to produce large
amounts of NO. Appropriate levels of NO can serve a neuroprotective
role, but large quantities of NO are neurotoxic (7). A high level of NO has been indicated
to serve as a major mediator in neurodegenerative diseases
(8,9). It was reported that prolonged NO
exposure could result in overproduction of cytoplasmic τ protein in
SH-SY5Y cells (10). NO also serves
an important role in the process of Parkinson's disease by
targeting the mitochondria, leading to inhibition of the
respiratory chain (11). A higher
concentration of NO reacts with O2•− to
generate a large amount of peroxynitrite (ONOO−).
ONOO− is a highly cytotoxic superoxide that can lead to
neuronal damage by oxidizing lipids, proteins and DNA (12). However, the roles of iNOS and NO in
the process of PND are not completely understood.
Ginkgolide B (GB) is one of the terpene lactone
components extracted from Gingko biloba (13) It has been reported that GB protects
SH-SY5Y cells against Aβ toxicity (14). GB can also inhibit oxidative stress
to protect neurons from cerebral ischemia injury (15). GB and its analog XQ-1H have been
reported to exert neuroprotective effects by downregulating iNOS
and NO in cerebral ischemia and reperfusion (16–18).
However, to the best of our knowledge, no previous studies have
investigated GB as a protective treatment for PND.
The present study hypothesized that iNOS was
involved in the process of PND by promoting the production of NO,
and the neuroprotective effect of GB on PND was associated with the
inhibition of iNOS-mediated production of NO. Therefore, the
present study used the specific iNOS inhibitor 1400W to investigate
the role of iNOS-mediated production of NO in the process of PND.
The effects of GB on PND, iNOS-mediated production of NO, neuronal
apoptosis and morphological alterations of neurons in the
hippocampus were also investigated. The results of the present
study may suggest a novel effective pretreatment for PND.
Materials and methods
Animals
Male C57B6/L mice (age, 10–12 weeks; weight, 23–28
g; n=96) were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd.. Mice were housed under controlled conditions
with 12-h light/dark cycles and were provided with a standard diet
and water ad libitum. The temperature was controlled at
26±1°C and the humidity was maintained at 50±10%. All experiments
were approved by the Animal Experiments and Experimental Animal
Welfare Committee of Capital Medical University (ethical review
number: 2018-0003; Beijing, China) and performed under the
regulations of the Medical Research Center of Beijing Chao-Yang
Hospital (Beijing, China).
Intraperitoneal administration of
1400W and GB
For the intervention study, 1400W and GB were
intraperitoneally administered before surgery. To determine the
effective dosage, three male C57B6/L mice (age, 10–12 weeks;
weight, 23–28 g) of each group received different doses of 1400W
(10 and 20 mg/kg) and GB (10 and 20 mg/kg) in the preliminary
experiment. These mice were housed under the same conditions as
aforementioned. The results demonstrated that the administration of
20 mg/kg 1400W at 30 min prior to surgery prevented surgery-induced
cognitive impairment. For pretreatment with GB, repeated
administration of GB (20 mg/kg/day) for 5 days prior to surgery
improved exploratory laparotomy-induced cognitive dysfunction (data
not shown). Subsequently, mice were randomly divided into the
following groups: i) Control (n=32, mice received no treatment);
ii) surgery (n=32); iii) surgery + GB [mice received GB (20
mg/kg/day) for 5 days prior to surgery, n=16]; and iv) surgery +
1400W [mice received 1400W (20 mg/kg) at 30 min prior to surgery,
n=16].
Abdominal surgery
The exploratory laparotomy was performed as
previously described (19,20). The mice were anesthetized with 3.0%
isoflurane for induction and 1.5% isoflurane for maintenance. A
1.5-cm vertical incision was made in the midline of the abdomen. A
sterile metal probe was inserted into the abdominal cavity and the
abdominal viscera was explored. The entire procedure lasted ~10
min. Subsequently, the peritoneum, abdominal muscles and skin were
sutured layer-by-layer using 4-0 silk thread sutures. The mice were
placed into a chamber for recovery from anesthesia. Following
recovery, the mice were placed back into their cages with ad
libitum access to water and food.
Open field test (OFT)
The OFT was performed as previously described
(21). The different groups of mice
were subjected to the OFT on postoperative days 1 and 3 (n=8). Each
mouse was gently placed in the center of the Plexiglas®
open field apparatus (40 cm3) for 5 min. Movement was
monitored and recorded using a video tracking system. The total
moving distance in the chamber and duration in the central area
were recorded to assess locomotor activity and exploration
(22).
Fear conditioning test (FCT)
The FCT was performed as previously described, with
minor adjustments (n=8 mice/group) (23,24).
All mice were trained for fear conditioning 2 h prior to surgery.
For fear conditioning, each mouse was placed into a conditioning
chamber, and a video and software (Xeye FCS; Beijing MacroAmbiton
S&T Development Co., Ltd.) were used to record and analyze the
movement of the mice. The mice were allowed to explore for 180 sec
before exposure to both tone and foot shock (tone: 2,500 Hz, 80 Db,
30 sec; foot shock: 0.7 mA, 2 sec), three times at 60-sec
intervals. Subsequently, the mouse was removed from the chamber and
returned to its cage. In addition, 70% alcohol was used to wipe the
grid floor during the interval between testing of different
animals.
The mice underwent the contextual and cued tests on
postoperative days 1 and 3. For the contextual test, each mouse was
placed into the same chamber for 360 sec without tone or foot
shock. For the cued test, each mouse was placed into the same
chamber for 180 sec with tone but without foot shock. There was a
2-h interval between the two tests. Cognitive function was
determined as the amount of time that freezing behavior was
recorded by the video tracking system. The contextual test
reflected hippocampus-dependent memory function and the cued test
reflected hippocampus-independent memory function (25).
Biochemical analysis
On postoperative day 1 and 3, mice were
transcardially perfused with ice-cold PBS under anesthesia (3.0%
isoflurane for induction and 1.5% isoflurane for maintenance).
Subsequently, the mice were euthanized via cervical dislocation,
and brain tissue was harvested and immediately stored at −80°C. The
cortex and hippocampus were homogenized in PBS. Following
centrifugation of the homogenate at 1,431 × g for 10 min at 4°C,
the supernatant was collected for biochemical analysis. The total
protein concentration of the supernatant was determined using the
bicinchoninic acid protein assay (cat. no. P0012S; Beyotime
Institute of Biotechnology). NO, malondialdehyde (MDA) and
superoxide dismutase (SOD) levels in the cortex and hippocampus
were measured using NO assay kit (nitrate reductase method; cat.
no. A012-1-2; Nanjing Jiancheng Bioengineering Institute), MDA
assay kit (TBA method; cat. no. A003-1-2; Nanjing Jiancheng
Bioengineering Institute), total SOD assay kit (hydroxylamine
method; cat. no. A001-1-2; Nanjing Jiancheng Bioengineering
Institute) according to the manufacturer's protocols. Biochemical
analyses were performed at 4°C. (n=6).
Western blotting
Total protein was extracted from hippocampal tissues
using Minute™ Total Protein Extraction kit (cat. SD-001/SN-002,
Invent Biotechnologies, Inc.) with a protease and phosphatase
inhibitor cocktail (Thermo Fisher Scientific, Inc.). The homogenate
was centrifuged at 13,147 × g for 2 min at 4°C. The supernatant was
obtained and the protein concentrations in the supernatant were
quantified using a BCA Protein assay reagent kit (Beyotime
Institute of Biotechnology). Proteins (30 µg per lane) were
separated on a 10% gel using SDS-PAGE and transferred onto PVDF
membranes. Following blocking with 5% skimmed milk for 1 h at 37°C,
the membranes were incubated overnight at 4°C with primary
antibodies in 5% milk targeted against iNOS, (n=6 mice/group)
(1:1,000; cat. no. ab178945; Abcam), Bax, (n=4), (1:1,000; cat. no.
ab32503; Abcam), Bcl-2, (n=4) (1:2,000; cat. no. ab182858; Abcam)
and β-actin (1:1,000; cat. no. ab8226; Abcam). After washing three
times with TBS-0.05% Tween-20, the membranes were incubated with
secondary antibodies (1:5,000; cat. nos. 31464 and 31450; Thermo
Fisher Scientific, Inc.) in TBS for 30 min at 37°C. Subsequently,
the membranes were incubated with chemiluminescent HRP substrate
(Thermo Fisher Scientific, Inc.). Protein bands were visualized
using the ChemiDoc XRS system (Version 3.0; Bio-Rad Laboratories,
Inc.). Protein expression was semi-quantified using Image Lab
software (Bio-Rad Laboratories, Inc.) with β-actin as the loading
control.
Hematoxylin and eosin staining
Mice were perfused with ice-cold PBS and sacrificed
by cervical dislocation. The brain tissue was immediately collected
and fixed with 4% paraformaldehyde for 24 h at room temperature.
After dehydration with alcohol (30–100%), the brain tissue was
embedded in paraffin. Subsequently, 4-µm thick coronal sections
were prepared. The brain sections were dewaxed with xylene,
followed by hydrating with gradient alcohol series (100–70%). The
brain sections were then placed in 0.5% hydrochloric acid for 10
sec and in water for 10 min. The slices were later stained with
hematoxylin for 5 min and counterstained with eosin for 3 min. An
optical microscope (Olympus Corporation) was used to observe the
slices, (n=3).
Statistical analysis
All experiments were performed once; the number of
mice in each group varied between three and eight. Data are
presented as the mean ± SEM. Statistical analyses were performed
using GraphPad Prism V7 (GraphPad Software, Inc.). Comparisons
among multiple groups were analyzed using one-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
1400W pretreatment improves abdominal
surgery-induced learning and memory impairments
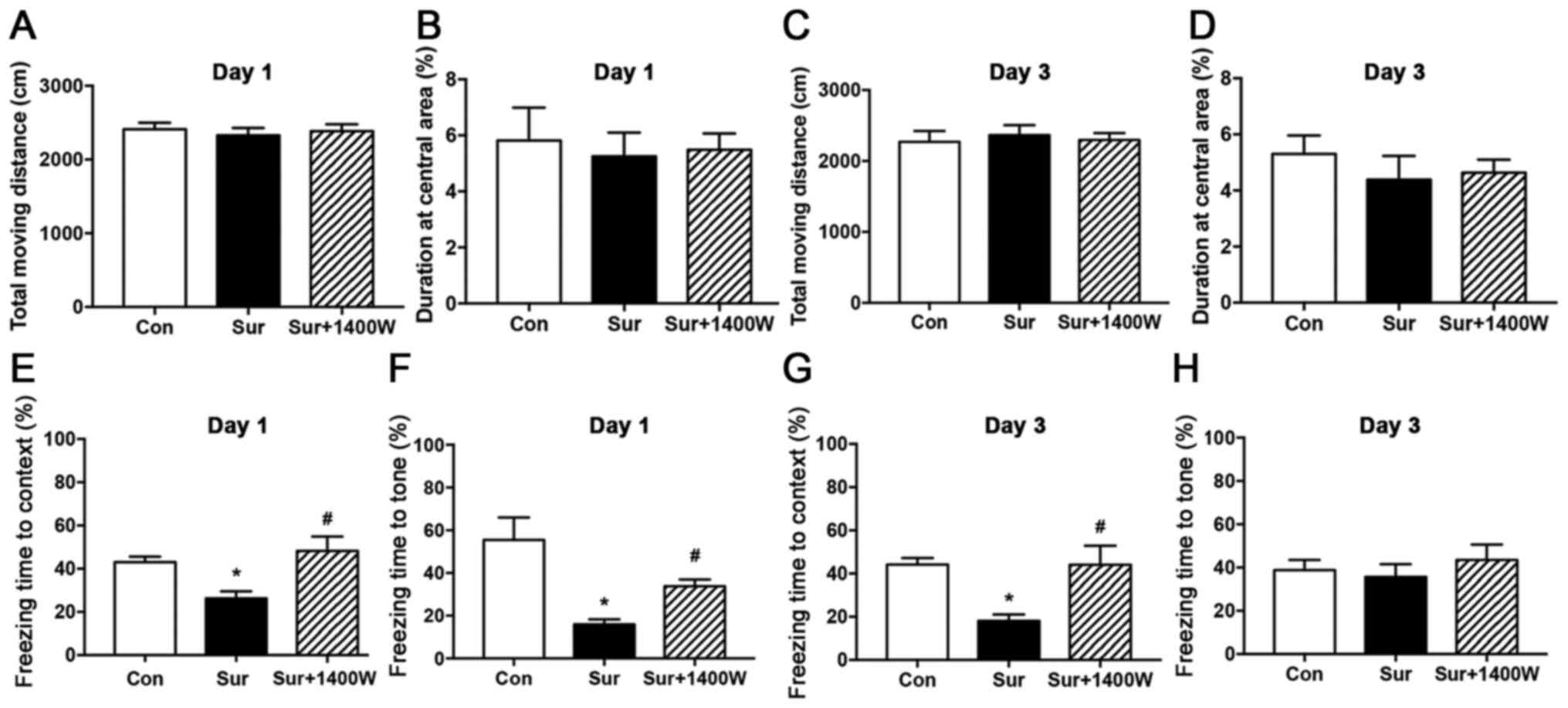
The OFT was performed to evaluate locomotor activity
and exploration of the mice prior to the FCT by recording the total
moving distance and duration at the central area. No significant
differences in the OFT results were identified among the three
groups on postoperative days 1 and 3, suggesting that locomotor
activity and exploration were not affected by surgery or
pretreatment with 1400W (P>0.05; Fig. 1A-D).
Subsequently, the FCT was conducted to evaluate
learning and memory capacities. The freezing time to context in the
surgery group was significantly lower on postoperative days 1 and 3
compared with that in the control group (P<0.05; Fig. 1E and G). Similarly, the freezing
time to tone was significantly lower in the surgery group compared
with that in the control group on postoperative day 1 (P<0.05;
Fig. 1F). No significant difference
in the freezing time to tone among the three groups was observed on
postoperative day 3 (P>0.05; Fig.
1H). Furthermore, compared with the surgery group, the surgery
+ 1400W group displayed significantly higher freezing times in both
the contextual and cued tests on postoperative day 1 (P<0.05;
Fig. 1E and F), and in the
contextual test on postoperative day 3 (P<0.05; Fig. 1G). The results suggested that 1400W
improved surgery-induced cognitive deficits.
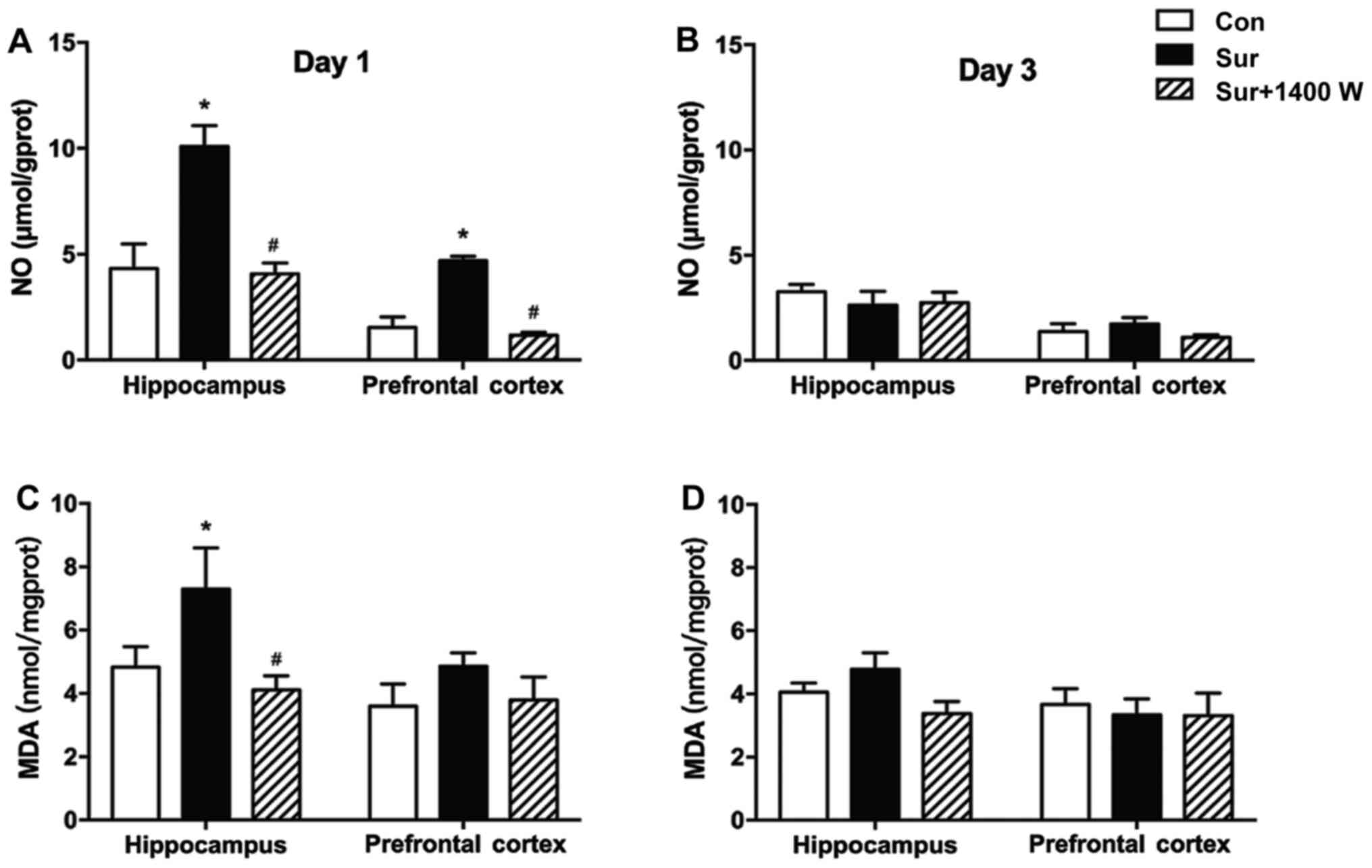
iNOS inhibitor 1400W pretreatment
decreases NO and MDA levels
Subsequently, whether surgery-induced learning and
memory function deficits were associated with iNOS-mediated NO
production in the hippocampus was investigated. NO levels in the
hippocampus and prefrontal cortex of the surgery group were
significantly higher compared with the levels in the control group
on postoperative day 1 (P<0.05; Fig.
2A). In addition, compared with the control group, the surgery
group exhibited a significant increase in the level of MDA in the
hippocampus on postoperative day 1 (P<0.05; Fig. 2C). No significant differences in NO
and MDA levels were observed among the three groups on
postoperative day 3 (P>0.05; Fig. 2B
and D). Compared with those in the surgery group, significantly
lower levels of NO and MDA were observed in the hippocampus of the
surgery + 1400W group on postoperative day 1. In addition, NO was
decreased in the prefrontal cortex in the surgery + 1400W group
compared with that in the surgery group (P<0.05; Fig. 2A and C).
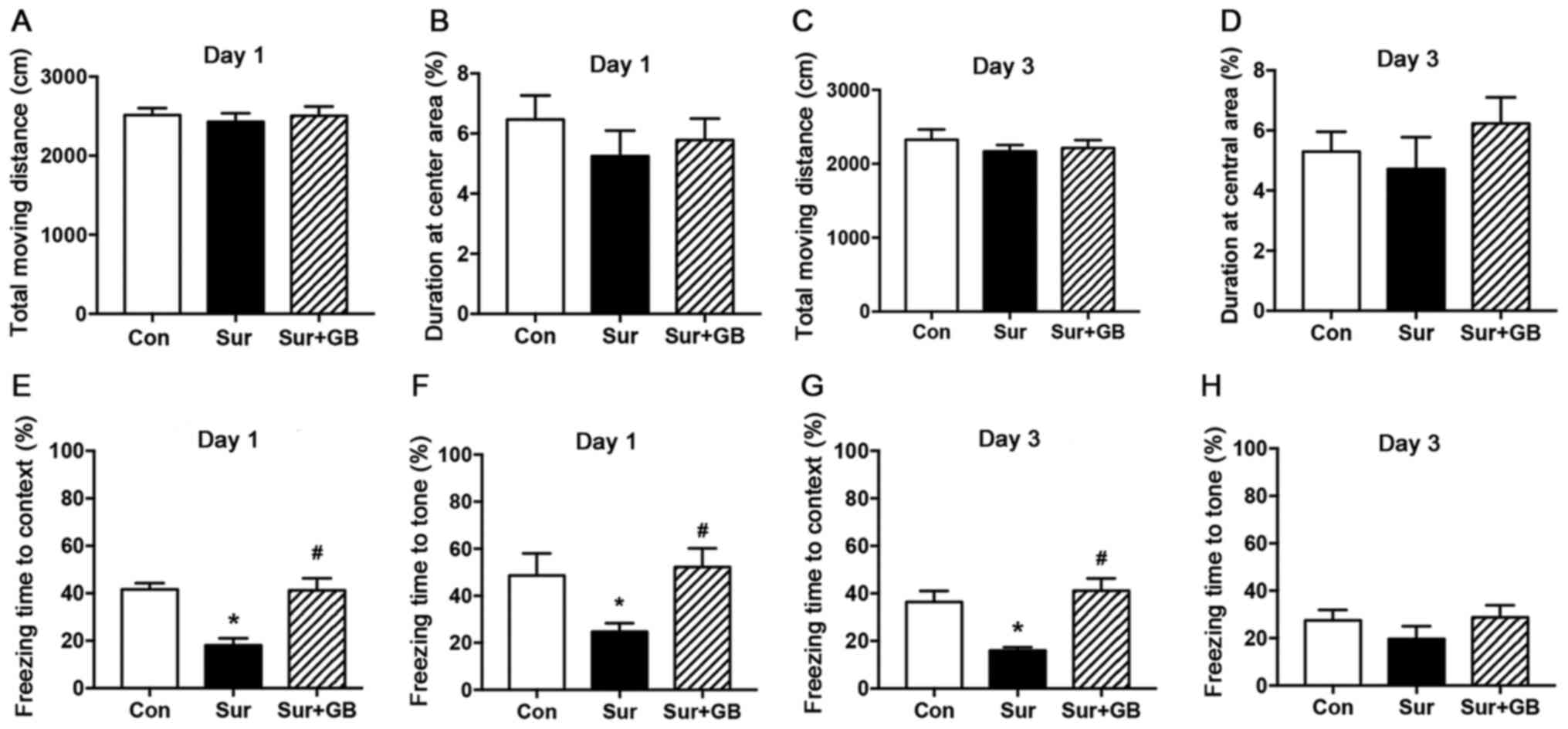
GB pretreatment attenuates abdominal
surgery-induced learning and memory impairments
An OFT was conducted to evaluate alterations in
locomotor activity and exploration. No significant differences in
the total moving distance and duration at the central area were
observed among the three groups (P>0.05; Fig. 3A-D).
The effects of GB pretreatment on learning and
memory capacities were evaluated by performing the FCT. Compared
with the control group, the surgery group displayed a significant
reduction in freezing time in both the contextual and cued tests on
postoperative day 1 (P<0.05; Fig. 3E
and F). On postoperative day 3, the freezing time in the
contextual test in the surgery group was significantly decreased
compared with that of the control group (P<0.05; Fig. 3G). Moreover, abdominal
surgery-induced cognitive deficits on postoperative day 1 were
significantly reversed by pretreatment with GB (P<0.05; Fig. 3E and F). In addition, on
postoperative day 3, GB pretreatment could increase the freezing
time in the contextual test (P<0.05; Fig. 3G), but had no impact on that in the
cued test (P>0.05; Fig. 3H).
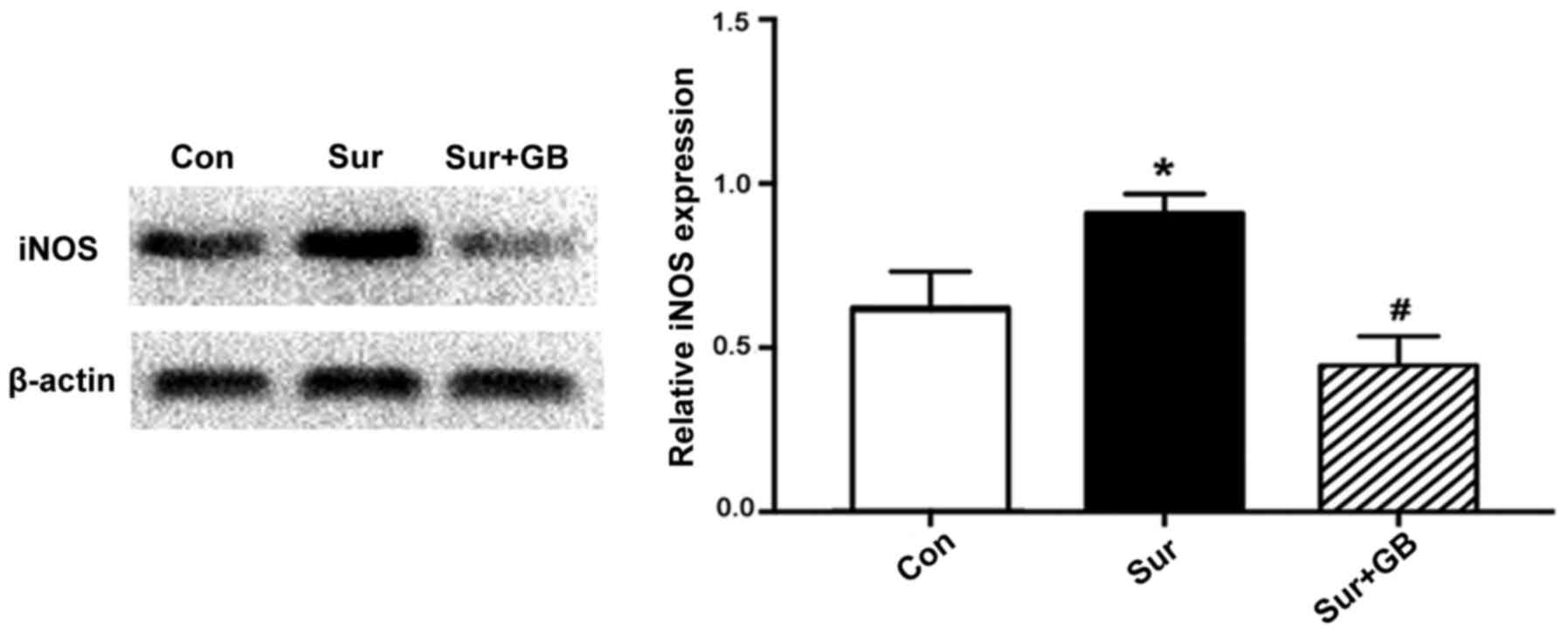
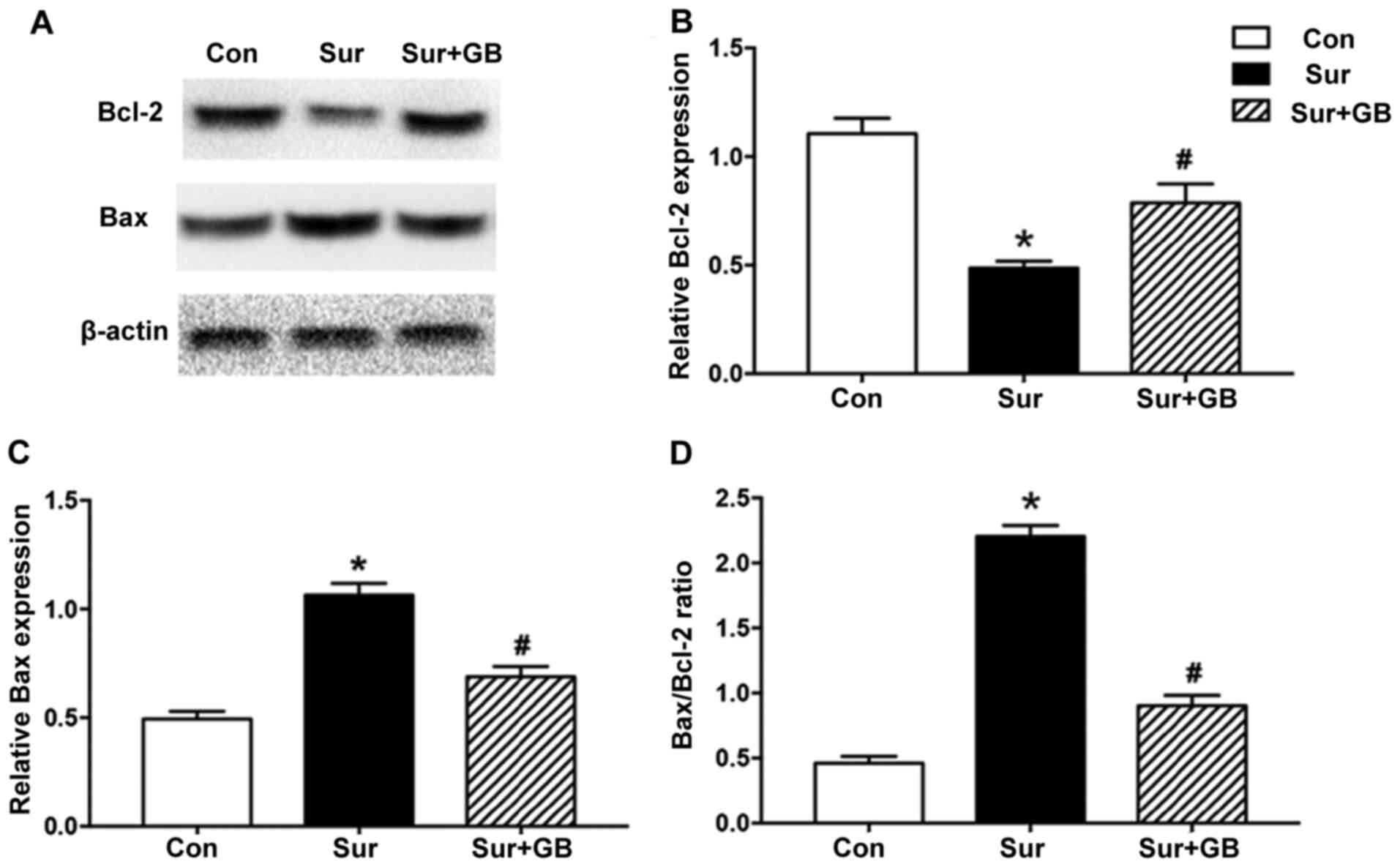
GB pretreatment inhibits
surgery-induced elevations in iNOS, NO and MDA, and alleviates
surgery-induced downregulation of SOD
The western blotting results demonstrated that
surgery significantly increased the protein expression levels of
iNOS in the hippocampus on postoperative day 1 compared with the
control group (P<0.05; Fig. 4).
GB pretreatment significantly decreased surgery-induced increases
in iNOS expression levels (P<0.05; Fig. 4). Subsequently, the effects of GB
pretreatment on the levels of NO, MDA and SOD on postoperative days
1 and 3 were assessed. GB pretreatment significantly alleviated
surgery-induced decreases in SOD levels in the hippocampus and
prefrontal cortex on postoperative day 1 (P<0.05; Fig. 5A). GB pretreatment displayed the
same effect on surgery-mediated alterations to SOD levels in the
hippocampus on postoperative day 3 (P<0.05; Fig. 5B). GB pretreatment significantly
decreased surgery-induced increases in NO and MDA levels in the
hippocampus on postoperative day 1 (P<0.05; Fig. 5C and E). GB pretreatment also
significantly inhibited surgery-induced upregulation of NO levels
on postoperative day 1 in the prefrontal cortex (P<0.05;
Fig. 5C). No significant
differences were indicated in the levels of NO and MDA among three
groups on postoperative day 3 (Fig. 5D
and F). The results indicated that GB treatment may alleviate
surgery-induced oxidative stress by downregulating iNOS
expression.
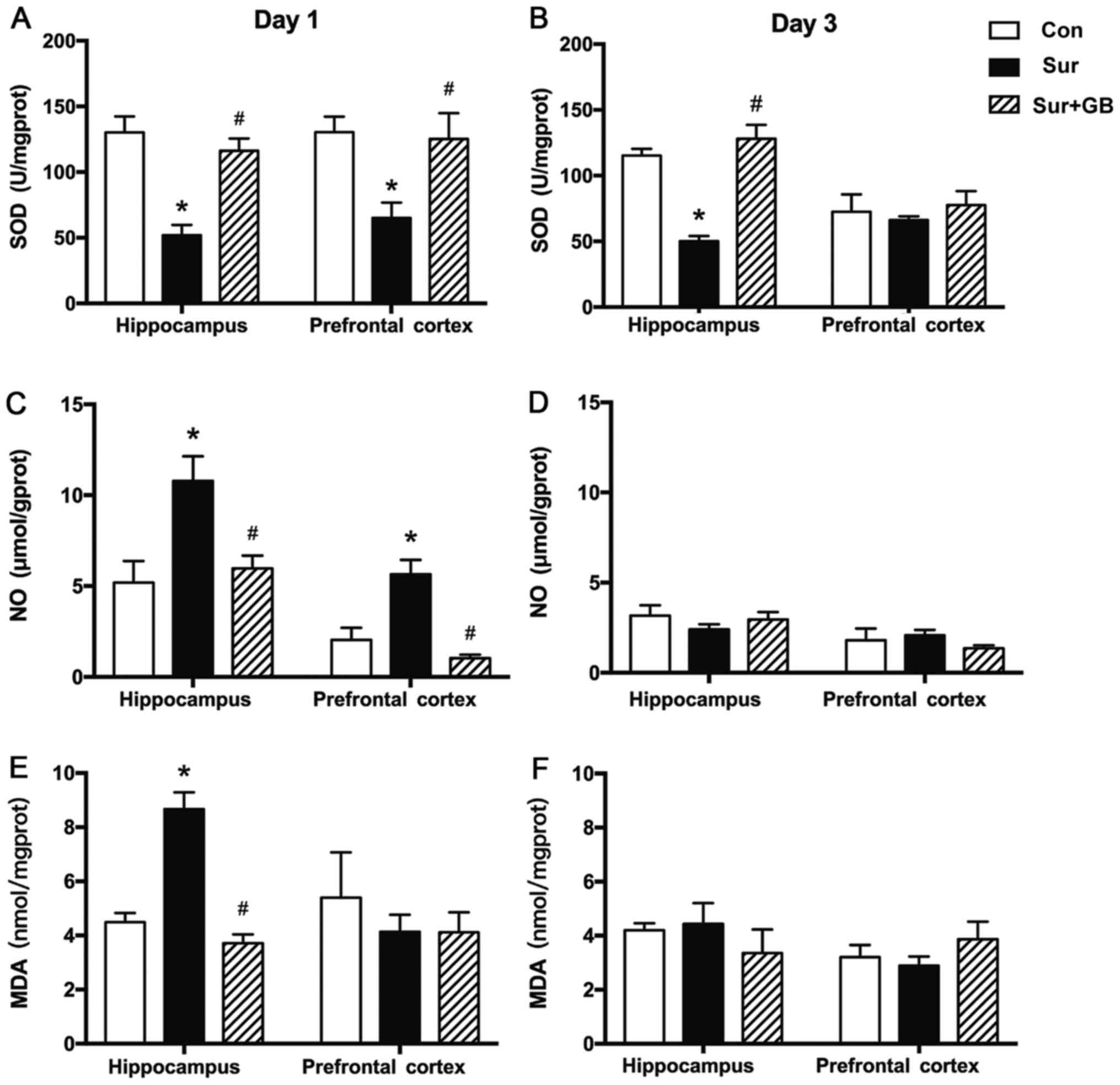 | Figure 5.Effect of GB pretreatment on SOD, NO
and MDA. GB pretreatment significantly reversed surgery-induced
decreases in SOD levels (A) in the hippocampus and prefrontal
cortex on postoperative day 1 and (B) in the hippocampus on
postoperative day 3. (C) Compared with the control group, surgery
resulted in significant overproduction of NO on postoperative day
1, which was significantly inhibited by pretreatment with GB. (D)
No significant differences in NO levels were observed among the
three groups on postoperative day 3. (E) Compared with the control
group, surgery resulted in significant overproduction of MDA in the
hippocampus on postoperative day 1, which was significantly
inhibited by pretreatment with GB. (F) No significant differences
in MDA levels were observed among the three groups on postoperative
day 3. Data are presented as the mean ± SEM (n=6 per group).
*P<0.05 vs. control; #P<0.05 vs. surgery. GB,
ginkgolide B; SOD, superoxide dismutase; NO, nitric oxide; MDA,
malondialdehyde; Con, control; Sur, surgery. |
GB pretreatment decreases the
Bax/Bcl-2 ratio and neuronal loss
The neuroprotective role of GB has been reported to
be associated with inhibition of the apoptotic protein Bax and
elevation of the anti-apoptotic protein Bcl-2 (17). To assess whether surgery altered the
Bax/Bcl-2 ratio in the hippocampus, the protein expression levels
of Bax and Bcl-2 were assessed via western blotting. Bcl-2
expression levels were significantly decreased in the surgery group
compared with those in the control group (P<0.05; Fig. 6A and B). By contrast, Bax expression
levels in the surgery group were significantly increased compared
with those in the control group (P<0.05; Fig. 6A and C). Compared with the surgery
group, pretreatment with GB significantly increased Bcl-2
expression levels on postoperative day 3, whereas GB pretreatment
significantly attenuated surgery-induced Bax upregulation
(P<0.05; Fig. 6A-C).
Furthermore, the ratio of Bax/Bcl-2, which indicates activation of
the proapoptotic signaling pathway (26), was significantly higher in the
surgery group compared with that in the control group (P<0.05;
Fig. 6D). Compared with the surgery
group, GB pretreatment significantly decreased the Bax/Bcl-2 ratio
(P<0.05; Fig. 6D). Thus, the
results suggested that GB pretreatment inhibited surgery-induced
apoptosis.
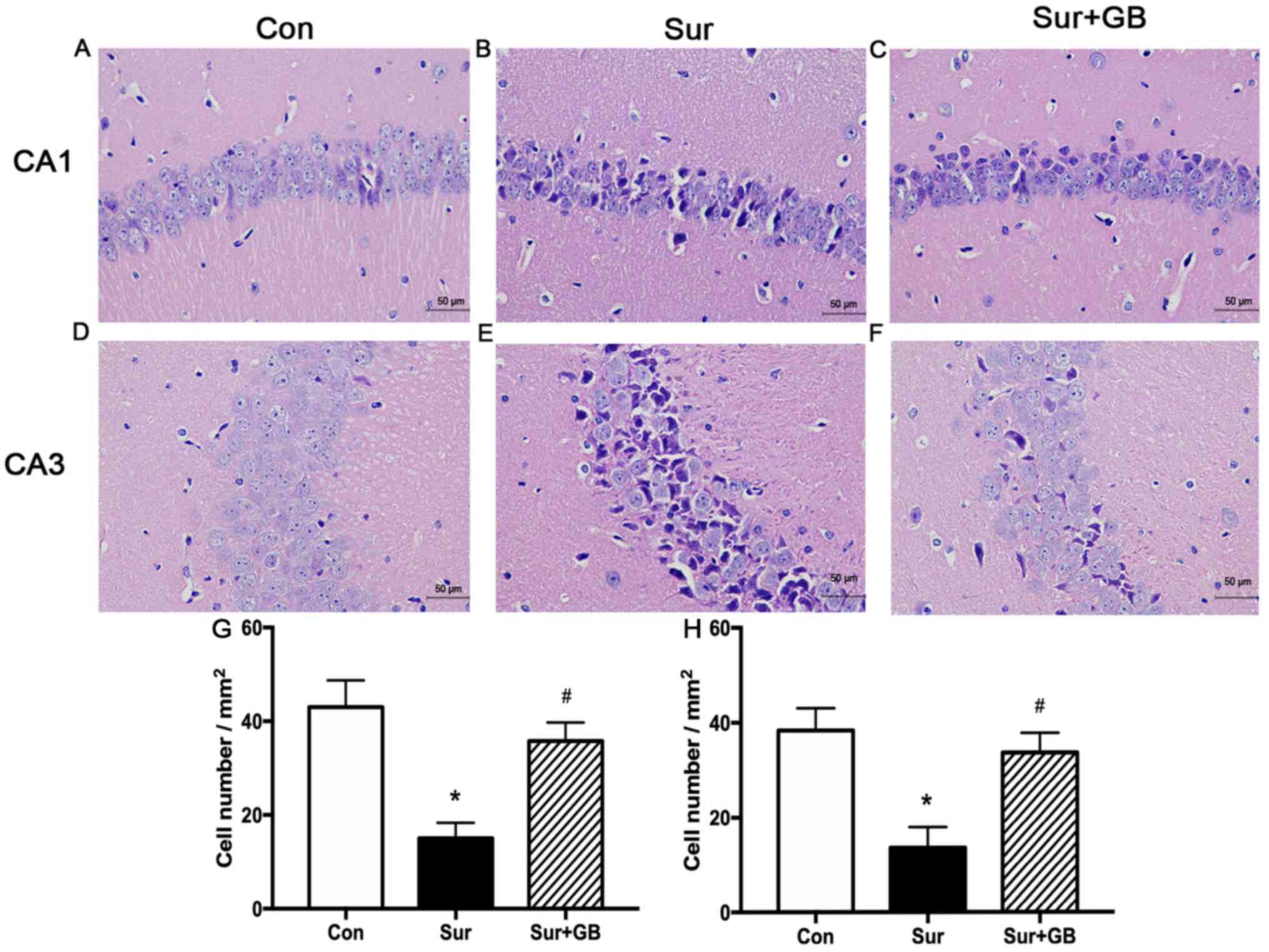
Hematoxylin and eosin staining was conducted to
evaluate morphological alterations of neurons in the hippocampus on
postoperative day 3. The neurons were round and tightly arranged in
the CA1 and CA3 regions in the control group (Fig. 7A and D). However, compared with
control group, the neurons in the CA1 and CA3 regions of the
surgery group displayed greater shrinkage, a looser arrangement and
darker staining (Fig. 7B and E). In
addition, compared with the surgery group, markedly fewer
dark-stained and paramorphic neurons were observed in the CA1 and
CA3 regions in the surgery + GB group (Fig. 7C and F). Compared with that in the
surgery group, a significantly higher number of normal neurons were
observed in the hippocampus of the surgery + GB group (P<0.05;
Fig. 7G and H). The results
indicated that pretreatment with GB reduced abdominal
surgery-induced neuronal histological abnormalities in the
hippocampal region.
Discussion
The results of the present study indicated an
association between iNOS-mediated overproduction of NO and
abdominal surgery-induced impairments in learning and memory.
Moreover, GB pretreatment improved cognitive dysfunction induced by
abdominal surgery under general anesthesia. Therefore, the present
study indicated that the protective role of GB against PND may be
associated with its ability to reduce iNOS production and
subsequent NO production, as well as its capacity to decrease
neuronal loss and apoptosis.
PND, as a common complication occurring after
surgery, has attracted increasing attention from perioperative
physicians. According to a previous study, a PND model was
constructed via abdominal laparotomy in the present study (27). PND can present in young and
middle-aged patients (28,29). Therefore, adult mice were used to
construct the PND model in the present study, according to a
previous study (30). To determine
the effects of 1400W and GB on surgery-induced learning and memory
impairment, the FCT, which has frequently been used in studies
investigating PND, was performed to assess the cognitive function
of mice in each group (24,31). An OFT was conducted to assess
exploratory and locomotor activities (21). The behavioral studies demonstrated
that surgery resulted in deficits in hippocampus-dependent learning
and memory without influencing exploratory and locomotor
activities. Consistent with the results reported by Sun et
al (32), the present study
demonstrated that hippocampus-dependent cognitive functions were
vulnerable to surgery under anesthesia in young adult mice.
Moreover, pretreatment with 1400W or GB significantly improved
surgery-induced cognitive dysfunction.
iNOS can be expressed in astrocytes, microglia,
neurons and endothelial cells in the central nervous system under
certain pathological conditions (33). The present study demonstrated higher
expression of iNOS and levels of NO in the surgery group on
postoperative day 1 compared with the control group. On
postoperative day 1, NO overproduction in the surgery group was
significantly reduced by pretreatment with the specific iNOS
inhibitor 1400W, which suggested that iNOS was involved in the
process of PND via mediation of NO overproduction.
As one of the most important antioxidants in the
central nervous system, SOD can protect cells from oxidative damage
by promoting the transformation of superoxide anions to oxygen
molecules and hydrogen peroxide (34). MDA is the final product of lipid
peroxidation induced by ROS (35).
In the present study, compared with the control group, the surgery
group displayed significantly decreased SOD activity in the
hippocampus and prefrontal cortex on postoperative day 1 and in the
hippocampus on postoperative day 3. Higher MDA content was found in
the hippocampus of the surgery group on postoperative day 1. These
changes indicated that anesthesia and surgery resulted in decreased
antioxidants and increased ROS. The results of the present study
were consistent with a previous study that reported a significant
decrease in SOD activity in the 24 h following surgery (5). Subsequently, increased superoxide
anion, a species of ROS, and NO induced by surgery could promote
the production of ONOO−, which could lead to neuronal
apoptosis and destruction (36,37).
In the present study, compared with the control
group, significant NO and MDA upregulation in the hippocampus in
the surgery group was observed on postoperative day 1, but not on
postoperative day 3. However, impaired cognitive results were
observed on postoperative days 1 and 3. Several reasons could
account for the aforementioned results. First, NO has been
indicated to be an unstable gas with a short half-life. However,
even in a very short period of time, large amounts of
ONOO- could be generated via the NO/superoxide anion
pathway (38). Second, in addition
to promoting lipid peroxidation, ONOO− could promote
nitrosylation of membrane protein thiols in the mitochondria,
mitochondrial damage, protein oxidation and DNA oxidation (39). As a result, other pathophysiological
mechanisms mediated by ONOO− may underlie PND on
postoperative day 3.
Numerous studies have indicated the protective role
of GB in the context of neurodegenerative diseases. Gu et al
(17) reported that GB could
partially reverse iNOS upregulation in a transient middle cerebral
artery occlusion mouse model. NF-κB is an important transcription
factor in regulating iNOS expression (40). A previous study demonstrated that GB
downregulated iNOS expression by inhibiting NF-kB activation
(41). In the present study,
surgery-induced iNOS upregulation and NO overproduction in the
hippocampus on postoperative day 1 were significantly inhibited by
pretreatment with GB. In addition, pretreatment with GB
significantly decreased surgery-induced downregulation of SOD in
the hippocampus and prefrontal cortex on postoperative day 1, and
in the hippocampus on postoperative day 3. Overproduction of MDA in
the hippocampus on postoperative day 1 was also inhibited by
pretreatment of GB. A previous study reported similar properties of
GB. GB pretreatment protected SH-5YSY cells against Aβ1-42 by
reducing lipid peroxidation and restoring antioxidant activities
(42). Furthermore, GB restored SOD
activity and decreased MDA content in hypoxia-exposed rats
(43). The present study
demonstrated a significantly higher ratio of proapoptotic Bax/Bcl-2
in the surgery group compared with that in the control group, which
was significantly inhibited by GB pretreatment. Surgery-induced
neuronal histological abnormalities in the CA1 and CA3 regions were
prevented by GB pretreatment. The results suggest that the
therapeutic effects of GB on PND were associated with the
inhibition of iNOS-mediated NO production, as well as the
alleviation of neuronal apoptosis and destruction, which were
possibly induced by the elevation of ONOO−.
Collectively, the results of the present study may provide novel
insight for the treatment of PND.
However, the present study had several limitations.
First, an inhibitor of iNOS, 1400W, was used to investigate the
role of iNOS in PND. However, employing iNOS gene knockout mice
would provide more powerful evidence. Second, without a GB
pretreatment and iNOS overexpression group, it cannot be concluded
from the results of the present study that iNOS mediated the
protective effect of GB on PND. In addition, testing relevant
indicators at different time points would aid in identifying the
dynamic alterations of experimental indexes post-surgery. However,
to reduce the number of animals used in the present study, relevant
indicators were assessed on postoperative days 1 and 3, according
to previous studies (30,44). Therefore, the regulatory mechanisms
underlying the effects of GB on iNOS during the process of PND
require further investigation.
In conclusion, the present study indicated that
surgery may cause PND by promoting elevations in iNOS/NO and ROS,
downregulating SOD and inducing neuronal damage. GB treatment may
serve a protective role in PND by inhibiting iNOS upregulation and
NO overproduction, inhibiting SOD downregulation and alleviating
neuronal damage. The results suggested that iNOS may serve as a
potential therapeutic target for PND, and GB may serve as a novel
drug for the prevention of PND. Future studies should focus on
further understanding the mechanisms underlying iNOS and NO in PND
to aid with the discovery of multiple therapeutic targets.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81541117 and
81371199).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AW and PH designed the present study. YH and RS
analyzed the data. TL and DL performed the experiments. TL was a
major contributor in writing the manuscript. DL and PH revised the
manuscript. CW and WS contributed new reagents, and performed the
data collection and interpretation. All authors confirm the
authenticity of all the raw data, and read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Animal
Experiments and Experimental Animal Welfare Committee of Capital
Medical University and performed under the regulations of the
Medical Research Center of Beijing Chao-Yang Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Evered L, Silbert B, Knopman DS, Scott DA,
DeKosky ST, Rasmussen LS, Oh ES, Crosby G, Berger M and Eckenhoff
RG; Nomenclature Consensus Working Group, : Recommendations for the
nomenclature of cognitive change associated with anaesthesia and
surgery-2018. Anesthesiology. 129:872–879. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang S, Hu H, Cai YH and Hua F: Effect of
parecoxib in the treatment of postoperative cognitive dysfunction:
A systematic review and meta-analysis. Medicine (Baltimore).
98:e138122019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Androsova G, Krause R, Winterer G and
Schneider R: Biomarkers of postoperative delirium and cognitive
dysfunction. Front Aging Neurosci. 7:1122015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Netto MB, de Oliveira Junior AN, Goldim M,
Mathias K, Fileti ME, da Rosa N, Laurentino AO, de Farias BX, Costa
AB, Rezin GT, et al: Oxidative stress and mitochondrial dysfunction
contributes to postoperative cognitive dysfunction in elderly rats.
Brain Behav Immun. 73:661–669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang J, Gao J, Guo G, Li S, Zhan G, Xie
Z, Yang C and Luo A: Anesthesia and surgery induce delirium-like
behavior in susceptible mice: The role of oxidative stress. Am J
Transl Res. 10:2435–2444. 2018.PubMed/NCBI
|
|
6
|
Dröge W: Free radicals in the
physiological control of cell function. Physiol Rev. 82:47–95.
2002. View Article : Google Scholar
|
|
7
|
Venturelli M, Pedrinolla A, Boscolo
Galazzo I, Fonte C, Smania N, Tamburin S, Muti E, Crispoltoni L,
Stabile A, Pistilli A, et al: Impact of nitric oxide
bioavailability on the progressive cerebral and peripheral
circulatory impairments during aging and Alzheimer's disease. Front
Physiol. 9:1692018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stephan BCM, Harrison SL, Keage HAD,
Babateen A, Robinson L and Siervo M: Cardiovascular disease, the
nitric oxide pathway and risk of cognitive impairment and dementia.
Curr Cardiol Rep. 19:872017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tse JKY: Gut microbiota, nitric oxide, and
microglia as prerequisites for neurodegenerative disorders. ACS
Chem Neurosci. 8:1438–1447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahashi M, Chin Y, Nonaka T, Hasegawa M,
Watanabe N and Arai T: Prolonged nitric oxide treatment induces tau
aggregation in SH-SY5Y cells. Neurosci Lett. 510:48–52. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chinta SJ and Andersen JK: Nitrosylation
and nitration of mitochondrial complex I in Parkinson's disease.
Free Radic Res. 45:53–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asiimwe N, Yeo SG, Kim MS, Jung J and
Jeong NY: Nitric oxide: Exploring the contextual link with
Alzheimer's disease. Oxid Med Cell Longev. 2016:72057472016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Xiong S, Liu P, Liu W, Wang Q,
Liu Y, Tan H, Chen X, Shi X, Wang Q and Chen T: Polymeric
nanoparticles-based brain delivery with improved therapeutic
efficacy of ginkgolide B in Parkinson's disease. Int J
Nanomedicine. 15:10453–10467. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma HS, Drieu K, Alm P and Westman J:
Role of nitric oxide in blood-brain barrier permeability, brain
edema and cell damage following hyperthermic brain injury. An
experimental study using EGB-761 and Gingkolide B pretreatment in
the rat. Acta Neurochir Suppl. 76:81–86. 2000.PubMed/NCBI
|
|
15
|
Liu Q, Jin Z, Xu Z, Yang H, Li L, Li G, Li
F, Gu S, Zong S, Zhou J, et al: Antioxidant effects of ginkgolides
and bilobalide against cerebral ischemia injury by activating the
Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones.
24:441–452. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng Y, Fang W, Li Y, Cen J, Fang F, Lv P,
Gong S and Mao L: Blood-brain barrier breakdown by PAF and
protection by XQ-1H due to antagonism of PAF effects. Eur J
Pharmacol. 616:43–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu JH, Ge JB, Li M, Wu F, Zhang W and Qin
ZH: Inhibition of NF-κB activation is associated with
anti-inflammatory and anti-apoptotic effects of ginkgolide B in a
mouse model of cerebral ischemia/reperfusion injury. Eur J Pharm
Sci. 47:652–660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang W, Sha L, Kodithuwakku ND, Wei J,
Zhang R, Han D, Mao L and Li Y: Attenuated blood-brain barrier
dysfunction by XQ-1H following ischemic stroke in hyperlipidemic
rats. Mol Neurobiol. 52:162–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosczyk HA, Sparkman NL and Johnson RW:
Neuroinflammation and cognitive function in aged mice following
minor surgery. Exp Gerontol. 43:840–846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian XS, Tong YW, Li ZQ, Li LX, Zhang T,
Ren TY, Zhou T, Wang HC, Zhan R, Sun Y, et al: Surgical stress
induces brain-derived neurotrophic factor reduction and
postoperative cognitive dysfunction via glucocorticoid receptor
phosphorylation in aged mice. CNS Neurosci Ther. 21:398–409. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seibenhener ML and Wooten MC: Use of the
open field maze to measure locomotor and anxiety-like behavior in
mice. J Vis Exp. 6:e524342015.PubMed/NCBI
|
|
22
|
Zhang Z, Li X, Li F and An L: Berberine
alleviates postoperative cognitive dysfunction by suppressing
neuroinflammation in aged mice. Int Immunopharmacol. 38:426–433.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saxe MD, Battaglia F, Wang JW, Malleret G,
David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER,
Santarelli L, et al: Ablation of hippocampal neurogenesis impairs
contextual fear conditioning and synaptic plasticity in the dentate
gyrus. Proc Natl Acad Sci USA. 103:17501–17506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Z, Dong Y, Wang H, Culley DJ,
Marcantonio ER, Crosby G, Tanzi RE, Zhang Y and Xie Z:
Age-dependent postoperative cognitive impairment and
Alzheimer-related neuropathology in mice. Sci Rep. 4:37662014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Winocur G, Wojtowicz JM, Sekeres M, Snyder
JS and Wang S: Inhibition of neurogenesis interferes with
hippocampus-dependent memory function. Hippocampus. 16:296–304.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Korsmeyer SJ, Shutter JR, Veis DJ, Merry
DE and Oltvai ZN: Bcl-2/Bax: A rheostat that regulates an
anti-oxidant pathway and cell death. Semin Cancer Biol. 4:327–332.
1993.PubMed/NCBI
|
|
27
|
Hovens IB, van Leeuwen BL, Mariani MA,
Kraneveld AD and Schoemaker RG: Postoperative cognitive dysfunction
and neuroinflammation; cardiac surgery and abdominal surgery are
not the same. Brain Behav Immun. 54:178–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Monk TG, Weldon BC, Garvan CW, Dede DE,
van der Aa MT, Heilman KM and Gravenstein JS: Predictors of
cognitive dysfunction after major noncardiac surgery.
Anesthesiology. 108:18–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shu AH, Wang Q and Chen XB: Effect of
different depths of anesthesia on postoperative cognitive function
in laparoscopic patients: A randomized clinical trial. Curr Med Res
Opin. 31:1883–1887. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiong C, Liu J, Lin D, Zhang J, Terrando N
and Wu A: Complement activation contributes to perioperative
neurocognitive disorders in mice. J Neuroinflammation. 15:2542018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Zhu S, Jin P, Huang Y, Dai Q, Zhu
Q, Wei P, Yang Z, Zhang L, Liu H, et al: Graphene oxide improves
postoperative cognitive dysfunction by maximally alleviating
amyloid beta burden in mice. Theranostics. 10:11908–11920. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun L, Dong R, Xu X, Yang X and Peng M:
Activation of cannabinoid receptor type 2 attenuates
surgery-induced cognitive impairment in mice through
anti-inflammatory activity. J Neuroinflammation. 14:1382017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou XY, Zhang F, Ying CJ, Chen J, Chen L,
Dong J, Shi Y, Tang M, Hu XT, Pan ZH, et al: Inhibition of iNOS
alleviates cognitive deficits and depression in diabetic mice
through downregulating the NO/sGC/cGMP/PKG signal pathway. Behav
Brain Res. 322:70–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clausen A, Doctrow S and Baudry M:
Prevention of cognitive deficits and brain oxidative stress with
superoxide dismutase/catalase mimetics in aged mice. Neurobiol
Aging. 31:425–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Greilberger J, Koidl C, Greilberger M,
Lamprecht M, Schroecksnadel K, Leblhuber F, Fuchs D and Oettl K:
Malondialdehyde, carbonyl proteins and albumin-disulphide as useful
oxidative markers in mild cognitive impairment and Alzheimer's
disease. Free Radic Res. 42:633–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi Q, Liu X, Wang N, Zheng X, Ran J, Liu
Z, Fu J and Zheng J: 1400W ameliorates acute hypobaric
hypoxia/reoxygenation-induced cognitive deficits by suppressing the
induction of inducible nitric oxide synthase in rat cerebral cortex
microglia. Behav Brain Res. 319:188–199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moldogazieva NT, Mokhosoev IM, Feldman NB
and Lutsenko SV: ROS and RNS signalling: Adaptive redox switches
through oxidative/nitrosative protein modifications. Free Radic
Res. 52:507–543. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chitnis T and Weiner HL: CNS inflammation
and neurodegeneration. J Clin Invest. 127:3577–3587. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hemanth Kumar K, Tamatam A, Pal A and
Khanum F: Neuroprotective effects of cyperus rotundus on SIN-1
induced nitric oxide generation and protein nitration: Ameliorative
effect against apoptosis mediated neuronal cell damage.
Neurotoxicology. 34:150–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ranjan R, Karpurapu M, Rani A, Chishti AH
and Christman JW: Hemozoin regulates iNOS expression by modulating
the transcription factor NF-κB in macrophages. Biochem Mol Biol J.
2:102016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Woo CW, Cheung F, Chan VW, Siow YL and O
K: Homocysteine stimulates inducible nitric oxide synthase
expression in macrophages: Antagonizing effect of ginkgolides and
bilobalide. Mol Cell Biochem. 243:37–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gill I, Kaur S, Kaur N, Dhiman M and
Mantha AK: Phytochemical ginkgolide B attenuates amyloid-β1-42
induced oxidative damage and altered cellular responses in human
neuroblastoma SH-SY5Y Cells. J Alzheimers Dis. 60 (Suppl
1):S25–S40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li W, Qinghai S, Kai L, Xue M, Lili N,
Jihua R, Zhengxiang L, Xiaoling L, Di G, Qi Y, et al: Oral
administration of ginkgolide B alleviates hypoxia-induced neuronal
damage in rat hippocampus by inhibiting oxidative stress and
apoptosis. Iran J Basic Med Sci. 22:140–145. 2019.PubMed/NCBI
|
|
44
|
Qian XL, Zhang W, Liu MZ, Zhou YB, Zhang
JM, Han L, Peng YM, Jiang JH and Wang QD: Dexmedetomidine improves
early postoperative cognitive dysfunction in aged mice. Eur J
Pharmacol. 746:206–212. 2015. View Article : Google Scholar : PubMed/NCBI
|