Introduction
Pulmonary fibrosis (PF) is a chronic, progressive
and fatal disease with an unclear etiology (1). The most common type of PF is
idiopathic PF, with an annual incidence of 16.3–17.4 per 100,000
individuals, as determined using broad case definitions in the USA
(1). PF is characterized by
repetitive alveolar epithelial cell injury, fibroblast activation
and increased extracellular matrix deposition, resulting in lung
function distortion (2–4). While anti-inflammatory and
antifibrinolytics agents and glucocorticoids are utilized for PF,
no effective lung fibrosis treatment exists and patients with PF
have a median survival of only 2–4 years (5). Therefore, elucidation of mechanisms
underlying PF and the identification of potential early detection
PF biomarkers are necessary.
Epithelial-mesenchymal transition (EMT),
characterized by loss of epithelial characteristics (E-cadherin
expression) and gain of mesenchymal features [vimentin and α-smooth
muscle actin (α-SMA) expression], serves a key role in fibrosis
pathogenesis (6). Transforming
growth factor-β1 (TGF-β1) has been implicated as a ‘master switch’
in the induction of fibrosis in various organs, including the lung
(7).
MicroRNAs (miRNAs or miRs) are a class of small,
non-coding RNAs that inhibit gene expression by binding to the
3′-untranslated regions (3′-UTRs) of target genes (8). miRNAs have been implicated in various
biological processes, including cell proliferation and
differentiation (9). Accumulating
evidence has shown that miRNAs are critical in the EMT and PF
processes (10). For example,
miR-26a overexpression has been found to inhibit TGF-β1-induced EMT
via the regulation of high-mobility group protein A2 in PF
(11). Wang et al (12) reported that a miR-483-5p inhibitor
serves a role in lung cancer by targeting RBM5 to induce apoptosis.
Another previous study indicated that miR-483-5p is upregulated in
serum from patients with systemic sclerosis and is a potential
fibrosis driver in systemic sclerosis (13). To the best of our knowledge,
however, the underlying role of miR-483-5p in PF development has
not been studied.
The present study aimed to determine the expression
level of miR-483-5p in normal lung tissues and PF tissues. Then,
the role of miR-483-5p in TGF-β1-induced EMT was examined in A549
cells. Finally, the mechanism of miR-483-5p in TGF-β1-induced EMT
was determined.
Materials and methods
Ethics statement
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Chongqing Medical
University (approval no. 2020-147) and was performed in accordance
with the Declaration of Helsinki. All patients provided written
informed consent.
Tissues, cell lines and cell
culture
A total of 12 PF tissue samples were collected from
patients undergoing lung biopsy (including 8 females and 4 males).
A total of 17 control lung tissue samples (including 10 females and
7 males) were obtained from the normal areas of the peripheral lung
removed during lung cancer resection. The average age of patients
with PF was 60.5 years and control subjects was 55.5 years.
Detailed information on the control subjects and patients with PF,
who were diagnosed with PF via pathology examinations, is provided
in Table SI. The inclusion
criteria for control subjects was individuals who underwent the
lung cancer resection and without other pulmonary disease, and the
normal areas of the peripheral lung were collect. All tissue
samples were collected between June 2020 and December 2020 at The
First Affiliated Hospital of Chongqing Medical University.
Human alveolar epithelial cells (A549) were
purchased from the Cell Bank of the Chinese Academy of Sciences.
The cells were maintained in RPMI-1640 (Hyclone; Cytiva) medium
containing 10% FBS (PAN-Biotech GmbH), 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C in a humidified 5% CO2
atmosphere. The A549 cells were treated with 10 ng/ml TGF-β1
(PeproTech, Inc.) at 37°C for 48 h to establish the EMT cell model,
as previously described (6,14).
Cell transfection
The A549 cells were transfected with either at the
final concentration of 50 nM miR-483-5p mimic or inhibitor
(Shanghai GenePharma Co., Ltd.) using Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The sequences of miR-483-5p mimic,
inhibitor, and corresponding negative control (NC) are provided in
Table SII. In brief, miRNA and
Lipofectamine 3000 were separately mixed with 250 µl RPMI-1640
serum-free medium. Then, both mixtures were combined and incubated
for 15 min at room temperature. Finally, the Lipofectamine 3000 and
miRNA mixture was added to the cells and incubated for 24 h at
37°C. After 24 h transfection, the cells were treated in the
presence or absence of 10 ng/ml TGF-β1 for 48 h at 37°C, then
collected and utilized for further experiments.
Lentivirus transfection
In order to establish stable genetic Rho GDP
dissociation inhibitor 1 (RhoGDI1) knockdown, lentivirus was
utilized as a vector to carry the interference sequence. In brief,
lentivirus vectors containing either the target gene or NC were
constructed by Hanbio Biotechnology Co., Ltd. The lentiviral
backbone used for short harpin (sh)RNA was
pHBLV-U6-MCS-CMV-ZsGreen-PGK-PRUO (3rd). Sequences of RhoGDI1 and
NC are listed in Table SII. In
order to measure the infection efficiency and select the cells,
each lentivirus vector expressed the green fluorescent protein and
puromycin resistance gene. The multiplicity of infection for A549
cells is 50. A549 cells (1×105 per well) in the
logarithmic growth phase were digested with trypsin and seeded in
six-well plates. The next day, the cells were incubated at 37°C in
2 ml RPMI-1640 complete medium containing lentivirus and 5 µg/ml
Polybrene. After 24 h, the medium was replaced with fresh complete
medium without both lentivirus and Polybrene, and cultured for
another 48 h at 37°C. Following this, expression of green
fluorescent protein was observed via a fluorescence microscope
(magnification, ×20). The cells were cultured with 1 µg/ml
puromycin to select stable knockdown lines. Finally, reverse
transcription-quantitative (RT-q)PCR and western blot assay were
utilized to determine the shRNA knockdown efficiency.
RT-qPCR
In order to detect the mRNA or miRNA levels, total
RNA from lung tissue or A549 cells was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The RNA
concentration was determined via a NanoDrop 2000
micro-spectrophotometer (Thermo Fisher Scientific, Inc.). Next, 1
µg total RNA was utilized to synthesize complementary (c)DNA via a
PrimeScript RT Reagent kit (Takara Biotechnology Co., Ltd.). The
gDNA elimination reaction was conducted at 42°C for 2 min and
reverse transcription was performed at 37°C for 15 min and at 85°C
for 5 sec. Subsequently, cDNA was amplified via the TB Green Premix
EX Taq II PCR kit (Takara Biotechnology Co., Ltd.). Amplification
cycle included an initial 30 sec incubation at 95°C for
denaturation, followed by 40 cycles of 5 sec at 95°C for annealing
and 30 sec at 60°C for elongation. Small nuclear RNA U6 was
utilized for the internal normalization of miR-483-5p, and GAPDH
was utilized as a control for mRNA expression. Relative
quantification was calculated via the 2−ΔΔCq method
(15). The primer sequences
utilized are listed in Table
SIII.
Western blot assay
Total protein was extracted from the A549 cells
using RIPA lysis buffer supplemented with protease and phosphatase
inhibitor. The proteins were quantified via a bicinchoninic acid
kit (Beyotime Institute of Biotechnology). Then, the proteins (25
µg) were separated via 10% SDS-PAGE and transferred onto PVDF
membranes (EMD Millipore). The membranes were blocked with 5%
non-fat milk at room temperature for 2 h, then incubated overnight
with specific primary antibodies at 4°C. The next day, membranes
were washed three times with Tris-buffered saline with 1% Tween-20,
then incubated with secondary antibodies (1:8,000; Zhong
Shan-Golden Bridge Biological Technology Co., Ltd.; cat. no.
ZB-2306) for 1 h at room temperature. The proteins were visualized
via an electrochemiluminescence kit (Wuhan Boster Biological
Technology Ltd.). Images were captured with the use of an automatic
Fusion FX Edge chemiluminescence image analysis system (Vilber
Lourmat Sa). The Fusion Capt software v.18.06 (Vilber Lourmat Sa)
was used for the densitometric analysis of the blot images. The
following primary antibodies were utilized: Anti-E-cadherin
(1:10,000; Abcam; cat. no. ab40772), anti-α-SMA (1:2,000; Abcam;
cat. no. ab32575), anti-vimentin (1:3,000; Abcam; cat. no.
ab92547), anti-RhoGDI1 (1:7,000; Abcam; cat. no. ab133248),
anti-Rac family small GTPase (Rac)1 (1:1,000; Cell Signaling
Technology, Inc.; cat. no. 4651), anti-Akt (1:10,000; Abcam; cat.
no. ab179463), anti-phosphorylated (p)-Akt (1:8,000; Abcam; cat.
no. ab81283), anti-p-PI3K (1:2,000; GeneTex, Inc.; cat. no.
GTx132597), anti-PI3K (1:1,000; Abcam; cat. no. ab191606) and
anti-GAPDH (1:10,000; Abcam; cat. no. ab181602).
Rac activity assay
Rac activity was determined via a Rac activation
assay kit (Cell Signaling Technology, Inc.) according to the
manufacturer's instructions. In brief, the cells were washed twice
with ice-cold PBS and were harvested using 1X lysis/binding/wash
buffer containing phenylmethanesulfonyl fluoride (PMSF). Then, the
GST-PAK1-PBD fusion protein was used to bind the GTP-bound Rac1,
which was immunoprecipitated via glutathione resin. Finally, bound
Rac1 protein was detected using western blot assay and anti-Rac1
antibodies.
Luciferase reporter assay
The potential target gene of miR-483-5p was
identified using TargetScan (Version 7.2; http://www.targetscan.org/). The luciferase reporter
assay was performed using a Dual-Luciferase Reporter Assay System
according to the manufacturer's instructions (Promega Corporation)
to validate the target association between miR-483-5p and RhoGDI1.
The RhoGDI1 wild-type (wt) and mutant (mut) 3′-UTRs were
constructed and cloned from genomic DNA and inserted into the
pSI-Check2 vector. The 293T cells were cultured in 96-well plates
and co-transfected with miR-483-5p mimics/NC and wt or mut
pSI-Check2-RhoGDI1-3′-UTR using Lipofectamine 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.). After 48 h, the luciferase
activity was detected via a dual-luciferase reporter system
(Promega Corporation). Luciferase activity was normalized to that
of Renilla.
Statistical analysis
Data were analyzed via SPSS 23.0 software (IBM
Corp.). Data are presented as the mean ± SD (n=3). The comparisons
between two groups were analyzed via unpaired Student's t-test.
Multiple comparisons were assessed via one-way ANOVA followed by
post hoc Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
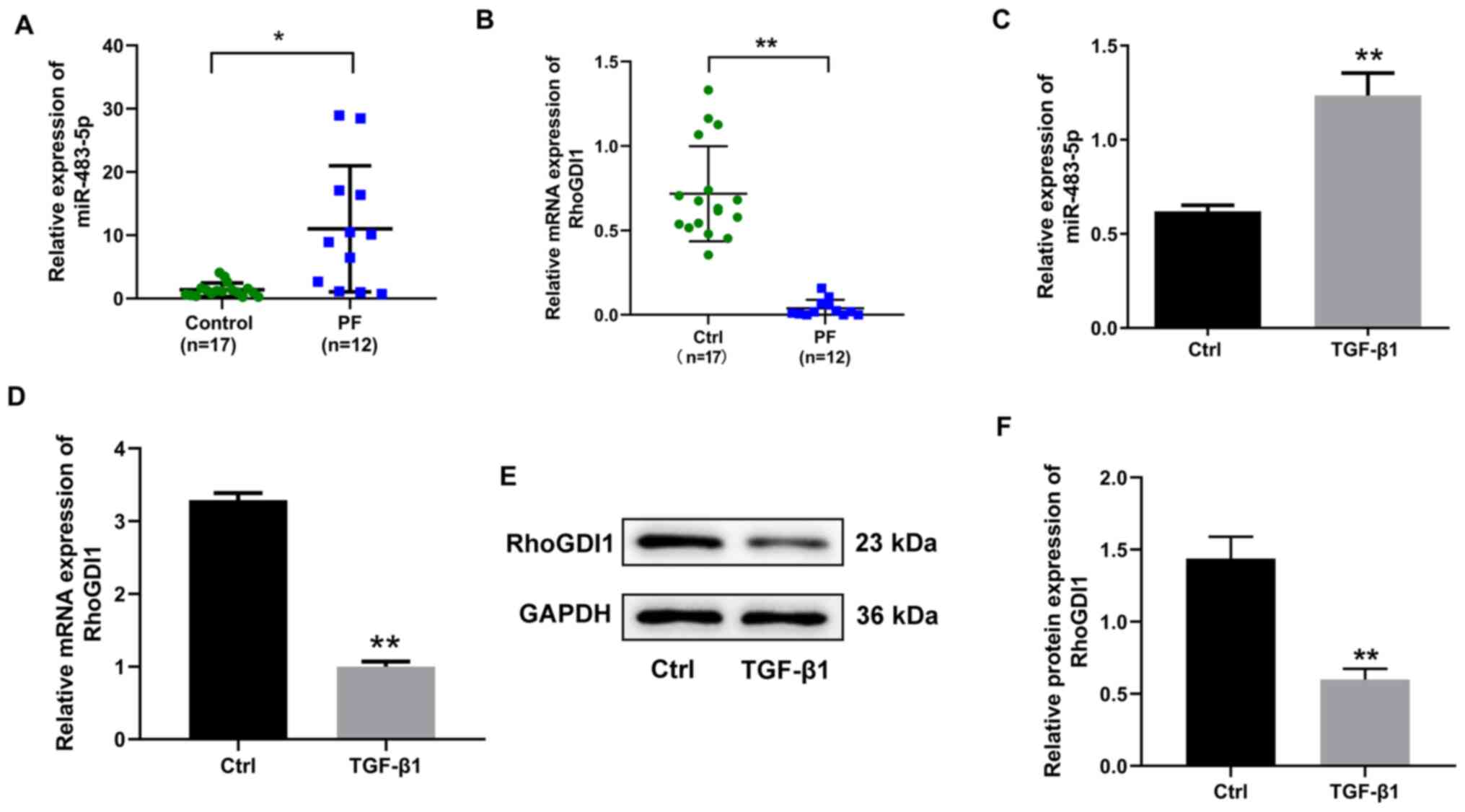
miR-483-5p expression is upregulated
and RhoGDI1 expression is downregulated in PF tissue and
TGF-β1-induced EMT in A549 cells
In order to investigate whether miR-483-5p
participated in PF development, the expression of miR-483-5p in PF
tissue and TGF-β1-induced EMT were detected. Expression of
miR-483-5p was significantly increased in PF compared with control
tissue (Fig. 1A). The expression of
miR-483-5p in TGF-β1 treated with A549 cells in vitro was
determined; TGF-β1 upregulated miR-483-5p expression compared with
the control group (Fig. 1C).
Subsequently, RhoGDI1 was predicted to be a miR-483-5p target gene
via online tools. In order to validate this prediction, the
expression of RhoGDI1 was detected in PF tissue and TGF-β1-treated
A549 cells and was observed to be significantly decreased at both
the mRNA and protein level in PF tissue and TGF-β1 treated A549
cells (Fig. 1B and D-F). Considered
together, these findings indicated that expression of miR-483-5p
was upregulated in both the PF tissue and TGF-β1-stimulated A549
cells, whereas the expression of RhoGDI1 was downregulated.
miR-483-5p promotes TGF-β1-induced
EMT
In order to assess the role of miR-483-5p in
TGF-β1-induced EMT in A549 cells, miR-483-5p mimics or inhibitors
were transfected into A549 cells, which were then treated with
TGF-β1. miRNA transfection efficiency was assessed via RT-qPCR.
Following 24 h transfection, the miR-483-5p level increased by ~90
times in the miR-483-5p mimics group compared with the mimic-NC
group (Fig. 2A). Additionally, the
miR-483-5p level decreased by ~6 times in the miR-483-5p
inhibitor-transfected compared with the inhibitor-NC group
(Fig. 2G). These results indicated
that the transfection was efficient.
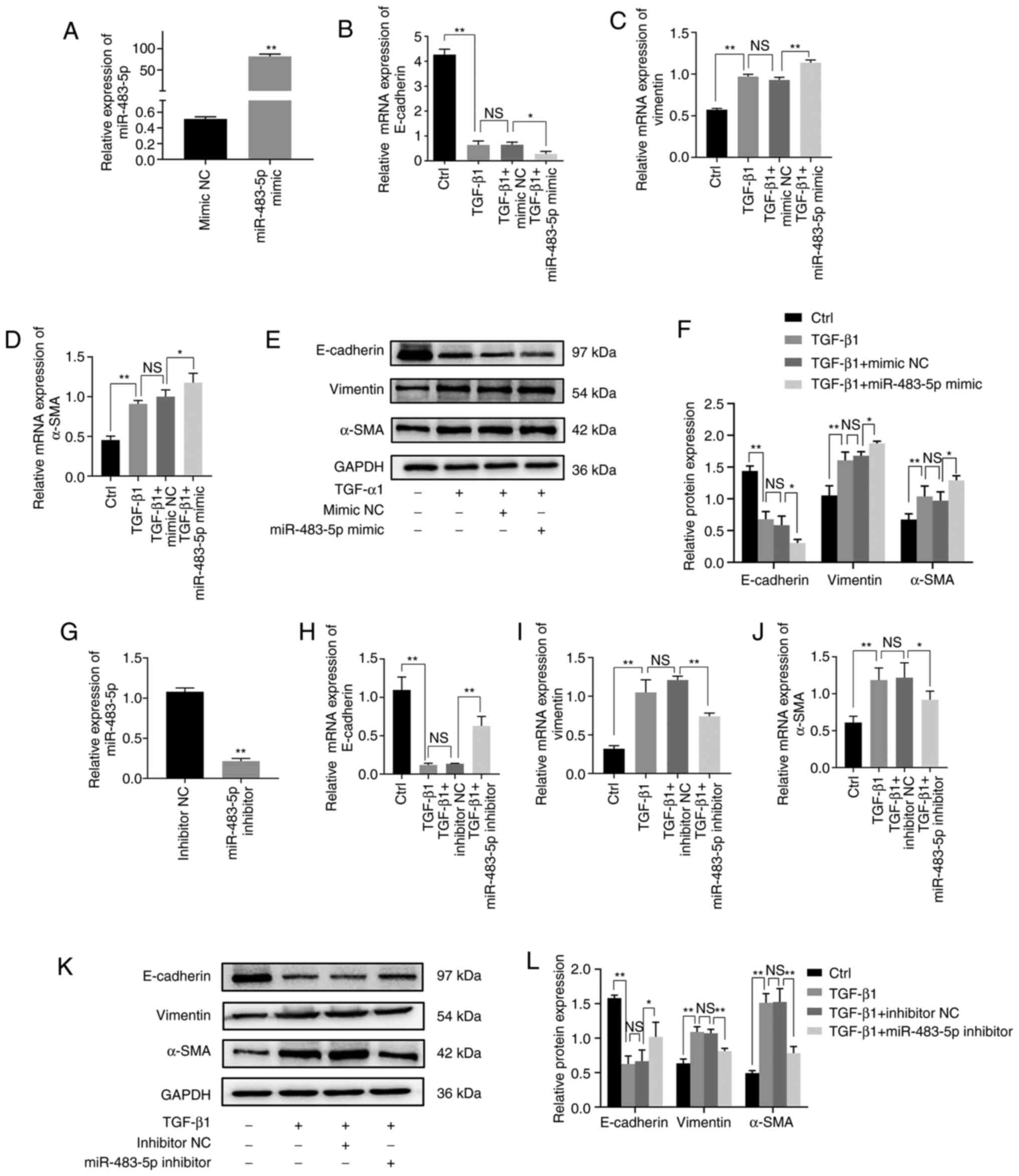 | Figure 2.Effect of miR-483-5p on
TGF-β1-induced EMT. (A) Relative expression of miR-483-5p in A549
cells after 24 h transfection with miR-483-5p mimic was detected
via RT-qPCR. **P<0.01 vs. mimic NC. Following treatment with
TGF-β1 for 48 h, relative of expression of (B) E-cadherin, (C)
vimentin and (D) α-SMA were determined by RT-qPCR and (E and F)
western blotting. A549 cells were transfected with miR-483-5p
inhibitor for 24 h, and then treated with TGF-β1 for 48 h.
*P<0.05; **P<0.01. (G) Relative expression of miR-483-5p in
A549 cells following transfection with inhibitor. **P<0.01 vs.
inhibitor NC. The relative of expression of (H) E-cadherin, (I)
vimentin and (J) α-SMA were determined by RT-qPCR and (K and L)
western blotting. Data are presented as the mean ± SD (n=3).
*P<0.05; **P<0.01. NS, not significant; EMT,
epithelial-mesenchymal transition; TGF-β1, transforming growth
factor-β1; α-SMA, α-smooth muscle actin; miR, microRNA; RT-qPCR,
reverse transcription-quantitative PCR; NC, negative control. |
In order to investigate the functional effect of
miR-483-5p on TGF-β1-induced EMT in A549 cells, EMT biomarkers,
including E-cadherin, vimentin and α-SMA, were detected via RT-qPCR
and western blot assay. Upregulation of miR-483-5p inhibited
TGF-β1-stimulated E-cadherin expression but increased the
expression of vimentin and α-SMA at both the mRNA and protein
levels (Fig. 2B-F). Downregulation
of miR-483-5p increased TGF-β1-induced E-cadherin expression but
decreased TGF-β1-induced vimentin and α-SMA expression at both the
mRNA and protein level (Fig. 2H-L).
Collectively, these findings suggested that upregulation of
miR-483-5p promoted TGF-β1-induced EMT, whereas downregulation of
miR-483-5p suppressed TGF-β1-induced EMT.
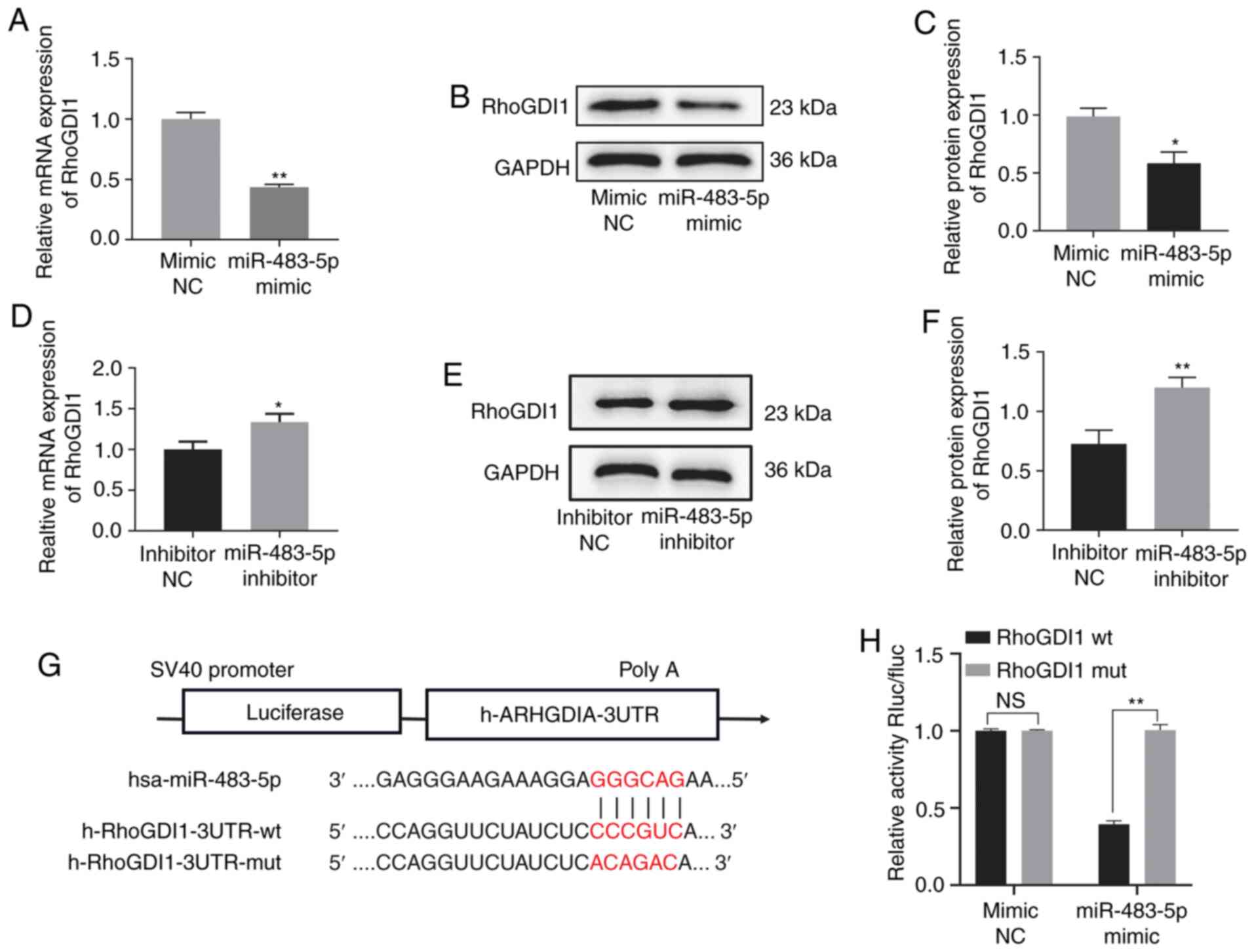
RhoGDI1 is a target gene of
miR-483-5p
The miR-483-5p target gene was predicted via the
online database TargetScan 7.2 and RhoGDI1 was identified as a
potential miR-483-5p target gene (Fig.
3G). Therefore, the association between miR-483-5p and RhoGDI1
was determined via RT-qPCR and western blot assay. Expression of
RhoGDI1 was decreased in the miR-483-5p mimic group compared with
the mimic-NC group at both the mRNA and protein level, but was
increased in the miR-483-5p inhibitor group compared with the
inhibitor-NC group, indicating a negative association between
miR-483-5p and RhoGDI1 (Fig. 3A-F).
In order to confirm RhoGDI1 as a miR-483-5p target, luciferase
reporter assays were performed. The results indicated that
luciferase activity of the RhoGDI1-wt vector was significantly
decreased by co-transfection with miR-483-5p mimics compared with
the RhoGDI1-mut group (Fig. 3H).
These findings suggested that RhoGDI1 was directly regulated by
miR-483-5p.
RhoGDI1 silencing eliminates the
effect of the miR-483-5p inhibitor on TGF-β1-induced EMT
In order to confirm that the miR-483-5p inhibitor
suppressed TGF-β1-induced EMT via a RhoGDI1-dependent pathway,
miR-483-5p inhibitor was transfected into A549 cells with RhoGDI1
knockdown. First, the knockdown efficiency in the A549 cells was
tested. Expression of RhoGDI1 was significantly downregulated in
the shRNA-RhoGDI1 group compared with the shRNA-NC group at both
the mRNA and protein levels, which demonstrated that the knockdown
was efficient (Fig. 4A-C). The
results of RT-qPCR and western blot assay indicated that the
expression of E-cadherin was decreased, but expression of vimentin
and α-SMA increased, in the TGF-β1 + miR-483-5p inhibitor +
shRNA-RhoGDI1 group compared with the TGF-β1 + miR-483-5p inhibitor
+ shRNA-NC group (Fig. 4D-H). These
findings indicated that RhoGDI1 knockdown eliminated the effect of
miR-483-5p inhibitor on TGF-β1-induced EMT.
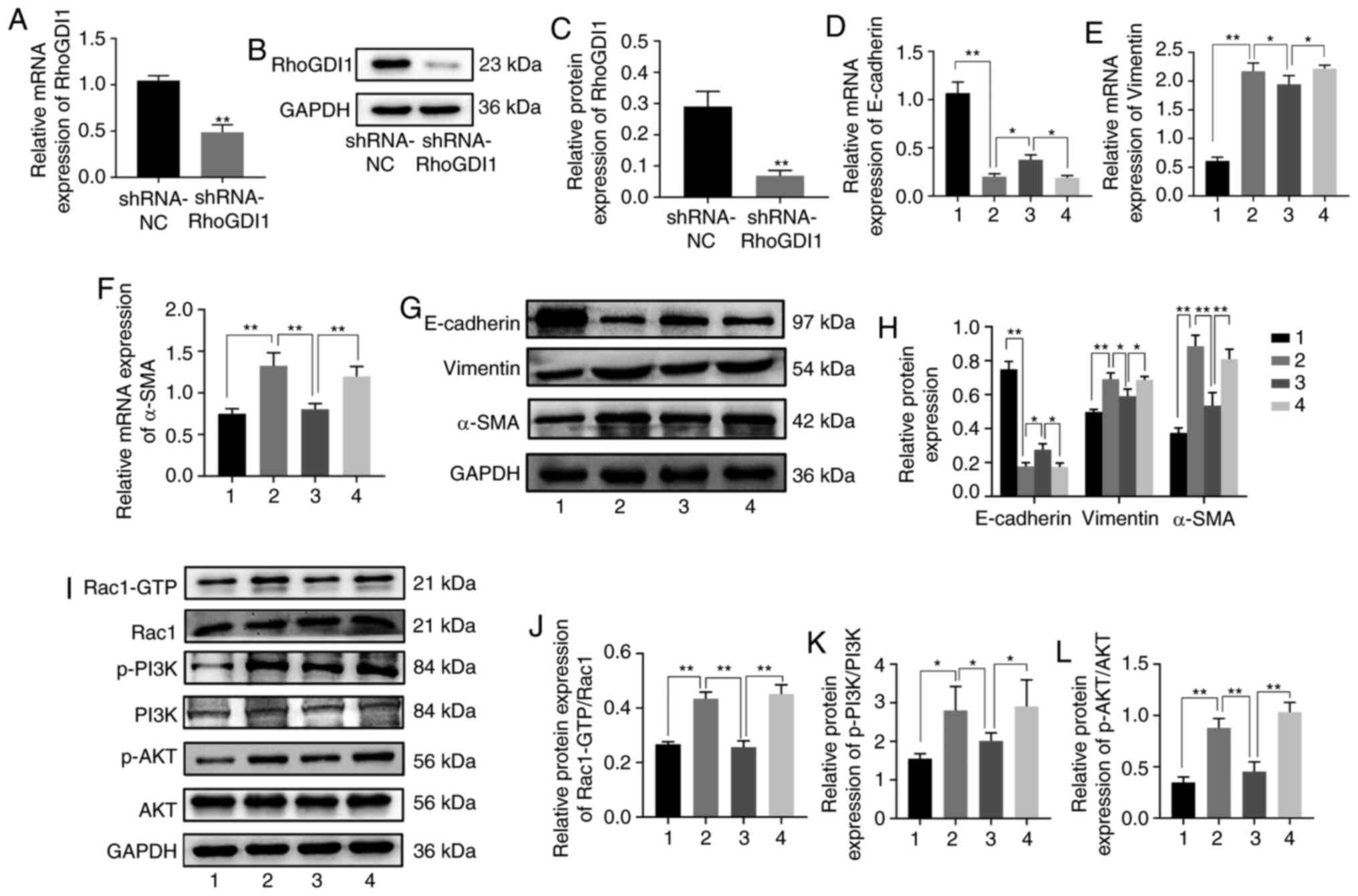 | Figure 4.miR-483-5p inhibitor inhibits
TGF-β1-induced EMT by targeting RhoGDI1 via the Rac1/PI3K/AKT
signaling pathway. The relative expression of RhoGDI1 in A549 cells
infected with lentivirus carrying the interference sequence of
RhoGDI1 was detected by (A) RT-qPCR and (B and C) western blotting.
**P<0.01 vs. shRNA-NC. A549 cells or A549 cells with
shRNA-RhoGDI1 silencing were transfected with miR-483-5p inhibitor
for 24 h, and then treated with TGF-β1 for 48 h. The relative of
expression of (D) E-cadherin, (E) vimentin and (F) α-SMA were
determined by RT-qPCR and (G and H) western blotting. (I)
Expression of (J) Rac1-GTP, Rac1, (K) p-PI3K, PI3K, (L) p-AKT and
AKT were determined by western blot analysis. Data are presented as
the mean ± SD (n=3). *P<0.05; **P<0.01. NS, not significant.
1=Ctrl, 2=TGF-β1 group, 3=TGF-β1+ miR-483-5p inhibitor+ shRNA-NC
group, 4=TGF-β1+ miR-483-5p inhibitor + shRNA-RhoGDI1 group. EMT,
epithelial-mesenchymal transition; RhoGDI1, Rho GDP dissociation
inhibitor 1; TGF-β1, transforming growth factor-β1; α-SMA, α-smooth
muscle actin; miR, microRNA; Rac1, Rac family small GTPase 1; RT-q,
reverse transcription-quantitative; p-, phosphorylated; sh, short
hairpin; NC, negative control; Ctrl, control. |
miR-483-5p inhibitor suppresses
TGF-β1-induced EMT via the Rac1/PI3K/AKT signaling pathway
In order to elucidate the mechanism underlying
suppression of TGF-β1-induced EMT by miR-483-5p inhibitor, the
RhoGDI1 downstream signaling pathway was detected. RhoGDI1 is a
negative Rho GTPase regulator (16), which belongs to the Ras superfamily
of small GTPases (17), and Rac1 is
a family member of Rho GTPase. The PI3K/AKT axis is a key Ras
effector (18). Therefore, the
expression levels of Rac1/PI3K/AKT pathway-associated indicators
were evaluated. Expressions of Rac1-GTP, p-PI3K and p-AKT were
significantly higher in the TGF-β1 group than in the control group
(Fig. 4I-L). However, in the TGF-β1
+ miR-483-5p inhibitor + shRNA-NC group, expression levels of
Rac1-GTP, p-PI3K, and p-AKT were lower than those in the TGF-β1
group (Fig. 4I-L). Furthermore,
expression of Rac1-GTP, p-PI3K, and p-AKT was significantly
increased in the TGF-β1 + miR-483-5p inhibitor + shRNA-RhoGDI1
group compared with the TGF-β1 + miR-483-5p inhibitor + shRNA-NC
group (Fig. 4I-L). Considered
together, these findings implied that miR-483-5p inhibition may
suppress TGF-β1-induced EMT via the Rac1/PI3K/AKT signaling pathway
in PF.
Discussion
PF is a progressive interstitial lung disease
characterized by alveolar epithelial injury, fibroblast activation
and extracellular matrix deposition (19). miRNAs are endogenous, small
non-coding RNA that modulate genes at the post-transcription level
in various physiological and pathological processes (20). Increasing evidence has indicated
that miRNAs drive the onset and progression of PF (21,22).
In the present study, upregulation of miR-483-5p was
observed in human PF tissue, and the functional role of miR-483-5p
and the underlying mechanism of its effect on PF were identified.
The results demonstrated that TGF-β1 increased expression of
miR-483-5p and decreased expression of RhoGDI1 in A549 cells. Also,
downregulation of miR-483-5p inhibited TGF-β1-induced EMT, which
was regulated by targeting RhoGDI1. Upregulation of miR-483-5p
promoted TGF-β1-induced EMT.
EMT serves a key role in organ fibrosis, including
kidney and lung fibrosis (23).
Moreover, TGF-β1 is an important cytokine for EMT induction
(24). In PF development, alveolar
epithelial cells take on the characteristics of mesenchymal cells
by undergoing EMT (25). Although
A549 is a cancer cell line, these cells possess normal
characteristics (26) of type II
alveolar epithelial cells (27),
such as their general morphology, and have been utilized to
investigate the mechanism of EMT in PF (28–32).
Therefore, the A549 cell line was utilized to establish an EMT
model in the present study. In general, downregulated E-cadherin
and upregulated vimentin and α-SMA are used in epithelial cells as
reliable markers of EMT occurrence. As A549 is an epithelial cell
line (27), it exhibits high basal
expression levels of E-cadherin, which is an epithelial cell marker
(6,28). In A549 cells treated with TGF-β1 at
10 ng/ml for 48 h, E-cadherin was significantly downregulated, and
vimentin and α-SMA were upregulated. Western blotting showed that
the E-cadherin strip was thicker in control groups than in groups
treated with TGF-β1. The reason for this result may be that the
final concentration of TGF-β1 was relatively high when compared
with the control group. These findings indicated that TGF-β1
induced EMT, which was consistent with a previous study (6). Furthermore, overexpression of
miR-483-5p promoted TGF-β1-induced EMT; by contrast, downregulation
of miR-483-5p inhibited TGF-β1-induced EMT.
Rho GDP dissociation inhibitors (RhoGDIs) are
involved in various cell processes, including migration, adhesion
and proliferation, via regulation of Rho GTPase family functions
(33). RhoGDIs contains three
members, namely RhoGDI1, RhoGDI2 and RhoGDI3. RhoGDI1, also known
as RhoGDIα or ARHGDIα, is ubiquitously expressed in many types of
cell (34) and has been studied in
different types of cancer. For instance, Song et al
(35) reported that downregulation
of RhoGDI1 promotes lung adenocarcinoma invasion and metastasis via
EMT regulation. Jiang et al (36) reported that decreased expression of
RhoGDI1 is correlated with nodal involvement and metastasis in
human breast cancer. In the present study, RhoGDI1 was
downregulated in PF tissue and TGF-β1-induced EMT in A549 cells. It
was also directly regulated by miR-483-5p. Furthermore, RhoGDI1
knockdown eliminated the effect of miR-483-5p inhibitor on
TGF-β1-induced EMT. These results indicated that RhoGDI1
participated in PF via the EMT process.
Rac1, a member of the Rho family of small GTPases,
is involved in a variety of dynamic biological cell processes,
including proliferation, EMT, cell-cell contact, motility and
invasiveness (37). Rac1 activity
is positively regulated by guanine nucleotide exchanges factors,
which favor GDP/GTP exchange, GTPase-activating proteins, which
favor the switching on/off of GTP/GDP, and guanosine nucleotide
dissociation inhibitor (GDI) that binds to GDP-bound forms,
preventing GDP/GTP exchange (off state) and sequestering small
GTPases in the cytoplasm to form an inactive pool (37). Therefore, RhoGDI1 negatively
regulates Rac1 by regulating the GDP/GTP exchange cycle (37). Numerous studies have indicated that
the PI3K/AKT signaling pathway is essential in TGF-β1-induced EMT
in PF (38,39), and PI3K activity can be stimulated
via Rac1-GTP (40). Feng et
al (41) reported that
miRNA-630 suppresses EMT by regulating forkhead box M1 via
inactivation of the Rac1/PI3K/AKT pathway in gastric cancer. Shen
et al (42) demonstrated
that cigarette smoke induces EMT by activating the Rac1/PI3K/AKT
signaling pathway in chronic obstructive pulmonary disease. Another
study indicated that neogenin-1 promotes EMT by upregulating zinc
finger E-box-binding homeobox 1 via activation of the Rac1/PI3K/AKT
pathway in gastric cancer (43). In
addition, Xu et al (44)
reported that Rac1 promotes a fibrogenic phenotype in fibroblasts
via PI3K/AKT, which results in fibrotic disease. Another study
demonstrated that endothelin-1 contributes to lung fibrosis through
endothelin-1 receptor via Rac/PI3K/AKT-dependent signaling pathway
(45). The results of the present
study indicate that TGF-β1 upregulated expression of GTP-Rac1,
p-PI3K and p-AKT, and that the miR-483-5p inhibitor downregulated
levels of GTP-Rac1, p-PI3K and p-AKT. Moreover, RhoGDI1 knockdown
reversed the effect of miR-483-5p inhibitor on the expression of
GTP-Rac1, p-PI3K and p-AKT, indicating that miR-483-5p inhibitor
suppressed TGF-β1-induced EMT by targeting RhoGDI1 via
Rac1/PI3K/AKT inactivation.
In conclusion, the present results suggested that
miR-483-5p promoted TGF-β1-induced EMT in PF via RhoGDI1
inhibition; this effect may be due to activation of the
Rac1/PI3K/AKT pathway. Therefore, miR-483-5p inhibition may provide
a novel approached for the prevention and treatment of lung
fibrosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Major Science and Technology Projects of China (grant no.
2018ZX10302302003).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
GH and SG designed the experiments and revised the
manuscript. JZ, GQ and DL analyzed the data and revised the
manuscript. GH and XW performed the experiments and wrote the
manuscript. XW, YW, YL and YC analyzed and interpreted the data. GH
and SG confirmed the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Chongqing Medical University
(approval no. 2020-147) and was performed in accordance with the
Declaration of Helsinki. All patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nalysnyk L, Cid-Ruzafa J, Rotella P and
Dirk E: Incidence and prevalence of idiopathic pulmonary fibrosis:
Review of the literature. Eur Respir Rev. 21:355–361. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rydell-Törmänen K, Andréasson K,
Hesselstrand R, Risteli J, Heinegård D, Saxne T and
Westergren-Thorsson G: Extracellular matrix alterations and acute
inflammation; developing in parallel during early induction of
pulmonary fibrosis. Lab Invest. 92:917–925. 2012. View Article : Google Scholar
|
|
3
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
King TE, Pardo A and Selman M: Idiopathic
pulmonary fibrosis. Lancet. 378:1949–1961. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Richeldi L, Collard HR and Jones MG:
Idiopathic pulmonary fibrosis. Lancet. 389:1941–1952. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang YC, Liu JS, Tang HK, Nie J, Zhu JX,
Wen LL and Guo QL: miR-221 targets HMGA2 to inhibit
bleomycin-induced pulmonary fibrosis by regulating
TGF-β1/Smad3-induced EMT. Int J Mol Med. 38:1208–1216. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Willis BC and Borok Z: TGF-beta-induced
EMT: Mechanisms and implications for fibrotic lung disease. Am J
Physiol Lung Cell Mol Physiol. 293:L525–L534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stefani G and Slack FJ: Small non-coding
RNAs in animal development. Nat Rev Mol Cell Biol. 9:219–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gai YP, Zhao HN, Zhao YN, Zhu BS, Yuan SS,
Li S, Guo FY and Ji XL: MiRNA-seq-based profiles of miRNAs in
mulberry phloem sap provide insight into the pathogenic mechanisms
of mulberry yellow dwarf disease. Sci Rep. 8:8122018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miao C, Xiong Y, Zhang G and Chang J:
MicroRNAs in idiopathic pulmonary fibrosis, new research progress
and their pathophysiological implication. Exp Lung Res. 44:178–190.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang H, Gu Y, Li T, Zhang Y, Huangfu L,
Hu M, Zhao D, Chen Y, Liu S, Dong Y, et al: Integrated analyses
identify the involvement of microRNA-26a in epithelial-mesenchymal
transition during idiopathic pulmonary fibrosis. Cell Death Dis.
5:e12382014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang F, Zhang X, Zhong X, Zhang M, Guo M,
Yang L, Li Y, Zhao J and Yu S: Effect of miR-483-5p on apoptosis of
lung cancer cells through targeting of RBM5. Int J Clin Exp Pathol.
11:3147–3156. 2018.PubMed/NCBI
|
|
13
|
Chouri E, Servaas NH, Bekker CPJ, Affandi
AJ, Cossu M, Hillen MR, Angiolilli C, Mertens JS, van den Hoogen
LL, Silva-Cardoso SL, et al: Serum microRNA screening and
functional studies reveal miR-483-5p as a potential driver of
fibrosis in systemic sclerosis. J Autoimmun. 89:162–170. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang H, He H, Ji H, Gao L, Mao J, Liu J,
Lin H and Wu T: Tanshinone IIA ameliorates bleomycin-induced
pulmonary fibrosis and inhibits transforming growth
factor-beta-β-dependent epithelial to mesenchymal transition. J
Surg Res. 197:167–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative Pcr and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin X, Yang B, Liu W, Tan X, Wu F, Hu P,
Jiang T, Bao Z, Yuan J, Qiang B, et al: Interplay between PCBP2 and
miRNA modulates ARHGDIA expression and function in glioma migration
and invasion. Oncotarget. 7:19483–19498. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hall A: Rho GTPases and the control of
cell behaviour. Biochem Soc Trans. 33:891–895. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Castellano E and Downward J: RAS
Interaction with PI3K: More than just another effector pathway.
Genes Cancer. 2:261–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strieter RM and Mehrad B: New mechanisms
of pulmonary fibrosis. Chest. 136:1364–1370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomankova T, Petrek M and Kriegova E:
Involvement of microRNAs in physiological and pathological
processes in the lung. Respir Res. 11:1592010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rajasekaran S, Rajaguru P and Sudhakar
Gandhi PS: MicroRNAs as potential targets for progressive pulmonary
fibrosis. Front Pharmacol. 6:2542015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Zhang Q, Zhou Y, Yang Z and Tan M:
Inhibition of miR-182-5p attenuates pulmonary fibrosis via
TGF-β/Smad pathway. Hum Exp Toxicol. 39:683–695. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stone RC, Pastar I, Ojeh N, Chen V, Liu S,
Garzon KI and Tomic-Canic M: Epithelial-mesenchymal transition in
tissue repair and fibrosis. Cell Tissue Res. 365:495–506. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang C, Zhu X, Hua Y, Zhao Q, Wang K,
Zhen L, Wang G, Lü J, Luo A, Cho WC, et al: YY1 mediates
TGF-β1-induced EMT and pro-fibrogenesis in alveolar epithelial
cells. Respir Res. 20:2492019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chapman HA: Epithelial-mesenchymal
interactions in pulmonary fibrosis. Annu Rev Physiol. 73:413–435.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mason RJ, Walker SR, Shields BA, Henson JE
and Williams MC: Identification of rat alveolar type II epithelial
cells with a tannic acid and polychrome stain. Am Rev Respir Dis.
131:786–788. 1985.PubMed/NCBI
|
|
27
|
Foster KA, Oster CG, Mayer MM, Avery ML
and Audus KL: Characterization of the A549 cell line as a type II
pulmonary epithelial cell model for drug metabolism. Exp Cell Res.
243:359–366. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan X, Dagher H, Hutton CA and Bourke JE:
Effects of PPAR gamma ligands on TGF-beta1-induced
epithelial-mesenchymal transition in alveolar epithelial cells.
Respir Res. 11:212010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kasai H, Allen JT, Mason RM, Kamimura T
and Zhang Z: TGF-beta1 induces human alveolar epithelial to
mesenchymal cell transition (EMT). Respir Res. 6:562005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu H, Königshoff M, Jayachandran A,
Handley D, Seeger W, Kaminski N and Eickelberg O: Transgelin is a
direct target of TGF-beta/Smad3-dependent epithelial cell migration
in lung fibrosis. FASEB J. 22:1778–1789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ando S, Otani H, Yagi Y, Kawai K, Araki H,
Fukuhara S and Inagaki C: Proteinase-activated receptor 4
stimulation-induced epithelial-mesenchymal transition in alveolar
epithelial cells. Respir Res. 8:312007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu Y, Tan J, Xie H, Wang J, Meng X and
Wang R: HIF-1α regulates EMT via the Snail and β-catenin pathways
in paraquat poisoning-induced early pulmonary fibrosis. J Cell Mol
Med. 20:688–697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho HJ, Kim JT, Baek KE, Kim BY and Lee
HG: Regulation of Rho GTPases by RhoGDIs in Human Cancers. Cells.
8:10372019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leonard D, Hart MJ, Platko JV, Eva A,
Henzel W, Evans T and Cerione RA: The identification and
characterization of a GDP-dissociation inhibitor (GDI) for the
CDC42Hs protein. J Biol Chem. 267:22860–22868. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song Q, Xu Y, Yang C, Chen Z, Jia C, Chen
J, Zhang Y, Lai P, Fan X, Zhou X, et al: miR-483-5p promotes
invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1
and ALCAM. Cancer Res. 74:3031–3042. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang WG, Watkins G, Lane J, Cunnick GH,
Douglas-Jones A, Mokbel K and Mansel RE: Prognostic value of rho
GTPases and rho guanine nucleotide dissociation inhibitors in human
breast cancers. Clin Cancer Res. 9:6432–6440. 2003.PubMed/NCBI
|
|
37
|
Kotelevets L and Chastre E: Rac1
signaling: From intestinal homeostasis to colorectal cancer
metastasis. Cancers (Basel). 12:6652020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tan WJ, Tan QY, Wang T, Lian M, Zhang L
and Cheng ZS: Calpain 1 regulates TGF-β1-induced
epithelial-mesenchymal transition in human lung epithelial cells
via PI3K/Akt signaling pathway. Am J Transl Res. 9:1402–1409.
2017.PubMed/NCBI
|
|
39
|
Yang W, Li X, Qi S, Li X, Zhou K, Qing S,
Zhang Y and Gao MQ: lncRNA H19 is involved in TGF-β1-induced
epithelial to mesenchymal transition in bovine epithelial cells
through PI3K/AKT Signaling Pathway. PeerJ. 5:e39502017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bokoch GM, Vlahos CJ, Wang Y, Knaus UG and
Traynor-Kaplan AE: Rac GTPase interacts specifically with
phosphatidylinositol 3-kinase. Biochem J. 315:775–779. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Feng J, Wang X, Zhu W, Chen S and Feng C:
MicroRNA-630 suppresses Epithelial-to-Mesenchymal transition by
regulating FoxM1 in gastric cancer cells. Biochemistry Mosc.
82:707–714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shen HJ, Sun YH, Zhang SJ, Jiang JX, Dong
XW, Jia YL, Shen J, Guan Y, Zhang LH, Li FF, et al: Cigarette
smoke-induced alveolar epithelial-mesenchymal transition is
mediated by Rac1 activation. Biochim Biophys Acta. 1840:1838–1849.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qu H, Sun H and Wang X: Neogenin-1
promotes cell proliferation, motility, and adhesion by
Up-regulation of Zinc Finger E-Box binding homeobox 1 via
activating the Rac1/PI3K/AKT pathway in gastric cancer cells. Cell
Physiol Biochem. 48:1457–1467. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu SW, Liu S, Eastwood M, Sonnylal S,
Denton CP, Abraham DJ and Leask A: Rac inhibition reverses the
phenotype of fibrotic fibroblasts. PLoS One. 4:e74382009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shi-Wen X, Chen Y, Denton CP, Eastwood M,
Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM,
Black CM, et al: Endothelin-1 promotes myofibroblast induction
through the ETA receptor via a rac/phosphoinositide
3-kinase/Akt-dependent pathway and is essential for the enhanced
contractile phenotype of fibrotic fibroblasts. Mol Biol Cell.
15:2707–2719. 2004. View Article : Google Scholar : PubMed/NCBI
|