Introduction
Non-small-cell lung cancer (NSCLC) accounts for ~85%
of all lung cancer cases and is a leading cause of
cancer-associated death worldwide (1). Despite the development of early
detection techniques, novel therapeutic strategies and standard
therapeutic procedures, the 5-year relative survival rate for lung
cancer is only 17%, and the survival rate of patients with tumors
is still poor due to a high proportion of early metastases,
recurrence after surgery, or the development of chemoresistance or
radioresistance (2). In the past
years, the main treatment options for lung cancer include surgery,
radiation therapy, chemotherapy and targeted therapy, despite of
poor prognosis (3). For decades,
the tumor microenvironment, which is considered an emerging
hallmark of cancer, including in lung cancer, has attracted
attention for its critical role in regulating cancer malignancies
(4).
The tumor microenvironment is characterized by the
complex interactions of various cell types, including
tumor-associated macrophages (TAMs), dendritic cell (DC) subsets,
cytotoxic and regulatory T cells (CTLs and Tregs, respectively),
natural killer (NK) cells, and myeloid-derived suppressor cells
(MDSCs) (3). Among patients, the
tumor microenvironment can vary dramatically and lead to different
therapeutic outcomes and prognoses due to the different
compositions of immune cell subsets (5,6). Among
these subsets of immune cells, TAMs play critical roles in
regulating the microenvironment through tumor-associated immune
cells, which accumulate in the tumor stroma and are associated with
poor therapeutic outcome and prognosis (7). Recruited macrophages adapt to their
environment by developing one of two major polarization phenotypes:
M1 (classical) and M2 (alternative). M1 macrophages produce
inflammatory cytokines such as IL-1, 6, 12 and 23, TNF-α, reactive
oxygen species and nitric oxide. However, M2 macrophages produce
IL-10, transforming growth factor-β, vascular endothelial growth
factor, and matrix metalloproteinase 9 and express argininase-1
(ARG-1), scavenger receptors (CD163 and CD204), and C-type lectin
(CD301) (8). Among TAMs, there is
an increase in the frequency of the pro-tumor M2 phenotype and
impaired antigen presentation and subsequent T cell stimulation
(9). Among TAMs inhibiting the M1
phenotype, which is characterized by antitumor functions exerted
through the expression of HLA-DR, inducible nitric oxide synthase
(iNOS) and TNF-α were found to promote malignancies in NSCLC
(10).
Accumulating evidence has shown that crosstalk
between tumor cells and macrophage polarization is involved in
tumor progression (11). Tumor
cells tightly promote TAMs by secreting several types of soluble
factors, such as Wnts, thus activating Wnt/β-catenin signaling
(12). Wnts that are secreted by
tumor cells bind to the Frizzled (Fzd) family and mediate
noncanonical Wnt signaling pathway activation in macrophages,
resulting in an M2-like phenotype (13), demonstrating that Wnt/β-catenin is
critical for TAM polarization. LGK-974, which is also known as
Wnt974, is a novel inhibitor of Porcupine, which is an
O-acyltransferase responsible for the palmitoylation of Wnt ligands
(14). In lung adenocarcinoma
(LUAD), LGK-974 was found to exert antitumor effects on tumor
growth and proliferation and extended the survival of mice with
advanced LUAD tumors by inhibiting ligand-driven Wnt signaling
(15). Although the antitumor
effects of LGK-974 via the direct inhibition of Wnt/β-catenin
signaling in lung cancer have been well researched, little is known
about whether LGK-974 affects tumors by regulating the
microenvironment through TAM polarization, which depends on
inhibiting Wnt/β-catenin signaling.
The present study used human monocytic THP-1 cells
and the NSCLC cell lines A549 and H1299 for TAM stimulation, in
order to explore the effects of LGK-974 on TAM polarization,
regulation of the microenvironment and subsequent NSCLC
malignancies. It was shown that LGK-974 indirectly affected NSCLC
by regulating TAM polarization. These findings provide novel
insight into the antitumor role of LGK-974.
Materials and methods
Cell culture and reagents
The human monocyte cell line THP-1, normal human
bronchial epithelial cells 16HBE, human NSCLC cell lines H1299 and
A549 were purchased from the Chinese Academy of Sciences in
Shanghai. THP-1 cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
with 5% CO2. Aiming to stimulate macrophage
polarization, 3×105 THP-1 cells were seeded in 6-well
plate with the addition of 200 nM phorbol 12-myristate 13-acetate
(PMA; Sigma-Aldrich; Merck KGaA). To obtain TAMs, THP-1 macrophages
were cultured using 50% original media and 50% conditioned media
(CM) from H1299 or A549 cell lines for another 48 h. TAMs and NSCLC
cell lines co-culture was conducted using the non-contact
co-culture Transwell system (Corning, Inc.). TAMs or THP-1 were
cultured in upper inserts and co-cultured with NSCLC cells
(1×105 cells per well) (16). After 48 h of co-culture, TAMs or
NSCLC cells were harvested for further analyses.
Migration assay
In total, 5×105 cultured cells were
seeded into 12-well plate and incubated at 37°C and 5%
CO2 overnight. When cells reached 100% confluence in
wells, a 200-µl pipette tip was used to obtain the cell-free lane.
Cells were washed with serum-free RPMI-1640 medium and imaged to
record the wound width at 0 h. Then, cells were cultured in
serum-free RPMI-1640 medium for 24 h. A ruler was used as a guide
to obtain a straight line. Images were taken at 0 and 24 h using a
X71 (U-RFL-T) fluorescence microscope (Olympus Corporation) at
magnification of ×1,000.
Invasion assays
H1299 or A549 cells (2×104) suspended in
RPMI-1640 medium without FBS were seeded into the 24-well Transwell
plate, which was precoated with Matrigel (Thermo Fisher Scientific,
Inc.) at 37°C for 4 h, in lower chambers for 24-h culture. Then,
the Transwell was plated into 24-well plate filled with CM from
TAMs supplemented with 10% FBS. After incubating at 37°C for 24 h,
the non-invading cells were removed using a cotton swab. The cells
that had penetrated through the filter were fixed using 4%
paraformaldehyde for 10 min at room temperature and stained with
0.05% crystal violet at room temperature for 30 min and counted
under a X71 (U-RFL-T) fluorescence microscope (Olympus Corporation)
at magnification of ×40. In total 10 randomly selected fields were
used to count the number of invading cells number in each
insert.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. For
RT-qPCR analysis, reverse transcription was performed from 1 µg
total RNA using RT kit (Thermo Fisher Scientific, Inc.) at 37°C for
1 h. qPCR was performed using the Fast start Universal SYBR Green
master mix (Roche Diagnostics). Then, 0.5 µl cDNA was used as the
template for each reaction under the following conditions: 40
cycles of 95°C for 30 sec; 55°C for 30 sec; and 72°C for 1 min.
ABI7500 (Applied Biosystems; Thermo Fisher Scientific, Inc.) was
used for qPCR. The primers used for qPCR were as follows: Actin
forward, 5′-CATGTACGTTGCTATCCAGGC-3′; actin reverse,
5′-CTCCTTAATGTCACGCACGAT-3′; CD68 forward,
5′-GGAAATGCCACGGTTCATCCA-3′; CD68 reverse,
5′-TGGGGTTCAGTACAGAGATGC-3′; CD163 forward,
5′-TTTGTCAACTTGAGTCCCTTCAC-3′; CD163 reverse,
5′-TCCCGCTACACTTGTTTTCAC-3′; CD206 forward,
5′-TCCGGGTGCTGTTCTCCTA-3′; CD206 reverse,
5′-CCAGTCTGTTTTTGATGGCACT-3′; Arg1 forward,
5′-GTGGAAACTTGCATGGACAAC-3′; Arg1 reverse,
5′-AATCCTGGCACATCGGGAATC-3′; CD86 forward,
5′-CTGCTCATCTATACACGGTTACC-3′; CD86 reverse,
5′-GGAAACGTCGTACAGTTCTGTG-3′; HLA-DR forward,
5′-AGTCCCTGTGCTAGGATTTTTCA-3′; HLA-DR reverse,
5′-ACATAAACTCGCCTGATTGGTC-3′; TNF-α forward,
5′-CCTCTCTCTAATCAGCCCTCTG-3′; TNF-α reverse,
5′-GAGGACCTGGGAGTAGATGAG-3′; IL-12 forward,
5′-ACCCTGACCATCCAAGTCAAA-3′; IL-12 reverse,
5′-TTGGCCTCGCATCTTAGAAAG-3′; iNOS forward,
5′-TTCAGTATCACAACCTCAGCAAG-3′; iNOS reverse,
5′-TGGACCTGCAAGTTAAAATCCC-3′; mannose receptor (MR) forward,
5′-GATGGCGTTCCTGTTACCTCT-3′; MR reverse,
5′-GCCCAGGCGAAAATATCTCAG-3′; IL-10 forward,
5′-GACTTTAAGGGTTACCTGGGTTG-3′; reverse,
5′-TCACATGCGCCTTGATGTCTG-3′; Fzd4 forward,
5′-CCTCGGCTACAACGTGACC-3′; Fzd4 reverse,
5′-TGCACATTGGCACATAAACAGA-3′; Fzd7 forward,
5′-GTGCCAACGGCCTGATGTA-3′; Fzd7 reverse,
5′-AGGTGAGAACGGTAAAGAGCG-3′; Fzd9 forward,
5′-TGCGAGAACCCCGAGAAGT-3′; Fzd9 reverse, 5′-GGGACCAGAACACCTCGAC-3′;
β-catenin forward, 5′-AAAGCGGCTGTTAGTCACTGG-3′; β-catenin reverse,
5′-CGAGTCATTGCATACTGTCCAT-3′; c-Myc forward,
5′-GGCTCCTGGCAAAAGGTCA-3′; c-Myc reverse,
5′-CTGCGTAGTTGTGCTGATGT-3′; Axin2 forward,
5′-CAACACCAGGCGGAACGAA-3′; and Axin2 reverse,
5′-GCCCAATAAGGAGTGTAAGGACT-3′. The qPCR results were analyzed and
expressed relative to the CT (threshold cycle) values and then
converted to fold changes, using the 2−∆∆Cq method;
2.0-fold change was considered as significant (17).
Western blotting
Cells (1×106) were lysed with 500 µl RIPA
buffer (cat. no. R0278; Sigma-Aldrich; Merck KGaA) containing 1X
protease cocktail (Thermo Fisher Scientific, Inc.), and then total
protein sample was denatured at 100°C for 15 min. The protein was
measured using the BCA method, and 20 µg total protein was
fractionated in each lane by performing electrophoresis using 6–12%
SDS-polyacrylamide gradient gel, and then the proteins from the gel
were transferred to PVDF membranes. After being blocked with 5%
non-fat dry milk for 1 h at room temperature, the membrane was
incubated with the primary antibodies at 4°C overnight, followed by
secondary antibody incubation at room temperature for 1 h. After
three washes with 0.1% TBS-Tween-20, membrane was imaged using an
ECL luminescence kit (MilliporeSigma) on X-ray file. The dilution
ratios of the primary antibodies were as follows: Anti-Arg-1
antibody (1:1,000 dilution; cat. no. A4923, ABclonal Biotech Co.,
Ltd.) and anti-β-actin antibody (1:1,000 dilution; cat. no. ab8226;
Abcam). The HRP-conjugated goat anti-mouse secondary antibody and
HRP-conjugated goat anti-rabbit secondary antibody (cat. nos.
ab205719 and ab205718; Abcam) were diluted at a ratio of 1:5,000.
Blots were semi-quantitatively analyzed using ImageJ software
(version-2.0; National Institutes of Health).
ELISA
The cytokines contained in the supernatant of
cultured medium, including TNF-α, IL-12 and IL-10 were examined by
ELISA kits: Human-TNF-α- ELISA-Kit (cat. no. ml077385),
Human-IL-10-ELISA-Kit, (cat. no. ml064299), Human-IL-12-ELISA-Kit,
(cat. no. ml058044), according to the manufacturer's instructions
(Shanghai Enzyme-linked Biotechnology Co., Ltd.).
Cell viability assays
Cells (5×103) were seeded in 96-well
plates for each well and allowed to attach overnight. Then, cells
were cultured using 50% RPMI-1640 medium and 50% CM supplemented
with 10% FBS for 1–5 days. Medium was refreshed every 2 days. For
each day, 10 µl tetrazolium salt Cell Counting Kit-8 (CCK-8;
Nanjing KeyGen Biotech Co., Ltd.) was added to each well and
allowed for an additional 1-h incubation at 37°C. Optical density
was measured at a wavelength of 450 nm (OD450).
Cell cycle distribution
Cells (1×106) were pelleted and washed by
pre-cold PBS three times and fixed using 75% ethyl alcohol (1 ml)
for 16 h at 4°C. Then, cells were pelleted and washed using
ice-cold PBS two times. RNase A (100 µl) and 400 µl PI
(Sigma-Aldrich; Merck KGaA) were added in the dark and incubated
for 30 min at room temperature. After 30 min incubation at 4°C,
cell cycle phases were measured using the FACS LSRII flow cytometer
(BD Biosciences) and the data were analyzed using FlowJo software
(FlowJo Treestar; version 9.7.4).
Colony formation
Cells (5×103) were suspended in 50%
original medium and 50% CM in a 12-well plate, with the addition of
antibiotic-antimycotic mixture (final concentration, 1%). Medium
was refreshed every 3 days. Cells were cultured for 14 days and
colonies were fixed using 4% paraformaldehyde at room temperature
for 10 min and stained with 0.05% crystal violet at room
temperature for 10 min and counted under a X71 (U-RFL-T)
fluorescence microscope (Olympus Corporation) at magnification of
×40. Then, colonies were identified as those containing >50
cells.
Statistical analysis
Statistical analysis was performed with SPSS version
15.1 (SPSS, Inc.). Data are expressed as mean ± SD (x±s). The
statistical significance of the difference between groups was
assessed by unpaired Student's t-tests and one-way ANOVA followed
by Bonferroni post hoc analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
LGK-974 regulates the polarization of
tumor-associated macrophages
In order to determine the effects of LGK-974 on the
polarization of tumor-associated macrophages co-cultured with lung
cancer cells, an in vitro model of tumor-associated
macrophages TAMs was utilized. The human monocyte cell line THP-1
pretreated with PMA for 24 h was cultured in CM from NSCLC cell
lines (A549 or H1299) or normal human bronchial epithelial cells
16HBE, which was considered as a negative control, to generate
TAMs. The polarization of TAMs was validated on the basis of marker
expression and cytokine profile. As shown in Fig. 1A-C, by detecting CD68, CD163, CD206,
Arginase 1, HLA-DR mRNA levels and Arginase 1 protein level,
co-culture with both A549 and H1299 demonstrated upregulated
expression levels of M1 markers (arginase 1, CD86, HLA-DR) and M2
markers (CD163, CD206) compared with that with 16HBE, without
disturbing pan-macrophage marker CD68. This indicates that both
A549 and H1299 induced TAMs of a mixed M1/M2 phenotype. The
addition of 10 nM of LGK-974 significantly decreased the expression
levels of all these markers, demonstrating that LGK-974 potentially
blocks the polarization stimulated by tumor cell co-culture.
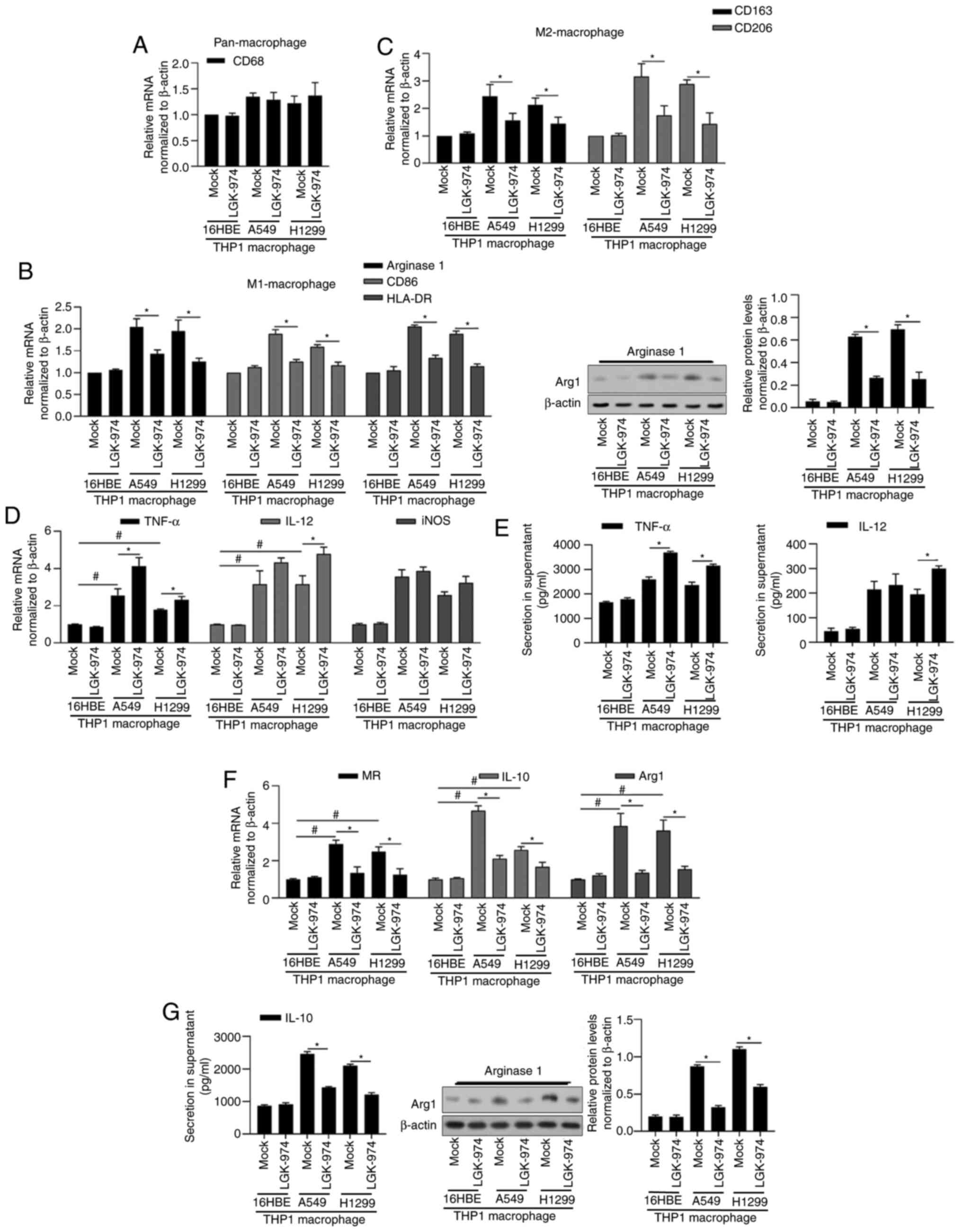 | Figure 1.Addition of LGK-974 regulates TAMs and
its inflammation-associated cytokines. After 24-h co-incubation
with 16HBE, A549 or H1299, total RNA or protein was isolated and
the expression levels of cell markers for pan-macrophage CD68 (A),
for M1-macrophage Arginase 1, CD86 and HLA-DR (B), and for
M2-macrophage CD163 and CD206 (C) were determined. (D-G) Expression
levels of functional markers for M1-macrophage (TNF-α, IL-12 and
iNOS) and for M2-macrophage (MR, IL-10 and Arg1) were measured by
reverse transcription-quantitative PCR. *P<0.05 vs. mock group;
#P<0.05 vs. mock group/16HBE. TAMS, tumor-associated
macrophages; iNOS, inducible nitric oxide synthase; MR, mannose
receptor; Arg1, argininase-1. |
In order to explore the roles of LGK-974 in
macrophage polarization, the expression of macrophage surface and
functional markers for M1 such as TNF-α, IL-12 and iNOS, for M2
such as MR, IL-10 and Arg1 were assessed. As shown in Fig. 1D and E, the addition of LGK-974
affected TNF-α, IL-12 and iNOS slightly, but significantly
decreased MR, IL-10 and Arg1 (Fig. 1F
and G), indicating that LGK-974 may inhibit M2-type phenotype
and M2-like function.
LGK-974 blocks Wnt signaling and
regulates the function of TAMs
LGK-974 specifically inhibits Wnt secretion, and
thus regulates Wnt/β-catenin pathway (18). The two main canonical Wnt signaling
inducers (19,20), Wnt3a and Wnt5a, were chosen to
investigate whether the effect of LGK-974 on macrophage
polarization is dependent on the inhibition of Wnt secretion. As
shown in Fig. 2A, the addition of
commercially available Wnt3a and Wnt5a recombinant proteins
reversed the decrease in IL-10 and Arg1 by LGK-974. Expectedly, the
addition of Wnt3a and Wnt5a increased MR, IL-10 and Arg1 (Fig. 2A). It was also observed that the
addition of Wnt3a and Wnt5a increased M1-macrophage markers,
including TNF-α, IL-12 and iNOS, which may be due to the promoting
effect of Wnt on M2-type phenotype (Fig. 2B).
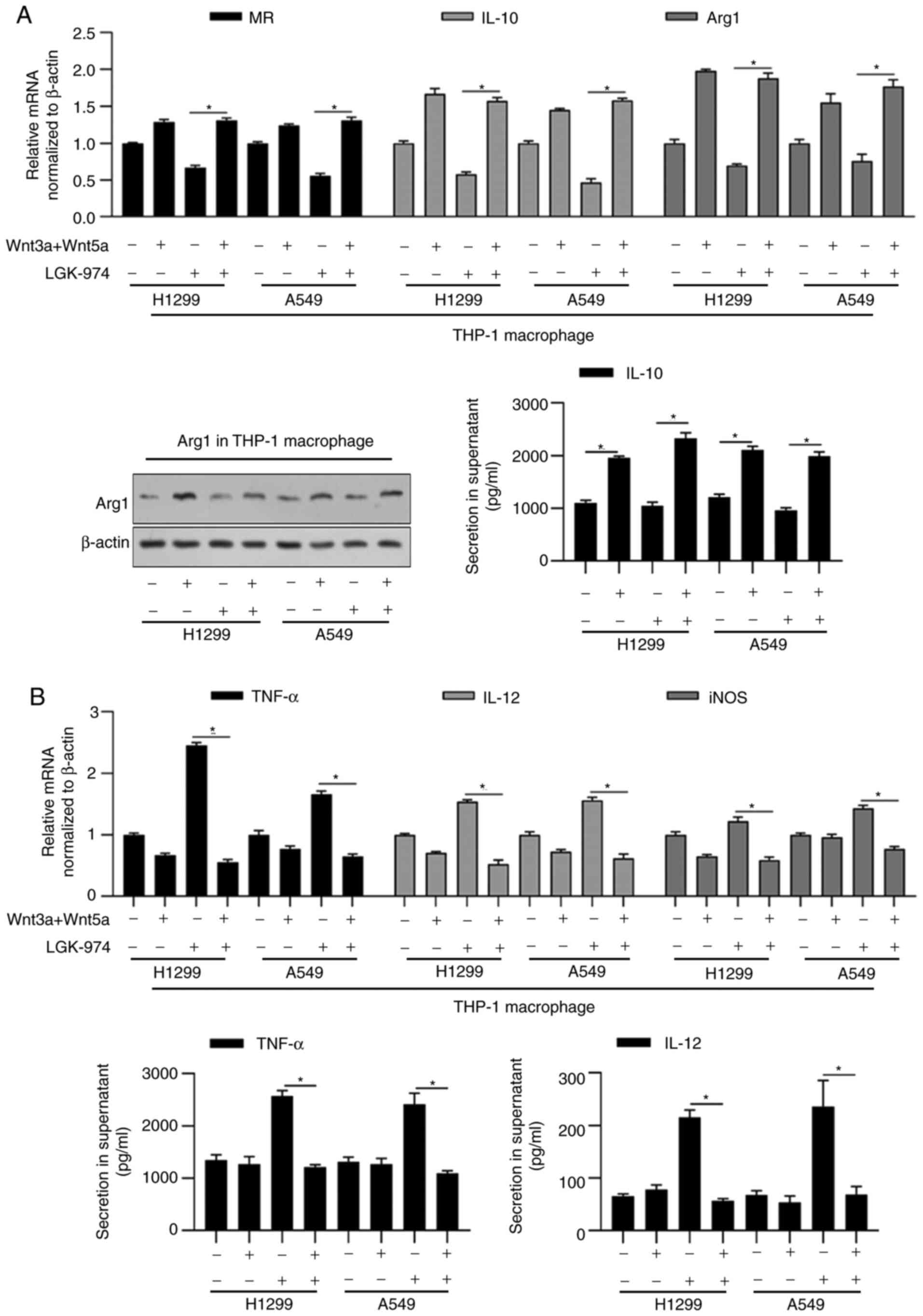 | Figure 2.LGK-974 regulates functional markers
for macrophage via blocking Wnt/β-catenin signaling. 16HBE, A549 or
H1299 cells were co-cultured with LGK-974 and/or Wnt3a/5aA for 24
h. Then, total RNA was isolated and the expression levels of
functional markers for MR, IL-10 and Arg1 (A), and functional
markers for M1-macrophage (TNF-α, IL-12 and iNOS) (B) were measured
by performing reverse transcription-quantitative PCR. Arg1 protein
level was detected by western blotting and IL-10 section in
supernatant was measured by performing ELISA (A, right panel).
TNF-α and IL-12 section in supernatant was measured by performing
ELISA (B, right panel). *P<0.05 vs. mock group. MR, mannose
receptor; iNOS, inducible nitric oxide synthase; Arg1,
argininase-1. |
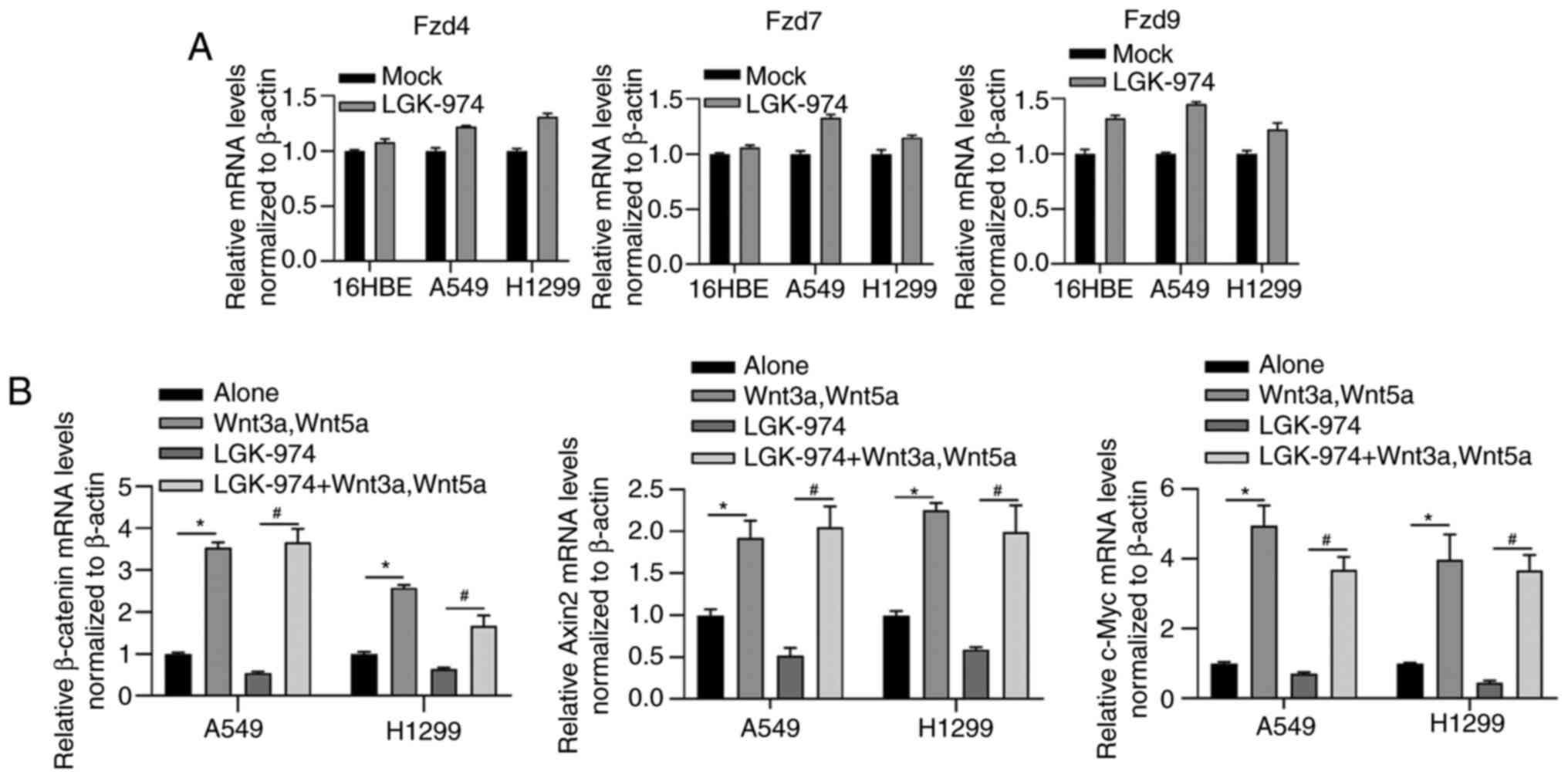
In order to assess whether LGK-974 modulates the
expression level of Wnt receptors, the mRNA levels of Fzd4, Fzd7
and Fzd9 in THP-1 macrophage co-cultured with 16HBE, A549 or H1299,
respectively, were examined. As shown in Fig. 3A, Fzd4, Fzd7 and Fzd9 were not
obviously affected by the addition of LGK-974, suggesting that Wnt
signaling could not be modified in LGK-974 regulation via
regulating Wnt receptors. The addition of LGK-974 significantly
decreased the mRNA levels of three downstream genes of the
Wnt/β-catenin signaling pathway including β-catenin, Axin2, and
c-Myc, which were reversed by co-culturing with Wnt3a and Wnt5a
(Fig. 3B), further indicated that
the regulatory role of LGK-974 was dependent on regulating Wnt
secretion.
LGK-974 modulates TAMs' effects on
proliferation, colony formation and invasion
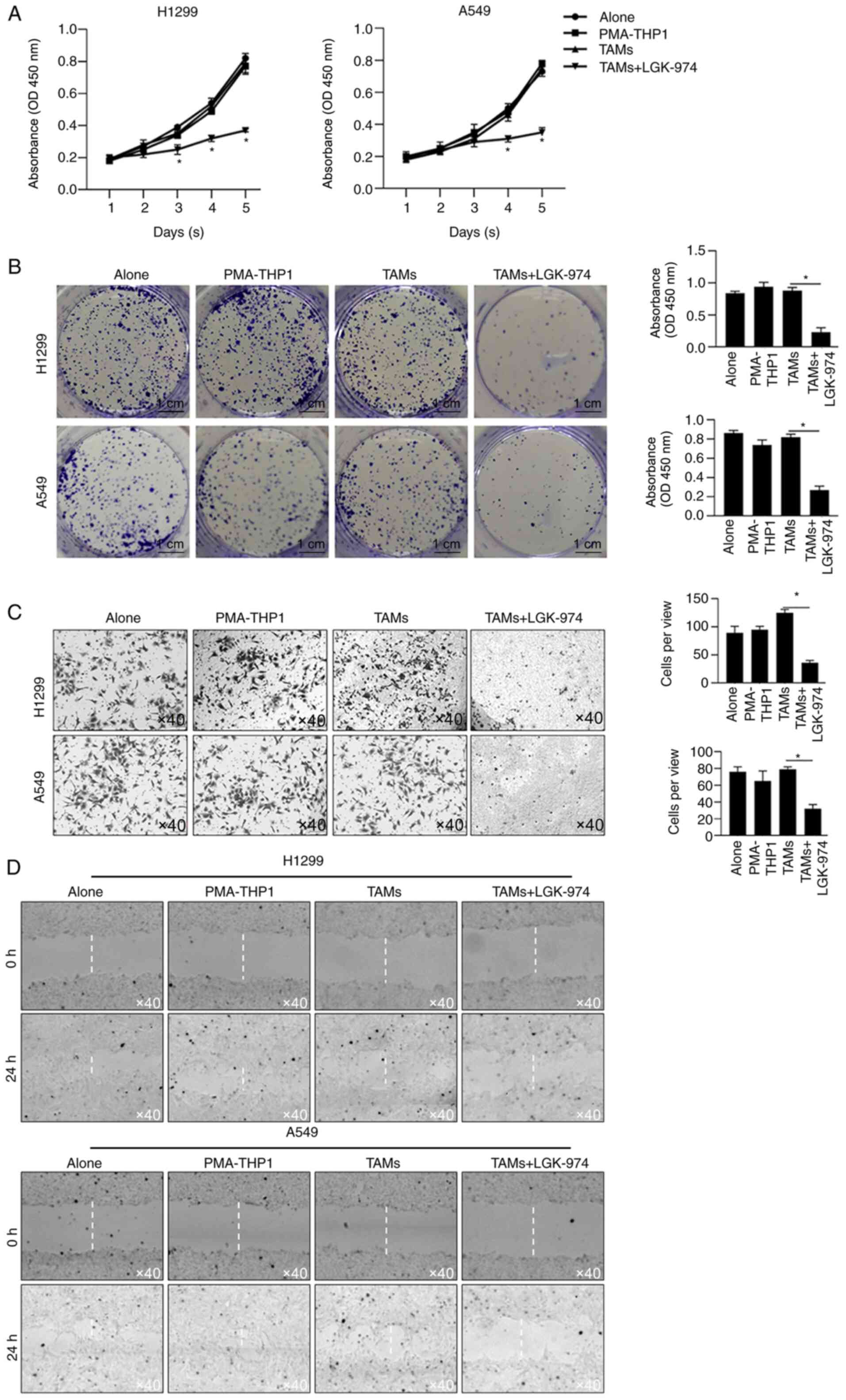
Since TAMs play critical roles in tumor malignant
behaviors, including proliferation, invasion, metastasis and
immunosuppression, the ability of TAMs modified by co-culture with
LGK-974 to regulate tumor cell proliferation was detected by a
CCK-8 assay and colony-formation assay. The results showed that the
cell viability of both H1299 and A549 cultured with CM, which was
collected from LGK-974-modified TAMs culture medium and contained
no LGK-974, were significantly decreased (Fig. 4A). Secondly, colony-formation assay
of H1299 and A549 cultured with CM from LGK-974-modified TAMs was
performed, which indicated that TAM-modified CM inhibited cell
proliferation without LGK-974. Consistently, CM from TAMs with the
addition of LGK-974 significantly inhibited the colony-formation
ability in these two cell lines (Fig.
4B). Thirdly, the invasion of tumor cells was also measured
using Transwell inserts with a Matrigel layer for 24 h. The results
showed that CM from LGK-974-modified TAMs significantly limited the
numbers of invaded cells (Fig. 4C).
Expectedly, by performing scratch assay, it was also observed that
CM from LGK-974-modified TAMs markedly limited cell migration
(Fig. 4D). Notably, CM from TAMs
without LGK-974 pre-modification slightly affected cell
proliferation, colony formation, invasion or migration, which is
potentially due to the high malignancies of H1299 and A549.
Wnt/β-catenin signaling is critical
for LGK-974's effects on TAMs
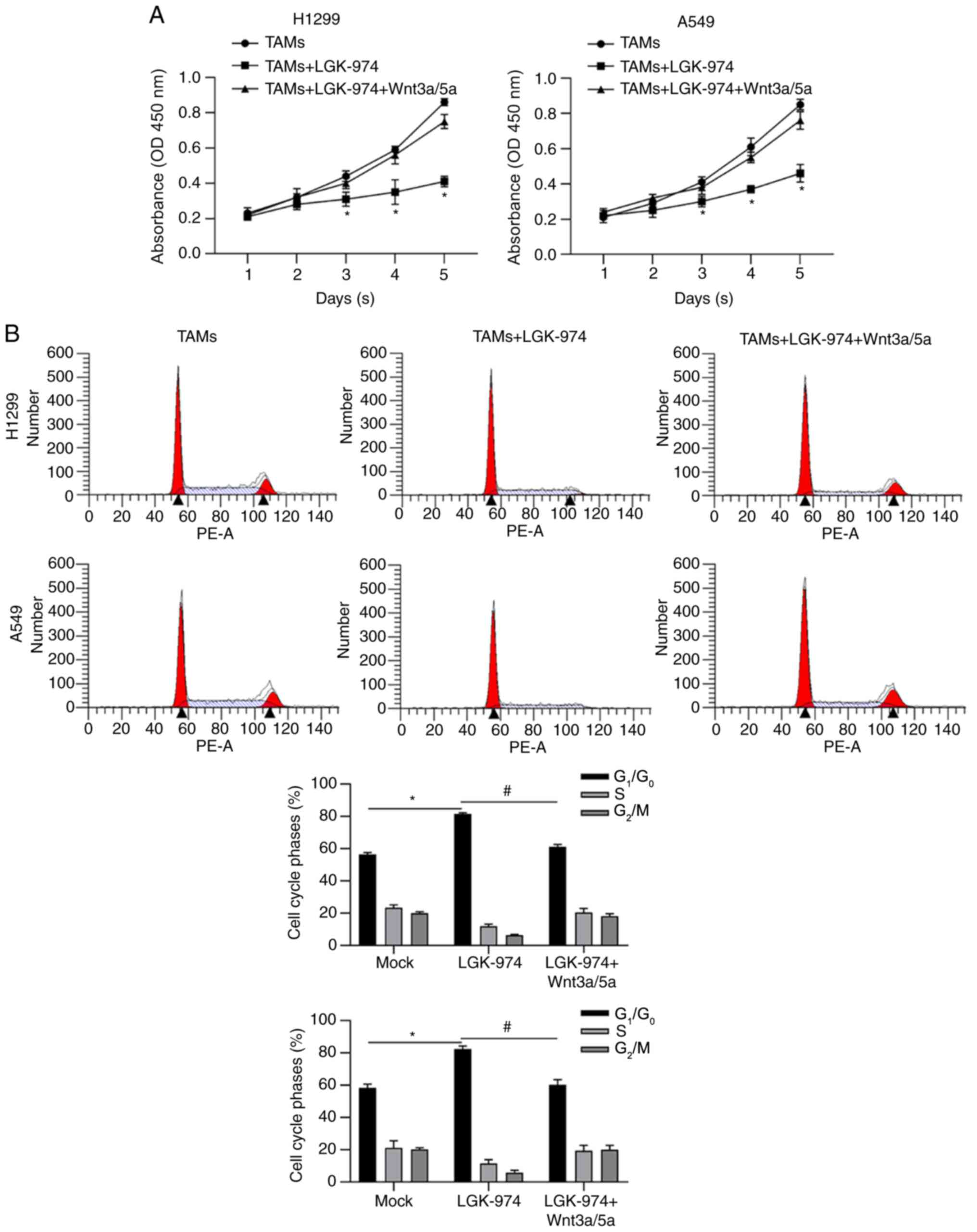
In order to further confirm whether LGK-974
regulates TAMs via inhibiting Wnt/β-catenin, Wnt3a and Wnt 5a in
were employed for TAM culturing. Consistent with previous results,
CM from TAMs with LGK-974 resulted in decreased cell viability. CM
from TAMs with LGK-974 and Wnt3a/5a significantly promoted cell
viability compared with that without the addition of Wnt3a/5a
(Fig. 5A). By detecting cell cycle
phase distribution, it was also observed that CM from TAMs with
LGK-974 significantly increased the proportion of
G1/G0 phase, which was reversed by the
addition of LGK-974 (Fig. 5B).
Taken together, all these data demonstrated that the modulation of
LGK-974 on TAMs is potentially via blocking Wnt/β-catenin by
inhibiting Wnt secretion.
Discussion
The present study results showed that LGK-974, a
novel inhibitor of Porcupine, which is an O-acyltransferase
responsible for the palmitoylation of Wnt ligands, regulated the
polarization of macrophages and thus regulated the microenvironment
and subsequently inhibited tumor malignancies, including
proliferation, colony-formation and invasion. Numerous reports have
described the antitumor effects of LGK-974 in several types of
cancer, including NSCLC. However, little is known as to whether
LGK-974 affects tumor malignancies indirectly by regulating
macrophage polarization. As expected, LGK-974 regulated macrophage
polarization by inhibiting Wnt/β-catenin signaling, and the
modified microenvironment significantly inhibited tumor
malignancies. Taken together, these results suggest that LGK-974
not only directly regulates tumor malignancies but also indirectly
regulates tumor malignancies by modifying macrophage polarization
and the microenvironment.
The porcupine-selective inhibitor LGK-974 blocks
Wnt/β-catenin signaling by inhibiting the secretion of Wnt ligands.
LGK-974 has been proven to block tumor growth and malignancies
in vivo (14). Giefing et
al (21) reported that in head
and neck squamous cell carcinoma cell lines with NOTCH1 mutations,
LGK-974 exerts antitumor effects by blocking Wnt signaling.
Wnt/β-catenin signaling is critical in promoting the proliferation
and maintenance of cancer stem-like cells (CSCs) in various cancer
types, including NSCLC (22). This
indicated that LGK-974 may affect stemness in CSCs derived from
NSCLC via blocking Wnt signaling and the absence of the
investigation of the effect of LGK-974 on stemness maintenance is a
limitation in the present study. In NSCLC, hyperactivation of Wnt
signaling is necessary for cancer progression and the maintenance
of self-renewal capacity, resulting in the poor prognosis of lung
cancer patients (23,24). Guimaraes et al (25) found that in NSCLC, LGK-974 treatment
significantly inhibited cell migration and invasion by blocking Wnt
signaling. In LUAD, despite intestinal toxicity, LGK-974 markedly
decreased cell viability, indicating that it is a promising
antitumor treatment (25).
TAMs not only affect the intrinsic characteristics
of tumor cells, but also affect the tumor microenvironment. TAMs
can stimulate the proliferation, migration and genetic instability
of tumor cells. TAMs act on the primary tumor site or secondary
localization site, and promote the invasion and the metastasis.
TAMs promote angiogenesis and lymph angiogenesis, as well as tissue
remodeling of fibrous tissue deposition (26). TAMs contribute to immunoregulation
observed in the tumor microenvironment. Therefore, TAMs targeting
may complement the action of checkpoint blockade inhibitors.
LGK-974 was accepted as a tumor inhibitor by blocking Wnt signaling
(19), without knowing its exact
role in regulating microenvironment via TAMs. The present study
focused on the effects of LGK-974 on TAMs polarization by
inhibiting Wnt signaling. However, molecular evaluation by
identifying TAM polarization in clinical sample set it is failed
was not performed, which is a limitation of the present study.
β-catenin is one of the critical components of
canonical Wnt signaling. In the inactivated state, β-catenin is at
a low level, localized in the cytoplasm and binds to a destruction
complex that is composed of APC, axins, CK1α and GSK3β (27). Canonical Wnt ligands interact with
specific receptors, such Fzd4, Fzd7 and Fzd9, and inactivate this
destruction complex, which leads to the nuclear translocation of
cytoplasmic β-catenin. In the nucleus, β-catenin then activates the
transcription factors LEF and TCF, resulting in the upregulation of
downstream target genes, including c-Myc and Axin2 (27). The present results showed that the
addition of LGK-974 notably decreased c-Myc and Axin2 without
disturbing the expression of Fzd4, Fzd7 or Fzd9, and this effect
was reversed by the addition of the Wnt ligands Wnt3a and Wnt5a,
which are known to promote the activation of Wnt/β-catenin
signaling. Interestingly, the addition of the Wnt ligands Wnt3a and
Wnt5a induced TAMs to adopt the M1 phenotype. However, the effects
of Wnt3a and Wnt5a on the malignant behaviors in A549 and H1299
were not demonstrated, which is a limitation in the present
study.
Overall, the present study shows that LGK-974 exerts
antitumor effects not only by blocking Wnt/β-catenin signaling in
cancer cells but also by regulating macrophage polarization and
thus modifying the tumor microenvironment. The present study
results are the first to demonstrate that LGK-974 indirectly
regulates lung cancer malignancies via TAM-derived Wnt ligands and
thus activates canonical Wnt/β-catenin signaling in TAMs, resulting
in the inhibition of cell proliferation, colony formation and
invasion. Blocking Wnt secretion from TAMs or inactivating Wnt
signaling in TAMs should be a novel strategy for NSCLC therapy in
the future.
Acknowledgements
The authors would like to thank Mr Hong Tao (Sichuan
University, Chengdu, China) for language editing and suggestion of
statistical analysis.
Funding
This study was supported by the program of National
Natural Science Foundation of China (grant no. 81960532).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YT, YS, CC and XK designed the experiments. YT, MJ,
AC, WQ and XH performed cell culture-associated experiments. JZ and
GX are responsible for data collection and performed the
statistical analysis. All authors read and approved the final
manuscript. YT, CC and XK confirmed the authenticity of all the raw
data
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lemjabbar-Alaoui H, Hassan OU, Yang YW and
Buchanan P: Lung cancer: Biology and treatment options. Biochim
Biophys Acta. 1856:189–210. 2015.PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pages F, Berger A, Camus M, Sanchez-Cabo
F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte
D, et al: Effector memory T cells, early metastasis, and survival
in colorectal cancer. N Engl J Med. 353:2654–2666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sautes-Fridman C, Cherfils-Vicini J,
Damotte D, Fisson S, Fridman WH, Cremer I and Dieu-Nosjean MC:
Tumor microenvironment is multifaceted. Cancer Metastasis Rev.
30:13–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki K, Kachala SS, Kadota K, Shen R, Mo
Q, Beer DG, Rusch VW, Travis WD and Adusumilli PS: Prognostic
immune markers in non-small cell lung cancer. Clin Cancer Res.
17:5247–5256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tamura R, Tanaka T, Yamamoto Y, Akasaki Y
and Sasaki H: Dual role of macrophage in tumor immunity.
Immunotherapy. 10:899–909. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kerkar SP and Restifo NP: Cellular
constituents of immune escape within the tumor microenvironment.
Cancer Res. 72:3125–3130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Welsh TJ, Green RH, Richardson D, Waller
DA, O'Byrne KJ and Bradding P: Macrophage and mast-cell invasion of
tumor cell islets confers a marked survival advantage in
non-small-cell lung cancer. J Clin Oncol. 23:8959–8967. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mantovani A and Allavena P: The
interaction of anticancer therapies with tumor-associated
macrophages. J Exp Med. 212:435–445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parchure A, Vyas N and Mayor S: Wnt and
hedgehog: Secretion of lipid-modified morphogens. Trends Cell Biol.
28:157–170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Ye YC, Chen Y, Zhao JL, Gao CC,
Han H, Liu WC and Qin HY: Crosstalk between hepatic tumor cells and
macrophages via Wnt/β-catenin signaling promotes M2-like macrophage
polarization and reinforces tumor malignant behaviors. Cell Death
Dis. 9:7932018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang
T, Kasibhatla S, Schuller AG, Li AG, Cheng D, et al: Targeting
Wnt-driven cancer through the inhibition of Porcupine by LGK974.
Proc Natl Acad Sci USA. 110:20224–20229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tammela T, Sanchez-Rivera FJ, Cetinbas NM,
Wu K, Joshi NS, Helenius K, Park Y, Azimi R, Kerper NR, Wesselhoeft
RA, et al: A Wnt-producing niche drives proliferative potential and
progression in lung adenocarcinoma. Nature. 545:355–359. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chatterjee S, Mookerjee A, Basu JM,
Chakraborty P, Ganguly A, Adhikary A, Mukhopadhyay D, Ganguly S,
Banerjee R, Ashraf M, et al: A novel copper chelate modulates tumor
associated macrophages to promote anti-tumor response of T cells.
PLoS One. 4:e70482009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak JK and Schmittgen DT: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jang J, Jung Y, Kim Y, Jho EH and Yoon Y:
LPS-induced inflammatory response is suppressed by Wnt inhibitors,
Dickkopf-1 and LGK974. Sci Rep. 7:416122017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nygren MK, Dosen G, Hystad ME, Stubberud
H, Funderud S and Rian E: Wnt3A activates canonical Wnt signalling
in acute lymphoblastic leukaemia (ALL) cells and inhibits the
proliferation of B-ALL cell lines. Br J Haematol. 136:400–413.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mikels AJ and Nusse R: Purified Wnt5a
protein activates or inhibits beta-catenin-TCF signaling depending
on receptor context. PLoS Biol. 4:e1152006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giefing M, Wierzbicka M, Szyfter K,
Brenner JC, Braakhuis BJ, Brakenhoff RH, Bradford CR, Sorensen JA,
Rinaldo A, Rodrigo JP, et al: Moving towards personalised therapy
in head and neck squamous cell carcinoma through analysis of next
generation sequencing data. Eur J Cancer. 55:147–157. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mei Y, Liu YB, Cao S, Tian ZW and Zhou HH:
RIF1 promotes tumor growth and cancer stem cell-like traits in
NSCLC by protein phosphatase 1-mediated activation of Wnt/β-catenin
signaling. Cell Death Dis. 9:9422018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang L, Cai J, Chen B, Wu S, Li R, Xu X,
Yang Y, Guan H, Zhu X, Zhang L, et al: Aberrantly expressed
miR-582-3p maintains lung cancer stem cell-like traits by
activating Wnt/β-catenin signalling. Nat Commun. 6:86402015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lei Z, Shi H, Li W, Yu D, Shen F, Yu X, Lu
D, Sun C and Liao K: miR185 inhibits non small cell lung cancer
cell proliferation and invasion through targeting of SOX9 and
regulation of Wnt signaling. Mol Med Rep. 17:1742–1752.
2018.PubMed/NCBI
|
|
25
|
Guimaraes P, Tan M, Tammela T, Wu K, Chung
A, Oberli M, Wang K, Spektor R, Riley RS, Viana CTR, et al: Potent
in vivo lung cancer Wnt signaling inhibition via
cyclodextrin-LGK974 inclusion complexes. J Control Release.
290:75–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kudo M: Immuno-oncology in hepatocellular
carcinoma: 2017 update. Oncology. 93 (Suppl 1):147–159. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|