Introduction
Chronic joint inflammation is the hallmark of
rheumatoid arthritis (RA), the most common type of autoimmune
disease (1,2). Although the origin of the disease
remains unclear, the immune system is known to affect the lining of
joints and cause a painful swelling that may eventually result in
bone erosion and joint deformity (3). The synovial lining of diarthrodial
joints is the site of the initial inflammatory process, and it
contains abundant cytokines and chemokines produced by several
immune cell types (4).
Fibroblast-like synoviocytes (FLS) are of mesenchymal origin in the
synovial lining, and RA FLS are important mediators of joint
destruction that have the ability to invade adjacent collagenous
structures and secrete factors that promote inflammation,
neovascularization, and cartilage degradation (5). Thus, strategies to control the
inflammatory effect of FLS may contribute to the prevention and
treatment of RA.
Ginkgo biloba extract (GBE), one of the most common
industrialized herbal medicines, has been clinically used in the
treatment of cardiovascular, cerebrovascular, and neurological
disorders due to its anti-inflammatory, antioxidant and
anti-apoptotic activities (6–8). The
major identified active ingredients of GBE include ginkgo flavonol
glycosides (GFGs) and ginkgolides (GGs) (9). GGs consist of ginkgolide A (GA),
ginkgolide B (GB), ginkgolide C (GC), ginkgolide J (GJ), and
ginkgolide K (GK) (10). GA has
been revealed to suppress the expression of pro-inflammatory
mediators [cyclooxygenase-2 (COX-2) and nitric oxide (NO)] and
pro-inflammatory cytokines [tumor necrosis factor (TNF)-α,
interleukin (IL)-6 and IL-1β] in LPS-treated mouse and human
macrophages (11). Other CGs have
exhibited anti-platelet-activating, anti-apoptotic, anti-oxidative,
neurotrophic and neuroimmunomodulatory effects by inhibition of the
mitogen-activated protein kinase (MAPK) and nuclear factor-κB
(NF-κB) signaling pathways (12).
Among these CGs, GB has the most obvious pharmacological
properties, while little is known about GJ (13,14).
Vitolo et al (15) reported
that GJ is capable of inhibiting the cell death of rodent
hippocampal neurons caused by Aβ(1–42). However, the
pharmacological effect of GJ on RA has yet to be investigated.
Bacterial LPS is capable of eliciting a strong immune response and
frequently used to induce symptoms of RA (16). In the present study, the protective
effect of GJ against inflammation induced by LPS in human synovial
cells SW982 as well as the underlying mechanisms were
investigated.
Materials and methods
Reagents
GJ was provided from Wanbangde Pharmaceutical Group
Co., Ltd. LPS, DMSO and DAPI were purchased from Sigma-Aldrich
(Merck KGaA). Antibodies were purchased from Santa Cruz
Biotechnology, Inc., and Abcam. The antibodies used in the present
study were as follows: COX-2 (cat. no. ab169782), inducible nitric
oxide synthase (iNOS; cat. no. ab178945), NLR family pyrin domain
containing 3 (NLRP3; cat. no. ab263899) and caspase-1 (cat. no.
ab207802; all 1:1,000; all from Abcam), IL-1β (cat. no. sc-515598),
phosphorylated (p)-p38 (cat. no. sc-166182), p38 (cat. no. sc-7972)
and GAPDH (cat. no. sc-365062; all 1:1,000; all from Santa Cruz
Biotechnology, Inc.). Other reagents were purchased from Beyotime
Institute of Biotechnology and Sangon Biotech Co., Ltd.
Cell culture and treatment
Human synovial cells SW982 were purchased from
American Type Culture collection and maintained in DMEM with 10%
fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S) in a
humidified atmosphere with 5% CO2 at 37°C. For the
experiments, cells were pretreated with or without various
concentrations of GJ (5, 10, 25 µM) for 24 h and then exposed to
LPS (1 µg/ml) for 12 h. Additionally, p38 activation was conducted
with hesperetin treatment (2 µM, cat. no. HY-N0168;
MedChemExpression) for 2 h at 37°C prior to other treatments.
Enzyme-linked immunosorbent assay
(ELISA)
After treatment, the culture medium was collected
and processed for ELISA. Culture medium (100 µl) was reacted with
the following ELISA kits: PGE2 (cat. no. SEKH-0414; Beijing
Solarbio Science & Technology Co., Ltd.), TNF-α (cat. no.
SEKH-0047; Beijing Solarbio Science & Technology Co., Ltd.),
IL-1β (cat. no. SEKH-0002; Beijing Solarbio Science &
Technology Co., Ltd.) and IL-18 (cat. no. SEKH-0028; Beijing
Solarbio Science & Technology Co., Ltd.) according to the
manufacturers' protocols. The absorbance was measured at 450 nm
using a microplate reader (Molecular Devices, LLC).
Assessment of NO
After treatment, the culture medium was collected
and processed for the Griess assay. Culture medium (50 µl) was
mixed with an equal volume of Griess reagent (Beijing Solarbio
Science & Technology Co., Ltd.) for 10 min at 37°C in the dark.
The absorbance was measured at 540 nm using a microplate reader
(Molecular Devices, LLC).
Western blot analysis
After treatment, cells were harvested and lysed in
ice-cold RIPA buffer (cat. no. P0013E; Beyotime Institute of
Biotechnology) and the supernatant was collected. The protein
concentration was measured using a BCA protein assay kit (cat. no.
P0010; Beyotime Institute of Biotechnology). Proteins (40 µg) were
separated by 12% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes. Membranes were blocked with 5% non-fat milk
for 1 h at room temperature and incubated with primary antibodies
at 4°C overnight, followed by incubation with HRP-conjugated goat
anti-rabbit IgG (1:1,000; cat. no. A0208; Beyotime Institute of
Biotechnology) at 37°C for 2 h. Protein bands were visualized using
the ECL assay kit (cat. no. P0018AM; Beyotime Institute of
Biotechnology). The density of each band was normalized to the
expression of the housekeeping gene GAPDH. ImageJ v1.8.0 (National
Institutes of Health) was used for semi-quantification.
Immunofluorescence analysis
Cells were fixed in 2% paraformaldehyde (cat. no.
P0099; Beyotime Institute of Biotechnology) for 15 min and
permeabilized in 0.1% Triton X-100 (cat. no. ST797; Beyotime
Institute of Biotechnology) for 20 min at room temperature,
followed by incubation with 2% bovine serum albumin (BSA; cat. no.
ST025; Beyotime Institute of Biotechnology) at room temperature for
60 min before proceeding to immunostaining. Then, cells were
incubated with anti-p65 antibody (1:500; cat. no. AF1234; Beyotime
Institute of Biotechnology) overnight at 4°C and Alexa Fluor
488-conjugated goat anti-rabbit IgG (1:250; cat. no. A0423;
Beyotime Institute of Biotechnology) for 5 min at room temperature.
DAPI (cat. no. C1005; Beyotime Institute of Biotechnology) was used
to co-stain nuclei for 5 min at room temperature. Fluorescence was
observed using a fluorescent microscope (Leica Microsystems,
Inc.).
p38 kinase activity assay
The plates were coated with 50 µl/well p38 substrate
ATF-2 and stored at 4°C overnight. Then, plates were blocked with
blocking buffer [BB, 0.05% Tween-20, 0.025% BSA and 0.02% NaN3 in
TBS] for a further 30 min at room temperature. Samples were diluted
in a kinase buffer (KB), which contained 12 ng/50 µl p38 MAPK, 50
mM Tris (pH 7.5), 10 mM MgCl2, 10 mM
b-glycerolphosphate, 100 µg/ml BSA, 1 mM dithiothreitol, 0.1 mM
Na3VO4 and 100 µM ATP. Each dilution was
pipetted into the wells and incubated for 1 h at 37°C. After the
incubation, 50 µl p-ATF-2 (Thr69/71) antibody (1:1,000, cat. no.
61584; Cell Signaling Technology, Inc.) was added into each well
for 4 h at room temperature and then TMB substrate (200 µl; cat.
no. P0209; Beyotime Institute of Biotechnology) was added in the
presence of peroxide-labeled conjugates for 10 min at room
temperature. The reaction was measured with an ELISA reader.
Statistical analysis
Statistical analysis was performed with SPSS 11.0
(SPSS, Inc.). All data are expressed as the means ± SD. Experiments
were performed independently in triplicate. One-way analysis of
variance followed by Tukey's post hoc test was used to determine
significant differences between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
GJ inhibits LPS-induced production of
cytokines in SW982 cells
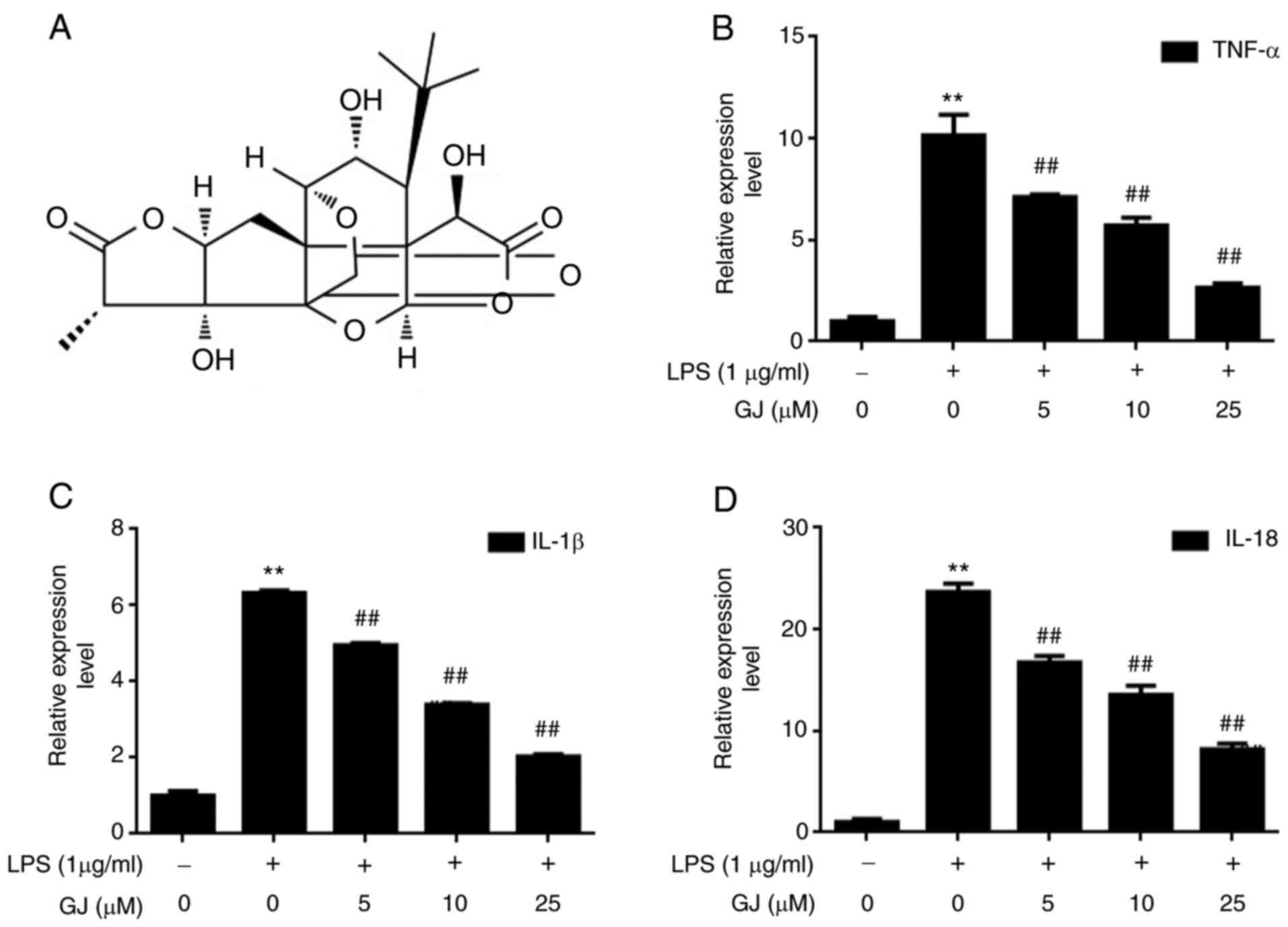
To determine the effects of GJ (Fig. 1A) on inflammatory cytokines induced
by LPS stimulation, the expression levels of TNF-α, IL-1β and IL-18
were assessed in SW982 cells. As revealed in Fig. 1B-D, the expression levels of TNF-α,
IL-1β and IL-18 were strongly induced by LPS stimulation, with an
approximate 5 to 10-fold increase. However, GJ pretreatment
significantly attenuated the effect of LPS, in a dose-dependent
manner. The results indicated that there is a robust effect of GJ
against inflammation in LPS-treated SW982 cells.
GJ inhibits LPS-induced activation of
NF-κB/NLRP3 signaling in SW982 cells
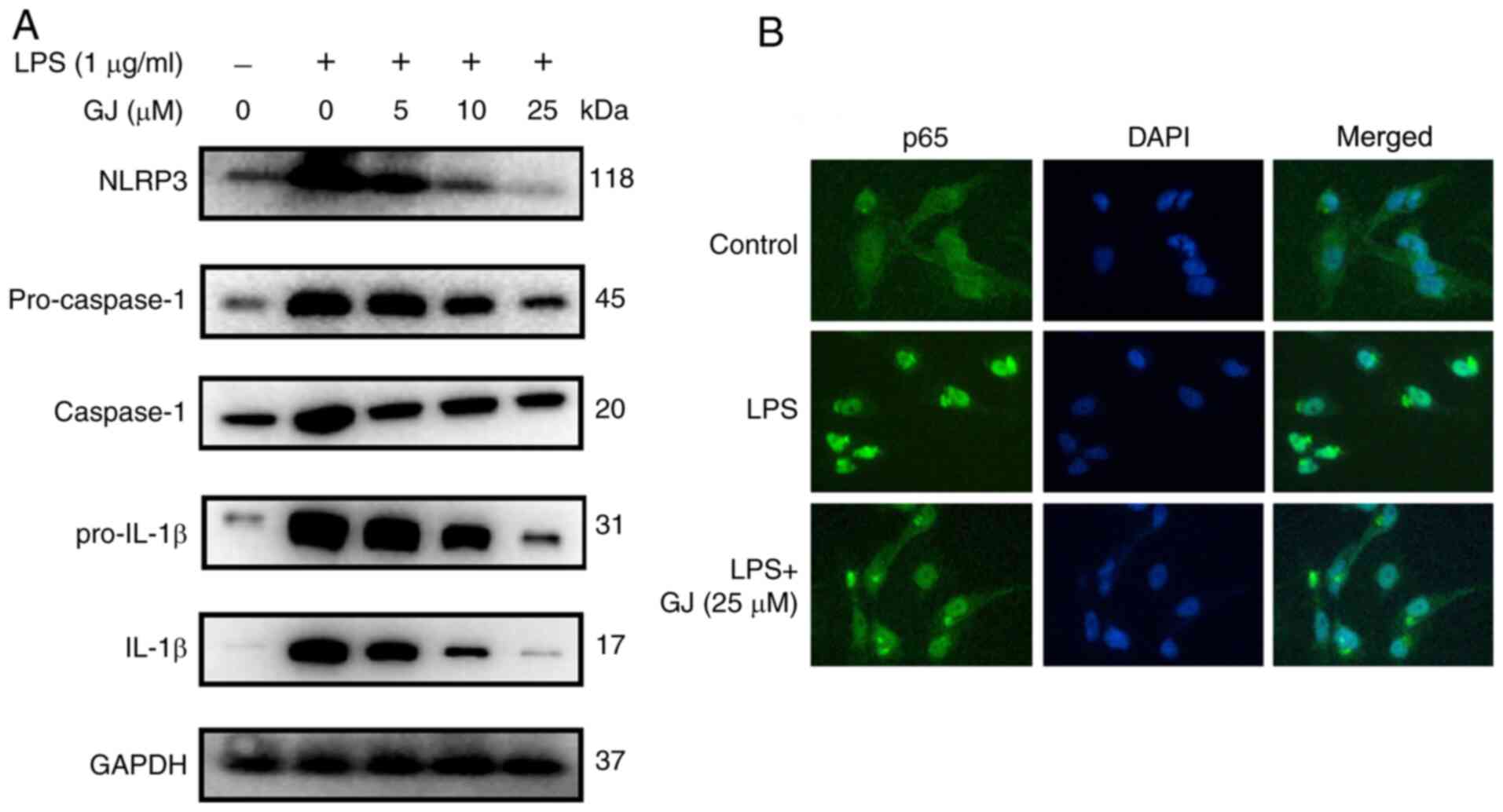
To determine whether the inhibitory effect of GJ on
cytokine production was associated with NF-κB/NLRP3 signaling
regulation, the expression levels of multiple factors that occur
during this signaling were assessed in SW982 cells. As revealed in
Fig. 2A and B, the upregulated
expression levels of NLRP3, pro-caspase-1, caspase-1, pro-IL-1β and
IL-1β as well as nucleus translocation of NF-κB were observed in
cells with LPS stimulation. However, GJ pretreatment notably
attenuated the effect of LPS, in a dose-dependent manner. The
results indicated that the anti-inflammatory effects of GJ were
also associated with the inactivation of the NF-κB/NLRP3 signaling
pathway in LPS-treated SW982 cells.
GJ inhibits LPS-induced activation of
PGE2/COX-2 and iNOS/NO in SW982 cells
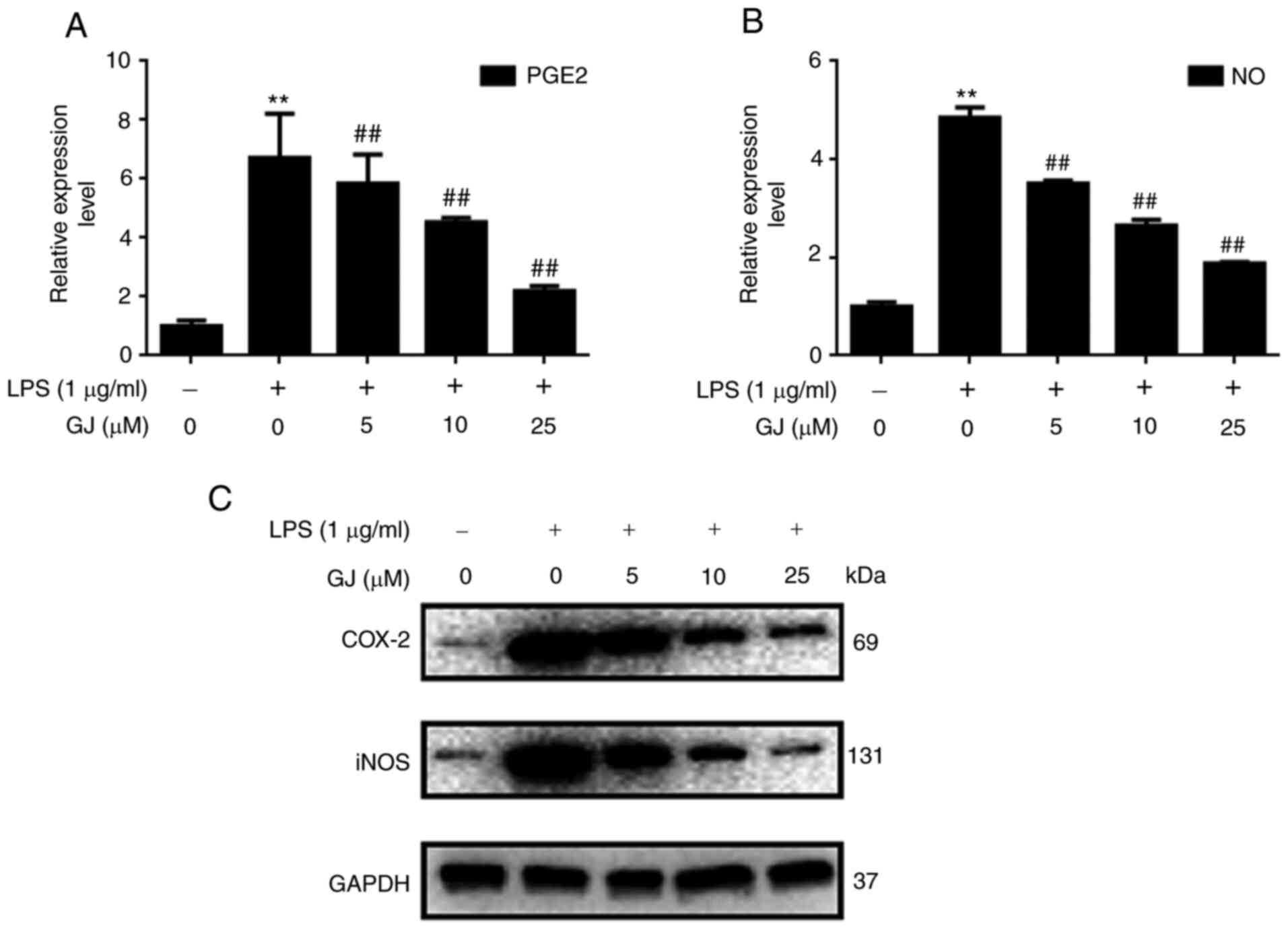
To determine whether GJ exerted inhibitory effects
on PGE2/COX-2 and iNOS/NO signaling, the expression levels of PGE2,
COX-2, iNOS and NO were assessed in SW982 cells. As revealed in
Fig. 3A-C, the expression levels of
PGE2, COX-2, iNOS and NO were notably upregulated in cells that
underwent LPS stimulation. However, GJ pretreatment notably
attenuated the effect of LPS, in a dose-dependent manner. The
results indicated that the anti-inflammatory effects of GJ were
associated with the suppression of PGE2/COX-2 and iNOS/NO
expression in LPS-treated SW982 cells.
GJ inhibits LPS-induced upregulation
and activation of p38 kinase in SW982 cells
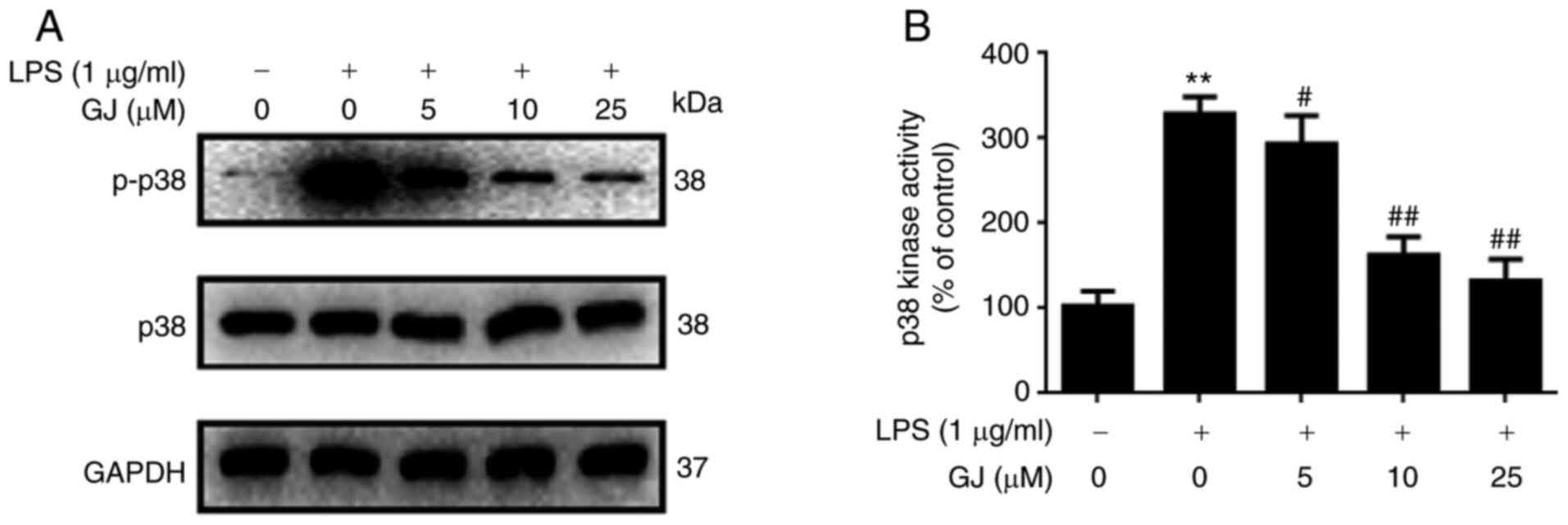
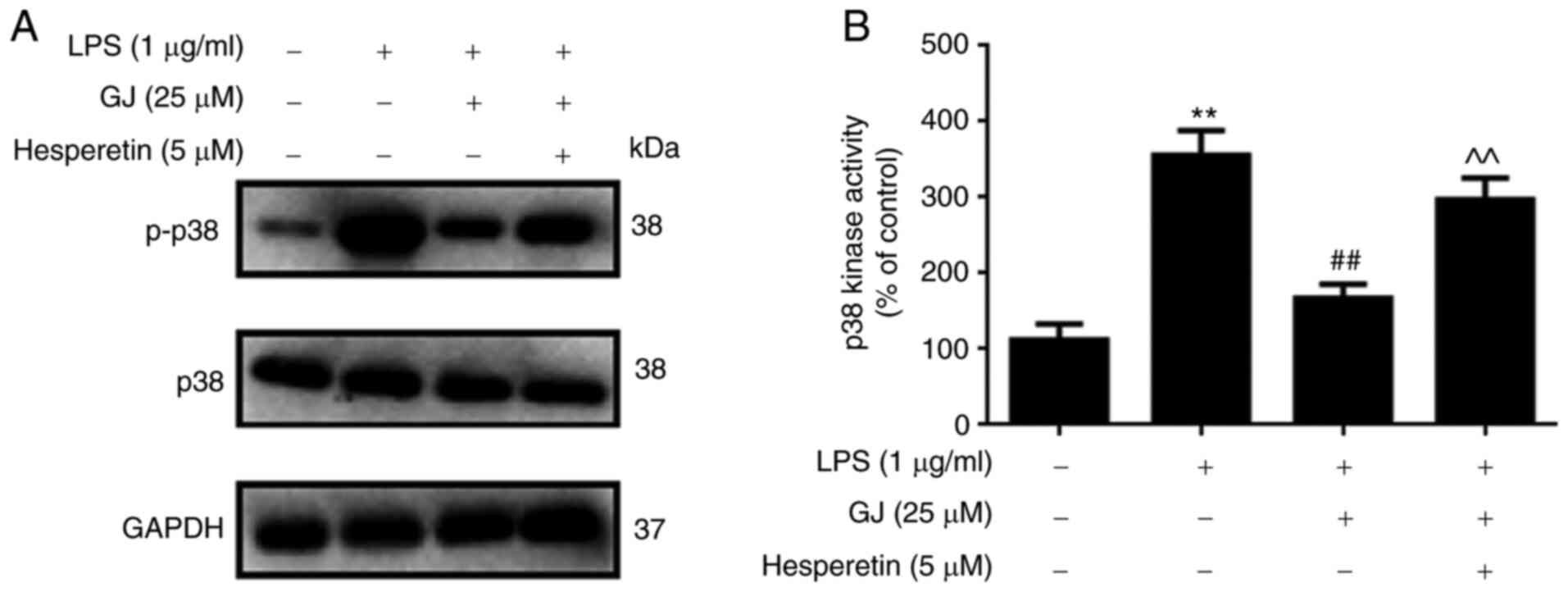
To determine whether p38 MAPK was involved in the
anti-inflammatory effects of GJ, the phosphorylation of p38 as well
as p38 kinase activity were assessed in SW982 cells. As revealed in
Fig. 4A and B, LPS stimulation
notably induced the phosphorylation of p38 as well as enhanced the
kinase activity of p38 in SW982 cells. However, GJ pretreatment
markedly attenuated these effects. The results indicated that p38
kinase may be involved in the anti-inflammatory effects of GJ in
LPS-treated SW982 cells.
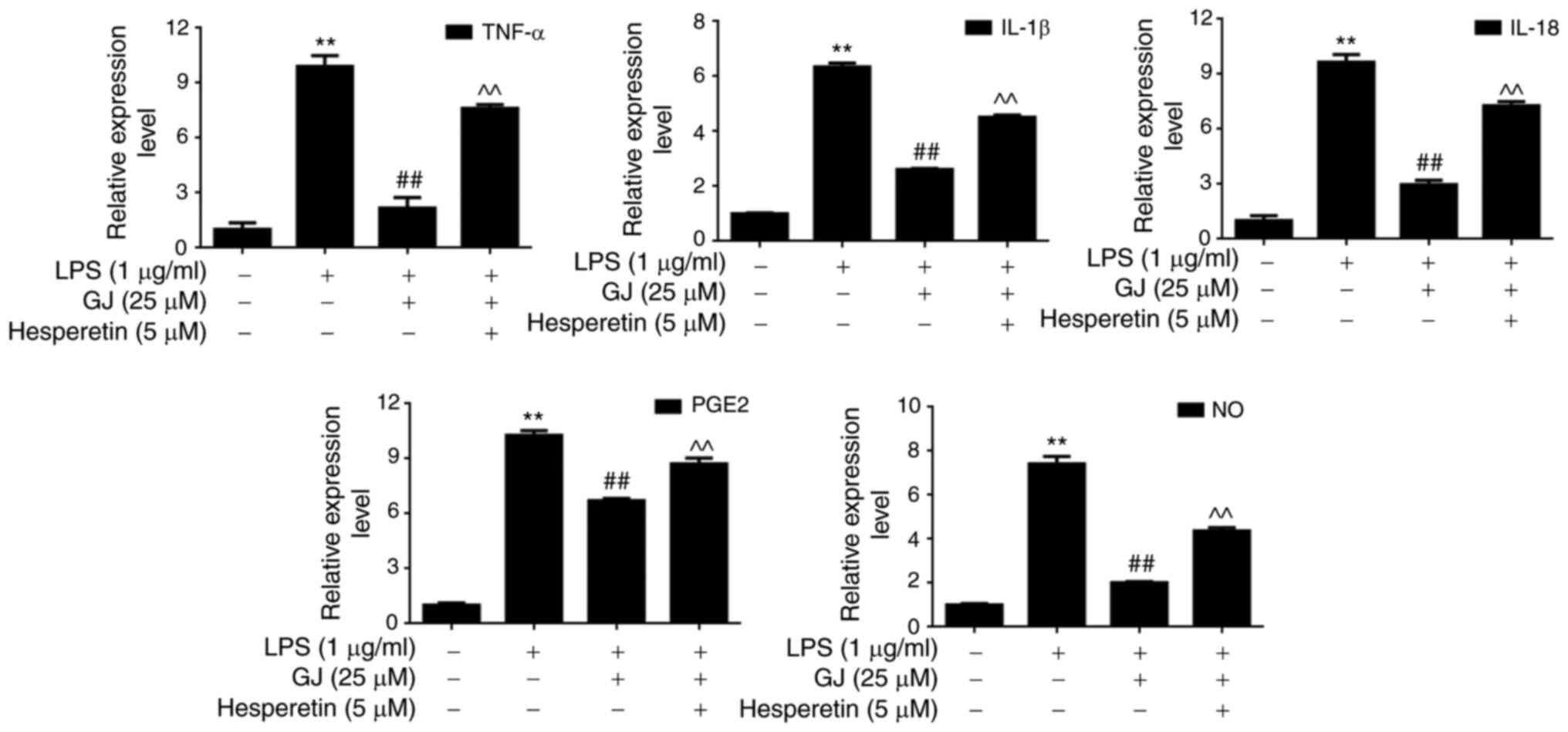
p38 activation attenuates the effects
of GJ on pro-inflammatory mediators in SW982 cells
To determine whether p38 MAPK contributed to the
anti-inflammatory effects of GJ, p38 was activated in SW982 cells.
As revealed in Fig. 5A and B, p38
activator (hesperetin) treatment induced the phosphorylation and
activation of p38 in cells treated with LPS combined with GJ
treatment, and this attenuated the anti-inflammatory effects of GJ
by induction of cytokines, PGE2 and NO production (Fig. 6). The results indicated that p38
kinase contributed to the anti-inflammatory effects of GJ in
LPS-treated SW982 cells.
Discussion
LPS, the main endotoxin component of gram-negative
bacterial cell walls, is known for its inducive effect that elicits
a strong immune response in the host (17). It stimulates cells to produce
pro-inflammatory factors, such as PGE2, free radicals and
cytokines, and thus leads to marked secondary inflammation in
tissues (18,19). Currently, LPS is used to establish
transient synovitis-osteoarthritis models of RA for therapeutic
research (20). Alsaleh et
al (21) constructed a model
using LPS-activated RA FLS to identify miRNAs that could play a
role in the anti-inflammatory response. Jin et al (22) used LPS-activated RA FLS as in
vitro model to identify the anti-inflammatory effect of
hyperoside against LPS-induced cell proliferation and migration,
cytokine production and MMP-9 secretion. Ginkgolide and bilobalide
are major trilactone constituents of Ginkgo biloba leaves and have
characteristic powerful anti-inflammatory properties (10). Although several studies examining
the anti-inflammatory effects of GA, GB and GC have been reported
(11–15); to date, there has been little
research exploring the relationship between GJ and RA inflammation,
and the exact mechanism of the anti-inflammatory effect of GJ
remains obscure. In the present study, using LPS-activated human
synovial cells SW982, it was revealed that GJ pretreatment could
attenuate LPS-induced production of pro-inflammatory mediators such
as cytokines, PGE2 and NO.
NO, produced from iNOS enzymes, is upregulated in
the process of inflammation and has pro-inflammatory and regulatory
effects. iNOS/NO signaling was previously revealed to be most
strongly activated in the synovial lining layer, subsynovium,
vascular smooth muscle and chondrocytes from patients with RA
(23). PGE2/COX-2 signaling also
contributed to LPS-induced FLS activation, which then resulted in a
release of pro-inflammatory cytokines such as TNF-α, IL-1β and
IL-18 (24). In addition,
activation of NF-κB/NLRP3 signaling also occurred following LPS
stimulation and contributed to a release of IL-1β and IL-18
(25). Activation of the three
important signaling pathways was significantly attenuated by GJ
pretreatment in a dose-dependent manner, indicating that GJ exerted
its anti-inflammatory effects mainly against
TNF-α/IL-1β/IL-18/NF-κB/NLRP3, PGE2/COX-2 and iNOS/NO signaling
pathways.
p38, also called cytokinin-specific binding protein
(CSBP), can be activated under inflammatory and stress stimuli, and
participates in autophagy, apoptosis and cell differentiation
(26). Accumulating evidence
suggests that p38 plays an important role in the process of
inflammation. p38 is involved in the production of proinflammatory
mediators such as TNF-α, IL-1β/IL-18, PGE2 and NO, as well as
NF-κB/NLRP3, COX-2 and iNOS (27).
The p38 signaling pathway has been strongly implicated in the
pathological process of RA, which contributes to the excessive
production of pro-inflammatory mediators in FLS, and then the
destruction of bone and cartilage. Thus, p38 signaling is
considered as a promising target for new drug development for RA
treatment (28). In the present
study, the phosphorylation of p38 occurred in FLS with LPS
stimulation, and it was attenuated by GJ pretreatment. In addition,
p38 activator (hesperetin) treatment reversed the protective effect
of GJ. The present data indicated that GJ exerted its effect by
targeting p38 signaling, and then inhibited the production of
pro-inflammatory mediators.
In conclusion, the results demonstrated the
protective effect and detailed mechanism of GJ on LPS-treated SW982
human synovial cells, which was achieved through the suppression of
the p38-dependent inflammatory signaling pathways
TNF-α/IL-1β/IL-18/NF-κB/NLRP3, PGE2/COX-2 and iNOS/NO induced by
LPS treatment. However, numerous questions remain to be answered.
For example, in vivo experiments are required to visualize
the effect of GJ against the inflammatory response. The present and
a future in vivo study may contribute to the pharmaceutical
potential of GJ and its derivatives in therapy for RA.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Young
Talent's Subsidy Project in Science and Education of the Department
of Public Health of Jiangsu Province (grant no. QNRC2016627), the
Six Talent Peaks Project of Jiangsu Province (grant no. WSW-047),
and the Six-one Scientific Research Project (grant no.
LGY2019087).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW, XZ and FZ designed the experiments. YZ, YC and
YL carried out the experiments. YZ, YC and KW analyzed the
experimental results. YZ and YC wrote the manuscript. All authors
read and approved the final manuscript. JW and XZ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pisetsky DS and Ward MM: Advances in the
treatment of inflammatory arthritis. Best Pract Res Clin Rheumatol.
26:251–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim Y, Oh HC, Park JW, Kim IS, Kim JY, Kim
KC, Chae DS, Jo WL and Song JH: Diagnosis and treatment of
inflammatory joint disease. Hip Pelvis. 29:211–222. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Firestein GS and McInnes IB:
Immunopathogenesis of rheumatoid arthritis. Immunity. 46:183–196.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tak PP and Breedveld FC: Current
perspectives on synovitis. Arthritis Res. 1:11–16. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshitomi H: Regulation of immune
responses and chronic inflammation by fibroblast-like synoviocytes.
Front Immunol. 10:1395. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zuo W, Yan F, Zhang B, Li J and Mei D:
Advances in the studies of Ginkgo biloba leaves extract on
aging-related diseases. Aging Dis. 8:812–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nash KM and Shah ZA: Current perspectives
on the beneficial role of Ginkgo biloba in neurological and
cerebrovascular Disorders. Integr Med Insights. 10:1–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Le Bars PL and Kastelan J: Efficacy and
safety of a Ginkgo biloba extract. Public Health Nutr. 3((4a)):
495–499. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao G, Lyu M, Wang Y, He S, Liu X, Ni J,
Li L, Fan G, Han J, Gao X, et al: Ginkgo flavonol glycosides or
Ginkgolides tend to differentially protect myocardial or cerebral
ischemia-reperfusion injury via regulation of TWEAK-Fn14 signaling
in heart and brain. Front Pharmacol. 10:735. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jaracz S, Malik S and Nakanishi K:
Isolation of ginkgolides A, B, C, J and bilobalide from G. biloba
extracts. Phytochemistry. 65:2897–2902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Wu Y, Yao X, Hao F, Yu C, Bao Y, Wu
Y, Song Z, Sun Y, Zheng L, et al: Ginkgolide A Ameliorates
LPS-induced inflammatory responses in vitro and in vivo. Int J Mol
Sci. 18:7942017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li C, Liu K, Liu S, Aerqin Q and Wu X:
Role of Ginkgolides in the inflammatory immune response of
neurological diseases: A review of current literatures. Front Syst
Neurosci. 14:452020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu F, Shi W, Zhou G, Yao H, Xu C, Xiao W,
Wu J and Wu X: Ginkgolide B functions as a determinant constituent
of Ginkgolides in alleviating lipopolysaccharide-induced lung
injury. Biomed Pharmacother. 81:71–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Shi Q, Nan W, Wang Y, Wang S,
Yang F and Li G: Ginkgolide B and bilobalide promote the growth and
increase β-catenin expression in hair follicle dermal papilla cells
of American minks. Biofactors. 45:950–958. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vitolo O, Gong B, Cao Z, Ishii H, Jaracz
S, Nakanishi K, Arancio O, Dzyuba SV, Lefort R and Shelanski M:
Protection against beta-amyloid induced abnormal synaptic function
and cell death by Ginkgolide J. Neurobiol Aging. 30:257–265. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshino S and Ohsawa M: The role of
lipopolysaccharide injected systemically in the reactivation of
collagen-induced arthritis in mice. Br J Pharmacol. 129:1309–1314.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bertani B and Ruiz N: Function and
Biogenesis of Lipopolysaccharides. EcoSal Plus.
8:10.1128/ecosalplus.ESP.0001-2018. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang CF, Chau YP, Kung HN and Lu KS: The
lipopolysaccharide-induced pro-inflammatory response in RAW264.7
cells is attenuated by an unsaturated fatty acid-bovine serum
albumin complex and enhanced by a saturated fatty acid-bovine serum
albumin complex. Inflamm Res. 61:151–160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yücel G, Zhao Z, El-Battrawy I, Lan H,
Lang S, Li X, Buljubasic F, Zimmermann WH, Cyganek L, Utikal J, et
al: Lipopolysaccharides induced inflammatory responses and
electrophysiological dysfunctions in human-induced pluripotent stem
cell derived cardiomyocytes. Sci Rep. 7:2935. 2017. View Article : Google Scholar
|
|
20
|
Cope PJ, Ourradi K, Li Y and Sharif M:
Models of osteoarthritis: The good, the bad and the promising.
Osteoarthritis Cartilage. 27:230–239. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alsaleh G, Suffert G, Semaan N, Juncker T,
Frenzel L, Gottenberg JE, Sibilia J, Pfeffer S and Wachsmann D:
Bruton's tyrosine kinase is involved in miR-346-related regulation
of IL-18 release by lipopolysaccharide-activated rheumatoid
fibroblast-like synoviocytes. J Immunol. 182:5088–5097. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin XN, Yan EZ, Wang HM, Sui HJ, Liu Z,
Gao W and Jin Y: Hyperoside exerts anti-inflammatory and
anti-arthritic effects in LPS-stimulated human fibroblast-like
synoviocytes in vitro and in mice with collagen-induced arthritis.
Acta Pharmacol Sin. 37:674–686. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grabowski PS, Wright PK, Van't Hof RJ,
Helfrich MH, Ohshima H and Ralston SH: Immunolocalization of
inducible nitric oxide synthase in synovium and cartilage in
rheumatoid arthritis and osteoarthritis. Br J Rheumatol.
36:651–655. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawashima M, Ogura N, Akutsu M, Ito K and
Kondoh T: The anti-inflammatory effect of cyclooxygenase inhibitors
in fibroblast-like synoviocytes from the human temporomandibular
joint results from the suppression of PGE2 production. J Oral
Pathol Med. 42:499–506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu Q, Gao Y, Zhao H, Wang Z and Wang J:
Galangin protects human rheumatoid arthritis fibroblast like
synoviocytes via suppression of the NF κB/NLRP3 pathway. Mol Med
Rep. 18:3619–3624. 2018.PubMed/NCBI
|
|
26
|
Herlaar E and Brown Z: p38 MAPK signalling
cascades in inflammatory disease. Mol Med Today. 5:439–447. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schieven GL: The p38alpha kinase plays a
central role in inflammation. Curr Top Med Chem. 9:1038–1048. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yong HY, Koh MS and Moon A: The p38 MAPK
inhibitors for the treatment of inflammatory diseases and cancer.
Expert Opin Investig Drugs. 18:1893–1905. 2009. View Article : Google Scholar : PubMed/NCBI
|