Introduction
Bladder cancer (BC) is one of the most common
malignant tumors of the urinary system and is characterized by high
morbidity and mortality rates (1).
A number of factors can induce BC, including smoking, environmental
exposure, exposure to carcinogens and genetic factors (1,2).
Surgical resection, chemotherapy and radiotherapy are the
conventional treatments for BC (3).
Initial tumor resection combined with adjuvant therapy increases
the 5-year survival rate (4);
however, BC has a high recurrence rate after surgery. It was
previously demonstrated that non-muscle invasive BC has tumor
recurrence and disease progression rates of up to 80 and 45%,
respectively (5). Therefore, it is
necessary to investigate the molecular mechanisms involved in the
development of BC in order to find novel diagnostic markers and
therapeutic targets.
Autophagy is a conservative self-degradation process
that plays an important role in the development and treatment of
cancers (6). It has been reported
that the induction of autophagy inhibited breast cancer progression
(7). MicroRNAs (miRs) are
non-coding single-stranded RNA molecules that regulate gene
expression by binding to the 3′-untranslated region (UTR) of
downstream targets (8). Due to this
characteristic, miRs have multiple functions, including regulating
cell autophagy (9). For example,
miR-16-5p, which acts as a tumor suppressor in BC, has been
reported to induce autophagy of cervical carcinoma cells (10,11).
It has also been observed that autophagy significantly increased in
non-small cell lung carcinoma cells transfected with miR-16-5p
mimics (12). These findings
indicate that miR-16-5p exerts an important regulatory effect on
the autophagy of cancer cells. However, whether miR-16-5p regulates
autophagy in BC cells requires further investigation.
It is predicted that caspase recruitment domain
family member 10 (BIMP1) may be a downstream target of miR-16-5p.
BIMP1, also known as caspase recruitment domain 10, is a member of
the CARMA family (13). BIMP1 has
been reported to be upregulated in BC cells and BIMP1 silencing can
inhibit activation of the NF-κB signaling pathway (14). The NF-κB signaling pathway is
activated in numerous types of cancer, including colorectal cancer,
prostate cancer and BC, and exerts a tumorigenic effect (15–17).
Moreover, blocking the NF-κB signaling pathway has been found to
induce autophagy (18–20). Therefore, the aim of the present
study was to investigate whether the miR-16-5p/BIMP1/NF-κB axis
exerts antitumor effects by regulating autophagy in BC.
Materials and methods
Cell lines
T24 and 5637 cells were purchased from Shanghai
Zhong Qiao Xin Zhou Biotechnology Co., Ltd. RPMI-1640 medium
(MilliporeSigma) containing 10% fetal bovine serum (Sangon Biotech
Co., Ltd.) was used for cell culture in an incubator at 37°C with
5% CO2.
Cell transfection
Cells were cultured to a density of ~90% and washed
once with PBS. The supernatant was discarded and 0.25% trypsin was
added to digest the cells. When the cells became round, the
complete medium (RPMI-1640 medium) was added to terminate the
reaction. Cells were then seeded in a 6-well plate
(2×105 cells per well) and placed in an incubator at
37°C in 5% CO2. After 24 h, the transfection experiments
were performed. The 5637 cells were transfected with miR-16-5p
inhibitor (50 pmol per well) or its negative control (NC; 50 pmol
per well) for 24 h. T24 cells were transfected with miR-16-5p
mimics (50 pmol per well) or its NC (50 pmol per well) for 24 h.
The transfections were performed with 6 µl
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.).
After transfection for 6 h, the T24 cells were
treated with the autophagy inhibitor 3-methyladenine (3-MA; 2.5 mM)
for 48 h. The 5637 cells were incubated with pyrrolidine
dithiocarbamate (PDTC; 20 µM) for 48 h at 37°C. For
co-transfection, miR-16-5p inhibitor (50 pmol per well) and BIMP1
small interfering (si)RNA (50 pmol per well) or its NC (50 pmol per
well) was co-transfected into 5637 cells for 24 h with
Lipofectamine 2000, according to the manufacturer's instructions.
All of the transfections were performed at room temperature. All
cells were transfected and dosed up for the appropriate duration,
and follow-up experiments were conducted immediately. The BIMP1 and
NC siRNA, miR-16-5p mimics, mimics NC and miR-16-5p inhibitor and
inhibitor NC were purchased from JTS Scientific Ltd. The sequences
used for the present study are presented in Table I.
 | Table I.Sequences used for transfection. |
Table I.
Sequences used for transfection.
| Sequence type | Sequence
(5′-3′) |
|---|
| BIMP1 siRNA | Forward:
GGAUGAGAACUACAUGAUCTT |
|
| Reverse:
GAUCAUGUAGUUCUCAUCCTT |
| siRNA NC | Forward:
UUCUCCGAACGUGUCACGUTT |
|
| Reverse:
ACGUGACACGUUCGGAGAATT |
| miR-16-5p
mimics | Forward:
UAGCAGCACGUAAAUAUUGGCG |
|
| Reverse:
CCAAUAUUUACGUGCUGCUAUU |
| Mimics NC | Forward:
UUCUCCGAACGUGUCACGUTT |
|
| Reverse:
ACGUGACACGUUCGGAGAATT |
| miR-16-5p
inhibitor |
CGCCAAUAUUUACGUGCUGCUA |
| Inhibitor NC |
UUGUACUACACAAAAGUACUG |
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from BC cells with TriPure
Isolation Reagent (BioTeke Corporation) and the concentration was
determined using a NanoDrop 2000 UV spectrophotometer (Thermo
Fisher Scientific, Inc.). Next, cDNA was obtained via reverse
transcription using Super M-MLV Reverse Transcriptase (BioTeke
Corporation), dNTPs (Beijing Solarbio Science & Technology Co.,
Ltd.) and RNase inhibitor (BioTeke Corporation). Finally, using the
cDNA template, primers, SYBR Green (Millipore Sigma) and 2X Power
Taq PCR MasterMix (BioTeke Corporation), qPCR was performed and the
relative mRNA expression level was calculated using the
2−ΔΔCq method (21). The
thermocycling conditions for qPCR were as follows: For miRNA, 94°C
for 4 min, followed by 40 cycles of 94°C for 15 sec, 60°C for 20
sec and 72°C for 15 sec; and for mRNA, 94°C for 5 min, followed by
40 cycles of 94°C for 15 sec, 60°C for 25 sec and 72°C for 30 sec.
miRNA and mRNA expression levels were normalized to ribosomal 5S
RNA and β-actin, respectively. The primers used for qPCR are
presented in Table II.
 | Table II.Primers used for qPCR. |
Table II.
Primers used for qPCR.
|
| Primers
(5′-3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| miR-16-5p |
TAGCAGCACGTAAATATTGGCG |
GCAGGGTCCGAGGTATTC |
| 5S rRNA |
GATCTCGGAAGCTAAGCAGG |
TGGTGCAGGGTCCGAGGTAT |
| BIMP1 |
GGTGCCGAGCCCTTCTACATT |
TCCAGGTCCCGCAGAGTGAG |
| β-actin |
CTTAGTTGCGTTACACCCTTTCTTG |
CTGTCACCTTCACCGTTCCAGTTT |
Cell proliferation assay
BC cell viability was analyzed via Cell Counting
Kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology). Briefly,
cells were seeded in a 96-well plate at a density of
4×103 per well. After transfection and treatments, the
supernatant was discarded and complete medium (100 µl) was added to
each well. Then, 10 µl CCK-8 was added to each well and cells were
incubated at 37°C for 1 h. Finally, the optical density (OD) value
at 450 nm was calculated using a microplate reader (BioTek
Instruments, Inc.).
Cell apoptosis assay
Cell apoptosis was detected by flow cytometry.
Briefly, after transfection and treatments, cells were washed once
with PBS and then centrifuged (300 × g) for 5 min at room
temperature and the supernatant was discarded. After washing twice
with PBS, cells were stained with Annexin V-FITC (5 µl) and
propidium iodide (5 µl). After incubation at room temperature in
the dark for 15 min, the apoptosis rate was detected by flow
cytometry (NovoCyte; ACEA Bioscience, Inc.) and analyzed by
NovoExpress (version 1.2.5; ACEA Biosciences, Inc.).
Western blotting
Total proteins were extracted using Western and IP
cell lysate (cat. no. P0013; Beyotime Institute of Biotechnology)
mixed with PMSF (cat. no. ST506; Beyotime Institute of
Biotechnology) and the total protein concentration was determined
using a BCA protein assay kit (Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. Proteins were
separated via SDS-PAGE (20–40 µg/lane; 8, 10 and 14%). After
electrophoresis, the separated proteins were transferred onto the
PVDF membranes and blocked with 5% skimmed milk for 1 h at room
temperature. The membranes were subsequently incubated with primary
antibodies overnight at 4°C, followed by incubation with
horseradish peroxidase (HRP)-conjugated secondary antibodies for 45
min at 37°C. Finally, the proteins were visualized with ECL
chemiluminescent reagent (Beyotime Institute of Biotechnology) and
the OD of the target proteins was analyzed using a gel image
processing system (Gel-Pro-Analyzer 4.0; Media Cybernetics, Inc.).
The following primary antibodies (1:1,000) used for western
blotting were purchased from ABclonal Biotech Co., Ltd.:
Microtubule-associated proteins 1A/1B light chain 3B (LC3; cat. no.
A19665), beclin 1 (cat. no. A7353), p62 (cat. no. A19700), IκBα
(cat. no. A1187), p-IκBα (ser32; cat. no. AP0707), NF-κB (cat. no.
A19653) and BIMP1 (cat. no. A7368). Histone H3 primary antibody
(1:2,000) was purchased from Abgent Biotech Co., Ltd. (cat. no.
AM8433) and β-actin primary antibody (1:1,000) was obtained from
Santa Cruz Biotechnology, Inc. (cat. no. sc-47778). The following
secondary antibodies (1:5,000) were purchased from Beyotime
Institute of Biotechnology: Goat anti-rabbit IgG (cat. no. A0208)
and goat anti-mouse IgG (cat. no. A0216).
mCherry-green fluorescent protein
(GFP)-LC3 puncta formation assay
Cells were seeded in 6-well plates at a density of
2×105 cells per well. The mCherry-GFP-LC3 plasmids (1 µg
per well) and miR-16-5p mimics, miR-16-5p inhibitor or its NC were
co-transfected into BC cells for 48 h at room temperature. The
co-transfection was performed with Lipofectamine 2000. Finally, the
puncta were observed under a fluorescence microscope
(magnification, ×400; Olympus Corporation). Yellow and red dots
represent autophagosomes and autolysosomes, respectively.
Electrophoretic mobility shift
assay
Nuclear proteins were extracted from BC cells using
the Nuclear Protein Extraction kit (Beyotime Institute of
Biotechnology). The protein lysate was obtained as previously
described for western blotting. Following centrifugation,
cytoplasmic protein extraction reagent (200 µl/20 µl cell pellet;
Beyotime Institute of Biotechnology) containing PMSF was added to
the pellet. The cell pellet was vigorously vortexed on the highest
setting for 5 sec and incubated on ice for 10–15 min. Subsequently,
cytoplasmic protein extraction reagent (10 µl; Beyotime Institute
of Biotechnology) was added and vortexed for 5 sec on the highest
setting and centrifuged for 5 min (4°C) at 12,000 × g. The pellet
was collected again and treated with nuclear protein extraction
reagent (50 µl; Beyotime Institute of Biotechnology) containing
PMSF. The tubes containing the pellet were vortexed on the highest
setting for 15–30 sec, every 1–2 min for a total duration of 30
min. Following centrifugation for 5 min (4°C) at 12,000 × g, the
supernatant was obtained, which only contained nuclear protein. The
concentration was detected using a BCA Protein assay kit (Beyotime
Institute of Biotechnology). After nuclear proteins were incubated
with the biotin-labeled probe for 20 min at room temperature,
electrophoresis was performed using 6.5% polyacrylamide gels. After
separation on polyacrylamide gels, nuclear proteins were
transferred onto nylon membranes and the membranes were
cross-linked under UV light for 30 min. After incubation in the
streptavidin-HRP (Viagene Biotech) reaction solution for 20 min at
room temperature, the membranes were visualized with the ECL
chemiluminescent reagent. Finally, the OD value was calculated.
Dual-luciferase reporter assay
The binding sites between miR-16-5p and BIMP1 were
determined using TargetScanHuman (version 7.2; http://www.targetscan.org/vert_72/). miR-16-5p
was searched and multiple genes that miR-16-5p may target were
obtained. UTRs between BIMP1 and miR-16-5p were also searched to
obtain the target binding sequence between miR-16-5p and BIMP1. The
luciferase reporter assay was performed using T24 cells. Briefly,
T24 cells were co-transfected with mutant (MUT) or wild-type (WT)
plasmid (1.25 µg per well) containing the 3′-UTR of BIMP1 and
miR-16-5p mimics (15 pmol per well) or its NC (15 pmol per well),
respectively. After 48 h, the cells were harvested and luciferase
activity was detected using a Luciferase Detection kit (Promega
Corporation) according to the manufacturer's protocols. Relative
luciferase activities were evaluated through the ratio of firefly
luciferase to Renilla luciferase.
Xenograft tumor model
The present study was approved by The China Medical
University Laboratory Animal Welfare and Ethical Committee
(approval no. KT2020037) and animal experiments were performed in
accordance with the Guidelines for the Care and Use of Laboratory
Animals (22). Twelve male BALB/c
nude mice (age, 6 weeks old; weight, 18±2 g) were purchased from
Huafukang Biotechnology Co., Ltd. and housed under the following
conditions: 12 h light/dark cycle, 45–55% humidity and a
temperature of 24±1°C. All mice had free access to food and water.
The mice (n=12) were randomly divided into the following two
groups: i) Mimics NC group (n=6), mice were injected subcutaneously
(right armpit) with T24 cells (1×107) transfected with
mimics NC; and ii) miR-16-5p mimics group (n=6), mice were injected
subcutaneously with T24 cells (1×107) transfected with
miR-16-5p mimics. The tumor volume was measured every 3 days from
day 6. The maximum xenograft tumor size was 334.75 mm3.
On day 24, mice were euthanized by an intraperitoneal injection of
sodium pentobarbital (150 mg/kg), the tumor weight was recorded and
tumor tissue samples were preserved in 4% paraformaldehyde in an
ultra-low temperature refrigerator at −70°C for subsequent
experiments.
Immunofluorescence staining
Tumor tissue samples were fixed using 4%
paraformaldehyde for 48 h at room temperature, embedded in paraffin
and cut into 5-µm sections. The sections were first blocked with
normal goat serum (stock solution, Beijing Solarbio Science &
Technology Co., Ltd.) for 15 min at room temperature. The tumor
sections were treated with the primary antibody, LC3 obtained from
Santa Cruz Biotechnology, Inc. (1:50; cat. no. sc-376404) at 4°C
overnight and then incubated with the secondary antibody
Cy3-labeled goat anti-mouse IgG obtained from Beyotime Institute of
Biotechnology (1:200; cat. no. A0521) at room temperature for 1 h.
Following DAPI staining, the cover slips were mounted and
visualized using a fluorescence microscope (magnification, ×400;
Olympus Corporation).
Statistical analysis
An unpaired t-test was used to compare the
differences between two groups. Differences among ≥3 groups were
analyzed by one-way ANOVA with Tukey's multiple comparisons test.
Data are expressed as the means ± SD. Data analysis was performed
using GraphPad Prism 8.0 (GraphPad Software, Inc.) and P<0.05
was considered to indicate a statistically significant
difference.
Results
miR-16-5p promotes autophagy in BC
cells
In order to investigate the role of miR-16-5p in the
autophagy of BC cells, the expression of miR-16-5p in 5637 and T24
cells was determined. It was found that the expression of miR-16-5p
in T24 cells was lower than that in 5637 cells (Fig. 1A). miR-16-5p was overexpressed in
T24 cells via transfection with miR-16-5p mimics, whereas miR-16-5p
expression was knocked down in 5637 cells via transfection with
miR-16-5p inhibitor (Fig. 1B). As
presented in Fig. 1C, miR-16-5p
overexpression significantly decreased the viability of BC cells,
whereas miR-16-5p knockdown significantly increased cell viability
when compared with the corresponding control group. As shown in
Fig. 1D, miR-16-5p overexpression
led to an increase in free yellow puncta and red puncta (indicating
autolysosomes) and the merged colors were more towards red,
indicating that miR-16-5p overexpression increased the formation of
autophagosomes and autolysosomes, and that autophagic flux was
promoted. However, no apparent yellow or red spots were observed in
the miR-16-5p inhibitor group (Fig.
1D). Furthermore, the expression levels of autophagy-related
proteins were detected via western blotting. The results
demonstrated that the LC3-II/I ratio and beclin 1 protein levels in
the miR-16-5p mimics group were significantly higher than those in
the mimics NC group, whereas miR-16-5p knockdown notably decreased
their levels as compared with the inhibitor NC (Fig. 1E). By contrast, the protein
expression of p62 was notably decreased by miR-16-5p mimics,
whereas its expression was significantly increased by transfection
with miR-16-5p inhibitor as compared with the corresponding control
group (Fig. 1E). Therefore, these
results indicated that miR-16-5p induced autophagy and this may be
achieved by increasing autophagic flux and the formation of
autophagosomes in BC cells.
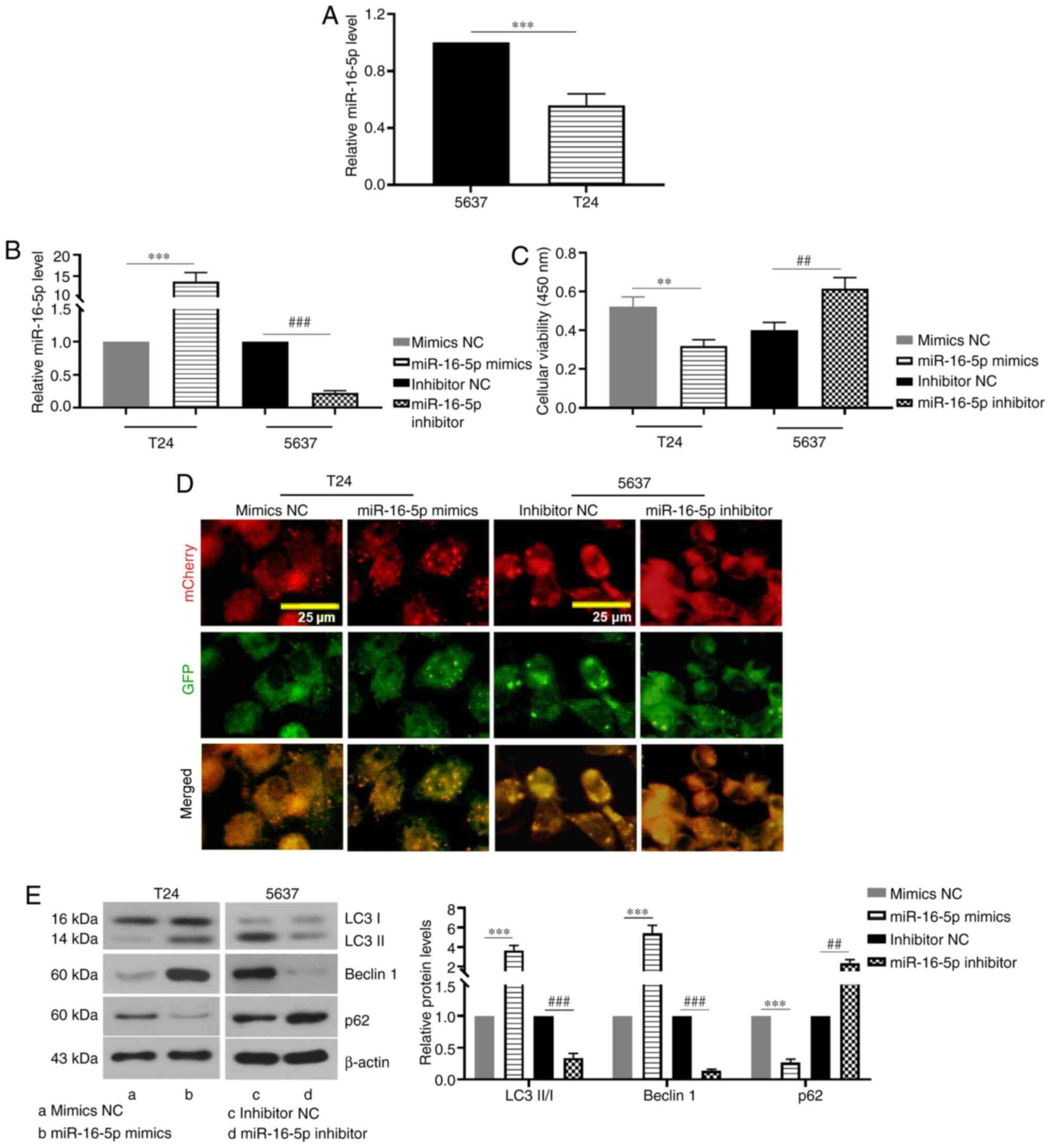 | Figure 1.miR-16-5p promotes autophagy in
bladder cancer cells. (A) Relative miR-16-5p expression levels in
5637 and T24 cells. (B) Relative miR-16-5p expression levels after
5637 and T24 cells were transfected with miR-16-5p mimics,
inhibitor or the NC. (C) Viability of 5637 and T24 cells after
transfection with miR-16-5p mimics, inhibitor or the NC. (D)
Representative fluorescence images of autophagosomes and
autolysosomes in 5637 and T24 cells after co-transfection with
mCherry-GFP-LC3 plasmid and miR-16-5p mimics, inhibitor or the NC
(magnification, ×400). (E) Relative LC3-II/I, beclin 1 and p62
protein expression levels in 5637 and T24 cells transfected with
miR-16-5p mimics, inhibitor or the NC (n=3). Data are presented as
the means ± SD. **P<0.01, ***P<0.001, ##P<0.01,
###P<0.001. miR, microRNA; NC, negative control; GFP,
green fluorescent protein; LC3, microtubule-associated proteins
1A/1B light chain 3B. |
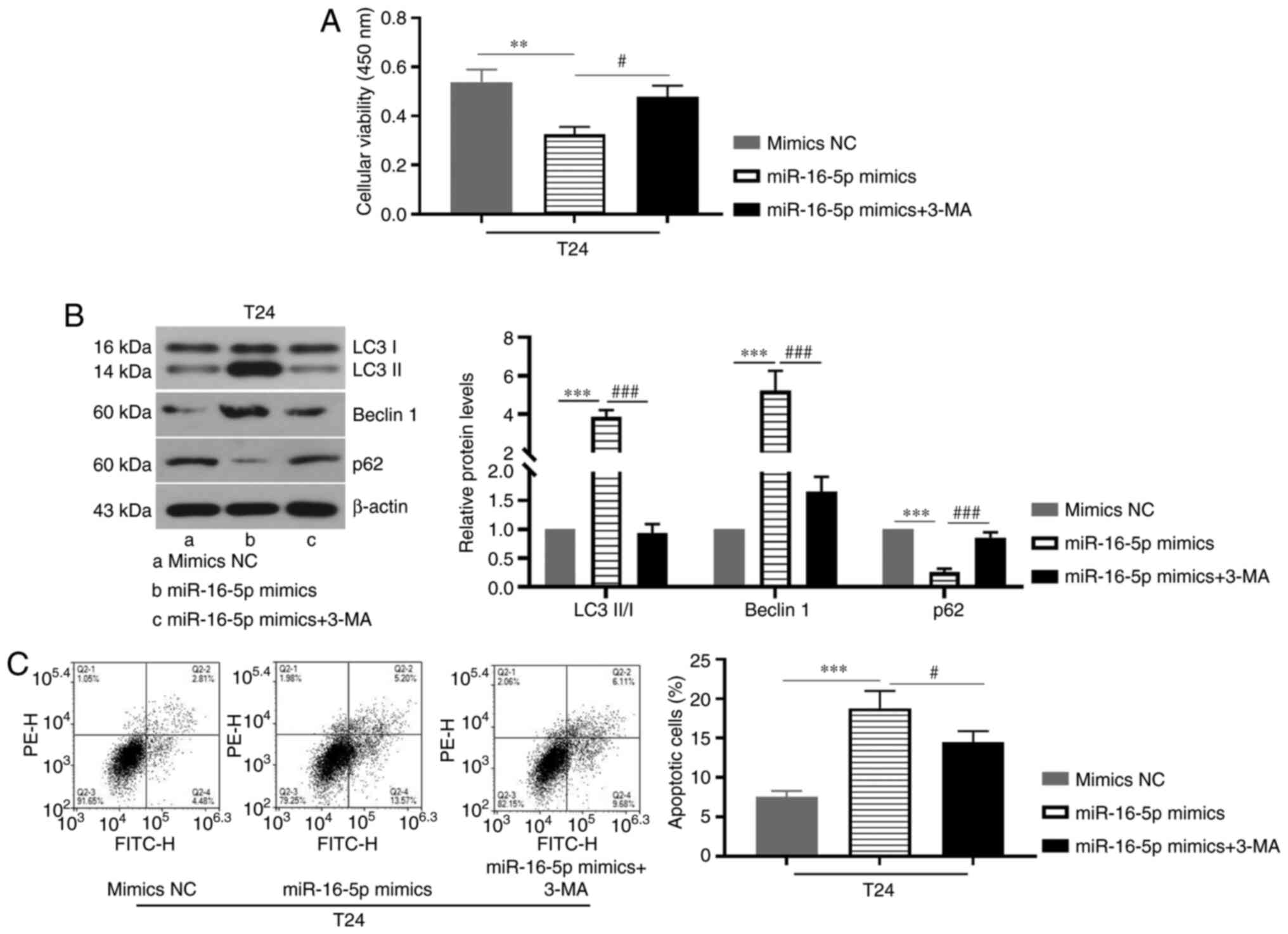
Inhibition of autophagy attenuates the
promoting effect of miR-16-5p overexpression on apoptosis of BC
cells
Subsequently, whether there is an association
between autophagy and apoptosis in BC was investigated. As
presented in Fig. 2A, miR-16-5p
overexpression significantly decreased cell viability, whereas 3-MA
treatment increased cell viability as compared with the miR-16-5p
mimics group. Furthermore, miR-16-5p overexpression significantly
increased LC3-II/I ratio and beclin 1 protein expression levels,
whereas it decreased p62 expression, which was reversed by
treatment with 3-MA (Fig. 2B).
Notably, 3-MA treatment also decreased miR-16-5p
overexpression-induced apoptosis in BC cells (Fig. 2C). Overall, these results suggested
that autophagy and apoptosis are interrelated in the progression of
BC. Specifically, the inhibition of autophagy could weaken the
promoting effect of miR-16-5p overexpression on apoptosis.
miR-16-5p overexpression inhibits the
BIMP1/NF-κB signaling pathway by directly targeting BIMP1 in BC
cells
According to the prediction of TargetScanHuman,
miR-16-5p may have binding sites with BIMP1 (Fig. 3A). In addition, the targeting
relationship between miR-16-5p and BIMP1 was verified using a
dual-luciferase reporter assay. The results showed that the
luciferase activity in the WT-BIMP1 + miR-16-5p mimics group was
significantly lower than that of the other three groups (Fig. 3A). In addition, the mRNA and protein
expression levels of BIMP1 were significantly reduced by miR-16-5p
mimics, whereas miR-16-5p knockdown increased BIMP1 expression
(Fig. 3B and C). Furthermore, the
overexpression of miR-16-5p increased the protein expression of
IκBα and decreased the expression of phosphorylated (p)-IκBα.
However, miR-16-5p knockdown decreased IκBα protein expression and
increased the expression of p-IκBα (Fig. 3D). When miR-16-5p was overexpressed,
NF-κB protein expression in the cytoplasm was significantly
increased, whereas NF-κB protein expression in the cell nucleus and
NF-κB activity was decreased (Fig. 3D
and E). However, miR-16-5p knockdown had the opposite effects
on the protein expression and activity of NF-κB (Fig. 3D and E). Taken together, the results
indicated that miR-16-5p negatively regulated the BIMP1/NF-κB
signaling pathway via binding to BIMP1 in BC cells.
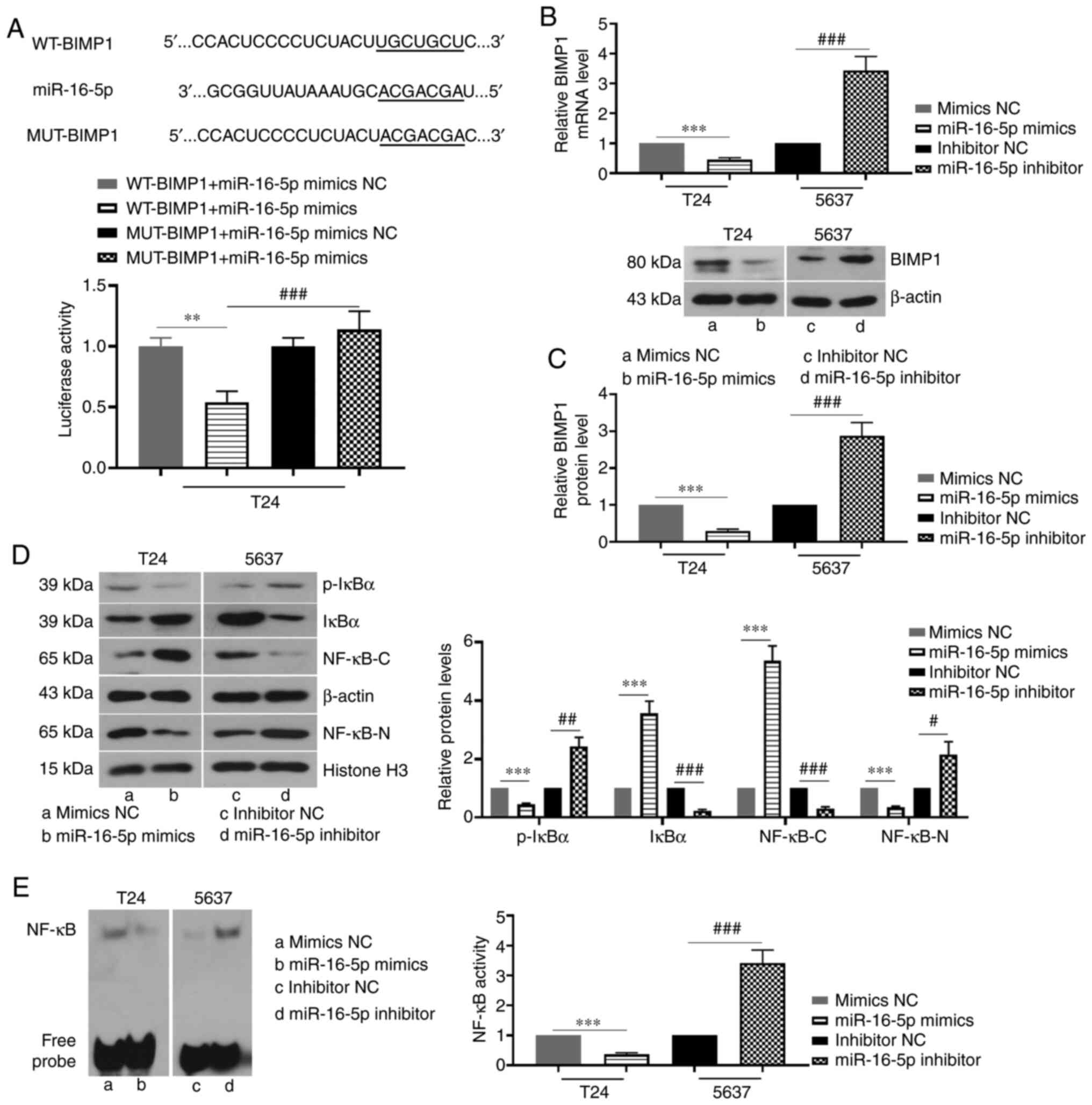 | Figure 3.miR-16-5p overexpression inhibits the
BIMP1/NF-κB signaling pathway by directly targeting BIMP1 in
bladder cancer cells. (A) Binding sites of miR-16-5p to the
3′-untranslated region of BIMP1 as predicted by TargetScanHuman
7.2, and the targeting relationship between them was verified by a
dual-luciferase reporter assay in T24 cells. (B and C) Relative
mRNA and protein expression levels of BIMP1 in 5637 and T24 cells
following transfection with miR-16-5p mimics, inhibitor or the NC.
(D) Relative protein expression levels of IκBα/p-IκBα (ser32) and
NF-κB in the cytoplasm (NF-κB-C) and nucleus (NF-κB-N) following
transfection with miR-16-5p mimics, inhibitor or the NC. (E) NF-κB
activity in 5637 and T24 cells after transfection with miR-16-5p
mimics, inhibitor or the NC, as detected by electrophoretic
mobility shift assay (n=3). Data are presented as the means ± SD.
**P<0.01, ***P<0.001, #P<0.05,
##P<0.01, ###P<0.001. miR, microRNA;
NC, negative control; p-, phosphorylated; WT, wild-type; MUT,
mutant; BIMP1, caspase recruitment domain family member 10. |
miR-16-5p promotes autophagy by
blocking the BIMP1/NF-κB signaling pathway in BC cells
After transfection with the miR-16-5p inhibitor,
5,637 cells were treated with small interfering (si)-BIMP1, the NC
or the NF-κB pathway inhibitor PDTC to investigate whether
miR-16-5p regulates autophagy in BC cells via the BIMP1/NF-κB
signaling pathway. The BIMP1 knockdown efficiency in 5637 cells was
first verified. The results showed that BIMP1 siRNA transfection
significantly decreased the BIMP1 protein level (Fig. 4A). As presented in Fig. 4B, miR-16-5p knockdown significantly
increased cell viability, whereas BIMP1 knockdown or PDTC treatment
reversed this effect. Furthermore, PDTC treatment increased the
LC3-II/I ratio and the protein expression levels of and beclin 1,
but decreased p62 expression when compared with the miR-16-5p
inhibitor group (Fig. 4C).
Similarly, BIMP1 knockdown also increased the LC3-II/I ratio and
beclin 1 protein expression levels and decreased p62 expression
when compared with the miR-16-5p inhibitor + si-NC group (Fig. 4C). Thus, the results suggested that
inhibition of the BIMP1/NF-κB signaling pathway reversed the
inhibitory effects of miR-16-5p knockdown on autophagy in BC
cells.
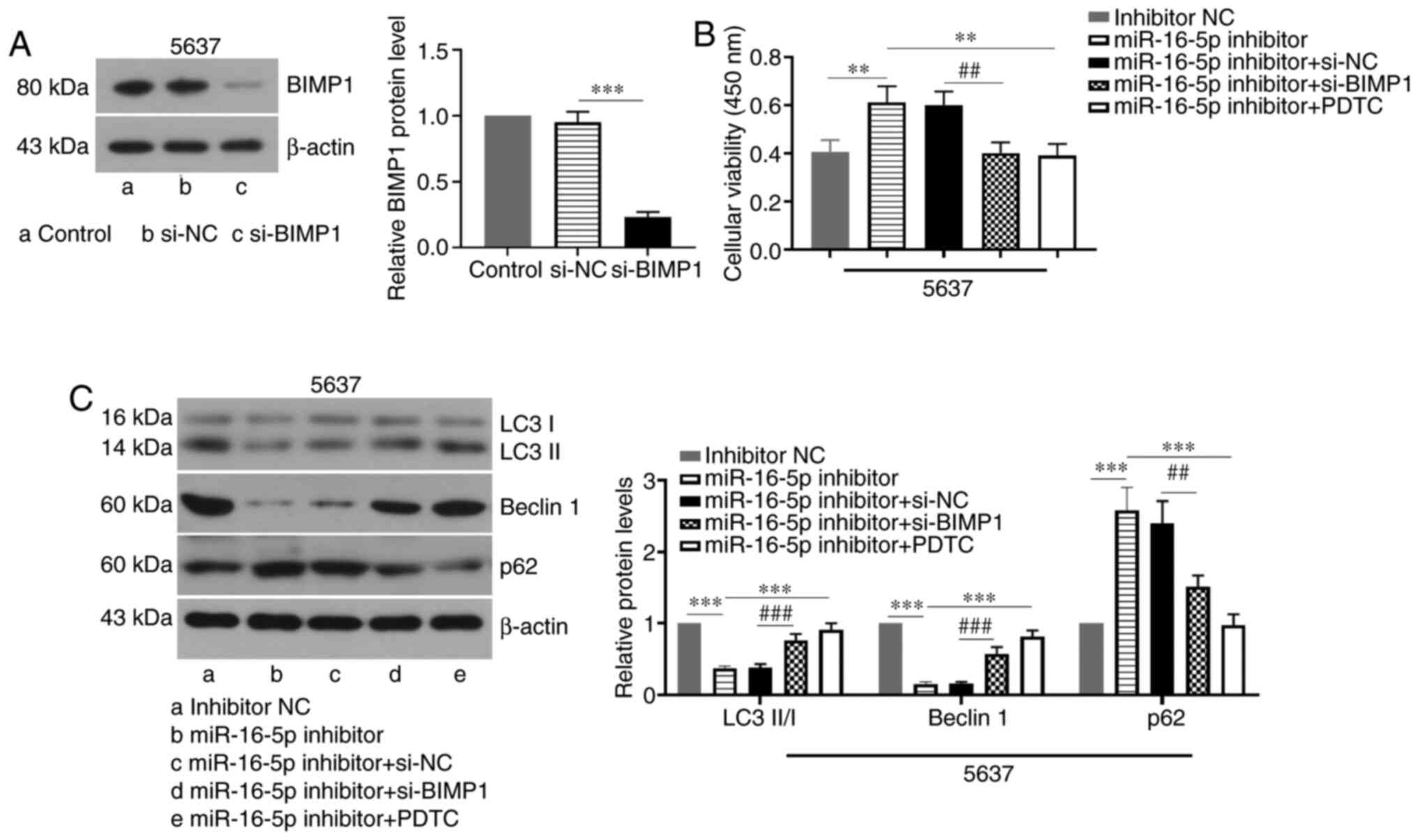 | Figure 4.miR-16-5p promotes autophagy by
blocking the BIMP1/NF-κB signaling pathway in bladder cancer cells.
(A) The protein level of BIMP1 in 5637 cells transfected with
si-BIMP1 or si-NC was evaluated by western blotting. (B and C)
Viability and protein expression levels of LC3-II/I, beclin 1 and
p62 in 5637 cells. The 5637 cells were co-transfected with
miR-16-5p inhibitor and BIMP1 siRNA or its NC, or cells were
transfected with miR-16-5p inhibitor followed by treatment with 20
µM PDTC (n=3). Data are presented as the means ± SD. **P<0.01,
***P<0.001, ##P<0.01, ###P<0.001.
miR, microRNA; NC, negative control; PDTC, pyrrolidine
dithiocarbamate; siRNA, small interfering RNA; LC3,
microtubule-associated proteins 1A/1B light chain 3B; BIMP1,
caspase recruitment domain family member 10. |
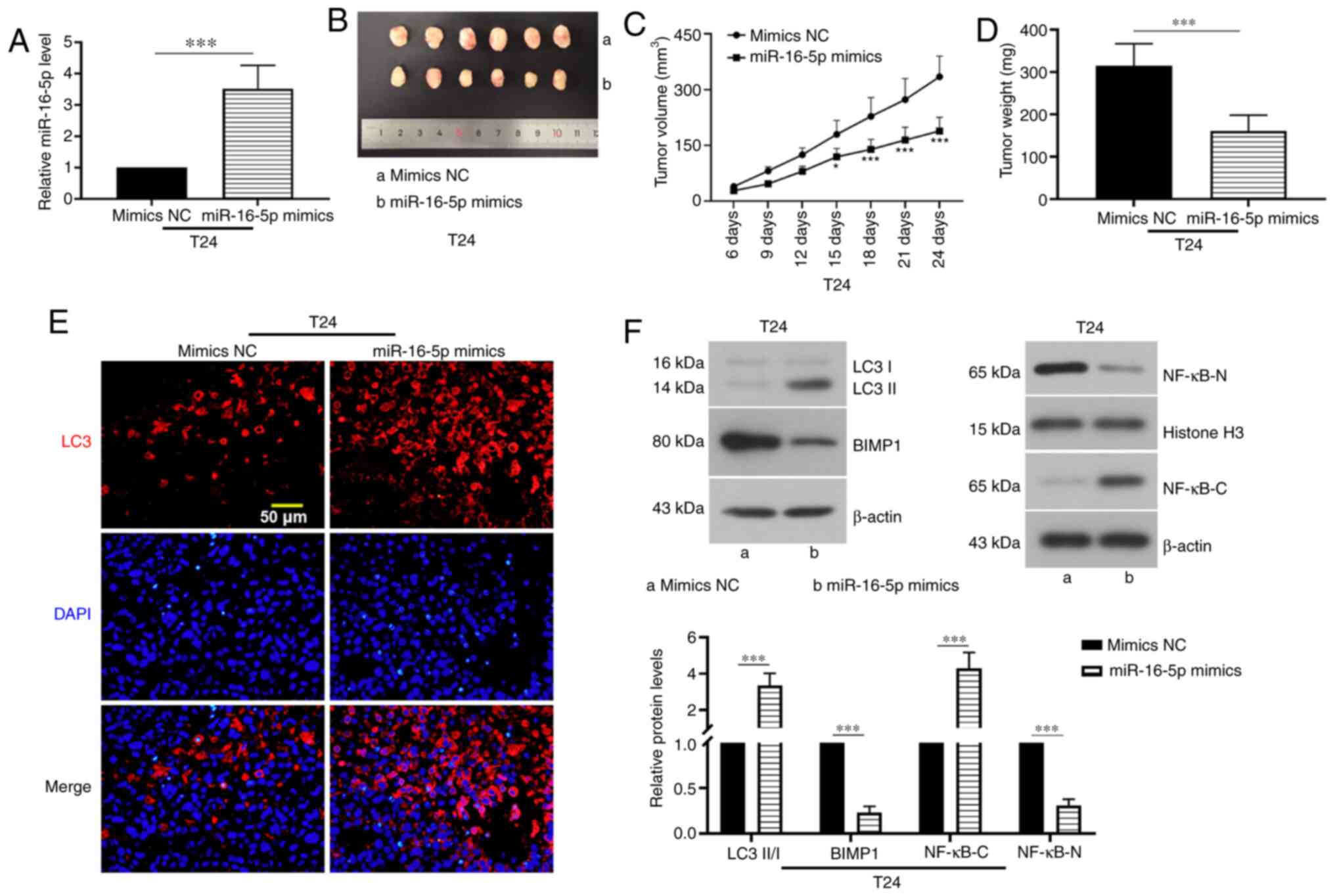
miR-16-5p overexpression suppresses
tumor growth in mice
T24 cells transfected with mimics NC or miR-16-5p
mimics were injected subcutaneously into the nude mice. The
expression of miR-16-5p was analyzed via RT-qPCR, which
demonstrated that the injection of cells transfected with miR-16-5p
mimics significantly increased miR-16-5p expression in tumor
tissues (Fig. 5A). Tumor growth was
detected every 3 days. The results showed that miR-16-5p
overexpression decreased tumor volume and weight (Fig. 5B-D). The results of
immunofluorescence staining showed that LC3 expression in the tumor
tissue samples was significantly increased by miR-16-5p
overexpression (Fig. 5E). In
addition, miR-16-5p overexpression increased the protein expression
level of p65 in the cytoplasm and the LC3-II/I ratio, whereas it
decreased the protein expression levels of BIMP1 and p65 protein in
nucleus (Fig. 5F). Taken together,
these data indicated that miR-16-5p overexpression inhibited tumor
growth.
Discussion
The present study elucidated the regulatory
mechanisms of miR-16-5p and the BIMP1/NF-κB signaling pathway in
the autophagy of BC cells. It was found that miR-16-5p induced BC
cell autophagy and further promoted apoptosis. miR-16-5p negatively
regulated the BIMP1/NF-κB signaling pathway by directly targeting
BIMP1. The induction of autophagy by miR-16-5p overexpression was
mediated through the BIMP1/NF-κB signaling pathway. These findings
provided novel insight into the molecular mechanism underlying BC
cell autophagy and indicated that miR-16-5p may be a target for the
treatment of BC.
With increasing numbers of studies into miRs,
various miRs have been found to be dysregulated during cancer
development, and they have been demonstrated to be involved in
cancer cell proliferation, apoptosis and autophagy, which suggests
that miRs may represent potential targets for cancer diagnosis and
treatment (23). It has been
reported that miR-16-5p was downregulated in BC tissues and cells,
and miR-16-5p silencing led to a decrease in BC cell viability
(11,24), which was consistent with the
findings of the present study.
In addition, overexpression of miR-16-5p induces
autophagy in non-small cell lung carcinoma cells (12). Autophagy is a highly conserved
process of self-degradation. By preventing the accumulation of
damaged proteins and organelles, autophagy reduces oxidative stress
and oncogenic signaling, thereby inhibiting cancer progression
(25). Although the role of
autophagy in inhibiting human cancer is unclear, previous findings
have suggested a role for autophagy stimulation in cancer
prevention and treatment (26). The
present study demonstrated that miR-16-5p induced autophagy in BC
cells, as evidenced by increased autophagic flux, LC3-II/I ratio
and beclin 1 protein expression, and decreased p62 expression in
miR-16-5p-overexpressing BC cells. In addition, increasing evidence
has demonstrated that there is crosstalk between autophagy and
apoptosis during cancer development. Specifically, it has been
reported that apoptotic inhibitors had no significant effect on
autophagy in colorectal cancer cells, whereas 3-MA treatment or
silencing autophagy-related gene 5 inhibits apoptosis in colorectal
cancer cells (27). In addition,
3-MA administration reduces troglitazone-induced apoptosis in BC
cells (28). The present results
demonstrated that 3-MA treatment decreased miR-16-5p
overexpression-induced apoptosis. These findings demonstrated that
autophagy may act as an upstream mediator of apoptosis and activate
apoptosis during the development of BC. Furthermore, it has been
reported that beclin 1 is an important mediator of autophagy and
apoptosis. Specifically, beclin 1 contains a Bcl-2 homology (BH)3
region, which physically interacts with Bcl-2/Bcl-xL proteins
(29). BH3 domains bind to BH3
receptors and inhibit the anti-apoptotic Bcl-2 proteins, including
Bcl-2, or activate the pro-apoptotic Bcl-2 family members, such as
Bax (30). Thus, treatment with the
autophagy inhibitor 3-MA may inhibit the apoptosis of BC cells by
suppressing beclin 1.
BIMP1 is a scaffold protein that plays an important
role in the development of cancer. It has been reported that BIMP1
is highly expressed in several types of cancer, including breast
cancer, BC and colorectal cancer (31–33).
Functional studies have revealed that BIMP1 is associated with
cancer cell proliferation, migration, invasion and apoptosis, but
whether BIMP1 regulates autophagy in BC cells remains largely
unknown (14,32). BIMP1 has been reported to be
associated with the NF-κB signaling pathway, and BIMP1
overexpression promotes breast cancer cell proliferation and
suppresses apoptosis by activating the NF-κB signaling pathway
(31). In addition, our previous
study revealed that BIMP1 knockdown inhibited NF-κB signaling in BC
cells (34).
The NF-κB signaling pathway is considered to be a
positive regulatory pathway in cancer development (35). During cancer progression, the
activation of the IκB protein by the IκB kinase complex leads to
phosphorylation-induced proteasomal degradation of the IκB protein.
The dimer (p50/p65) then forms and enters the nucleus to bind to
the κB site in the target gene promoter or enhancer and further
regulates gene expression (36).
The NF-κB signaling pathway is a negative regulator of autophagy in
several types of cancer. For example, baicalein induces autophagy
in breast cancer cells by inhibiting the NF-κB signaling pathway
(37). Dihydroartemisinin
stimulates the induction of autophagy in human multiple myeloma,
colorectal and cervical cancer cell lines via repression of the
NF-κB signaling pathway (19).
Taken together, these findings indicate that the BIMP1/NF-κB
signaling pathway suppresses autophagy.
Additionally, the present study demonstrated that
miR-16-5p directly targeted BIMP1 and suppressed its expression,
suggesting that miR-16-5p is able to block the BIMP1/NF-κB
signaling pathway by targeting BIMP1. It was demonstrated that
BIMP1 inhibition or the blockade of the NF-κB signaling pathway
restored the effects of miR-16-5p knockdown on cell autophagy,
indicating that miR-16-5p induces autophagy in BC cells through
blocking the BIMP1/NF-κB signaling pathway. However, a single miRNA
can target multiple downstream genes. For example, AKT3 has been
demonstrated to be a target gene of miR-16-5p (38), while AKT3 knockdown in glioblastoma
multiforme cells induces autophagy w9). Therefore, miR-16-5p may
also induce autophagy in BC cells by targeting AKT3, indicating
that miR-16-5p may participate in the regulation of BC cell
autophagy through various pathways, and the miR-16-5p/BIMP1/NF-κB
axis may be one of multiple potential pathways. Collectively, these
findings provide initial evidence that the miR-16-5p/BIMP1/NF-κB
axis may serve as a potential therapeutic target for BC.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Scientific Research Project of the Education Department of Liaoning
Province (grant no. QN2019008), China Medical University's 2018
Youth Support Program (Natural Science; grant no. QGZ2018041), the
Shenyang Plan Project of Science and Technology (grant no.
F19-112-4-098), the National Key R&D Plan Key Research Projects
of Precision Medicine (grant no. 2017YFC0908000) and China Medical
University's 2019 Discipline Promotion Program.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH and XM were involved in the study design and
wrote the manuscript. ZQ, HZ and ZG performed the experiments. YJ,
ZL and CK confirmed the authenticity of all the raw data and
conducted the statistical analysis. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The China Medical
University Laboratory Animal Welfare and Ethical Committee
(approval no. KT2020037) and animal experiments were performed in
accordance with the Guidelines for the Care and Use of Laboratory
Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benhamou S, Bonastre J, Groussard K,
Radvanyi F, Allory Y and Lebret T: A prospective multicenter study
on bladder cancer: The COBLAnCE cohort. BMC Cancer. 16:8372016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Racioppi M, D'Agostino D, Totaro A, Pinto
F, Sacco E, D'Addessi A, Marangi F, Palermo G and Bassi PF: Value
of current chemotherapy and surgery in advanced and metastatic
bladder cancer. Urol Int. 88:249–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blick C, Ramachandran A, McCormick R,
Wigfield S, Cranston D, Catto J and Harris AL: Identification of a
hypoxia-regulated miRNA signature in bladder cancer and a role for
miR-145 in hypoxia-dependent apoptosis. Br J Cancer. 113:634–644.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Rhijn BW, Burger M, Lotan Y, Solsona
E, Stief CG, Sylvester RJ, Witjes JA and Zlotta AR: Recurrence and
progression of disease in non-muscle-invasive bladder cancer: From
epidemiology to treatment strategy. Eur Urol. 56:430–442. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng Y, Li Z, Xie J, Wang P, Zhu J, Li Y
and Wang Y: MiRNA-224-5p inhibits autophagy in breast cancer cells
via targeting Smad4. Biochem Biophys Res Commun. 506:793–798. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vishnoi A and Rani S: MiRNA biogenesis and
regulation of diseases: An overview. Methods Mol Biol. 1509:1–10.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang N, Wu J, Qiu W, Lyu Q, He J, Xie W,
Xu N and Zhang Y: MiR-15a and miR-16 induce autophagy and enhance
chemosensitivity of camptothecin. Cancer Biol Ther. 16:941–948.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang QQ, Liu B and Yuan T: MicroRNA-16
inhibits bladder cancer proliferation by targeting Cyclin D1. Asian
Pac J Cancer Prev. 14:4127–4130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Zhang Y, Wu Q, Wang YB and Wang W:
miR-16 mimics inhibit TGF-β1-induced epithelial-to-mesenchymal
transition via activation of autophagy in non-small cell lung
carcinoma cells. Oncol Rep. 39:247–254. 2018.PubMed/NCBI
|
|
13
|
Xia ZX, Li ZX, Zhang M, Sun LM, Zhang QF
and Qiu XS: CARMA3 regulates the invasion, migration, and apoptosis
of non-small cell lung cancer cells by activating NF-кB and
suppressing the P38 MAPK signaling pathway. Exp Mol Pathol.
100:353–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang S, Zhang C, Liu W, Zheng W, Zhang Y,
Wang S, Huang D, Liu X and Bai Z: MicroRNA-24 upregulation inhibits
proliferation, metastasis and induces apoptosis in bladder cancer
cells by targeting CARMA3. Int J Oncol. 47:1351–1360. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patel M, Horgan PG, McMillan DC and
Edwards J: NF-κB pathways in the development and progression of
colorectal cancer. Transl Res. 197:43–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Kuang Y, Wang Y, Xu Q and Ren Q:
Notch-4 silencing inhibits prostate cancer growth and EMT via the
NF-κB pathway. Apoptosis. 22:877–884. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang C, Li A, Yang S, Qiao R, Zhu X and
Zhang J: CXCL5 promotes mitomycin C resistance in non-muscle
invasive bladder cancer by activating EMT and NF-κB pathway.
Biochem Biophys Res Commun. 498:862–868. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao G: Autophagy and NF-kappaB: Fight for
fate. Cytokine Growth Factor Rev. 18:233–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu W, Chen SS, Zhang JL, Lou XE and Zhou
HJ: Dihydroartemisinin induces autophagy by suppressing NF-κB
activation. Cancer Lett. 343:239–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He HJ, Bing H and Liu G: TSR2 Induces
laryngeal cancer cell apoptosis through inhibiting NF-κB signaling
pathway. Laryngoscope. 128:E130–E134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Research Council, . Committee for
the Update of the Guide for the Care and Use of Laboratory Animals.
Guide for the Care and Use of Laboratory Animals. 8th edition.
National Academies Press; Washington, DC: 2011, PubMed/NCBI
|
|
23
|
Wang F, Wu H, Fan M, Yu R, Zhang Y, Liu J,
Zhou X, Cai Y, Huang S, Hu Z and Jin X: Sodium butyrate inhibits
migration and induces AMPK-mTOR pathway-dependent autophagy and
ROS-mediated apoptosis via the miR-139-5p/Bmi-1 axis in human
bladder cancer cells. FASEB J. 34:4266–4282. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Li S, Li Y, Cheng B, Tan B and Wang
G: Puerarin inhibits proliferation and induces apoptosis by
upregulation of miR-16 in bladder cancer cell line T24. Oncol Res.
26:1227–1234. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
White E, Mehnert JM and Chan CS:
Autophagy, metabolism, and cancer. Clin Cancer Res. 21:5037–5046.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levy JMM, Towers CG and Thorburn A:
Targeting autophagy in cancer. Nat Rev Cancer. 17:528–542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Won SJ, Yen CH, Liu HS, Wu SY, Lan SH,
Jiang-Shieh YF, Lin CN and Su CL: Justicidin A-induced autophagy
flux enhances apoptosis of human colorectal cancer cells via class
III PI3K and Atg5 pathway. J Cell Physiol. 230:930–946. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan S, Yang X, Chen T, Xi Z and Jiang X:
The PPARγ agonist Troglitazone induces autophagy, apoptosis and
necroptosis in bladder cancer cells. Cancer Gene Ther. 21:188–193.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maiuri MC, Le Toumelin G, Criollo A, Rain
JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K,
Tavernarakis N, et al: Functional and physical interaction between
Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 26:2527–2539.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galonek HL and Hardwick JM: Upgrading the
BCL-2 network. Nat Cell Biol. 8:1317–1319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao T, Miao Z, Wang Z, Xu Y, Wu J, Liu X,
You Y and Li J: CARMA3 overexpression accelerates cell
proliferation and inhibits paclitaxel-induced apoptosis through
NF-κB regulation in breast cancer cells. Tumour Biol. 34:3041–3047.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Man X, Liu T, Jiang Y, Zhang Z, Zhu Y, Li
Z, Kong C and He J: Silencing of CARMA3 inhibits bladder cancer
cell migration and invasion via deactivating β-catenin signaling
pathway. Onco Targets Ther. 12:6309–6322. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Qian L, Li X and Yan J:
MicroRNA-195 inhibits colorectal cancer cell proliferation,
colony-formation and invasion through targeting CARMA3. Mol Med
Rep. 10:473–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Man X, He J, Kong C, Zhu Y and Zhang Z:
Clinical significance and biological roles of CARMA3 in human
bladder carcinoma. Tumour Biol. 35:4131–4136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Naugler WE and Karin M: NF-kappaB and
cancer-identifying targets and mechanisms. Curr Opin Genet Dev.
18:19–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan W, Ma X, Zhao X and Zhang S: Baicalein
induces apoptosis and autophagy of breast cancer cells via
inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des Devel Ther.
12:3961–3972. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ruan L and Qian X: MiR-16-5p inhibits
breast cancer by reducing AKT3 to restrain NF-κB pathway. Biosci
Rep. Aug 23–2019.(Epub ahead of print). doi: 10.1042/BSR20191611.
View Article : Google Scholar
|
|
39
|
Paul-Samojedny M, Pudelko A, Kowalczyk M,
Fila-Daniłow A, Suchanek-Raif R, Borkowska P and Kowalski J:
Knockdown of AKT3 and PI3KCA by RNA interference changes the
expression of the genes that are related to apoptosis and autophagy
in T98G glioblastoma multiforme cells. Pharmacol Rep. 67:1115–1123.
2015. View Article : Google Scholar : PubMed/NCBI
|