Introduction
Coronavirus disease 2019 (COVID-19) was first
identified in December 2019, and it has caused an outbreak of viral
pneumonia worldwide (1,2). The severe acute respiratory syndrome
(SARS) coronavirus 2 (SARS-CoV-2), responsible for COVID-19, has
potentially severe adverse health effects, such as acute
respiratory distress syndrome, difficult-to-correct metabolic
acidosis and coagulation dysfunction (3,4).
Although several SARS-CoV-2 vaccines have been developed, due to
the increasing demand for vaccines, insufficient vaccine production
(5), rapid virus mutation (6) and other reasons, SARS-CoV-2 is still
endangering human health. The current public health emergency is
similar to the SARS outbreak caused by the SARS-CoV in 2002–2003
(7). Moreover, recent research has
shown that the SARS-CoV-2 genome shared a sequence homology with
the SARS-CoV genome (8).
There are four major structural proteins of
SARS-CoV-2, including: Nucleocapsid protein, membrane glycoprotein,
small envelope glycoprotein and spike glycoprotein (9) Previous studies have reported that
angiotensin-converting enzyme 2 (ACE2), to which the SARS-CoV spike
protein binds, mediates SARS-CoV by binding to the S1 domain of the
SARS-CoV S protein and promoting viral replication (10,11).
As one of the metallopeptidases, ACE2 serves an essential role in
mediating the angiotensin II to angiotensin-(1–7)
conversion (12). Furthermore, ACE2
receptors can limit some harmful effects of angiotensin II
production, such as increased inflammation (13). The enhanced production of
angiotensin 1–7 also inhibits the ACE2/angiotensin 1–7/MAS axis and
decreasing angiotensin II production (14). Before being confirmed as the
functional cellular receptor for SARS-CoV, ACE2 had been
extensively studied in heart disease, hypertension and diabetes
(11). Moreover, using a
computational model, Xu et al (8) revealed that the SARS-CoV-2 spike
protein has a significant binding affinity to human ACE2, despite
replacing four out of five important interface amino acid residues
to SARS-CoV. Another study also reported that SARS-CoV-2 used the
same cell entry receptor, ACE2, as SARS-CoV in HeLa cells (15).
The ocular surface may be a mode of transmission of
SARS-CoV-2, but it remains poorly understood. Previous reports
(16–18) have confirmed that SARS-CoV-2 could
cause conjunctivitis. Indeed, the initial symptom of several
patients infected by SARS-CoV-2 is conjunctivitis (19). A recent study revealed the presence
of conjunctivitis is 11.6% in patients with COVID-19 (20). The tissues in contact with air on
the ocular surface are primarily the corneal epithelium and
conjunctival epithelium. The cornea and conjunctiva are developed
from the ectoderm (21). Moreover,
the conjunctival epithelial cells migrate to the corneal epithelial
cells at the limbus (22,23). Previous studies have confirmed the
presence of SARS-CoV-2 in tear and conjunctival swabs from patients
with COVID-19 (23,24). In addition, the lack of ocular
protection increases the risk of contracting SARS-CoV-2 (25).
The present study focused on the relationship
between SARS-CoV-2 and the human corneal epithelium, and aimed to
investigate the host inflammatory response signature caused by
SARS-CoV-2 in human corneal epithelial cells (HCECs).
Materials and methods
Clinical specimens
Healthy corneas were obtained from organ donors who
agreed to donate their corneas after they died. The cornea had been
thoroughly tested to ensure its use was safe and it was healthy.
The six healthy corneas used in the study were the remaining
peripheral corneal tissues after penetrating keratoplasty had been
performed. All corneas were healthy without any infection or
trauma. All six corneal specimens were collected from The
Affiliated Hospital of Qingdao University (Shandong, China) between
January 2020 and November 2020. The Ethics Committee of The
Affiliated Hospital of Qingdao University approved the use of the
corneas at The Affiliated Hospital of Qingdao University. This
research adhered to the principles described in the Declaration of
Helsinki. Written informed consent was obtained from individuals or
their next of kin. The demographic information for postmortem eyes
is presented in Table I.
Immunofluorescence staining was used to examine ACE2 expression in
the corneas.
 | Table I.Demographic information for
postmortem eyes. |
Table I.
Demographic information for
postmortem eyes.
| Case no. | Age, years | Sex | Ethnicity |
|---|
| N1 | 62 | Male | Asian |
| N2 | 48 | Female | Asian |
| N3 | 54 | Female | Asian |
| N4 | 36 | Male | Asian |
| N5 | 68 | Male | Asian |
| N6 | 43 | Male | Asian |
SARS-CoV-2 spike protein
The SARS-CoV-2 spike protein was purchased from Sino
Biological, Inc. (cat. no. MB14JA2203).
In vitro experiments
HCECs were provided by the Ocular Surface Laboratory
at the Zhongshan Ophthalmic Center. HCECs were from a primary cell
culture. The Ethics Committee of The Affiliated Hospital of Qingdao
University approved the use of HCECs at The Affiliated Hospital of
Qingdao University. This research adhered to the principles
described in the Declaration of Helsinki.
Cells were cultured to 80% confluence in DMEM
(HyClone; Cytiva) containing 12% fetal bovine serum (HyClone;
Cytiva) and 1% penicillin and streptomycin, and were then
stimulated with SARS-CoV-2 spike protein at two different final
concentration for 16 h at 37°C; 10 µg/ml (26) was selected as a lower concentration,
and a larger concentration of 50 µg/ml was chosen in order to show
the different effects of the concentrations. The negative control
group was untreated. After 16 h, reverse transcription-quantitative
(RT-q)PCR and western blotting were conducted.
Immunofluorescence
ACE2 expression in the normal human cornea was
evaluated via immunofluorescent staining of frozen sections of
corneas after embedding them in an optimum cutting temperature
(OCT) compound (Leica Microsystems, Inc.). Corneas were then
immediately frozen in liquid nitrogen after embedding in OCT
compound (27). Frozen corneal
slices (7 µm) were cut using a freezing-microtome (Leica
Microsystems GmbH). Slices were blocked with 10% blocking buffer
containing rabbit serum (Beijing Solarbio Science & Technology
Co., Ltd.) for 30 min at 37°C and were then stained with rabbit
anti-human ACE2 antibody (1:100; cat. no. bs-1004R; BIOSS)
overnight at 4°C. Subsequently, slices were incubated with donkey
anti-rabbit secondary antibody (1:500; cat. no. ab150061; Abcam)
for 1 h and with DAPI solution (Beijing Solarbio Science &
Technology Co., Ltd.) for another 10 min at room temperature. The
slices were observed and images were captured under a Zeiss
Axiovert microscope (Carl Zeiss AG; magnification, ×40).
RT-qPCR
Total RNA was extracted from HCECs using the RNAiso
Plus kit (Takara Biotechnology Co., Ltd.), and cDNA was obtained by
RT of total RNA using the Primescript RT kit (Takara Biotechnology
Co., Ltd.) according to the manufacturer's protocol. The mRNA
expression levels of IL-8, TNF-α, IL-6, gasdermin D (GSDMD) and
IL-1β in HCECs were detected as described previously (27). RT-qPCR was performed using an
Eppendorf Mastercycler and SYBR-Green (Takara Biotechnology Co.,
Ltd.). The following thermocycling parameters were used for the
amplification: Initial denaturation at 95°C for 30 sec, followed by
40 cycles of 95°C for 5 sec, 60°C for 30 sec, and a final stage of
95°C for 15 sec, 60°C for 30 sec and 95°C for 15 sec. The primer
pairs that were used are shown in Table II. All the primers were designed by
Takara Biotechnology Co., Ltd. Relative transcription levels were
calculated using the relative standard curve method that compares
the amount of target normalized to the housekeeping gene β-actin.
Relative gene expression was calculated using the 2−∆∆Cq
method (28). Data are presented as
the mean ± SD for relative mRNA levels.
 | Table II.Nucleotide sequences of human primers
used for reverse transcription-quantitative PCR. |
Table II.
Nucleotide sequences of human primers
used for reverse transcription-quantitative PCR.
| Genes | Primer sequence
(5′-3′) |
|---|
| hIL-8 | F: CGG CAA TAG CTC
TGT AT |
| hIL-8 | R: CCT TGA AAC TTT
GCC TCA |
| hTNF-α | F: TTC TGT CTA CTG
AAC TTC GGG GTG ATC GGT CC |
| hTNF-α | R: GTA TGA GAT AGC
AAA TCG GCT GAC GGT GTG GG |
| hIL-6 | F: CAC AAG TCC GGA
GAG GAG AC |
| hIL-6 | R: CAG AAT TGC CAT
TGC ACA AC |
| hGSDMD | F: TGA ATG TGT ACT
CGC TGA GTG TGG |
| hGSDMD | R: CAG CTG CTG CAG
GAC TTT GTG |
| hIL-1β | F: GCT GAT GGC CCT
AAA CAG ATG AA |
| hIL-1β | R: TCC ATG GCC ACA
ACA ACT GAC |
| β-actin | F: GCT CCT CCT GAG
CGC AAG |
| β-actin | R: CAT CTG CTG GAA
GGT GGA CA |
Western blot analysis
HCECs stimulated with the SARS-CoV-2 spike protein
were analysed using western blotting, as described previously
(27). Total protein of HCECs was
extracted using the tissue protein lysate (radioimmunoprecipitation
assay buffer:phenylmethanesulfonyl:phosphatase inhibitor, 100:1:1;
Beijing Solarbio Science & Technology Co., Ltd.). Protein
concentration was measured using a bicinchoninic acid protein assay
reagent (Beijing Solarbio Science & Technology Co., Ltd.).
Total protein samples (10 µg) were separated by SDS-PAGE on 12%
gels and transferred to PVDF membranes (EMD Millipore), which were
blocked in 5% bovine serum albumin (Beyotime Institute of
Biotechnology) at room temperature for 2 h. Blots were incubated
with anti-ACE2 (1:100; cat. no. bs-1004R; BIOSS), anti-GSDMD
(1:100; cat. no. sc-393581; Santa Cruz Biotechnology, Inc.),
anti-IL-1β (1:1,000; cat. no. AF-401-NA; R&D Systems, Inc.) and
anti-GAPDH (1:2,000; cat. no. E-AB-20059; Elabscience, Inc.) at 4°C
overnight, followed by incubation with HRP-linked anti-rabbit
(1:500; cat. no. ab150061; Abcam) antibody at room temperature for
2 h. The bands were visualized with Western ECL Blotting Substrates
(Bio-Rad Laboratories, Inc.). Digital images were obtained using
the Vilber Solo 4S chemiluminescence imaging system (Vilber
Lourmat).
Statistical analysis
All data were analyzed with SPSS 25 software (IBM
Corp.). The data are presented as the mean ± SD of ≥3 independent
experiments. The statistical analysis was performed using a one-way
ANOVA followed by LSD-t test, which was used for analysis between
two groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
ACE2 is expressed in normal human
corneas and HCECs
Immunofluorescence staining was performed to examine
the expression level of the ACE2 receptor in normal human corneas.
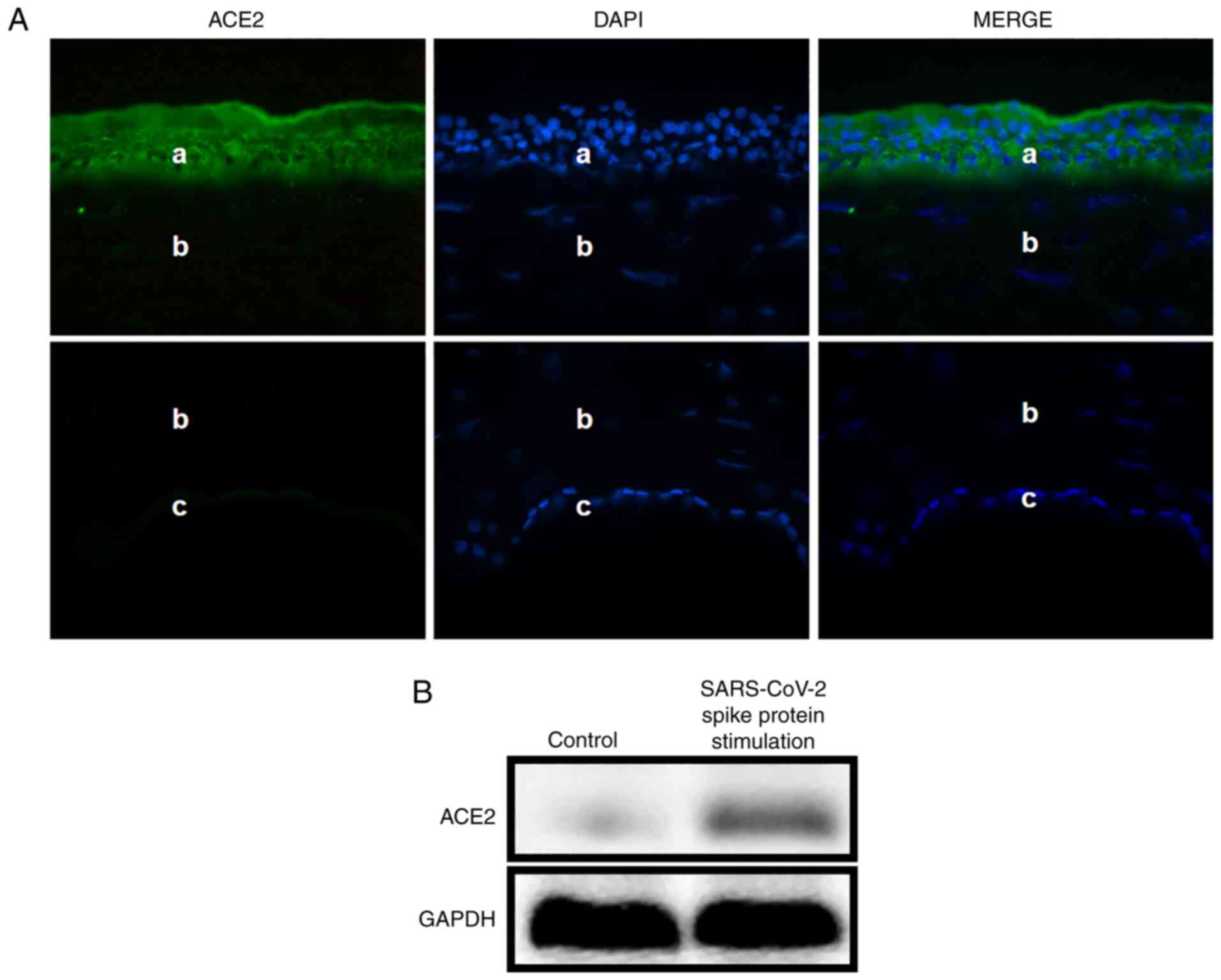
The results demonstrated that the ACE2 protein (green; Fig. 1A) was expressed in the epithelium of
normal human corneas (a, epithelium; b, stroma; c, endothelial
cells). Western blotting then was used to examine ACE2 expression
in HCECs. The protein expression level of ACE2 was higher after the
stimulation with SARS-CoV-2 spike protein compared with the control
group (Fig. 1B).
SARS-CoV-2 spike protein suppresses
the host inflammatory response in HCECs
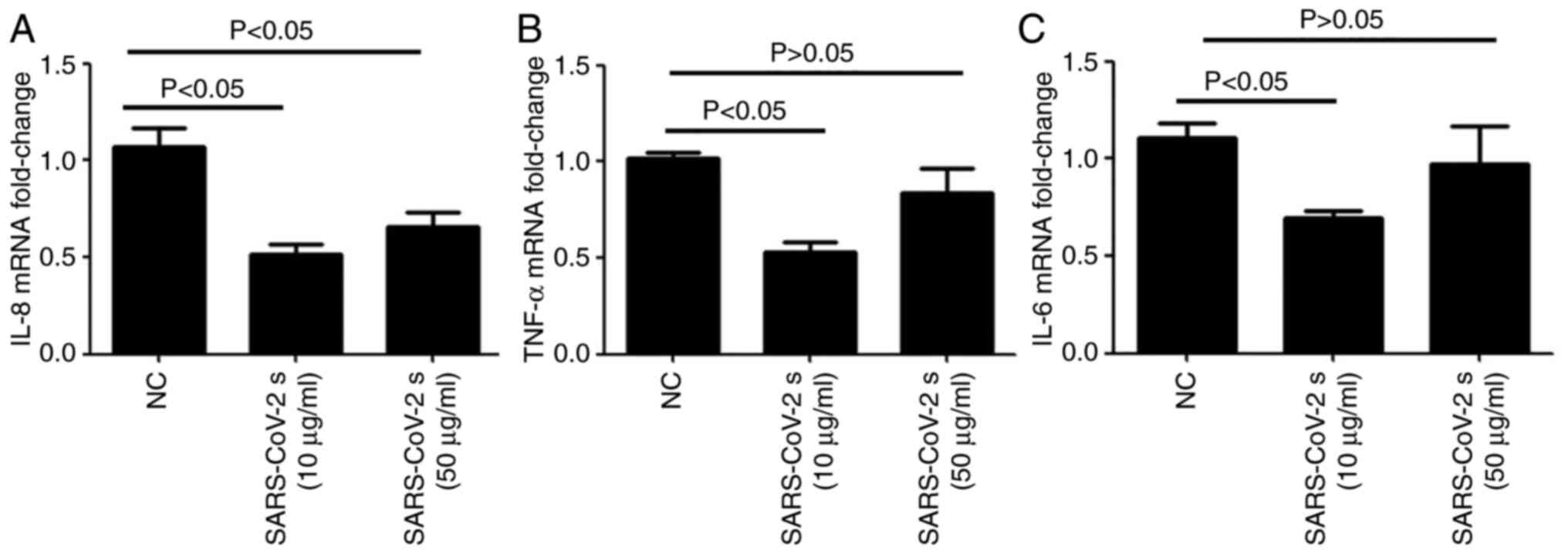
RT-qPCR was conducted to examine the expression
levels of proinflammatory factors in HCECs after stimulation with
the SARS-CoV-2 spike protein. Compared with the negative control
group, the mRNA expression levels of IL-8 (Fig. 2A), TNF-α (Fig. 2B) and IL-6 (Fig. 2C) were decreased by stimulation with
the SARS-CoV-2 spike protein.
SARS-CoV-2 spike protein-induced
pyroptosis in HCECs
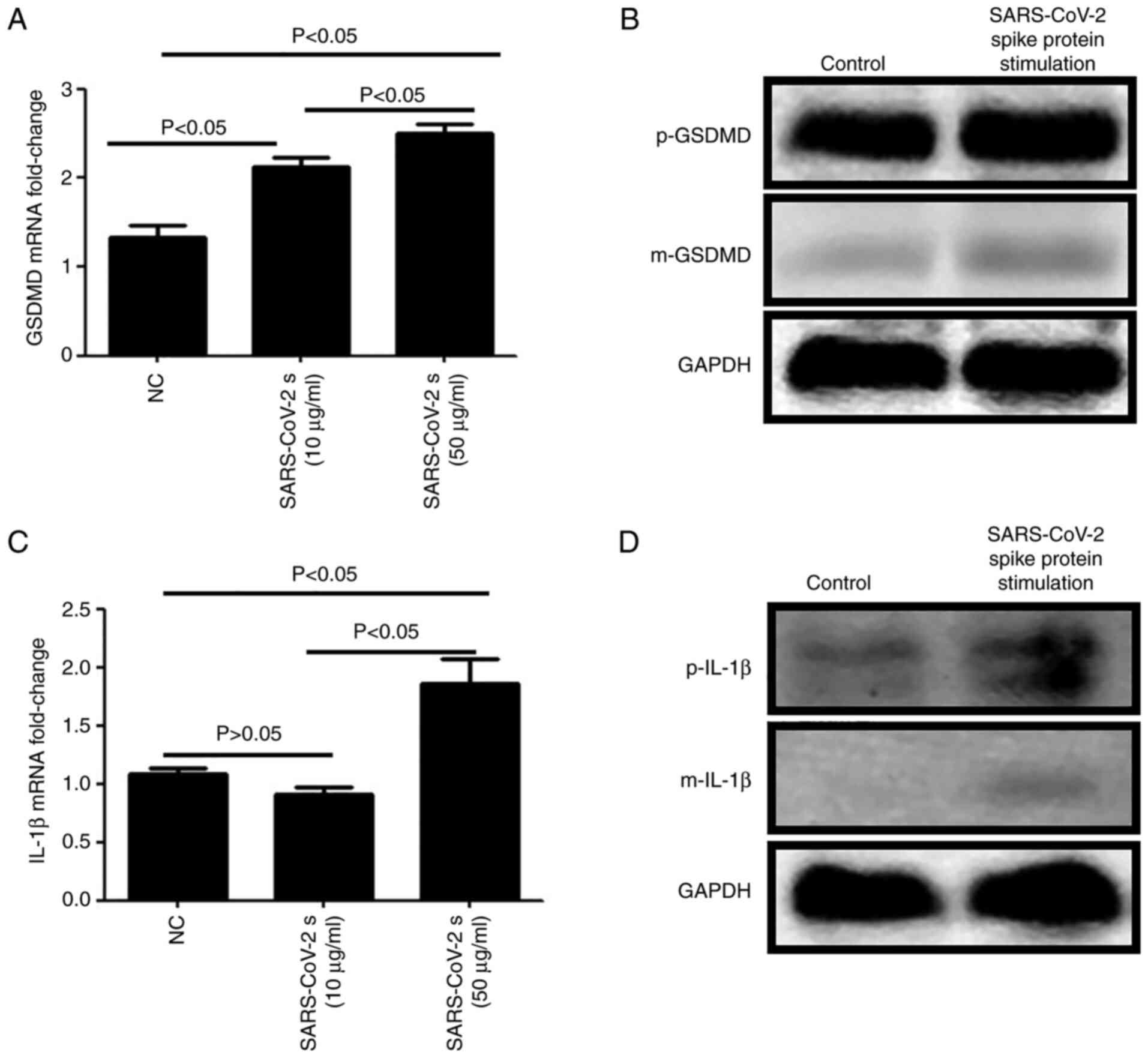
RT-qPCR and western blotting were used to examine
GSDMD and IL-1β expression in HCECs. It was found that the mRNA
(Fig. 3A) and protein (Fig. 3B) expression levels of precursor
(p)-GSDMD and mature (m)-GSDMD were higher following stimulation
with SARS-CoV-2 spike protein at the concentrations of 10 or 50
µg/ml. With regards to IL-1β, the mRNA (Fig. 3C) and protein (Fig. 3D) expression levels of p-IL-1β and
m-IL-1β were notably increased after stimulation with 50 µg/ml
SARS-CoV-2 spike protein.
Discussion
In total, >8,000 patients were diagnosed with
SARS-CoV between 2002–2003. Different from COVID-19, the symptoms
on the ocular surface were rarely identified in SARS (29). Since the discovery of COVID-19 in
2019, >100,000,000 people worldwide have been infected with
SARS-CoV-2. According to findings by Menachery et al
(30), the SARS-CoV spike
protein-bound to the ACE2 receptor, and replicated efficiently in
human airway cells. Moreover, Liu et al (31) reported that the SARS-CoV spike
protein weakly bound to ACE2 receptors in eyes. These results were
consistent with the clinical manifestation of SARS. Although the
expression levels of key cytokines, including membrane-associated
transmembrane serine protease 2 and ACE2 receptor, have been well
studied in respiratory tract cells (32), the relationship between SARS-CoV and
ACE2 in ocular cells is not fully understood.
The ocular surface, consisting of conjunctival
epithelium and corneal epithelium, is continuously exposed to the
environment (33). It has been
reported that SARS-CoV-2 can be transmitted via mucous membranes,
such as the conjunctiva (34).
Previous studies (16,17,35)
also have shown that conjunctivitis is an initial clinical
manifestation when an individuals is infected by SARS-CoV-2.
However, whether and how SARS-CoV-2 can invade the corneal
epithelium is yet to be fully elucidated.
Based on the present results, it was suggested that
the SARS-CoV-2 spike protein could induce the host inflammatory
response by binding to ACE2 in HCECs. The SARS-CoV-2 spike protein
binds to the ACE2 receptor and acts as a starting link to mediate
the invasion and spread of the virus (10,11).
Moreover, the findings of the present study indicated that the
SARS-CoV-2 spike protein inhibited the release of proinflammatory
factors in the host, which may make it more challenging for the
host to eliminate the virus in the early stages of infection.
Furthermore, a previous study revealed that IL-6, IL-8 and TNF-α
were highly upregulated in patients with SARS (36). In the present study, when the
concentration of SARS-CoV-2 spike protein was 10 µg/ml, the mRNA
expression levels of IL-8, TNF-α and IL-6 were lower in HCECs
compared with those stimulated with 50 μg/ml SARS-CoV-2 spike
protein. Thus, the results demonstrated that SARS-CoV-2 could
inhibit the release of proinflammatory factors and escaped immune
clearance when the viral load was small. However, when the virus
content increased, the virus could not escape the immunity of the
body, and the expression levels of the proinflammatory factors
began to increase.
Pyroptosis is a form of programmed cell death caused
by inflammatory bodies (37). It
can resist intracellular infection by eliminating damaged cells,
thus eliminating pathogens (38).
Pyroptosis is dependent on the family of caspases (39) and activation of the pore-forming
effector protein GSDMD (40). The
precursor-GSDMD protein is 53 kDa in length and is cleaved to
produce two major domains: 30 kDa N-terminal fragment of GSDMD
(GSDMD-NT) and 20 kDa C-terminal fragment of GSDMD (GSDMD-CT).
GSDMD-NT is the main functional domain and is also known as the
m-GSDMD (41). The m-GSDMD can
cause plasma membrane rupture, resulting in the release of
intracellular substances and proinflammatory mediators, such as
IL-1β (42). At the same time,
GSDMD also serves an important role in IL-1β maturation (41). The present study revealed that when
the SARS-CoV-2 spike protein invaded cells, pyroptosis was induced
as a cellular defense mechanism in the early stage. However, even
when viral load was low (spike protein at a final concentration of
10 µg/ml), cells still began the process of pyroptosis. These
findings indicated that although SARS-CoV-2 can inhibit the release
of proinflammatory factors in the host to some extent, the body can
still eliminate the virus via pyroptosis.
At present, there are numerous research areas for
investigations into the SARS-CoV-2 spike protein, ACE2 and
pyroptosis. Future studies will aim to determine the complete
mechanism of how SARS-CoV-2 invades the human body, and the body's
response to the invasion of the virus. However, due to the COVID-19
pandemic, experiments can only be conducted to a limited extent. In
the future, morphological research and flow cytometry will be
conducted to perform multi-dimensional evaluation of pyroptosis,
and experiments will be conducted in vivo, along with
possible long-term follow-up research.
In conclusion, the present study demonstrated that
the SARS-CoV-2 spike protein suppressed the host inflammatory
response and induced pyroptosis in HCECs. These findings highlight
the importance of ocular surface protection in response to the
SARS-CoV-2 infection. Moreover, blocking the ACE2 receptor in HCECs
may be an effective method to reduce the infection rate of
COVID-19.
Acknowledgements
Not applicable.
Funding
This study was supported by the Shandong Qingdao
Outstanding Health Professional Development Fund and PhD Research
Foundation of Affiliated Hospital of Jining Medical University
(grant no. 2021-BS-003).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GZ, LL and H Yang contributed to data acquisition,
analysis and interpretation, and drafted and critically revised the
manuscript. GL, SY, CG, LW and H Yan contributed to data
acquisition, analysis and interpretation, and critically revised
the manuscript. CC and MH contributed to conception, design, data
acquisition, analysis and interpretation, and drafted and
critically revised the manuscript. CC, GZ, LL and H Yang confirm
the authenticity of all the raw data. GZ, LL and H Yang contributed
equally to this work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The Ethics Committee of The Affiliated Hospital of
Qingdao University approved the use of the corneas and approved the
use of HCECs. Written informed consent was obtained from
individuals or their next to kin.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kannan S, Shaik Syed Ali P, Sheeza A and
Hemalatha K: COVID-19 (Novel Coronavirus 2019) - recent trends. Eur
Rev Med Pharmacol Sci. 24:2006–2011. 2020.PubMed/NCBI
|
|
2
|
Pascarella G, Strumia A, Piliego C, Bruno
F, Del Buono R, Costa F, Scarlata S and Agrò FE3: COVID-19
diagnosis and management: A comprehensive review. J Intern Med.
288:192–206. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li H, Liu SM, Yu XH, Tang SL and Tang CK:
Coronavirus disease 2019 (COVID-19): Current status and future
perspectives. Int J Antimicrob Agents. 55:1059512020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palacios Cruz M, Santos E, Velázquez
Cervantes MA and León Juárez M: COVID 19, a worldwide public health
emergency. Rev Clin Esp. 221:55–61. 2020.(In English and Spanish).
View Article : Google Scholar
|
|
5
|
Ortiz-Prado E, Espín E, Vásconez J,
Rodríguez-Burneo N, Kyriakidis NC and López-Cortés A: Vaccine
market and production capabilities in the Americas. Trop Dis Travel
Med Vaccines. 7:11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar A, Dowling WE, Román RG, Chaudhari
A, Gurry C, Le TT, Tollefson S, Clark CE, Bernasconi V and
Kristiansen PA: Status Report on COVID-19 Vaccines Development.
Curr Infect Dis Rep. 23:9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu B, Guo H, Zhou P and Shi ZL:
Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol.
19:141–154. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu X, Chen P, Wang J, Feng J, Zhou H, Li
X, Zhong W and Hao P: Evolution of the novel coronavirus from the
ongoing Wuhan outbreak and modeling of its spike protein for risk
of human transmission. Sci China Life Sci. 63:457–460. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Astuti I and Ysrafil: Severe Acute
Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of
viral structure and host response. Diabetes Metab Syndr.
14:407–412. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Moore MJ, Vasilieva N, Sui J, Wong
SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough
TC, et al: Angiotensin-converting enzyme 2 is a functional receptor
for the SARS coronavirus. Nature. 426:450–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Turner AJ, Hiscox JA and Hooper NM: ACE2:
From vasopeptidase to SARS virus receptor. Trends Pharmacol Sci.
25:291–294. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song W, Gui M, Wang X and Xiang Y: Cryo EM
structure of the SARS coronavirus spike glycoprotein in complex
with its host cell receptor PLoS Pathog. 14:e10072362018.PubMed/NCBI
|
|
13
|
Verdecchia P, Cavallini C, Spanevello A
and Angeli F: The pivotal link between ACE2 deficiency and
SARS-CoV-2 infection. Eur J Intern Med. 76:14–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santos RAS, Sampaio WO, Alzamora AC,
Motta-Santos D, Alenina N, Bader M and Campagnole-Santos MJ: The
ACE2/Angiotensin-(1–7)/MAS Axis of the Renin Angiotensin System:
Focus on Angiotensin-(1–7). Physiol Rev. 98:505–553. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou P, Yang XL, Wang XG, Hu B, Zhang L,
Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al: A pneumonia outbreak
associated with a new coronavirus of probable bat origin. Nature.
579:270–273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia J, Tong J, Liu M, Shen Y and Guo D:
Evaluation of coronavirus in tears and conjunctival secretions of
patients with SARS CoV 2 infection. J Med Virol. 92:589–594. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Panoutsopoulos AA: Conjunctivitis as a
Sentinel of SARS-CoV-2 Infection: A Need of Revision for Mild
Symptoms. SN Compr Clin Med. 19:1–6. 2020.PubMed/NCBI
|
|
18
|
Aiello F, Gallo Afflitto G, Mancino R, Li
JO, Cesareo M, Giannini C and Nucci C: Coronavirus disease 2019
(SARS CoV 2) and colonization of ocular tissues and secretions: A
systematic review. Eye (Lond). 34:1206–1211. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Loffredo L, Pacella F, Pacella E, Tiscione
G, Oliva A and Violi F: Conjunctivitis and COVID 19: A meta
analysis. J Med Virol. 92:1413–1414. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Güemes-Villahoz N, Burgos-Blasco B,
García-Feijoó J, Sáenz-Francés F, Arriola-Villalobos P,
Martinez-de-la-Casa JM, Benítez-Del-Castillo JM and Herrera de la
Muela M: Conjunctivitis in COVID 19 patients: Frequency and
clinical presentation. Graefes Arch Clin Exp Ophthalmol.
58:2501–2507. 2020. View Article : Google Scholar
|
|
21
|
Ramos T, Scott D and Ahmad S: An update on
ocular surface epithelial stem cells: Cornea and conjunctiva. Stem
Cells Int. 2015:6017312015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pearson AA: The development of the
eyelids. Part I. External features. J Anat. 130:33–42.
1980.PubMed/NCBI
|
|
23
|
Zhang H, Hara M, Seki K, Fukuda K and
Nishida T: Eyelid fusion and epithelial differentiation at the
ocular surface during mouse embryonic development. Jpn J
Ophthalmol. 49:195–204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ho D, Low R, Tong L, Gupta V,
Veeraraghavan A and Agrawal R: COVID 19 and the Ocular Surface: A
Review of transmission and manifestations. Ocul Immunol Inflamm.
28:726–734. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chu DK, Akl EA, Duda S, Solo K, Yaacoub S,
Schünemann HJ, Chu DK, Akl EA, El-harakeh A, Bognanni A, et al
COVID-19 Systematic Urgent Review Group Effort (SURGE) study
authors, : Physical distancing, face masks, and eye protection to
prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A
systematic review and meta-analysis. Lancet. 395:1973–1987. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leth-Larsen R, Zhong F, Chow VTK, Holmskov
U and Lu J: The SARS coronavirus spike glycoprotein is selectively
recognized by lung surfactant protein D and activates macrophages.
Immunobiology. 212:201–211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao G, Hu M, Li C, Lee J, Yuan K, Zhu G
and Che C: Osteopontin contributes to effective neutrophil
recruitment, IL 1β production and apoptosis in Aspergillus
fumigatus keratitis. Immunol Cell Biol. 96:401–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Darnell MER, Subbarao K, Feinstone SM and
Taylor DR: Inactivation of the coronavirus that induces severe
acute respiratory syndrome, SARS-CoV. J Virol Methods. 121:85–91.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Menachery VD, Yount BL Jr, Debbink K,
Agnihothram S, Gralinski LE, Plante JA, Graham RL, Scobey T, Ge XY,
Donaldson EF, et al: A SARS-like cluster of circulating bat
coronaviruses shows potential for human emergence. Nat Med.
21:1508–1513. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu L, Sun Y, Pan X, Shen W, Liu ZY and
Liu YP: Expression of SARS CoVs protein functional receptor ACE2 in
human cornea and conjunctiva. Ophthalmic Res. 22:561–564. 2004.
|
|
32
|
Barnett BP, Wahlin K, Krawczyk M, Spencer
D, Welsbie D, Afshari N and Chao D: Potential of ocular
transmission of SARS-CoV-2: A Review. Vision (Basel).
4:42020.PubMed/NCBI
|
|
33
|
Kugadas A and Gadjeva M: Impact of
microbiome on ocular health. Ocul Surf. 14:342–349. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu CW, Liu XF and Jia ZF: 2019-nCoV
transmission through the ocular surface must not be ignored.
Lancet. 395:e392020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheema M, Aghazadeh H, Nazarali S, Ting A,
Hodges G, McFarlane A, Kanji J, Zelyas N, Damji K and Solarte C:
Keratoconjunctivitis as the initial medical presentation of the
novel coronavirus disease 2019 (COVID 19). Can J Ophthalmol.
55:e125–e129. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang W, Ye L, Ye L, Li B, Gao B, Zeng Y,
Kong L, Fang X, Zheng H, Wu Z, et al: Up-regulation of IL-6 and
TNF-alpha induced by SARS-coronavirus spike protein in murine
macrophages via NF-kappaB pathway. Virus Res. 128:1–8. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kovacs SB and Miao EA: Gasdermins:
Effectors of Pyroptosis. Trends Cell Biol. 27:673–684. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem
Sci. 42:245–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu YJ, Zheng L, Hu YW and Wang Q:
Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta.
476:28–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Man SM, Karki R and Kanneganti TD:
Molecular mechanisms and functions of pyroptosis, inflammatory
caspases and inflammasomes in infectious diseases. Immunol Rev.
277:61–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao W, Yang H, Lyu L, Zhang J, Xu Q,
Jiang N, Liu G, Wang L, Yan H and Che C: GSDMD, an executor of
pyroptosis, is involved in IL-1β secretion in Aspergillus fumigatus
keratitis. Exp Eye Res. 202:1083752021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miao EA, Rajan JV and Aderem A:
Caspase-1-induced pyroptotic cell death. Immunol Rev. 243:206–214.
2011. View Article : Google Scholar : PubMed/NCBI
|