Introduction
Thyroid cancer is the most common malignancy of the
endocrine system worldwide, and its incidence has shown a
significant increase over the past 30 years (1,2).
Thyroid carcinomas are classified as differentiated or
undifferentiated according to their histological type. Anaplastic
thyroid carcinoma (ATC) is an undifferentiated subtype of thyroid
cancer with a high risk of invasion, recurrence and metastasis
(3). Generally, 90% of patients
with ATC pass away within 6 months of diagnosis, accounting for
14–39% of all thyroid cancer-related deaths annually worldwide
(4). Despite therapeutic advances
in diagnosis and clinical treatment, multiple studies have shown no
obvious improvement in the survival rate of patients with ATC
(3,5), perhaps due to the insufficient
understanding of the underlying mechanism of cancer metastasis and
the lack of effective therapeutic targets for ATC.
Cancer stem cells (CSCs), also known as
tumour-initiating cells, are a small subpopulation of cancer cells
with the properties of multidirectional differentiation and
metastasis, unlimited proliferation and self-renewal (6). An increasing number of studies have
indicated that CSCs are closely related to chemoradiotherapy
tolerance, tumour metastasis and recurrence (7–9). The
clinical implication of the CSC model is that it may be possible to
eradicate the tumour by removing all CSCs or other factors that
promote the characteristics of CSCs (10), which provides a largely novel idea
for targeted treatment of thyroid cancer. The tumour
microenvironment is crucial in regulating the plasticity of CSCs
(11). However, the effect of the
tumour microenvironment on the stemness of thyroid cancer cells is
unclear. Tumour-associated macrophages (TAMs) are vital immune
cells in the tumour microenvironment that have been shown to be
associated with poor prognosis in a variety of malignant tumours
(12,13), especially M2-like TAMs, which
directly communicate with CSCs to promote their stemness and/or
subsequent oncogenic properties, thereby triggering tumour invasion
and metastasis (14,15). Therefore, the present study focused
on investigating the role of M2-like TAMs in cancer stemness and
thyroid cancer metastasis.
The insulin-like growth factor (IGF) system plays an
important role in regulating the development and growth of mammals
(16). Notably, the two main
receptors of this system, IGF-1 receptor (IGF-1R) and insulin
receptor (IR), which occurs in two isoforms (IR-A and IR-B), are
usually overexpressed in tumour cells, supporting their biological
significance in cancer development (17,18).
There is extensive crosstalk between IR-A and IGF-1R, and the
pleiotropy of IR-A/IGF-1R signals is mediated by a variety of
downstream pathways, including the PI3K/AKT and ERK pathways
(19). IGF-1 and IGF-2 activate
downstream signalling pathways and participate in the regulation of
stemness, epithelial-mesenchymal transition (EMT), proliferation
and metastasis of tumour cells by binding to receptors IGF-1R and
IR-A (20). Studies have shown that
upregulation of IGF-1 by TAMs increases the proliferation and
migration of cancer cells (21,22).
In addition, high expression of IRs and IGF was observed in thyroid
cancer cells (23). Therefore, the
present study hypothesized that upregulation of IGF by M2-like TAMs
promotes stemness and metastasis by activating IR-A/IGF-1R-mediated
PI3K/AKT/mTOR signalling in human ATC.
In the present study, the data showed that M2-like
TAMs were enriched in human ATC tissues and that M2-like
TAM-secreted IGF promoted the metastasis and stemness of ATC cells
by activating the IR-A/IGF1R-mediated PI3K/AKT/mTOR signalling
pathway. These data could improve the understanding of ATC
progression and provide promising therapeutic targets for the
treatment of ATC.
Materials and methods
Tissue samples
Tissues from 12 patients with thyroid adenoma (seven
women and five men; mean age, 48.08±15.43 years old, range 22–68
years old), and tissues from 12 patients with ATC (six women and
six men; mean age, 52.25±16.99 years old, range 19–73 years old)
were acquired from the Third Affiliated Hospital of Kunming Medical
University, also known as Yunnan Cancer Hospital (Kunming, China),
collected from February 2019 to January 2020. Informed consent was
obtained from all participants and the Research Ethics Committee of
the Yunnan Cancer Hospital approved this study (approval no.
KY2020220). The inclusion criteria were undifferentiated thyroid
carcinoma patients with complete data. Patients with severe chronic
diseases (such as renal insufficiency) and severe liver disease
were excluded. Tissue samples were collected during surgery and
immediately stored in liquid nitrogen and 4% paraformaldehyde.
Cell culture and treatment
The human monocyte cell line THP-1 was purchased
from the American Type Culture Collection. The human anaplastic
thyroid cancer cell line C643 was purchased from The Cell Bank of
Type Culture Collection of the Chinese Academy of Sciences. Both
cell lines were maintained in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) containing 10% foetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (Beijing Solarbio Science & Technology
Co., Ltd.) in a humidified atmosphere at 37°C with 5%
CO2.
C643 cells were incubated with serum-free medium
containing 100 ng/ml IGF-1 or IGF-2 (Sigma-Aldrich; Merck KGaA) for
24 h at 37°C. To block IGF-1 and IGF-2 signals, cells were treated
with serum-free containing anti-IGF-1 (cat. no. ab40657, Abcam) and
anti-IGF-2 (cat. no. ab63984, Abcam) antibodies for 2 h at 37°C,
and then co-cultured with M2-like TAMs. To block the PI3K/AKT
pathway, cells were pre-incubated with serum-free medium containing
PI3K/AKT pathway inhibitor LY294002 (20 µM; Sigma-Aldrich; Merck
KGaA) for 2 h, and then co-cultured with M2-like TAMs for 24 h at
37°C.
Preparation of M2-like
macrophages
THP-1 cells (1×106/well) were cultured in
6-well plates. To generate M2-like TAMs, 320 nM PMA (Sigma-Aldrich;
Merck KGaA) was used to treat THP-1 cells for 6 h, and then the
cells were treated with PMA plus 20 ng/ml IL-4 (Sigma-Aldrich;
Merck KGaA) and 20 ng/ml IL-13 (Sigma-Aldrich; Merck KGaA) for
another 18 h (24).
Flow cytometry analysis
Following washing, trypsin digestion and
centrifugation (1,000 × g, 4°C, 5 min), the M2-like TAMs were
resuspended in 100 µl PBS (1×106 cells) and stained with
5 µl mouse anti-human CD14-FITC, CD68-FITC, CD206-PE and CD163-FITC
antibodies for 30 min at 4°C. The stained cells were then analysed
using a FACSCalibur flow cytometer (BD Biosciences) and Cell Quest
3.3 software (BD Biosciences). Antibodies against CD14-FITC (cat.
no.367115; 1:500), CD68-FITC (cat. no. 333805; 1:500), CD206-PE
(cat. no. 321105; 1:500) and CD163-FITC (cat. no. 333617; 1:500)
were purchased from BioLegend, Inc.
M2-like TAMs and C643 cell
co-culture
A 0.4-µm Transwell chamber (Corning, Inc.) was used
in the co-culture assay. Briefly, M2-like TAMs (2×105)
were seeded into the upper chamber and co-cultured with C643 cells
(2×105/well) in 6-well plates. After 24 h of co-culture
at 37 °C, the upper chamber was discarded, and C643 cells in the
lower chamber were collected and used for subsequent
experiments.
Transwell invasion assay
A 24-well Transwell cell culture chamber (Corning,
Inc.) coated with Matrigel (BD Biosciences) for 30 min at 37°C was
used to investigate cell invasion ability. To assess invasion, C643
cells were harvested after co-culture with M2-like TAMs for 24 h at
37°C. Then, the C643 cells were diluted to 1×105/ml in
200 µl serum-free RPMI-1640 medium and added to the upper chamber.
RPMI-1640 medium (600 µl) containing 10% FBS was placed in the
lower chamber. After incubation for 24 h at 37°C, the invaded cells
in the lower chamber were fixed with 4% paraformaldehyde at 25°C
for 30 min and stained with 0.1% crystal violet at 25°C for 15 min.
The invaded cells were imaged and counted at ×200 magnification
using a light microscope in five different fields for each
chamber.
Sphere formation assay
C643 cells were harvested after co-culture with
M2-like TAMs for 24 h. Then, C643 cells (4×104/well)
were plated in ultra-low-attachment 24-well plates (Corning, Inc.)
and maintained in serum-free DMEM-F12 (Sigma-Aldrich; Merck KGaA)
containing B27 supplement minus vitamin A (Gibco; Thermo Fisher
Scientific, Inc.), 20 ng/ml epidermal growth factor (R&D
Systems) and 20 ng/ml basic fibroblast growth factor (R&D
Systems). After 2 weeks, cell spheroids with a diameter >75 µm
were counted at ×100 magnification using a light microscope.
Western blot analysis
Cellular lysates were prepared using
radioimmunoprecipitation assay lysis buffer (Wuhan Boster
Biological Technology, Ltd.) supplemented with protease inhibitors
(Roche Diagnostics) according to the manufacturer's protocols. A
bicinchoninic acid protein assay kit (Wuhan Boster Biological
Technology, Ltd.) was used to determine the protein concentration.
Equal amounts of protein (30 µg of lysates) were separated via 10%
SDS-PAGE, and then separated proteins were transferred onto PVDF
membranes (EMD Millipore). The membranes were then blocked with 5%
non-fat milk at room temperature for 1 h, followed by incubation at
4°C overnight with primary antibodies against E-cadherin (cat. no.
ab15148; 1:500), N-cadherin (cat. no. ab18203; 1:500), Vimentin
(cat. no. ab137321; 1:1,000), CD133 (cat. no. ab19898; 1:1,000),
Oct4 (cat. no. ab18976; 1:500), Sox2 (cat. no. ab97959; 1:500),
IGF1R (cat. no. ab131476; 1:500), phosphorylated (p)-IGF1R (cat.
no. ab39398; 1:1,000), IR (cat. no. ab137747; 1:1,000), p-IR (cat.
no. ab60946; 1:1,000), AKT (cat. no. ab18785; 1:500), p-AKT (cat.
no. ab38449; 1:500), mTOR (cat. no. ab2732; 1:2,000), p-mTOR (cat.
no. ab84400; 1:1,000) and GAPDH (cat. no. ab9485; 1:2,000). All
antibodies were purchased from Abcam. After the membrane was
incubated with HRP-conjugated goat anti-rabbit immunoglobulin G
secondary antibody (cat. no. BA1054; 1:2,000; Wuhan Boster
Biological Technology, Ltd.) at room temperature for 1 h, the
signals were developed using an enhanced chemiluminescence reagent
(EMD Millipore) according to the manufacturer's instructions.
Image-Pro Plus 6.0 software (Media Cybernetics, Inc.) was used to
semi-quantify the protein bands, and GAPDH was used as a loading
control.
Reverse transcription-quantitative
(RT-q) PCR analysis
Isolation of total RNA from cultured cells was
performed with TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Briefly, RT was performed according to
the manufacturer's protocol and cDNA was generated using a
PrimeScript™ RT reagent kit (Takara Biotechnology Co., Ltd.). qPCR
was conducted using SYBR Green P0.remix Ex Taq II (Takara
Biotechnology Co., Ltd.) in an Applied Biosystems 7500 Fast
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). qPCR was performed under the following conditions: 95°C for
30 sec, followed by 40 cycles of 95°C for 3 sec and 60°C for 30
sec. The 2−∆∆Cq method was used for
comparative quantitation (25).
GAPDH was used as an internal normalization control. The
primer sequences used were as follows: IGF1 forward,
5′-CAGCAGTCTTCCAACCCAAT-3′ and reverse, 5′-CCACACACGAACTGAAGAGC-3′;
IGF2 forward, 5′-CGGACAACTTCCCCAGATAC-3′ and reverse,
5′-GTCTTGGGTGGGTAGAGCAA-3′; and GAPDH forward,
5′-CCAGGTGGTCTCCTCTGA-3′ and reverse,
5′-GCTGTAGCCAAATCGTTGT-3′.
ELISA for IGF-1 and IGF-2
C643 cells were co-cultured with M2-like TAMs and
C643 cells alone were used as a control group. After 24 h of
co-culture at 37°C, the supernatant was collected and centrifuged
at 200 × g for 5 min at 4°C. Then, following the instructions of
the Human IGF Signaling Antibody Array (cat. no. ab197446; Abcam),
the optical density of the collected supernatant was detected at
450 nm using a microplate reader, and the concentration of IGF-1 or
IGF-2 was calculated.
Immunohistochemistry (IHC)
staining
The tissues were fixed in 4% paraformaldehyde for 24
h at 4°C and dehydrated with a gradient of ethanol (100, 95, 80 and
70%). The tissues were embedded in paraffin and sectioned at a
thickness of 3 µm. The sections were soaked in 3%
H2O2 for 10 min and blocked with 5%
non-immune goat serum (cat. no. 5425; Cell Signaling Technology,
Inc.) at room temperature for 10 min, followed by incubation with
anti-CD68 (cat. no. ab213363; 1:2,000; Abcam) or anti-CD206 (cat.
no. 91992; 1:400; Cell Signaling Technology, Inc.) antibody at 4°C
for ~12 h. Subsequently, the sections were washed with PBS buffer
three times and incubated with secondary antibodies (cat. no. 8114;
1:2,000; Cell Signaling Technology, Inc.) at 25°C for 30 min. Then,
the sections were stained with DAB reagent (Sigma-Aldrich; Merck
KGaA) for 5 min at room temperature and stained with haematoxylin
for 2 min at room temperature. The histomorphological changes were
observed and imaged under a light microscope (Leica DM LB2; Leica
Microsystems, Inc.).
Statistical analysis
Statistical evaluation was performed using SPSS 20.0
software (IBM Corp.). The values are presented as the mean ±
standard deviation. Differences among multiple groups were compared
by one-way analysis of variance followed by Tukey's post hoc test
and differences between two groups were compared by unpaired
Student's t-test (for parametric data). P<0.05 was considered to
indicate a statistically significant difference.
Results
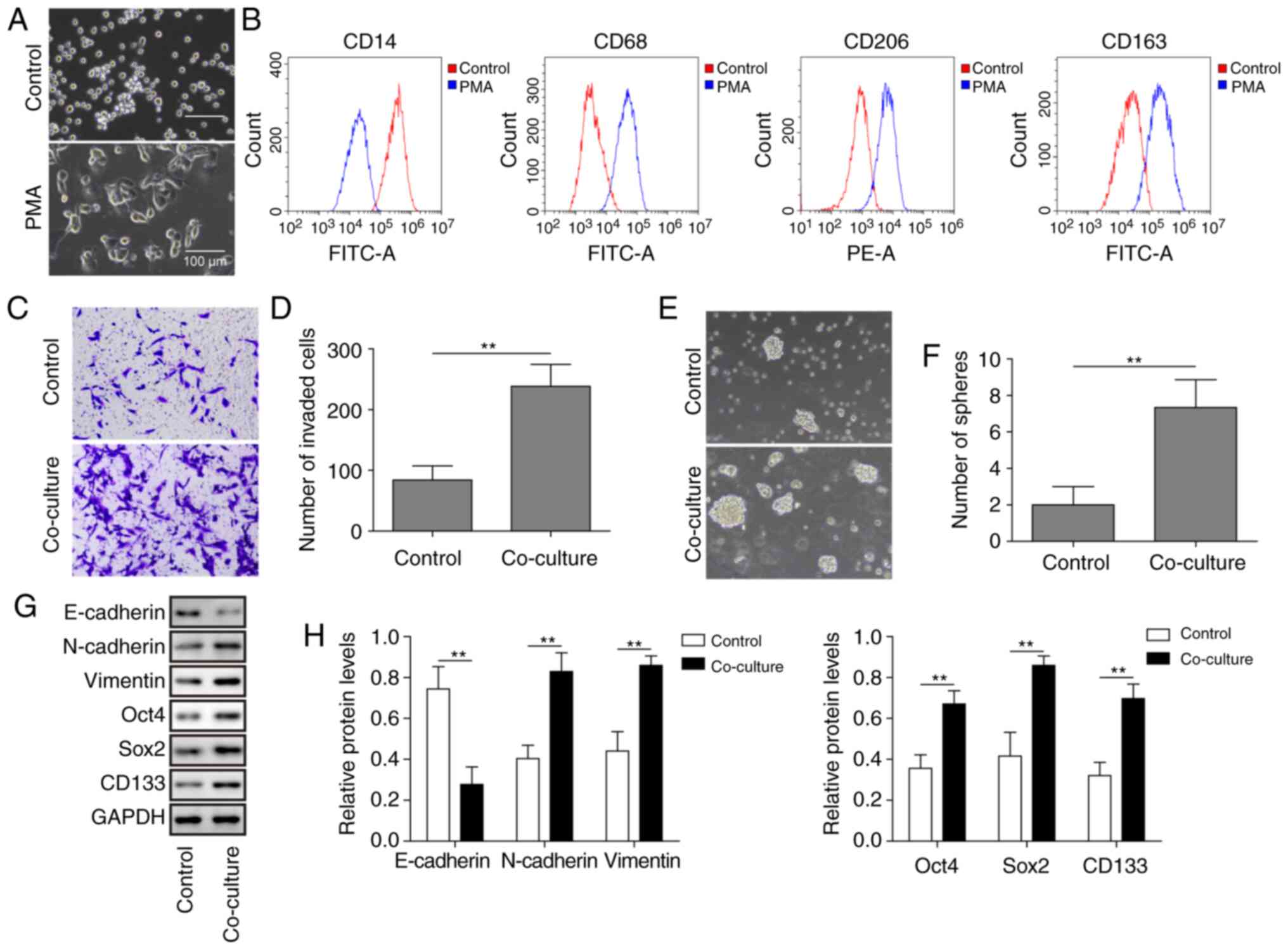
M2-like TAMs promote invasion and
stemness of human ATC cells
Human THP-1 cells are widely used as a model for
macrophage differentiation (26).
To investigate the role of M2-like TAMs in ATC, the THP-1 cells
were first treated with PMA, and then IL-4 and IL-13 were
continuously used. As shown in Fig.
1A, M2-like macrophages were larger and more stacked in
morphology. The THP-1 macrophages treated with PMA plus IL-4 and
IL-13 exhibited notable expression of M2-like TAM surface markers,
including CD68, CD206 and CD163, whereas the expression of CD14 (a
monocyte marker) was markedly decreased (Fig. 1B). These results indicated that the
M2-like TAM model was successful. M2-like TAMs were then used in
co-culture with C643 cells. The results revealed that after
co-culture with M2-like TAMs, the invasion capacity of ATC cells
was significantly increased (Fig. 1C
and D). Furthermore, a sphere formation assay and western
blotting were performed to assess cancer stemness. The results
revealed that sphere formation and the expression of
stemness-related markers Oct4, Sox2 and CD133 in ATC cells were
significantly increased following co-culture with M2-like TAMs
(Fig. 1E-H). EMT is considered to
be an essential part of the process of tumour cell metastatic
dissemination (27). Thus, the
present study investigated whether M2-like TAMs were involved in
EMT. As shown in Fig. 1G and H,
co-culture with M2-like TAMs significantly decreased the expression
of the epithelial marker E-cadherin and upregulated the expression
of the mesenchymal markers N-cadherin and Vimentin in ATC cells.
Taken together, these results indicated that M2-like TAMs
accelerated the invasion and increased the stemness of ATC
cells.
M2-like TAMs activate IR-A/IGF-1R
signalling in human ATC cells by secreting IGF-1 and IGF-2
To further investigate the underlying mechanisms by
which M2-like TAMs regulate cancer stemness and invasion of human
ATC, RT-qPCR, ELISA and western blot assays were performed to
discover the potential molecular mechanism. Significant induction
of IGF-1 and IGF-2 mRNA expression was found in the supernatants of
the co-culture group (Fig. 2A and
B). ELISA results also confirmed that the secretion of IGF-1
and IGF-2 was significantly increased in the supernatant of the
co-cultured cells compared with that of C643 cells alone (as a
control group) (Fig. 2C and D). In
addition, the western blotting results showed that ATC cells
exhibited higher expression of p-IR-A and p-IGF1R after co-culture
with M2-like TAMs (Fig. 2E and F),
indicating that the M2-like TAMs activated IR-A/IGF-1R signalling
in the ATC cells.
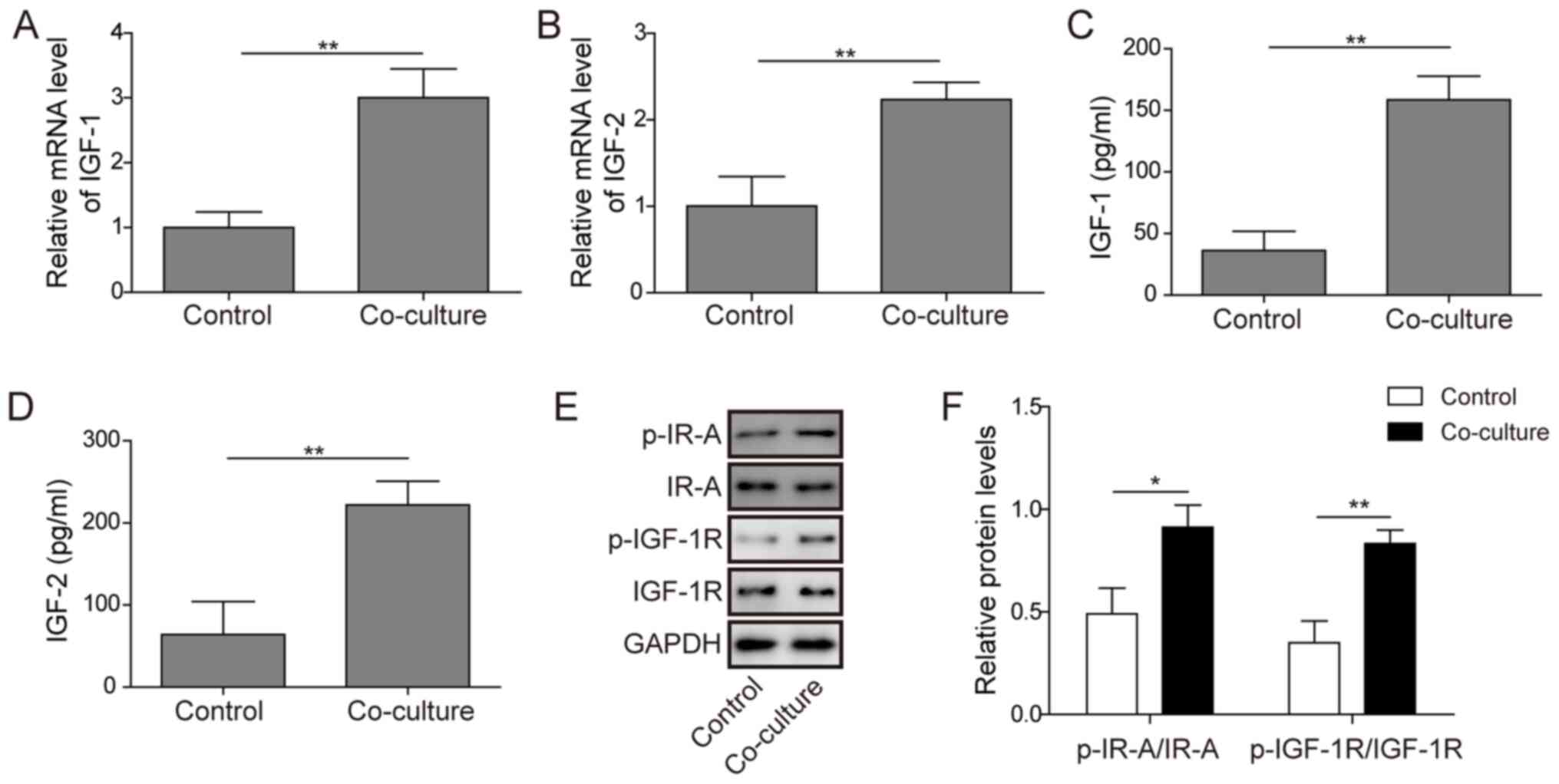 | Figure 2.Effects of M2-like TAM-secreted IGF-1
and IGF-2 on the activation of IR-A/IGF-1R signalling in anaplastic
thyroid carcinoma cells. (A and B) The IGF-1 and IGF-2 mRNA levels
in C643 cells, M2-like TAMs and co-cultured cells were measured by
reverse transcription-quantitative PCR. (C and D) The
concentrations of IGF-1 and IGF-2 in supernatants obtained from
C643 cells, M2-like TAMs and co-cultured cells were measured by
ELISA. (E and F) The protein expression levels of IR-A, IGF-1R,
p-IR-A and p-IGF-1R in C643 cells were measured by western blotting
after co-culture with M2-like TAMs for 24 h. C643 cells were
cultured alone as the control groups. The experiment was repeated
three times, and the results are shown as the mean ± standard
deviation. *P<0.05 and **P<0.01. TAM, tumour-associated
macrophage; IGF, insulin-like growth factor; IR, insulin receptor;
IGF-1R, IGF1 receptor; p-, phosphorylated. |
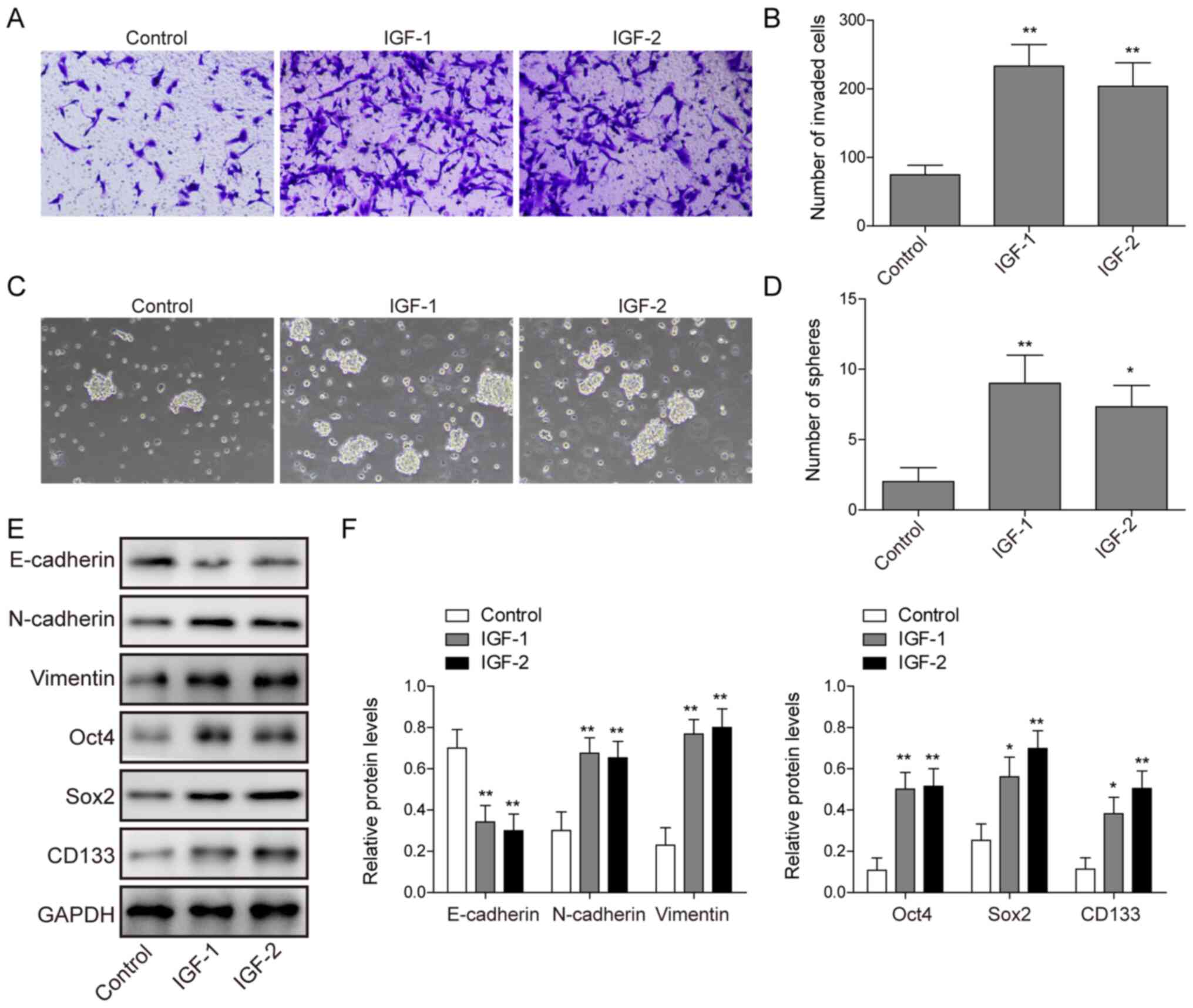
Exogenous IGF-1/IGF-2 promotes
invasion and stemness of C643 cells
Next, exogenous IGF-1 or IGF-2 were used to treat
C643 cells. As shown in Fig. 3A-D,
it was found that exogenous IGF-1 or IGF-2 promoted the invasion
and stemness in C643 cells. Furthermore, exogenous IGF-1 or IGF-2
increased the protein expression levels of N-cadherin, Vimentin,
Oct4, Sox2 and CD133, and decreased the protein expression level of
E-cadherin in C643 cells (Fig. 3E and
F). Taken together, these data suggested that exogenous
IGF-1/IGF-2 promoted the invasion and stemness of C643 cells.
Blockade of IGF1/IGF2 inhibits the
effect of M2-like TAMs on the invasion and stemness of human ATC
cells
After observing that M2-like TAMs accelerated the
invasion and increased the stemness of C643 cells and
simultaneously promoted the production of IGF-1 and IGF-2, the
present study next assessed whether blockade of IGF-1/IGF-2 would
suppress the M2-like TAM-induced alteration of invasion and
stemness of C643 cells. An IGF-1-/IGF-2-neutralizing antibody was
used to block the IGF pathway, and the blockade of IGF-1 and IGF-2
inhibited the invasion and stemness induced by M2-like TAMs in C643
cells (Fig. 4A-D). In addition,
blockade of IGF-1 and IGF-2 reversed the increase in N-cadherin,
Vimentin, Oct4, Sox2 and CD133 protein expression and the decrease
in E-cadherin protein expression induced by M2-like TAMs in C643
cells (Fig. 4E and F). These data
indicated that blocking IGF-1/IGF-2 suppressed the alteration of
invasion and stemness of C643 cells induced by M2-like TAMs.
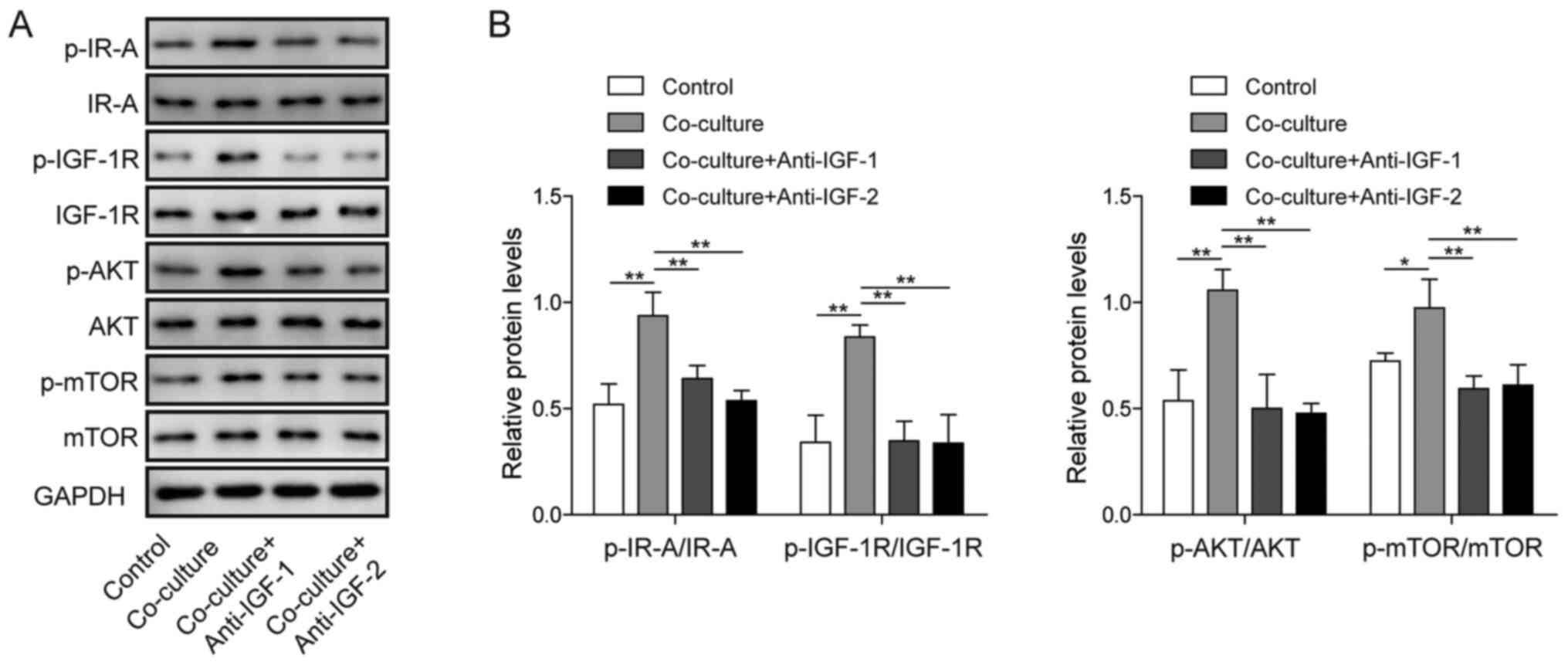
Blockade of IGF1/IGF2 inhibits
IR-A/IGF-1R-mediated PI3K/AKT/mTOR signalling in the co-culture
system
Next, the effects of blocking IGF1/IGF2 on the
IR-A/IGF-1R-mediated PI3K/AKT signalling pathway in the co-culture
system were examined. As shown in Fig.
5A and B, blockade of IGF1 and IGF2 significantly attenuated
the protein expression of p-IR-A, p-IGF1R, p-AKT and p-mTOR.
Although the expression of IR-A, IGF1R, AKT and mTOR showed no
obvious change in any group, the ratios of p-IR-A/IR-A,
p-IGF1R/IGF1R, p-AKT/AKT and p-mTOR/mTOR were markedly decreased
following the blockade of IGF1/IGF2 in the co-culture system. These
results indicated that IR-A/IGF-1R-mediated PI3K/AKT signalling was
activated in the co-culture system and that blocking IGF1/IGF2
inhibited its activation.
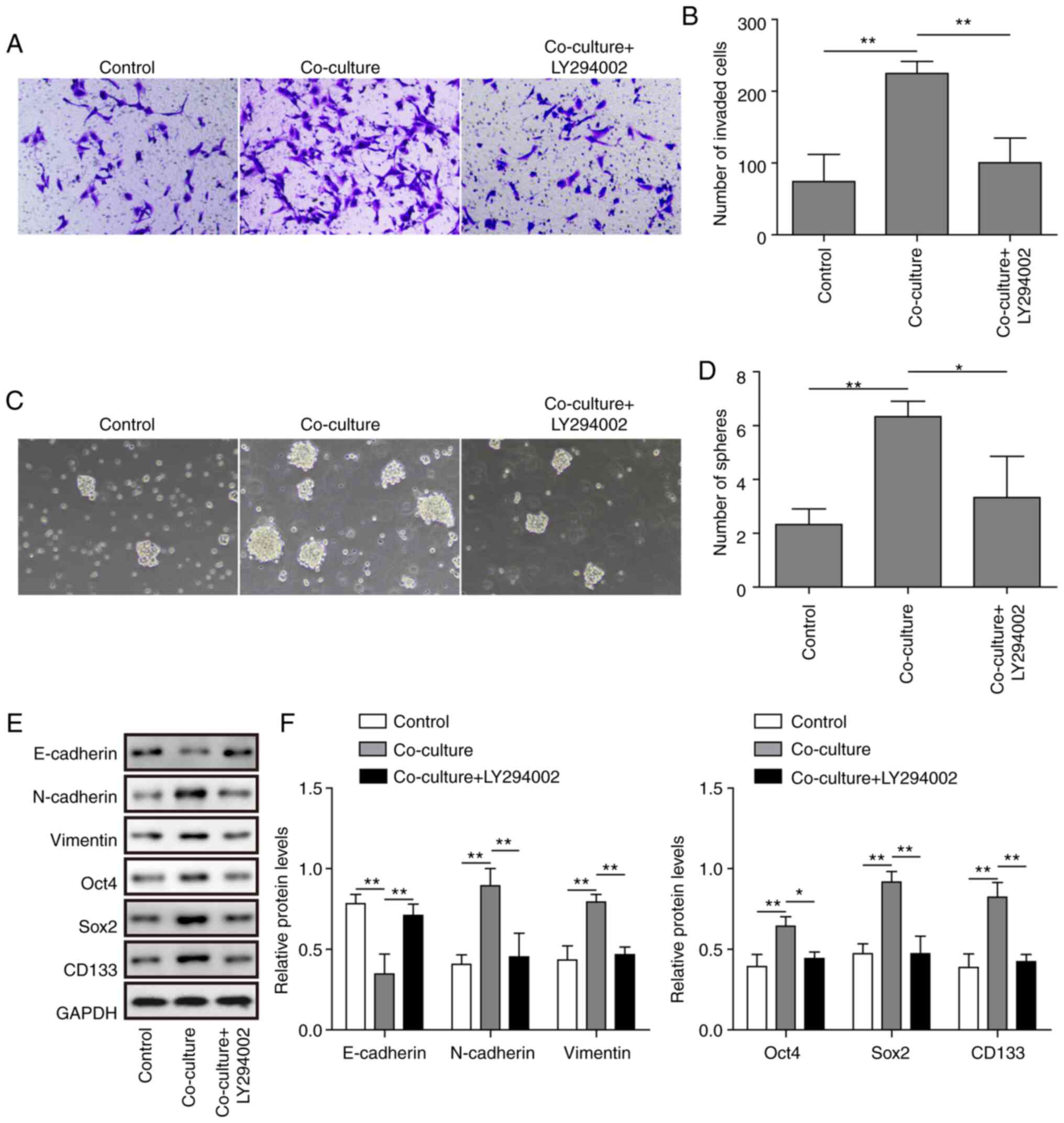
PI3K/AKT/mTOR pathway is critical for
the M2-like TAM-induced invasion and stemness of C643 cells
As the present study observed activation of the
PI3K/AKT/mTOR signalling pathway in the co-culture system, it was
next investigated whether the PI3K/AKT/mTOR pathway was involved in
cell invasion and stemness in the co-culture system. The PI3K/AKT
pathway inhibitor LY294002 was used, and inhibition of the PI3K/AKT
pathway significantly inhibited the invasion and stemness of the
co-culture system (Fig. 6A-D). In
addition, inhibition of the PI3K/AKT pathway reversed the increase
in N-cadherin, Vimentin, Oct4, Sox2 and CD133 protein expression
and the decrease in E-cadherin protein expression in the co-culture
system (Fig. 6E and F). These
results indicated that inhibition of the PI3K/AKT pathway inhibited
the M2-like TAM-induced invasion and stemness of ATC cells.
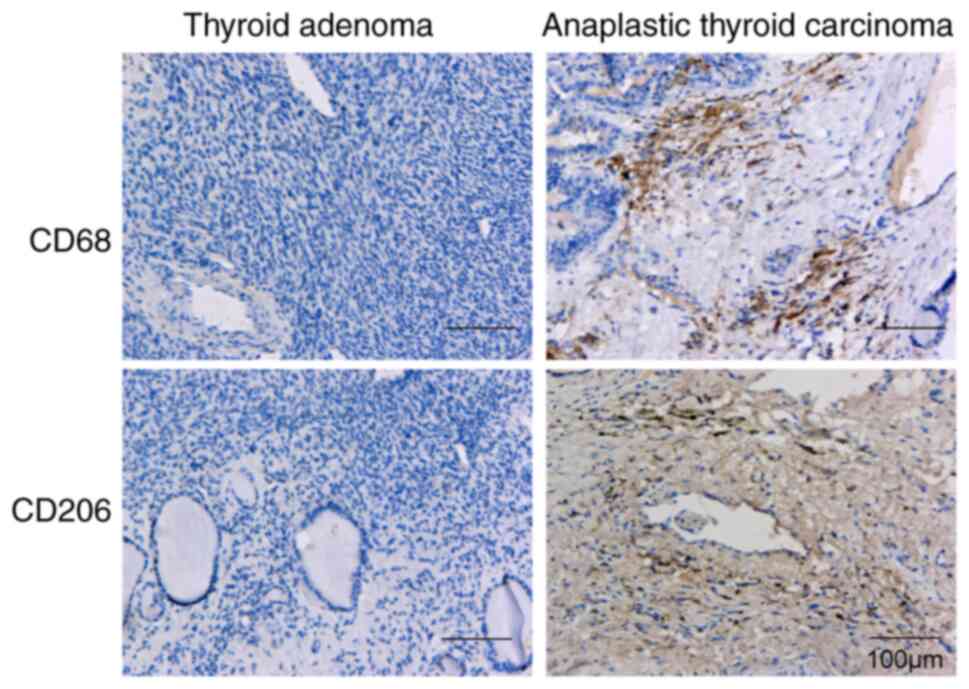
Expression of CD68 and CD206 is
notably increased in ATC tissues
Next, IHC was performed to detect the expression of
the M2-like TAM markers CD68 and CD206 in ATC tissues and thyroid
adenoma tissues. The results showed that the expression of CD68 and
CD206 in ATC tissues was markedly increased compared with that in
thyroid adenoma tissues (Fig. 7),
indicating that the M2-like TAMs clustered with the ATC
tissues.
Discussion
ATC remains one of the most fatal human
malignancies, despite significant progress in diagnosis and
treatment. Cancer stemness and distant metastasis are the main
causes of poor survival in patients with ATC (4,28).
Therefore, it is urgent to understand the underlying mechanism of
the occurrence and metastasis of ATC to obtain effective
treatments. Recent studies have shown that TAMs are one of the key
immune cells in the tumour microenvironment that affect the degree
of tumour malignancy (29,30). In the present study, it was found
that the upregulation of IGF-1/IGF-2 induced by M2-like TAMs
accelerated the metastasis and increased the stemness of ATC cells
by activating the IR-A/IGF1R-mediated PI3K/AKT/mTOR signalling
pathway.
Macrophages have functional plasticity and can be
differentiated into differentially polarized TAMs under different
microenvironmental stimuli (31).
Macrophages differentiate into M1-like TAMs after exposure to
lipopolysaccharides and IFN-γ, and have antitumour activities. When
macrophages are exposed to Th2 cytokines, such as IL-4 and IL-13,
they are polarized to M2 macrophages and promote tumour growth
(32,33). M2-like TAMs have been reported to
play a vital role in promoting tumour progression, and
identification of M2-like TAMs during tumour progression is
considered an appealing approach for cancer treatment (34). In thyroid cancers, an increased
density of M2-like TAMs was associated with decreased survival
(35). Caillou et al
(36) confirmed the presence of a
high number of TAMs in most ATC cases. In the present study, it was
observed that when co-cultured with M2-like TAMs, ATC cells
exhibited increased invasion, stemness and EMT. These findings
indicated that M2-like TAMs may play a positive role in the
promotion of ATC development. The TAM markers CD68 and CD206 were
detected in ATC tissues and thyroid adenoma tissues. In addition,
the IHC results showed that the expression of CD68 and CD206 in ATC
tissues was notably increased compared with that in thyroid adenoma
tissues, suggesting that the M2-like TAMs clustered with the ATC
tissues.
IGFs are often present in large quantities in the
tumour microenvironment and can be secreted by tumour stroma and/or
malignant cells. Elevated circulating IGF levels are associated
with a higher cancer risk, and experimental evidence has suggested
that IGFs contribute to the growth, metastasis and chemoresistance
of various cancers (37–39). In the present study, it was found
that M2-like TAMs significantly increased the induction of IGF-1
and IGF-2 in ATC cells. More importantly, IGFs have been reported
to be overexpressed in both thyroid cancer cell lines and tissues
(40). Thus, in the present study
it was demonstrated that M2-like TAMs promoted the progression of
ATC by secreting IGFs. The biological effects of insulin and IGFs
are mediated by the activation of their cell surface receptors,
which possess tyrosine kinase activity (41). In the present study, high expression
of p-IR-A and p-IGF1R in ATC cells were observed after co-culture
with M2-like TAMs. Exogenous IGF-1/IGF-2 promoted the invasion and
stemness of C643 cells, whereas an IGF1-/IGF2-neutralizing antibody
reversed the alteration of invasion, stemness and EMT. These
results indicated that M2-like TAM-secreted IGFs may be key factors
in the promotion of ATC progression via the IR-A/IGF-1R signalling
pathway.
Activation of the PI3K/AKT pathway has been linked
to cancer cell proliferation, survival and apoptosis by regulating
downstream molecules (42). Fu
et al (43) demonstrated
that metallothionein 1G suppressed cell growth and invasiveness by
regulating the PI3K/AKT signalling pathway in thyroid cancer. In
the present study, it was found that M2-like TAMs increased ATC
cell invasion, stemness and EMT by activating the PI3K/AKT pathway.
Ligand binding to IGF-IR can trigger a variety of signalling
pathways, such as the PI3K/AKT pathway, which is involved in the
transmission of cell survival signals (44,45).
Ma et al (46) reported that
IGF-1 enhanced cell proliferation and invasion by activating the
IGF-1/PI3K/AKT signalling pathway in pancreatic cancer cells.
Targeting AKT or ERK signalling reversed IGF-1-induced EMT in
gastric cancer (47). The present
study revealed that blocking IGF1/IGF2 inhibited the activation of
the IR-A/IGF-1R-mediated PI3K/AKT/mTOR signalling pathway in the
co-culture system. Taken together, these results suggested that
M2-like TAM-secreted IGFs promote ATC tumour progression by
activating IR-A/IGF-1R-mediated PI3K/AKT/mTOR signalling.
In summary, the present study revealed that M2-like
TAM-secreted IGF-1 and IGF-2 promoted cancer invasion, stemness and
EMT of ATC via the IR-A/IGF-1R-mediated PI3K/AKT/mTOR signalling
pathway. These data provided novel insights into the molecular
mechanism underlying ATC progression, and currently available
IGF1/IGF2 inhibitors may be a therapeutic strategy for ATC
intervention.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant from the Joint
Program of Yunnan Province and Kunming Medical University [grant
no. 2017FE467(−080)] and the National Natural Science Foundation of
China (grant no. 81860312).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZYD was the guarantor of integrity for the entire
study. ZYD and ZPF conceived the study, and ZYD and JL designed the
study. PJL, LJ, ZXY and FH performed the experiments. ZXY acquired
the data, CL performed data analysis and FKC conducted the
statistical analysis. JL prepared and edited the manuscript. ZYD
and ZPF reviewed the manuscript. ZYD and CL confirmed the
authenticity of all the raw data. All authors read and reviewed the
final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all participants
and The Research Ethics Committee of the Yunnan Cancer Hospital
approved this study (approval no. KY2020220; Kunming, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TAMs
|
tumour-associated macrophages
|
|
ATC
|
anaplastic thyroid carcinoma
|
|
CSCs
|
cancer stem cells
|
|
IGF
|
insulin-like growth factor
|
|
IR
|
insulin receptor
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen QT, Lee EJ, Huang MG, Park YI,
Khullar A and Plodkowski RA: Diagnosis and treatment of patients
with thyroid cancer. Am Health Drug Benefits. 8:30–40.
2015.PubMed/NCBI
|
|
3
|
Molinaro E, Romei C, Biagini A, Sabini E,
Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini
A, Torregrossa L, et al: Anaplastic thyroid carcinoma: From
clinicopathology to genetics and advanced therapies. Nat Rev
Endocrinol. 13:644–660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taccaliti A, Silvetti F, Palmonella G and
Boscaro M: Anaplastic thyroid carcinoma. Front Endocrinol
(Lausanne). 3:842012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saini S, Tulla K, Maker AV, Burman KD and
Prabhakar BS: Therapeutic advances in anaplastic thyroid cancer: A
current perspective. Mol Cancer. 17:1542018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blanpain C: Cancer Stem Cells. Radiother
Oncol. 106:S1982019.
|
|
7
|
Chang JC: Cancer stem cells: Role in tumor
growth, recurrence, metastasis, and treatment resistance. Medicine
(Baltimore). 95 (Suppl 1):S20–S25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang R and Rofstad EK: Cancer stem cells
(CSCs), cervical CSCs and targeted therapies. Oncotarget.
8:35351–35367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mertins SD: Cancer stem cells: A systems
biology view of their role in prognosis and therapy. Anticancer
Drugs. 25:353–367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kreso A and Dick JE: Evolution of the
cancer stem cell model. Cell Stem Cell. 14:275–291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Magee JA, Piskounova E and Morrison SJ:
Cancer stem cells: Impact, heterogeneity, and uncertainty. Cancer
Cell. 21:283–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Yan Y, Yang Y, Wang L, Li M and
Wang J, Liu X, Duan X and Wang J: High Infiltration of
Tumor-Associated Macrophages Influences Poor Prognosis in Human
Gastric Cancer Patients, Associates With the Phenomenon of EMT.
Medicine (Baltimore). 95:e26362016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steyaert S, Van Dorpe J, Hoorens A, Van
Biesen W and Van Laecke S: Intravenous immunoglobulins modify
relapsing membranous glomerulonephritis after kidney
transplantation: A case report. Acta Clin Belg. 73:229–232. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raggi C, Mousa HS, Correnti M, Sica A and
Invernizzi P: Cancer stem cells and tumor-associated macrophages: A
roadmap for multitargeting strategies. Oncogene. 35:671–682. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rother KI and Accili D: Role of insulin
receptors and IGF receptors in growth and development. Pediatr
Nephrol. 14:558–561. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Novosyadlyy R, Lann DE, Vijayakumar A,
Rowzee A, Lazzarino DA, Fierz Y, Carboni JM, Gottardis MM, Pennisi
PA, Molinolo AA, et al: Insulin-mediated acceleration of breast
cancer development and progression in a nonobese model of type 2
diabetes. Cancer Res. 70:741–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yakar S, Leroith D and Brodt P: The role
of the growth hormone/insulin-like growth factor axis in tumor
growth and progression: Lessons from animal models. Cytokine Growth
Factor Rev. 16:407–420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boucher J, Kleinridders A and Kahn CR:
Insulin receptor signaling in normal and insulin-resistant states.
Cold Spring Harb Perspect Biol. 6:a0091912014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuo YC, Chu JS, Lee KL, DrewV J, Zhuang W,
Chen C, Ho Y and Huang Y: Abstract 5739: Nicotine promotes
stemness-related properties and cell migration/metastasis through
IGF-1R regulation in triple negative breast cancer. Cancer Res.
77:57392017.
|
|
21
|
Liu L and Wang X, Li X, Wu X, Tang M and
Wang X: Upregulation of IGF1 by tumor-associated macrophages
promotes the proliferation and migration of epithelial ovarian
cancer cells. Oncol Rep. 39:818–826. 2018.PubMed/NCBI
|
|
22
|
Wang X, Zhu Q, Lin Y, Wu L, Wu X, Wang K,
He Q, Xu C, Wan X and Wang X: Crosstalk between TEMs and
endothelial cells modulates angiogenesis and metastasis via
IGF1-IGF1R signalling in epithelial ovarian cancer. Br J Cancer.
117:1371–1382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vella V, Pandini G, Sciacca L, Mineo R,
Vigneri R, Pezzino V and Belfiore A: A novel autocrine loop
involving IGF-II and the insulin receptor isoform-A stimulates
growth of thyroid cancer. J Clin Endocrinol Metab. 87:245–254.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao
YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, et al:
Tumor-associated macrophage-induced invasion and angiogenesis of
human basal cell carcinoma cells by cyclooxygenase-2 induction. J
Invest Dermatol. 129:1016–1025. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chanput W, Mes JJ and Wichers HJ: THP-1
cell line: An in vitro cell model for immune modulation approach.
Int Immunopharmacol. 23:37–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wong CE, Yu JS, Quigley DA, To MD, Jen KY,
Huang PY, Del Rosario R and Balmain A: Inflammation and Hras
signaling control epithelial-mesenchymal transition during skin
tumor progression. Genes Dev. 27:670–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ain KB: Anaplastic thyroid carcinoma: A
therapeutic challenge. Semin Surg Oncol. 16:64–69. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Li D, Cang H and Guo B: Crosstalk
between cancer and immune cells: Role of tumor-associated
macrophages in the tumor microenvironment. Cancer Med. 8:4709–4721.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei C, Yang C, Wang S, Shi D, Zhang C, Lin
X, Liu Q, Dou R and Xiong B: Crosstalk between cancer cells and
tumor associated macrophages is required for mesenchymal
circulating tumor cell-mediated colorectal cancer metastasis. Mol
Cancer. 18:642019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Caras I, Tucureanu C, Lerescu L, Pitica R,
Melinceanu L, Neagu S and Salageanu A: Influence of tumor cell
culture supernatants on macrophage functional polarization: In
vitro models of macrophage-tumor environment interaction. Tumori.
97:647–654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Genin M, Clement F, Fattaccioli A, Raes M
and Michiels C: M1 and M2 macrophages derived from THP-1 cells
differentially modulate the response of cancer cells to etoposide.
BMC Cancer. 15:5772015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mehibel M, Singh S, Chinje EC, Cowen RL
and Stratford IJ: Effects of cytokine-induced macrophages on the
response of tumor cells to banoxantrone (AQ4N). Mol Cancer Ther.
8:1261–1269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tariq M, Zhang JQ, Liang GK, He QJ, Ding L
and Yang B: Gefitinib inhibits M2-like polarization of
tumor-associated macrophages in Lewis lung cancer by targeting the
STAT6 signaling pathway. Acta Pharmacol Sin. 38:1501–1511. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ryder M, Ghossein RA, Ricarte-Filho JC,
Knauf JA and Fagin JA: Increased density of tumor-associated
macrophages is associated with decreased survival in advanced
thyroid cancer. Endocr Relat Cancer. 15:1069–1074. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Caillou B, Talbot M, Weyemi U,
Pioche-Durieu C, Al Ghuzlan A, Bidart JM, Chouaib S, Schlumberger M
and Dupuy C: Tumor-associated macrophages (TAMs) form an
interconnected cellular supportive network in anaplastic thyroid
carcinoma. PLoS One. 6:e225672011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang C, Su K, Zhang Y, Zhang W, Zhao Q,
Chu D and Guo R: IR-A/IGF-1R-mediated signals promote
epithelial-mesenchymal transition of endometrial carcinoma cells by
activating PI3K/AKT and ERK pathways. Cancer Biol Ther. 20:295–306.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kalet BT, O'Donoghue LE and Duval DL:
Abstract 3008: IGF2 mRNA binding protein 1 drives growth,
metastasis and chemoresistance in osteosarcoma. Cancer Res.
73:30082013.
|
|
39
|
Li B, Tsao SW, Chan KW, Ludwig DL,
Novosyadlyy R, Li YY, He QY and Cheung ALM: Id1-induced IGF-II and
its autocrine/endocrine promotion of esophageal cancer progression
and chemoresistance - implications for IGF-II and IGF-IR-targeted
therapy. Clin Cancer Res. 20:2651–2662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ciampolillo A, De Tullio C, Perlino E and
Maiorano E: The IGF-I axis in thyroid carcinoma. Curr Pharm Des.
13:729–735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sasako T and Ueki K: Insulin/IGF-1
signaling and aging. Nihon Rinsho. 74:1435–1440. 2016.PubMed/NCBI
|
|
42
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fu J, Lv H, Guan H, Ma X, Ji M, He N, Shi
B and Hou P: Metallothionein 1G functions as a tumor suppressor in
thyroid cancer through modulating the PI3K/Akt signaling pathway.
BMC Cancer. 13:4622013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Samani AA and Brodt P: The receptor for
the type I insulin-like growth factor and its ligands regulate
multiple cellular functions that impact on metastasis. Surg Oncol
Clin N Am. 10289–312. (viii)2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A,
Takahashi H, Wakasugi T, Funahashi H, Sato M and Takeyama H: IGF-1
mediates PTEN suppression and enhances cell invasion and
proliferation via activation of the IGF-1/PI3K/Akt signaling
pathway in pancreatic cancer cells. J Surg Res. 160:90–101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li H, Xu L, Li C, Zhao L, Ma Y, Zheng H,
Li Z, Zhang Y, Wang R, Liu Y, et al: Ubiquitin ligase Cbl-b
represses IGF-I-induced epithelial mesenchymal transition via ZEB2
and microRNA-200c regulation in gastric cancer cells. Mol Cancer.
13:1362014. View Article : Google Scholar : PubMed/NCBI
|