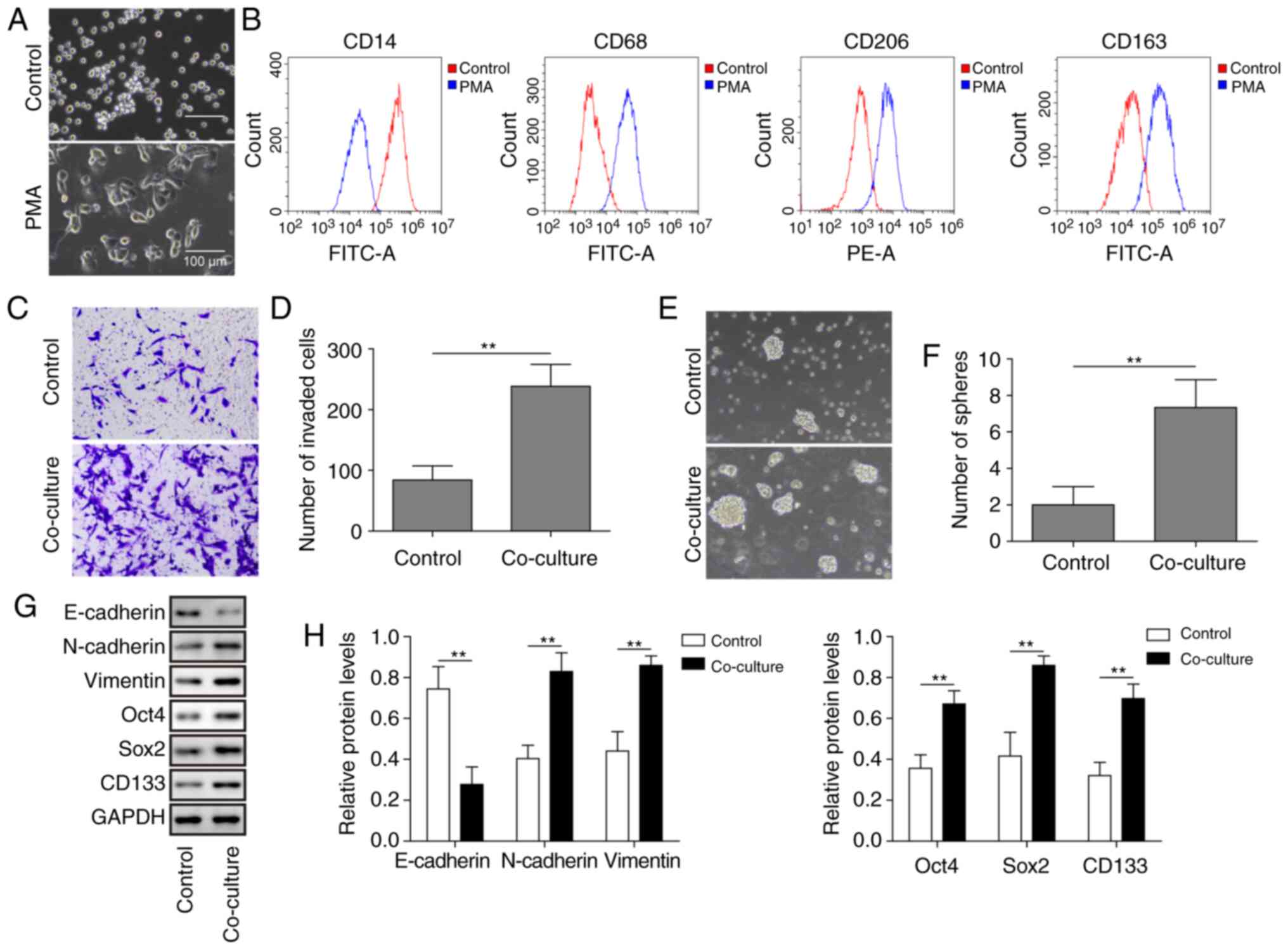
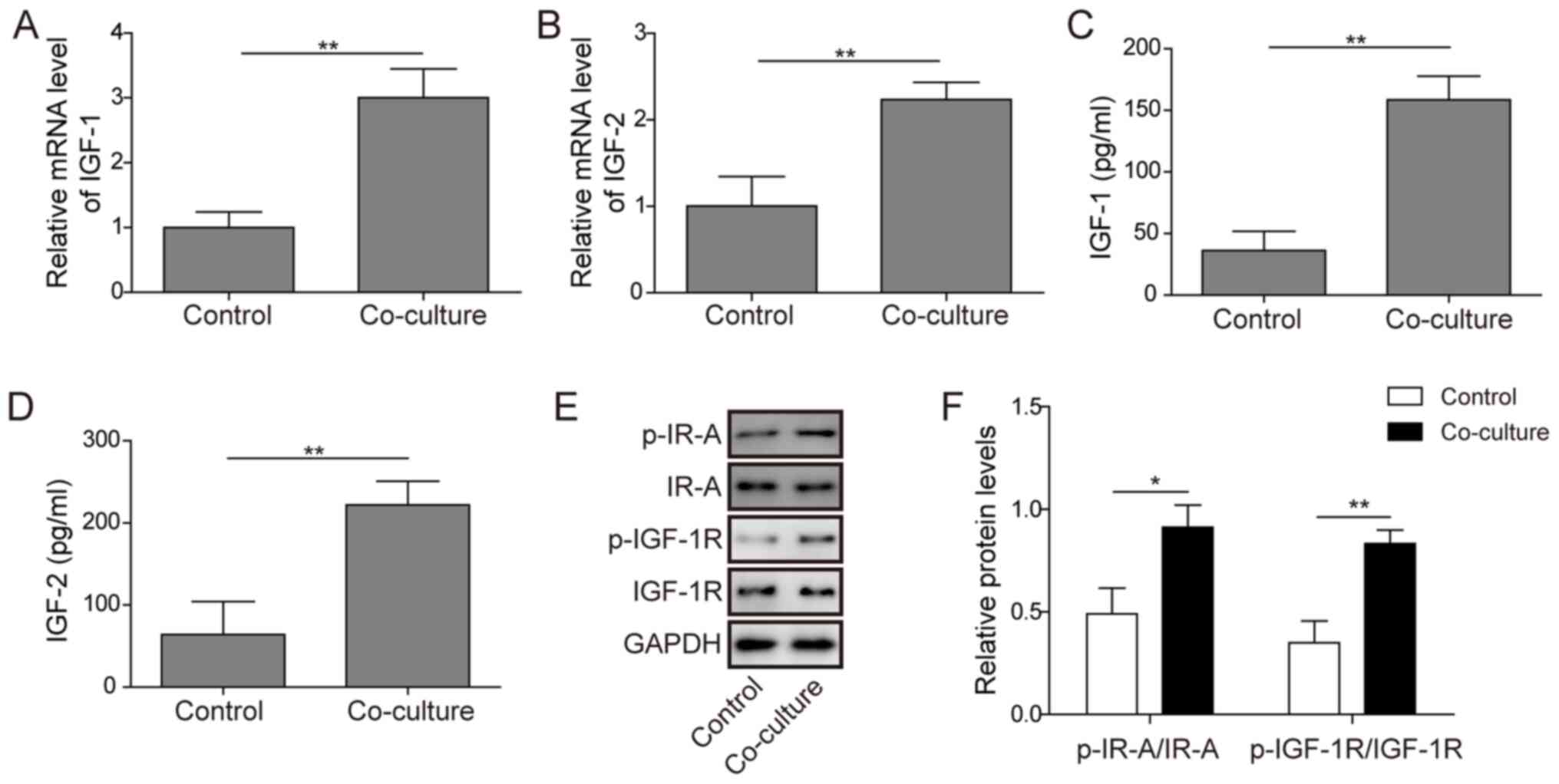
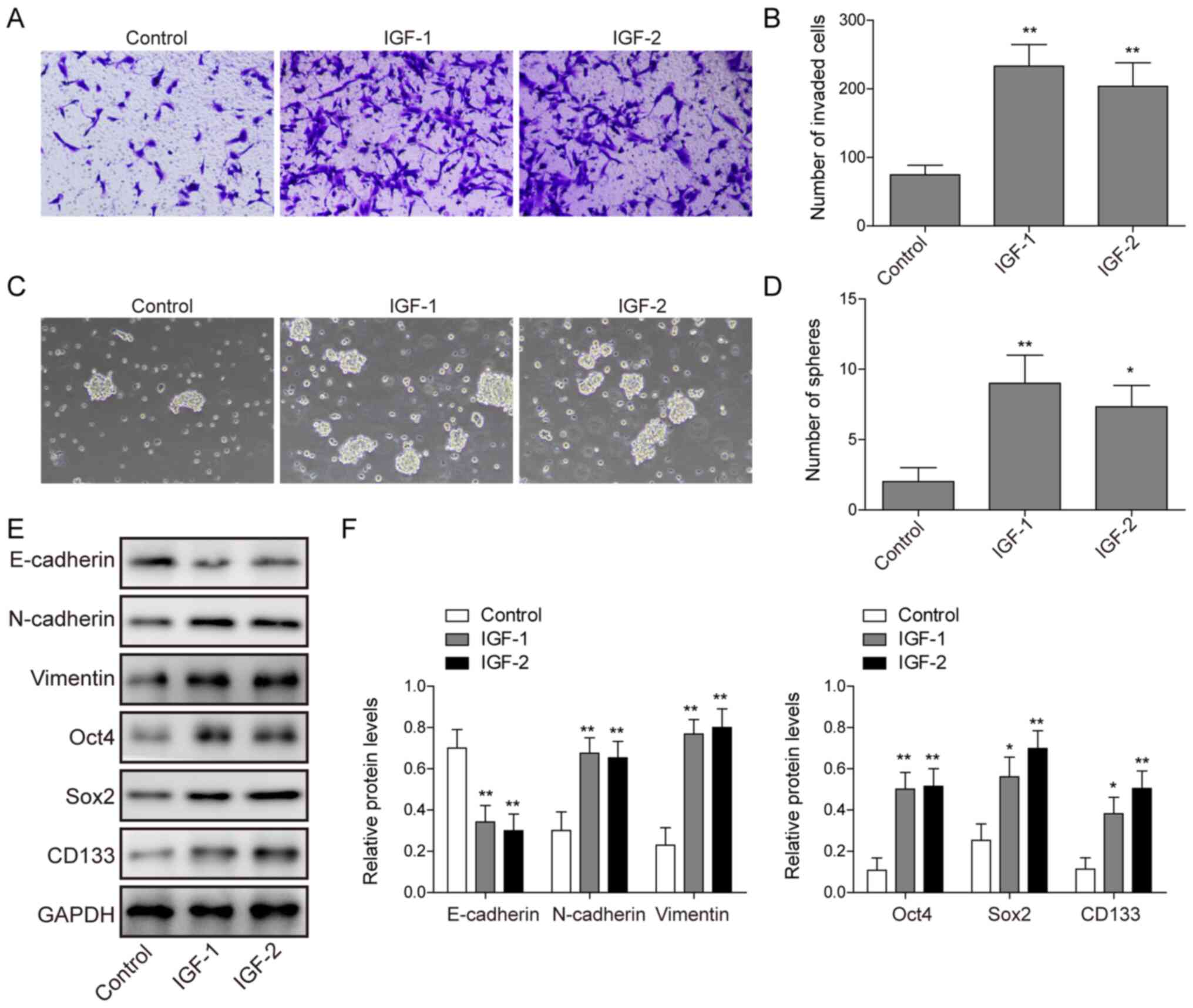
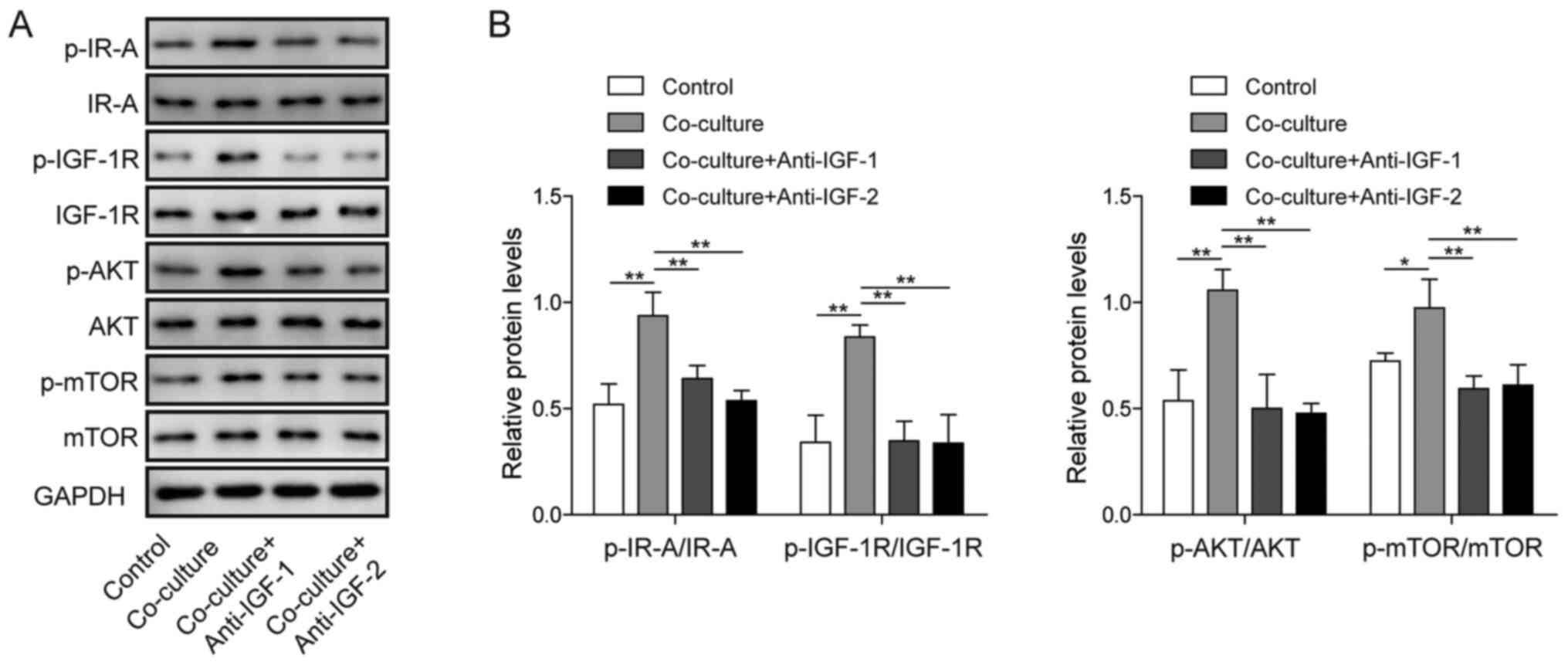
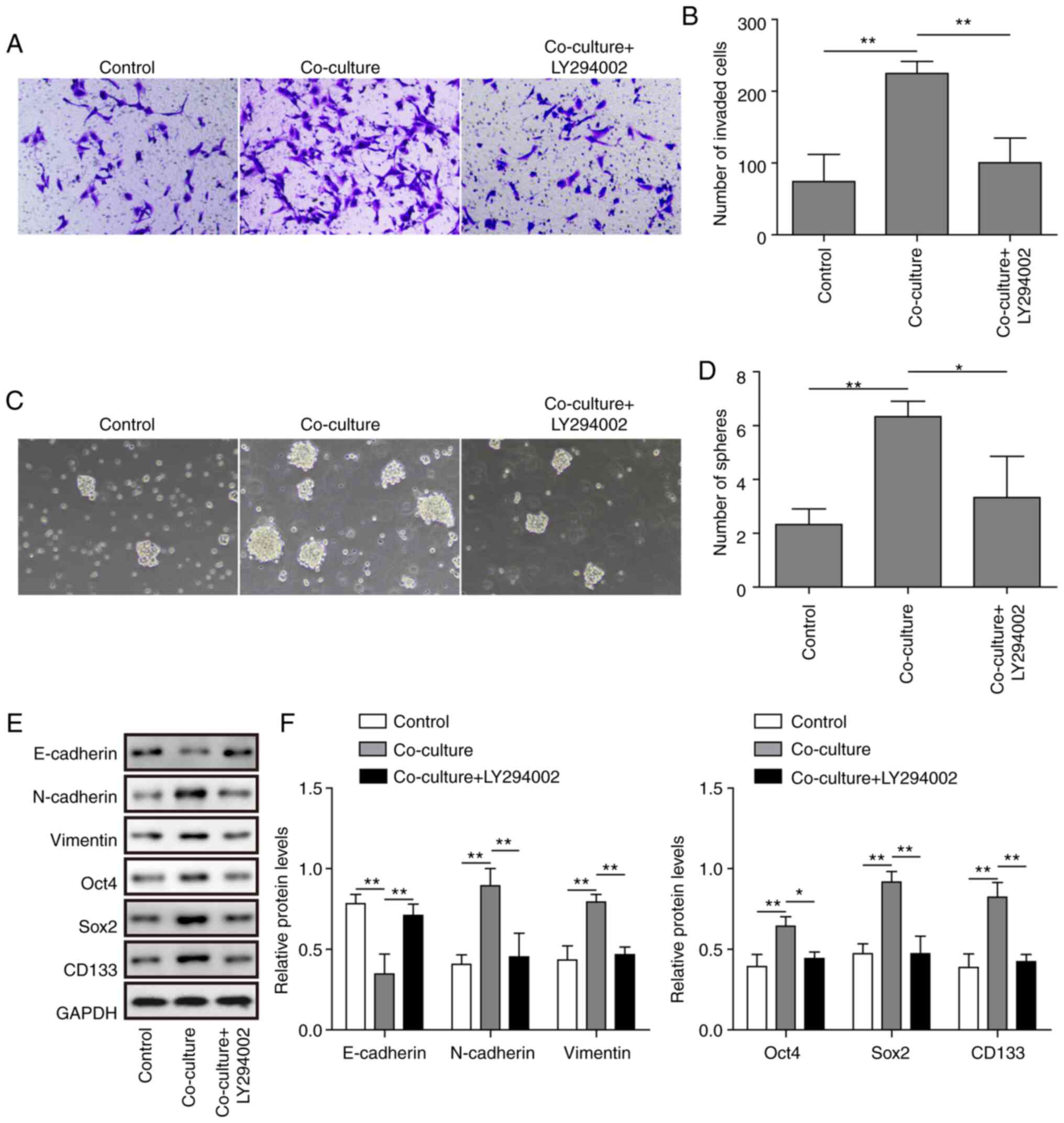
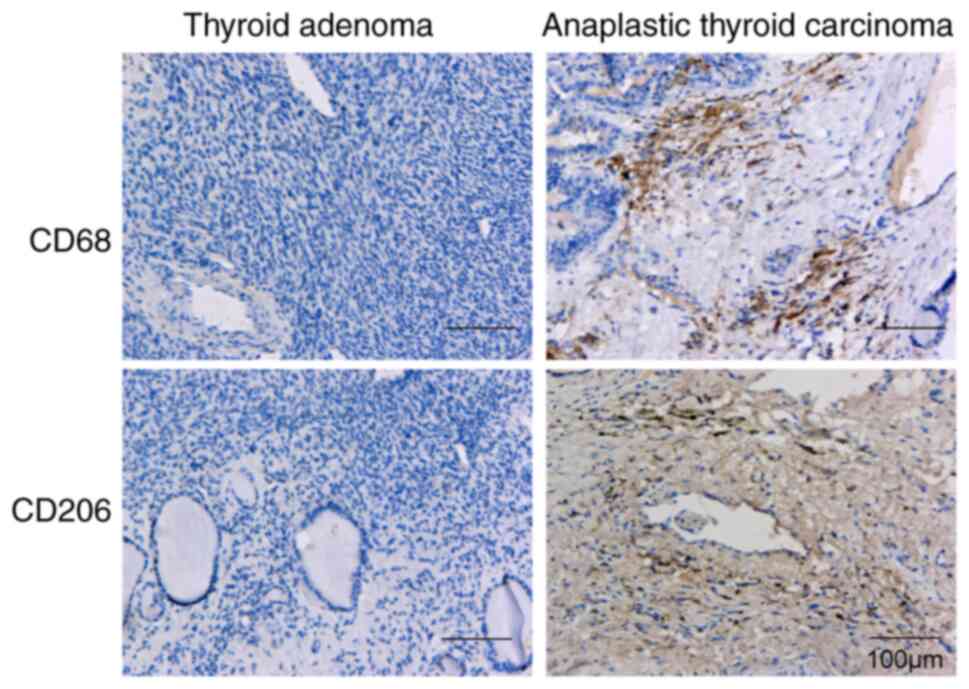
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen QT, Lee EJ, Huang MG, Park YI,
Khullar A and Plodkowski RA: Diagnosis and treatment of patients
with thyroid cancer. Am Health Drug Benefits. 8:30–40.
2015.PubMed/NCBI
|
|
3
|
Molinaro E, Romei C, Biagini A, Sabini E,
Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini
A, Torregrossa L, et al: Anaplastic thyroid carcinoma: From
clinicopathology to genetics and advanced therapies. Nat Rev
Endocrinol. 13:644–660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taccaliti A, Silvetti F, Palmonella G and
Boscaro M: Anaplastic thyroid carcinoma. Front Endocrinol
(Lausanne). 3:842012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saini S, Tulla K, Maker AV, Burman KD and
Prabhakar BS: Therapeutic advances in anaplastic thyroid cancer: A
current perspective. Mol Cancer. 17:1542018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blanpain C: Cancer Stem Cells. Radiother
Oncol. 106:S1982019.
|
|
7
|
Chang JC: Cancer stem cells: Role in tumor
growth, recurrence, metastasis, and treatment resistance. Medicine
(Baltimore). 95 (Suppl 1):S20–S25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang R and Rofstad EK: Cancer stem cells
(CSCs), cervical CSCs and targeted therapies. Oncotarget.
8:35351–35367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mertins SD: Cancer stem cells: A systems
biology view of their role in prognosis and therapy. Anticancer
Drugs. 25:353–367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kreso A and Dick JE: Evolution of the
cancer stem cell model. Cell Stem Cell. 14:275–291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Magee JA, Piskounova E and Morrison SJ:
Cancer stem cells: Impact, heterogeneity, and uncertainty. Cancer
Cell. 21:283–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Yan Y, Yang Y, Wang L, Li M and
Wang J, Liu X, Duan X and Wang J: High Infiltration of
Tumor-Associated Macrophages Influences Poor Prognosis in Human
Gastric Cancer Patients, Associates With the Phenomenon of EMT.
Medicine (Baltimore). 95:e26362016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steyaert S, Van Dorpe J, Hoorens A, Van
Biesen W and Van Laecke S: Intravenous immunoglobulins modify
relapsing membranous glomerulonephritis after kidney
transplantation: A case report. Acta Clin Belg. 73:229–232. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raggi C, Mousa HS, Correnti M, Sica A and
Invernizzi P: Cancer stem cells and tumor-associated macrophages: A
roadmap for multitargeting strategies. Oncogene. 35:671–682. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rother KI and Accili D: Role of insulin
receptors and IGF receptors in growth and development. Pediatr
Nephrol. 14:558–561. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Novosyadlyy R, Lann DE, Vijayakumar A,
Rowzee A, Lazzarino DA, Fierz Y, Carboni JM, Gottardis MM, Pennisi
PA, Molinolo AA, et al: Insulin-mediated acceleration of breast
cancer development and progression in a nonobese model of type 2
diabetes. Cancer Res. 70:741–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yakar S, Leroith D and Brodt P: The role
of the growth hormone/insulin-like growth factor axis in tumor
growth and progression: Lessons from animal models. Cytokine Growth
Factor Rev. 16:407–420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boucher J, Kleinridders A and Kahn CR:
Insulin receptor signaling in normal and insulin-resistant states.
Cold Spring Harb Perspect Biol. 6:a0091912014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuo YC, Chu JS, Lee KL, DrewV J, Zhuang W,
Chen C, Ho Y and Huang Y: Abstract 5739: Nicotine promotes
stemness-related properties and cell migration/metastasis through
IGF-1R regulation in triple negative breast cancer. Cancer Res.
77:57392017.
|
|
21
|
Liu L and Wang X, Li X, Wu X, Tang M and
Wang X: Upregulation of IGF1 by tumor-associated macrophages
promotes the proliferation and migration of epithelial ovarian
cancer cells. Oncol Rep. 39:818–826. 2018.PubMed/NCBI
|
|
22
|
Wang X, Zhu Q, Lin Y, Wu L, Wu X, Wang K,
He Q, Xu C, Wan X and Wang X: Crosstalk between TEMs and
endothelial cells modulates angiogenesis and metastasis via
IGF1-IGF1R signalling in epithelial ovarian cancer. Br J Cancer.
117:1371–1382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vella V, Pandini G, Sciacca L, Mineo R,
Vigneri R, Pezzino V and Belfiore A: A novel autocrine loop
involving IGF-II and the insulin receptor isoform-A stimulates
growth of thyroid cancer. J Clin Endocrinol Metab. 87:245–254.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao
YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, et al:
Tumor-associated macrophage-induced invasion and angiogenesis of
human basal cell carcinoma cells by cyclooxygenase-2 induction. J
Invest Dermatol. 129:1016–1025. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chanput W, Mes JJ and Wichers HJ: THP-1
cell line: An in vitro cell model for immune modulation approach.
Int Immunopharmacol. 23:37–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wong CE, Yu JS, Quigley DA, To MD, Jen KY,
Huang PY, Del Rosario R and Balmain A: Inflammation and Hras
signaling control epithelial-mesenchymal transition during skin
tumor progression. Genes Dev. 27:670–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ain KB: Anaplastic thyroid carcinoma: A
therapeutic challenge. Semin Surg Oncol. 16:64–69. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Li D, Cang H and Guo B: Crosstalk
between cancer and immune cells: Role of tumor-associated
macrophages in the tumor microenvironment. Cancer Med. 8:4709–4721.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei C, Yang C, Wang S, Shi D, Zhang C, Lin
X, Liu Q, Dou R and Xiong B: Crosstalk between cancer cells and
tumor associated macrophages is required for mesenchymal
circulating tumor cell-mediated colorectal cancer metastasis. Mol
Cancer. 18:642019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Caras I, Tucureanu C, Lerescu L, Pitica R,
Melinceanu L, Neagu S and Salageanu A: Influence of tumor cell
culture supernatants on macrophage functional polarization: In
vitro models of macrophage-tumor environment interaction. Tumori.
97:647–654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Genin M, Clement F, Fattaccioli A, Raes M
and Michiels C: M1 and M2 macrophages derived from THP-1 cells
differentially modulate the response of cancer cells to etoposide.
BMC Cancer. 15:5772015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mehibel M, Singh S, Chinje EC, Cowen RL
and Stratford IJ: Effects of cytokine-induced macrophages on the
response of tumor cells to banoxantrone (AQ4N). Mol Cancer Ther.
8:1261–1269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tariq M, Zhang JQ, Liang GK, He QJ, Ding L
and Yang B: Gefitinib inhibits M2-like polarization of
tumor-associated macrophages in Lewis lung cancer by targeting the
STAT6 signaling pathway. Acta Pharmacol Sin. 38:1501–1511. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ryder M, Ghossein RA, Ricarte-Filho JC,
Knauf JA and Fagin JA: Increased density of tumor-associated
macrophages is associated with decreased survival in advanced
thyroid cancer. Endocr Relat Cancer. 15:1069–1074. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Caillou B, Talbot M, Weyemi U,
Pioche-Durieu C, Al Ghuzlan A, Bidart JM, Chouaib S, Schlumberger M
and Dupuy C: Tumor-associated macrophages (TAMs) form an
interconnected cellular supportive network in anaplastic thyroid
carcinoma. PLoS One. 6:e225672011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang C, Su K, Zhang Y, Zhang W, Zhao Q,
Chu D and Guo R: IR-A/IGF-1R-mediated signals promote
epithelial-mesenchymal transition of endometrial carcinoma cells by
activating PI3K/AKT and ERK pathways. Cancer Biol Ther. 20:295–306.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kalet BT, O'Donoghue LE and Duval DL:
Abstract 3008: IGF2 mRNA binding protein 1 drives growth,
metastasis and chemoresistance in osteosarcoma. Cancer Res.
73:30082013.
|
|
39
|
Li B, Tsao SW, Chan KW, Ludwig DL,
Novosyadlyy R, Li YY, He QY and Cheung ALM: Id1-induced IGF-II and
its autocrine/endocrine promotion of esophageal cancer progression
and chemoresistance - implications for IGF-II and IGF-IR-targeted
therapy. Clin Cancer Res. 20:2651–2662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ciampolillo A, De Tullio C, Perlino E and
Maiorano E: The IGF-I axis in thyroid carcinoma. Curr Pharm Des.
13:729–735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sasako T and Ueki K: Insulin/IGF-1
signaling and aging. Nihon Rinsho. 74:1435–1440. 2016.PubMed/NCBI
|
|
42
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fu J, Lv H, Guan H, Ma X, Ji M, He N, Shi
B and Hou P: Metallothionein 1G functions as a tumor suppressor in
thyroid cancer through modulating the PI3K/Akt signaling pathway.
BMC Cancer. 13:4622013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Samani AA and Brodt P: The receptor for
the type I insulin-like growth factor and its ligands regulate
multiple cellular functions that impact on metastasis. Surg Oncol
Clin N Am. 10289–312. (viii)2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A,
Takahashi H, Wakasugi T, Funahashi H, Sato M and Takeyama H: IGF-1
mediates PTEN suppression and enhances cell invasion and
proliferation via activation of the IGF-1/PI3K/Akt signaling
pathway in pancreatic cancer cells. J Surg Res. 160:90–101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li H, Xu L, Li C, Zhao L, Ma Y, Zheng H,
Li Z, Zhang Y, Wang R, Liu Y, et al: Ubiquitin ligase Cbl-b
represses IGF-I-induced epithelial mesenchymal transition via ZEB2
and microRNA-200c regulation in gastric cancer cells. Mol Cancer.
13:1362014. View Article : Google Scholar : PubMed/NCBI
|