Introduction
The incidence of gallbladder and biliary tract
cancer has increased by 76% between 1990 and 2017 on a global scale
(1). Due to the low early detection
rate, gallbladder cancer (GBC) often undergoes local invasion and
lymph node metastasis (2). Most
patients with GBC are diagnosed at advanced stages and are
unresectable (3). These patients
tend to relapse despite having received standard chemotherapy and
radiotherapy. Therefore, the overall survival of GBC is extremely
low, ranging from 13.2–19 months (4,5). A
recent study revealed that a value of 65 IU/ml CA 19-9 may be
helpful in evaluating the prognosis of GBC (6). Currently, there is no effective
chemotherapy or targeted therapy for the treatment of GBC. Novel
immunotherapeutic drugs, such as immune checkpoint inhibitors of
anti-programmed cell death protein-1 antibody and anti-programmed
cell death-ligand 1 antibody, have shown limited efficacy in the
clinical intervention of GBC (7,8).
Therefore, additional efforts should be made to identify novel
targets and to determine the in-depth mechanism to advance the
understanding and the curative effect of GBC.
Estrogen-related receptor-α (ERRα) is a member of
the orphan nuclear receptors (9)
and belongs to the ERR family, which consists of ERRα, ERRβ and
ERRγ (10). ERRα was identified on
the basis of the structural similarity between its DNA binding
domain and human estrogen receptor (ER) α; however, ERRα does not
bind to natural estrogens or estrogen-like molecules (11). ERRα is involved in various
biological processes and activities, including energy metabolism
and cell proliferation and invasion, by binding to estrogen-related
response elements and estrogen response elements (EREs) (12). A number of orphan nuclear receptors
are activated by the peroxisome proliferator-activated receptor γ
(PPARγ) coactivator (PGC) family, including PGC-1α, PGC-1β and PRC
(13). In the absence of specific
ligands, ERRα can be activated by PGC-1 family members, such as
PGC-1α (14) and PGC-1β (15). Moreover, as wild-type PGC-1α (PGC-1α
WT) can activate other receptors, such as ERRβ and ERRγ,
researchers have reported that some peptides (such as L3-09) can
bind to ERRα specifically. Herein, the investigators replaced L2
and L3 motifs with L3-09 peptides to generate PGC-1α 2×9, in an
attempt to selectively activate ERRα (16). Moreover, a 3X ERE-TATA luciferase
reporter was applied to measure the activity of ERs and ERRs,
including ERRα (12).
As one of the most important signaling transduction
pathways in mammalian cells, the PI3K/AKT signaling pathway
functions to inhibit cellular apoptosis and promote proliferation
by interacting with multiple downstream effectors (17). LY294002 has been proved to
specifically inhibit the activity of the PI3K (18,19),
whereas recombinant human insulin-like growth factor-I (IGF-I) can
be applied to activate the PI3K/AKT signaling pathway (20). The binding of IGF-I to IGF-I
receptor (IGF-IR) functions to induce receptor autophosphorylation
and to elevate the tyrosine kinase activity of IGF-IR, thereby
leading to the activation of the 85-kDa subunit of PI3K by
recruiting and phosphorylating intracellular insulin receptor
substrate-1 (21–23). AKT is then activated via recruitment
to cellular membranes by the PI3K lipid (24). Previous studies have reported that
ERRα triggered PI3K/AKT phosphorylation by enhancing the
transcription of Nectin-4, thereby promoting the growth and
metastasis of GBC (25,26).
The present study aimed to investigate whether
PI3K/AKT phosphorylation could positively activate the activity and
expression of the PGC-1/ERRα axis. To that end, LY294002 and IGF-I
were used to specifically inhibit and trigger PI3K/AKT
phosphorylation, respectively. Moreover, a 3X ERE-TATA luciferase
reporter was applied to measure the degree of ERRα activation.
XCT-790 is a specific inverse agonist of ERRα. PGC-1α 2×9 and
XCT-790 were used to specifically enhance and inhibit the activity
of ERRα, respectively.
Materials and methods
Cell culture
The NOZ human GBC cell line was purchased from
Shanghai Key Laboratory of Biliary Tract Diseases, and was cultured
in William's medium E (Genom Biotech Pvt., Ltd.) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) in the humidified
incubator containing 5% CO2 at 37°C.
Chemicals
LY294002 (cat. no. S1105) was purchased from Selleck
Chemicals to inhibit PI3K phosphorylation. Recombinant human IGF-I
(cat. no. 291-G1) was acquired from R&D Systems, Inc. to
promote PI3K phosphorylation. XCT-790 (cat. no. HY-10426) was
purchased from MedChemExpress to inhibit the activity of ERRα. The
concentration gradient was set to detect the values of
IC50 or half maximal effective concentration
(EC50) of the chemicals in NOZ cells. Inhibition curves
with concentration gradients ranging from 0.1–20 µM (treatment for
72 h at 37°C) and 0.6–40 µM (treatment for 72 h at 37°C) for
LY294002 and XCT-790, respectively, were drawn to determine the
IC50 values of LY294002 and XCT-790 in NOZ cells. Based
on the IC50 values, the final concentrations of 7 µM
LY294002 and 6 µM XCT-790 were cocultured with NOZ cells at 37°C
for 72 h in indicated experiments. The activation curve with
concentration gradients ranging from 0.1–100 ng/ml (treatment for
72 h at 37°C) for IGF-I was drawn to determine the EC50
value. Based on the EC50 value, 13 ng/ml IGF-I was
cocultured with NOZ cells at 37°C for 72 h in indicated assays.
Cell Counting Kit (CCK)-8 assay
The viability of GBC cells was determined using a
CCK-8 assay (Dojindo Molecular Technologies, Inc.) according to the
manufacturer's protocol. The cells were seeded into the 96-well
plate at the density of 1×103 cells each well. Then, 24
h later, 10 µl CCK-8 solution and 90 µl complete medium were
co-cultured with NOZ cells for 2 h at 37°C. The absorbance value
(optical density) of NOZ cells was detected on a microplate reader
at the wavelength of 450 nm (Bio-Tek Instruments, Inc.).
Colony formation assay
The biological effects of LY294002 and IGF-I on the
colony formation ability of NOZ cells were tested. In brief, the
NOZ cells were seeded into 6-well plate at a density of 500 cells
each well. After 6 h, 7 µM LY294002 and 13 ng/ml IGF-I were added
into the medium for co-incubation with NOZ cells for 72 h at 37°C.
Subsequently, LY294002 and IGF-I were removed, leaving the NOZ
cells cultured at 37°C with the medium for 1 week. The cloning foci
were fixed using 4% PFA (paraformaldehyde) for 20 min and were
stained using 0.1% crystal violet for 20 min, both at room
temperature. The colonies with >50 cells were counted under a
light microscope (magnification, ×20).
Transwell invasion assay
The 8-µm Transwell filters (BD Biosciences) and
24-well Transwell chambers were used to detect the invasive
capacity of cells. In total, 70 µl 1 mg/ml Matrigel (BD
Biosciences) was added onto the upper chamber at 37°C overnight.
Then, the upper chamber with Matrigel-coated membrane was seeded
with 4×104 NOZ cells in 200 µl serum-free medium.
Moreover, 500 µl basal medium containing 15% FBS was added into the
lower chamber. Following the 20-h co-culturing in an incubator
containing 5% CO2 at 37°C, the cells that invaded to the
lower layer were fixed using 4% paraformaldehyde for 20 min and
were then stained using crystal violet for another 20 min, both at
room temperature. In total, five random fields were chosen to count
the invaded cells using a light microscope (magnification, ×20) in
order to determine the invasive capacity of NOZ cells. The assays
were carried out in triplicate.
Antibodies and western blot
analysis
Primary antibodies, including rabbit anti-PI3K p85
(1:1,000; cat. no. 4257), anti-AKT (1:1,000; cat. no. 4691) and
anti-phosphorylated (p)-AKT (Ser473; 1:2,000; cat. no. 4060) were
purchased from Cell Signaling Technology, Inc. Rabbit anti-ERRα
primary antibody (1:500; cat. no. NBP1-47254) was purchased from
Novus Biologicals, LLC. Rabbit anti-PGC-1α (1:500; cat. no.
ab191838), PGC-1β (1:1,000; cat. no. ab176328) and p-PI3K p85α
(p-Y607; 1:1,000; cat. no. ab182651) were purchased from Abcam.
Goat anti-rabbit HRP-conjugated secondary antibody (1:5,000; cat.
no. S0001) was obtained from Affinity Biosciences.
Total proteins were extracted from each group of
cells using RIPA lysis buffer (Cell Signaling Technology, Inc.),
and a BCA protein quantification kit (Thermo Fisher Scientific,
Inc.) was used to quantify the concentration of protein. A total of
30 µg protein was separated via 10–15% SDS-PAGE and the proteins
were then transferred onto PVDF membranes (MilliporeSigma). For the
testing of non-phosphorylated antibody, 5% non-fat dry milk was
used to block the PVDF membrane at room temperature for 1 h; for
the testing of phosphorylated antibody, 5% BSA (Suzhou Yacoo
Science Co., Ltd.) was used to block the membranes at room
temperature for 1 h. The incubation with primary antibody at 4°C
lasted 12 h, followed by the 2-h co-incubation with HRP-conjugated
secondary antibody (1:5,000) at room temperature. The intensities
of the signals were determined using a Gel Doc 2000 system (Bio-Rad
Laboratories, Inc.) after being visualized with an
electrochemiluminescence kit (Wuhan Boster Biological Technology
Ltd.).
RNA interference
The short hairpin (sh)RNA sequences to specifically
knockdown ERRα, PGC-1α and PGC-1β were 5′-GCGAGAGGAGUAUGUUCUA-3′,
5′-GAUGUGAACGACUUGGAUACA −3′ and 5′-UGUAGUUCUGUACAACUUCGG−3′,
respectively. The sequence for negative control (scrambled
sequence) was 5′-TTCTCCGAACGTGTCACGT-3′. All sequences were
constructed by Genomeditech Biotechnology, and were inserted into
the PGMLV-SC5 lentivirus core vector (Genomeditech Biotechnology).
In serum-free medium, the concentrated viruses with a MOI of 40
were then infected into the NOZ cells using ViaFect™ transfection
reagent (Promega Corporation) following the manufacturer's
instructions at 37°C. The supernatant was replaced with complete
culture medium after 24 h. Subsequent experimentations were
performed after 120 h.
Construction of plasmids and
transfection
pGL3-Basic-3X ERE-TATA-luc that contains triple the
AGGTCANNNTGACCT, plasmids with WT PGC-1α [pCDNA3.1(+)-3 ×
Flag-C-M-PGC-1α-WT] and mutant-type (MT) PGC-1α [pCDNA3.1(+)-3 ×
Flag-CM-PGC-1α-2×9] were synthesized by Genomeditech Biotechnology,
in accordance with the protocol described in a previous study
(16). A total of 2 µg constructed
plasmids were then transfected into the NOZ cells using ViaFect
Transfection reagent at 37°C. The supernatant was replaced with
complete culture medium after 24 h. The expression level was
analyzed via western blot analysis after 120 h. Moreover, the empty
vector-infected cells (Mock-transfected) were used as the
control.
Dual luciferase reporter gene
assay
pGL3-Basic-3X ERE-TATA-luc was applied to detect
ERRα activity. pRL-TK plasmids (25 ng; Genomeditech Biotechnology)
containing PGC-1α WT or PGC-1α 2×9 (250 ng) and pGL3-Basic-3X
ERE-TATA-luc (250 ng) were transfected into NOZ cells using 1.5 µl
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 48 h. The activity of Renilla
luciferase and firefly luciferase was detected on a luminometer
using a SEAP Reporter Gene assay kit (Abcam; cat. no. ab133077).
The empty vector-infected cells were used as the internal control.
Finally, the results were expressed as the ratio of firefly
luciferase activity/Renilla luciferase activity.
Statistical analysis
Quantitative data are presented as the mean ± SD
based on triplicated experiments. An unpaired Student's t-test was
used to compare the inter-group difference between two groups using
GraphPad Prism 8.0 software (GraphPad Software, Inc.) for
statistical analyses. Comparative data among multiple groups were
analyzed using one-way ANOVA followed by Tukey's test, using SPSS
19.0 for Windows (IBM Corp.). The suppression curves for IGF-I,
LY294002 and XCT-790 were plotted according to the results of seven
differential concentrations. P<0.05 was considered to indicate a
statistically significant difference.
Results
Sensitivity of NOZ cells to IGF-I and
LY294002
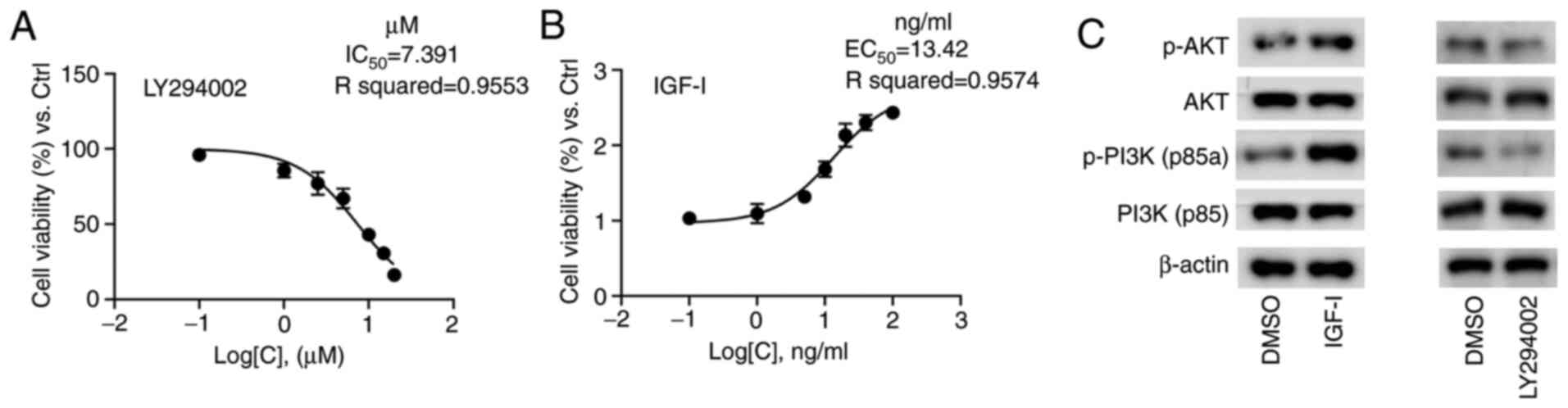
The concentration gradients ranging from 0.1–20 µM
were set to draw the inhibition curve, which demonstrated that the
IC50 value of LY294002 was 7.39 µM in NOZ cells
(Fig. 1A). Similarly, the
activation curve for IGF-I was drawn to determine that the value of
EC50 was 13.42 ng/ml (Fig.
1B). The final concentrations of 7 µM for LY294002 and 13 ng/ml
for IGF-I were applied in the subsequent assays, and no obvious
cytotoxicity was observed. The results of western blot analysis
revealed that the protein expression levels of p-PI3K p85a and
p-AKT were notably elevated in the NOZ cells cultured with IGF-I
(Fig. 1C), indicating that IGF-I
effectively activated the PI3K/AKT signaling pathway via PI3K/AKT
phosphorylation. Conversely, LY294002 markedly reduced the
expression levels of p-PI3K p85a and p-AKT (Fig. 1C), suggesting that LY294002
effectively diminished the PI3K/AKT signaling pathway via PI3K/AKT
dephosphorylation.
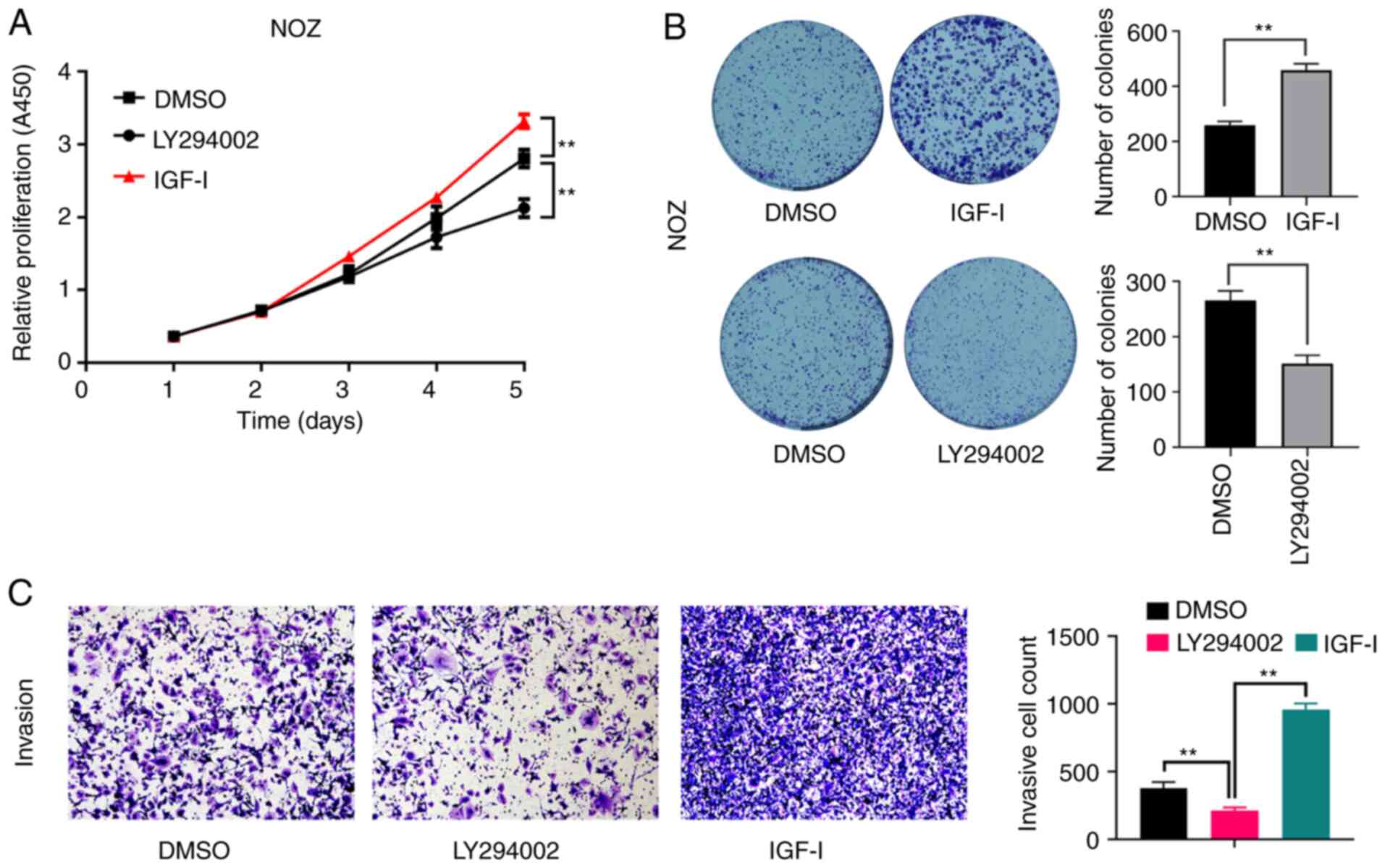
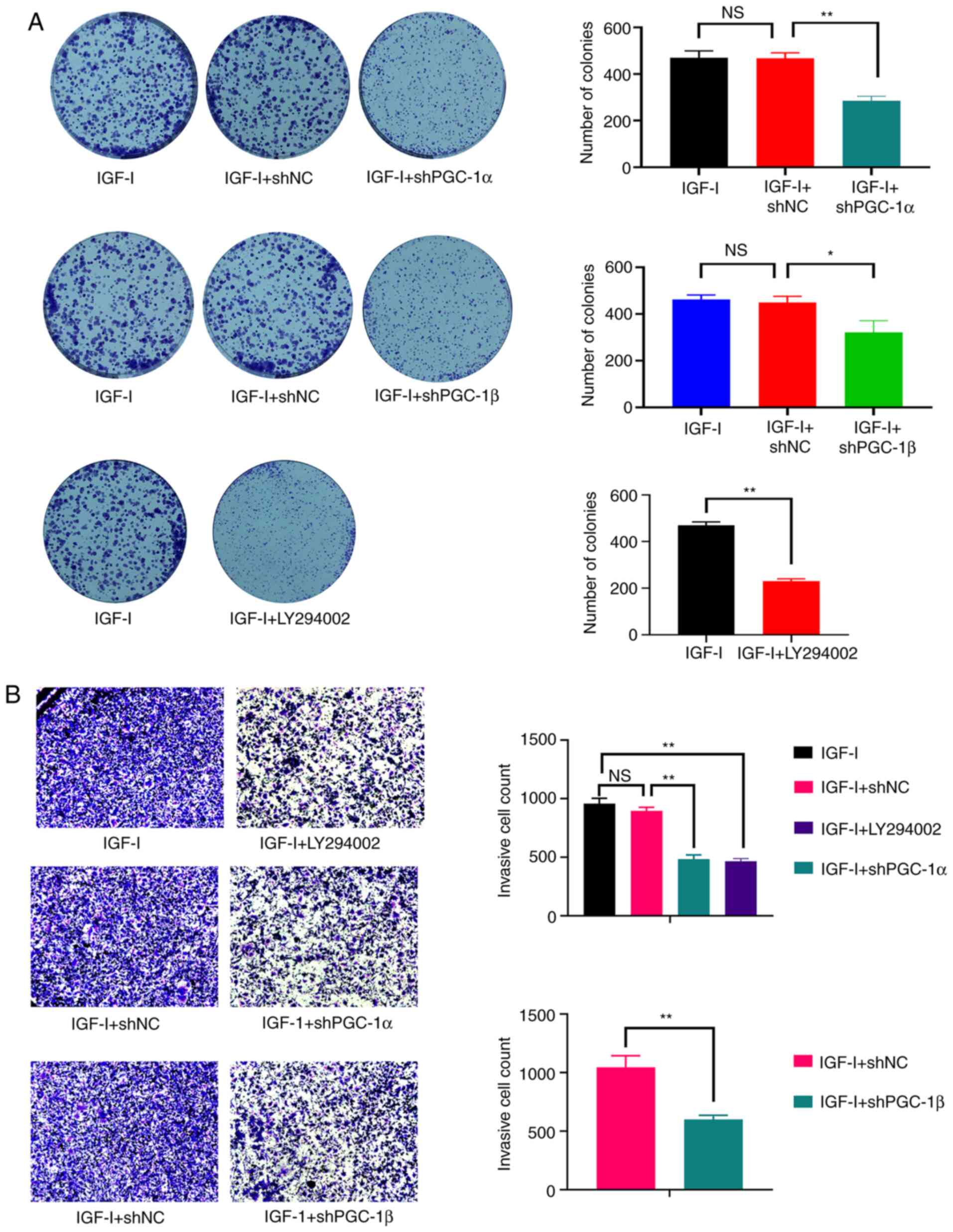
Consistently, the proliferative capacity (Fig. 2A), colony formation ability
(Fig. 2B) and the invasive capacity
(Fig. 2C) of NOZ cells were
significantly enhanced by IGF-I, but were significantly inhibited
by LY294002.
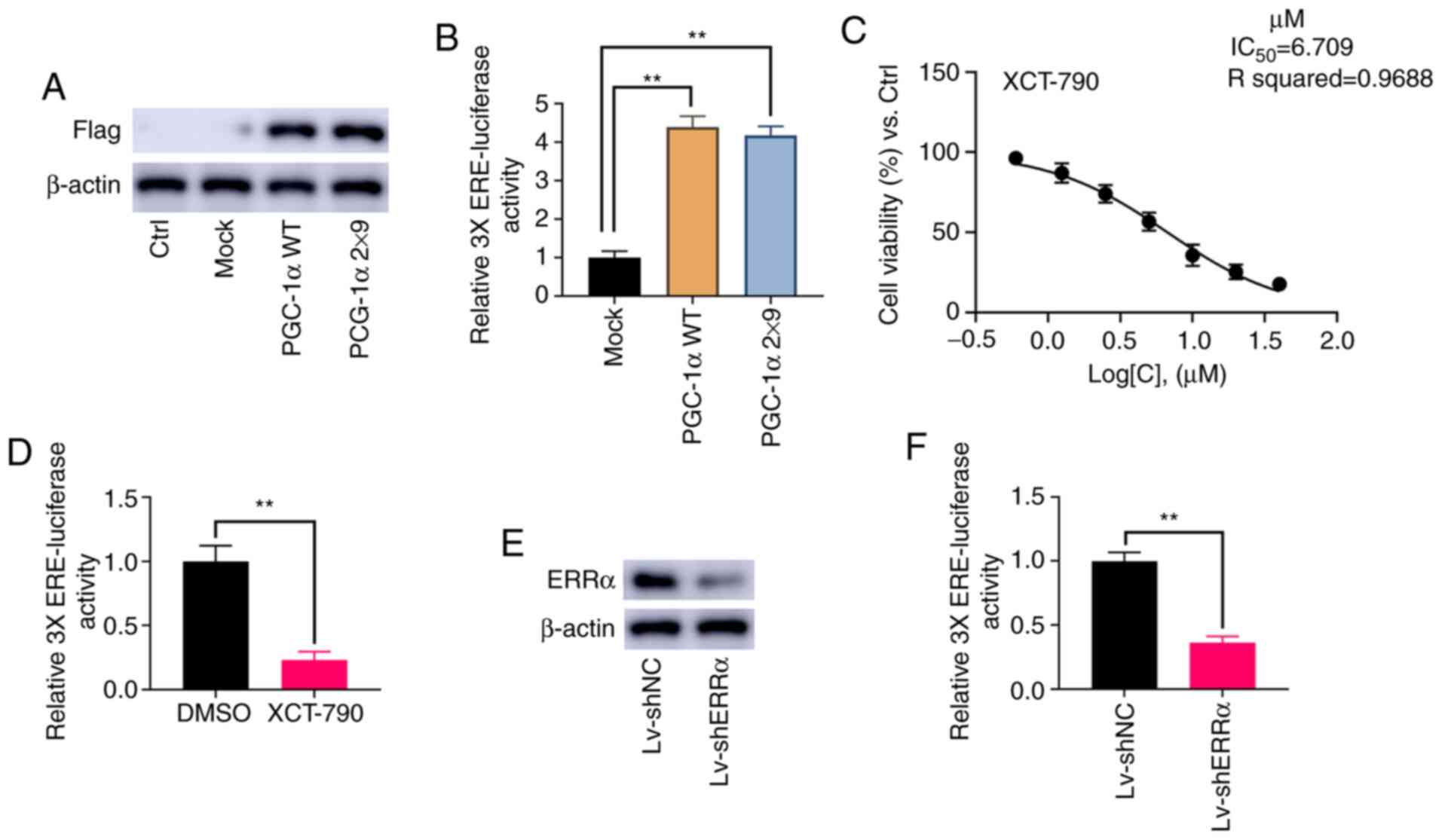
Detection of ERRα activation
PGC-1α can activate ERRα, as well other receptors
(16). To specifically and
selectively activate ERRα, the current study followed the protocol
described by Gaillard et al (16) and Chang et al (27), replacing both L2 and L3 motifs in WT
PGC-1α with L3-09 peptides to generate PGC-1α 2×9. PGC-1α and
PGC-1α 2×9 were successfully overexpressed in NOZ cells (Fig. 3A). As shown in Fig. 3B, the relative activity of 3X ERE
TATA dual luciferase reporter was significantly increased by PGC-1α
and PGC-1α 2×9 (P<0.01).
As a specific inverse agonist of ERRα, XCT-790 can
inhibit the activation of ERRα (28). The results demonstrated that the
IC50 value of XCT-790 in NOZ cells was 6.71 µM (Fig. 3C), and therefore, a final
concentration at 6 µM XCT-790 was applied in subsequent assays. As
presented in Fig. 3D, 6 µM XCT-790
significantly inhibit the activation of 3X ERE TATA dual luciferase
reporter (P<0.01). Moreover, it was found that the knockdown of
ERRα significantly reduced the activation of 3X ERE TATA dual
luciferase reporter (P<0.01; Fig. 3E
and F). These results indicated that the relative 3X ERE TATA
luciferase activity was consistent with the activity of ERRα.
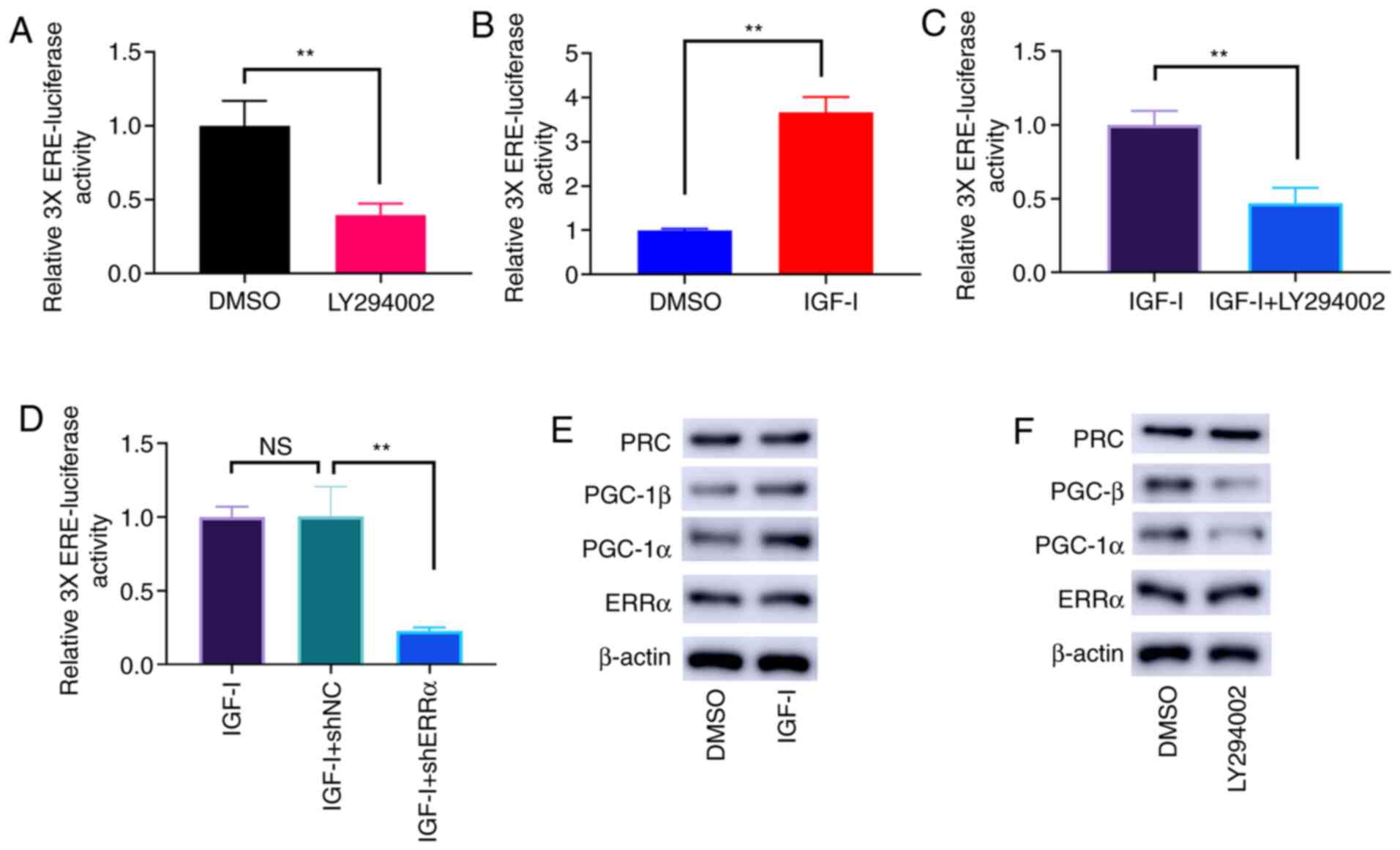
PI3K/AKT phosphorylation triggers the
ERRα activity
The dephosphorylation of PI3K/AKT by LY294002 led to
the lower activities of ERRα (Fig.
4A). Conversely, PI3K/AKT phosphorylation induced by IGF-I
enhanced the activities of ERRα (Fig.
4B), and this effect was offset by LY294002 (Fig. 4C) and ERRα knockdown (Fig. 4D). Nevertheless, the protein
expression level of ERRα was not affected by PI3K/AKT
phosphorylation. As potential coactivators of ERRα, PGC-1α and
PGC-1β expression was notably elevated by PI3K/AKT phosphorylation
(Fig. 4E). Conversely,
dephosphorylation of PI3K/AKT by LY294002 reduced the protein
expression levels of PGC-1α and PGC-1β (Fig. 4F). However, the protein expression
level of PGC-related coactivator (PRC) was not affected by PI3K/AKT
phosphorylation (Fig. 4E and
F).
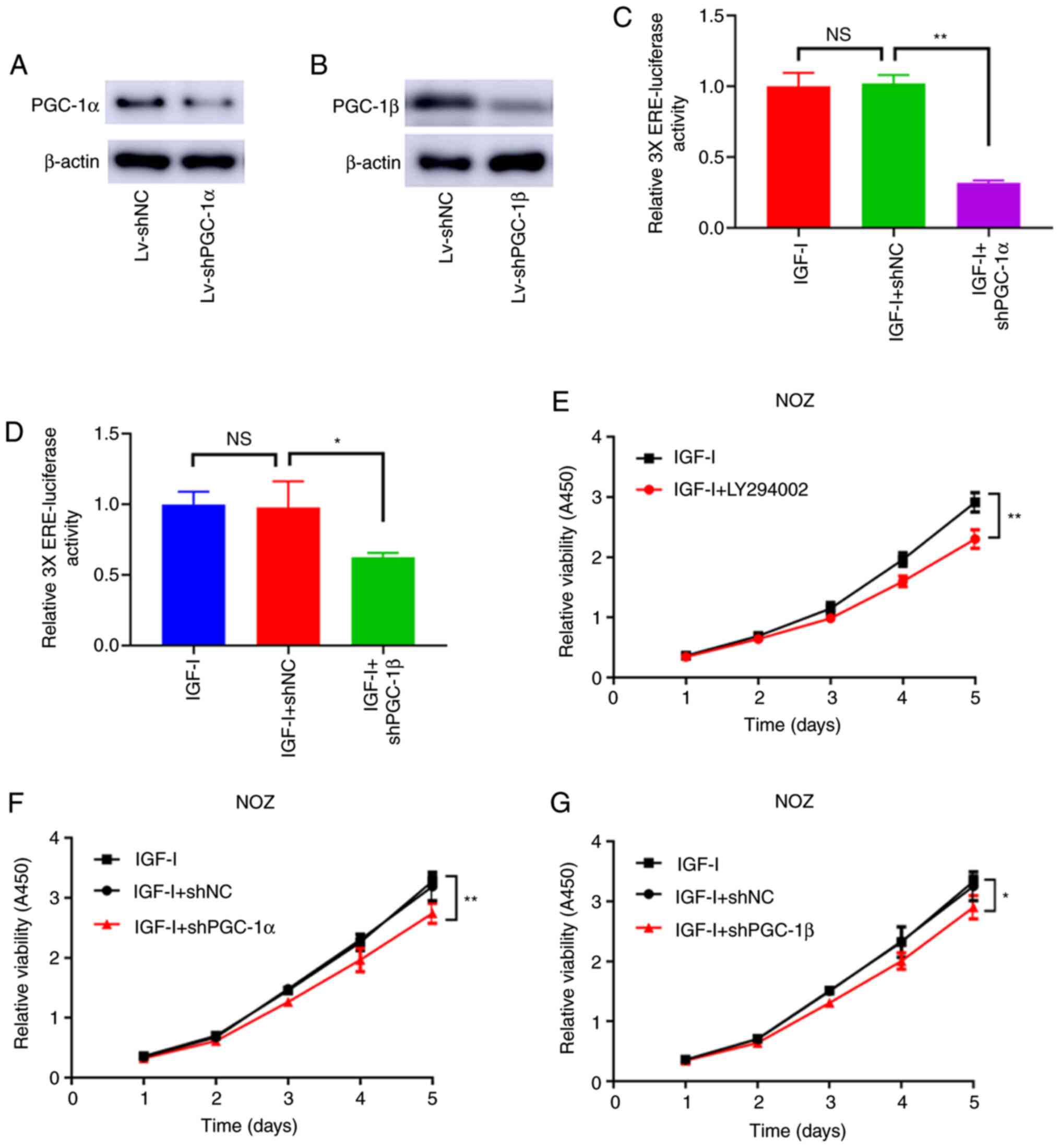
PGC-1α and PGC-1β mediate the
activation of ERRα enhanced by IGF-I
As shown in Fig. 5A and
B, PGC-1α and PGC-1β were effectively knocked down by
Lv-shPGC-1α and Lv-shPGC-1β. Moreover, the loss of PGC-1α and
PGC-1β antagonized the increased ERRα activity caused by IGF-I
(Fig. 5C and D). Similarly, the
enhanced cell viability caused by IGF-I was antagonized by the
knockdown of PGC-1α and PGC-1β and the treatment of LY294002
(Fig. 5E-G). The effect of LY294002
treatment and the knockdown of PGC-1α and PGC-1β also antagonized
the increased colony formation and invasive ability of NOZ cells
(Fig. 6A and B). Therefore, the
activation effect of PI3K/AKT on ERRα was attributable to its
ability of elevating PGC-1α and PGC-1β expression.
Discussion
The vast majority of GBC cases are diagnosed at the
advanced stages, and the low 5-year survival rate of patients with
advanced GBC is aggravated by low sensitivity to chemoradiotherapy
and targeted therapy (29).
Moreover, the molecular mechanisms that underlie the onset and
progression of GBC continue to defy the medical community (30). Thus, additional efforts are required
to develop novel effective targeted therapies, which are considered
to be the key to improve the prognosis and the quality of life of
patients with GBC.
Our previous study reported that ERRα enhanced the
transcription of Nectin-4, thereby triggering the PI3K/AKT
signaling pathway to promote the growth and metastasis of GBC
(25). As an orphan nuclear
receptor in the nucleus, ERRα bears structural resemblance to ERα.
Nevertheless, ERRα cannot be activated by estrogen (11). The majority of the genes under the
regulation of ERRα are distinct from those mediated by ERα. The
PGC-1 family serves as co-activators to activate ERRα, which, once
activated, can regulate the expression levels of genes that are
involved in the tricarboxylic acid cycle, lipid metabolism and
oxidative phosphorylation (27).
Accumulating evidence has shown that ERRα may be involved in a wide
variety of cancer types (31).
Therefore, in-depth examination into the molecular mechanisms that
affect the activity of ERRα could shed light on ERRα targets. For
example, in a recent study, Yang et al (15) revealed that F-box and leucine-rich
repeat protein 10 increased ERRα enrichment at the promoter region
of its target genes by promoting the mono-ubiquitylation of ERRα.
However, additional, novel pathogenesis mechanisms are yet to be
elucidated.
The primary aim of the present study was to validate
whether PI3K/AKT phosphorylation affects and regulates ERRα
activity in GBC cells to form a positive feedback loop. To that
end, IGF-I and LY294002 were used to enhance and inhibit PI3K/AKT
phosphorylation in NOZ cells, respectively. The present results
demonstrated that the bioactivity of ERRα was upregulated and
downregulated, respectively, and hence a positive feedback loop of
ERRα/PI3K/AKT could be established.
The genes in the PI3K/AKT pathway show the highest
frequency of aberrant expression in human cancer (17,32).
The activated PI3K/AKT pathway functions to enhance the
transformation, proliferation and invasion of cancerous cells.
Moreover, the aberrant overexpression or activation of PI3K/AKT has
been reported in various malignancies, including GBC, and is
associated with an improved proliferative capacity and invasive
potential of cancerous cells (17).
Therefore, the PI3K/AKT signaling pathway is an ideal target to
provide a promising approach for the prevention and clinical
therapy of cancer cases. The PI3K/AKT signaling pathway exerts an
anti-apoptotic effect mainly by influencing a variety of downstream
effector molecules, such as CREB regulated transcription
coactivator 1, ribosomal protein S6 kinase B1, S6 Rb and eukaryotic
translation initiation factor 4E (17,32).
At present, the PI3K/AKT signaling pathway and its related genes
can be suppressed by applying gene intervention methods or via the
treatment of small-molecule compound drugs. Blocking the activation
of a variety of downstream anti-apoptotic effector molecules and
promoting cell apoptosis are regarded as effective means to treat
cancer (33). In the present study,
it was found that PI3K/AKT phosphorylation activated ERRα, but does
not promote the amplification of ERRα, which indicated that the
activity of ERRα depends on the binding state rather than the total
amount. The abundant factors in the ERRα/PI3K/AKT circuit are
regarded as potential targets for the targeted therapy of GBC.
Therefore, a novel combination therapy using the antagonist of ERRα
and the inhibitors of PI3K/AKT signaling has a promising prospect
to improve the prognosis of patients with GBC.
The present study demonstrated that PGC-1α and
PGC-1β were downstream targets of the PI3K/AKT signaling pathway,
and that the PGC-1 family acted as the nuclear transcription
co-activator that mediates multiple cellular pathways, among which
the regulation of metabolism (34)
and tissue-specific functions (13,35–37)
are most prominent. The PGC-1 family consists of PGC-1α, PGC-1β and
PRC (13). The PGC-1 family serves
a critical role in the regulation of mitochondrial biogenesis and
bioenergetics. Furthermore, PGC-1 co-activators are essential to
sustain tumor survival and growth (38). PGC-1α activity is regulated by a
number of post-translational modifications, such as methylation,
phosphorylation and acetylation (39). PGC-1α and PGC-1β bind to multiple
nuclear transcription factors or hormone receptors, including ER,
ERR and thyroid hormone receptor. The presence of PGC-1α and PGC-1β
is required for the activity of ERRα (36). In NOZ cells, the phosphorylated
PI3K/AKT function could elevate the activity of PGC-1α and PGC-1β,
and thereby enhance ERRα activity.
In summary, the present study reported the
sensitivity and dosage of LY294002 and IGF-I in inhibiting and
activating the PI3K/AKT signaling pathway in NOZ cells,
respectively. The experimental results of dual luciferase reporter
gene assay indicated that ERRα was positively regulated by PI3K/AKT
phosphorylation. Furthermore, PGC-1α and PGC-1β were shown to
mediate the activation of ERRα stimulated by PI3K/AKT
phosphorylation. Thus, the combined inhibition of multiple targets
in the positive feedback loop of ERRα/PI3K/AKT may present
significant potential to provide promising anti-cancer
solutions.
Acknowledgements
The authors would like to thank Dr Chingyi Chang at
Duke University for providing guidance in designing 3X ERE-TATA-luc
and PGC-1α-2×9.
Funding
This work was supported by the following Funds:
Natural Science Foundation of Jiangsu Province (grant no.
BK20181129), The Science Foundation of Health Commission of Wuxi
(grant no. Q201714) and The Project of Public Health Research
Center at Jiangnan University (grant no. JUPH201829).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW, MY and HJ designed the study, analyzed the data,
performed the experiments and wrote the manuscript. LW and HJ
performed the critical revision of the manuscript and supervised
the study. All authors read and approved the final manuscript. LW
and HJ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ouyang G, Liu Q, Wu Y, Liu Z, Lu W, Li S,
Pan G and Chen X: The global, regional, and national burden of
gallbladder and biliary tract cancer and its attributable risk
factors in 195 countries and territories, 1990 to 2017: A
systematic analysis for the Global Burden of Disease Study 2017.
Cancer. 127:2238–2250. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baiu I and Visser B: Gallbladder cancer.
JAMA. 320:12942018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Kong Z, Xu X, Yun X, Chao J, Ding
D, Li T, Gao Y, Guan N, Zhu C and Qin X: ARRB1 drives gallbladder
cancer progression by facilitating TAK1/MAPK signaling activation.
J Cancer. 12:1926–1935. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma A, Sharma KL, Gupta A, Yadav A and
Kumar A: Gallbladder cancer epidemiology, pathogenesis and
molecular genetics: Recent update. World J Gastroenterol.
23:3978–3998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu YP, Jin YP, Wu XS, Yang Y, Li YS, Li
HF, Xiang SS, Song XL, Jiang L, Zhang YJ, et al: LncRNA-HGBC
stabilized by HuR promotes gallbladder cancer progression by
regulating miR-502-3p/SET/AKT axis. Mol Cancer. 18:1672019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim M, Kim H, Han Y, Sohn H, Kang JS, Kwon
W and Jang JY: Prognostic value of carcinoembryonic antigen (CEA)
and carbohydrate antigen 19-9 (CA 19-9) in gallbladder cancer; 65
IU/ml of CA 19-9 is the new cut-off value for prognosis. Cancers
(Basel). 13:10892021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Liu F, Zhang F, Zhou W, Jiang X,
Yang Y, Qu K, Wang Y, Ma Q, Wang T, et al: Genomic ERBB2/ERBB3
mutations promote PD-L1-mediated immune escape in gallbladder
cancer: A whole-exome sequencing analysis. Gut. 68:1024–1033. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Wu X, Wu H, Gu Y, Shao Y, Shao Q,
Zhu F, Li X, Qian X, Hu J, et al: Camrelizumab plus gemcitabine and
oxaliplatin (GEMOX) in patients with advanced biliary tract cancer:
A single-arm, open-label, phase II trial. J Immunother Cancer.
8:e0012402020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Giguère V, Yang N, Segui P and Evans RM:
Identification of a new class of steroid hormone receptors. Nature.
331:91–94. 1988. View
Article : Google Scholar
|
|
10
|
Deblois G and Giguère V: Functional and
physiological genomics of estrogen-related receptors (ERRs) in
health and disease. Biochim Biophys Acta. 1812:1032–1040. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SY, Yang CS, Lee HM, Kim JK, Kim YS,
Kim YR, Kim JS, Kim TS, Yuk JM, Dufour CR, et al: ESRRA
(estrogen-related receptor α) is a key coordinator of
transcriptional and post-translational activation of autophagy to
promote innate host defense. Autophagy. 14:152–168. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deblois G, Hall JA, Perry MC, Laganière J,
Ghahremani M, Park M, Hallett M and Giguère V: Genome-wide
identification of direct target genes implicates estrogen-related
receptor alpha as a determinant of breast cancer heterogeneity.
Cancer Res. 69:6149–6157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo C, Widlund HR and Puigserver P: PGC-1
coactivators: Shepherding the mitochondrial biogenesis of tumors.
Trends Cancer. 2:619–631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huss JM, Kopp RP and Kelly DP: Peroxisome
proliferator-activated receptor coactivator-1alpha (PGC-1alpha)
coactivates the cardiac-enriched nuclear receptors estrogen-related
receptor-alpha and -gamma. Identification of novel leucine-rich
interaction motif within PGC-1alpha. J Biol Chem. 277:40265–40274.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Y, Li S, Li B, Li Y, Xia K, Aman S,
Yang Y, Ahmad B, Zhao B and Wu H: FBXL10 promotes ERRα protein
stability and proliferation of breast cancer cells by enhancing the
mono-ubiquitylation of ERRα. Cancer Lett. 502:108–119. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gaillard S, Grasfeder LL, Haeffele CL,
Lobenhofer EK, Chu TM, Wolfinger R, Kazmin D, Koves TR, Muoio DM,
Chang CY, et al: Receptor-selective coactivators as tools to define
the biology of specific receptor-coactivator pairs. Mol Cell.
24:797–803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mayer IA and Arteaga CL: The PI3K/AKT
pathway as a target for cancer treatment. Annu Rev Med. 67:11–28.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang F, Xie HY, Yang LF, Zhang L, Zhang
FL, Liu HY, Li DQ and Shao ZM: Stabilization of MORC2 by estrogen
and antiestrogens through GPER1-PRKACA-CMA pathway contributes to
estrogen-induced proliferation and endocrine resistance of breast
cancer cells. Autophagy. 16:1061–1076. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia P, Gütl D, Zheden V and Heisenberg CP:
Lateral inhibition in cell specification mediated by mechanical
signals modulating TAZ activity. Cell. 176:1379–1392.e14. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu Y, Tao F, Zhou MT and Tang KF: The
signaling pathways that mediate the anti-cancer effects of caloric
restriction. Pharmacol Res. 141:512–520. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Girnita L, Worrall C, Takahashi S,
Seregard S and Girnita A: Something old, something new and
something borrowed: Emerging paradigm of insulin-like growth factor
type 1 receptor (IGF-1R) signaling regulation. Cell Mol Life Sci.
71:2403–2427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
LeRoith D, Werner H, Beitner-Johnson D and
Roberts CT Jr: Molecular and cellular aspects of the insulin-like
growth factor I receptor. Endocr Rev. 16:143–163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Myers MG Jr, Backer JM, Sun XJ, Shoelson
S, Hu P, Schlessinger J, Yoakim M, Schaffhausen B and White MF:
IRS-1 activates phosphatidylinositol 3′-kinase by associating with
src homology 2 domains of p85. Proc Natl Acad Sci USA.
89:10350–10354. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vanhaesebroeck B and Alessi DR: The
PI3K-PDK1 connection: More than just a road to PKB. Biochem J.
346:561–576. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Yang M, Guo X, Yang Z, Liu S, Ji Y
and Jin H: Estrogen-related receptor-α promotes gallbladder cancer
development by enhancing the transcription of Nectin-4. Cancer Sci.
111:1514–1527. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Liu S, Wang L, Wu Y, Hao J, Wang
Z, Lu W, Wang XA, Zhang F, Cao Y, et al: A novel PI3K/AKT signaling
axis mediates Nectin-4-induced gallbladder cancer cell
proliferation, metastasis and tumor growth. Cancer Lett.
375:179–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang CY, Kazmin D, Jasper JS, Kunder R,
Zuercher WJ and McDonnell DP: The metabolic regulator ERRα, a
downstream target of HER2/IGF-1R, as a therapeutic target in breast
cancer. Cancer Cell. 20:500–510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Theodoris CV, Zhou P, Liu L, Zhang Y,
Nishino T, Huang Y, Kostina A, Ranade SS, Gifford CA, Uspenskiy V,
et al: Network-based screen in iPSC-derived cells reveals
therapeutic candidate for heart valve disease. Science.
371:eabd07242021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramaswamy A, Ostwal V, Sharma A, Bhargava
P, Srinivas S, Goel M, Patkar S, Mandavkar S, Jadhav P, Parulekar
M, et al: Efficacy of capecitabine plus irinotecan vs irinotecan
monotherapy as second-line treatment in patients with advanced
gallbladder cancer: A multicenter phase 2 randomized clinical trial
(GB-SELECT). JAMA Oncol. 7:436–439. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nepal C, Zhu B, O'Rourke CJ, Bhatt DK, Lee
D, Song L, Wang D, Van Dyke A, Choo-Wosoba H, Liu Z, et al:
Integrative molecular characterization of gallbladder cancer
reveals microenvironment-associated subtypes. J Hepatol.
74:1132–1144. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ranhotra HS: Estrogen-related receptor
alpha and cancer: Axis of evil. J Recept Signal Transduct Res.
35:505–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mercurio L, Albanesi C and Madonna S:
Recent updates on the involvement of PI3K/AKT/mTOR molecular
cascade in the pathogenesis of hyperproliferative skin disorders.
Front Med (Lausanne). 8:6656472021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song M, Bode AM, Dong Z and Lee MH: AKT as
a therapeutic target for cancer. Cancer Res. 79:1019–1031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Villena JA: New insights into PGC-1
coactivators: Redefining their role in the regulation of
mitochondrial function and beyond. FEBS J. 282:647–672. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin J, Handschin C and Spiegelman BM:
Metabolic control through the PGC-1 family of transcription
coactivators. Cell Metab. 1:361–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Patten IS and Arany Z: PGC-1 coactivators
in the cardiovascular system. Trends Endocrinol Metab. 23:90–97.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li S, Liu C, Li N, Hao T, Han T, Hill DE,
Vidal M and Lin JD: Genome-wide coactivation analysis of PGC-1alpha
identifies BAF60a as a regulator of hepatic lipid metabolism. Cell
Metab. 8:105–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vernier M and Giguère V: Aging, senescence
and mitochondria: The PGC-1/ERR axis. J Mol Endocrinol. 66:R1–R14.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chambers JM and Wingert RA: PGC-1α in
disease: Recent renal insights into a versatile metabolic
regulator. Cells. 9:22342020. View Article : Google Scholar : PubMed/NCBI
|