Introduction
Pulmonary fibrosis is a disease induced by
interstitial pneumonia; however, to the best of our knowledge, the
mechanism underlying the occurrence of this disease is yet to be
elucidated (1,2). At present, injury to epithelial cells,
cellular senescence and an abnormal immune response are considered
to contribute to lung fibrosis development (3–5).
Moreover, the excessive accumulation of fibroblasts and the
increased secretion of extracellular matrix promotes the fibrosis
of lung tissues (6). However, the
specific molecular mechanism underlying lung fibrosis requires
further investigations.
Non-coding RNAs are types of RNA that cannot encode
proteins, and previous studies have reported that non-coding RNA
could participate in, and regulate various physiological processes,
such as cell differentiation, aging and the cell cycle (7). Long non-coding RNAs (lncRNAs) are a
type of non-coding RNA that are >200 nucleotides in length, and
the lncRNAs have been reported to regulate the proliferation of
multiple types of cells (8). The
upregulated lncRNA HOXA distal transcript antisense (HOTTIP) was
revealed to promote the development of prostate cancer and lung
cancer (9,10). A previous study reported that the
expression levels of HOTTIP were upregulated during the development
of hepatic fibrosis, and higher levels of HOTTIP induced the
fibrosis of hepatic tissue by downregulating the expression levels
of microRNA (miRNA/miR)-148a (11).
Furthermore, the upregulated expression level of the lncRNA HOTTIP
enhanced the fibrosis of liver tissues by inducing the activation
of hepatic stellate cells (12).
However, to the best of our knowledge, whether lncRNA HOTTIP can
affect the occurrence and development of fibrosis of lung tissues
remains unknown.
starBase was used to predict targets of lncRNA
HOTTIP. Previous studies have revealed that the upregulated
expression levels of miR-744-5p promoted the proliferation and
metastasis of ovarian cancer and non-small cell lung cancer cells
(13,14). In addition, analysis using starBase
revealed that miR-744-5p could target and regulate the expression
of polypyrimidine tract binding protein 1 (PTBP1). It has been
observed that the expression levels of PTBP1 are upregulated in
pulmonary fibrosis tissues from mice (15). However, to the best of our
knowledge, whether the lncRNA HOTTIP could promote the fibrosis of
lung tissues by regulating the miR-744-5p/PTBP1 axis is yet to be
elucidated.
The present study established in vitro and
in vivo pulmonary fibrosis models to investigate the role of
HOTTIP in pulmonary fibrosis. Moreover, the binding relationship
between miR-774-5p and lncRNA HOTTIP or PTBP1 was determined. The
current results revealed that the molecular functions of the lncRNA
HOTTIP were achieved by regulating the miR-774-5p/PTBP1 signaling
axis, which suggested the importance of the lncRNA
HOTTIP/miR-774-5p/PTBP1 signaling pathway during the development of
lung fibrosis.
Materials and methods
Cell lines and culture
A549 cells were obtained from the American Type
Culture Collection and cultured in RPMI-1640 medium (Hyclone;
Cytiva) supplemented with 10% FBS (Gibco; Thermo Fisher Scientifc,
Inc.). The cells were maintained at 37°C in a humidified atmosphere
containing 5% CO2. A total of 2 ng/ml TGF-β1 (cat. no.
PHG9214; Gibco; Thermo Fisher Scientific, Inc.) was used to treat
A549 cells at 37°C for 48 h to induce fibrosis. Cells incubated
with normal medium were used as the control.
Animal studies
A total of 24 male mice (weight, 18–20 g; age, 8
weeks) were purchased from the Shanghai Animal Experiment Center of
the Chinese Academy of Sciences. All mice were housed under
controlled temperature (~22°C) and humidity (~55%) with 12/12 h
light cycle and free access to water and food. Bleomycin (BLM;
Thermo Fisher Scientific, Inc.) was used to induce the fibrosis of
lung tissues in mice. After accommodation for 1 week, mice were
divided into the following experimental groups: i) Control group,
in which mice were intravenously injected with 1 ml normal saline;
ii) BLM group, in which mice were intravenously injected with BLM
(5 mg/kg/day); iii) BLM + short hairpin (sh)RNA-negative control
(NC) group, in which mice were intravenously injected with BLM and
shRNA-NC (Shanghai GeneChem Co., Ltd.); and iv) BLM +
shRNA-HOTTIP-1 group, in which mice were injected with BLM and
shRNA-HOTTIP-1 (Shanghai GeneChem Co., Ltd.). After 2 weeks, mice
were euthanized by inhalation of 5% isoflurane (cat. no. HR135327;
Hairui Chemical) for 30 sec prior to cervical dislocation, as
previously described (16,17); death of mice was verified by the
lack of heartbeat and a cold body. Experimental protocols were
approved by the Ethics Committee of Tianjin Medical University
General Hospital (Tianjin, China).
Lung tissues were subsequently collected and fixed
in 10% formaldehyde solution (Sigma-Aldrich; Merck KGaA) for 24 h
at room temperature, embedded in paraffin and cut into 5-µm
sections. Then, the sections were stained with hematoxylin and
eosin (H&E; Thermo Fisher Scientific, Inc.) at room temperature
for 5 min for observation of histological injury or Masson's
trichrome dye (Thermo Fisher Scientific, Inc.) at room temperature
for 8 min for observation of lung fibrosis at room temperature. The
images were captured by a light microscope (magnification, ×400;
Olympus Corporation).
Cell transfection
Short hairpin (sh)RNAs targeting HOTTIP
(shRNA-HOTTIP-1/2) and its negative control (shRNA-NC), miR-774-5p
mimic, mimic-NC, overexpression (oe)-PTBP1 plasmid, oe-NC plasmid,
miR-744-5p inhibitor and inhibitor-NC were obtained from Shanghai
GeneChem Co., Ltd. PTBP1 overexpression plasmid (oe-PTBP1)
pcDNA3.1-LINC00885 was commercially constructed by Shanghai
GenePharma Co., Ltd.; empty pcDNA 3.1 vector (oe-NC) was used as
the control. A549 cells were transfected with shRNA-HOTTIP-1/2 (500
ng/µl), shRNA-NC plasmid (500 ng/µl), miR-774-5p mimic (40 nM),
mimic-NC (40 nM), oe-PTBP1 (15 nM), oe-NC (15 nM), miR-744-5p
inhibitor (40 nM) and inhibitor-NC (40 nM) using
Lipofectamine® 2000 reagent (Thermo Fisher Scientific,
Inc.) at 37°C according to the manufacturer's protocol. At 48 h
post transfection, cells were harvested for subsequent
experiments.
5′-ethynl-2′-deoxyuridine (EdU)
assay
The EdU assay was performed using a Click-iT EdU
Imaging kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Fluorescence was detected using a
fluorescence microscope (magnification, ×100; Olympus
Corporation).
Wound healing assay
Cells were plated into six-well plates
(1×105 cells/well) before the experiments and allowed to
growth to 90% confluence in RPMI-160 medium with 10% FBS at 37°C.
Then a scratch was made using a 10-µl sterile pipette tip in the
cell monolayer. The cells were washed twice to remove debris and
incubated with RPMI-160 medium without FBS for 48 h at 37°C. The
scratch was imaged with an inverted light microscope
(magnification, ×100; Olympus Corporation) at 0 h, and again
following 48 h of incubation. The width of the scratch was
calculated using ImageJ software (version 1.46; National Institutes
of Health).
Dual luciferase reporter assay
starBase (starbase.sysu.edu.cn) was used to predict the
potential binding sites between lncRNA HOTTIP and miR-744-5p, which
were verified by dual luciferase reporter assay. Briefly, the
wild-type (WT) and mutant (MUT) 3′-untranslated region (UTR) of
HOTTIP was obtained from Shanghai GeneChem Co., Ltd and cloned into
a pGL3-promoter (Promega Corporation). Point mutations in the
binding site was generated using the QuikChange Site-Directed
Mutagenesis kit (Stratagene; Agilent Technologies) according to the
manufacturer's protocol. A549 cells were co-transfected with 50 ng
WT-HOTTIP or MUT-HOTTIP and 20 µM miR-744-5p mimic or mimic NC
using Lipofectamine 2000 reagent (Thermo Fisher Scientific, Inc.).
Following 48-h transfection, the relative luciferase activity was
measured using a Dual-Luciferase Reporter assay system (Promega
Corporation), according to the manufacturer's protocol. Firefly
luciferase activity was normalized to Renilla luciferase
activity.
Immunofluorescence assay
Paraffin sections (5-µm thick) were de-waxed with
xylene, rehydrated with ethanol and microwaved for 15 min for
antigen retrieval. After cooling, the sections were blocked with
H2O2 for 10 min at room temperature. After
blocking with 5% goat serum (cat. no. 16210072; Gibco; Thermo
Fisher Scientific, Inc.) at 37°C for 30 min, sections were
incubated with the following primary antibodies at 4°C overnight:
Anti-α-smooth muscle actin (α-SMA; 1:200; cat. no. ab5694; Abcam)
and anti-E-cadherin (1:200; cat. no. ab231303; Abcam). Afterwards,
the sections were incubated with FITC-labeled goat anti-mouse
secondary antibody (1:200; cat. no. IF-0051; Beijing Dingguo
Changsheng Biotechnology Co., Ltd.) for 1 h at 37°C. Cell nuclei
were stained with DAPI (Invitrogen; Thermo Fisher Scientific, Inc.)
for 2 min at room temperature. Fluorescence was detected using a
fluorescence microscope (magnification, ×200; Olympus
Corporation).
Western blotting
Total protein from A549 cells was extracted using
RIPA lysis buffer (Beyotime Institute of Biotechnology) and
quantified using a BCA assay. The proteins (30 µg/lane) were
subsequently separated via 10% SDS-PAGE (Beyotime Institute of
Biotechnology) and transferred onto the PVDF membranes (EMD
Millipore). After blocking in 10% non-fat milk for 1 h at 37°C, the
membranes were then incubated with the following primary antibodies
at 4°C overnight: Anti-α-SMA (1:1,000; cat. no. ab5694; Abcam),
anti-collagen I (1:1,000; cat. no. ab34710; Abcam), anti-collagen
III (1:1,000; cat. no. ab6310; Abcam), anti-fibronectin 1 (FN1;
1:1,000; cat. no. ab2413; Abcam), anti-E-cadherin (1:1,000; cat.
no. ab231303; Abcam) and anti-GAPDH (1:1,000; cat. no. ab9485;
Abcam). Following the primary antibody incubation, the membranes
were incubated with a polyclonal goat anti-rabbit (1:5,000; cat.
no. ab98512; Abcam) or anti-mouse (1:5,000; cat. no. ab97040;
Abcam) HRP-conjugated secondary antibody for 2 h at room
temperature. Protein bands were visualized using an enhanced
chemiluminescence reagent (Pierce; Thermo Fisher Scientific, Inc.).
ImageJ software (1.46; National Institutes of Health) was used to
quantify the protein bands.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from A549 cells was extracted using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
Total RNA was reverse transcribed into cDNA using a PrimeScript RT
Reagent kit (Takara Bio, Inc.) according to the manufacturer's
protocol. qPCR was subsequently performed on an ABI 7500 Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
with SYBR Premix Ex Taq reagent (Takara Bio, Inc.). qPCR was
performed as follows: 60°C for 2 min, 95°C for 25 sec and 40 cycles
of 95°C for 10 sec and 60°C for 30 sec. The primers sequences used
for the qPCR are listed in Table I.
The expression levels of target genes were analyzed using the
2−∆∆Cq method (18).
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| Long non-coding HOXA
distal transcript antisense RNA | F:
CCTAAAGCCACGCTTCTTTG |
|
| R:
TGCAGGCTGGAGATCCTACT |
| MicroRNA-774-5p | F:
CTGTTGCCACTAACCTCAACCT |
|
| R:
TGCGGGGCTAGGGCTAACAGCA |
| Polypyrimidine tract
binding protein 1 | F:
AGCGCTGCGTCGCTGCGCACGTGGGAAG |
|
| R:
AACAATGGAATCTGGGGATGGACTATTC |
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
| GAPDH | F:
ATGACATCAAGAAGGTGGTG |
|
| R:
CATACCAGGAAATGAGCTTG |
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6 software (GraphPad Software, Inc.). All experiments were
repeated in triplicate and data are presented as the mean ± SD.
Statistical differences between groups were determined using a
one-way ANOVA followed by a Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Knockdown of lncRNA HOTTIP suppresses
the TGF-β1-induced fibrosis of A549 cells
TGF-β1 was used to induce the fibrosis of A549
cells. The expression levels of the lncRNA HOTTIP were
significantly upregulated in A549 cells following stimulation with
TGF-β1 compared with the control group (Fig. 1A). The expression of HOTTIP was
knocked down in A549 cells via transfection with shRNAs. RT-qPCR
analysis revealed that the expression levels of the lncRNA HOTTIP
were significantly downregulated following the transfection with
shRNA-HOTTIP-1/2 compared with the shRNA-NC group (Fig. 1B), and the knockdown efficiency of
shRNA-HOTTIP-1 was more significant compared with shRNA-HOTTIP-2.
Therefore, cells transfected with shRNA-HOTTIP-1 were selected for
use in subsequent assays.
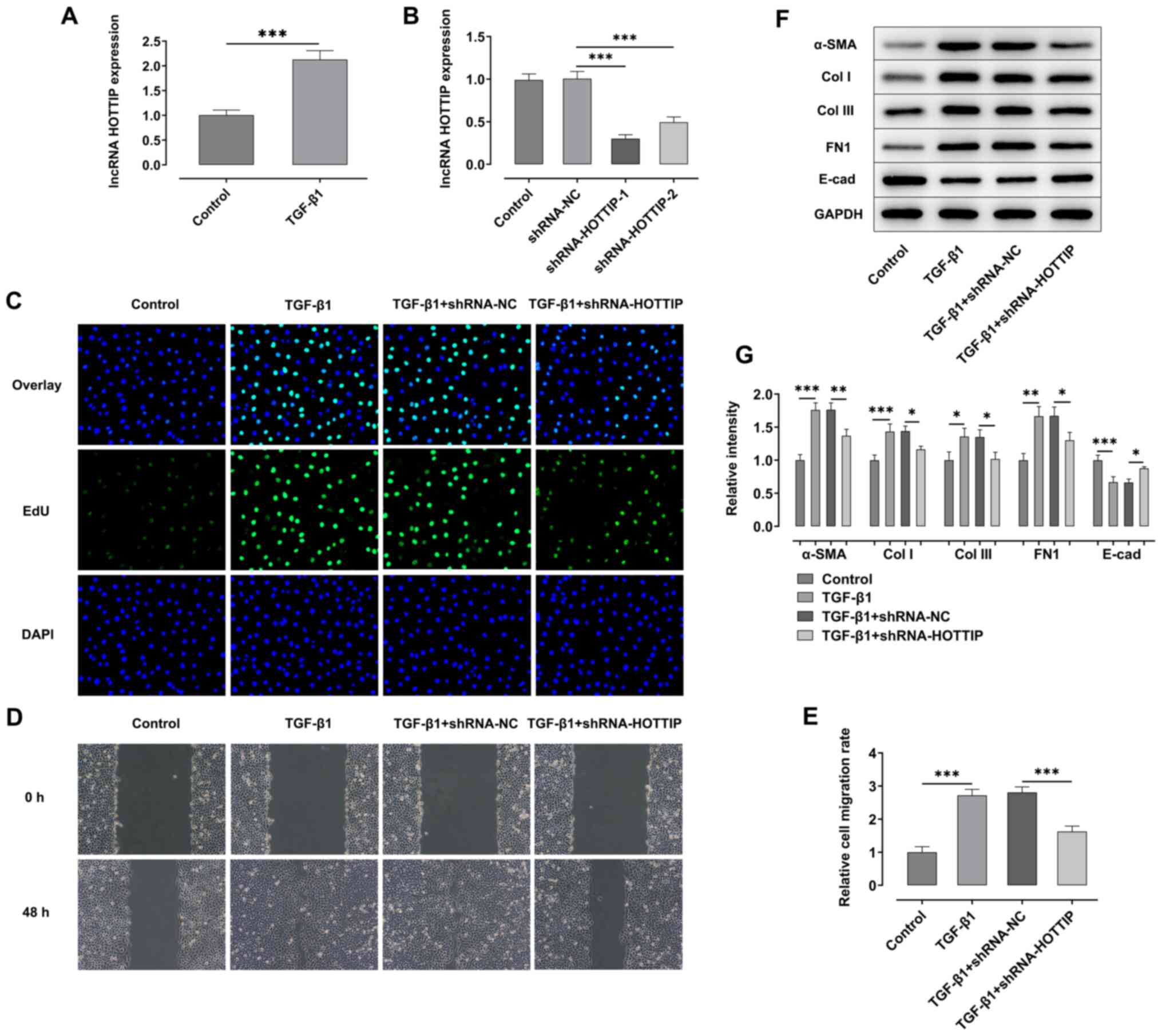 | Figure 1.Knockdown of lncRNA HOTTIP relieves
the TGF-β1 induced fibrosis of A549 cells. (A) RT-qPCR was used to
analyze the expression levels of lncRNA HOTTIP in A549 cells
stimulated with TGF-β1. (B) Expression levels of lncRNA HOTTIP were
analyzed by RT-qPCR following transfection with shRNA-HOTTIP-1/2.
(C) EdU assay was performed to determine the proliferation of A549
cells following stimulation with TGF-β1 with or without
shRNA-HOTTIP transfection. Magnification, ×100. (D) Wound healing
assay was performed to detect the migration of A549 cells following
stimulation with TGF-β1 with or without shRNA-HOTTIP transfection.
Magnification, ×100. (E) Semi-quantification of results from wound
healing assay. (F) Western blotting was performed to analyze the
expression levels of α-SMA, Col I, Col III, FN1 and E-cad in A549
cells following stimulation with TGF-β1 with or without
shRNA-HOTTIP transfection. (G) Protein expression was quantified.
*P<0.05, **P<0.01, ***P<0.001. lncRNA, long non-coding
RNA; HOTTIP, HOXA distal transcript antisense RNA; RT-qPCR, reverse
transcription-quantitative PCR; shRNA, short hairpin RNA; NC,
negative control; α-SMA, α-smooth muscle actin; Col I, collagen I;
Col III, collagen III; E-cad, E-cadherin; FN1, fibronectin 1; EdU,
5-ethynyl-2′-deoxyuridine. |
EdU and wound healing assays were performed to
detect the proliferation and migration of A549 cells, respectively.
As shown in Fig. 1C-E, the
proliferation and migration of A549 cells were both significantly
increased following stimulation with TGF-β1 compared with the
control group. However, the proliferation and migration of A549
cells were suppressed following the concomitant knockdown of HOTTIP
in the TGF-β1 + shRNA-HOTTIP group compared with the TGF-β1 +
shRNA-NC group. Moreover, the expression levels of α-SMA, collagen
I (Col I), collagen III (Col III) and FN1 were significantly
upregulated, while expression levels of E-cadherin (E-cad) were
significantly downregulated in TGF-β1 group, compared with the
control group. However, the expression levels of α-SMA, collagen I,
collagen III and FN1 were downregulated, while the expression level
of E-cadherin was significantly upregulated following the knockdown
of HOTTIP in TGF-β1 stimulated cells compared with the TGF-β1 +
shRNA-NC group (Fig. 1F-G).
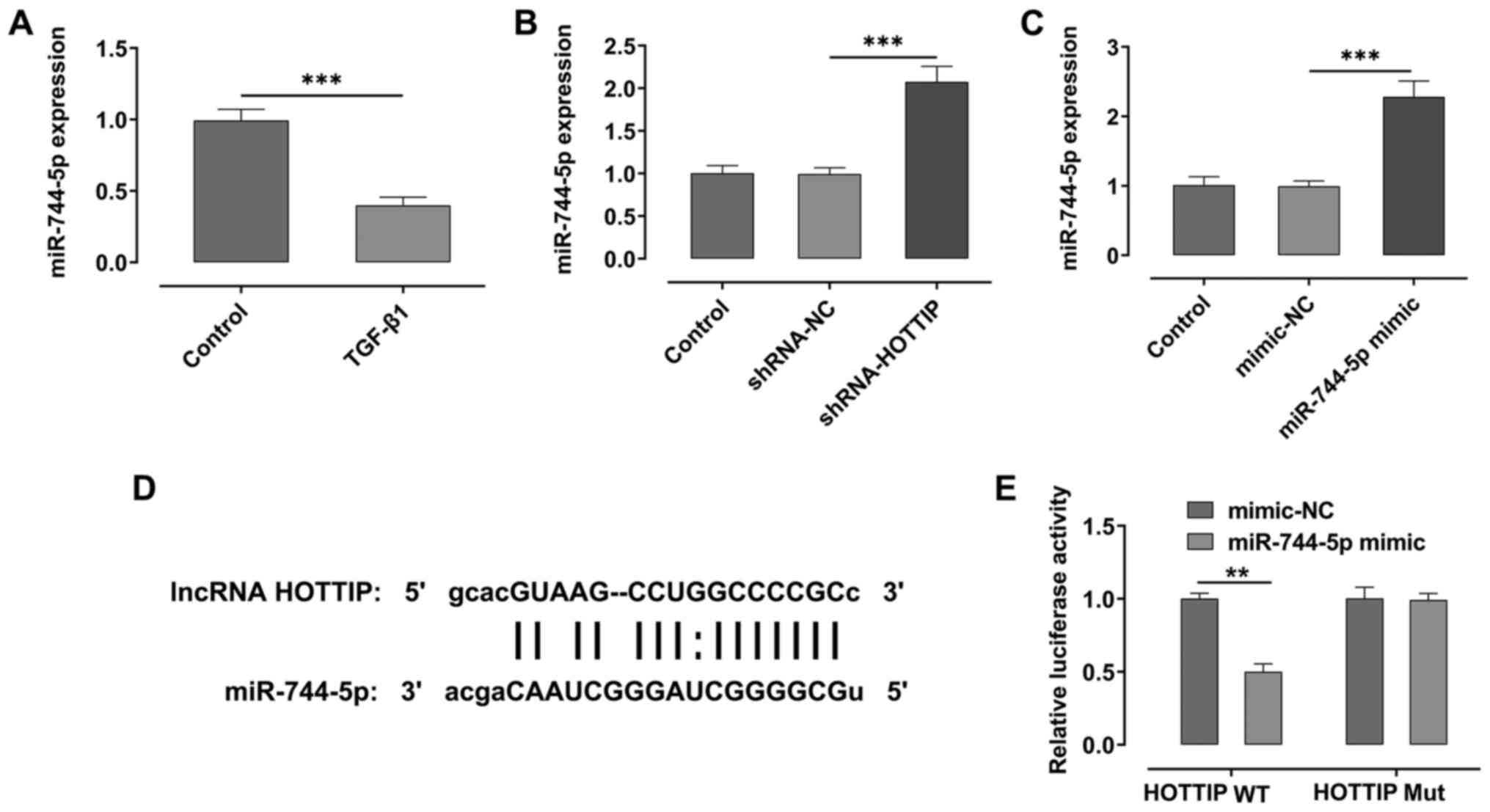
HOTTIP targets and downregulates the
expression levels of miR-744-5p
Using the starBase database, it was predicted that
HOTTIP had the potential to target miR-774-5p. The expression
levels of miR-774-5p were significantly downregulated following
stimulation with TGF-β1 compared with the control group (Fig. 2A). Moreover, the expression levels
of miR-774-5p were significantly upregulated following the
knockdown of HOTTIP compared with the shRNA-NC group (Fig. 2B). Subsequently, miR-744-5p was
overexpressed in A549 cells. As shown in Fig. 2C, the expression levels of
miR-744-5p in cells transfected with the miR-744-5p mimic were
significantly upregulated compared with the mimic-NC group. The
potential binding sites between the lncRNA HOTTIP and miR-774-5p
were then identified (Fig. 2D). The
results of the dual luciferase reporter assay indicated that the
relative luciferase activity was suppressed in the lncRNA HOTTIP
wild-type (WT) and miR-774-5p mimic group, compared with HOTTP WT
and mimic-NC group (Fig. 2E).
Knockdown of HOTTIP suppresses the
TGF-β1-induced fibrosis of A549 cells by regulating the expression
of miR-744-5p
In the following experiments, the expression of
miR-744-5p in HOTTIP-knockdown A549 cells was also successfully
knocked down using a miR-744-5p inhibitor (Fig. 3A). The results demonstrated that the
combined TGF-β1 and shRNA-HOTTIP-induced inhibition of
proliferation and migration of A549 cells were both partially
rescued following the concurrent knockdown of miR-744-5p (Fig. 3B-D). Furthermore, the expression
levels of α-SMA, Col I, Col III and FN1 were upregulated, while the
expression levels of E-cad were downregulated, in the TGF-β1 +
shRNA-HOTTIP + miR-744-5p inhibitor group compared with the TGF-β1
+ shRNA-HOTTIP + inhibitor-NC group (Fig. 3E).
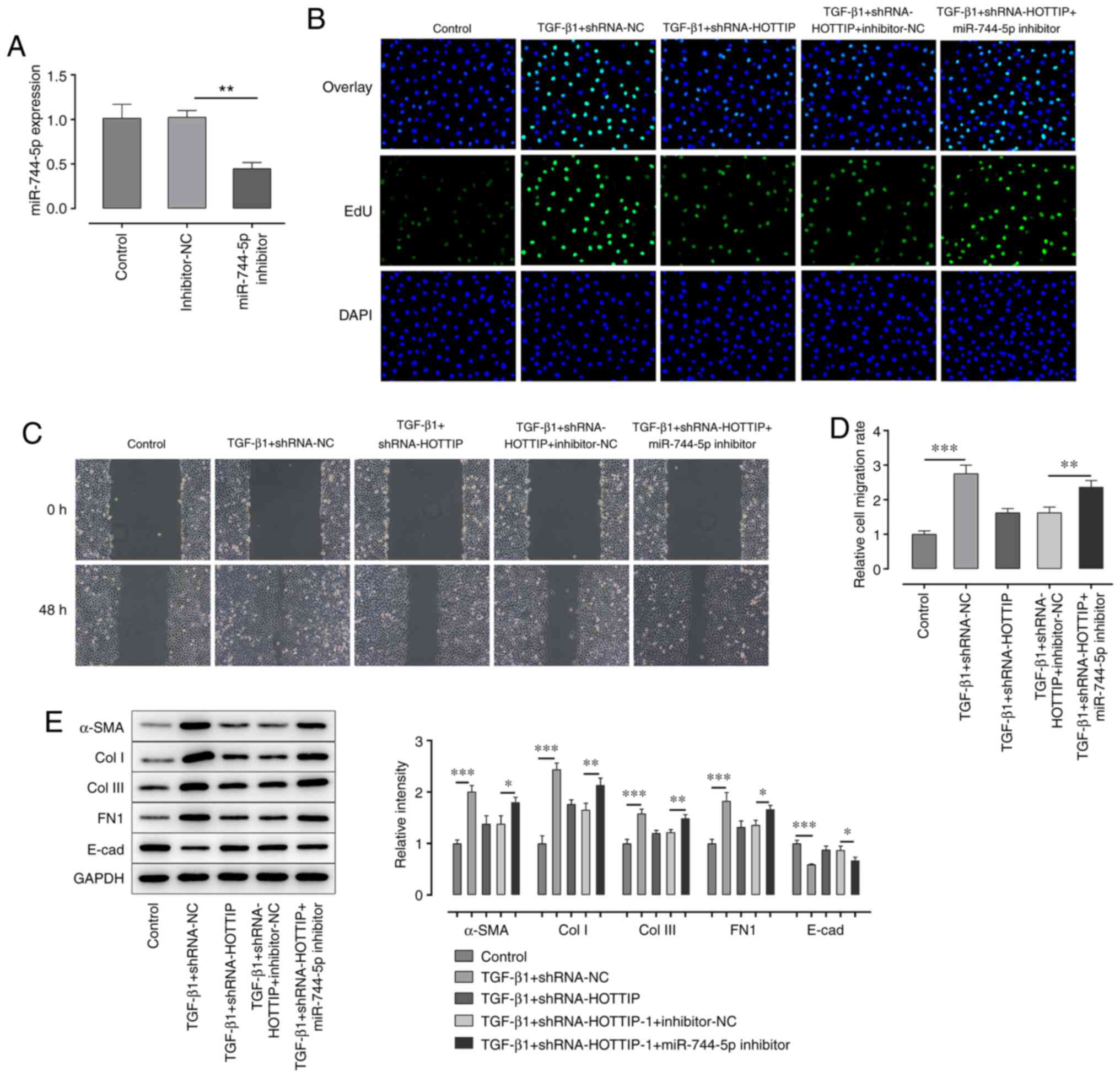 | Figure 3.Knockdown of miR-744-5p expression
rescues the long non-coding RNA HOTTIP-induced suppression of
proliferation and migration. (A) Expression levels of miR-744-5p in
A549 cells following transfection with the miR-744-5p inhibitor
were analyzed using reverse transcription-quantitative PCR. (B) EdU
assay was performed to detect the proliferation of A549 cells
following stimulation with TGF-β1 and transfection with
shRNA-HOTTIP and miR-744-5p inhibitor. Magnification, ×100. (C)
Wound healing assays were performed to determine the migration of
A549 cells following stimulation with TGF-β1 and transfection with
shRNA-HOTTIP and miR-744-5p inhibitor. Magnification, ×100. (D)
Semi-quantification of the results of the wound healing assay. (E)
Western blotting was performed to analyze the expression levels of
α-SMA, Col I, Col III, FN1 and E-cad in A549 cells following
stimulation with TGF-β1 and transfection with shRNA-HOTTIP and
miR-744-5p inhibitor. *P<0.05, **P<0.01, ***P<0.001. miR,
microRNA; EdU, EdU, 5-ethynyl-2′-deoxyuridine; α-SMA, α-smooth
muscle actin; Col I, collagen I; Col III, collagen III; E-cad,
E-cadherin; FN1, fibronectin 1; shRNA, short hairpin RNA; NC,
negative control. |
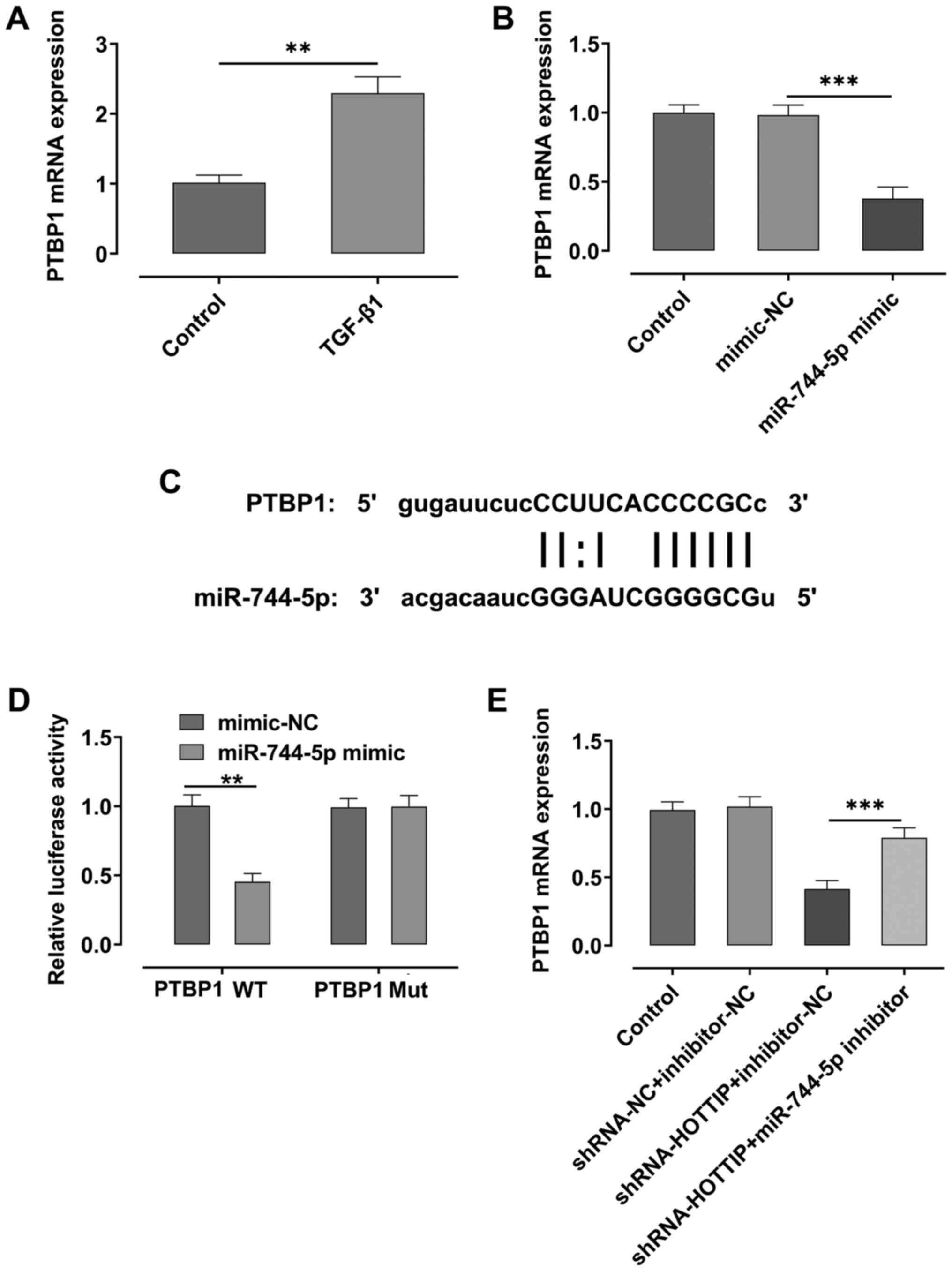
miR-744-5p regulates the expression
levels of PTBP1
RT-qPCR analysis demonstrated that the expression
levels of PTBP1 were significantly upregulated after A549 cells
were stimulated with TGF-β1 compared with the control group
(Fig. 4A). However, the expression
levels of PTBP1 were significantly downregulated following the
overexpression of miR-744-5p compared with the mimic-NC group
(Fig. 4B). Using starBase,
miR-744-5p was predicted to share a binding site with PTBP1
(Fig. 4C), and the results of the
dual luciferase reporter assay demonstrated that the relative
luciferase activity was suppressed in the PTBP1 WT and miR-744-5p
mimic group, compared with PTBP1 WT and mimic-NC group (Fig. 4D). Moreover, the expression levels
of PTBP1 were downregulated in HOTTIP-knockdown A549 cells,
compared with the control; however, the expression levels of PTBP1
were rescued upon the concurrent knockdown of miR-744-5p in the
cells (Fig. 4E).
HOTTIP regulates TGF-β1-induced
fibrosis of A549 cells by modulating the miR-744-5p/PTBP1 signaling
axis
To determine the effect of PTBP1 on the development
of fibrosis in A549 cells, PTBP1 was overexpressed in A549 cells.
As shown in Fig. 5A, the expression
levels of PTBP1 were significantly upregulated in A549 cells in the
oe-PTBP1 group compared with the oe-NC group. The results of EdU
and wound healing assays demonstrated that the proliferation and
migration of A549 cells were suppressed following the
overexpression of miR-744-5p in TGF-β1-induced A549 cells compared
with the TGF-β1 + mimic-NC group (Fig.
5B-D). However, the proliferation and migration of these cells
were rescued following the concurrent overexpression of PTBP1.
Similarly, the expression levels of α-SMA, Col I, Col III and FN1
were upregulated, while the expression levels of E-cad were
downregulated, in the TGF-β1 + miR-744-5p mimic + oe-PTBP1 group
compared with the TGF-β1 + miR-744-5p mimic + oe-NC group (Fig. 5E).
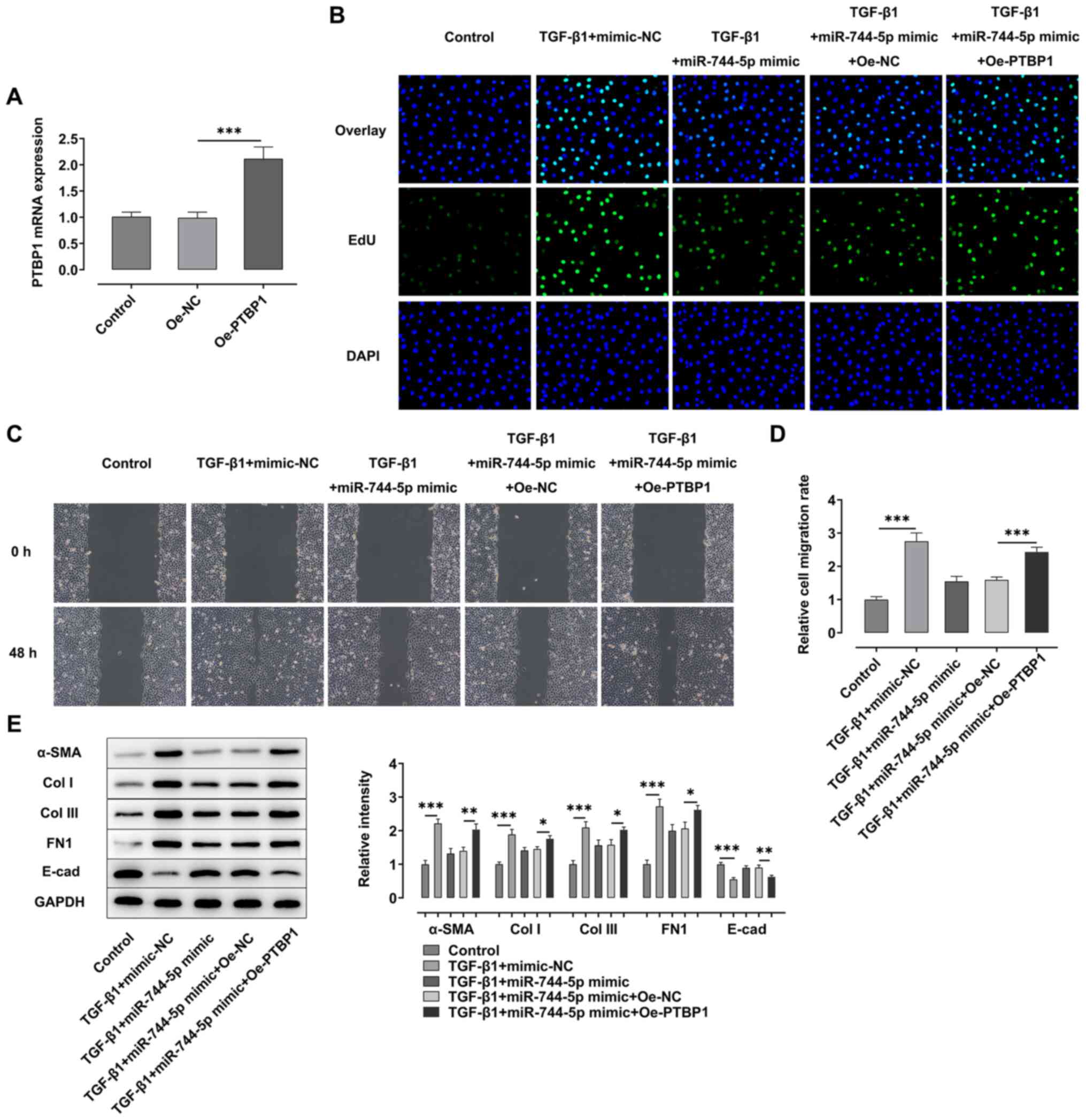 | Figure 5.Long non-coding RNA HOTTIP regulates
TGF-β1-induced fibrosis of A549 cells by modulating the
miR-744-5p/PTBP1 signaling axis. (A) RT-qPCR was performed to
analyze the expression levels of PTBP1 in A549 cells transfected
with oe-PTBP1. (B) EdU assay was performed to detect the
proliferation of A549 cells following stimulation with TGF-β1 and
transfection with miR-744-5p mimic and oe-PTBP1. Magnification,
×100. (C) Wound healing assay was performed to analyze the
migration of A549 cells following stimulation with TGF-β1 and
transfection with miR-744-5p mimic and oe-PTBP1. Magnification,
×100. (D) Semi-quantification of the results of the wound healing
assay. (E) Western blotting was performed to analyze the expression
levels of α-SMA, Col I, Col III, FN1 and E-cad in A549 cells
following stimulation with TGF-β1 and transfection with miR-744-5p
mimic and oe-PTBP1. *P<0.05, **P<0.01, ***P<0.001. HOTTIP,
HOXA distal transcript antisense RNA; miR, microRNA; PTBP1,
polypyrimidine tract binding protein 1; oe, overexpression; α-SMA,
α-smooth muscle actin; Col I, collagen I; Col III, collagen III;
E-cad, E-cadherin; FN1, fibronectin 1; NC, negative control. |
Knockdown of HOTTIP relieves the
BLM-induced lung fibrosis of mice
BLM was used to induce fibrosis in mice. Next,
shRNA-NC and shRNA-HOTTIP were injected into the mice to determine
the effect of lncRNA HOTTIP on the development of lung fibrosis.
After the euthanasia of these mice, H&E and Masson's trichrome
staining were performed to analyze the fibrosis in the lung tissues
of mice. The results demonstrated that the number of nodules was
decreased, and the fibrosis of lung tissues was relieved after the
knockdown of HOTTIP (Fig. 6A).
Compared with the control group, BLM induced increased expression
of HOTTIP and PTBP1 and decreased expression of miR-744-5p
(Fig. 6B-D). In addition, the
expression levels of HOTTIP and PTBP1 were significantly
downregulated, while the expression levels of miR-744-5p were
significantly upregulated in the lung tissues of the BLM +
shRNA-HOTTIP group compared with the BLM + shRNA-NC group.
Immunofluorescence analysis exhibited upregulated expression of
α-SMA and downregulated expression of E-cad in BLM group, compared
with the control group. In addition, the results also illustrated
that the expression of α-SMA was downregulated, while the
expression of E-cad was upregulated, in the lung tissues from the
BLM + shRNA-HOTTIP group compared with the BLM + shRNA-NC group
(Fig. 6E and F). Furthermore, BLM
increased expression of α-SMA, Col I, Col III and FN1, and
decreased expression of E-cad, compared with the control group.
Additionally, the expression levels of α-SMA, Col I, Col III and
FN1 were significantly downregulated, while the expression levels
of E-cadherin were significantly upregulated in the lung tissues
from the BLM + shRNA-HOTTIP group compared with the BLM + shRNA-NC
group (Fig. 6G and H).
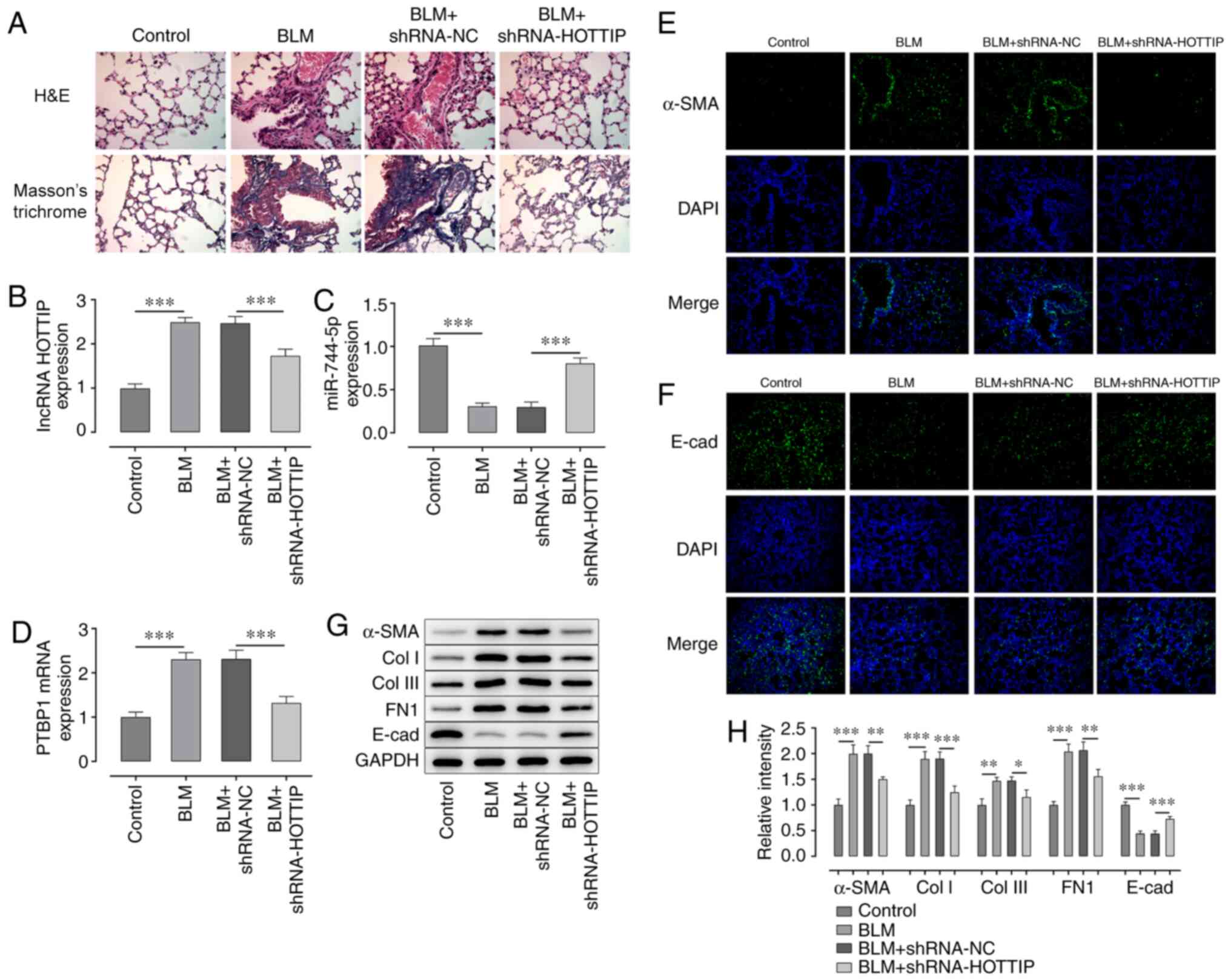 | Figure 6.Knockdown of lncRNA HOTTIP relieves
lung tissue fibrosis in mice. (A) Fibrosis of lung tissues of mice
was detected using H&E and Masson's trichrome staining.
Magnification, ×400. Expression levels of (B) lncRNA HOTTIP and (C)
miR-774-5p in lung tissues of mice treated with BLM with or without
shRNA-HOTTIP transfection were analyzed using RT-qPCR. (D) RT-qPCR
was performed to analyze the expression levels of PTBP1 in lung
tissues of mice treated with BLM with or without shRNA-HOTTIP
transfection. Immunofluorescence was performed to analyze the
expression levels of (E) α-SMA and (F) E-cadherin in lung tissues
of mice treated with BLM with or without shRNA-HOTTIP transfection.
Magnification, ×200. (G) Western blotting was used to determine the
expression levels of α-SMA, Col I, Col III, FN1 and E-cad in lung
tissues of mice treated with BLM with or without shRNA-HOTTIP
transfection. (H) Semi-quantification of western blotting results
from part (G) *P<0.05, **P<0.01, ***P<0.001. lncRNA, long
non-coding RNA; HOTTIP, HOXA distal transcript antisense RNA;
H&E, hematoxylin and eosin; miR, microRNA; BLM, bleomycin;
shRNA, short hairpin RNA; NC, negative control; PTBP1,
polypyrimidine tract binding protein 1; SMA, α-smooth muscle actin;
Col I, collagen I; Col III, collagen III; E-cad, E-cadherin; FN1,
fibronectin 1. |
Discussion
Pulmonary fibrosis is a progressive and irreversible
disease characterized by lung tissue remodeling and collagen
deposition (19). The development
of lung fibrosis has also been found to eventually lead to the loss
of lung function (20). The
incidence and mortality rates of pulmonary fibrosis have gradually
increased (21). However, to the
best of our knowledge, the molecular mechanism underlying the
pathogenesis of pulmonary fibrosis remains unknown (19). Therefore, further investigating the
molecular mechanisms underlying the occurrence and development of
pulmonary fibrosis has great significance for the clinical
treatment of pulmonary fibrosis.
Previous studies have revealed that the expression
of lncRNA was associated with the development of fibrosis in lung
tissues (22,23). The expression levels of lncRNA
HOTTIP were found to be upregulated during the development of
hepatic fibrosis (12). The
upregulated expression levels of lncRNA HOTTIP induced the fibrosis
of liver tissues by downregulating the expression levels of
miR-148a and upregulating the expression levels of TGF-β receptor
(TGFBR)1 and TGFBR2 (11).
Moreover, the upregulated expression levels of HOTTIP were
discovered to activate hepatic stellate cells by upregulating the
expression of serum response factor (12). The knockdown of lncRNA HOTTIP also
relieved high glucose-induced inflammation and fibrosis in mice
(24). However, whether lncRNA
HOTTIP could influence the development of lung fibrosis is yet to
be elucidated. A549 cells have been widely used as model human
respiratory epithelial cells that can be stimulated with TGF-β1
(25,26); thus, TGF-β1-induced A549 cells were
used as the in vitro pulmonary fibrosis model in the present
study. The present study revealed that the knockdown of the lncRNA
HOTTIP suppressed the proliferation and migration of A549 cells and
downregulated the expression levels of fibrosis-related proteins
(α-SMA, Col I, Col III and FN1). The in vivo assay results
also demonstrated that knockdown of the lncRNA HOTTIP relieved lung
fibrosis in mice. These results suggested that knockdown of the
lncRNA HOTTIP may alleviate the fibrosis of A549 cells.
The present findings also suggested that the lncRNA
HOTTIP may exert its effects by regulating the miR-744-5p/PTBP1
signaling axis. Previous studies have revealed that the expression
levels of miR-744-5p were associated with the occurrence and
development of multiple types of cancer (4,27). For
instance, the upregulated expression of miR-744-5p inhibited the
proliferation and migration of ovarian cancer cells (28), and higher levels of miR-744-5p also
repressed the proliferation and invasion of lung cancer cells by
regulating the expression of paired box 2 (13). However, the present study reported
that the lncRNA HOTTIP targeted and negatively regulated
miR-744-5p, and the knockdown of miR-744-5p partially reversed the
HOTTIP-induced inhibitory effect on the proliferation and migration
of A549 cells. As enhanced cell proliferation and migration suggest
the occurrence and development of fibrosis (29), the present results indicated that
the lncRNA HOTTIP enhanced the fibrosis of A549 cells by
downregulating the expression of miR-744-5p. Furthermore, the
expression levels of PTBP1 have been associated with the
development of pulmonary fibrosis (15). The present results revealed that
miR-744-5p targeted and downregulated the expression of PTBP1. It
was also found that the overexpression of PTBP1 partially reversed
the inhibitory effects of miR-744-5p on the proliferation and
migration of A549 cells.
However, the present study has several limitations.
First, even though A549 cells have been extensively used to
establish in vitro pulmonary fibrosis models, further in
vitro studies should be performed with other appropriate cell
lines, such as the human lung fibroblast cell line HLF, to validate
the present results, which will be taken into consideration in
future studies. Second, the current study mainly focused on the
role and the molecular mechanism of HOTTIP on the cellular level
and animal level; however, the expression of lncRNA HOTTIP in
clinical cases of pulmonary fibrosis needs to be analyzed in future
studies to support the present findings from cell line and animal
studies.
In conclusion, the findings of the present study
suggested that the lncRNA HOTTIP may enhance the fibrosis of lung
tissues by downregulating the expression of miR-744-5p and
upregulating the expression of PTBP1. The current results suggested
that the lncRNA HOTTIP/miR-744-5p/PTBP1 signaling pathway may serve
a crucial role during the development of lung fibrosis. The results
of the current study also provided a potential novel target and
strategy for the clinical treatment of lung fibrosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW conceptualized and designed the experiments. JL,
WC and ZZ conducted the experiments and obtained the data. JL and
YZ analyzed the data and interpreted the results. JL and WC wrote
the manuscript. QW revised the manuscript. All authors read the
final version of the manuscript. QW and JL confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Experimental protocols were approved by the Ethics
Committee of Tianjin Medical University General Hospital (Tianjin,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rangarajan S, Bone NB, Zmijewska AA, Jiang
S, Park DW, Bernard K, Locy ML, Ravi S, Deshane J, Mannon RB, et
al: Metformin reverses established lung fibrosis in a bleomycin
model. Nat Med. 24:1121–1127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Selman M, Pardo A and Kaminski N:
Idiopathic pulmonary fibrosis: Aberrant recapitulation of
developmental programs? PLoS Med. 5:e622008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao Z, Lis R, Ginsberg M, Chavez D, Shido
K, Rabbany SY, Fong GH, Sakmar TP, Rafii S, Ding BS, et al:
Targeting of the pulmonary capillary vascular niche promotes lung
alveolar repair and ameliorates fibrosis. Nat Med. 22:154–162.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hofmann P, Sommer J, Theodorou K, Kirchhof
L, Fischer A, Li Y, Perisic L, Hedin U, Maegdefessel L, Dimmeler S
and Boon RA: Long non-coding RNA H19 regulates endothelial cell
aging via inhibition of STAT3 signalling. Cardiovasc Res.
115:230–242. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inchingolo R, Varone F, Sgalla G and
Richeldi L: Existing and emerging biomarkers for disease
progression in idiopathic pulmonary fibrosis. Expert Rev Respir
Med. 13:39–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang H, Gu Y, Li T, Zhang Y, Huangfu L,
Hu M, Zhao D, Chen Y, Liu S, Dong Y, et al: Integrated analyses
identify the involvement of microRNA-26a in epithelial-mesenchymal
transition during idiopathic pulmonary fibrosis. Cell Death Dis.
5:e12382014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balakirev ES and Ayala FJ: Pseudogenes:
Are they ‘junk’ or functional DNA? Annu Rev Genet. 37:123–151.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Charles Richard JL and Eichhorn PJA:
Platforms for Investigating lncRNA Functions. SLAS Technol.
23:493–506. 2018.PubMed/NCBI
|
|
9
|
Malek R, Gajula RP, Williams RD, Nghiem B,
Simons BW, Nugent K, Wang H, Taparra K, Lemtiri-Chlieh G, Yoon AR,
et al: TWIST1-WDR5-Hottip Regulates Hoxa9 chromatin to facilitate
prostate cancer metastasis. Cancer Res. 77:3181–3193. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Y, Hu B, Wang Q, Ye M, Qiu Q, Zhou Y,
Zeng F, Zhang X, Guo Y and Guo L: Long non-coding RNA HOTTIP
promotes BCL-2 expression and induces chemoresistance in small cell
lung cancer by sponging miR-216a. Cell Death Dis. 9:852018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng G, Qu H, Li F, Ma W and Yang H:
Propofol attenuates sepsis-induced acute kidney injury by
regulating miR-290-5p/CCL-2 signaling pathway. Braz J Med Biol Res.
51:e76552018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng J, Mao Y, Dong P, Huang Z and Yu F:
Long noncoding RNA HOTTIP mediates SRF expression through sponging
miR-150 in hepatic stellate cells. J Cell Mol Med. 23:1572–1580.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen S, Shi F, Zhang W, Zhou Y and Huang
J: miR-744-5p inhibits non-small cell lung cancer proliferation and
invasion by directly targeting PAX2. Technol Cancer Res Treat.
18:15330338198769132019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kleemann M, Schneider H, Unger K, Sander
P, Schneider EM, Fischer-Posovszky P, Handrick R and Otte K:
miR-744-5p inducing cell death by directly targeting HNRNPC and
NFIX in ovarian cancer cells. Sci Rep. 8:90202018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu T, Yan W, Wu Q, Xu Q, Yuan J, Li Y, Li
P, Pan H and Ni C: miR-326 inhibits inflammation and promotes
autophagy in Silica-Induced pulmonary fibrosis through targeting
TNFSF14 and PTBP1. Chem Res Toxicol. 32:2192–2203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Yang P, Zhang B, Ding Y, Lei S,
Hou Y, Guan X and Li Q: Hepatic NPC1L1 overexpression attenuates
alcoholic autophagy in mice. Mol Med Rep. 20:3224–3232.
2019.PubMed/NCBI
|
|
17
|
Zhou J, Zhou Z, Ji P, Ma M, Guo J and
Jiang S: Effect of fecal microbiota transplantation on experimental
colitis in mice. Exp Ther Med. 17:2581–2586. 2019.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mora AL, Rojas M, Pardo A and Selman M:
Emerging therapies for idiopathic pulmonary fibrosis, a progressive
age-related disease. Nat Rev Drug Discov. 16:755–772. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lagares D, Ghassemi-Kakroodi P, Tremblay
C, Santos A, Probst CK, Franklin A, Santos DM, Grasberger P,
Ahluwalia N, Montesi SB, et al: ADAM10-mediated ephrin-B2 shedding
promotes myofibroblast activation and organ fibrosis. Nat Med.
23:1405–1415. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Richeldi L, Collard HR and Jones MG:
Idiopathic pulmonary fibrosis. Lancet. 389:1941–1952. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei L, Chen J, Huang J, Lu J, Pei S, Ding
S, Kang L, Xiao R and Zeng Q: Functions and regulatory mechanisms
of metastasis-associated lung adenocarcinoma transcript 1. J Cell
Physiol. 234:134–151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leti F, Legendre C, Still CD, Chu X,
Petrick A, Gerhard GS and DiStefano JK: Altered expression of
MALAT1 lncRNA in nonalcoholic steatohepatitis fibrosis regulates
CXCL5 in hepatic stellate cells. Transl Res. 190:25–39.e21. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu XJ, Gong Z, Li SJ, Jia HP and Li DL:
Long non-coding RNA Hottip modulates high-glucose-induced
inflammation and ECM accumulation through miR-455-3p/WNT2B in mouse
mesangial cells. Int J Clin Exp Pathol. 12:2435–2445.
2019.PubMed/NCBI
|
|
25
|
Xi Y, Tan K, Brumwell AN, Chen SC, Kim YH,
Kim TJ, Wei Y and Chapman HA: Inhibition of
epithelial-to-mesenchymal transition and pulmonary fibrosis by
methacycline. Am J Respir Cell Mol Biol. 50:51–60. 2014.PubMed/NCBI
|
|
26
|
Ohbayashi M, Kubota S, Kawase A, Kohyama
N, Kobayashi Y and Yamamoto T: Involvement of
epithelial-mesenchymal transition in methotrexate-induced pulmonary
fibrosis. J Toxicol Sci. 39:319–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan Q, Fan Y, Liu Z, Wang X, Jia M, Geng
Z, Zheng J and Lu X: miR-744-5p mediates lncRNA HOTTIP to regulate
the proliferation and apoptosis of papillary thyroid carcinoma
cells. Exp Cell Res. 392:1120242020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao LG, Wang J, Li J and Li QF:
miR-744-5p inhibits cellular proliferation and invasion via
targeting ARF1 in epithelial ovarian cancer. Kaohsiung J Med Sci.
36:799–807. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Y, Zhang F, Pan Z, Luo H, Liu K and
Duan X: lncRNA NR_003923 promotes cell proliferation, migration,
fibrosis, and autophagy via the miR-760/miR-215-3p/IL22RA1 axis in
human Tenon's capsule fibroblasts. Cell Death Dis. 10:5942019.
View Article : Google Scholar : PubMed/NCBI
|