Introduction
Intracerebral hemorrhage (ICH) is a type of stroke
associated with higher morbidity and mortality rates (1). It is a significant cause of death and
disability, with an incidence rate of 24.6 per 100,000 person-years
and a fatality rate of 40% in 21 countries between 1980 and 2008
(2). ICH mainly results from the
rupturing of small arterioles due to chronic hypertension (3). However, the pathophysiology of ICH is
highly complex and involves multiple mechanisms. The poor outcome
of ICH is attributed to both direct damages caused by hemorrhage
and secondary injuries, such as brain edema, blood-brain barrier
(BBB) disruption, inflammation and neuronal apoptosis (4). ICH also influences brain function,
especially cognitive abilities, and may even cause cognitive
decline or impairment (5).
Indications for surgical treatment of ICH are restricted to a few
clinically-relevant survival advantages (6). Although studies have recently focused
on the pharmacological treatment of ICH, no effective regimen has
been established so far (7).
Neural stem cells (NSCs) are characterized by
self-renewal and multipotent differentiation (8). They can differentiate into neurons,
astrocytes and oligodendrocytes, depending on the specific stimuli
received (9). NSCs located in the
lateral subventricular zone (SVZ) of the adult mammalian brain
generate new neurons and glia throughout life (10). Additionally, NSCs play an important
role in replenishing neural cells, neurotrophy and
neuro-immunoregulation (11),
promoting the recovery of motion and sensory and cognitive
functions following any injury (12). Interestingly, ICH stimulates NSC
proliferation and differentiation in the SVZ (13,14),
and the nascent neurons then migrate to the damaged brain region to
replace the dead neurons. Since neurogenesis plays a pivotal role
in facilitating neurological recovery after stroke (15), NSC-based therapy has gained
considerable attention for treating hemorrhagic stroke. A study
demonstrated that NSC transplantation could promote the functional
recovery of ICH rats (16). It has
also been reported that endogenous NSCs are activated in the brain
of experimental ICH rats and help neurons achieve self-repair
(17). Neurotrophic factors, such
as brain-derived neurotrophic factor (BDNF) and its receptor
(TrκB), promote the proliferation and differentiation of endogenous
NSCs (18) and may augment lesion
repair.
Traditional Chinese medicine (TCM) has been used to
improve health, prevent diseases, and treat serious illnesses for
thousands of years in China and other Asian countries (19,20).
The formulations consist of multiple herbs that target several
disease components by following the TCM theory (21). Tongfu Xingshen capsules (TXC) are
composed of senna leaf, giant knotweed rhizome, tabasheer, snake
gourd seed and artificial bezoar to purge fu-organs to arouse the
spirit, clear heat and resolve phlegm. It was first prescribed by
Professor Liu of Guangdong Hospital of Traditional Chinese Medicine
for treating hemorrhagic stroke as part of the
‘Tongfu-Xiedu-Xingnao-Kaiqiao’ treatment model. TXC can promote
recovery after cerebral hemorrhage, improve capillary permeability
of the hemorrhagic area, and alleviate cerebral edema. The
absorption of hematoma can relieve pressure on the brain tissue,
improve cerebral microcirculation and oxygen supply, and eventually
restore neurological functions (22–25).
In the present study, the mechanisms underlying the therapeutic
effect of TXC was assessed in a rat model of ICH, with a specific
focus on the neurological function score and the proliferation and
differentiation of endogenous NSCs.
Materials and methods
Chemicals and reagents
Tongfu Xingshen capsules (TXC; 0.4 g/granule) were
provided by the Pharmacy Department of Guangdong Hospital of
Traditional Chinese Medicine. Evans Blue (EB), BrdU, formamide and
sodium pentobarbital were purchased from Guangzhou Weijia
Technology Co. Ltd. All chemicals and solvents were of an
analytical grade.
Establishment of an ICH model and drug
administration
Male SPF grade SD rats (age, 6 weeks) weighing
between 200 and 250 g were purchased from the Institute of
Experimental Animals, Chinese Academy of Medical Sciences (Beijing,
China), and acclimatized in standard cages under controlled
temperature (23±3°C) and a regular 12 h:12 h light-dark cycle for 7
days before the procedure. All experimental animal protocols were
approved and performed following the Institutional Animal Care
Guidelines (approval no. 2016040; Experimental Animal Ethics
Committee, Guangdong Provincial Hospital of Chinese Medicine,
Guangzhou, China).
The animals were divided into the sham-operated,
untreated model, low dose TXC-treated, and high dose TXC-treated
groups, and ICH was induced through autologous intracerebral blood
infusion. In brief, the animals were all anesthetized using
pentobarbital (50 mg/kg i.p.), and 50 µl blood was drawn from the
tail artery. The rats were positioned in a stereotaxic frame
(Stoelting Instruments), and a cranial burr hole (1 mm) was drilled
near the right coronal suture 3.0 mm lateral to the midline. A
microsyringe was stereotaxically inserted into the right basal
ganglia (coordinates: 0.2 mm anterior, 6.0 mm ventral, and 3.0 mm
lateral to the bregma), and autologous whole blood was injected at
the rate of 10 µl/min (26–28). In the sham-operated control, the
blood was also drawn from the tail artery before the needle was
inserted without injecting any blood. The rats were
intragastrically administered 1 ml/100 g normal saline
(sham-operated and untreated controls), 1 g TXC/kg/day (high dose;
0.1 g/ml with normal saline), or 0.5 g TXC/kg/day (low dose; 0.05
g/ml with normal saline), as appropriate. For testing neurological
function score, brain water content and vascular permeability test,
96 SD rats were divided as aforementioned (n=24 per group). Each
group was further subdivided into the 1, 7, 14 and 28-day groups
(n=6 per group). For immunofluorescence and reverse
transcription-quantitative PCR (RT-qPCR), 72 animals were similarly
divided into 7, 14 and 28-day subgroups in each treatment
group.
BrdU labeling
BrdU (50 mg/kg) was intraperitoneally injected at a
concentration of 5 mg/ml in 0.9% NaCl, once a day. Except for the
1-day group, the other groups received daily injections for 5 days
after the model was established.
Tissue collection
From each rat, 6-µm thick serial coronal sections of
the brain were cut to include the entire SVZ (80–90 slides per
animal, and 3 sections per slide). Every tenth slide was stained
using hematoxylin and eosin at room temperature following standard
protocols. Briefly, brain sections were stained with hematoxylin
for 5 min and differentiated with 1% hydrochloric acid alcohol for
5 sec at room temperature. Following immersion in ammonia-water for
10 sec, sections were dyed with eosin for 3 min, followed by
mounting with neutral balsam. After sealing, the SVZ structures
were observed at ×40 magnification under an Olympus BX61 light
microscope, and adjacent sections with similar SVZ structures were
used for double immunofluorescence staining.
Behavioral testing
Neurological tests were performed 24 h post-stroke
and were scored according to the modified Bederson scale as
follows: 0, no deficits; 1, flexed forepaw; 2, an inability to
resist the lateral push; 3, circling; 4, agitated circling; and 5,
unresponsive to stimulation/stupor (29). The neurobehavioral score of 1–5 was
considered as a successful model.
Evan's Blue dye extravasation
assay
Evan's Blue dye extravasation test was performed, as
previously described (30). The
harvested brains were quickly separated into the left anterior,
right anterior, left posterior and right posterior segments,
weighed and digested using 3 ml formamide in a 60°C water bath for
24 h. The homogenates were centrifuged at 10,000 × g at room
temperature for 20 min, and the absorbance of the supernatant at
630 nm was measured using a spectrophotometer. The Evans blue
content was calculated as mg/g against a standard curve.
Brain water content
Rats were decapitated under deep anesthesia
(pentobarbital; 50 mg/kg i.p.), which was validated based on the
fact that there was no pain reaction, no stimulation reaction,
general muscle relaxation, and smooth breathing, and the brains
were immediately removed. The posterior brains were weighed using
an analytical microbalance to obtain the wet weight (WW). The
samples were then dried at 75°C for 5 days, and the dry weight (DW)
was determined. Brain water content was calculated as (WW-DW)/WW
×100%.
Double immunofluorescence
NeuN/BrdU and GFAP/BrdU double staining were
performed on the 14-day and 28-day samples, while the 7-day samples
were stained using Nestin and BrdU. In brief, the sections were
incubated overnight with rat anti-BrdU (cat. no. ab6326) and mouse
anti-Nestin (cat. no. ab6142), rabbit anti-NeuN (cat. no. ab177487)
or rabbit anti-GFAP (all 1:200; cat. no. ab33922; all Abcam), as
appropriate, at 4°C. After probing with the relevant secondary
antibody (all 1:500; Alexa Fluor®488 donkey anti-rat,
cat. no. A21208; anti-mouse, cat. no. A32744 or anti-rabbit IgG,
cat. no. A32754; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h in the dark, the slides were washed and
observed at ×400 magnification under a Nikon Ti2-E light
microscope. ImageJ software (v2.1.4.7; National Institutes of
Health) was used to measure the number of positive cells. The
number of cells in the SVZ region of 3 non-overlapping fields were
counted in each section, and the mean was calculated.
RNA preparation and RT-qPCR
analysis
Total RNA was extracted from the brain tissues using
the TRIzol reagent, following the manufacturer's instructions
(Takara Biotechnology Co., Ltd.). The following primers were used
to perform the RT-qPCR: Rat-BDNF forward primer,
GATTAGGTGGCTTCATAGGAGAC; rat-BDNF reverse primer,
CGAACAGAAACAGAGGAGAGATT; rat-TrκB forward primer,
GATGTTCCAGCCACTGTGAACC; rat-TrκB reverse primer,
TCCACCACCCTGTTGCTGTA. Rat-GADPH (forward,
5′-GATGTTCCAGCCACTGTGAACC-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′) was used as the internal control. The
purity of RNA samples was assessed via NanoDrop, and samples with
ratios between 1.80 and 2.01 were used for cDNA synthesis using the
GoScript™ Reverse Transcription system, according to the
manufacturer's protocol (Promega Corporation). The qPCR reaction
was performed in 20 µl with 5 µl template DNA and 500 nM primers.
The PCR condition was as follows: Initial denaturation, 95°C for 10
min, 40 cycles of amplification (95°C for 10 sec and 60°C for 30
sec) and cooling period, 50°C for 5 sec. Each sample was analyzed
in triplicate. iTaq™ Universal SYBR® Green Supermix
(Bio-Rad Laboratories, Inc.) was used according to the
manufacturer's instruction. The PCR results were analyzed in a
Real-Time PCR system (Bio-Rad Laboratories, Inc.; cat. no. CFX96).
The specificity of the primers was tested by analyzing the
dissolution curve. The mRNA of each target gene was homogenized,
and the phase of mRNA of each target gene was calculated by Cq
value for the relative expression level (2−ΔΔCq method:
ΔΔ Cq=(Cq target gene -Cq GAPDH)
treatment group- (Cq target gene -Cq
GAPDH) control group) (31).
Statistical analysis
The data were analyzed using SPSS 21 software (IBM
Corp.) and are expressed as the mean ± SD. Differences were
analyzed using one-way analysis of variance ANOVA with post hoc
Bonferroni test. P<0.05 was considered to indicate a
statistically significant difference.
Results
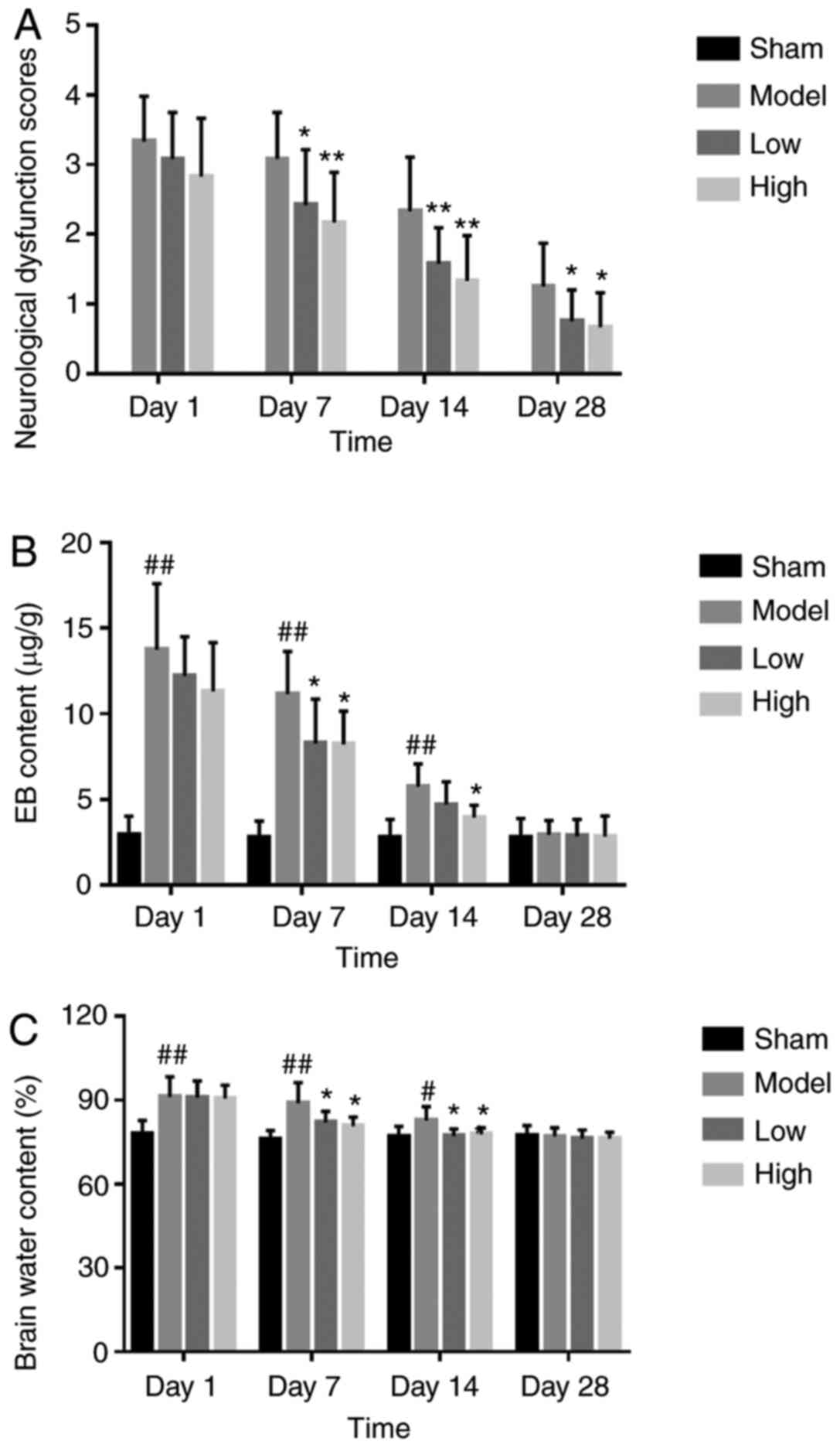
TXC alleviates neurological deficit
and structural damage following ICH
ICH resulted in left hemiplegia, severe hemiplegia
in the left forelimb and clockwise rear-ended rotation within 7
days of induction, and the symptoms were observed over 14 and 28
days. Compared with the untreated model group, both low and high
dosages of TXC improved gait symptoms and decreased hemiplegia
(Fig. 1A). Furthermore, TCX
significantly decreased the extravasation of EB from brain tissues
after 7, 14 and 28 days of stroke, compared with the untreated
model group (Fig. 1B). Finally, the
brain water content in the ipsilateral hemicerebrum substantially
increased in the model group, compared with the sham-operated
animals, and was significantly decreased by low and high dose TXC
treatment at all time points (Fig.
1C).
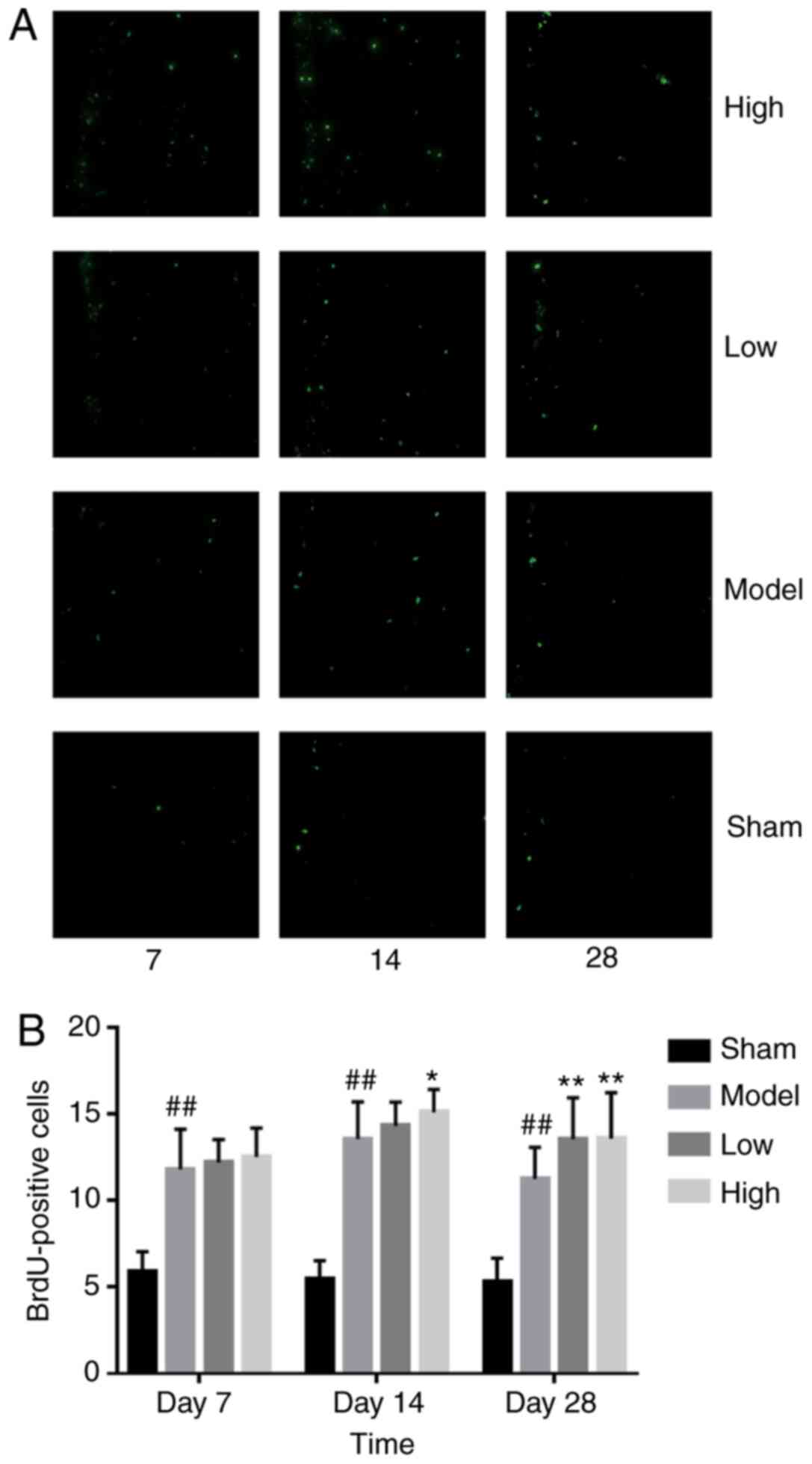
TXC promotes the proliferation and
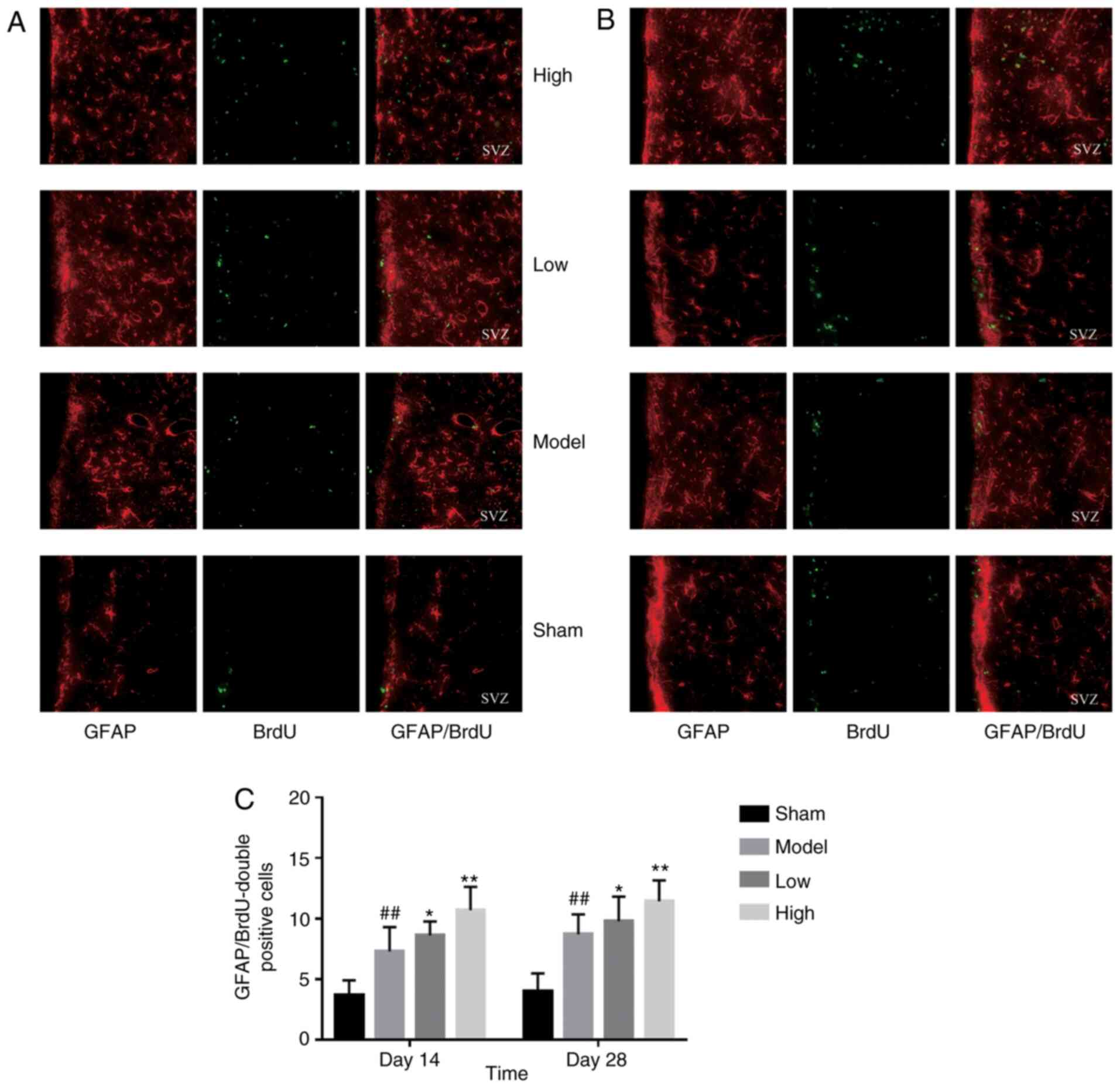
differentiation of endogenous NSCs in SVZ
ICH injury significantly increased the number of
proliferating BrdU-positive cells in the SVZ region, increased by
low or high dose TXC-treated (Fig.
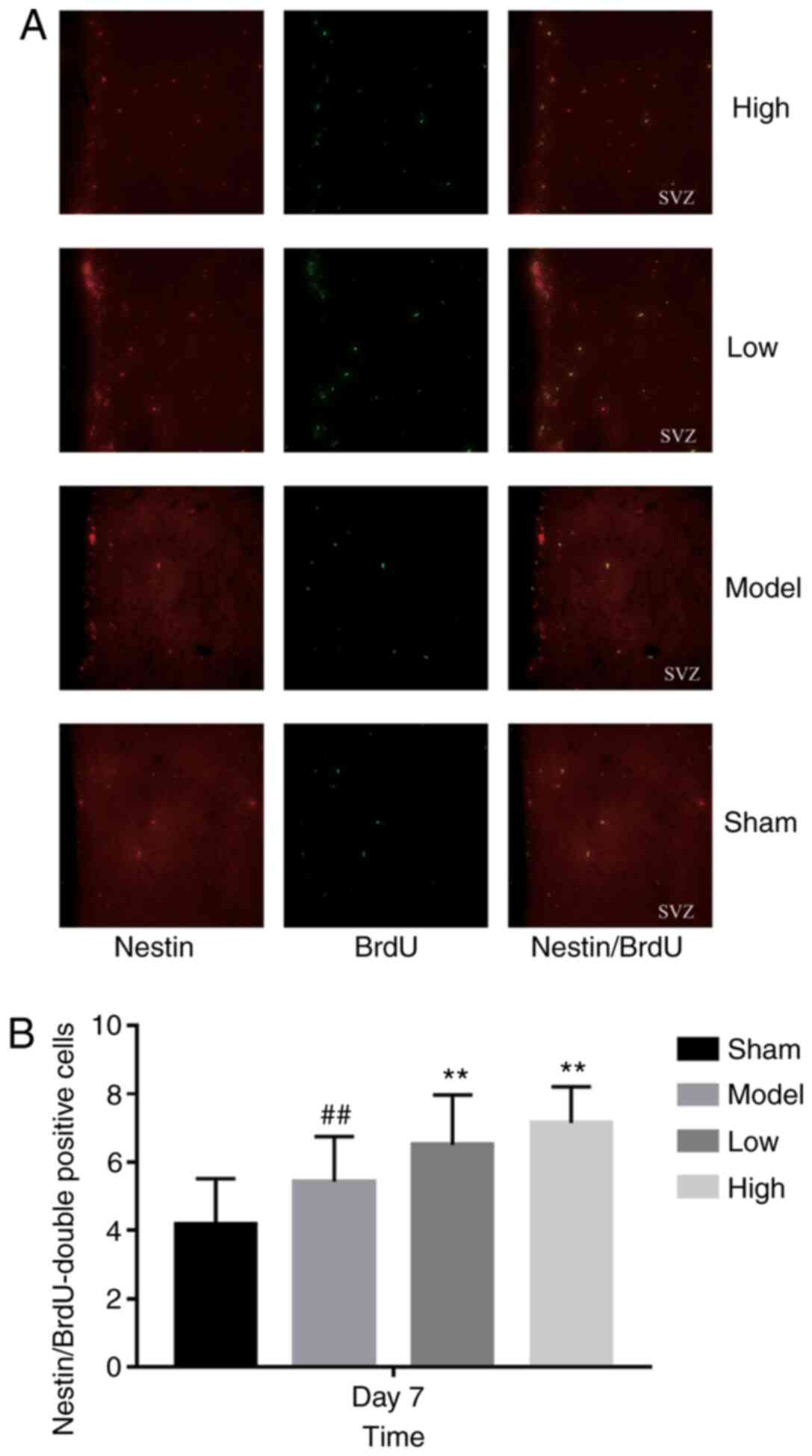
2). As shown in Fig. 3, the
total number of proliferating NSCs (Nestin- and BrdU-positive
cells) was markedly increased after TXC treatment within 7 days
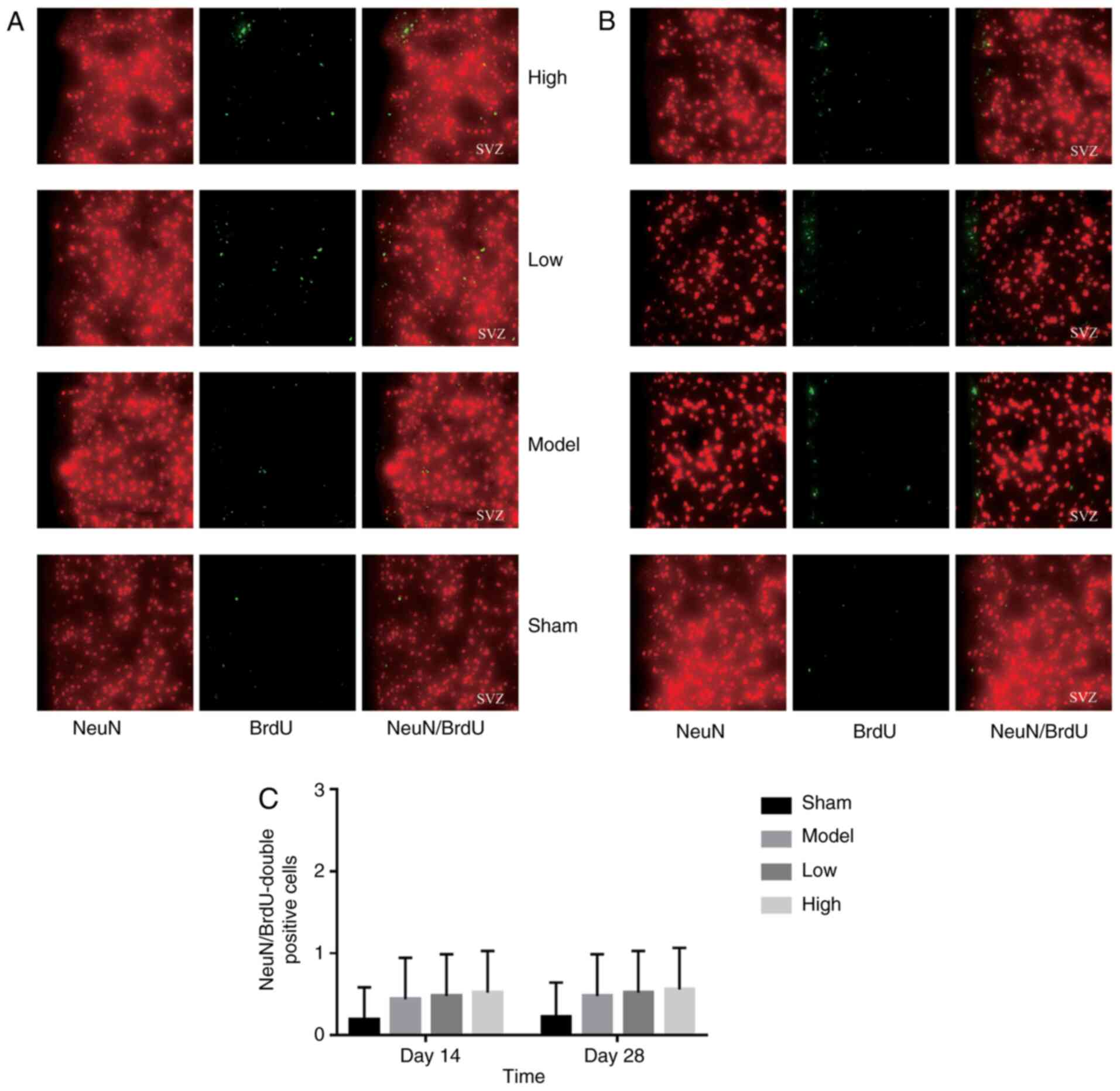
post-stroke. However, the proportion of actively cycling neuron
(NeuN- and BrdU-positive) cells was similar in both the untreated
and treated groups, both at 14 and 28 days post-stroke (Fig. 4). Furthermore, TXC treatment also
increased the number of proliferating glial cells (GFAP- and
BrdU-positive) in the SVZ region after 14 and 28 days post-stroke
(Fig. 5).
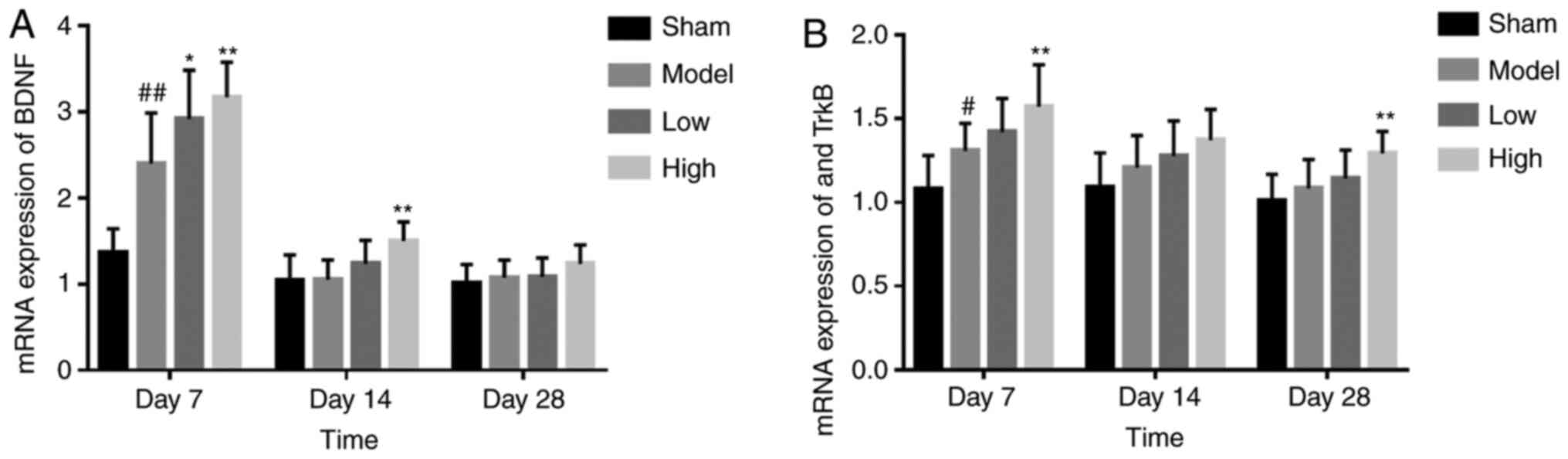
TXC upregulates BDNF and TrκB after
ICH
The BDNF mRNA levels were significantly higher in
the brain tissues of the TXC-treated animals compared with the
untreated controls at 7 and 14 days post-stroke. The upregulation
on day 14 was particularly notable in the high dose TXC group.
However, there was no significant difference in BDNF expression
between the untreated and treated groups after 28 days (Fig. 6A). TrκB mRNA was also upregulated in
the model group compared with the sham-operated group after 7 days
(P<0.05), and this effect was augmented further by high-dose TXC
treatment on day 7 and day 28 (Fig.
6B).
Discussion
It was found that EB extravasation in the brain was
most significant on the first-day post-stroke and decreased along
with time. Brain edema following ICH is mainly attributed to the
disruption of the BBB (32).
However, currently, drugs that can significantly improve BBB
function after stroke have not yet been identified. TXC
significantly inhibited EB extravasation, indicating that it can
improve cerebral vascular permeability and alleviate brain edema of
the affected rats in a dose-dependent manner during ICH.
A few BrdU-positive cells were detected in the
ependymal epithelium of the SVZ region at different time points in
the sham-operated group, which was consistent with the small number
of resting endogenous NSCs previously reported in this region
(13). In the present study, ICH
significantly increased the number of proliferating Nestin-positive
NSCs on the 7th day, which further enhanced the TXC treatment
effect. Furthermore, significant glial cell proliferation was
observed in the TXC-treated animals on the 14 and 28th days after
TXC administration. TXC promoted NSC proliferation and
differentiation into astroglial cells but not into neurons.
However, the proliferation and activation of astrocytes were also
beneficial for ICH. Astrocytes act as a ‘double-edged sword’
inactivating NSCs after stroke. During the early stage of stroke,
astrocytes can restrict the diffusion of inflammatory factors by
secreting various neurotrophic factors that protect nascent
neurons. During the later stages, excessive proliferation and
activation of astrocytes can mechanically hinder neural
regeneration by forming an extensive network (33).
Additionally, TXC further augmented BDNF and TrκB
levels, which likely increased NSC proliferation and
differentiation in the SVZ, although the exact mechanism is still
unclear. Studies have shown that BDNF and its receptor, TrκB, are
upregulated following cerebral ischemia and epilepsy (34,35).
Brain transplantation of human NSCs overexpressing BDNF provided
differentiation and survival abilities to the grafted human NSCs
and renewed angiogenesis in the host brain and functional recovery
of ICH animals (36). The
simultaneous increase in astrocyte proliferation and BDNF/TrκB
expression after ICH suggests that activated glial cells secrete
neurotrophic factors, which may drive pathological changes
associated with ICH and promote neuronal survival. However, BDNF
protein is mainly expressed in activated microglia around hematoma.
In contrast, BDNF mRNA is mainly expressed in neurons and partially
expressed in activated microglia around hematoma (37), which may be closely associated with
the secretion of various neurotrophic factors by activated glial
cells (38).
Taken together, the present findings demonstrated
that TXC could significantly improve neurological deficits,
absorption of brain edema, BBB integrity and NSC proliferation and
differentiation into glial cells by upregulating the
neuroprotective factors, BDNF and TrκB. This study showed that TXC
might be an effective treatment method using an ICH rat model and
provided a novel perspective on the treatment and prevention of ICH
in patients. However, further clinical studies will be necessary to
confirm these results.
Acknowledgements
The authors would like to thank Professor Xiao Cheng
(Second Clinical Medical College; Guangzhou University of Chinese
Medicine, Guangzhou, China) for editing this manuscript, critical
reading and providing suggestions.
Funding
The present study was supported by the Guangdong
Natural Science Foundation (grant nos. 2015A030310436 and
2018A0303130053 to LQ).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LQ and YZ conceived and designed the experiments. ZC
and SL performed the experiments. LH, YS, HC and HM analyzed the
data. The manuscript was drafted by ZC and SL and revised by all
authors. ZC and SL confirm the authenticity of all the raw data.
All authors have read and approved the manuscript and have
contributed significantly to the study.
Ethics approval and consent to
participate
All experimental animal protocols were approved and
performed following the Institutional Animal Care Guidelines
(approval nr. 2016040; Experimental Animal Ethics Committee,
Guangdong Provincial Hospital of Chinese Medicine, Guangzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang AP, Hsu YH, Wu MS, Tsai HH, Su CY,
Ling TY, Hsu SH and Lai DM: Potential of stem cell therapy in
intracerebral hemorrhage. Mol Biol Rep. 47:4671–4680. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gregorio T, Pipa S, Cavaleiro P, Atanásio
G, Albuquerque I, Chaves PC and Azevedo L: Prognostic models for
intracerebral hemorrhage: Systematic review and meta-analysis. Bmc
Med Res Methodol. 18:1452018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
An SJ, Kim TJ and Yoon BW: Epidemiology,
risk factors, and clinical features of intracerebral hemorrhage: An
update. J Stroke. 19:3–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu TY, Sharma G, Strbian D, Putaala J,
Desmond PM, Tatlisumak T, Davis SM and Meretoja A: Natural history
of perihematomal edema and impact on outcome after intracerebral
hemorrhage. Stroke. 48:873–879. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garcia PY, Roussel M, Bugnicourt JM, Lamy
C, Canaple S, Peltier J, Loas G, Deramond H and Godefroy O:
Cognitive impairment and dementia after intracerebral hemorrhage: A
cross-sectional study of a hospital-based series. J Stroke
Cerebrovasc Dis. 22:80–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pias-Peleteiro J, Campos F, Castillo J and
Sobrino T: Endothelial progenitor cells as a therapeutic option in
intracerebral hemorrhage. Neural Regen Res. 12:558–561. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z, Zhou F, Dou Y, Tian X, Liu C, Li
H, Shen H and Chen G: Melatonin alleviates intracerebral
hemorrhage-induced secondary brain injury in rats via suppressing
apoptosis, inflammation, oxidative stress, DNA damage, and
mitochondria injury. Transl Stroke Res. 9:74–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu YD, Zhao Q, Zhang XR, Xiong LL, Zhang
ZB, Zhang P, Zhang RP and Wang TH: Comparison of the properties of
neural stem cells of the hippocampus in the tree shrew and rat in
vitro. Mol Med Rep. 17:5676–5683. 2018.PubMed/NCBI
|
|
9
|
Suksuphew S and Noisa P: Neural stem cells
could serve as a therapeutic material for age-related
neurodegenerative diseases. World J Stem Cells. 7:502–511. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuentealba LC, Obernier K and
Alvarez-Buylla A: Adult neural stem cells bridge their niche. Cell
Stem Cell. 10:698–708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dooley D, Vidal P and Hendrix S:
Immunopharmacological intervention for successful neural stem cell
therapy: New perspectives in CNS neurogenesis and repair. Pharmacol
Ther. 141:21–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stenudd M, Sabelstrom H and Frisen J: Role
of endogenous neural stem cells in spinal cord injury and repair.
JAMA Neurol. 72:235–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen J, Xie L, Mao X, Zhou Y, Zhan R,
Greenberg DA and Jin K: Neurogenesis after primary intracerebral
hemorrhage in adult human brain. J Cereb Blood Flow Metab.
28:1460–1468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masuda T, Isobe Y, Aihara N, Furuyama F,
Misumi S, Kim TS, Nishino H and Hida H: Increase in neurogenesis
and neuroblast migration after a small intracerebral hemorrhage in
rats. Neurosci Lett. 425:114–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang P, Cai L, Zhang G, Bian Z and Han G:
The role of the miR-17–92 cluster in neurogenesis and angiogenesis
in the central nervous system of adults. J Neurosci Res.
95:1574–1581. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wakai T, Narasimhan P, Sakata H, Wang E,
Yoshioka H, Kinouchi H and Chan PH: Hypoxic preconditioning
enhances neural stem cell transplantation therapy after
intracerebral hemorrhage in mice. J Cereb Blood Flow Metab.
36:2134–2145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui M, Ge H, Zeng H, Yan H, Zhang L, Feng
H and Chen Y: Repetitive transcranial magnetic stimulation promotes
neural stem cell proliferation and differentiation after
intracerebral hemorrhage in mice. Cell Transplant. 28:568–584.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schabitz WR, Berger C, Kollmar R, Seitz M,
Tanay E, Kiessling M, Schwab S and Sommer C: Effect of
brain-derived neurotrophic factor treatment and forced arm use on
functional motor recovery after small cortical ischemia. Stroke.
35:992–997. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bochorakova H, Paulova H, Slanina J, Musil
P and Taborska E: Main flavonoids in the root of scutellaria
baicalensis cultivated in Europe and their comparative
antiradical properties. Phytother Res. 17:640–644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang CZ, Mehendale SR, Calway T and Yuan
CS: Botanical flavonoids on coronary heart disease. Am J Chin Med.
39:661–671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu X, Lu R and Zhao S: Simultaneous
quantitation of six aconitum alkaloids and three flavonoids in the
herb couple of radix aconiti lateralis-radix glycyrrhizae
(Fuzi-Gancao) by UHPLC-ESI-MS/MS. Pharmacogn Mag. 13:425–429. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Liu M, Lu B and Sun J: Effect of
tongfu xingshen liquid on the expression of HO-1 mRNA and HSP70 in
cerebral tissue of rats with intracerebral hemorrhage. J Chengdu
Unversity of Tarditional Chin Med. 2:27–29. 2004.(In Chinese).
PubMed/NCBI
|
|
23
|
Hui QX, Zhi ZR and Hua L: Study on
qualitative and quantitative methods of tongfu xingshen capsule.
Chin J Exp Traditional Med Formulae. 7:22–24. 2001.(In
Chinese).
|
|
24
|
Cai LM, Xun LB and Bo SJ: Effect of tongfu
xingshen liquid enema on cerebral edema and cerebral vascular
permeability in rats with intracerebral hemorrhage. Chin J
Information on TCM. 11:210–212. 2004.(In Chinese).
|
|
25
|
Jingbo S, Rong H, Peixin H and Yan H:
Effects of tongfu xingshen capsule on the animal model of stroke
with tanre fushi syndrome in rats. Chin J Integrated Traditional
Chin Western Medicine First Aid. 06:341–343. 2001.(In Chinese).
|
|
26
|
Nath FP, Jenkins A, Mendelow AD, Graham DI
and Teasdale GM: Early hemodynamic changes in experimental
intracerebral hemorrhage. J Neurosurg. 65:697–703. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu YY, Niu L, Gao L, Zhang GL, Li J, Deng
JP, Qu YZ, Zhao ZW and Gao GD: Ferrous chelator 2,2′-dipyridyl
attenuates cerebral vasospasm after experimental subarachnoid
haemorrhage in rabbits. J Int Med Res. 38:583–592. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manaenko A, Chen H, Zhang JH and Tang J:
Comparison of different preclinical models of intracerebral
hemorrhage. Acta Neurochir Suppl. 111:9–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bechet S, Hill F, Gilheaney O and Walshe
M: Diagnostic accuracy of the modified evan's blue dye test in
detecting aspiration in patients with tracheostomy: A systematic
review of the evidence. Dysphagia. 31:721–729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin JH, Jie WJ, Qiang X, Fen ZJ and Yu XX:
Influence factors and mechanism of Borneol on blood brain barrier
permeability. Chin J Chin Materia Medica. 42:2200–2207. 2017.(In
Chinese).
|
|
33
|
Hongjiang L, Zhaoliang S, Xitao Y and
Dongfu F: Effect of astrocyte activation on the regeneration of
optic nerve after injury. Chin J Minimally Invasive Neurosurgery.
21:283–285. 2016.(In Chinese).
|
|
34
|
Hagihara H, Hara M, Tsunekawa K, Nakagawa
Y, Sawada M and Nakano K: Tonic-clonic seizures induce division of
neuronal progenitor cells with concomitant changes in expression of
neurotrophic factors in the brain of pilocarpine-treated mice.
Brain Res Mol Brain Res. 139:258–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee TH, Yang JT, Kato H, Wu JH and Chen
ST: Expression of brain-derived neurotrophic factor
immunoreactivity and mRNA in the hippocampal CA1 and cortical areas
after chronic ischemia in rats. J Neurosci Res. 76:705–712. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee HJ, Lim IJ, Lee MC and Kim SU: Human
neural stem cells genetically modified to overexpress brain-derived
neurotrophic factor promote functional recovery and neuroprotection
in a mouse stroke model. J Neurosci Res. 88:3282–3294. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Heng W: Expression and significance of
brain-derived neurotrophic factor protein and mRNA in rats with
intracerebral hemorrhage. Chin J Geriatric Heart Brain Vessel Dis.
08:564–567. 2006.(In Chinese).
|
|
38
|
Ohta K, Ohta M, Mizuta I, Fujinami A,
Shimazu S, Sato N, Yoneda F, Hayashi K and Kuno S: The novel
catecholaminergic and serotoninergic activity enhancer
R-(−)-1-(benzofuran-2-yl)-2-propylaminopentane up-regulates
neurotrophic factor synthesis in mouse astrocytes. Neurosci Lett.
328:205–208. 2002. View Article : Google Scholar : PubMed/NCBI
|