It is estimated that there will be >1.9 million
new cases of cancer in the United States in 2021, accompanied by
>608,000 deaths (1). Cancer of
the digestive system has the second-highest number of new cases and
cancer deaths (1). It is widely
accepted that the normal function of the digestive system is
essential for food digestion, residue excretion, nutrient
absorption, and toxic substance discharge. These functions are
dependent on the transport of large amounts of water, ions, and
nutrients across the epithelium. These physiological processes are
achieved via the uneven distribution of ions mediated by ion
channels (2), such as absorption of
glucose by sodium-glucose cotransporters in the small intestine
(3). In addition to controlling the
distribution of chloride inside and outside of the cell to maintain
water-electrolyte balance, chloride channels also contribute to the
regulation of intracellular volume and pH (4). Numerous studies have found that
chloride intracellular channels (CLICs) have crucial roles in
tumors of the digestive system and should be considered a potential
diagnostic and therapeutic target for cancer (5–9).
Therefore, the present review focused on CLICs as novel biomarkers
for digestive system tumors.
In mammals, the CLIC family has seven members,
CLIC1-6. Of these, CLIC5 has two alternative splice variants,
CLIC5A and CLIC5B. CLICs exist as soluble globular proteins that
can form ion channels in organelles and plasma membranes (10). However, to date, only crystal
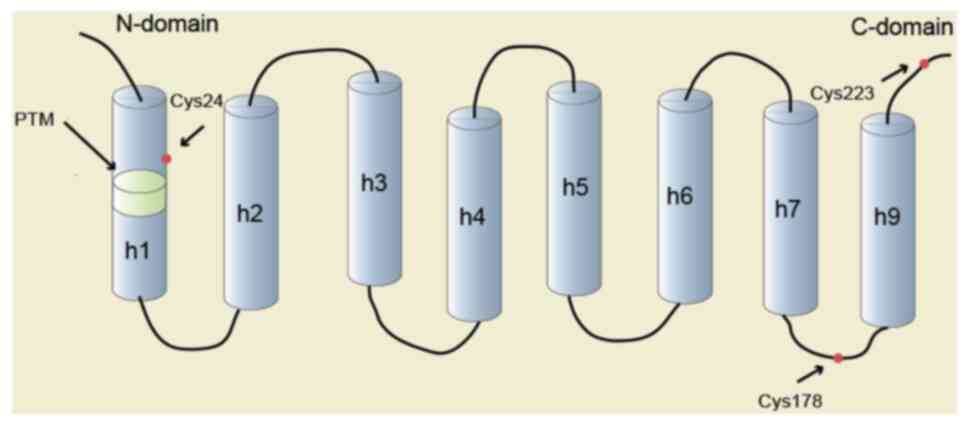
structures of soluble forms of CLICs have been obtained. CLICs
comprise an N-terminal thioredoxin-like domain, which contains a
mixture of α-helices and β-sheets, and an α-helical C-terminal
domain. The N-terminal domain includes a putative transmembrane
region (PTM) (4). In the
three-dimensional folded structure, there is a similarity between
soluble CLICs and ω class glutathione (GSH)-S-transferases (GSTs).
CLICs, similar to ω class GST proteins, include a conserved
glutaredoxin-like site and a reactive cysteine residue (Cys24 in
CLIC1 and Cys35 in CLIC4) in mammals (11), suggesting that the function of CLICs
can be regulated in a redox-dependent manner. Furthermore, the CLIC
structure includes an elongated cleft (or groove), similar to ω
class GSTs, which can bind to glutathione (10). However, CLIC proteins have a very
low affinity for glutathione (4).
It can therefore be inferred that CLIC proteins use GSH-binding
sites to target the CLICs to specific subcellular sites (4). The structure of CLIC1 is presented in
Fig. 1. In addition to the
N-terminus, C-terminus and PTM, which are common among CLICs, the
secondary structure of CLIC1 is also shown. The primary tissues
with physiological CLIC protein expression and digestive system
tumors presenting abnormal expression of CLIC proteins are
presented in Table I (12–36).
CLICs not only have significant roles in cancer
directly through chloride but also through interacting with other
proteins and affecting cell signaling. Each CLIC member has unique
functions that have been associated with hallmarks of cancer,
including cell cycle, apoptosis, angiogenesis, migration and
metastasis.
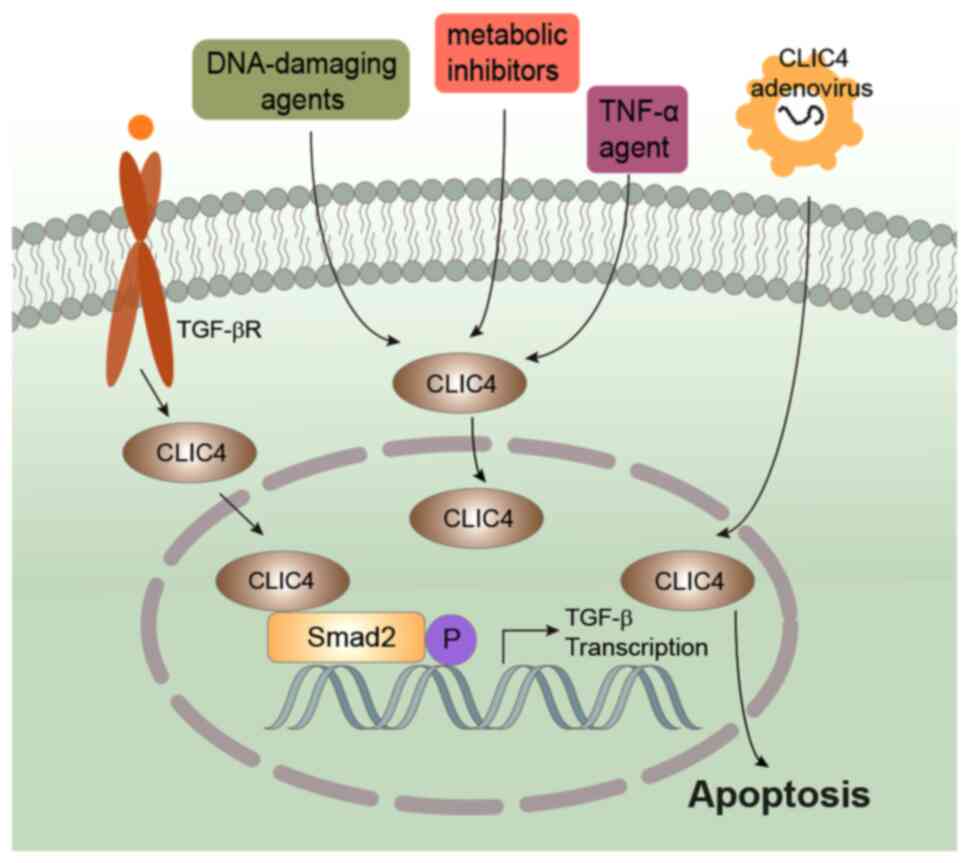
CLIC4 and CLIC5 participate in apoptosis. CLIC4 acts
as an apoptotic effector and has a substantial role in p53 and
c-Myc-mediated apoptosis (27). p53
overexpression or DNA damage mediates CLIC4 upregulation and
induces apoptosis (27).
Mechanistically, mitochondrial membrane potential is reduced by
CLIC4 overexpression, followed by cytochrome c release into the
cytoplasm and the activation of caspases to induce apoptosis
(27). CLIC4 downregulation reduces
p53-induced, but not Bax-induced apoptosis, indicating that the two
pro-apoptotic proteins function independently (27). CLIC4 downregulation enhances
autophagy and contributes to mitochondrial and endoplasmic
reticulum stress-induced apoptosis under starvation (64). It has been shown that endogenous
CLIC4 from the cytoplasm translocates into the nucleus under
starvation, as well as after treatment with DNA-damaging agents
(etoposide, adriamycin and mitomycin), metabolic inhibitors
(cycloheximide and actinomycin D), camptothecin, tumor necrosis
factor-α and transforming growth factor-β (Fig. 2) (64,65).
CLIC4 nuclear translocation is a response to stress and may
contribute to the initiation of apoptosis-related nuclear
alterations (65).
CLIC5 is located at the inner mitochondrial membrane
and CLIC4 in the outer mitochondrial membrane. CLIC5 has a direct
role in the regulation of mitochondrial reactive oxygen species
(ROS) generation (66).
Mitochondria serve a significant role in lysosomal-mediated cell
death (67). The ETS variant
transcription factor 6 (ETV6)/RUNX family transcription factor 1
fusion gene results in childhood precursor B-cell acute
lymphoblastic leukemia (68). The
loss of ETV6 transcriptional repressor induces CLIC5 upregulation,
ultimately leading to a decrease in lysosome-mediated apoptosis,
indicating that CLIC5 activity facilitates an environment of
oxidative stress induced by DNA damage accumulation, thereby
contributing to the development of leukemogenesis (40).
The function of angiogenesis involves CLIC1, CLIC4
and CLIC5. The downregulation of CLIC1 reduces endothelial
migration, capillary-like network formation, branching
morphogenesis and capillary-like sprouting (23). Endothelial cell migration and
adhesion depend on an appropriate amount of integrin expression
(69). CLIC1 has an essential role
in the regulation of the cell surface expression of various
integrins in angiogenesis, such as αVβ3, αVβ5 and subunits β1 and
α3. In CLIC1−/− mice, CLIC1 knockdown resulted in a mild
platelet dysfunction characterized by prolonged bleeding, and P2Y12
receptor signaling-related ADP stimulation led to a reduction in
platelet activation (30). The
activation of G(12/13) pathways by ADP regulates fibrinogen
receptor activation in platelets and dense granule release
(70).
The expression of CLIC4 is required at multiple
stages of angiogenesis. CLIC4 is necessary for endothelial cell
hollowing, a process required for vessel formation during ischemia
and embryogenesis (71). CLIC4
promotes endothelial cell proliferation and regulates endothelial
morphogenesis (38,72). CLIC4 downregulation was demonstrated
to decrease cell proliferation, capillary-like sprouting, lumen
formation and capillary network formation, all of which was
promoted by CLIC4 upregulation (25). CLIC4−/− mice exhibited
defective angiogenesis in vivo (38,71,73).
Compared with wild-type mice, CLIC4−/− mice demonstrated
abnormal collateral circulation in response to ischemic injury
(71,73), and the native cerebral collateral
density was reduced, leading to severe infarctions (71), smaller kidneys with fewer glomeruli,
less dense peritubular capillary networks (74), and retinal angiogenesis defects
(38). Furthermore,
CLIC4/CLIC5A-mediated ezrin, radixin, moesin activation is
necessary for the maintenance of the glomerular capillary
architecture (75).
CLIC1 and CLIC4 bridge the cortical actin
cytoskeleton and the plasma membrane for cytokinesis (76). The downregulation of CLIC4 and CLIC1
result in abnormal blebbing at the polar cortex and regression of
the cleavage furrow during late cytokinesis, ultimately forming
multinucleated cells (76). CLIC2
is decreased in most endothelial cells in blood vessels of cancer
tissue (77). In human umbilical
vein endothelial cells (HUVECs), CLIC2 downregulation helps human
cancer cells to transmigrate through a HUVEC monolayer (77). CLIC4 is implicated in various
actin-based processes, such as integrin trafficking and cell
adhesion (78). Mechanistically,
CLIC4 regulates the Ras homolog family member A/mouse homolog of
diaphanous 2-regulated signaling network to integrate cortical
actin assembly and membrane protrusion by binding to profilin-1
(78). In addition, in HeLa and
MDA-MB-231 cells, CLIC4 downregulation suppresses cell spreading,
cell-matrix adhesion and integrin signaling (82). CLIC4 is recruited to β1 integrin at
the plasma membrane and RAB35-positive endosomes by
lysophosphatidic acid stimulation. Furthermore, CLIC4 impedes the
RAB35-dependent regulation of β1 integrin trafficking by decreasing
RAB35 activity (82). In renal
glomerular podocyte foot processes, CLIC5A, one of two alternative
splicing variants of CLIC5, is a component of the ezrin
(EZR)/sodium-hydrogen exchanger regulatory factor 2
(NHERF-2)/podocalyxin cytoskeletal complex (79). Furthermore, at the cell cortex,
similar to EZR, CLIC5A may have an essential role in the assembly
and/or maintenance of F-actin-based structures (83). CLIC5A is a component of the
EZR-NHERF2-podocalyxin complex in glomeruli. In CLIC5-deficient
mice, the cytoskeletal association of EZR and NHERF2 was diminished
(79). Mechanistically, the
interaction of CLIC5A with PI(4,5)P2-generating kinases led to
clustered plasma membrane PI(4,5)P2 accumulation, followed by the
promotion of EZR activation and actin-dependent cell surface
remodeling (79).
In esophageal squamous cell carcinoma (ESCC), CLIC1
expression is upregulated in both cell lines (TE2, TE5, TE8, TE9,
TE15, KYSE70, KYSE150, KYSE170, and KYSE790), and tissue samples
(5). Furthermore, CLIC1 is present
in the cytoplasm of cancer cells (5). CLIC1 knockdown inhibited the
proliferation of tumor cells, and CLIC1 regulated apoptosis through
the Toll-like receptor 2/JNK pathway (5). Furthermore, cell cycle arrest was
found to occur in the sub-G1phase following CLIC1 knockdown
(5). However, as previously
mentioned, CLIC1 is only observed at the plasma membrane of G2/M
phase cells, and blockade of CLICK1 by chloride channel blockers
IAA-94 and anthracene-9-carboxylic acid resulted in the arrest of
CHO-K1 cells in the G2/M phase of the cell cycle (16). Whether this contradictory result was
due to the different cell lines or different mechanisms requires
further investigation. In addition, patients with strong expression
of CLIC1 exhibited a significantly lower 5-year overall survival
than those with weak expression of CLIC1 (sample size, 61)
(5). In a cohort of 45 patients
with ESCC, CLIC3 was found downregulated, CLIC4 was upregulated and
CLIC2 unchanged, as detected by quantitative PCR and western
blotting (5). The specific
functions of CLICs in ESCC, as well as differences in expression,
require further study. Squamous cell carcinoma is a common type of
esophageal cancer, and CLIC4 has been demonstrated to inhibit the
growth of squamous cell carcinoma, and the degree of reduction in
CLIC4 coincided with the progression of squamous cell tumors from
benign to malignant (5). However,
it remains unclear whether CLIC4 is involved in the development and
progression of ESCC.
In GC, CLIC1 is upregulated and correlates with
lymph node metastasis, lymphatic invasion, perineural invasion, and
poor patient prognosis (6,84,85).
Following CLIC1 knockdown in GC cells SGC-7901, the expression
levels of integrin α3, αv and β1 in vivo, as well as the
phosphorylation of PI3K/AKT, ERK, and p38, were found to be
decreased, while integrin α1 was found to be increased; it was
therefore hypothesized that the mechanism of CLIC1 in the
progression of GC may be associated with the regulation of integrin
family proteins, leading to the sequential regulation of PI3K/AKT,
mitogen-activated protein kinase (MAPK)/ERK, and MAPK/p38 pathways
(86). CLIC1 upregulation could
mediate, at least partly, the ability to enhance invasion and
metastasis of GC following the knockdown of proteasome activator
subunit 2 (87). As aforementioned,
one of the hallmarks of malignancy is angiogenesis, which provides
blood supply to the tumor tissue (36). One of the functions of CLICs is
their participation in angiogenesis (41,47).
Unregulated angiogenesis leads to a constant hypoxia-reoxidation
(H-R) state, thus increasing ROS production, providing a substrate
for further undifferentiation (88). Similarly, CLIC1 regulates GC cell
migration and invasion through the ROS-mediated p38 MAPK signaling
pathway (84). This is further
confirmed by the fact that blocking CLIC proteins with chloride
channel blocker IAA-94 reduces ROS production (89). CLIC1 is closely associated with GC
resistance to vincristine, and exosome-mediated transfer of CLIC1
can induce the development of vincristine resistance in
vitro, possibly through the upregulation of P-glycoprotein and
Bcl-2 (90). Tissue microarray
analysis using 107 GC specimens revealed that CLIC3 was inversely
correlated with pathological tumor depth, that a lower CLIC3
expression was linked to a worse prognosis, and that CLIC3
functioned as a chloride channel on the plasma membrane of GC cells
(91).
In hepatitis B virus X protein-positive HepG2 cells,
CLIC1 protein accumulation was found to promote hepatocellular
carcinoma (HCC) development (92).CLIC1 has been reported to be
upregulated in HCC (93), and CLIC1
overexpression in liver tumor tissues was significantly correlated
with tumor size, metastasis, and pTNM stage. In addition, CLIC1
overexpression was found to be associated with poor prognosis
(7,93). In mouse hepatoma ascites, CLIC1 is
expressed in the cytoplasm and plasma membrane in both the Hca-F
lymphatic metastasis cell line with a high metastatic potential and
the Hca-P lymphatic metastasis cell line with a low metastatic
potential. Furthermore, two-dimensional difference-gel
electrophoresis revealed that CLIC1 expression was higher in both
Hca-F cells and plasma membranes, compared with Hca-P (94). Consistently, another study found
that the expression of CLIC1 mRNA and protein in Hca-F cells was
higher compared with that in Hca-P cells (2 and 1.6-fold,
respectively), and the migration and invasion ability were
significantly decreased following CLIC1 downregulation, thus
demonstrating that CLIC1 may be a key factor in the development of
lymphatic metastasis (95).
Furthermore, CLIC1 is upregulated in HCC tissues with portal vein
tumor thrombus (96). CLIC1
overexpression was confirmed to decrease maspin expression and
increase vascular endothelial growth factor (VEGF), matrix
metalloproteinase (MMP) 2, MMP12, and MMP13 expression (96). These results revealed that, in HCC,
CLIC1 upregulation was significantly associated with vascular
invasion, and that CLIC1 may control the mechanism of HCC
invasiveness by targeting maspin (96). CLIC1 downregulation by RNA
interference significantly enhanced the expression of tumor
metastasis genes annexin A7 and gelsolin in vitro;
conversely, annexin A7 and gelsolin downregulation enhanced the
expression of CLIC1 in vitro and in vivo (97). These data illustrated that CLIC1 may
function by regulating the expression of annexin A7 and gelsolin in
the migration and invasion of liver cancer (97). Furthermore, at the transcriptome
level, microRNA (miR)-124 directly reduced CLIC1 expression,
further inhibiting cell migration, and invasion in HCC cells, but
without affecting cell proliferation (98). Alternatively, the levels of CLIC1
can be regulated by miR-122-5P (7).
It is not clear whether CLICs act as ion channels to
affect liver cancer. Of note, the chloride channel blocker
4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS) can
effectively inhibit the proliferation of liver cancer cells
(99). DIDS induces G1 arrest by
downregulating the protein expression levels of cyclin D1 and
cyclin E (99). DIDS reduces the
protein expression levels of α-fetoprotein, suggesting that it may
be able to improve the prognosis of HCC in patients (99). CLIC1 has a vital role, similar to
that of proto-oncogene, that may be targeted in the development of
novel tumor treatments with chloride channel blocker IAA-94
(100). However, the high
concentration of IAA-94 required for its function, as well as its
poor specificity for CLIC1, have affected its use as a
CLIC1-specific drug for cancer therapy. Further therapeutic
development requires a specific and potent CLIC1 inhibitor. If the
structure of soluble CLIC1 were characterized, the rational design
of small molecules or peptides for CLIC1 inhibition would provide
new avenues for blocking the function of CLIC1 (62). Function inhibition may also be
achieved by hyperpolarization using native CLIC1 chloride channels,
suggesting a therapeutic modality that does not require gene
therapy (101).
CLICs are involved in cytoskeleton formation, while
cell deformation is required during tumor invasion and metastasis
(102). CLIC2 knockdown in HUVECs
allows human cancer cells to migrate through HUVEC monolayers
(77). CLIC2 was found to be
downregulated in fibrotic and advanced HCC tissues (77). CLIC5 can be used as a biological
indicator to predict the prognosis of HCC together with EZR and
podocalyxin-like (PODXL) (proteins associated with invasion,
migration, and poor prognosis of various types of cancer). It has
been found that CLIC5 forms a complex with EZR and PODXL, and that
it is required for podocyte structure and function (103). In HCC, EZR, PODXL, and CLIC5 are
overexpressed (103). Furthermore,
migration and invasion were found to be decreased when the
expression of CLIC5 and PODXL was inhibited in Huh7 cells (103).
In human GBC, CLIC1 expression is upregulated
compared with normal tissues, and high CLC1 expression is
associated with histological grade, TNM stage, perineural invasion
(P<0.05), and decreased overall survival (P<0.001) (104). In fact, CLIC1 expression and
histological grade are independent risk factors for overall
survival (104). CLIC1 knockdown
promoted apoptosis and inhibited proliferation, migration and
invasion of GBC cells (105).
CLIC1 overexpression promoted GBC-SD18L cell motility and invasion
and, conversely, CLIC1 knockdown significantly reduced the in
vitro GBC-SD18H cell motility and invasion, indicating that
CLIC1 may play an essential role in the metastasis of gallbladder
cancer (8). At the transcript
level, CLIC1 is a direct target gene of hsa-miR-372 and miR-122
(7,106). In GBC, hsa-miR-372 is
downregulated and correlates with the aggressive and progressive
behavior of tumors by affecting CLIC1 expression (106). Urothelial cancer associated 1
(UCA1) was found to promote bile duct carcinoma (BDC) cell
migration and invasiveness, while miR-122 inhibited their
progression (84). CLIC1, as a
downstream target gene of miR-122, has the opposite effect. The
ERK/MAPK signaling pathway is activated following the upregulation
of long non-coding RNA UCA1 (84).
UCA1 promoted the metastasis of BDC cells and the activation of the
ERK/MAPK pathway by regulating the expression of miR-122 and its
downstream gene CLIC1, therefore expanding the options for targeted
treatment of cholangiocarcinoma (107). In addition, in GBC, serum
carbohydrate antigen 19-9 concentration is positively correlated
with the expression levels of troponin T1, slow skeletal type,
MMP-9 and CLIC3 (108). Of note,
metformin markedly inhibits the proliferation and viability of GBC
cells, promotes apoptosis and increases the number of early
apoptotic cells (109). Metformin
has been further shown to exert growth inhibitory effects by
inhibiting p-AKT activity and the Bcl-2 family (85). Of note, either the dysfunction or
downregulation of CLIC1 could partially reduce the antitumor effect
of metformin, whereas upregulation of CLIC1 could increase drug
sensitivity (110).
In the pancreas, inhibition of CLIC4 increases
β-cell survival, likely due to increased levels of Bcl-2, Bcl-xl,
and Bad phosphorylation, and the overexpression of Bcl-2 or Bcl-xl
in β-cells increases their resistance to cytokine-induced apoptosis
(111). Using in silico
modeling to construct an interactome of the CLIC gene family,
CLIC1, CLIC3, and CLIC4 have been identified as prognostic markers
of overall survival in pancreatic ductal adenocarcinoma (PDAC)
compared with healthy controls. Among them, the expressions of
CLIC1-CLIC3, CLIC4-CLIC5, and CLIC5-CLIC6 have been found to be
positively correlated (112).
Similar to these findings, CLIC1 protein expression was
significantly increased in tumor samples from patients with
resected PDAC compared with normal tissues (67.1% vs. 25.7%,
respectively; P<0.001) (113).
In addition, CLIC1 overexpression was found to be associated with a
higher histological grade, larger tumor size, and worse overall
survival [hazard ratio, 5.822; 95% confidence interval (CI),
1.329–15.628; P=0.016) (113).
Furthermore, the treatment of pancreatic cancer cell lines with
CLIC1-targeting small interfering RNA oligonucleotides
significantly reduced cell proliferation, anchorage-independent
growth, and cell migration on soft agar (9). CLIC3 drives pancreatic cancer
invasiveness by cooperating with RAB25 to regulate α5β1 integrin
recycling from late endosomes to the plasma membrane (114). Similarly, CLIC3 predicts lymph
node metastasis and poor prognosis in inoperable cases of PDAC
(12). CLIC4 and Indian hedgehog
have been found to be significantly correlated with tumor grade,
lymph node metastasis, tumor invasion, and poor overall survival
(115). Of note, HOXA distal
transcript (HOTTIP), a long non-coding RNA, is upregulated in PDAC
(92), and, by identifying
canonical HOTTIP/HOXA13 targets, CLIC5 was found to be crucial for
PDAC cell growth and cell invasion (116). However, the oncogenic pathway
mediated by HOTTIP is not fully understood.
CLICs have an integral role in the process of
tumorigenesis by participating in various physiological processes.
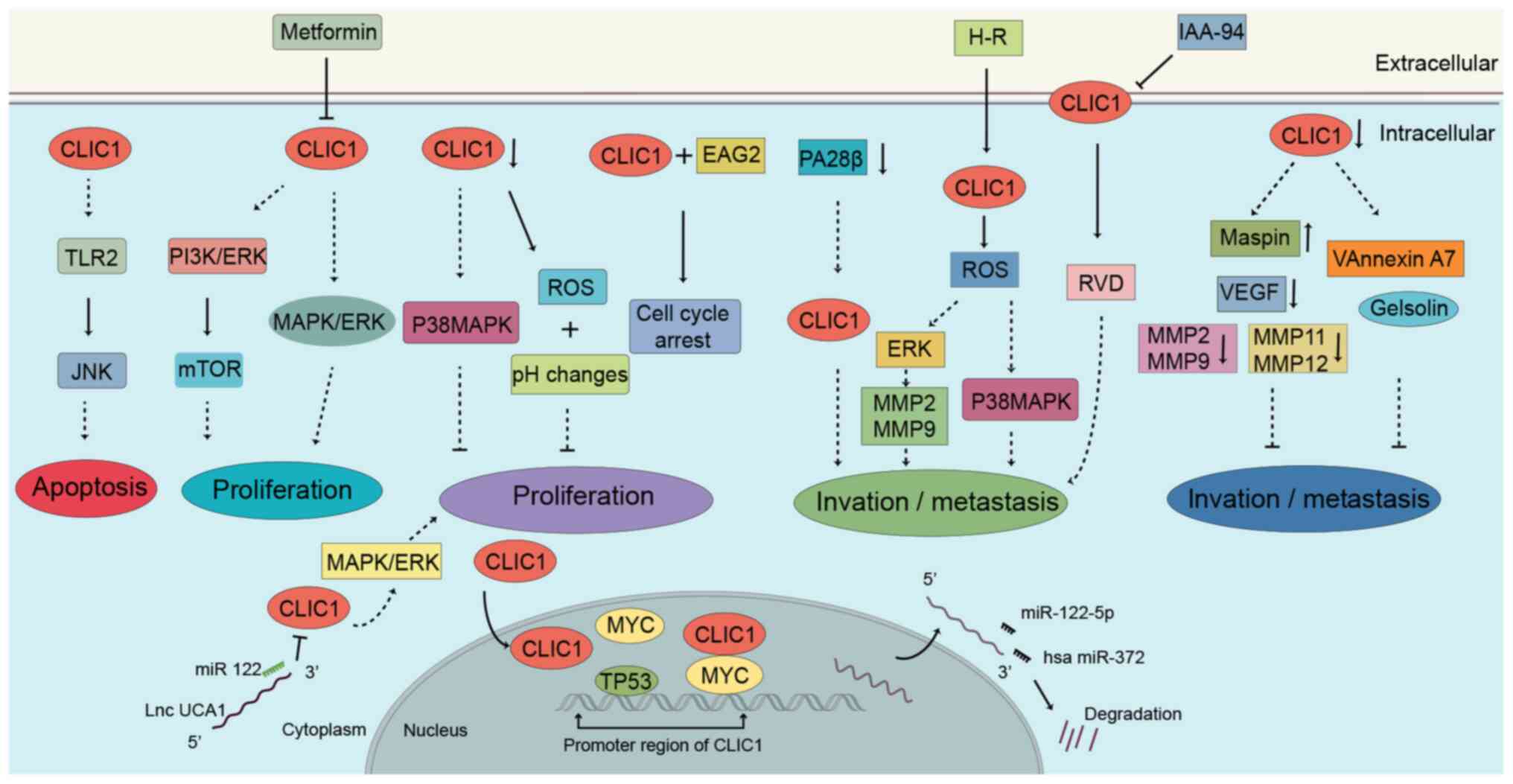
In particular, the role of CLIC1 in cancer has been summarized in
Fig. 3. The expression levels of
CLICs can be used as a diagnostic and prognostic marker for
digestive system tumors. However, there is currently a lack of
studies based on CLIC knockout mice in digestive system tumors. The
understanding of the role of CLICs in health and tumors is
incomplete. It is also necessary to clarify whether CLICs function
as chloride channels or as proteins under specific disease
conditions, and to understand their interactions and exact
molecular basis of the complex signaling network activated by
CLICs. Subsequently, the potential functional continuity of CLICs
between the cytoplasm and membrane needs to be explored. The
development of conformation-specific drug inhibitors and CLIC
protein activity modulators may herald new and more effective
avenues for the treatment of cancer.
Not applicable.
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 82073087), the
Guizhou Provincial Department of Education Youth Science and
Technology Talents Growth Project [grant no. QIAN-JIAO-HE KY ZI
(2018)236], and the Zunyi Medical University 2017 New Academic
Cultivation and Innovation Exploration Special Project [grant no.
Qian-Ke-He-Ping-Tai-Ren-Cai (2017)5733-072].
Not applicable.
HW wrote the manuscript. JA and CL participated in
information collection, analysis, and organization. SH and JW
primarily revised and finalized the manuscript. BT revised the
manuscript for clarity and style and critically revised the article
for important intellectual content. All authors have read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pácha J: Development of intestinal
transport function in mammals. Physiol Rev. 80:1633–1667. 2000.
View Article : Google Scholar
|
|
3
|
Poulsen SB, Fenton RA and Rieg T:
Sodium-glucose cotransport. Curr Opin Nephrol Hypertens.
24:463–469. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Argenzio E and Moolenaar WH: Emerging
biological roles of Cl-intracellular channel proteins. J Cell Sci.
129:4165–4174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi T, Shiozaki A, Nako Y, Ichikawa
D, Kosuga T, Shoda K, Arita T, Konishi H, Komatsu S, Kubota T, et
al: Chloride intracellular channel 1 as a switch among tumor
behaviors in human esophageal squamous cell carcinoma. Oncotarget.
9:23237–23252. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma PF, Chen JQ, Wang Z, Liu JL and Li BP:
Function of chloride intracellular channel 1 in gastric cancer
cells. World J Gastroenterol. 18:3070–3080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang X, Liu Y, Wang G, Yao Y, Mei C, Wu
X, Ma W and Yuan Y: Up-regulation of CLIC1 activates MYC signaling
and forms a positive feedback regulatory loop with MYC in
Hepatocellular carcinoma. Am J Cancer Res. 10:2355–2370.
2020.PubMed/NCBI
|
|
8
|
Wang JW, Peng SY, Li JT, Wang Y, Zhang ZP,
Cheng Y, Cheng DQ, Weng WH, Wu XS, Fei XZ, et al: Identification of
metastasis-associated proteins involved in gallbladder carcinoma
metastasis by proteomic analysis and functional exploration of
chloride intracellular channel 1. Cancer Lett. 281:71–81. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu J, Dong Q, Zhang B, Wang X, Ye B, Zhang
F, Song X, Gao G, Mu J, Wang Z, et al: Chloride intracellular
channel 1 (CLIC1) is activated and functions as an oncogene in
pancreatic cancer. Med Oncol. 32:6162015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harrop SJ, DeMaere MZ, Fairlie WD,
Reztsova T, Valenzuela SM, Mazzanti M, Tonini R, Qiu MR, Jankova L,
Warton K, et al: Crystal structure of a soluble form of the
intracellular chloride ion channel CLIC1 (NCC27) at 1.4-A
resolution. J Biol Chem. 276:44993–45000. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Littler DR, Harrop SJ, Goodchild SC, Phang
JM, Mynott AV, Jiang L, Valenzuela SM, Mazzanti M, Brown LJ, Breit
SN and Curmi PM: The enigma of the CLIC proteins: Ion channels,
redox proteins, enzymes, scaffolding proteins? FEBS Lett.
584:2093–2101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dozynkiewicz MA, Jamieson NB, Macpherson
I, Grindlay J, van den Berghe PV, von Thun A, Morton JP, Gourley C,
Timpson P, Nixon C, et al: Rab25 and CLIC3 collaborate to promote
integrin recycling from late endosomes/lysosomes and drive cancer
progression. Dev Cell. 22:131–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chou SY, Hsu KS, Otsu W, Hsu YC, Luo YC,
Yeh C, Shehab SS, Chen J, Shieh V, He GA, et al: CLIC4 regulates
apical exocytosis and renal tube luminogenesis through retromer-
and actin-mediated endocytic trafficking. Nat Commun. 7:104122016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Al Khamici H, Brown LJ, Hossain KR, Hudson
AL, Sinclair-Burton AA, Ng JP, Daniel EL, Hare JE, Cornell BA,
Curmi PM, et al: Members of the chloride intracellular ion channel
protein family demonstrate glutaredoxin-like enzymatic activity.
PLoS One. 10:e1156992015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Board PG, Coggan M, Watson S, Gage PW and
Dulhunty AF: CLIC-2 modulates cardiac ryanodine receptor
Ca2+ release channels. Int J Biochem Cell Biol.
36:1599–1612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valenzuela SM, Mazzanti M, Tonini R, Qiu
MR, Warton K, Musgrove EA, Campbell TJ and Breit SN: The nuclear
chloride ion channel NCC27 is involved in regulation of the cell
cycle. J Physiol. 529:541–552. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang JY, Jung JY, Cho SW, Choi HJ, Kim SW,
Kim SY, Kim HJ, Jang CH, Lee MG, Han J and Shin CS: Chloride
intracellular channel 1 regulates osteoblast differentiation. Bone.
45:1175–1185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fernández-Salas E, Sagar M, Cheng C, Yuspa
SH and Weinberg WC: p53 and tumor necrosis factor alpha regulate
the expression of a mitochondrial chloride channel protein. J Biol
Chem. 274:36488–36497. 1999. View Article : Google Scholar
|
|
19
|
Shanks RA, Larocca MC, Berryman M, Edwards
JC, Urushidani T, Navarre J and Goldenring JR: AKAP350 at the Golgi
apparatus. II. Association of AKAP350 with a novel chloride
intracellular channel (CLIC) family member. J Biol Chem.
277:40973–40980. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan L, Yu W and Zhu X: Interaction of
Sedlin with chloride intracellular channel proteins. FEBS Lett.
540:77–80. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qian Z, Okuhara D, Abe MK and Rosner MR:
Molecular cloning and characterization of a mitogen-activated
protein kinase-associated intracellular chloride channel. J Biol
Chem. 274:1621–1627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edwards JC, Cohen C, Xu W and Schlesinger
PH: c-Src control of chloride channel support for osteoclast HCl
transport and bone resorption. J Biol Chem. 281:28011–28022. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tung JJ and Kitajewski J: Chloride
intracellular channel 1 functions in endothelial cell growth and
migration. J Angiogenes Res. 2:232010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gaudet P, Livstone MS, Lewis SE and Thomas
PD: Phylogenetic-based propagation of functional annotations within
the Gene Ontology consortium. Brief Bioinform. 12:449–462. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tung JJ, Hobert O, Berryman M and
Kitajewski J: Chloride intracellular channel 4 is involved in
endothelial proliferation and morphogenesis in vitro. Angiogenesis.
12:209–220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rønnov-Jessen L, Villadsen R, Edwards JC
and Petersen OW: Differential expression of a chloride
intracellular channel gene, CLIC4, in transforming growth
factor-beta1-mediated conversion of fibroblasts to myofibroblasts.
Am J Pathol. 161:471–480. 2002. View Article : Google Scholar
|
|
27
|
Fernández-Salas E, Suh KS, Speransky VV,
Bowers WL, Levy JM, Adams T, Pathak KR, Edwards LE, Hayes DD, Cheng
C, et al: mtCLIC/CLIC4, an organellular chloride channel protein,
is increased by DNA damage and participates in the apoptotic
response to p53. Mol Cell Biol. 22:3610–3620. 2002. View Article : Google Scholar
|
|
28
|
Salao K, Jiang L, Li H, Tsai VW, Husaini
Y, Curmi PM, Brown LJ, Brown DA and Breit SN: CLIC1 regulates
dendritic cell antigen processing and presentation by modulating
phagosome acidification and proteolysis. Biol Open. 5:620–630.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiu MR, Jiang L, Matthaei KI,
Schoenwaelder SM, Kuffner T, Mangin P, Joseph JE, Low J, Connor D,
Valenzuela SM, et al: Generation and characterization of mice with
null mutation of the chloride intracellular channel 1 gene.
Genesis. 48:127–136. 2010.PubMed/NCBI
|
|
30
|
Tang T, Lang X, Xu C, Wang X, Gong T, Yang
Y, Cui J, Bai L, Wang J, Jiang W and Zhou R: CLICs-dependent
chloride efflux is an essential and proximal upstream event for
NLRP3 inflammasome activation. Nat Commun. 8:2022017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Domingo-Fernández R, Coll RC, Kearney J,
Breit S and O'Neill LAJ: The intracellular chloride channel
proteins CLIC1 and CLIC4 induce IL-1β transcription and activate
the NLRP3 inflammasome. J Biol Chem. 292:12077–12087. 2017.
View Article : Google Scholar
|
|
32
|
Dulhunty AF, Pouliquin P, Coggan M, Gage
PW and Board PG: A recently identified member of the glutathione
transferase structural family modifies cardiac RyR2 substate
activity, coupled gating and activation by Ca2+ and ATP.
Biochem J. 390:333–343. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suginta W, Karoulias N, Aitken A and
Ashley RH: Chloride intracellular channel protein CLIC4 (p64H1)
binds directly to brain dynamin I in a complex containing actin,
tubulin and 14-3-3 isoforms. Biochem J. 359:55–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suh KS, Mutoh M, Mutoh T, Li L, Ryscavage
A, Crutchley JM, Dumont RA, Cheng C and Yuspa SH: CLIC4 mediates
and is required for Ca2+-induced keratinocyte
differentiation. J Cell Sci. 120:2631–2640. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Berryman MA and Goldenring JR: CLIC4 is
enriched at cell-cell junctions and colocalizes with AKAP350 at the
centrosome and midbody of cultured mammalian cells. Cell Motil
Cytoskeleton. 56:159–172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seco CZ, Oonk AM, Domínguez-Ruiz M,
Draaisma JM, Gandía M, Oostrik J, Neveling K, Kunst HP, Hoefsloot
LH, del Castillo I, et al: Progressive hearing loss and vestibular
dysfunction caused by a homozygous nonsense mutation in CLIC5. Eur
J Hum Genet. 23:189–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ling CK, Santos LL, Zhou W and Dimitriadis
E: Chloride intracellular channel 4 is dysregulated in endometrium
of women with infertility and alters receptivity. Biochem Biophys
Res Commun. 531:490–496. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ulmasov B, Bruno J, Gordon N, Hartnett ME
and Edwards JC: Chloride intracellular channel protein-4 functions
in angiogenesis by supporting acidification of vacuoles along the
intracellular tubulogenic pathway. Am J Pathol. 174:1084–1096.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Berryman M and Bretscher A: Identification
of a novel member of the chloride intracellular channel gene family
(CLIC5) that associates with the actin cytoskeleton of placental
microvilli. Mol Biol Cell. 11:1509–1521. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pierchala BA, Muñoz MR and Tsui CC:
Proteomic analysis of the slit diaphragm complex: CLIC5 is a
protein critical for podocyte morphology and function. Kidney Int.
78:868–882. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ponnalagu D, Rao SG, Farber J, Xin W,
Hussain AT, Shah K, Tanda S, Berryman MA, Edwards JC and Singh H:
Data supporting characterization of CLIC1, CLIC4, CLIC5 and DmCLIC
antibodies and localization of CLICs in endoplasmic reticulum of
cardiomyocytes. Data Brief. 7:1038–1044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tavasoli M, Li L, Al-Momany A, Zhu LF,
Adam BA, Wang Z and Ballermann BJ: The chloride intracellular
channel 5A stimulates podocyte Rac1, protecting against
hypertension-induced glomerular injury. Kidney Int. 89:833–847.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Redhead C, Sullivan SK, Koseki C, Fujiwara
K and Edwards JC: Subcellular distribution and targeting of the
intracellular chloride channel p64. Mol Biol Cell. 8:691–704. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Griffon N, Jeanneteau F, Prieur F, Diaz J
and Sokoloff P: CLIC6, a member of the intracellular chloride
channel family, interacts with dopamine D(2)-like receptors. Brain
Res Mol Brain Res. 117:47–57. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rojo de la Vega M, Chapman E and Zhang DD:
NRF2 and the hallmarks of cancer. Cancer Cell. 34:21–43. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Prevarskaya N, Skryma R and Shuba Y: Ion
channels in cancer: Are cancer hallmarks oncochannelopathies?
Physiol Rev. 98:559–621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Leanza L, Romio M, Becker KA, Azzolini M,
Trentin L, Managò A, Venturini E, Zaccagnino A, Mattarei A,
Carraretto L, et al: Direct pharmacological targeting of a
mitochondrial ion channel selectively kills tumor cells in vivo.
Cancer Cell. 31:516–531.e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Valenzuela SM, Martin DK, Por SB, Robbins
JM, Warton K, Bootcov MR, Schofield PR, Campbell TJ and Breit SN:
Molecular cloning and expression of a chloride ion channel of cell
nuclei. J Biol Chem. 272:12575–12582. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cromer BA, Gorman MA, Hansen G, Adams JJ,
Coggan M, Littler DR, Brown LJ, Mazzanti M, Breit SN, Curmi PM, et
al: Structure of the Janus protein human CLIC2. J Mol Biol.
374:719–731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Edwards JC: A novel p64-related
Cl-channel: Subcellular distribution and nephron segment-specific
expression. Am J Physiol. 276:F398–F408. 1999.PubMed/NCBI
|
|
51
|
Sachs G, Shin JM, Vagin O, Lambrecht N,
Yakubov I and Munson K: The gastric H,K ATPase as a drug target:
Past, present, and future. J Clin Gastroenterol. 41 (Suppl
2):S226–S242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Edwards JC, Tulk B and Schlesinger PH:
Functional expression of p64, an intracellular chloride channel
protein. J Membr Biol. 163:119–127. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tulk BM, Schlesinger PH, Kapadia SA and
Edwards JC: CLIC-1 functions as a chloride channel when expressed
and purified from bacteria. J Biol Chem. 275:26986–26993. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Valdivieso ÁG and Santa-Coloma TA: The
chloride anion as a signalling effector. Biol Rev Camb Philos Soc.
94:1839–1856. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bortner CD, Sifre MI and Cidlowski JA:
Cationic gradient reversal and cytoskeleton-independent volume
regulatory pathways define an early stage of apoptosis. J Biol
Chem. 283:7219–7229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kunzelmann K: Ion channels in regulated
cell death. Cell Mol Life Sci. 73:2387–2403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Miyazaki H, Shiozaki A, Niisato N, Ohsawa
R, Itoi H, Ueda Y, Otsuji E, Yamagishi H, Iwasaki Y, Nakano T, et
al: Chloride ions control the G1/S cell-cycle checkpoint by
regulating the expression of p21 through a p53-independent pathway
in human gastric cancer cells. Biochem Biophys Res Commun.
366:506–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lai ZF, Chen YZ and Nishi K: Modulation of
intracellular Cl-homeostasis by lectin-stimulation in Jurkat T
lymphocytes. Eur J Pharmacol. 482:1–8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hiraoka K, Miyazaki H, Niisato N, Iwasaki
Y, Kawauchi A, Miki T and Marunaka Y: Chloride ion modulates cell
proliferation of human androgen-independent prostatic cancer cell.
Cell Physiol Biochem. 25:379–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nakajima KI and Marunaka Y: Intracellular
chloride ion concentration in differentiating neuronal cell and its
role in growing neurite. Biochem Biophys Res Commun. 479:338–342.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Heimlich G and Cidlowski JA: Selective
role of intracellular chloride in the regulation of the intrinsic
but not extrinsic pathway of apoptosis in Jurkat T-cells. J Biol
Chem. 281:2232–2241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Francisco MA, Wanggou S, Fan JJ, Dong W,
Chen X, Momin A, Abeysundara N, Min HK, Chan J, McAdam R, et al:
Chloride intracellular channel 1 cooperates with potassium channel
EAG2 to promote medulloblastoma growth. J Exp Med.
217:e201909712020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Neurohr GE, Terry RL, Lengefeld J, Bonney
M, Brittingham GP, Moretto F, Miettinen TP, Vaites LP, Soares LM,
Paulo JA, et al: Excessive cell growth causes cytoplasm dilution
and contributes to senescence. Cell. 176:1083–1097.e18. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhong J, Kong X, Zhang H, Yu C, Xu Y, Kang
J, Yu H, Yi H, Yang X and Sun L: Inhibition of CLIC4 enhances
autophagy and triggers mitochondrial and ER stress-induced
apoptosis in human glioma U251 cells under starvation. PLoS One.
7:e393782012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Suh KS, Mutoh M, Nagashima K,
Fernandez-Salas E, Edwards LE, Hayes DD, Crutchley JM, Marin KG,
Dumont RA, Levy JM, et al: The organellular chloride channel
protein CLIC4/mtCLIC translocates to the nucleus in response to
cellular stress and accelerates apoptosis. J Biol Chem.
279:4632–4641. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ponnalagu D, Gururaja Rao S, Farber J, Xin
W, Hussain AT, Shah K, Tanda S, Berryman M, Edwards JC and Singh H:
Molecular identity of cardiac mitochondrial chloride intracellular
channel proteins. Mitochondrion. 27:6–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kim R, Emi M and Tanabe K: Role of
mitochondria as the gardens of cell death. Cancer Chemother
Pharmacol. 57:545–553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Neveu B, Spinella JF, Richer C, Lagacé K,
Cassart P, Lajoie M, Jananji S, Drouin S, Healy J, Hickson GR, et
al: CLIC5: A novel ETV6 target gene in childhood acute
lymphoblastic leukemia. Haematologica. 101:1534–1543. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sales A, Ende K, Diemer J, Kyvik AR,
Veciana J, Ratera I, Kemkemer R, Spatz JP and Guasch J: Cell
Type-dependent integrin distribution in adhesion and migration
responses on protein-coated microgrooved substrates. ACS Omega.
4:1791–1800. 2019. View Article : Google Scholar
|
|
70
|
Jin J, Mao Y, Thomas D, Kim S, Daniel JL
and Kunapuli SP: RhoA downstream of G(q) and G(12/13) pathways
regulates protease-activated receptor-mediated dense granule
release in platelets. Biochem Pharmacol. 77:835–844. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chalothorn D, Zhang H, Smith JE, Edwards
JC and Faber JE: Chloride intracellular channel-4 is a determinant
of native collateral formation in skeletal muscle and brain. Circ
Res. 105:89–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bohman S, Matsumoto T, Suh K, Dimberg A,
Jakobsson L, Yuspa S and Claesson-Welsh L: Proteomic analysis of
vascular endothelial growth factor-induced endothelial cell
differentiation reveals a role for chloride intracellular channel 4
(CLIC4) in tubular morphogenesis. J Biol Chem. 280:42397–42404.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lucitti JL, Tarte NJ and Faber JE:
Chloride intracellular channel 4 is required for maturation of the
cerebral collateral circulation. Am J Physiol Heart Circ Physiol.
309:H1141–H1150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Edwards JC, Bruno J, Key P and Cheng YW:
Absence of chloride intracellular channel 4 (CLIC4) predisposes to
acute kidney injury but has minimal impact on recovery. BMC
Nephrol. 15:542014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tavasoli M, Al-Momany A, Wang X, Li L,
Edwards JC and Ballermann BJ: Both CLIC4 and CLIC5A activate ERM
proteins in glomerular endothelium. Am J Physiol Renal Physiol.
311:F945–F957. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Uretmen Kagiali ZC, Saner N, Akdag M,
Sanal E, Degirmenci BS, Mollaoglu G and Ozlu N: CLIC4 and CLIC1
bridge plasma membrane and cortical actin network for a successful
cytokinesis. Life Sci Alliance. 3:e2019005582019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ueno Y, Ozaki S, Umakoshi A, Yano H,
Choudhury ME, Abe N, Sumida Y, Kuwabara J, Uchida R, Islam A, et
al: Chloride intracellular channel protein 2 in cancer and
non-cancer human tissues: Relationship with tight junctions. Tissue
Barriers. 7:15937752019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Argenzio E, Klarenbeek J, Kedziora KM,
Nahidiazar L, Isogai T, Perrakis A, Jalink K, Moolenaar WH and
Innocenti M: Profilin binding couples chloride intracellular
channel protein CLIC4 to RhoA-mDia2 signaling and filopodium
formation. J Biol Chem. 293:19161–19176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Al-Momany A, Li L, Alexander RT and
Ballermann BJ: Clustered PI(4,5)P2 accumulation and
ezrin phosphorylation in response to CLIC5A. J Cell Sci.
127:5164–5178. 2014.PubMed/NCBI
|
|
80
|
Oh ES, Seiki M, Gotte M and Chung J: Cell
adhesion in cancer. Int J Cell Biol. 2012:9656182012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Conway JRW and Jacquemet G: Cell matrix
adhesion in cell migration. Essays Biochem. 63:535–551. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Argenzio E, Margadant C, Leyton-Puig D,
Janssen H, Jalink K, Sonnenberg A and Moolenaar WH: CLIC4 regulates
cell adhesion and β1 integrin trafficking. J Cell Sci.
127:5189–5203. 2014.PubMed/NCBI
|
|
83
|
Berryman M, Bruno J, Price J and Edwards
JC: CLIC-5A functions as a chloride channel in vitro and associates
with the cortical actin cytoskeleton in vitro and in vivo. J Biol
Chem. 279:34794–34801. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhao W, Lu M and Zhang Q: Chloride
intracellular channel 1 regulates migration and invasion in gastric
cancer by triggering the ROS-mediated p38 MAPK signaling pathway.
Mol Med Rep. 12:8041–8047. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chen CD, Wang CS, Huang YH, Chien KY,
Liang Y, Chen WJ and Lin KH: Overexpression of CLIC1 in human
gastric carcinoma and its clinicopathological significance.
Proteomics. 7:155–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li BP, Mao YT, Wang Z, Chen YY, Wang Y,
Zhai CY, Shi B, Liu SY, Liu JL and Chen JQ: CLIC1 promotes the
progression of gastric cancer by regulating the MAPK/AKT pathways.
Cell Physiol Biochem. 46:907–924. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zheng DL, Huang QL, Zhou F, Huang QJ, Lin
JY and Lin X: PA28β regulates cell invasion of gastric cancer via
modulating the expression of chloride intracellular channel 1. J
Cell Biochem. 113:1537–1546. 2012.PubMed/NCBI
|
|
88
|
Krock BL, Skuli N and Simon MC:
Hypoxia-induced angiogenesis: Good and evil. Genes Cancer.
2:1117–1133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ponnalagu D and Singh H: Anion channels of
mitochondria. Handb Exp Pharmacol. 240:71–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhao K, Wang Z, Li X, Liu JL, Tian L and
Chen JQ: Exosome-mediated transfer of CLIC1 contributes to the
vincristine-resistance in gastric cancer. Mol Cell Biochem.
462:97–105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kawai S and Fujii T, Shimizu T, Sukegawa
K, Hashimoto I, Okumura T, Nagata T, Sakai H and Fujii T:
Pathophysiological properties of CLIC3 chloride channel in human
gastric cancer cells. J Physiol Sci. 70:152020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li WH, Miao XH, Qi ZT, Ni W, Zhu SY and
Fang F: Proteomic analysis of differently expressed proteins in
human hepatocellular carcinoma cell lines HepG2 with transfecting
hepatitis B virus X gene. Chin Med J (Engl). 122:15–23.
2009.PubMed/NCBI
|
|
93
|
Zhang S, Wang XM, Yin ZY, Zhao WX, Zhou
JY, Zhao BX and Liu PG: Chloride intracellular channel 1 is
overexpression in hepatic tumor and correlates with a poor
prognosis. APMIS. 121:1047–1053. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Song MY, Tang JW, Sun MZ, Liu SQ and Wang
B: Localization and expression of CLIC1 in hepatocarcinoma ascites
cell lines with high or low potentials of lymphatic spread.
Zhonghua Bing Li Xue Za Zhi. 39:463–466. 2010.(In Chinese).
PubMed/NCBI
|
|
95
|
Li RK, Zhang J, Zhang YH, Li ML, Wang M
and Tang JW: Chloride intracellular channel 1 is an important
factor in the lymphatic metastasis of hepatocarcinoma. Biomed
Pharmacother. 66:167–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wei X, Li J, Xie H, Wang H, Wang J, Zhang
X, Zhuang R, Lu D, Ling Q, Zhou L, et al: Chloride intracellular
channel 1 participates in migration and invasion of hepatocellular
carcinoma by targeting maspin. J Gastroenterol Hepatol. 30:208–216.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang J, Li M, Song M, Chen W, Mao J, Song
L, Wei Y, Huang Y and Tang J: Clic1 plays a role in mouse
hepatocarcinoma via modulating Annexin A7 and Gelsolin in vitro and
in vivo. Biomed Pharmacother. 69:416–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yue X, Cui Y, You Q, Lu Y and Zhang J:
MicroRNA-124 negatively regulates chloride intracellular channel 1
to suppress the migration and invasion of liver cancer cells. Oncol
Rep. 42:1380–1390. 2019.PubMed/NCBI
|
|
99
|
Wang R, Kang B, Hu R, Huang Y, Qin Z, Du J
and Lin X: ClC-3 chloride channel protein induces G1 arrest in
hepatocellular carcinoma Hep3B cells. Oncol Rep. 40:472–478.
2018.PubMed/NCBI
|
|
100
|
Li X and Weinman SA: Chloride channels and
hepatocellular function: Prospects for molecular identification.
Annu Rev Physiol. 64:609–633. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chernet BT and Levin M: Transmembrane
voltage potential of somatic cells controls oncogene-mediated
tumorigenesis at long-range. Oncotarget. 5:3287–3306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Liu Z, Lee SJ, Park S, Konstantopoulos K,
Glunde K, Chen Y and Barman I: Cancer cells display increased
migration and deformability in pace with metastatic progression.
FASEB J. 34:9307–9315. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Flores-Téllez TN, Lopez TV, Vásquez Garzón
VR and Villa-Treviño S: Co-Expression of Ezrin-CLIC5-podocalyxin is
associated with migration and invasiveness in hepatocellular
carcinoma. PLoS One. 10:e01316052015. View Article : Google Scholar
|
|
104
|
Ding Q, Li M, Wu X, Zhang L, Wu W, Ding Q,
Weng H, Wang X and Liu Y: CLIC1 overexpression is associated with
poor prognosis in gallbladder cancer. Tumour Biol. 36:193–198.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
He YM, Zhang ZL, Liu QY, Xiao YS, Wei L,
Xi C and Nan X: Effect of CLIC1 gene silencing on proliferation,
migration, invasion and apoptosis of human gallbladder cancer
cells. J Cell Mol Med. 22:2569–2579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhou N, Cheng W, Peng C, Liu Y and Jiang
B: Decreased expression of hsa-miR-372 predicts poor prognosis in
patients with gallbladder cancer by affecting chloride
intracellular channel 1. Mol Med Rep. 16:7848–7854. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kong L, Wu Q, Zhao L, Ye J, Li N and Yang
H: Upregulated lncRNA-UCA1 contributes to metastasis of bile duct
carcinoma through regulation of miR-122/CLIC1 and activation of the
ERK/MAPK signaling pathway. Cell Cycle. 18:1212–1228. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Guo Z, Neilson LJ, Zhong H, Murray PS,
Zanivan S and Zaidel-Bar R: E-cadherin interactome complexity and
robustness resolved by quantitative proteomics. Sci Signal.
7:rs72014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Gu X, Li B, Jiang M, Fang M, Ji J, Wang A,
Wang M, Jiang X and Gao C: RNA sequencing reveals differentially
expressed genes as potential diagnostic and prognostic indicators
of gallbladder carcinoma. Oncotarget. 6:20661–20671. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu Y, Wang Z, Li M, Ye Y, Xu Y, Zhang Y,
Yuan R, Jin Y, Hao Y, Jiang L, et al: Chloride intracellular
channel 1 regulates the antineoplastic effects of metformin in
gallbladder cancer cells. Cancer Sci. 108:1240–1252. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Patel D, Ythier D, Brozzi F, Eizirik DL
and Thorens B: Clic4, a novel protein that sensitizes β-cells to
apoptosis. Mol Metab. 4:253–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Magouliotis DE, Sakellaridis N, Dimas K,
Tasiopoulou VS, Svokos KA, Svokos AA and Zacharoulis D: In silico
Transcriptomic analysis of the chloride intracellular channels
(CLIC) interactome identifies a molecular panel of seven prognostic
markers in patients with pancreatic ductal adenocarcinoma. Curr
Genomics. 21:119–127. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Jia N, Dong S, Zhao G, Gao H, Li X and
Zhang H: CLIC1 overexpression is associated with poor prognosis in
pancreatic ductal adenocarcinomas. J Cancer Res Ther. 12:892–896.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Macpherson IR, Rainero E, Mitchell LE, van
den Berghe PV, Speirs C, Dozynkiewicz MA, Chaudhary S, Kalna G,
Edwards J, Timpson P, et al: CLIC3 controls recycling of late
endosomal MT1-MMP and dictates invasion and metastasis in breast
cancer. J Cell Sci. 127:3893–3901. 2014.PubMed/NCBI
|
|
115
|
Zou Q, Yang Z, Li D, Liu Z and Yuan Y:
Association of chloride intracellular channel 4 and Indian hedgehog
proteins with survival of patients with pancreatic ductal
adenocarcinoma. Int J Exp Pathol. 97:422–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wong CH, Li CH, He Q, Chan SL, Tong JH, To
KF, Lin LZ and Chen Y: Ectopic HOTTIP expression induces
noncanonical transactivation pathways to promote growth and
invasiveness in pancreatic ductal adenocarcinoma. Cancer Lett.
477:1–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang P, Zhang C, Yu P, Tang B, Liu T, Cui
H and Xu J: Regulation of colon cancer cell migration and invasion
by CLIC1-mediated RVD. Mol Cell Biochem. 365:313–321. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Petrova DT, Asif AR, Armstrong VW, Dimova
I, Toshev S, Yaramov N, Oellerich M and Toncheva D: Expression of
chloride intracellular channel protein 1 (CLIC1) and tumor protein
D52 (TPD52) as potential biomarkers for colorectal cancer. Clin
Biochem. 41:1224–1236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wang P, Zeng Y, Liu T, Zhang C, Yu PW, Hao
YX, Luo HX and Liu G: Chloride intracellular channel 1 regulates
colon cancer cell migration and invasion through ROS/ERK pathway.
World J Gastroenterol. 20:2071–2078. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Milton RH, Abeti R, Averaimo S, DeBiasi S,
Vitellaro L, Jiang L, Curmi PM, Breit SN, Duchen MR and Mazzanti M:
CLIC1 function is required for beta-amyloid-induced generation of
reactive oxygen species by microglia. J Neurosci. 28:11488–11499.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Deng YJ, Tang N, Liu C, Zhang JY, An SL,
Peng YL, Ma LL, Li GQ, Jiang Q, Hu CT, et al: CLIC4, ERp29, and
Smac/DIABLO derived from metastatic cancer stem-like cells stratify
prognostic risks of colorectal cancer. Clin Cancer Res.
20:3809–3817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Chiang PC, Chou RH, Chien HF, Tsai T and
Chen CT: Chloride intracellular channel 4 involves in the reduced
invasiveness of cancer cells treated by photodynamic therapy.
Lasers Surg Med. 45:38–47. 2013. View Article : Google Scholar : PubMed/NCBI
|