Introduction
Psoriasis is one of the most common types of
inflammatory-related skin diseases, which is characterized by
uncontrolled proliferation and the poor differentiation of
keratinocytes (1–3). Psoriasis is most commonly caused by
genetic, immune and infection factors (4). For example, a diet with poor nutrition
or low intake of omega-3 fatty acids can stimulate the development
of psoriasis (5). However, to the
best of our knowledge, the underlying mechanisms of the occurrence
and development of psoriasis have not yet been fully elucidated,
and the current treatment methods available for patients with
psoriasis remain unsatisfactory. Therefore, the development of more
effective strategies for the treatment of psoriasis is the current
focus of research.
In the affected skin areas of psoriasis, the
expression levels of chemokines [such as IL-8 and C-X-C motif
chemokine ligand 1 (CXCL1)], proinflammatory cytokines (such as
IL-1β) and antimicrobial peptides [such as S100 calcium binding
protein A7 (S100A7), β-defensin 2 and cathelicidin antimicrobial
peptide (LL-37)] are upregulated, leading to the hyperproliferation
of epithelial cells (6,7). Keratinocytes are the predominant type
of epithelial cells of the epidermis (8). Emerging evidence has shown that
treatment with M5 can induce psoriasis-like changes in cultured
keratinocytes, including increased cell proliferation and
inflammation, and poor differentiation (9). Thus, in the present study, in order to
mimic abnormal proliferation and differentiation in keratinocytes
in vitro, keratinocytes were stimulated with M5.
It has been reported that the NF-κB and JNK
signaling pathways play crucial roles in the immune system and
inflammatory response (10,11). For instance, salidroside, which is
an extract of Sedum rosea, a perennial herb (12), can inhibit inflammation and
keratinocyte proliferation by downregulating NF-κB and
STAT3-related signaling pathways, thus improving the symptoms of
psoriasis (12).
Cinnamaldehyde (CIN) is a Traditional Chinese
medicine that is derived from the dried bark of the Cinnamomum
lauraceae (Cinnamomum cassia Presl) plant (13). CIN was previously reported to exert
potent fungicidal, antitumor, immunological and anti-inflammatory
activities (14). In addition, CIN
has been found to downregulate the expression levels of the
proinflammatory cytokines, TNF-α, IL-1β and IL-6, in
lipopolysaccharide (LPS)-stimulated macrophages (15). CIN has also been discovered to
inhibit bleomycin-induced idiopathic pulmonary fibrosis in mice by
inhibiting the production of inflammatory cytokines and reactive
oxygen species (ROS) (16).
However, to the best of our knowledge, the biological effect of CIN
on psoriasis, particularly regarding its effect on keratinocyte
proliferation, inflammation and differentiation, remains unclear.
Therefore, the present study aimed to investigate the biological
role of CIN in keratinocytes exposed to M5 to mimic abnormal
proliferation and differentiation, and whether it can act as a
therapeutic agent against psoriasis via the NF-κB and JNK signaling
pathways.
Materials and methods
Cell lines and culture
Normal human epidermal keratinocytes (NHEKs) were
obtained from ScienCell Research Laboratories, Inc. NHEKs were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.), 100
U/ml penicillin (Thermo Fisher Scientific, Inc.) and 100 mg/ml
streptomycin (Thermo Fisher Scientific, Inc.), and maintained in an
incubator at 37°C in the presence of 5% CO2. NHEKs were
treated with 10 ng/ml M5 [IL-1α, IL-17A, IL-22, oncostatin M (OSM)
and TNF-α; PeproTech China] for 24 h at 37°C to mimic abnormal
proliferation and differentiation of keratinocytes (9).
Cell Counting Kit-8 (CCK-8) assay
CIN (MedChemExpress) was dissolved with DMSO. NHEKs
were treated with CIN (0, 5, 10, 20, 40 or 80 µM) for 24 h at 37°C.
A CCK-8 assay was used to evaluate the viability of NHEKs. Briefly,
NHEKs (5×103 cells/well) were plated into a 96-well
plate. Then, 10 µl CCK-8 reagent (Dojindo Molecular Technologies,
Inc.) was added to each well and further incubated for 2 h at 37°C.
The absorbance of each well was measured at a wavelength of 450 nm
using a spectrophotometer (Bio-Rad Laboratories, Inc.) (17).
5-Ethynyl-2′-deoxyuridine (EdU)
staining
An EdU DNA Proliferation in vitro Detection
kit (Guangzhou RiboBio Co., Ltd.) was used to evaluate the
proliferation of NHEKs, according to the manufacturer's protocol.
Briefly, NHEKs were seeded into 24-well plates (2.5×105
cells/well) and incubated with 50 µM EdU at room temperature for 2
h. Following the incubation, cells were stained with Apollo
staining solution for 30 min at 37°C in the dark. EdU-positive
cells were observed using a fluorescence microscope (Olympus
Corporation) (18).
Cell cycle distribution analysis
Flow cytometry was used to evaluate the cell cycle
distribution of NHEKs. Briefly, cells were fixed in 70% ethanol
overnight at 4°C, then incubated with 10 µl propidium iodide/RNase
staining buffer (BD Biosciences) for 30 min at 37°C in the dark.
The cell cycle distribution of NHEKs was analyzed using a FACScan™
flow cytometer (BD Biosciences). FlowJo software (version 10.6.2;
FlowJo LLC) was used to analyze the data. Flow cytometry was
performed according to methods outlined in previous studies
(19,20).
Western blotting
RIPA lysis buffer (Beyotime Institute of
Biotechnology) was used to extract the protein from cells. Total
protein concentration was determined using a BCA protein assay kit
(cat. no. AS1086; Aspen Biotechnology Co., Ltd.) and 30 µg protein
per lane was separated via SDS-PAGE on 10% gel. The separated
proteins were subsequently transferred onto PVDF membranes (EMD
Millipore) and blocked with 5% skimmed milk powder diluted in TBS
with 0.1% Tween-20 at room temperature for 1 h. The membranes were
then incubated with the following primary antibodies at 4°C
overnight: Anti-cyclin E1 (1:1,000; cat. no. ab33911), anti-CDK2
(1:1,000; cat. no. ab32147), anti-CDK inhibitor 1B (p27Kip1;
1:1,000; cat. no. ab32034), anti-keratin 1 (1:1,000; cat. no.
ab185628), anti-filaggrin (1:1,000; cat. no. sc-66192),
anti-loricrin (1:1,000; cat. no. ab137533), anti-keratin 5
(1:1,000; cat. no. ab64081), anti-keratin 10 (1:1,000; cat. no.
ab237775), anti-inhibitor of NF-κB (IκBα; 1:1,000; cat. no.
ab32518), anti-phosphorylated (p)-IκBα (1:1,000; cat. no. ab92700),
anti-p65 (1:1,000; cat. no. ab140751), anti-p-p65 (1:1,000; cat.
no. ab239882), anti-JNK (1:1,000; cat. no. ab199380), anti-p-JNK
(1:1,000; cat. no. ab131499) and anti-β-actin (1:1,000; cat. no.
ab8226). β-actin was used as an internal control. Following the
primary antibody incubation, the membranes were incubated with an
anti-rabbit secondary antibody (1:5,000; cat. no. ab96899) at room
temperature for 1 h. Protein bands were visualized with an ECL kit
(cat. no. AS1059-3; Aspen Biotechnology Co., Ltd.) and
densitometric analysis was performed using AlphaEaseFC software
(version 4.0; ProteinSimple). Anti-filaggrin was purchased from
Santa Cruz Biotechnology, Inc., while all other antibodies were
obtained from Abcam. All procedures were carried out according to
previous studies (19,21).
ROS analysis
The production of ROS was detected using a ROS assay
kit (Beyotime Institute of Biotechnology). Briefly, NHEKs
(1×105 cells/well) were plated into 6-well plates for 24
h. Then, the cells were treated with CIN (5 or 10 µM) for 24 h at
37°C. Following which, NHEKs were stained with
2′-7′-Dichlorofluorescin diacetate for 20 min at 37°C.
Subsequently, the cells were collected and gently washed. The
fluorescence intensity was subsequently detected using a FACScan™
flow cytometer (BD Biosciences). FlowJo software (version 10.6.2;
FlowJo LLC) was used to analyze the data. All procedures were
carried out according to a previous study (22).
ELISA
Malondialdehyde (MDA) assay kit (cat. no. A003-1)
and Reduced glutathione (GSH) assay kit (cat. no. A006-2-1) were
purchased from Nanjing Jiancheng Bioengineering Institute. These
specific ELISA kits were used to determine the concentrations of
MDA and GSH in NHEKs, according to the manufacturer's instructions
(23).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using TRIpure
Total RNA Extraction Reagent (ELK Biotechnology Co., Ltd.)
according to the manufacturer's protocol. Total RNA was reverse
transcribed into cDNA using an EntiLink™ 1st Strand cDNA Synthesis
kit (ELK Biotechnology Co., Ltd.), according to the manufacturer's
protocol. qPCR was subsequently performed on StepOne™ Real-Time PCR
instrument (Thermo Fisher Scientific, Inc.) using an EnTurbo™ SYBR
Green PCR SuperMix kit (ELK Biotechnology Co., Ltd.). The following
qPCR thermocycling conditions were used: 3 min at 95°C, followed by
40 cycles of 10 sec at 95°C, 30 sec at 58°C, and 30 sec at 72°C.
The primers used for qPCR are listed in Table I. β-actin was used as the internal
control. mRNA levels were quantified using the 2−ΔΔCq
method (24). All procedures were
carried out according to a previous study (23).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer sequences
(5′à3′) |
|---|
| IL-1β | F:
ACGATGCACCTGTACGATCACT |
|
| R:
GAGAACACCACTTGTTGCTCCA |
| IL-8 | F:
ACTGAGAGTGATTGAGAGTGGAC |
|
| R:
AACCCTCTGCACCCAGTTTTC |
| CXCL1 | F:
AACCGAAGTCATAGCCACACTC |
|
| R:
CTTCTCCTAAGCGATGCTCAAA |
| S100A7 | F:
GCACAAATTACCTCGCCGAT |
|
| R:
GACATTTTATTGTTCCTGGGGTC |
| β-defensin2 | F:
ATGTCATCCAGTCTTTTGCCC |
|
| R:
TGCGTATCTTTGGACACCATAG |
| LL-37 | F:
CGTGCTATAGATGGCATCAACC |
|
| R:
GCCCGTCCTTCTTGAAGTCA |
| β-actin | F:
GTCCACCGCAAATGCTTCTA |
|
| R:
TGCTGTCACCTTCACCGTTC |
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 7.0; GraphPad Software, Inc.). The CCK-8
assay was repeated five times, while all other experiments were
repeated three times. All data are presented as the mean ± SD.
Statistical differences between at least three groups were
determined using a one-way ANOVA followed by a Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
CIN treatment inhibits the
proliferation of M5-stimulated NHEKs
To determine the cytotoxic effect of CIN on NHEKs, a
CCK-8 assay was used. As shown in Fig.
1A, treatment with 5 or 10 µM CIN exerted a small effect on the
viability of NHEKs. However, treatment with 20, 40 or 80 µM CIN
significantly reduced the viability of NHEKs. Therefore, 5 and 10
µM CIN were selected as the optimal drug doses for use in
subsequent experiments. The effects of CIN on the viability and
proliferation of M5-treated NHEKs were subsequently investigated.
The results of the CCK-8 and EdU staining assays indicated that M5
significantly increased the viability and proliferation of NHEKs,
respectively, compared with the control group; however, these
effects were reversed by CIN treatment (Fig. 1B and C). These data suggested that
CIN may inhibit the viability and proliferation of M5-stimulated
NHEKs.
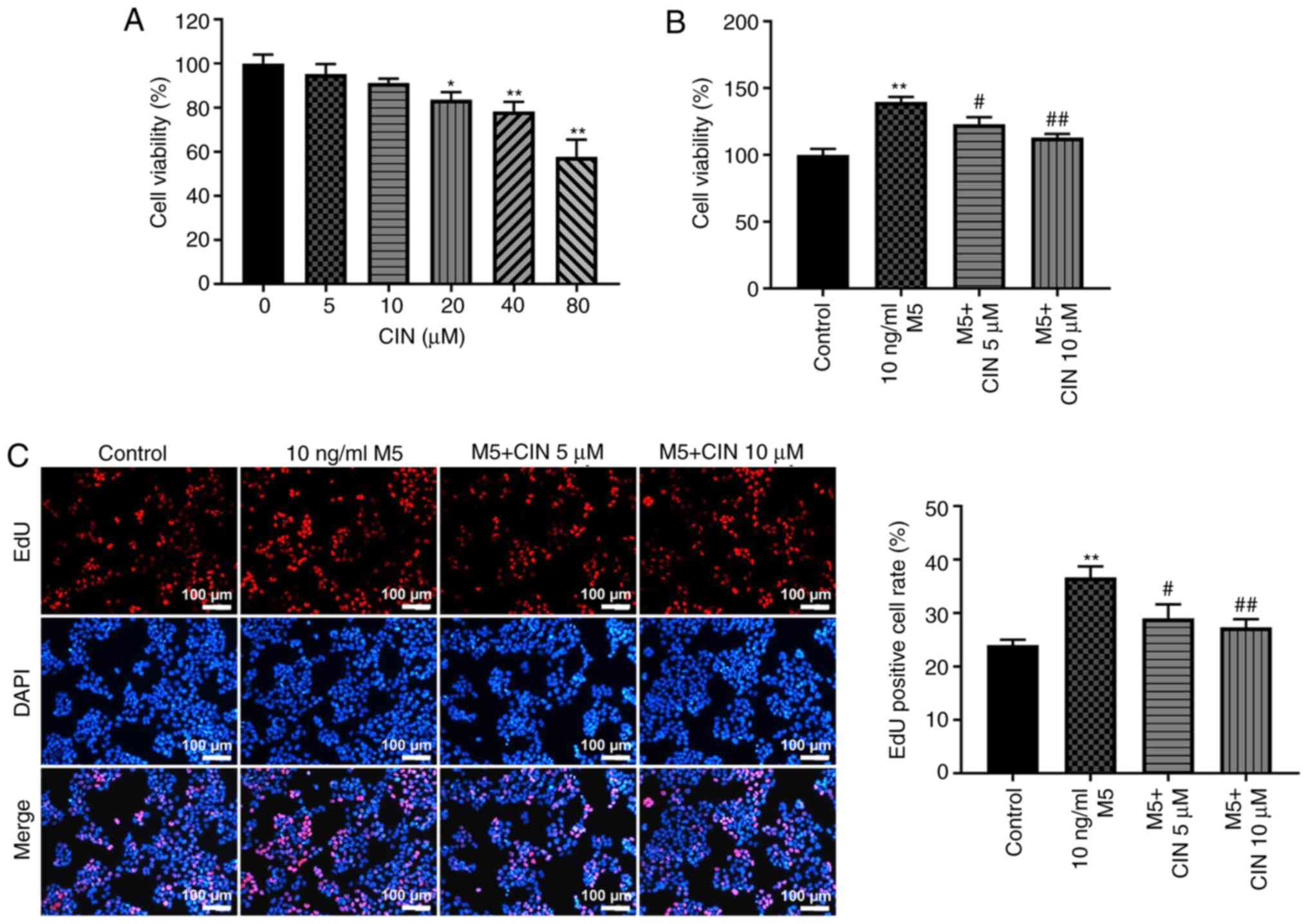 | Figure 1.CIN inhibits the proliferation of
M5-stimulated NHEKs. (A) CIN was dissolved with DMSO. NHEKs were
treated with CIN (0, 5, 10, 20, 40 or 80 µM) for 24 h. Cell
viability was detected by CCK-8 assay. (B) NHEKs were treated with
CIN (5 or 10 µM) for 24 h, and then treated with 10 ng/ml M5 for 24
h. Cell viability was detected by CCK-8 assay. (C) EdU staining
assay was used to detect cell proliferation. (Scale bar, 100 µm).
n=3. *P<0.05, **P<0.01 vs. control group;
#P<0.05, ##P<0.01 vs. 10 ng/ml M5
group. CIN, cinnamaldehyde; NHEKs, normal human epidermal
keratinocytes; CCK-8, Cell Counting Kit-8; Edu,
5-Ethynyl-2′-deoxyuridine. |
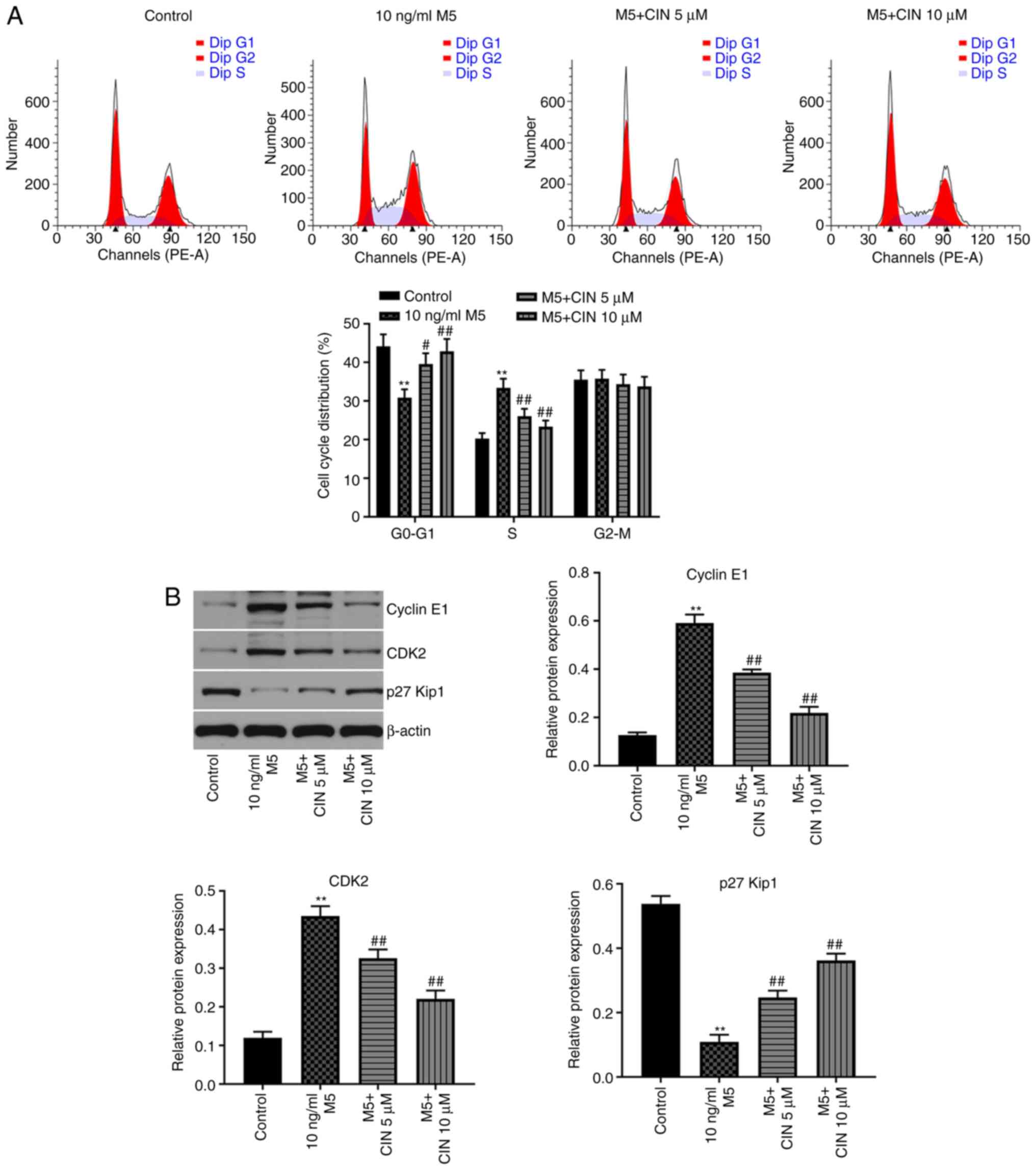
CIN induces cell cycle arrest in
M5-stimulated NHEKs
Next, to evaluate the effect of CIN on the cell
cycle distribution of M5-stimulated NHEKs, flow cytometry was used.
As shown in Fig. 2A, the percentage
of NHEKs in the G0/G1 phase was significantly
decreased following stimulation with M5, while the number of cells
in the S phase increased; however, these changes were reversed by
CIN treatment. In addition, the results of the western blotting
analysis revealed that M5 notably upregulated the levels of cyclin
E1 and CDK2, and downregulated the expression of p27Kip1, compared
with the control group (Fig. 2B).
CIN significantly downregulated the expression levels of cyclin E1
and CDK2, and upregulated the expression levels of p27Kip1 in
M5-stimulated NHEKs compared with the M5 treatment group (Fig. 2B). These results suggested that CIN
may induce cell cycle arrest in M5-stimulated NHEKs.
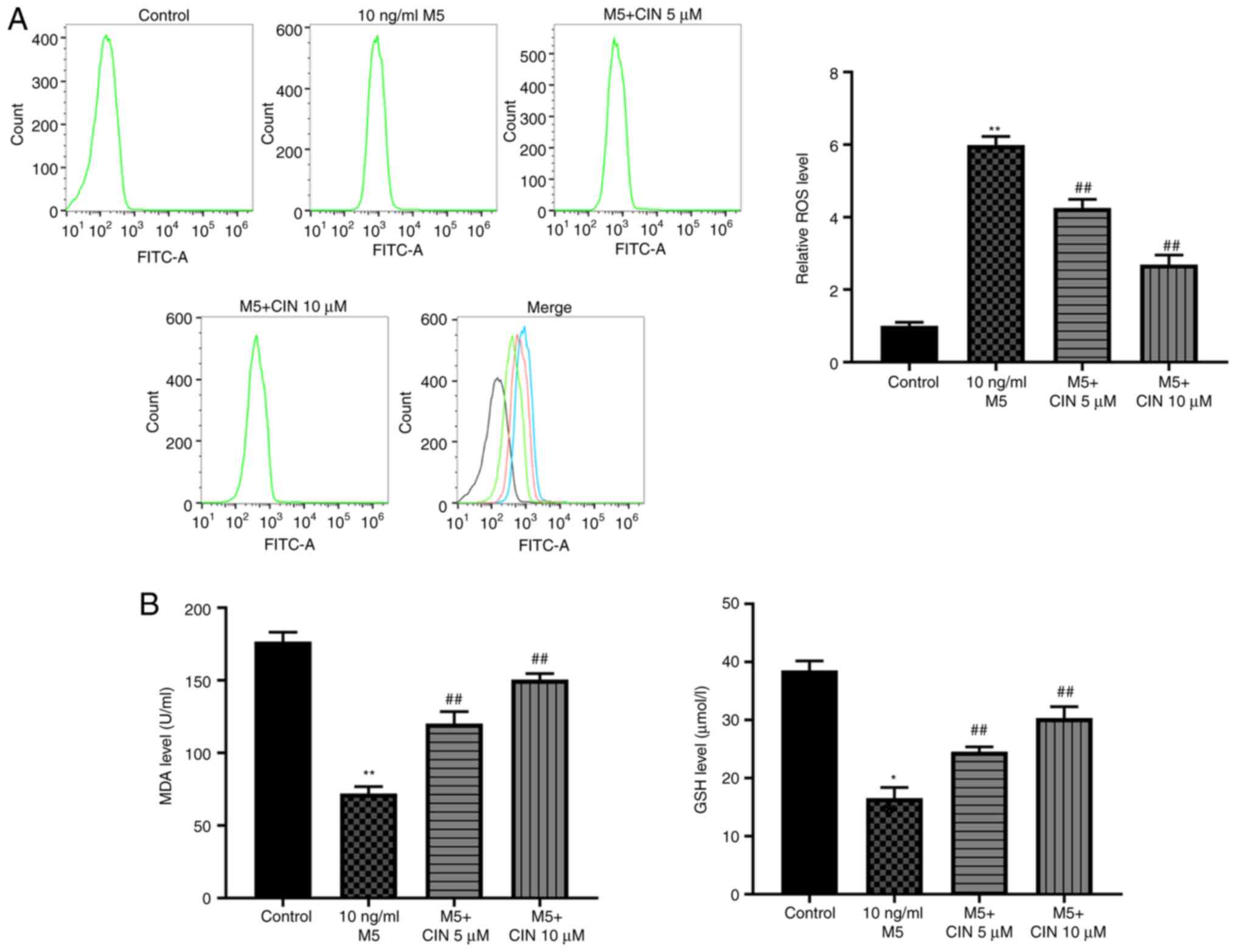
CIN attenuates M5-induced oxidative
stress damage in NHEKs
To determine whether CIN could protect NHEKs against
M5-induced oxidative stress, the production of intracellular ROS
and the levels of MDA and GSH were detected. As shown in Fig. 3A and B, M5 stimulation significantly
increased ROS production, and decreased the levels of MDA and GSH
in NHEKs compared with the control group, suggesting that M5 may
induce oxidative stress in NHEKs. However, treatment with CIN
significantly reversed M5-induced oxidative stress in NHEKs
(Fig. 3A and B). Taken together,
these data indicated that CIN may attenuate M5-induced oxidative
stress damage in NHEKs.
CIN promotes the differentiation of
M5-stimulated NHEKs
Western blotting was performed to determine whether
CIN could affect the differentiation of M5-treated NHEKs. Compared
with the control group, M5 stimulation significantly downregulated
the expression levels of keratin 1, filaggrin, loricrin, keratin 5
and keratin 10 in NHEKs; however, these changes were significantly
reversed by CIN treatment (Fig.
4A-F). These data suggested that CIN may promote the
differentiation of NHEKs following M5 exposure.
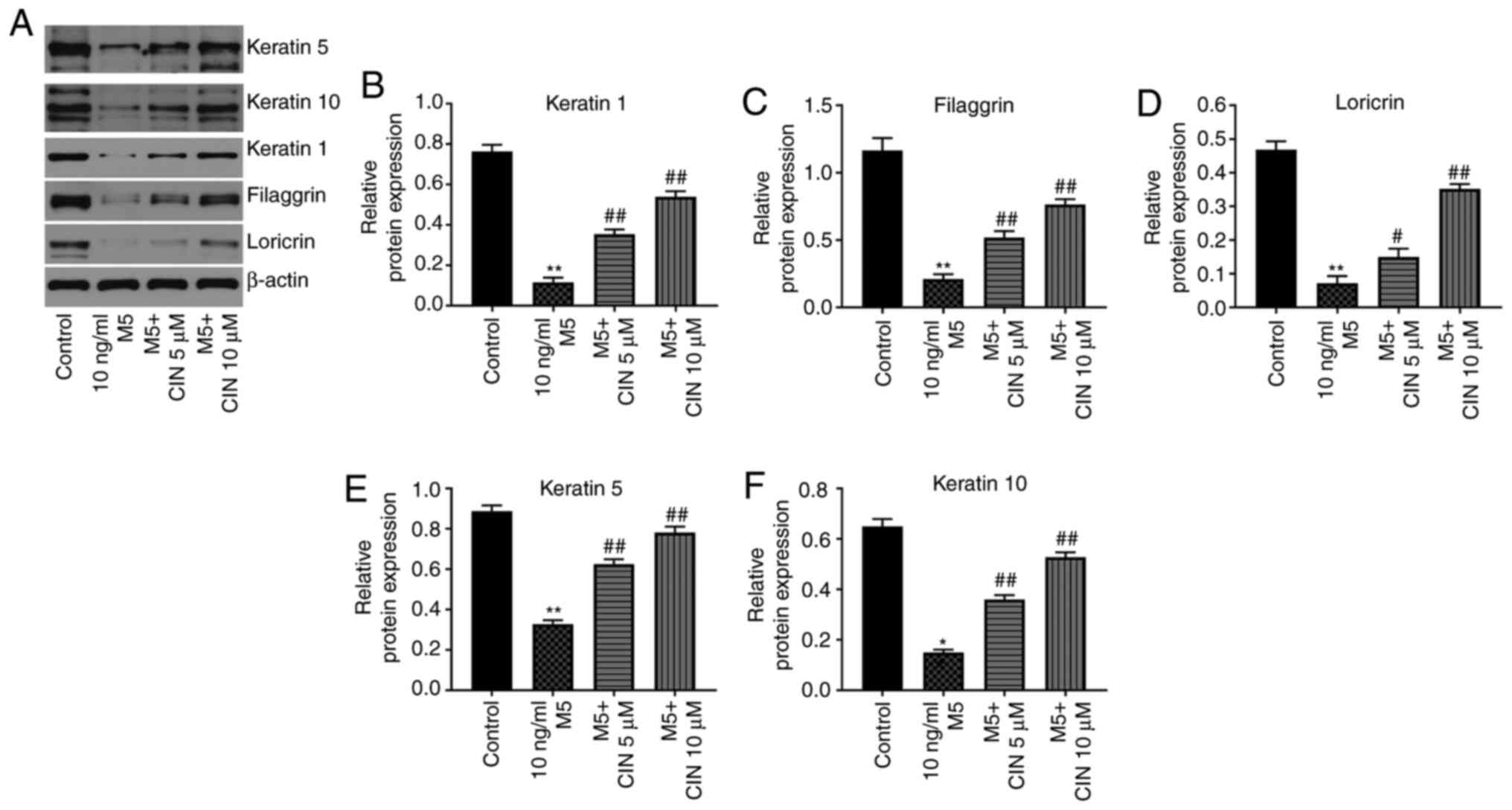 | Figure 4.CIN promotes the differentiation of
M5-stimulated NHEKs. NHEKs were treated with CIN (5 or 10 µM) for
24 h, and then treated with 10 ng/ml M5 for 24 h. (A-F) Western
blotting was used to detect the expression levels of keratin 5,
keratin 10, keratin 1, filaggrin and loricrin in NHEKs. The
relative expression levels of keratin 1, filaggrin, loricrin,
keratin 5 and keratin 10 in NHEKs were normalized to β-actin. n=3.
*P<0.05, **P<0.01 vs. control group; #P<0.05,
##P<0.01 vs. 10 ng/ml M5 group. CIN, cinnamaldehyde;
NHEKs, normal human epidermal keratinocytes. |
CIN attenuates inflammatory injury in
M5-stimulated NHEKs
It was previously reported that M5 promoted the
production of inflammatory mediators, such as cytokines (IL-1β),
chemokines (IL-8 and CXCL1) and antimicrobial peptide pairs
(S100A7, β-defensin 2 and LL-37) in keratinocytes (6). To validate whether CIN could attenuate
the inflammatory response in NHEKs following M5 exposure, RT-qPCR
was performed. As shown in Fig.
5A-C, M5 stimulation significantly upregulated the expression
levels of IL-1β, IL-8, CXCL1, S100A7, β-defensin 2 and LL-37 in
NHEKs compared with the control group; however, these M5-induced
changes were significantly decreased following CIN treatment. These
findings suggested that CIN may attenuate inflammatory injury in
M5-stimulated NHEKs.
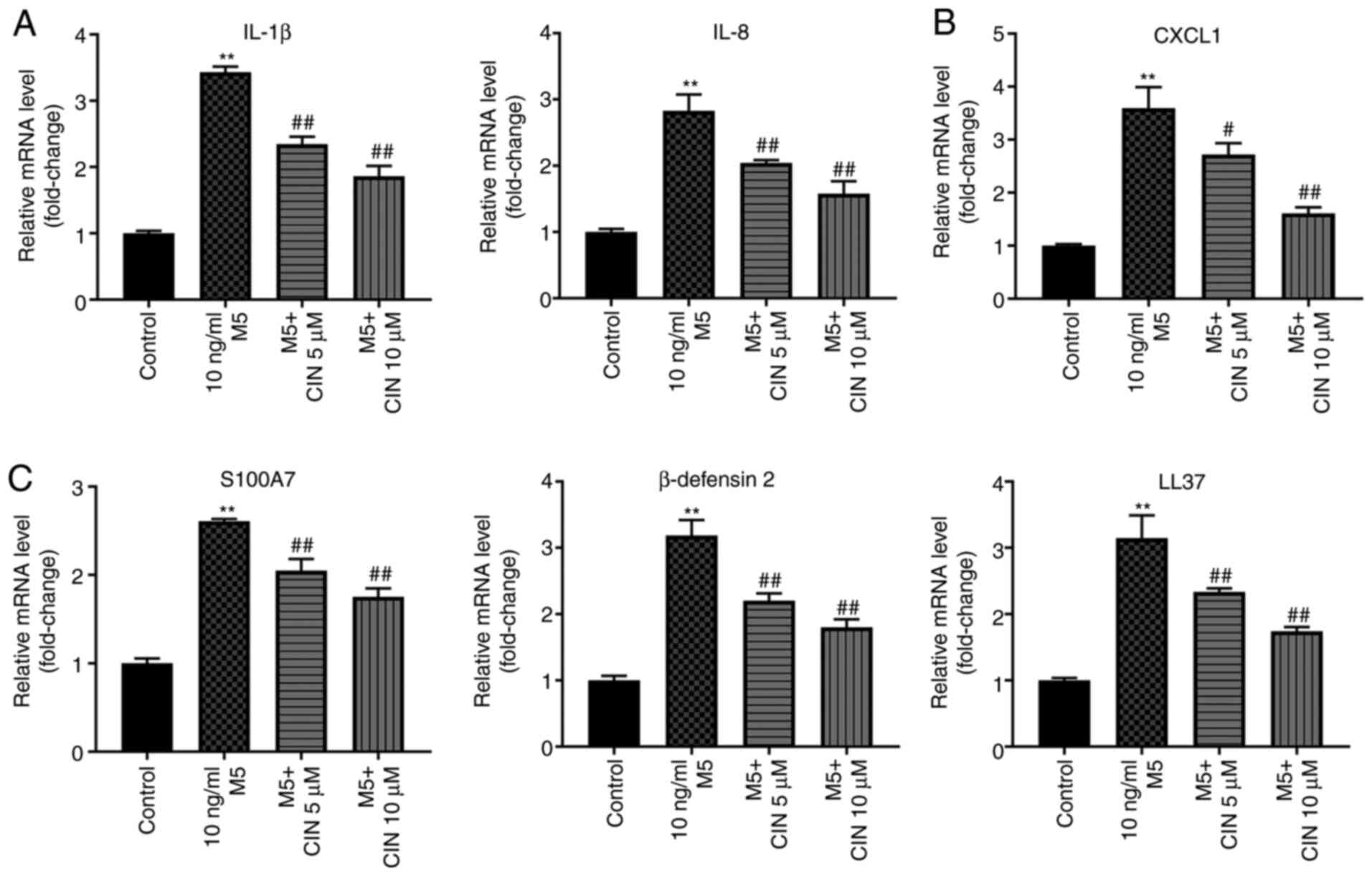 | Figure 5.CIN attenuates inflammatory injury in
M5-stimulated NHEKs. NHEKs were treated with CIN (5 or 10 µM) for
24 h, and then treated with 10 ng/ml M5 for 24 h. Reverse
transcription-quantitative PCR was performed to measure the
expression levels of (A) IL-1β, IL-8, (B) CXCL1, (C) S100A7,
β-defensin2 and LL-37 in NHEKs. n=3. **P<0.01 vs. control group;
#P<0.05, ##P<0.01 vs. 10 ng/ml M5
group. CIN, cinnamaldehyde; NHEKs, normal human epidermal
keratinocytes; CXCL1, C-X-C motif chemokine ligand 1; S100A7, S100
calcium binding protein A7; LL-37, cathelicidin antimicrobial
peptide. |
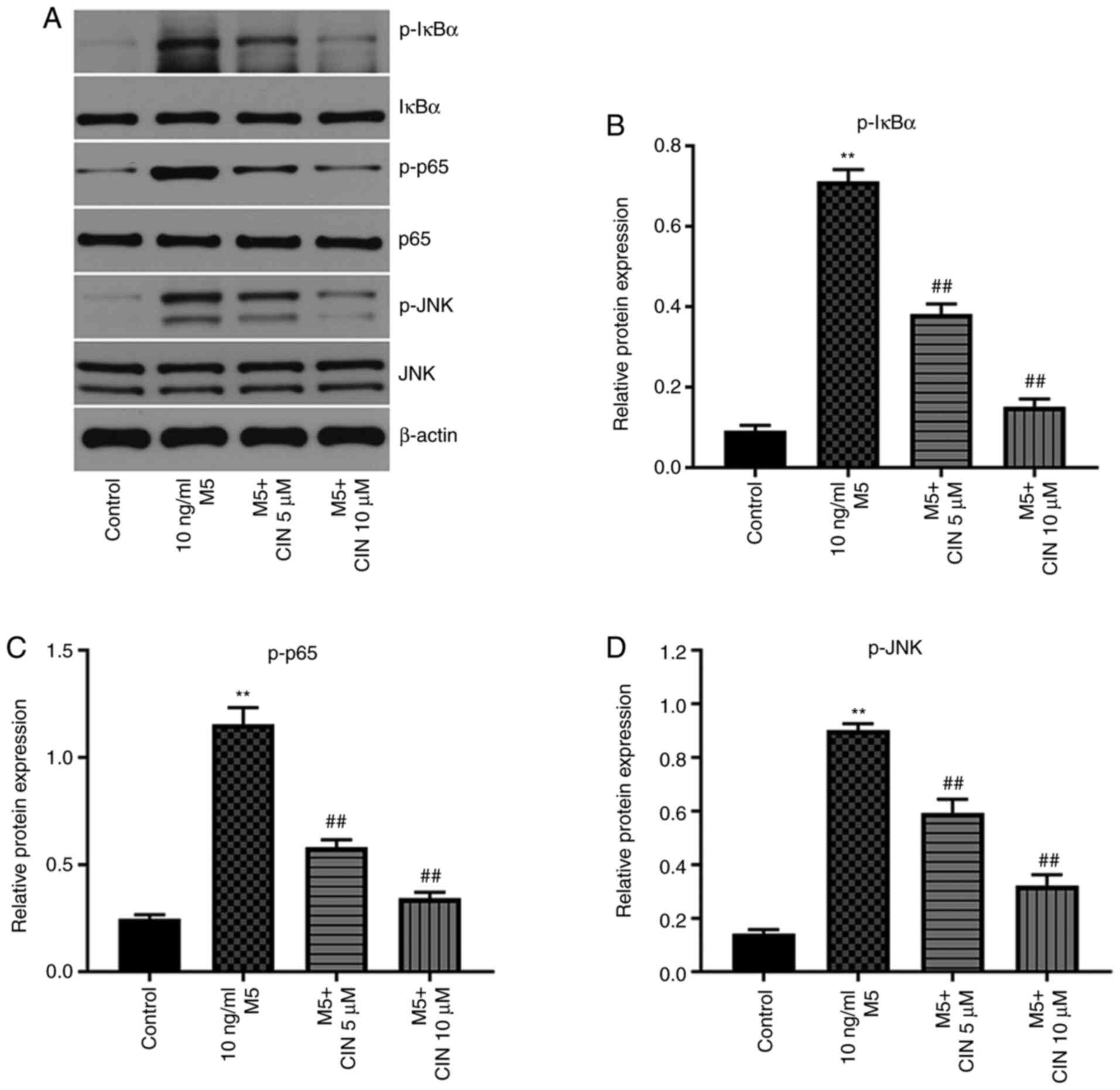
CIN inhibits the proliferation and
associated inflammation of M5-treated NHEKs by downregulating the
NF-κB and JNK signaling pathways
Previous studies have shown that the NF-κB and JNK
signaling pathways play important roles in psoriasis (25,26).
Thus, western blotting was used to analyze the expression levels of
p-IκBα, p-p65 and p-JNK in NHEKs. M5 stimulation significantly
upregulated the expression levels of p-IκBα, p-p65 and p-JNK in
NHEKs compared with the control group; however, these M5-induced
changes were significantly reversed by CIN treatment (Fig. 6A-D). These results indicated that
CIN may inhibit the proliferation and associated inflammation of
M5-treated NHEKs by downregulating the NF-κB and JNK signaling
pathways.
Discussion
Psoriasis is a common, chronic, relapsing
inflammatory skin disease (27–29).
Numerous previous studies have indicated that certain Traditional
Chinese medicines may exert promising therapeutic effects in
psoriasis (30). For instance, Xu
et al (12) reported that
salidroside inhibited the inflammatory response and
hyperproliferation of keratinocytes. In addition, Liu et al
(31) found that cimifugin could
inhibit oxidative stress and the inflammation of TNF-α-stimulated
keratinocytes. He et al (32) demonstrated that triptolide inhibited
the proliferation and cell cycle distribution of IL-22-stimulated
HaCaT cells. In addition, Wu et al (33) reported that diosgenin inhibited the
proliferation of HaCaT cells following LPS/IL-22 exposure via
inducing cell cycle arrest. However, to the best of our knowledge,
the role of CIN in the progression of psoriasis remains unclear.
The results of the present study found that CIN could inhibit the
proliferation and associated inflammatory response of M5-stimualted
NHEKs, in addition to inducing cell cycle arrest, which was
consistent with the findings of previous studies (30–33).
Accumulating evidence has shown that the NF-κB and
JNK signaling pathways play an important role in the pathogenesis
of psoriasis (25,26). For example. Yang et al
(25) demonstrated that Datura
metel attenuated imiquimod-induced psoriasis-like dermatitis
via inhibition of the NF-κB signaling pathway. Liu et al
(31) showed that cimifugin
inhibited oxidative stress and inflammation in TNF-α-treated
keratinocytes by inactivating the NF-κB and JNK signaling pathways.
Consistent with these previous findings, the present study
confirmed that CIN could inhibit the phosphorylation of IκBα and
p65 in M5-stimulated NHEKs, indicating that CIN may inhibit the
NF-κB signaling pathway in M5-stimulated NHEKs. In addition, JNK
has been suggested to represent a potential target for the
treatment of psoriasis (34). In
addition, Hammouda et al (34) reported that JNK promoted the
occurrence of psoriasis. Consistent with these findings, the
current results revealed that M5 markedly upregulated the
expression levels of p-JNK in NHEKs. However, treatment with CIN
significantly downregulated the expression levels of p-JNK in
M5-stimulated NHEKs. Taken together with the aforementioned
findings, these data indicated that CIN may inhibit the
proliferation and associated inflammatory response of NHEKs
following M5 exposure via inhibition of the NF-κB and JNK signaling
pathways.
It has been reported that keratinocyte proliferation
is inhibited and differentiation is promoted during the treatment
of psoriasis (35). In the present
study, CIN markedly upregulated the expression levels of keratin 1,
filaggrin, loricrin, keratin 5 and keratin 10 in NHEKs. Thus, these
findings indicated that CIN promoted keratinocyte
differentiation.
However, there are some limitations of the current
study. Additional in-depth and detailed studies that further
research the underlying mechanisms by which CIN regulates the NF-κB
and JNK signaling pathways are required. In addition, in order to
further verify the relationship between the NF-κB and JNK signaling
pathways and psoriasis, NF-κB and JNK signaling inhibitors will be
used in future studies to obtain more complete information. Thus,
further experiments are needed in the future.
In conclusion, the findings of the present study
suggested that CIN may inhibit the proliferation and associated
inflammation of M5-stimulated NHEKs via inhibition of the NF-κB and
JNK signaling pathways. Therefore, CIN may represent a potential
agent for the treatment of psoriasis.
Acknowledgements
Not applicable.
Funding
The present study was supported by Chinese National
Natural Science Foundation (grant no. 81703105).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZD made major contributions to the conception,
design and manuscript drafting of this study. JL, HQ and LW were
responsible for data acquisition, analysis and interpretation, as
well as manuscript revision. ML made substantial contributions to
the conception and design of this study, and revised the manuscript
critically for important intellectual content. All authors agreed
to be accountable for all aspects of the work. All authors have
read and approved the final manuscript. All authors confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schleicher SM: Psoriasis: Pathogenesis,
assessment, and therapeutic update. Clin Podiatr Med Surg.
33:355–366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakajima K and Sano S: Mouse models of
psoriasis and their relevance. J Dermatol. 45:252–263. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee J, Song K, Hiebert P, Werner S, Kim TG
and Kim YS: Tussilagonone ameliorates psoriatic features in
keratinocytes and imiquimod-induced psoriasis-like lesions in mice
via NRF2 activation. J Invest Dermatol. 140:1223–1232.e4. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Girolomoni G, Strohal R, Puig L, Bachelez
H, Barker J, Boehncke WH and Prinz JC: The role of IL-23 and the
IL-23/T(H) 17 immune axis in the pathogenesis and treatment of
psoriasis. J Eur Acad Dermatol Venereol. 31:1616–1626. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madden SK, Flanagan KL and Jones G: How
lifestyle factors and their associated pathogenetic mechanisms
impact psoriasis. Clin Nutr. 39:1026–1040. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rabeony H, Petit-Paris I, Garnier J,
Barrault C, Pedretti N, Guilloteau K, Jegou JF, Guillet G, Huguier
V, Lecron JC, et al: Inhibition of keratinocyte differentiation by
the synergistic effect of IL-17A, IL-22, IL-1α, TNFα and oncostatin
M. PLoS One. 9:e1019372014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niyonsaba F, Ushio H, Nakano N, Ng W,
Sayama K, Hashimoto K, Nagaoka I, Okumura K and Ogawa H:
Antimicrobial peptides human beta-defensins stimulate epidermal
keratinocyte migration, proliferation and production of
proinflammatory cytokines and chemokines. J Invest Dermatol.
127:594–604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang D, Wang Y, Xia Y, Huo J, Zhang Y,
Yang P, Zhang Y and Wang X: Repression of miR-142-3p alleviates
psoriasis-like inflammation by repressing proliferation and
promoting apoptosis of keratinocytes via targeting Sema3A. Mol Cell
Probes. 52:1015732020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen LTH, Ahn SH, Nguyen UT and Yang IJ:
Dang-Gui-Liu-Huang Tang a traditional herbal formula, ameliorates
imiquimod-induced psoriasis-like skin inflammation in mice by
inhibiting IL-22 production. Phytomedicine. 47:48–57. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matulewicz N and Karczewska-Kupczewska M:
Insulin resistance and chronic inflammation. Postepy Hig Med Dosw
(Online). 70:1245–1258. 2016.PubMed/NCBI
|
|
12
|
Xu F, Xu J, Xiong X and Deng Y:
Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in
psoriasis-associated oxidative stress via SIRT1 activation. Redox
Rep. 24:70–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun L, Liu LN, Li JC, Lv YZ, Zong SB, Zhou
J, Wang ZZ, Kou JP and Xiao W: The essential oil from the twigs of
Cinnamomum cassia Presl inhibits oxytocin-induced uterine
contraction in vitro and in vivo. J Ethnopharmacol. 206:107–114.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klibanov AM and Giannousis PP: Geometric
specificity of alcohol dehydrogenases and its potential for
separation of trans and cis isomers of unsaturated aldehydes. Proc
Natl Acad Sci USA. 79:3462–3465. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim ME, Na JY and Lee JS:
Anti-inflammatory effects of trans-cinnamaldehyde on
lipopolysaccharide-stimulated macrophage activation via MAPKs
pathway regulation. Immunopharmacol Immunotoxicol. 40:219–224.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan L, Song F, Li H, Li Y, Li J, He QY,
Zhang D, Wang F, Zhang M, Zhao H, et al: Submicron emulsion of
cinnamaldehyde ameliorates bleomycin-induced idiopathic pulmonary
fibrosis via inhibition of inflammation, oxidative stress and
epithelial-mesenchymal transition. Biomed Pharmacother.
102:765–771. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye F, Zhang J, Zhang Q, Zhang J and Chen
C: Preliminary study on the mechanism of long noncoding RNA SENCR
regulating the proliferation and migration of vascular smooth
muscle cells. J Cell Physiol. 235:9635–9643. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu J, Liu B, Wang Z, Wang D, Ni H, Zhang
L and Wang Y: Exosomes from nicotine-stimulated macrophages
accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC
migration and proliferation. Theranostics. 9:6901–6919. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Farahzadi R, Fathi E and Vietor I:
Mesenchymal stem cells could be considered as a candidate for
further studies in cell-based therapy of alzheimer's disease via
targeting the signaling pathways. ACS Chem Neurosci. 11:1424–1435.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fathi E and Vietor I: Mesenchymal stem
cells promote caspase expression in Molt-4 leukemia cells via
GSK-3α/Β and ERK1/2 signaling pathways as a therapeutic strategy.
Curr Gene Ther. 21:81–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fathi E, Farahzadi R, Javanmardi S and
Vietor I: L-carnitine extends the telomere length of the cardiac
differentiated CD117+−expressing stem cells. Tissue
Cell. 67:1014292020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Li XM, Bai Z, Chi BX, Wei Y and
Chen X: Curcumol induces cell cycle arrest in colon cancer cells
via reactive oxygen species and Akt/GSK3β/cyclin D1 pathway. J
Ethnopharmacol. 210:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fathi E, Valipour B, Sanaat Z, Nozad
Charoudeh H and Farahzadi R: Interleukin-6, −8, and TGF-β secreted
from mesenchymal stem cells show functional role in reduction of
telomerase activity of leukemia cell Via Wnt5a/β-catenin and p53
pathways. Adv Pharm Bull. 10:307–314. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang BY, Cheng YG, Liu Y, Liu Y, Tan JY,
Guan W, Guo S and Kuang HX: Datura Metel L. Ameliorates
imiquimod-induced psoriasis-like dermatitis and inhibits
inflammatory cytokines production through TLR7/8-MyD88-NF-κB-NLRP3
Inflammasome Pathway. Molecules. 24:21572019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hotamisligil GS and Davis RJ: Cell
signaling and stress responses. Cold Spring Harb Perspect Biol.
8:a0060722016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng Y, Chang C and Lu Q: The inflammatory
response in psoriasis: A comprehensive review. Clin Rev Allergy
Immunol. 50:377–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gooderham MJ, Papp KA and Lynde CW:
Shifting the focus - the primary role of IL-23 in psoriasis and
other inflammatory disorders. J Eur Acad Dermatol Venereol.
32:1111–1119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oliveira Mde F, Rocha Bde O and Duarte GV:
Psoriasis: Classical and emerging comorbidities. An Bras Dermatol.
90:9–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin W, Yu Q, Qin Y, Dai L, Xiao J, Jiao L,
Liu S, Ye S, Zhang J and Chen M: To explore the clinical efficacy
of Traditional Chinese Medicine bath in the treatment of psoriasis
vulgaris with blood-heat syndrome and its effect on related
cytokines based on different temperature and different
concentration. Medicine. 99:e201722020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu A, Zhao W, Zhang B, Tu Y, Wang Q and
Li J: Cimifugin ameliorates imiquimod-induced psoriasis by
inhibiting oxidative stress and inflammation via NF-κB/MAPK
pathway. Biosci Rep. 40:BSR202004712020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He Q, Zhang B, Hu F, Long J, Shi Q, Pi X,
Chen H and Li J: Triptolide inhibits the proliferation of HaCaT
cells induced by IL22 via upregulating miR-181b-5p. Drug Des Devel
Ther. 14:2927–2935. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu S, Zhao M, Sun Y, Xie M, Le K, Xu M and
Huang C: The potential of Diosgenin in treating psoriasis: Studies
from HaCaT keratinocytes and imiquimod-induced murine model. Life
Sci. 241:1171152020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hammouda MB, Ford AE, Liu Y and Zhang JY:
The JNK signaling pathway in inflammatory Skin disorders and
cancer. Cells. 9:8572020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C, Zong J, Li Y, Wang X, Du W and Li
L: miR-744-3p regulates keratinocyte proliferation and
differentiation via targeting KLLN in psoriasis. Exp Dermatol.
28:283–291. 2019. View Article : Google Scholar : PubMed/NCBI
|