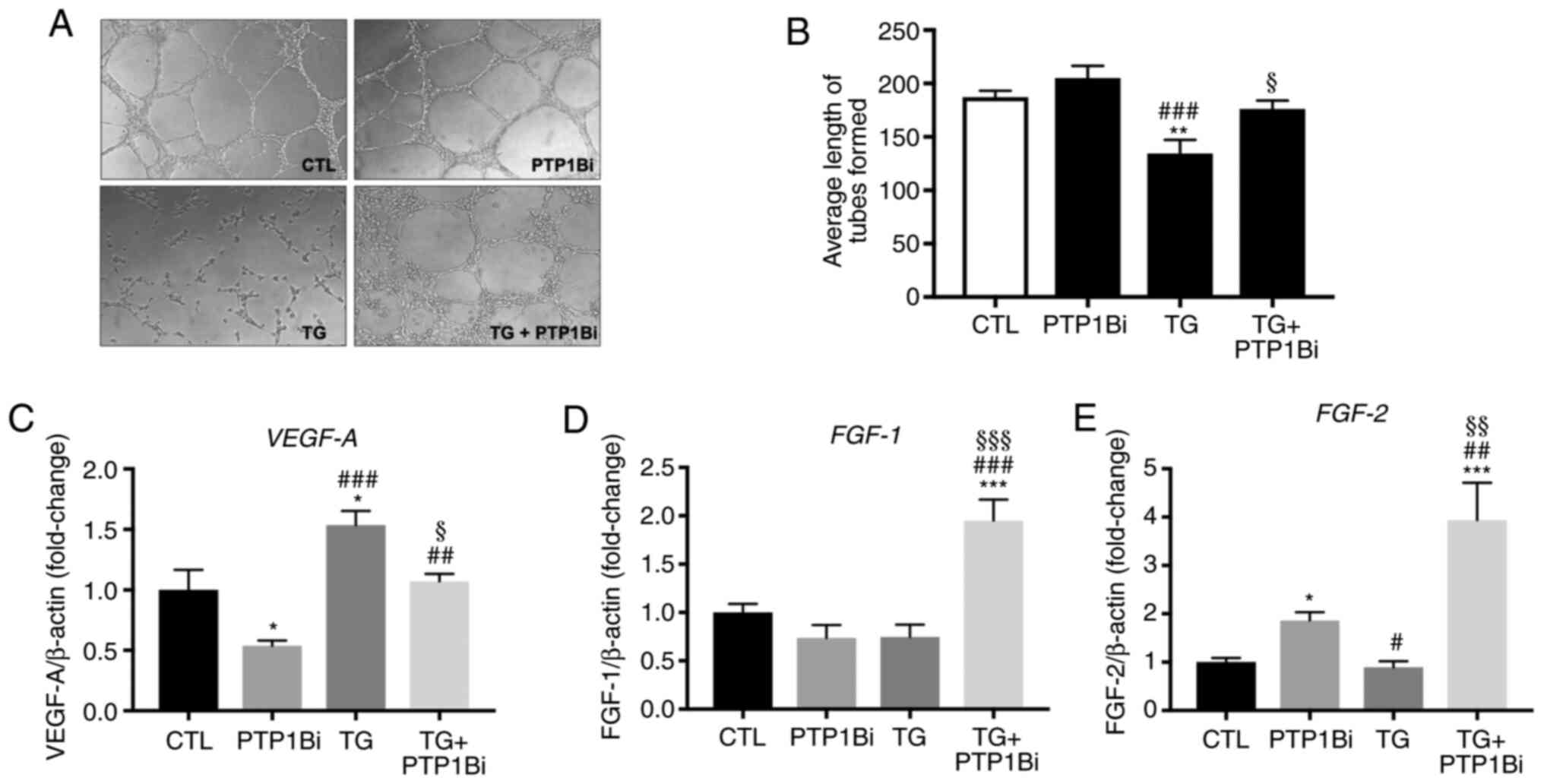
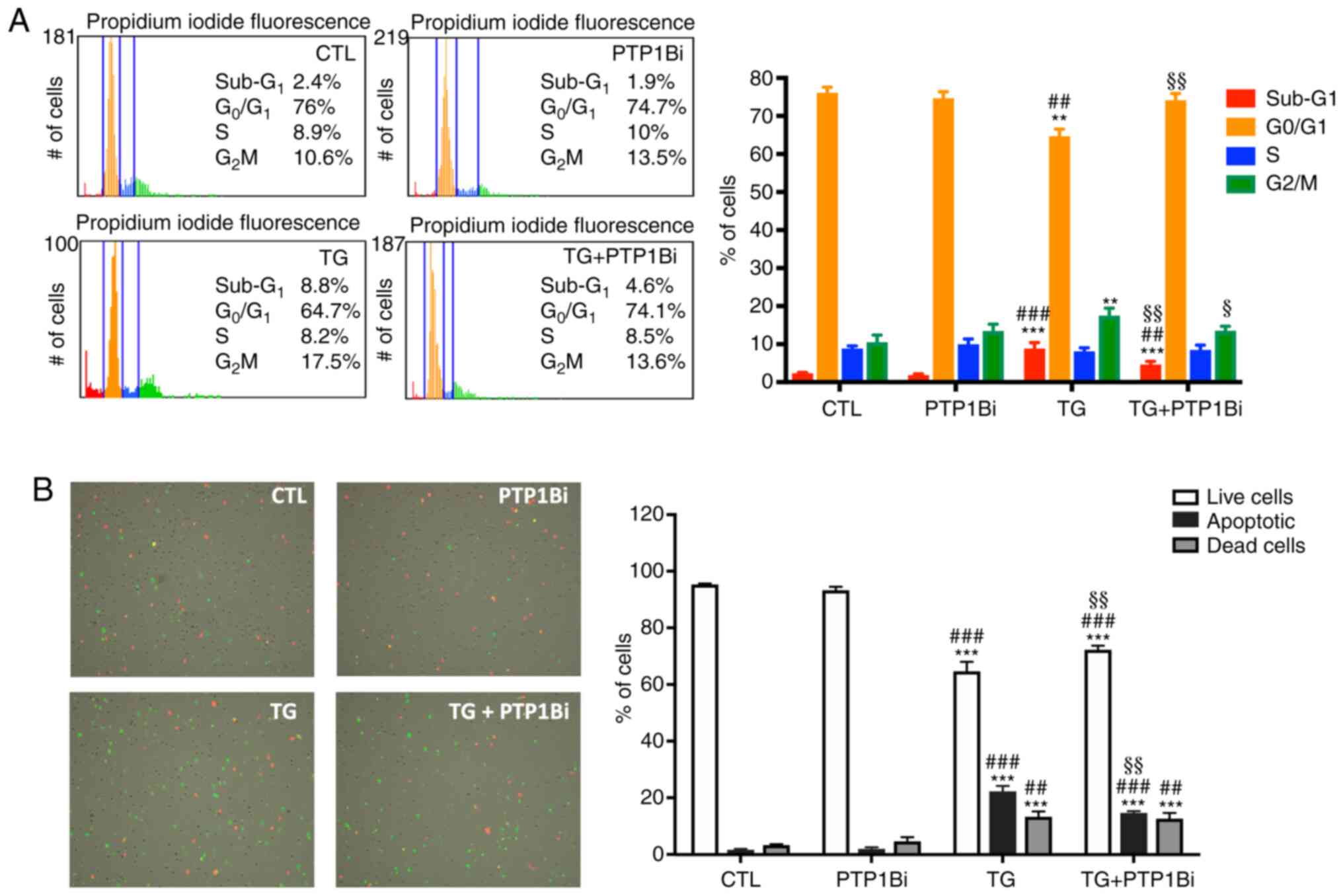
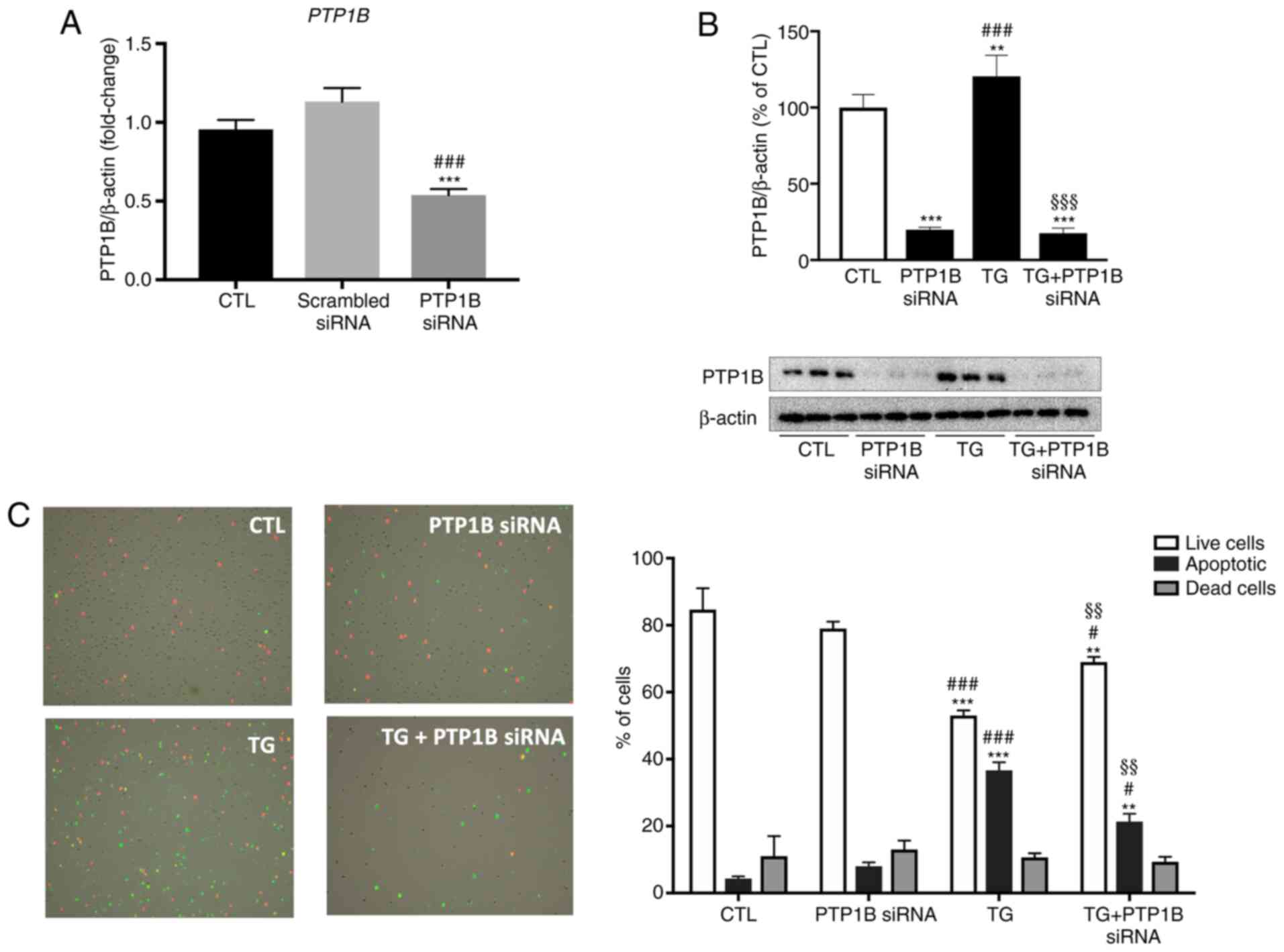
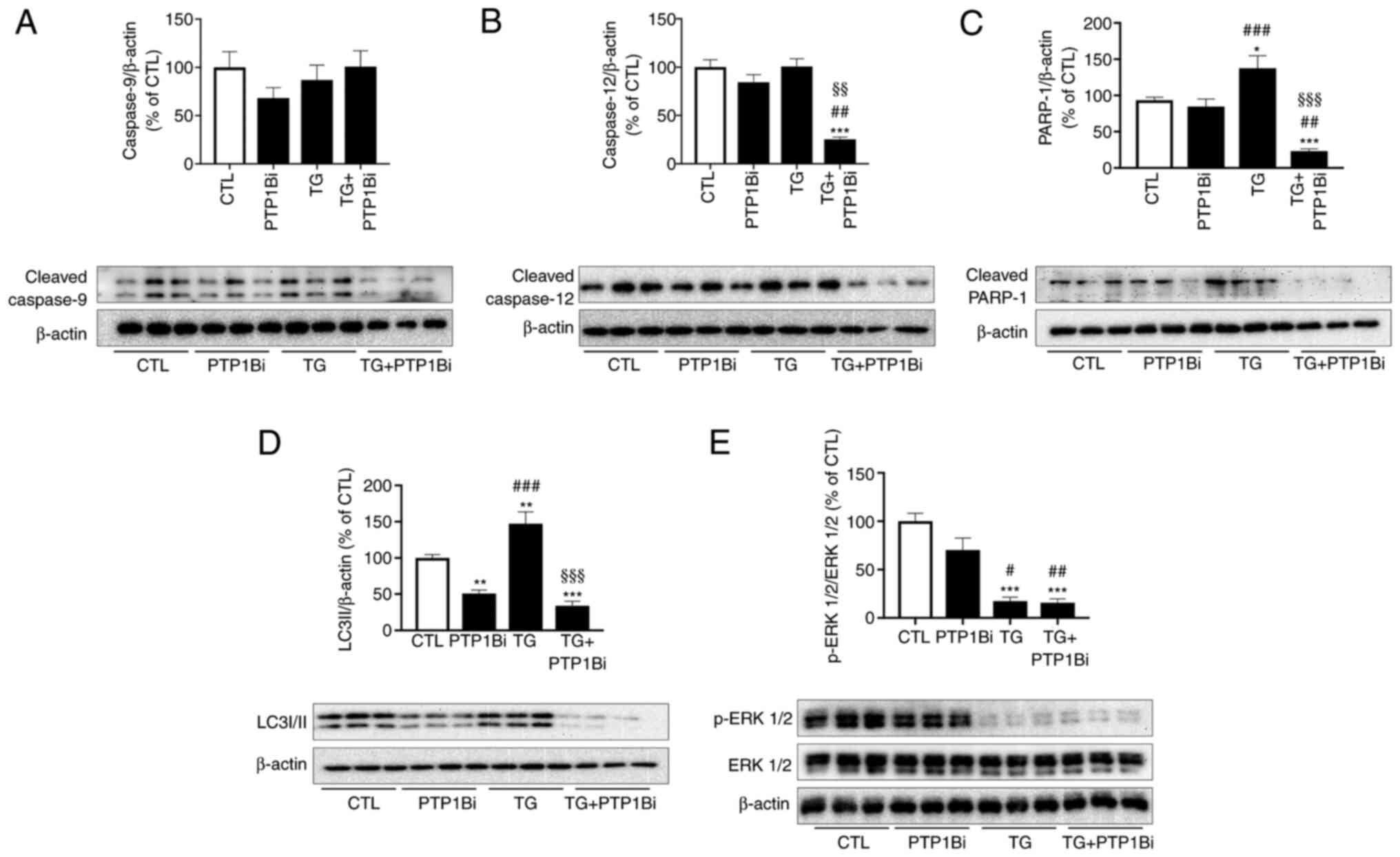
|
1
|
Maamoun H, Benameur T, Pintus G, Munusamy
S and Agouni A: Crosstalk between oxidative stress and endoplasmic
reticulum (ER) stress in endothelial dysfunction and aberrant
angiogenesis associated with diabetes: A focus on the protective
roles of heme oxygenase (HO)-1. Front Physiol. 10:702019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang HN, Xu QQ, Thakur A, Alfred MO,
Chakraborty M, Ghosh A and Yu XB: Endothelial dysfunction in
diabetes and hypertension: Role of microRNAs and long non-coding
RNAs. Life Sci. 213:258–268. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cersosimo E and DeFronzo RA: Insulin
resistance and endothelial dysfunction: The road map to
cardiovascular diseases. Diabetes Metab Res Rev. 22:423–436. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Potenza MA, Gagliardi S, Nacci C, Carratu
MR and Montagnani M: Endothelial dysfunction in diabetes: From
mechanisms to therapeutic targets. Curr Med Chem. 16:94–112. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muniyappa R, Iantorno M and Quon MJ: An
integrated view of insulin resistance and endothelial dysfunction.
Endocrinol Metab Clin North Am. 37:685–711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bakke J and Haj FG: Protein-tyrosine
phosphatase 1B substrates and metabolic regulation. Semin Cell Dev
Biol. 37:58–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tonks NK, Diltz CD and Fischer EH:
Purification of the major protein-tyrosine-phosphatases of human
placenta. J Biol Chem. 263:6722–6730. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galic S, Klingler-Hoffmann M,
Fodero-Tavoletti MT, Puryer MA, Meng TC, Tonks NK and Tiganis T:
Regulation of insulin receptor signaling by the protein tyrosine
phosphatase TCPTP. Mol Cell Biol. 23:2096–2108. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abdelsalam SS, Korashy HM, Zeidan A and
Agouni A: The role of protein tyrosine phosphatase (PTP)-1B in
cardiovascular disease and its interplay with insulin resistance.
Biomolecules. 9:2862019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vercauteren M, Remy E, Devaux C, Dautreaux
B, Henry JP, Bauer F, Mulder P, van Huijsduijnen RH, Bombrun A,
Thuillez C and Richard V: Improvement of peripheral endothelial
dysfunction by protein tyrosine phosphatase inhibitors in heart
failure. Circulation. 114:2498–2507. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gomez E, Vercauteren M, Kurtz B,
Ouvrard-Pascaud A, Mulder P, Henry JP, Besnier M, Waget A, Van
Huijsduijnen RH, Tremblay ML, et al: Reduction of heart failure by
pharmacological inhibition or gene deletion of protein tyrosine
phosphatase 1B. J Mol Cell Cardiol. 52:1257–1264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Agouni A, Tual-Chalot S, Chalopin M, Duluc
L, Mody N, Martinez MC, Andriantsitohaina R and Delibegović M:
Hepatic protein tyrosine phosphatase 1B (PTP1B) deficiency protects
against obesity-induced endothelial dysfunction. Biochem Pharmacol.
92:607–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gogiraju R, Schroeter MR, Bochenek ML,
Hubert A, Münzel T, Hasenfuss G and Schäfer K: Endothelial deletion
of protein tyrosine phosphatase-1B protects against pressure
overload-induced heart failure in mice. Cardiovasc Res.
111:204–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agouni A, Mody N, Owen C, Czopek A, Zimmer
D, Bentires-Alj M, Bence KK and Delibegović M: Liver-specific
deletion of protein tyrosine phosphatase (PTP) 1B improves
obesity-and pharmacologically-induced endoplasmic reticulum stress.
Biochem J. 438:369–378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Owen C, Lees EK, Grant L, Zimmer DJ, Mody
N, Bence KK and Delibegović M: Inducible liver-specific knockdown
of protein tyrosine phosphatase 1B improves glucose and lipid
homeostasis in adult mice. Diabetologia. 56:2286–2296. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Villalobos-Labra R, Subiabre M, Toledo F,
Pardo F and Sobrevia L: Endoplasmic reticulum stress and
development of insulin resistance in adipose, skeletal, liver, and
foetoplacental tissue in diabesity. Mol Aspects Med. 66:49–61.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maamoun H, Abdelsalam SS, Zeidan A,
Korashy HM and Agouni A: Endoplasmic reticulum stress: A critical
molecular driver of endothelial dysfunction and cardiovascular
disturbances associated with diabetes. Int J Mol Sci. 20:16582019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Flamment M, Hajduch E, Ferré P and
Foufelle F: New insights into ER stress-induced insulin resistance.
Trends Endocrinol Metab. 23:381–390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Özcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi
NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH and Hotamisligil
GS: Endoplasmic reticulum stress links obesity, insulin action, and
type 2 diabetes. Science. 306:457–461. 2004. View Article : Google Scholar
|
|
20
|
Özcan U, Yilmaz E, Özcan L, Furuhashi M,
Vaillancourt E, Smith RO, Görgün CZ and Hotamisligil GS: Chemical
chaperones reduce ER stress and restore glucose homeostasis in a
mouse model of type 2 diabetes. Science. 313:1137–1140. 2006.
View Article : Google Scholar
|
|
21
|
Kassan M, Galán M, Partyka M, Saifudeen Z,
Henrion D, Trebak M and Matrougui K: Endoplasmic reticulum stress
is involved in cardiac damage and vascular endothelial dysfunction
in hypertensive mice. Arterioscler Thromb Vasc Biol. 32:1652–1661.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galán M, Kassan M, Choi SK, Partyka M,
Trebak M, Henrion D and Matrougui K: A novel role for epidermal
growth factor receptor tyrosine kinase and its downstream
endoplasmic reticulum stress in cardiac damage and microvascular
dysfunction in type 1 diabetes mellitus. Hypertension. 60:71–80.
2012. View Article : Google Scholar
|
|
23
|
Ghemrawi R, Battaglia-Hsu SF and Arnold C:
Endoplasmic reticulum stress in metabolic disorders. Cells.
7:632018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwarz DS and Blower MD: The endoplasmic
reticulum: Structure, function and response to cellular signaling.
Cell Mol Life Sci. 73:79–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pandey VK, Mathur A and Kakkar P: Emerging
role of unfolded protein response (UPR) mediated proteotoxic
apoptosis in diabetes. Life Sci. 216:246–258. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Battson ML, Lee DM and Gentile CL:
Endoplasmic reticulum stress and the development of endothelial
dysfunction. Am J Physiol Heart Circul Physiol. 312:H355–H367.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Minamino T, Komuro I and Kitakaze M:
Endoplasmic reticulum stress as a therapeutic target in
cardiovascular disease. Circ Res. 107:1071–1082. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bilekova S, Sachs S and Lickert H:
Pharmacological targeting of endoplasmic reticulum stress in
pancreatic beta cells. Trends Pharmacol Sci. 42:85–95. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng L, Zampetaki A, Margariti A, Pepe AE,
Alam S, Martin D, Xiao Q, Wang W, Jin ZG, Cockerill G, et al:
Sustained activation of XBP1 splicing leads to endothelial
apoptosis and atherosclerosis development in response to disturbed
flow. Proc Natl Acad Sci USA. 106:8326–8331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maamoun H, Zachariah M, McVey JH, Green FR
and Agouni A: Heme oxygenase (HO)-1 induction prevents Endoplasmic
Reticulum stress-mediated endothelial cell death and impaired
angiogenic capacity. Biochem Pharmacol. 127:46–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang J, Wan L, Lu H and Li X: High
expression of active ATF6 aggravates endoplasmic reticulum
stress-induced vascular endothelial cell apoptosis through the
mitochondrial apoptotic pathway. Mol Med Rep. 17:6483–6489.
2018.PubMed/NCBI
|
|
32
|
Choy JC, Granville DJ, Hunt DW and McManus
BM: Endothelial cell apoptosis: Biochemical characteristics and
potential implications for atherosclerosis. J Mol Cell Cardiol.
33:1673–1690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Osman A, El-Gamal H, Pasha M, Zeidan A,
Korashy HM, Abdelsalam SS, Hasan M, Benameur T and Agouni A:
Endoplasmic reticulum (ER) stress-generated extracellular vesicles
(Microparticles) self-perpetuate ER stress and mediate endothelial
cell dysfunction independently of cell survival. Front Cardiovasc
Med. 7:5847912020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abdullahi A, Stanojcic M, Parousis A,
Patsouris D and Jeschke MG: Modeling acute ER stress in vivo and in
vitro. Shock. 47:506–513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Andersen HS, Olsen OH, Iversen LF,
Sørensen AL, Mortensen SB, Christensen MS, Branner S, Hansen TK,
Lau JF, Jeppesen L, et al: Discovery and SAR of a novel selective
and orally bioavailable nonpeptide classical competitive inhibitor
class of protein-tyrosine phosphatase 1B. J Med Chem. 45:4443–4459.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Agouni A, Mostefai HA, Porro C, Carusio N,
Favre J, Richard V, Henrion D, Martínez MC and Andriantsitohaina R:
Sonic hedgehog carried by microparticles corrects endothelial
injury through nitric oxide release. FASEB J. 21:2735–2741. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Coquerel D, Neviere R, Delile E, Mulder P,
Marechal X, Montaigne D, Renet S, Remy-Jouet I, Gomez E, Henry JP,
et al: Gene deletion of protein tyrosine phosphatase 1B protects
against sepsis-induced cardiovascular dysfunction and mortality.
Arterioscler Thromb Vasc Biol. 34:1032–1044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Fan Y, Song Y, Han X, Fu M, Wang
J, Cui X, Cao J, Chen L, Hu K, et al: Angiotensin II induces
apoptosis of cardiac microvascular endothelial cells via regulating
PTP1B/PI3K/Akt pathway. In vitro Cell Dev Biol Animal. 55:801–811.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lavandero S, Chiong M, Rothermel BA and
Hill JA: Autophagy in cardiovascular biology. J Clin Invest.
125:55–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thiebaut PA, Delile E, Coquerel D, Brunel
JM, Renet S, Tamion F and Richard V: Protein tyrosine phosphatase
1B regulates endothelial endoplasmic reticulum stress; role in
endothelial dysfunction. Vascul Pharmacol. 109:36–44. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun HJ, Wu ZY, Nie XW and Bian JS: Role of
endothelial dysfunction in cardiovascular diseases: The link
between inflammation and hydrogen sulfide. Front Pharmacol.
21:15682020. View Article : Google Scholar
|
|
43
|
Lubrano V and Balzan S: Roles of LOX-1 in
microvascular dysfunction. Microvasc Res. 105:132–140. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Grant L, Shearer KD, Czopek A, Lees EK,
Owen C, Agouni A, Workman J, Martin-Granados C, Forrester JV,
Wilson HM, et al: Myeloid-cell protein tyrosine phosphatase-1B
deficiency in mice protects against high-fat diet and
lipopolysaccharide-induced inflammation, hyperinsulinemia, and
endotoxemia through an IL-10 STAT3-dependent mechanism. Diabetes.
63:456–470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thompson D, Morrice N, Grant L, Sommer SL,
Ziegler K, Whitfield P, Mody N, Wilson HM and Delibegović M:
Myeloid protein tyrosine phosphatase 1B (PTP1B) deficiency protects
against atherosclerotic plaque formation in the
ApoE(−/−) mouse model of atherosclerosis with
alterations in IL10/AMPKα pathway. Mol Metab. 6:845–853. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Legeay S, Fautrat P, Norman JB, Antonova
G, Kennard S, Bruder-Nascimento T, Patel VS, Faure S and de
Chantemèle EJ: Selective deficiency in endothelial PTP1B protects
from diabetes and endoplasmic reticulum stress-associated
endothelial dysfunction via preventing endothelial cell apoptosis.
Biomed Pharmacother. 127:1102002020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim JA, Jang HJ and Hwang DH: Toll-like
receptor 4-induced endoplasmic reticulum stress contributes to
impairment of vasodilator action of insulin. Am J Physiol
Endocrinol Metab. 309:E767–E776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dixit M, Loot AE, Mohamed A, Fisslthaler
B, Boulanger CM, Ceacareanu B, Hassid A, Busse R and Fleming I:
Gab1, SHP2, and protein kinase A are crucial for the activation of
the endothelial NO synthase by fluid shear stress. Circ Res.
97:1236–1244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Karbach S, Wenzel P, Waisman A, Munzel T
and Daiber A: eNOS uncoupling in cardiovascular diseases-the role
of oxidative stress and inflammation. Curr Pharm Des. 20:3579–3594.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shukla N, Freeman N, Gadsdon P, Angelini
GD and Jeremy JY: Thapsigargin inhibits angiogenesis in the rat
isolated aorta: Studies on the role of intracellular calcium pools.
Cardiovasc Res. 49:681–689. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang J, Li L, Li J, Liu Y, Zhang CY,
Zhang Y and Zen K: Protein tyrosine phosphatase 1B impairs diabetic
wound healing through vascular endothelial growth factor receptor 2
dephosphorylation. Arterioscler Thromb Vasc Biol. 35:163–174. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Y, Li Q, Youn JY and Cai H: Protein
phosphotyrosine phosphatase 1B (PTP1B) in calpain-dependent
feedback regulation of vascular endothelial growth factor receptor
(VEGFR2) in endothelial cells: Implications in VEGF-dependent
angiogenesis and diabetic wound healing. J Biol Chem. 292:407–416.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Besnier M, Galaup A, Nicol L, Henry JP,
Coquerel D, Gueret A, Mulder P, Brakenhielm E, Thuillez C, Germain
S, et al: Enhanced angiogenesis and increased cardiac perfusion
after myocardial infarction in protein tyrosine phosphatase
1B-deficient mice. FASEB J. 28:3351–3361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jeon YM, Lee S, Kim S, Kwon Y, Kim K,
Chung CG, Lee S, Lee SB and Kim HJ: Neuroprotective effects of
protein tyrosine phosphatase 1B inhibition against ER
stress-induced toxicity. Mol Cells. 40:280–290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gu F, Nguyên DT, Stuible M, Dubé N,
Tremblay ML and Chevet E: Protein-tyrosine phosphatase 1B
potentiates IRE1 signaling during endoplasmic reticulum stress. J
Biol Chem. 279:49689–49693. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hotamisligil GS and Davis RJ: Cell
signaling and stress responses. Cold Spring Harb Perspect Biol.
8:a0060722016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Darling NJ and Cook SJ: The role of MAPK
signalling pathways in the response to endoplasmic reticulum
stress. Biochim Biophys Acta. 1843:2150–2163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang S, Chen X, Nair S, Sun D, Wang X and
Ren J: Deletion of protein tyrosine phosphatase 1B obliterates
endoplasmic reticulum stress-induced myocardial dysfunction through
regulation of autophagy. Biochim Biophys Acta Mol Basis Dis.
1863:3060–3074. 2017. View Article : Google Scholar : PubMed/NCBI
|