Introduction
Spinal cord injury (SCI) is characterized by
permanent motor and sensory deficits followed by secondary injury
mechanisms, including prolonged inflammation and oxidative stress
that leads to sustained and widespread cell death and tissue damage
(1). Unfortunately, SCI remains
incurable at present and the major treatments are limited to
reducing secondary complications after initial traumatic injury and
maximizing residual function through rehabilitation (2). Recently, cell transplantation,
including neural stem cells, progenitor cells, olfactory
ensheathing cells, oligodendrocyte precursor cells and mesenchymal
stem cells, has been considered as a potential and viable option
for the treatment of SCI (3). In
addition, it has been revealed in a previous study that tanshinone
IIA may enhance the therapeutic effect of bone marrow mesenchymal
stem cell transplant on SCI by promoting the differentiation of
neuronal cells (4). Therefore, the
present study aimed to illuminate the underlying molecular
mechanisms of microRNA-128 (miR-128) on the differentiation and
apoptosis of neuronal cells, which demonstrated a potent
ameliorating effect on SCI.
miRs, a group of non-coding small RNAs,
post-transcriptionally orchestrate gene expression that has been
implicated in pathogenic processes of SCI including inflammation,
oxidation, demyelination, and apoptosis (5). miR-128 has been previously reported to
exert a significant regulatory effect on SCI (6). miR-128 expression has been reported to
be downregulated in cells treated with SCI, whereas the
upregulation of miR-128 promotes cell viability of microglia cells
in mice, thereby reducing neuropathic pain following SCI (6). Although rarely documented, Unc-51 like
autophagy activating kinase 1 (ULK1) is predicted to be the
downstream target gene of miR-128 through the bioinformatic website
TargetScanHuman 7.2 (http://www.targetscan.org/vert_72/). As a
serine/threonine kinase, ULK1 plays a key role in the regulation of
autophagy activation in mice (7).
The marked elevation of mRNA expression of ULK1 in clear cell renal
cell carcinoma was been previously found to be associated with
unfavorable clinical prognosis (8).
Notably, the decreased expression of ULK1 contributes to the
downregulation of goat Sertoli cell marker gene Fas ligand (FasL)
(9). A previous study reported that
FasL participated in the apoptosis of virus-infected cells and that
the activation of FasL serves a key role in apoptosis and
inflammatory response (10). The
activation of FasL also exerts significant influences on apoptosis,
inflammatory response, and glial proliferation in a number of
nervous system diseases following SCI (11). Therefore, in this context, the
purpose of the present study was to investigate the anti-apoptotic
effect of miR-128 on neuronal cells following SCI by targeting the
ULK1/FasL axis.
Materials and methods
Ethical statement
All animal experiments were performed following
ratification by the Animal Ethics Committee of the First Affiliated
Hospital of Harbin Medical University (approval no. 2019010)
conforming to Principles and Procedures of the National Academy of
Animal Health Care Guidelines. All efforts were made to minimize
animal suffering and minimize the number of animals used.
Establishment of rat models of
SCI
A total of 100 adult specified-pathogens free-grade
Sprague-Dawley male rats weighing 200–220 g and 12–14 weeks old
(Beijing Vital River Laboratory Animal Technology Co., Ltd.) were
used in the present study. These rats were housed at 25±2°C and
humidity of 50±10% with a 12-h light/dark cycle and given free
access to water and food. Of them, 10 out of 100 rats were chosen
as sham-operated rats and the remaining rats were adopted for the
generation of rat models of SCI. Rats were anesthetized with 3%
pentobarbital sodium via intraperitoneal injection [cat. no. P3761;
Sigma-Aldrich; Merck KGaA; 30 mg/kg (1 ml/kg according to body
weight)]. Following dissection of the paravertebral muscles,
laminectomy was performed on the spinal segment (T9-T11).
Subsequently, rats with SCI were subjected to an aneurysm clip at
the T10 level for 60 sec. Subsequent to trauma, SCI rats
immediately underwent intrathecal injection with lentiviral vectors
containing miR-128 and ULK1 at the dose of 10 µl and 2 nmol
(12). Finally, the incision was
sutured layer by layer with silk sutures. Sham-operated rats only
underwent laminectomy. All rats were injected with penicillin and
analgesics for 3 days post-operation and then received manual
bladder expression thrice a day. The success rate of modeling was
89% and no sham-operated rats succumbed (the remaining mice were
used for experimental reserve). Following successful modeling, rats
experienced spinal cord hemorrhage, delayed hindlimb extension and
tail swing. Rats received manual bladder expression once a day
until bladder function was restored. The Basso, Beattie and
Bresnahan (BBB) scale of rats was performed on miR-128 treated on
days 1, 7, 14, 21 and 28 following SCI. At the 28th day
post-operation with the completion of behavioral tests, 0.2 ml
whole blood was obtained from each rat and the rats were euthanized
by asphyxia with 25% capacity volume/min of CO2 gas
displacement rate. The spinal cord tissues were harvested for the
related tests. Lentiviral vectors (LVs) including LV-control,
LV-miR-128, LV-sponge miR-128 (lentiviral vector-mediated
inhibition of miR-128), short hairpin RNA against negative control
(sh-NC) and shRNA against ULK1 (sh-ULK1) were constructed by
Genomeditech.
BBB scale
The hindlimb motor function of rats was evaluated on
days 1, 7, 14, 21 and 28 post-operation using the BBB scoring
system (13,14). In an open field (125 cm × 125 cm),
after rats adapted to the environment, the locomotor function was
observed and scored (5 min). The observation was performed in a
double-blinded manner with two trained and non-experimental staff.
A total of three average recorded values were taken as the BBB
score. BBB was a system that followed the recovery of hindlimb
function from a score of 0 (no observed hindlimb movements), to a
score of 21 (a normal ambulating rodent).
Dual-luciferase reporter gene
assay
Bioinformatic websites RAID version 2.0 (http://www.rna-society.org/raid2/), miRDB version
5.0 (http://www.mirdb.org/miRDB/download.html),
TargetScanHuman version 7.2 (http://www.targetscan.org/vert_72/) and miRWalk
version 2021 (http://mirwalk.umm.uni-heidelberg.de/) were employed
to predict the targeted relationship among miR-128, ULK1 and FasL.
Venn map online website (http://bioinformatics.psb.ugent.be/webtools/Venn)
was adopted for the validation of predicted results. The
dual-luciferase reporter gene assay was employed to verify the
targeting relationship between miR-128 and ULK1, as well as between
ULK1 and FasL. Briefly, pmirGLO firefly luciferase-expressing
vectors (Promega Corporation) was used to construct the wild type
(WT) and complementary sequence mutated (MUT) site with miR-128
(PGLO-ULK1 WT and PGLO-ULK1 MUT). FasL WT and MUT (PGLO-FasL WT and
PGLO-FasL MUT) and ULK1 overexpression (oe) vector and the
corresponding NC vector were constructed. The reporter plasmids
were co-transfected with the plasmids containing oe and NC of
miR-128 and ULK1 into 293T cells respectively using the
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) based on the manufacturer's
protocol. At 48 h post-transfection, Cells were lysed and
centrifuged at 6,500 × g for 1 min at 4°C, followed by harvesting
of the supernatant. The dual-luciferase reporter assay system
(E1910, Promega Corporation) was employed to detect the luciferase
activity. Relative luciferase activity=firefly
luciferase/Renilla luciferase.
Chromatin immunoprecipitation (ChIP)
assay
The enrichment of FasL by ULK1 was evaluated by a
ChIP kit (EMD Millipore). Briefly, 1% formaldehyde was added to fix
the 70–80% confluent cells at room temperature for 10 min to allow
the formation of DNA-protein cross-links in the cells.
Subsequently, cells were lysed in 1 ml lysate buffer. Next,
sonication (10 sec per time) was performed at intervals of 10 sec
(15 cycles) to fractionate large fragments into appropriate size,
followed by centrifugation at 6,500 × g for 1 min at 4°C. The
supernatant was then collected, divided into three tubes and then
added with the positive control antibody RNA polymerase II (cat.
no. ab36010; 1:1,000; Abcam), the NC antibody of normal mouse
immunoglobulin G (IgG; cat. no. ab6789; 1:1,000; Abcam), or the
target protein-specific rabbit anti-ULK1 antibody (cat. no. ab2851;
Abcam), followed by overnight incubation at 4°C. Protein
Agarose/Sepharose was supplemented to precipitate the endogenous
DNA-protein complex. Subsequent to transient centrifugation at
6,500 × g for 1 min at 4°C, the supernatant was discarded and the
non-specific complexes were washed. The complex was de-crosslinked
at 65°C and the DNA fragment was recovered by phenol/chloroform
extraction and purification. The binding of ULK1 to the FasL
promoter region was assessed by the specific primers in FasL
promoter region using western blotting as detailed in the following
section.
Hematoxylin and eosin (H&E) and
Nissl staining
Spinal cord tissue located at 0.5 cm above and below
the injury was attained 28 days post-surgery. The spinal cord
tissues were fixed in 4% neutral paraformaldehyde (PFA) for 24 h at
room temperature, routinely dehydrated with graded ethanol series
(100% for 5 min, 95% for 3 min, 90% for 3 min), cleared using
xylene and, embedded in paraffin, and sectioned into 4 µm-thick
sections. The sections were then dewaxed twice with xylene (each
time for 5 min) and rehydrated, followed by 5-min hematoxylin
staining and 20-sec differentiation with hydrochloric acid alcohol.
Subsequent to 5-min l% eosin staining, morphological changes in
spinal cord tissue structure were observed under a light microscope
(magnification, ×200; Olympus BX51; Olympus Corporation).
For Nissl staining the sections were stained with 1%
toluidine blue staining solution for 10 min, followed by
conventional dehydration using gradient ethanol and xylene clearing
and neutral resin mounting. Quantitative analysis of the number of
surviving neurons was implemented under a light microscope
(magnification, ×200; Olympus BX51; Olympus Corporation). In all
~3–5 rats were randomly attained in each group for statistical
analysis.
Immunofluorescence
The frozen sections were air-dried at room
temperature, fixed with −20°C pre-chilled acetone for 6 min and
water-bathed with 50% formamide/2X citrate hybridization solution
at 65°C for 2 h. Subsequently, the sections were washed using 2X
saline sodium citrate solution for 3 times, followed by 30-min
immersion in 0.3% Triton at ambient temperature. The sections were
washed in 2 M HCl and finally washed twice in 0.1 mol/l boric acid
buffer (pH 8.0; 5 min/time). Finally, the sections were blocked
with 10% sheep serum at 37°C for 1 h. Each section was then added
dropwise with neuronal nuclear antigen (cat. no. ab128886),
neurofilament-200 (cat. no. ab82259), glial fibrillary acidic
protein (1:100; cat. no. ab7260; all from Abcam) for overnight
incubation at 4°C. The 0.01 M phosphate-buffered saline instead of
the primary antibody was adopted as a NC. IgG was then added
dropwise to the sections for 45-min incubation at room temperature.
The sections were mounted with anti-fluorescent quencher and
observed under Leica SP5 confocal microscope (magnification, × 200;
Leica Microsystems GmbH).
Terminal deoxynucleotidyl
transferase-mediated dUTp nick end-labeling (TUNEL) staining
A one-step TUNEL apoptosis assay kit (Roche
Diagnostics GmbH) was employed to determine the morphological
changes of cell apoptosis 28 days post-operation. Apoptotic cells
were TUNEL positive in a Nikon ECLIPSE Ti inverted microscope
(Nikon Corporation) and the nuclei of apoptotic cells were stained
brownish-yellow. A total of 10 high-power fields were randomly
observed, followed by calculation of the mean number of positive
cells using BI-2000 image-analysis system (BI-2000; Visitech
Systems).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA extraction from spinal cord tissues of
rats from each group was implemented as per the instructions of
TRIzol® (Thermo Fisher Scientific, Inc.). All primers
were synthesized by Augct Biotechnology Co., Ltd. (Table I) according to the manufacturer's
protocols. Fluorescence quantitative PCR was performed in
accordance with the protocols of SYBR Premix Ex TaqTM II kit (cat.
no. RR820A; Xingzhi Biotech, Co. Ltd.). The reaction system was 50
µl: SYBR® Premix Ex Taq II (2X) 25 µl, PCR upstream
primer 2 µl, PCR downstream primer 2 µl, ROX reference dye (50X) 1
µl, DNA template 4 µl, and ddH2O 16 µl. PCR was
performed on ABI PRISM 7300 (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reaction conditions were as follows: 10 min
of pre-denaturation at 94°C, 15 sec of denaturation at 94°C and 30
sec of annealing at 55°C. After 40 cycles, amplification was
performed for 1 min at 72°C. The relative expression was calculated
using the 2−ΔΔCq method (15) and standardized by U6 (for miR-128)
and GAPDH (for ULK1; cat. no. abs830032; Absin Bioscience, Inc.).
The experiment was performed three times.
 | Table I.The primer sequences used in reverse
transcription-quantitative PCR. |
Table I.
The primer sequences used in reverse
transcription-quantitative PCR.
| Target gene | Primer sequence
(5′-3′) |
|---|
| miR-128 | F:
5′-GGTCACAGTGAACCGGTC-3′ |
|
| R:
5′-GTGCAGGGTCCGAGGT-3′ |
| ULK1 | F:
5′-CCCCAACCTTTCGGACTT-3′ |
|
| R:
5′-CCAACAGGGTCAGCAAACTC-3′ |
| U6 | F:
5′-GCGCGTCGTGAAGCGTTC-3′ |
|
| R:
5′-GTGCAGGGTCCGAGGT-3′ |
| GAPDH | F:
5′-CATCACTGCCACCCAGAAGA-3′ |
|
| R:
5′-TCCACGACCGACACGTTG-3′ |
Western blot analysis
The total protein content was extracted from the
tissue according to the manufacturer's protocols provided by
high-efficiency radioimmunoprecipitation assay-like buffer (cat.
no. R0010, Beijing Solarbio Science & Technology Co., Ltd.).
The estimation of protein concentration was implemented using a
bicinchoninic acid kit (cat. no. 20201ES76; Shanghai Yeasen
Biotechnology Co., Ltd.). The protein was uploaded (30 µg per
well), separated by 10% polyacrylamide gel electrophoresis, and
electro-blotted onto a polyvinylidene fluoride membrane. Subsequent
to 1-h membrane blocking with 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) at ambient temperature, overnight
membrane incubation was performed at 4°C with diluted primary
antibodies to Cleaved caspase-3 (cat. no. ab2302; 1:1,000), FAS
cell surface death receptor (Fas) (cat. no. ab82419; 1:1,000), FasL
(cat. no. ab15285; 1:1,000) and GAPDH (cat. no. ab181602; 1:1,000).
The membrane was washed thrice with Tris-buffered saline with 0.05%
Tween 20, each time for 5 min and subsequently re-probed with
horseradish peroxidase-tagged goat anti-rabbit IgG (cat. no.
ab150077; 1:10,000 Abcam). An electrogenerated chemiluminescence
(ECL) developer (Shanghai Lianshui Biotechnology Co., Ltd.) was
adopted to develop the membrane. ImageJ 1.48u software (National
Institutes of Health) was applied for the quantitative protein
analysis. Relative protein expression was analyzed by the ratio of
the gray value of the target band to that of GAPDH.
Enzyme-linked immunosorbent assay
(ELISA)
The 0.2 ml orbital whole blood was attained from
each rats and centrifuged at 1,500 × g at room temperature for 10
min to collect the serum of rats. ELISA was performed in accordance
with the manufacturer's protocols (cat. no. ER2101; Wuhan Msk
Biotechnology Co., Ltd.) and a standard curve was drawn to
determine the levels of inflammatory factors IL-1β, IL-6 and TNF-α
in the serum of rats.
Neuron injury models
Primary spinal cord neurons were isolated from
healthy SPF-level SD rats (Beijing Vital River Laboratory Animal
Technology Co., Ltd.) on day 14–17 after gestation. Briefly, spinal
cords were dissected in medium without serum and trypsinized
(0.125%) at 37°C for 15 min. The cell suspension was prepared and
incubated into 24-well plates coated with 0.1 g/l polylysine at a
cell density of 4×105 cells/ml under a
humidity-saturated environment with 5% CO2 at 37°C. The
medium was renewed every 2 days. On the 3rd day of culture,
cytarabine was supplemented at a final mass concentration of 5 mg/l
to inhibit the growth of mixed cells. Following 5 days of culture
in medium without serum, the neuronal cells were treated with 30 mm
H2O2 for 24 h to construct neuron injury
models. Then, 24 h before modeling, cells were transfected with
inhibitor NC (25 nM), miR-128 inhibitor (25 nM), oe-NC (25 nM), or
oe-ULK1 (25 nM) for 48 h using the HG transgene reagent
(Genomeditech), respectively The above vectors were all constructed
by Genomeditech. The sequences are shown in Table II. The neuronal cells were then
incubated for 48 h after transfection for subsequent detection.
 | Table II.Gene inhibitor and overexpression
sequence. |
Table II.
Gene inhibitor and overexpression
sequence.
| Group | Sequence |
|---|
| Inhibitor NC |
ACACUGCUGGGAAAGGUAGGG |
| miR-128
inhibitor |
AGUGUCACUUGGCCAGAGAAA |
| oe-NC |
GAATTCCACCACACTGGACTAGTGGATCC |
| oe-ULK1 |
ATGGAGCCGGGCCGCGGCGGCGTCGAGACCGTGGGCAAGTTCGAGTTCTCTCGCAAGGACCTGATTGGACACGGCGCCTTCGCGGTGGTCTTCAAGGGTCGACACCGCGAGAAGCACGACCTGGAGGTGGCCGTCAAATGCATTAACAAGAAGAACCTTGCCAAGTCCCAAACACTGCTGGGAAAGGAAATCAAAATCCTGAAGGAACTAAAGCACGAAAACATCGTGGCGCTGTATGACTTCCAGGAAATGGCTAATTCTGTCTACCTGGTCATGGAGTATTGTAATGGTGGAGACCTGGCTGACTACCTGCACACTATGCGCACACTGAGTGAAGACACTGTCAGGCTTTTCCTACAGCAGATCGCTGGCGCCATGCGGCTGCTGCACAGCAAGGGCATCATCCACCGGGACCTGAAGCCCCAAAACATCCTGCTGTCCAACCCTGGGGGCCGCCGGGCCAACCCCAGCAACATCCGAGTCAAGATTGCTGACTTTGGATTCGCTCGGTACCTCCAGAGCAACATGATGGCGGCCACACTCTGTGGTTCTCCTATGTACATGGCTCCTGAGGTCATTATGTCCCAGCACTACGATGGAAAGGCTGACCTGTGGAGCATTGGCACCATTGTCTACCAGTGTCTGACAGGGAAGGCCCCTTTTCAGGCCAGCAGCCCTCAGGATTTGCGCCTGTTTTATGAGAAGAACAAGACACTAGTTCCTGCCATCCCCCGGGAGACATCAGCTCCCCTGCGGCAGCTGCTCCTGGCTCTGTTGCAGCGGAACCACAAGGACCGCATGGACTTTGATGAATTTTTCCACCACCCTTTCTTGGATGCCAGCACCCCCATCAAGAAATCCCCACCTGTGCCTGTGCCCTCATATCCAAGCTCAGGGTCTGGCAGCAGCTCCAGCAGCAGCTCTGCCTCCCACCTGGCCTCTCCACCGTCCCTGGGGGAGATGCCACAGCTACAGAAGACCCTTACCTCCCCAGCCGATGCTGCTGGCTTTCTTCAGGGCTCCCGGGACTCTGGTGGCAGCAGCAAAGACTCCTGTGACACAGATGACTTTGTCATGGTCCCAGCCCAGTTTCCAGGTGATCTAGTTGCTGAGGCAGCCAGTGCCAAGCCCCCACCTGATAGCCTGCTGTGTAGTGGGAGCTCATTGGTGGCCTCTGCTGGCCTAGAGAGCCACGGCCGTACCCCCTCTCCCTCTCCGACCTGCAGCAGCTCTCCCAGCCCCTCTGGCCGGCCTGGCCCCTTCTCCAGCAACAGGTACGGTGCCTCGGTCCCCATTCCTGTCCCCACTCAGGTGCACAATTACCAGCGCATCGAGCAAAACCTGCAATCGCCCACTCAACAGCAGACAGCCAGGTCCTCTGCCATCCGAAGGTCAGGGAGCACCAGCCCCCTGGGCTTTGGCCGGGCCAGCCCATCACCCCCCTCCCACACCGATGGAGCCATGCTGGCCAGGAAGCTGTCACTTGGAGGTGGCCGTCCCTACACACCTTCTCCCCAAGTGGGAACCATCCCAGAGCGACCCAGCTGGAGCAGAGTGCCCTCCCCACAAGGAGCTGATGTGCGGGTTGGCAGGTCACCACGACCCGGTTCCTCTGTGCCTGAGCACTCTCCAAGAACCACTGGGCTGGGCTGCCGCCTGCACAGTGCCCCTAACCTGTCCGACTTCCATGTTGTGCGTCCCAAGCTGCCTAAGCCCCCAACAGACCCACTGGGAGCCACCTTTAGCCCACCCCAGACCAGCGCACCCCAGCCATGCCCAGGGCTACAGTCTTGCCGGCCACTGCGTGGCTCACCTAAGCTGCCTGACTTCCTACAGCGGAGTCCCCTACCCCCCATCCTAGGCTCTCCTACCAAGGCCGGGCCCTCCTTTGACTTCCCCAAAACCCCCAGCTCTCAGAATTTGCTGACCCTGTTGGCTAGGCAGGGGGTAGTAATGACACCACCTCGGAACCGTACACTGCCTGACCTCTCCGAGGCCAGTCCTTTCCATGGCCAGCAGCTGGGCTCTGGCCTTCGGCCCGCTGAAGACACCCGGGGTCCCTTTGGACGGTCCTTCAGCACCAGCCGCATTACGGACCTGCTGCTTAAGGCTGCATTTGGGACTCAGGCCTCTGACTCAGGCAGCACAGACAGCCTACAGGAGAAACCTATGGAGATTGCTCCCTCTGCTGGCTTTGGAGGGACTCTGCATCCAGGAGCTCGTGGTGGAGGGGCCAGCAGCCCAGCACCTGTGGTATTTACTGTAGGCTCCCCACCCAGTGGTGCCACCCCACCCCAGAGTACCCGTACCAGAATGTTCTCAGTGGGCTCTTCCAGCTCCCTGGGCTCTACTGGCTCCTCCTCTGCCCGCCACTTAGTGCCTGGGGCCTGTGGAGAGGCCCCGGAGCTTTCTGCCCCAGGCCACTGCTGTAGCCTTGCTGACCCCCTTGCTGCCAACTTGGAGGGGGCTGTGACCTTCGAGGCTCCTGACCTCCCAGAGGAGACCCTCATGGAGCAAGAGCACACGGAAACCCTACACAGTCTGCGCTTCACACTAGCGTTTGCACAGCAAGTTCTGGAGATTGCAGCCCTGAAGGGAAGTGCCAGTGAGGCCGCCGGTGGCCCTGAGTACCAGCTCCAGGAAAGTGTGGTGGCTGACCAGATCAGTCAGTTGAGCCGAGAGTGGGGCTTTGCAGAGCAACTGGTTCTGTACTTGAAGGTGGCTGAGCTGCTGTCCTCAGGCCTACAGACTGCCATTGACCAGATTCGAGCTGGCAAACTCTGCCTTTCATCTACTGTGAAGCAGGTGGTACGCAGACTAAATGAGCTGTACAAGGCCAGCGTGGTATCCTGCCAGGGCCTCAGCTTGCGACTTCAGCGCTTCTTTCTGGACAAACAACGGCTGCTGGACGGGATCCATGGTGTCACTGCAGAGCGGCTCATCCTCAGCCATGCTGTGCAAATGGTACAATCAGCTGCCCTTGATGAGATGTTCCAGCACCGAGAGGGCTGTGTACCGAGATATCACAAAGCCCTGCTATTGCTGGAGGGGTTGCAGCACACTCTCACGGACCAGGCAGACATTGAGAACATTGCCAAATGCAAGCTGTGCATTGAGAGGAGACTCTCGGCCCTGCTGAGTGGTGTCTATGCCTGA |
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT)
assay
Cells were prepared into 1×104 cell/ml
cell suspension using Dulbecco's modified Eagle's medium containing
10% fetal bovine serum (BioSer) and cultured in a 96-well plate.
Then, 8 wells (100 µl) were set for each group and culture was
implemented in a 37°C cell incubator with 5% CO2.
Subsequent to 24-h culture, the plate was removed and 10 µl MTT
(Sigma-Aldrich; Merck KGaA) was added into each well for 2-h
culture. The optical density value at 570 nm was read using an
enzyme-linked immunoassay (cat. no. NYW-96M; Beijing. Noahway
Instruments Co., Ltd.). The cell survival rate was calculated as
follows: Cell survival rate=(experimental group OD-blank group
OD)/(control group OD-blank group OD)x100%.
Flow cytometry
Cells were trypsinized in the absence of ethylene
diamine tetraacetic acid and attained into a flow tube. Subsequent
to cell centrifugation, the supernatant was discarded. As per the
protocols of Annexin V-fluorescein isothiocyanate (FITC) apoptosis
detection kit (cat. no. C1065; Beyotime Institute of
Biotechnology), Annexin V-FITC, phosphatidyl inositol (PI),
hydroxyethyl piperazine ethylsulfonic acid buffer was added to
Annexin V-FITC/PI staining solution in the proportion of 1:2:50.
Cells at a concentration of 1×106 cells per 100 µl of
the staining solution were resuspended, incubated at ambient
temperature for 15 min in the dark and added with 1 ml of
4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid buffer. Flow
cytometer (Bio-Rad ZE5; Bio-Rad Laboratories, Inc.) was employed to
determine the excitation wavelength at 488 nm. The excitation
wavelength at 525 or 620 nm was employed to detect FITC or PI
fluorescence for cell apoptosis, respectively. The samples (n=10)
were randomly selected in each group. Apoptosis index (AI)=number
of apoptotic cells/(number of apoptotic cells + normal cells).
Statistical analysis
Statistical analysis was performed using SPSS 21.0
(IBM Corp.). Measurement data were summarized by mean ± standard
deviation or inter-quartile range. An unpaired t-test was
performed for the variance of data consistent with the normal
distribution and homogeneity of variance. One-way analysis of
variance (ANOVA) was conducted for comparison among multiple
groups, followed by Tukey's post-hoc test. Data at different
time-points were compared using repeated-measures ANOVA, followed
by Bonferroni post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-128 is downregulated following
SCI
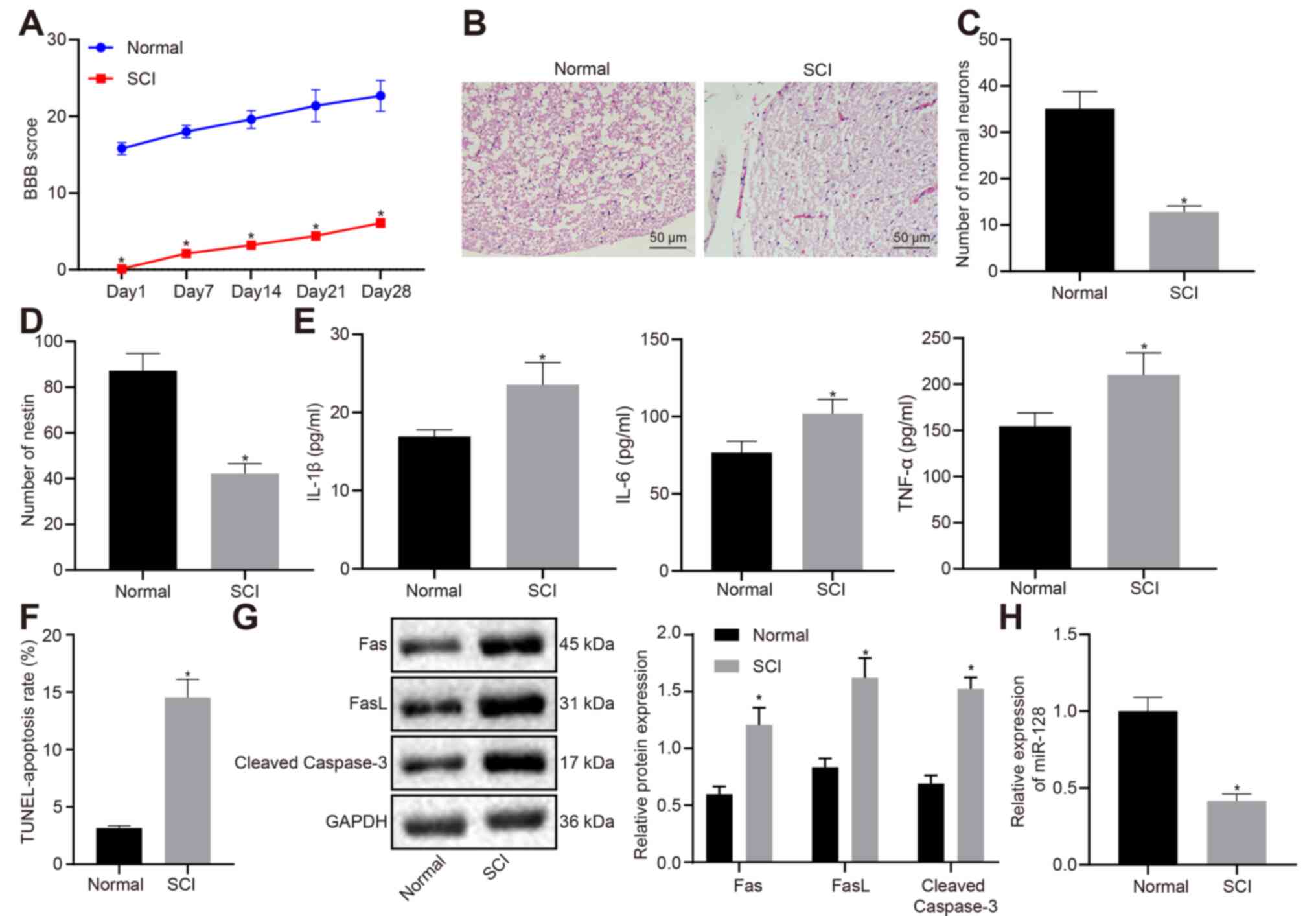
Rat models with SCI were induced and the BBB score
was calculated from the normal rats and SCI rats on day 1, 3, 5, 7
and 14 after modeling (Fig. 1A). A
markedly lower BBB score was seen in rats with SCI when compared
with normal rats. The results of H&E staining (Fig. 1B) showed that there were no
significant pathological changes in normal rats, whereas rats with
SCI exhibited distinct white matter edema in the spinal cord,
microvascular rupture or swelling in the gray matter with focal
bleeding, degeneration, and necrosis. Nissl staining (Fig. 1C) and immunofluorescence (Fig. 1D) indicated that neurons and
nestin-positive cells were both markedly reduced in rats with SCI
compared to normal rats. ELISA (Fig.
1E) demonstrated that the levels of IL-1β, IL-6 and TNF-α in
serum notably rose in rats with SCI in comparison with those in
normal rats. TUNEL results (Fig.
1F) revealed that apoptotic cells in rats with SCI were
significantly increased in rats with SCI in comparison with those
in normal rats. Meanwhile, western blot analysis demonstrated that
the expression of apoptosis-related factors, Fas, FasL and cleaved
caspase-3 rose markedly in rats with SCI compared with those in
normal rats (Fig. 1G). The above
results implied that neurons in rats with SCI exhibited promoted
apoptosis, accompanied by inflammation. RT-qPCR (Fig. 1H) demonstrated that miR-128
expression in rats with SCI markedly declined compared with that in
normal rats.
Overexpression of miR-128 attenuates
SCI in rats
To confirm whether the changes in miR-128 expression
affected the apoptosis and inflammatory response of rats with SCI,
vectors including LV-control, LV-miR-128, or LV-sponge miR-128 were
first constructed and injected into rats with SCI. As shown in
Fig. 2A, RT-qPCR revealed that SCI
rats injected with LV-sponge miR-128 exhibited markedly
downregulated miR-128 levels, but an opposite trend was observed in
SCI rats injected with LV miR-128 compared with SCI rats infected
with LV-control (P<0.05). SCI Rats expressing LV-miR-128 showed
a significantly higher BBB score while rats transfected with
LV-sponge miR-128 showed significantly lower BBB scores in
comparison with rats with SCI expressing LV-control (P<0.05;
Fig. 2B). Results from H&E
staining revealed that SCI rats infected with LV-miR-128 showed
alleviated pathological changes but SCI rats infected with
LV-sponge miR-128 exhibited aggravated pathological changes when
compared to rats infected with LV-control (P<0.05; Fig. 2C). The results of Nissl staining
(Fig. 2D) and immunofluorescence
(Fig. 2E) indicated that the number
of normal neurons and nestin-positive cells of SCI rats injected
with LV-miR-128 was significantly increased, but an opposite trend
was observed in SCI rats injected with LV-sponge miR-128 in
comparison with rats injected with LV-control (P<0.05). The
results of ELISA (Fig. 2F)
indicated that the serum levels of IL-1β, IL-6 and TNF-α in SCI
rats infected with LV-miR-128 were significantly lower in
comparison with those transduced with LV-control, whereas SCI rats
infected with LV-sponge miR-128 showed a significant increase
(P<0.05). TUNEL staining (Fig.
2G) demonstrated that SCI rats infected with LV-miR-128 showed
a strikingly reduction in cell apoptosis while SCI rats infected
with LV-sponge miR-128 showed a notable increase in apoptosis
compared to rats injected with LV-control (P<0.05). Western blot
analysis (Fig. 2H) demonstrated
that Fas, FasL and cleaved caspase-3 expression in SCI rats
infected with LV-miR-128 declined, while an opposite trend was
observed in SCI rats infected with LV-sponge miR-128 in compared
with those transduced with LV-control (P<0.05). These above
results suggested that the highly expressed miR-128 could inhibit
apoptosis and the inflammatory response in SCI rats.
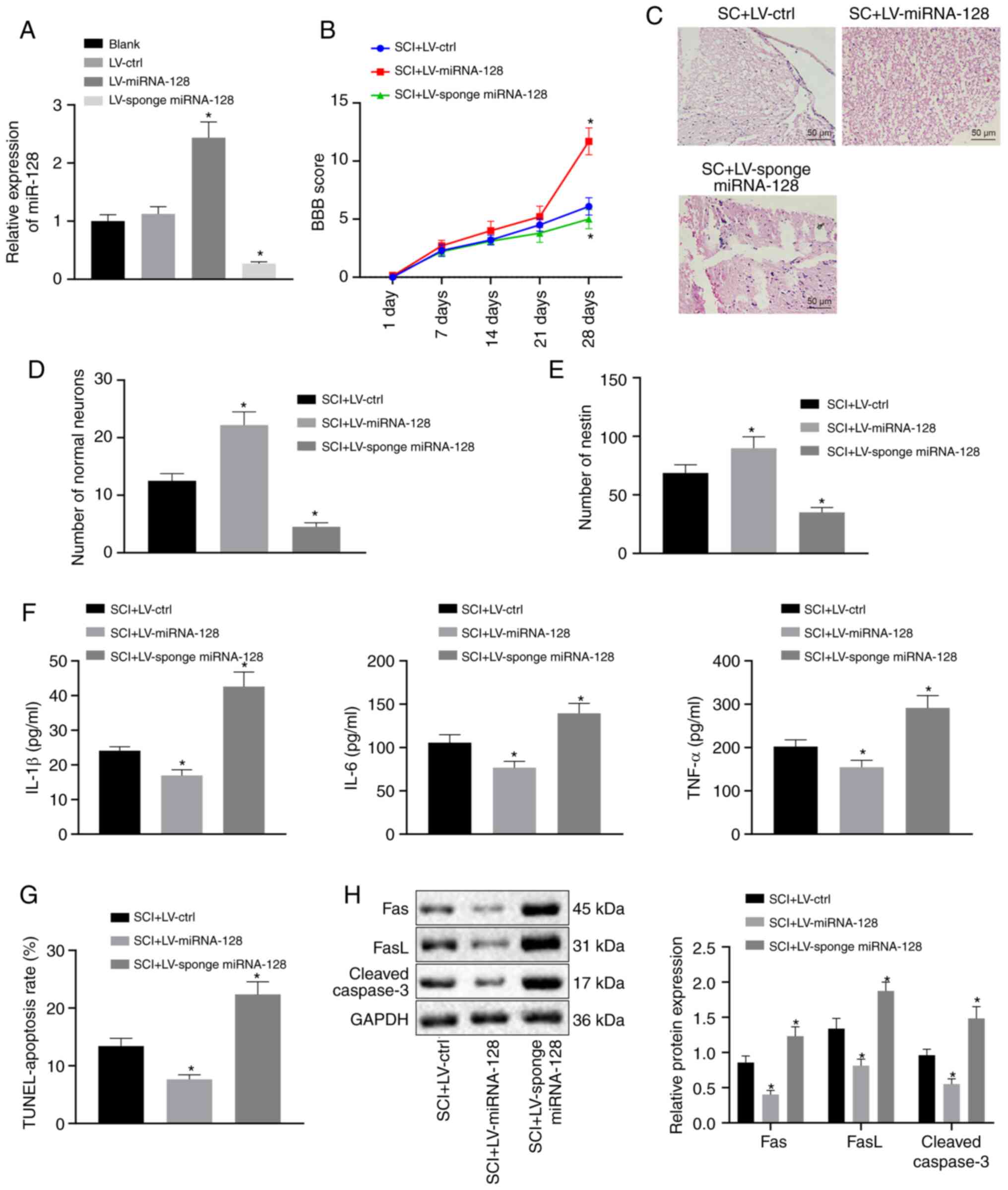 | Figure 2.Upregulation of miR-128 can alleviate
SCI in rats. Vectors containing LV-control, LV-miR-128, or
LV-sponge miR-128 were injected into rats with SCI (n=10/group).
(A) miR-128 expression was assessed by reverse
transcription-quantitative PCR. *P<0.05 vs. SCI rats injected
with LV-control. (B) Locomotor functions of rats indicated by BBB
score. (C) Hematoxylin and eosin staining of morphological changes
of neuronal cells (magnification, ×200). (D) The survival of
neuronal cells in rat spinal cord determined by Nissl staining. (E)
The number of nestin-positive cells evaluated by
immunofluorescence. (F) The levels of inflammatory factors in rat
serum detected by ELISA. (G) Cell apoptosis was assessed by TUNEL
staining. (H) The expression of apoptosis-related factor protein in
rat spinal cord determined by western blot analysis. An unpaired
t-test was performed for comparisons of data between two
groups. ANOVA was performed for comparisons of data among multiple
groups, followed by Tukey's post-hoc test. BBB score was used as
the comparisons of different time points using repeated-measures
analysis of ANOVA, followed by Bonferroni post-hoc test. *P<0.05
vs. SCI rats infected with LV-control. miR, microRNA; SCI, spinal
cord injury; LV, Lentiviral vector; ctrl, control; BBB, Basso,
Beattie and Bresnahan; ANOVA, analysis of variance. |
ULK1 is targeted by miR-128 and
silencing of ULK1 relieves SCI in rats
RNAInter, miRDB, TargetScan and miRWalk were
employed to predict the target genes of miR-128. The results
revealed that there were three genes appearing in the intersection,
which consisted of ULK1, ABCB9 and SGMS1 (Fig. 3A). To further verify the predicted
results, vectors containing LV-control, LV-miR-128, or LV-sponge
miR-128 were injected into SCI rats. RT-qPCR and western blot
analysis demonstrated that ULK1 expression was upregulated in rats
with SCI (P<0.05; Fig. 3B and
C). The binding site between ULK1 and miR-128 was predicted
using the bioinformatic website TargetScan Human version 7.2
(Fig. 3D). Results from
dual-luciferase report assay confirmed that luciferase activity in
the ULK1 WT was inhibited by miR-128 mimic, while ULK1 MUT was not
affected (P<0.05; Fig. 3E).
Furthermore, RT-qPCR and western blot analysis indicated that ULK1
expression was markedly repressed in SCI rats infected with
LV-miR-128 but was elevated in SCI rats injected with LV-sponge
miR-128 (P<0.05; Fig. 3F and G).
All these results suggested that miR-128 could target and repress
the expression of ULK1.
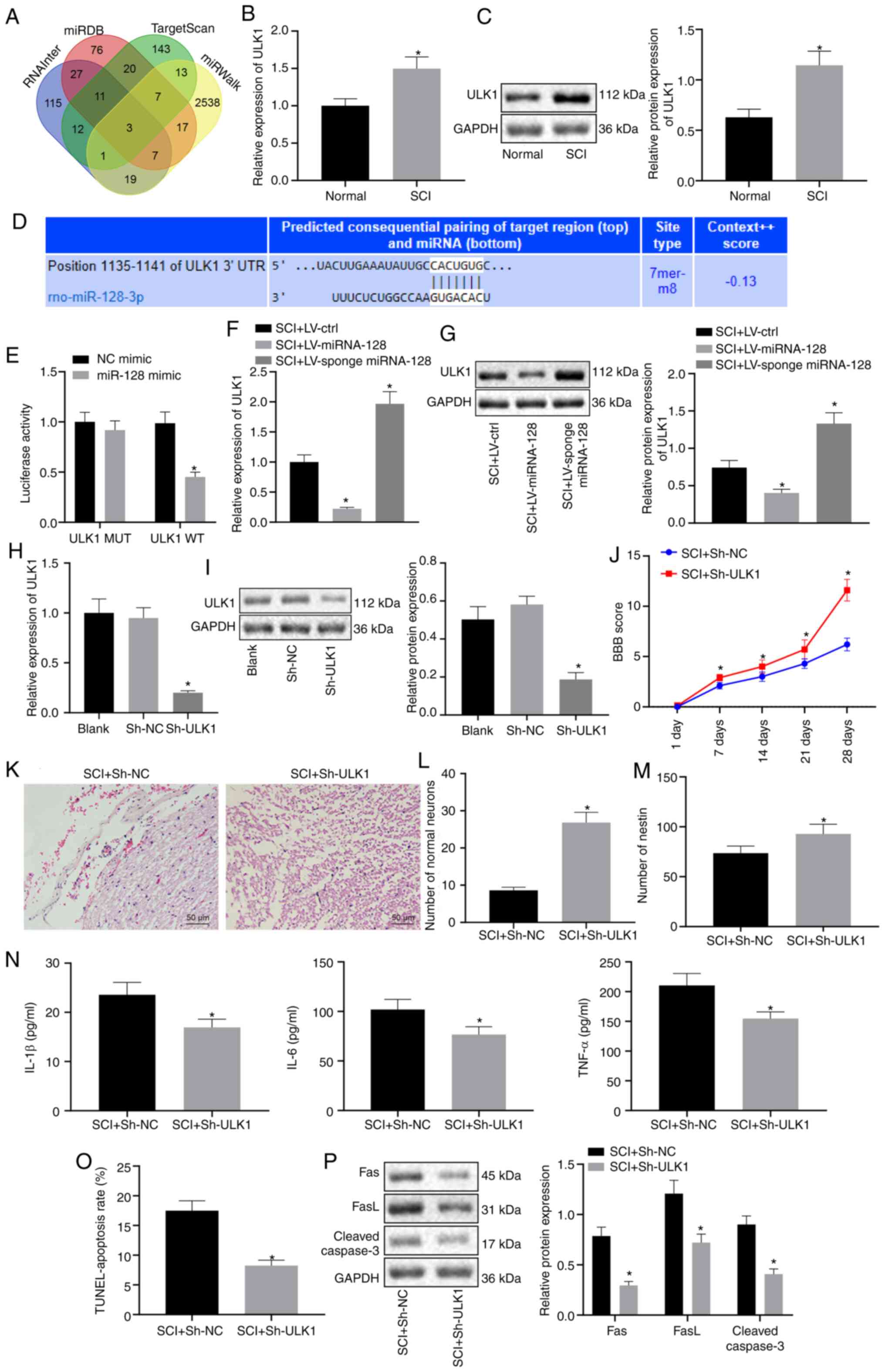 | Figure 3.miR-128 inhibits the expression of
ULK1 and knockdown of ULK1 attenuates SCI in rats. Vectors
containing LV-control, LV-miR-128, or LV-sponge miR-128 were
constructed and injected into rats with SCI in A-G, while vectors
containing sh-NC or sh-ULK1 were injected into rats with SCI in H-P
(n=10/group). (A) Venn diagram of the target genes of miR-128
predicted using RNAInter, miRDB, TargetScan and miRWalk. (B) The
mRNA expression of ULK1 in normal rats and rats with SCI detected
using RT-qPCR. *P<0.05 vs. normal rats. (C) Protein expression
of ULK1 detected by western blot analysis. *P<0.05 vs. normal
rats. (D) The binding site of miR-128 and ULK1 predicted using
TargetScanHuman 7.2. (E) The targeting relationship between ULK1
and miR-128 measured by dual-luciferase report assay. *P<0.05
vs. NC mimic. (F) The mRNA expression of ULK1 examined by RT-qPCR.
*P<0.05 vs. SCI rats injected with LV-control. (G) The protein
expression of ULK1 measured using western blot analysis. *P<0.05
vs. SCI rats infected with LV-control. (H) The mRNA expression of
ULK1 measured using RT-qPCR. *P<0.05 vs. Blank or sh-NC. (I) The
protein expression of ULK1 expressing sh-NC and sh-ULK1 evaluated
by western blot analysis. *P<0.05 vs. Blank or sh-NC. (J)
Locomotor functions of rats evaluated by BBB score. (K) H&E
staining of morphological changes of neurons (magnification, ×200).
(L) Survival of neuronal cells detected by Nissl staining. (M) The
number of nestin-positive cells detected by immunofluorescence. (N)
The content of inflammatory factors in rat serum determined by
ELISA. (O) Apoptosis of rat spinal cord cells determined using
TUNEL. (P) The protein expression of apoptosis-related factor in
rats with SCI evaluated by western blot analysis. J-P, *P<0.05
vs. SCI rats transfected with sh-NC. An unpaired t-test was
performed for comparisons of data between two groups. ANOVA was
performed for comparisons of data among multiple groups, followed
by Tukey's post-hoc test. BBB score was used as comparisons of data
at different time points using repeated-measures analysis of ANOVA,
followed by Bonferroni post-hoc test. miR, microRNA; ULK1, Unc-51
like autophagy activating kinase 1; SCI, spinal cord injury;
RT-qPCR, reverse transcription-quantitative PCR; NC, negative
control; LV, Lentiviral vector; ctrl, control; sh, short hairpin;
ANOVA, analysis of variance. |
To further explore the effect of the changes in ULK1
expression on SCI rats, vectors containing sh-NC or sh-ULK1 was
constructed and injected into SCI rats (Fig. 3H and I). Results showed that SCI
rats transfected with sh-ULK1 exhibited significant higher BBB
scores (P<0.05; Fig. 3J). In
addition, H&E staining revealed that SCI rats transfected with
sh-ULK1 exhibited less severe pathological changes (P<0.05;
Fig. 3K).
The results from Nissl staining (Fig. 3L) and immunofluorescence (Fig. 3M) demonstrated that the number of
normal neurons and nestin-positive cells were notably increased in
SCI rats transfected with sh-ULK1 (P<0.05). ELISA demonstrated
that the levels of IL-1β, IL-6 and TNF-α in SCI rats transfected
with sh-ULK1 exhibited a notable decline (P<0.05; Fig. 3N). The results of TUNEL (Fig. 3O) showed that reduced apoptosis was
observed in SCI rats transfected with sh-ULK1 (P<0.05). Western
blot analysis and the results showed that protein expression of
Fas, FasL and Cleaved Caspase-3 in SCI rats transfected with
sh-ULK1 was significantly lowered (P<0.05; Fig. 3P). Taken together, these results
suggested that miR-128 could inhibit the expression of ULK1, thus
relieving SCI in rats.
Elevation of miR-128 downregulates
ULK1, thereby attenuating SCI via FasL repression in vitro
To verify the miR-128/ULK1 regulatory mechanism
in vitro, fetal rat spinal cord neuronal cells were isolated
and cultured. The isolated spinal cord neuronal cells began to
adhere following 3–6 h of culture, accompanied by protruded cells.
Following 2–3 d of culture, the cell began to bulge forcefully and
the connection between cells became reticular. Spinal cord neuronal
cells were successfully isolated and validated by
immunofluorescence testing for nestin antibody staining (Fig. 4A and B). The neuronal cells were
transfected with inhibitor NC + sh-NC, miR-128 inhibitor + sh-NC,
or miR-128 inhibitor + sh-ULK1. RT-qPCR and western blot analysis
(Fig. 4C and D) demonstrated that
miR-128 expression in neuronal cells transfected with miR-128
inhibitor + sh-NC was significantly reduced, while the mRNA and
protein expression of ULK1 was significantly increased in
comparison with neuronal cells transfected with inhibitor NC +
sh-NC. In addition, miR-128 expression in neuronal cells
transfected with miR-128 inhibitor + sh-ULK1 remained unchanged,
whereas mRNA and protein expression of ULK1 were both downregulated
in comparison with those in neuronal cells transfected with miR-128
inhibitor + sh-NC. The results of MTT assay (Fig. 4E) demonstrated that the neuronal
cell viability was markedly reduced by miR-128 inhibitor, which was
negated by additional treatment with sh-ULK1. Flow cytometry
demonstrated that the apoptosis rate of neuronal cells was
augmented by miR-128 inhibitor, which was annulled by further
sh-ULK1 treatment (Fig. 4F). As
reflected by western blot analysis, the protein expression of Fas,
FasL and cleaved caspase-3 was significantly enhanced in neuronal
cells following miR-128 inhibitor treatment, which was abrogated by
additional sh-ULK1 treatment (Fig.
4G). Meanwhile, the ChIP assay revealed that there was
significant enrichment of ULK1 and FasL (Fig. 4H). Results from the dual-luciferase
report assay showed that the cells transfected with oe-NC or
oe-ULK1 had no significant difference in fluorescence intensity
after co-transfection with FasL MUT. Compared with the cells
transfected with oe-NC, cells transfected with oe-ULK1- and FasL WT
exhibited an increase in fluorescence intensity (Fig. 4I). Together, the elevation of
miR-128 could downregulate ULK1, thereby attenuating SCI via FasL
downregulation.
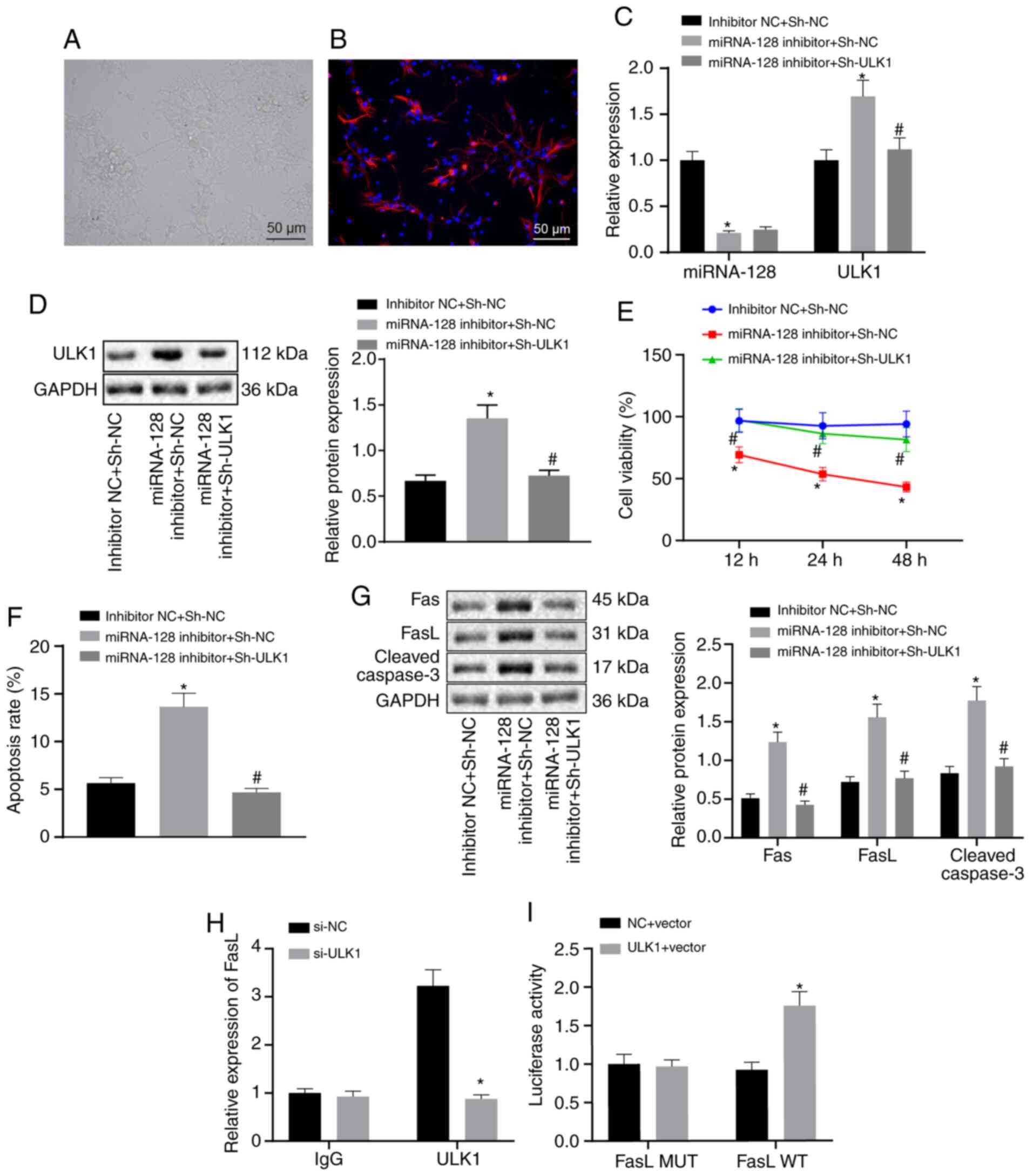 | Figure 4.Upregulation of miR-128 repressed
ULK1 expression and thereby attenuating SCI via the downregulation
of FasL. The neuronal cells were transfected with inhibitor NC and
sh-NC, miR-128 inhibitor and sh-NC or miR-128 inhibitor and
sh-ULK1. (A) The morphology of spinal cord neurons observed under
an inverted microscope (magnification, ×200). (B) Neuronal cell
nestin-positive expression determined by immunofluorescence
(magnification, ×200). (C) The expression of miR-128 and ULK1
detected using reverse transcription-quantitative PCR. (D) The
protein expression of ULK1 measured by western blot analysis. (E)
Cell proliferation assessed by MTT assay. (F) Cell apoptosis
determined by flow cytometry. (G) The protein expression of
apoptosis-related factors determined by western blot analysis. (H)
The enrichment of ULK1 and FasL determined by ChIP assay. (I) The
regulatory relationship of ULK1 on FasL assessed using
dual-luciferase reporter assay. *P<0.05 vs. cells co-transfected
with inhibitor NC and sh-NC or #P<0.05 vs. cells
co-transfected with miR-128 inhibitor and sh-NC, or cells
co-transfected with si-NC. An unpaired t-test was performed
for comparisons of data between two groups. ANOVA was conducted for
comparison of data among multiple groups, followed by Tukey's
post-hoc test. miR, microRNA; ULK1, Unc-51 like autophagy
activating kinase 1; SCI, spinal cord injury; FasL, Fas ligand; NC,
negative control; sh, short hairpin; WT, wild type; MUT, mutated;
ChIP, Chromatin immunoprecipitation; IgG, immunoglobulin G. |
Discussion
SCI causes permanent disruption and significantly
destroys the central nervous system function, curbing the
capability of patients to regenerate lost tissues (16). The differentiation of neural stem
cells can contribute to neuronal differentiation, thereby
attenuating SCI (17). A previous
study demonstrated that the aberrant expression of miRNA following
SCI has emerged as a potent research priority (18). The present study found evidence to
support the hypothesis that the upregulation of miR-128 contributed
to inhibited cell apoptosis and inflammation, as well as improved
motor function following SCI via elevation of FasL by targeting and
suppressing ULK1.
Initially, the results of the present study
demonstrated that miR-128 was poorly expressed in rats with SCI and
neuronal cells from SCI. The overexpression of miR-128 resulted in
inhibited neuronal cell apoptosis but contributed to improved motor
function and inflammation, thereby alleviating SCI as evidenced by
higher BBB score and abundant normal neurons and nestin-positive
cells. However, it was also evidenced that the expression levels of
IL-1β, IL-6 and TNF-α, as well as protein expression of Fas, FasL
and cleaved caspase-3, were notably decreased. A similar finding
was mentioned in a previous study, whereby in conditioned
medium-SCI-treated mouse microglia BV2, miR-128 expression is
downregulated and upregulation of miR-128 contributes to an
improved survival rate of microglia cells and reduces the
concentration of inflammatory cytokines (TNF-α, IL-1β and IL-6)
(6).
In addition, miR-128 was associated with the
development of the central nervous system and spinal cord
neuroepithelial cells (19).
Consistent with the results of the present study, SCI gives rise to
increased neuronal apoptosis, resulting in irreversible
neurological dysfunction of the spinal cord (20). The reduced neuronal death following
SCI has been demonstrated to be positively correlated to improved
motor function recovery (21). The
inhibition of neuronal cell apoptosis has been shown to be
favorable to protect the damaged spinal cord neurons (22). Increased expression of TNF-α, IL-6
and IL-1β is indicative of a more severe degree of SCI, whereas the
increased expression of apoptotic protein caspase-3 aggravates
inflammation and apoptosis, which further results in repressed
functional recovery (23). Fas/FasL
signal transduction pathway is an important pathway to mediate cell
apoptosis (24). The activation of
the Fas/FasL system has been shown to contribute to ovarian cell
apoptosis and impels oocyte capacity (25). Meanwhile, knockdown of FasL been
shown to inhibit miR-21-induced apoptosis of keloid fibroblasts
(26). In addition, an increased
number of nestin-positive cells is indicative of attenuated SCI
(27).
The results of previous study showed that ULK1 was
negatively targeted by miR-128 and silencing of ULK1 resulted in
attenuated SCI in rats, accompanied by inhibited neuronal cell
apoptosis, improved motor function and inflammation, thereby
alleviating SCI. These results were indicated by higher BBB score
and more normal neurons, but notably decreased expression levels of
IL-1β, IL-6 and TNF-α, as well as protein expression of Fas, FasL,
cleaved caspase-3. A previous study demonstrated that miR-372 can
inhibit human pancreatic adenocarcinoma cell proliferation,
migration, invasion, and autophagy by suppressing the expression of
ULK1 (28). In addition, the
overexpression of miR-26a-5p reduces ULK1 expression, whereas the
inhibition of miR-26a-5p can elevate ULK1 expression (29). Absence of ULK1 results in an
increased number of viable neurons in the hippocampus of traumatic
brain injury in mice, thereby reducing nerve inflammation (7). The knockdown of ULK1 enhances the cell
viability of neurons in a Parkinson's disease model (30).
In addition, the present study found that ULK1
positively regulated FasL expression and the downregulation of FasL
contributed to attenuating SCI. Following the activation of hypoxic
microglia, overexpression of FasL has been demonstrated to lead to
neuronal apoptosis (31). miR-411
inhibits acute SCI by suppressing FasL expression in rats (32). Activation of Fas/FasL has a
significant function in mediating apoptosis, inflammatory response
and neurodegeneration following SCI (11). Another study also noted that FasL
expression is increased in acute SCI and its repression could
attenuate acute SCI via downregulation in rats (32).
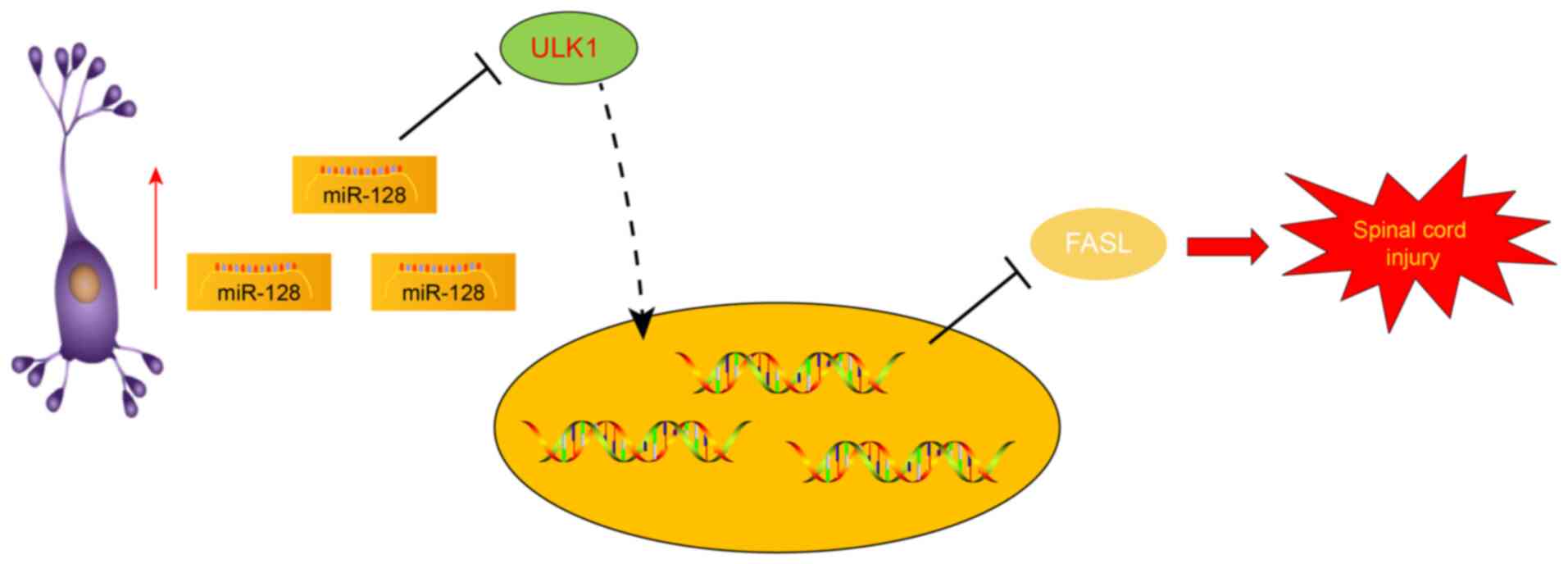
In conclusion, the present study revealed that the
upregulation of miR-128 could suppress ULK1 to inhibit the
apoptosis of neuronal cells, thereby alleviating SCI via the
downregulation of FasL (Fig. 5).
The current study provided further insights into the pathological
mechanism of SCI and proposed a novel feasible option for the
treatment of SCI. However, the potential molecular mechanism of
miR-128 is expected to be implemented in the clinical setting in a
future study.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and RLiu designed the study. ZP, YZ and RLi
collated the data, carried out data analyses and produced the
initial draft of the manuscript. YW and RLiu contributed to
drafting the manuscript. ZP and YZ confirmed the authenticity of
all the raw data. All authors have read and approved the final
submitted manuscript.
Ethics approval and consent to
participate
All animal experiments were implemented after
ratification by the Animal Ethics Committee of the First Affiliated
Hospital of Harbin Medical University (approval no. 2019010)
conforming to Principles and Procedures of the National Academy of
Animal Health Care Guidelines. All efforts were made to minimize
animal suffering and the minimum number of animals used.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SCI
|
spinal cord injury
|
|
miR
|
microRNA
|
|
TUNEL
|
Terminal deoxynucleotidyl
transferase-mediated dUTp nick end-labeling
|
|
BBB
|
Basso, Beattie and Bresnahan
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
H&E
|
Hematoxylin and eosin
|
|
ULK1
|
Unc-51 like autophagy activating
kinase 1
|
|
LV
|
Lentiviral vector
|
|
sh-NC
|
short hairpin RNA against negative
control
|
|
sh-ULK1
|
shRNA against ULK1
|
|
WT
|
wild type
|
|
MUT
|
mutated
|
|
oe
|
overexpression
|
|
ChIP
|
Chromatin immunoprecipitation
|
|
IgG
|
immunoglobulin G
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
phosphatidyl inositol
|
|
ANOVA
|
analysis of variance
|
References
|
1
|
Saxena T, Loomis KH, Pai SB, Karumbaiah L,
Gaupp E, Patil K, Patkar R and Bellamkonda RV: Nanocarrier-mediated
inhibition of macrophage migration inhibitory factor attenuates
secondary injury after spinal cord injury. ACS Nano. 9:1492–1505.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramer LM, Ramer MS and Bradbury EJ:
Restoring function after spinal cord injury: Towards clinical
translation of experimental strategies. Lancet Neurol.
13:1241–1256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Assinck P, Duncan GJ, Hilton BJ, Plemel JR
and Tetzlaff W: Cell transplantation therapy for spinal cord
injury. Nat Neurosci. 20:637–647. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang XM, Ma J, Sun Y, Yu BQ, Jiao ZM,
Wang D, Yu MY, Li JY and Fu J: Tanshinone IIA promotes the
differentiation of bone marrow mesenchymal stem cells into
neuronal-like cells in a spinal cord injury model. J Transl Med.
16:1932018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ning B, Gao L, Liu RH, Liu Y, Zhang NS and
Chen ZY: microRNAs in spinal cord injury: Potential roles and
therapeutic implications. Int J Biol Sci. 10:997–1006. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Z, Xu J, Zhu R and Liu L:
Down-regulation of miRNA-128 contributes to neuropathic pain
following spinal cord injury via activation of P38. Med Sci Monit.
23:405–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei HL, Ma SQ and Li CX: Deficiency of
unc-51 like kinase 1 (Ulk1) protects against mice traumatic brain
injury (TBI) by suppression of p38 and JNK pathway. Biochem Biophys
Res Commun. 503:467–473. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu J, Zhu L, Zheng LP, Cui Q, Zhu HH, Zhao
H, Shen ZJ, Dong HY, Chen SS, Wu WZ and Tan JM: Overexpression of
ULK1 represents a potential diagnostic marker for clear cell renal
carcinoma and the antitumor effects of SBI-0206965. EBioMedicine.
34:85–93. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pang J, Han L, Liu Z, Zheng J, Zhao J,
Deng K, Wang F and Zhang Y: ULK1 affects cell viability of goat
Sertoli cell by modulating both autophagy and apoptosis. In Vitro
Cell Dev Biol Anim. 55:604–613. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krzyzowska M, Orlowski P, Baska P, Bodera
P, Zdanowski R and Stankiewicz W: Role of Fas/FasL signaling in
regulation of anti-viral response during HSV-2 vaginal infection in
mice. Immunobiology. 219:932–943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu WR and Fehlings MG: Fas/FasL-mediated
apoptosis and inflammation are key features of acute human spinal
cord injury: Implications for translational, clinical application.
Acta Neuropathol. 122:747–761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao CL, Cui HA and Zhang XR: MiR-543-5p
inhibits inflammation and promotes nerve regeneration through
inactivation of the NF-kB in rats after spinal cord injury. Eur Rev
Med Pharmacol Sci. 23 (3 Suppl):S39–S46. 2019.
|
|
13
|
von Leden RE, Khayrullina G, Moritz KE and
Byrnes KR: Age exacerbates microglial activation, oxidative stress,
inflammatory and NOX2 gene expression, and delays functional
recovery in a middle-aged rodent model of spinal cord injury. J
Neuroinflammation. 14:1612017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takenaga M, Ohta Y, Tokura Y, Hamaguchi A,
Nakamura M, Okano H and Igarashi R: Lecithinized superoxide
dismutase (PC-SOD) improved spinal cord injury-induced motor
dysfunction through suppression of oxidative stress and enhancement
of neurotrophic factor production. J Control Release. 110:283–289.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li H, Ham TR, Neill N, Farrag M, Mohrman
AE, Koenig AM and Leipzig ND: A hydrogel bridge incorporating
immobilized growth factors and neural stem/progenitor cells to
treat spinal cord injury. Adv Healthc Mater. 5:802–812. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Fan C, Xiao Z, Zhao Y, Zhang H, Sun
J, Zhuang Y, Wu X, Shi J, Chen Y and Dai J: A collagen microchannel
scaffold carrying paclitaxel-liposomes induces neuronal
differentiation of neural stem cells through Wnt/β-catenin
signaling for spinal cord injury repair. Biomaterials. 183:114–127.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He J, Zhao J, Peng X, Shi X, Zong S and
Zeng G: Molecular Mechanism of MiR-136-5p targeting NF-kB/A20 in
the IL-17-mediated inflammatory response after spinal cord injury.
Cell Physiol Biochem. 44:1224–1241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Farrell BC, Power EM and Mc Dermott KW:
Developmentally regulated expression of Sox9 and microRNAs 124, 128
and 23 in neuroepithelial stem cells in the developing spinal cord.
Int J Dev Neurosci. 29:31–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shao Z, Lv G, Wen P, Cao Y, Yu D, Lu Y, Li
G, Su Z, Teng P, Gao K, et al: Silencing of PHLPP1 promotes
neuronal apoptosis and inhibits functional recovery after spinal
cord injury in mice. Life Sci. 209:291–299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng G, Zhan Y, Wang H, Luo Z, Zheng F,
Zhou Y, Wu Y, Wang S, Wu Y, Xiang G, et al: Carbon monoxide
releasing molecule-3 alleviates neuron death after spinal cord
injury via inflammasome regulation. EBioMedicine. 40:643–654. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Zhou L, Zheng X and Liu W: Effects
of dexamethasone on autophagy and apoptosis in acute spinal cord
injury. Neuroreport. 29:1084–1091. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong ZX, Feng SS, Chen SZ, Chen ZM and
Chen XW: Inhibition of MSK1 promotes inflammation and apoptosis and
inhibits functional recovery after spinal cord injury. J Mol
Neurosci. 68:191–203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang M and Su P: The role of the Fas/FasL
signaling pathway in environmental toxicant-induced testicular cell
apoptosis: An update. Syst Biol Reprod Med. 64:93–102. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li CY, Li ZB, Kong QQ, Han X, Xiao B, Li
X, Chang ZL and Tan JH: Restraint-induced corticotrophin-releasing
hormone elevation triggers apoptosis of ovarian cells and impairs
oocyte competence via activation of the Fas/FasL system. Biol
Reprod. 99:828–837. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Ren L, Liu W and Xiao Z: MiR-21
regulates the apoptosis of keloid fibroblasts by caspase-8 and the
mitochondria-mediated apoptotic signaling pathway via targeting
FasL. Biochem Cell Biol. 96:548–555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu PG, Hu SL, Hu R, Wu N, Chen Z, Meng H,
Lin JK and Feng H: Functional recovery in rat spinal cord injury
induced by hyperbaric oxygen preconditioning. Neurol Res.
34:944–951. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen H, Zhang Z, Lu Y, Song K, Liu X, Xia
F and Sun W: Downregulation of ULK1 by microRNA-372 inhibits the
survival of human pancreatic adenocarcinoma cells. Cancer Sci.
108:1811–1819. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng L, Lin S and Lv C: MiR-26a-5p
regulates cardiac fibroblasts collagen expression by targeting
ULK1. Sci Rep. 8:21042018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Zhang J and Yang C: UNC-51-like
kinase 1 blocks S6k1 phosphorylation contributes to
neurodegeneration in Parkinson's disease model in vitro. Biochem
Biophys Res Commun. 459:196–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Dong LY, Li YJ, Hong Z and Wei
WS: miR-21 represses FasL in microglia and protects against
microglia-mediated neuronal cell death following hypoxia/ischemia.
Glia. 60:1888–1895. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gong ZM, Tang ZY and Sun XL: miR-411
suppresses acute spinal cord injury via downregulation of Fas
ligand in rats. Biochem Biophys Res Commun. 501:501–506. 2018.
View Article : Google Scholar : PubMed/NCBI
|