Introduction
Liver ischemia/reperfusion (I/R) injury is a common
physiological and pathological process in liver surgery and liver
transplantation. Clinically, liver I/R is often manifested as
damage to liver function, increased incidence of postoperative
complications, and mortality (1).
Activation of Kupffer cells, oxidative stress, apoptosis, and
activation of inflammatory signaling pathways are involved in the
process of liver I/R injury (2). At
present, commonly used clinical treatments include ischemic
preconditioning, pharmacological preconditioning, and ischemic
postconditioning, among others (3),
but the effect is limited. Therefore, studies on the mechanism of
liver I/R injury and its preventive measures are of great clinical
significance for reducing the complications of liver surgery,
improving the success rate of liver transplantation, and promoting
the recovery of postoperative liver function.
MicroRNAs (miRNAs or miRs) are a class of highly
conserved, endogenous, small non-coding RNAs that play important
regulatory roles in a variety of biological processes and usually
inhibit the expression of target genes by binding to the
3′-untranslated regions (3′-UTRs) of target mRNAs (4). In liver I/R injury, miRNAs, such as
miR-125b, miR-128-3p, miR-142-3p, miR-450b-5p, and miR-27a,
participate in the regulation of liver I/R injury through
inflammatory responses, oxidative stress, apoptosis, autophagy, and
energy metabolism (5–9).
miR-140-5p, also known as miR-140, was first
identified in zebrafish in 2005 (10). In 2006, Tuddenham et al
reported that miR-140-5p was a cartilage-specific miRNA in mouse
cells (11). In 2009, Miyaki et
al revealed that miR-140-5p was highly expressed in
differentiated human articular chondrocytes (12), while later studies reported that
miR-140-5p was also expressed in other human tissues. miR-140-5p
has been revealed to be expressed at low levels in various tumor
tissues and play an antitumor role (13–18).
miR-140-5p has been revealed to inhibit inflammatory response
(19–21) and oxidative stress in non-tumor
diseases (22,23), and also be involved in the
regulation of brain I/R injury (24).
The calpain family members are calcium-dependent
proteases that regulate numerous cellular functions by truncating
bound proteins, leading to changes in their properties and
functions (25). Overactivation of
calpain has been revealed to be involved in numerous diseases. The
activity and expression of calpain-1 (CAPN1) has been revealed to
increase after ischemia in the heart, liver, brain and other
organs, while CAPN1 inhibition has been reported to alleviate organ
I/R injury (26–28).
In the present study, the expression and effect of
miR-140-5p in mouse liver tissues following I/R injury and in
H/R-induced AML12 cells were investigated. It was predicted that
CAPN1 may be a target of miR-140-5p, and thus the regulatory effect
of miR-140-5p on CAPN1 was demonstrated. Our novel insights into
miR-140-5p may provide a new therapeutic option for liver I/R
injury.
Materials and methods
Animals and liver I/R model
Male C57BL/6 mice (n=54) weighing 20–24 g (6–8 weeks
old) were purchased from Huaxin Laboratory Animal Co., Ltd. All the
mice were housed under a controlled temperature of 25±2°C, a
humidity of 55±5%, a 12-h light/dark cycle, and free access to
water and food. Animals were allowed to adapt to the environment
for one week before the experiment. Animals were maintained in
accordance with the Guidelines for the Care and Use of Laboratory
Animals (29). The present study
was approved (approval no. 2018-KY-78) by the Medical Ethics
Committee of the First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China).
A 70% hepatic ischemia model was established as
previously described (30).
Briefly, mice were anesthetized by intraperitoneal injection of
pentobarbital sodium (60 mg/kg). Then, the abdominal cavity was
incised along the mid-abdominal line, and microvascular clamps were
used to clip the portal vein, hepatic artery, and bile duct
resulting in 70% liver ischemia. Immediately after occlusion, the
color of the liver lobes changed from dark red to light red,
confirming successful ischemia. After 1 h of ischemia, the clamp
was removed and reperfusion was performed at different time-points.
The sham group underwent the same procedure without the blood
vessel clamps. The mice were anesthetized by intraperitoneal
injection of an overdose of sodium pentobarbital (100 mg/kg). Blood
and liver tissue samples were collected for further analysis after
3, 6, 12, and 24 h of reperfusion. To determine the effect of
miR-140-5p on liver I/R injury, 10 nmol/g of miR-140-5p agonist and
miR-140-5p negative control (NC) (Guangzhou RiboBio Co., Ltd.) were
injected into mice via the tail vein 48 h before I/R.
Cell culture and cell
transfection
The mouse hepatic cell line AML12 was purchased from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China),
and cultured in Dulbecco's Modified Eagle's Medium/Nutrient Mixture
F-12 Medium (DMEM/F12; cat. no. PM150312; Procell Life Science
& Technology Co., Ltd.) supplemented with 10% fetal bovine
serum (FBS; cat. no. 10270-106; Gibco; Thermo Fisher Scientific,
Inc.), 1X ITS (cat. no. ITSS-10201; Cyagen Biotechnology, Inc.), 40
ng/ml dexamethasone (cat. no. D8040; Beijing Solarbio Science &
Technology, Inc.), and 1% penicillin and streptomycin (cat. no.
P1400; Beijing Solarbio Science & Technology, Inc.), in an
incubator containing 5% CO2 at 37°C. AML12 cells were
transfected with 50 nM miR-140-5p mimics (sense,
5′-CAGUGGUUUUACCCUAUGGUAG-3′ and antisense,
5′-CUACCAUAGGGUAAAACCACUG-3′); 50 nM miR-140-5p mimics NC (sense,
5′-UCACAACCUCCUAGAAAGAGUA-3′ and antisense,
5′-UCUACUCUUUCUAGGAGGUUGU-3′); 50 nM miR-140-5p inhibitor
(5′-CUACCAUAGGGUAAAACCACUG-3′), and 50 nM miR-140-5p inhibitor NC
(5′-UCUACUCUUUCUAGGAGGUUGU-3′) (Hanbio Biotechnology Co., Ltd.) for
48 h at 37°C using Lipofectamine 3000 (cat. no. L300-015;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The CAPN1 overexpression pcDNA3.1
plasmid and pcDNA3.1 vector (Hanbio Biotechnology Co., Ltd.) were
transfected alone or co-transfected with miR-140-5p mimics using
Lipofectamine 3000 according to the manufacturer's instructions.
Cells were then subjected to hypoxia/reoxygenation (H/R) 48 h
post-transfection.
Cell H/R model
AML12 cells were first cultured under normal oxygen
concentrations and in a 10% FBS DMEM/F12 medium. To establish the
H/R model, cells were washed twice with PBS (cat. no. P1020;
Beijing Solarbio Science & Technology, Inc.), placed in
DMEM/F12 medium without glucose and serum, and were incubated in a
tri-gas incubator (Eppendorf GmbH) with 5% CO2, 94%
N2 and 1% O2 at 37°C. After 12 h of hypoxia,
the cells were cultured under normal conditions for 3, 6 and 12 h
for reoxygenation.
Cell counting Kit-8 (CCK-8) assay
Cell viability was assessed using the CCK-8 assay
kit [cat. no. HB-CCK8-2; Hanbio Biotechnology (Shanghai) Co.,
Ltd.]. Briefly, ~5,000 cells from miR-140-5p mimics, mimics NC,
miR-140-5p inhibitor and inhibitor NC groups were seeded into
individual wells of 96-well plates, and H/R was performed. After
reoxygenation, 10 µl CCK-8 reagent was added to the AML12 cell
medium, and cells were incubated at 37°C for 1 h. Thereafter, the
absorbance was measured at 450 nm using a microplate reader (Thermo
Fisher Scientific, Inc.).
Lactate dehydrogenase (LDH) release
assay
An LDH release assay was used to assess H/R-induced
cell injury as previously described (31). Cellular supernatants from the H/R,
transfection, and control groups were collected for the LDH assay
(cat. no. A020-2-2; Nanjing Jiancheng Bioengineering Institute),
which was performed according to the manufacturer's instructions.
Optical density (OD) values were measured at 450 nm using a
microplate reader (Thermo Fisher Scientific, Inc.).
Flow cytometric analysis
Cell apoptosis was determined using an Annexin
V-FITC/PI apoptosis detection kit (cat. no. F6012; US
Everbright®, Inc.) according to the manufacturer's
instructions. Briefly, AML12 cells were harvested and resuspended
with 100 µl binding buffer after being washed twice with cold PBS.
Cells were then incubated with 5 µl Annexin V-FITC and 5 µl PI for
15 min in the dark at room temperature. Thereafter, 400 µl binding
buffer was added post-incubation. Apoptosis cells was analyzed
using a flow cytometric system (BD FACSCanto II, BD Biosciences).
BD FACSDiva Software v.3.0.1 (BD Biosciences) was used to analyze
the results.
Prediction of miR-140-5p targets
StarBase version 2.0 (http://starbase.sysu.edu.cn/index.php), miRDB
(http://mirdb.org/index.html), and
TargetScan version 7.2 (http://www.targetscan.org) were used to predict
potential targets and binding sites of miR-140-5p. Genes predicted
by the three databases were considered as potential targets.
Dual-luciferase reporter assay
A dual-luciferase reporter assay was performed to
confirm the interaction between miR-140-5p and CAPN1 3′-UTR.
Luciferase reporter plasmids (pGL3 backbone) containing wild-type
(WT)-CAPN1 or mutant (MUT)-CAPN1 were constructed by Hanbio
Biotechnology Co., Ltd. AML-12 cells were co-transfected with
miR-140-5p mimics or inhibitors with WT-CAPN1 or MUT-CAPN1 using
Lipofectamine 3000. After transfection for 48 h, luciferase
activity was detected using a dual-luciferase reporter assay kit
(cat. no. F6075; US Everbright®. Inc.) according to the
manufacturer's instructions. Relative luciferase activity was
normalized to Renilla luciferase activity.
Alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) assays
ALT and AST levels were assessed in mouse serum
using commercial kits (cat. no. C009-2-1 and C010-2-1; Nanjing
Jiancheng Bioengineering Institute) according to the manufacturer's
instructions.
Hematoxylin and eosin (H&E)
staining
Liver tissues were fixed in 10% formaldehyde
solution at room temperature for 24 h and embedded in paraffin. The
tissue was cut into 5-µm sections and stained with an H&E
staining kit (cat. no. G1120; Beijing Solarbio Science &
Technology, Inc.) at room temperature for 5 min according to the
manufacturer's instructions. Images were captured using a light
microscope (Olympus Corporation).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
TUNEL staining was performed using One Step TUNEL
Apoptosis Assay kit (cat. no. C1086; Beyotime Institute of
Biotechnology) according to the manufacturer's instructions. The
paraffin-embedded tissue was cut into 5-µm sections and stained
with TUNEL reaction mixture containing terminal deoxynucleotidyl
transferase (TdT), fluorescent labeling solution, and TUNEL
detection liquid at room temperature for 1 h in the dark. DAPI (10
µg/ml) was used to stain the nucleus at room temperature for 10 min
followed by the addition of an anti-fluorescence quenching
solution. Images were captured using a fluorescence microscope
(Olympus Corporation). For each sample, >5 areas were randomly
selected for analysis. TUNEL-positive cells in the tissues were
quantified using Image-Pro Plus (version 6.0, Media Cybernetics,
Inc.).
Western blot analysis
Liver tissues or cells were lysed in
radioimmunoprecipitation assay lysis buffer (cat. no. R0010;
Beijing Solarbio Science & Technology, Inc.) according to the
manufacturer's instructions. The protein concentration was
estimated using a bicinchoninic acid (BCA) protein assay kit (cat.
no. PC0020; Beijing Solarbio Science & Technology, Inc.). Equal
amounts of 30 µg protein sample were separated using 10 or 12%
sodium dodecyl sulfate polyacrylamide gel electrophoresis, and the
gel was transferred to 0.45-µm polyvinylidene difluoride (PVDF)
membranes. The membranes were blocked with 5% skim milk for 1 h at
room temperature and incubated overnight at 4°C with primary
antibodies against CAPN1 (1:1,000; cat. no. 10538-1-AP; ProteinTech
Group, Inc.), Bcl-2 (1:800; cat. no. 12789-1-AP; ProteinTech Group,
Inc.), Bax (1:1,000; cat. no. 50599-2-Ig; ProteinTech Group, Inc.),
cleaved caspase-3 (1:1,000; cat. no. WL02117; Wanleibio Co., Ltd.),
and GAPDH (1:5,000; cat. no. 60004-1-Ig; ProteinTech Group, Inc.).
The membranes were washed with 1% TBST (0.05% Tween-20; cat. no.
CR10301; Monad Biotech Co., Ltd.) before and after incubation with
horseradish peroxidase-conjugated goat anti-rabbit antibody
(1:5,000; SA00001-2; ProteinTech Group, Inc.) for 1 h at room
temperature. Protein expression was detected using a ChemiDoc™ MP
Imaging System (Bio-Rad Laboratories, Inc.). Image Lab™ software
(version 5.2.1; Bio-Rad Laboratories, Inc.) was used to quantify
protein expression levels. The relative protein expression was
normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the liver tissues and
AML12 cells using the Triquick reagent (cat. no. R1100; Beijing
Solarbio Science & Technology, Inc.) according to the
manufacturer's instructions, and reverse transcribed into cDNA
using the HiScript II Q RT SuperMix (cat. no. R223-01; Vazyme
Biotech Co., Ltd.) or miRNA First Strand cDNA Synthesis (cat. no.
B532453; Sangon Biotech Co., Ltd.). qPCR analysis was performed
using the ChamQ Universal SYBR qPCR Master Mix (cat. no. Q711-02;
Vazyme Biotech Co., Ltd.) or miRNA Universal SYBR qPCR Master Mix
(cat. no. MQ101-02; Vazyme Biotech Co., Ltd.) according to the
manufacturer's instructions. The thermocycling conditions were 95°C
for 30 sec, 95°C for 5 sec, and 60°C for 35 sec (40 cycles). The
expression of mRNA and miRNA was normalized to the expression of
GAPDH and U6, respectively. Fold change was calculated by the
2−∆∆Cq method (32). The
sequences of the primers used in this experiment are provided in
Table I.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′-3′) |
|---|
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
| miR-140-5p | F:
CGCGCAGTGGTTTTACCCTA |
|
| R:
AGTGCAGGGTCCGAGGTATT |
| IL-6 | F:
AGAGACTTCCATCCAGTTGCC |
|
| R:
TCCTCTGTGAAGTCTCCTCTCC |
| IL-1β | F:
GCTTCAGGCAGGCAGTATCA |
|
| R:
AGTCACAGAGGATGGGCTCT |
| TNF-α | F:
AGCCGATGGGTTGTACCTTG |
|
| R:
ATAGCAAATCGGCTGACGGT |
Statistical analysis
SPSS software (version 22.0; IBM Corp.) was used for
statistical analyses. All experimental results were independently
repeated at least three times. Data are represented as the mean ±
standard deviation (SD). The unpaired Student's t- test was
used to analyze the differences between the two groups. One-way
analysis of variance (ANOVA) with post-hoc Tukey's multiple
comparison test was used to compare differences among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
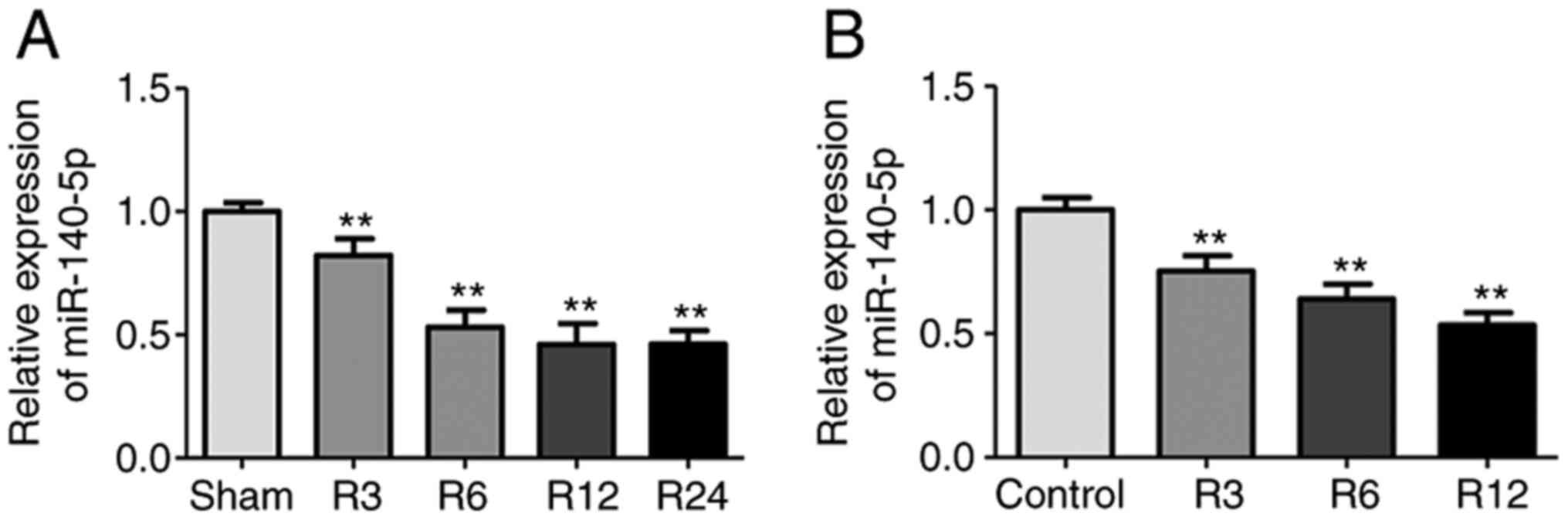
miR-140-5p is decreased in the liver
I/R injury mouse model and in AML12 cells subjected to H/R
To explore the potential role of miR-140-5p in liver
I/R, a mouse model of liver I/R injury and AML12 cells subjected to
H/R were established. Compared with the sham group, the miR-140-5p
expression was significantly downregulated in the liver tissues 6,
12, and 24 h after reperfusion, but there were no statistical
differences among these time-points (Fig. 1A), thus 6 h was selected as the
time-point for further studies. miR-140-5p expression was
significantly downregulated in AML12 cells subjected to H/R
compared with the control group. Furthermore, miR-140-5p expression
decreased gradually as reoxygenation time increased, with the
lowest level observed at 12 h (Fig.
1B). Therefore, 12 h was selected as the time-point for
reoxygenation in the following studies. These results indicated
that miR-140-5p may be involved in liver I/R injury.
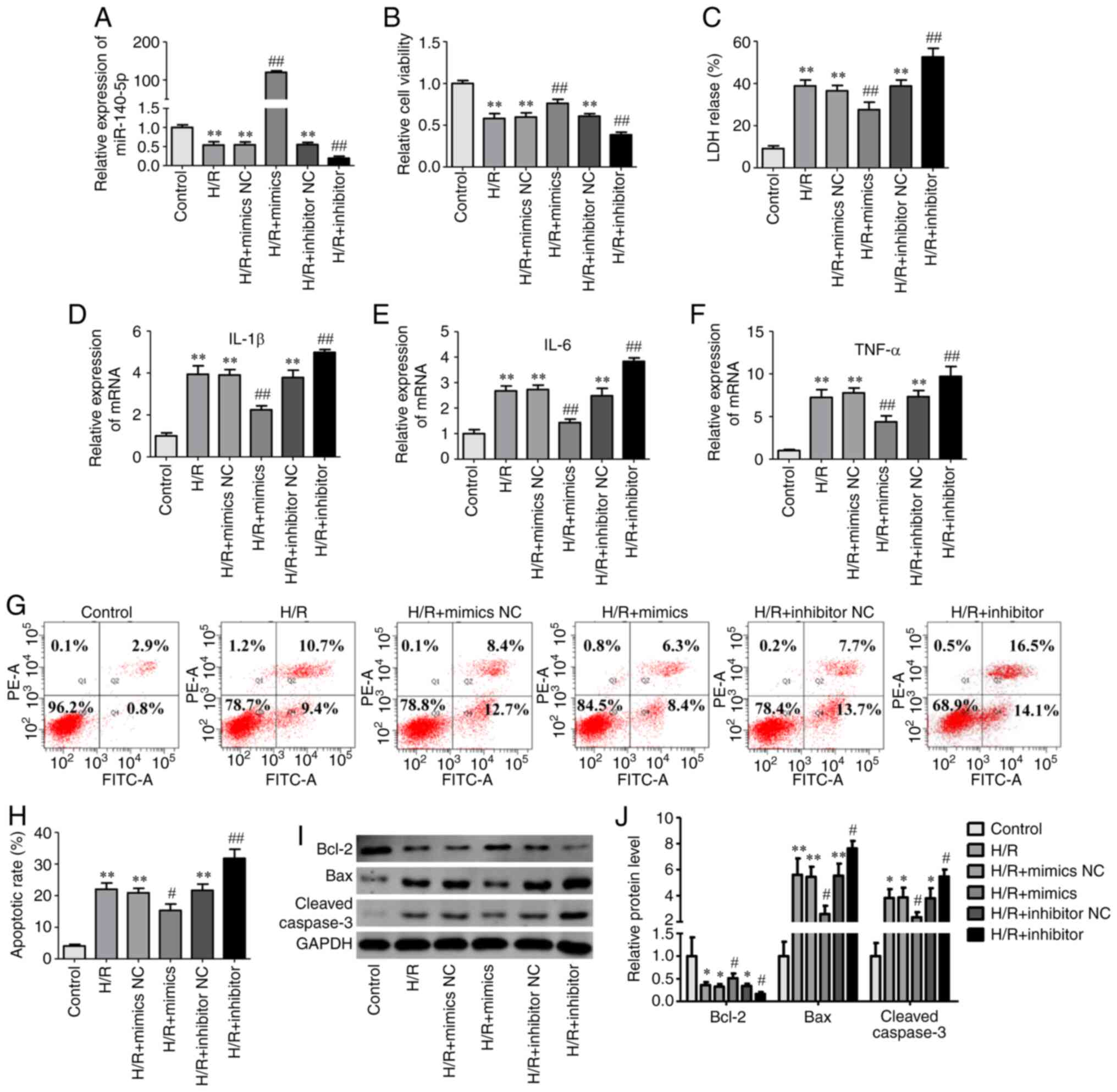
miR-140-5p regulates cell injury in
AML12 cells exposed to H/R
To investigate the effect of miR-140-5p on liver I/R
injury, an H/R model was established using AML12 cells. Cell
viability, LDH release, apoptosis, and expression of inflammatory
cytokines were used to evaluate cell injury. miR-140-5p
mimic/inhibitor and NC groups had no effect on cell viability in
normal conditions (Fig. S1).
miR-140-5p expression was decreased in AML12 cells subjected to H/R
but significantly increased after transfection with miR-140-5p
mimics and significantly decreased after transfection with
miR-140-5p inhibitor (Fig. 2A).
Compared with the control group, the viability of AML12 cells
significantly decreased after H/R (Fig.
2B), while miR-140-5p overexpression significantly increased
the viability of cells exposed to H/R (Fig. 2B). Compared with the control group,
LDH release (Fig. 2C) and
inflammatory cytokines interleukin (IL)-1β, IL-6, and tumor
necrosis factor (TNF)-α (Fig. 2D-F)
significantly increased after H/R. Overexpression of miR-140-5p
significantly decreased LDH release (Fig. 2C) and the expression of inflammatory
cytokines (Fig. 2D-F). Flow
cytometry and western blotting were performed to evaluate cellular
apoptosis. Flow cytometric results indicated that miR-140-5p
overexpression significantly decreased cells apoptosis compared
with the H/R group (Fig. 2G and H).
The levels of apoptosis-related proteins, cleaved caspase-3 and Bax
significantly increased in AML12 cells exposed to H/R, while
anti-apoptosis protein Bcl-2 levels significantly decreased
compared with those in the control group (Fig. 2I and J). Overexpression of
miR-140-5p reversed these changes in apoptosis-related protein
expression (Fig. 2I and J).
However, compared with the H/R group, inhibition of miR-140-5p
expression further reduced cell activity (Fig. 2B) and increased LDH release
(Fig. 2C), the expression of
inflammatory cytokines (Fig. 2D-F),
and cell apoptosis (Fig. 2G-J).
These results indicated that miR-140-5p may protect AML12 cells
against H/R-induced injury.
miR-140-5p overexpression is decreased
liver injury in an I/R injury mouse model
To further elucidate the effect of miR-140-5p on
liver I/R injury, mice were pretreated with a miR-140-5p agonist or
NC via the tail vein before I/R. RT-qPCR analysis revealed that the
miR-140-5p agonist significantly upregulated miR-140-5p expression
in the liver tissues compared with that in the I/R group (Fig. 3A). Liver injury was determined by
employing serum ALT and AST assays, H&E staining, TUNEL assays,
and apoptosis-related protein and tissue inflammation analysis.
Serum ALT and AST levels were significantly decreased in the I/R+
miR-140-5p agonist group compared with those in the I/R group
(Fig. 3B and C). H&E staining
revealed that the miR-140-5p agonist reduced the necrotic area
compared with the I/R group (Fig. 3D
and E). A TUNEL assay revealed a decrease in TUNEL-positive
cells (Fig. 3F and G), and western
blot analysis revealed decreased levels of pro-apoptosis proteins
cleaved-caspase 3 and Bax, and increased anti-apoptosis protein
Bcl-2 in the I/R+ miR-140-5p agonist group compared with those in
the I/R group (Fig. 3H and I).
miR-140-5p agonist also inhibited inflammatory cytokines IL-1β,
IL-6, and TNF-α (Fig. 3J-L). These
results indicated that miR-140-5p effectively alleviated liver
injury caused by I/R.
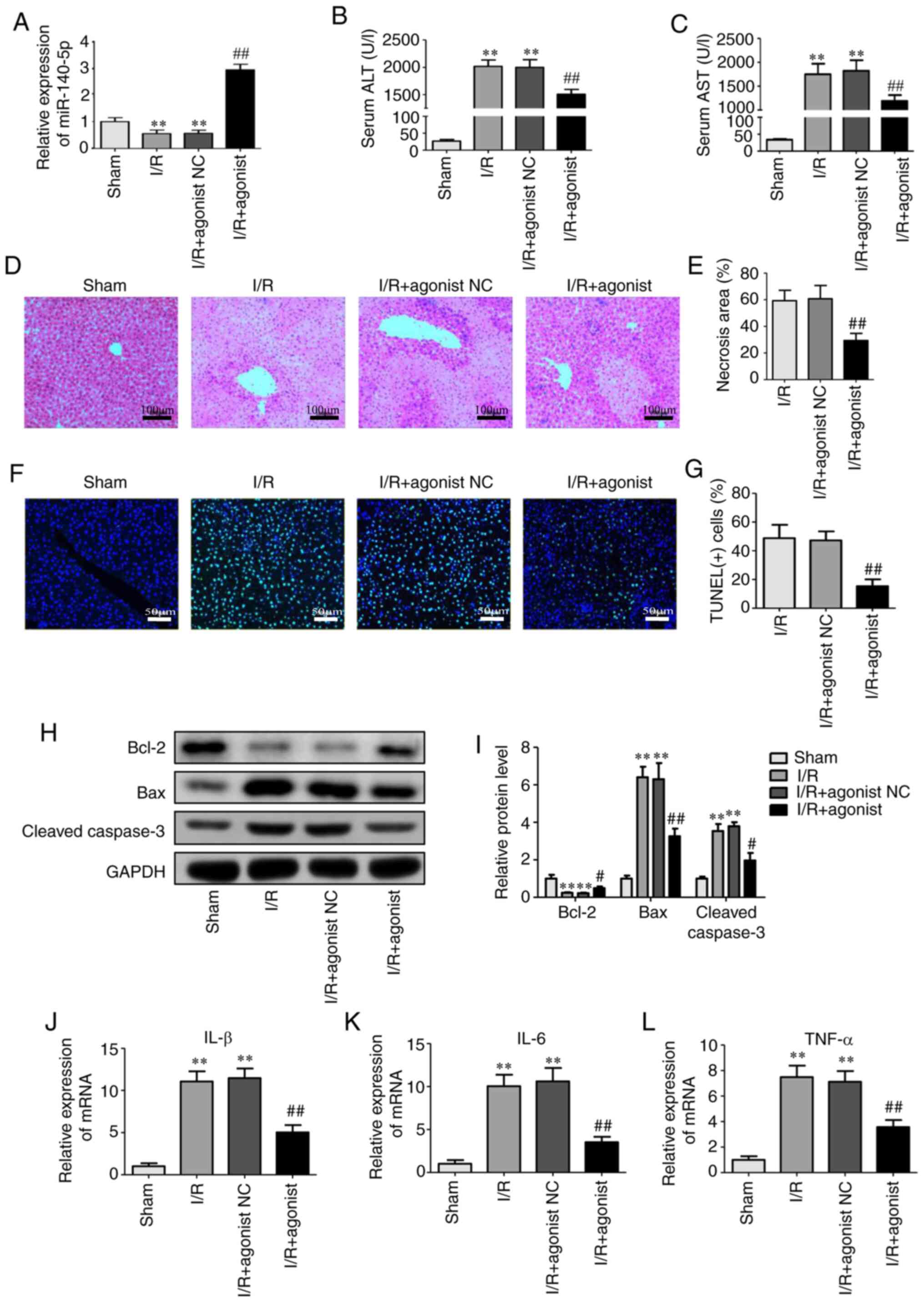 | Figure 3.miR-140-5p overexpression decreases
liver injury in an I/R injury mouse model. (A) Relative miR-140-5p
levels in the liver tissues after injection of miR-140-5p agonist.
(B) Serum levels of alanine aminotransferase. (C) Serum levels of
aspartate aminotransferase. (D) Hematoxylin and eosin staining of
liver sections (Scale bar=100 µm) and (E) necrotic area analysis.
(F) TUNEL staining of hepatocyte apoptosis and (G) quantification
of TUNEL(+) cells. Scale bar=50 µm (H) Western blot analysis and
(I) quantification of Bax, Bcl-2 and cleaved caspase-3. (J-L)
Inflammatory cytokines IL-1β, IL-6, and tumor necrosis factor-α was
detected by reverse transcription-quantitative polymerase chain
reaction. **P<0.01 vs. the sham group; #P<0.05 and
##P<0.01 vs. the I/R group. miR, microRNA; I/R,
ischemia/reperfusion; ALT, alanine aminotransferase; AST, aspartate
aminotransferase; TUNEL, terminal deoxynucleotidyl transferase dUTP
nick end labeling; IL, interleukin; TNF, tumor necrosis factor. |
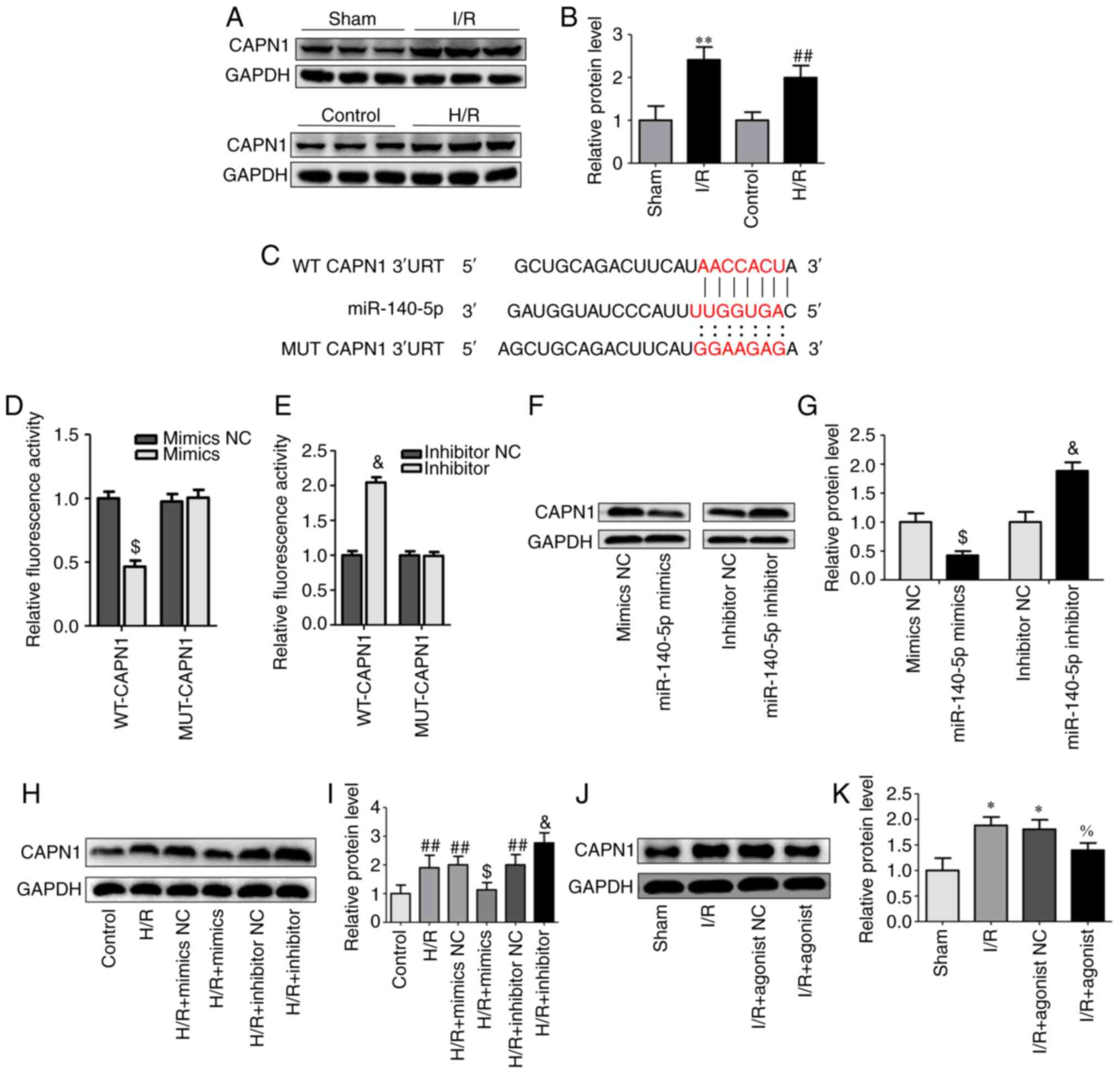
miR-140-5p directly targets CAPN1
To explore the mechanism of miR-140-5p in liver I/R,
StarBase, miRDB, and TargetScan were used to predict potential
target genes of miR-140-5p. A total of 26 target genes were
predicted using the three databases. By detecting the changes in
the mRNA expression of target genes after the transfection of
miR-140-5p mimics and mimics NC in AML12 cells, 8 of the 26 genes
were differentially expressed and, combined with literature reports
(15,33–37),
CAPN1 was identified as the target gene of miR-140-5p for
validation and subsequent verification was performed (Fig. S2). First, the expression of CAPN1
was detected in the mouse liver tissues after I/R injury and in
AML12 cells subjected to H/R, and CAPN1 was significantly increased
in the I/R and H/R groups (Fig. 4A and
B). Then, the interaction between miR-140-5p and CAPN1 was
verified; AML12 cells were co-transfected with miR-140-5p mimics or
inhibitors with WT-CAPN1 or MUT-CAPN1 luciferase reporter vectors
(Fig. 4C). The luciferase reporter
assay revealed that overexpression of miR-140-5p attenuated the
relative luciferase activity of the reporter containing the WT
CAPN1-3′-UTR site but not that of the MUT-type CAPN1-3′-UTR
(Fig. 4D). However, miR-140-5p
inhibition enhanced the luciferase activity of the reporter
containing the WT CAPN1-3′-UTR site but not that of the MUT-type
CAPN1-3′-UTR (Fig. 4E). To further
determine the effect of miR-140-5p on CAPN1, miR-140-5p mimics,
inhibitors, and NCs were transfected into AML12 cells, and CAPN1
expression was analyzed. Western blot analyses demonstrated that
CAPN1 expression was significantly decreased in the miR-140-5p
mimic group (Fig. 4F and G) and
increased in the miR-140-5p inhibitor group compared with that in
the respective NC group (Fig. 4F and
G). Similar results were observed in AML12 cells exposed to H/R
and in mice; miR-140-5p mimics inhibited the expression of CAPN1
induced by H/R whereas miR-140-5p inhibitor further increased the
expression of CAPN1 induced by H/R (Fig. 4H and I), and miR-140-5p agonist
decreased I/R-induced CAPN1 protein expression (Fig. 4J and K). These results indicated a
targeted relationship between miR-140-5p and CAPN1.
CAPN1 overexpression abrogates the
effect of miR-140-5p on H/R-induced cell injury
To determine the effect of CAPN1 on miR-140-5p
during H/R-induced cell injury, miR-140-5p mimics were transfected
alone or co-transfected with CAPN1 overexpression plasmid into
AML12 cells before H/R. Results revealed that the expression of
CAPN1 was significantly higher in AML12 cells than that of the
vector group after transfection of CAPN1-overexpressing plasmid
(Fig. S3), and compared with the
miR-140-5p mimic group, overexpression of CAPN1 abrogated the
effects of miR-140-5p on H/R-induced cell injury, which manifested
as decreased cell viability (Fig.
5A) and Bcl-2 protein expression (Fig. 5H and I), while LDH release (Fig. 5B), inflammatory cytokine expression
(Fig. 5C-E), the apoptotic rate
(Fig. 5F and G), and pro-apoptosis
proteins cleaved caspase-3 and Bax expression (Fig. 5H and I) were increased. These
results indicated that miR-140-5p alleviated cell injury by
inhibiting CAPN1 in AML12 cells.
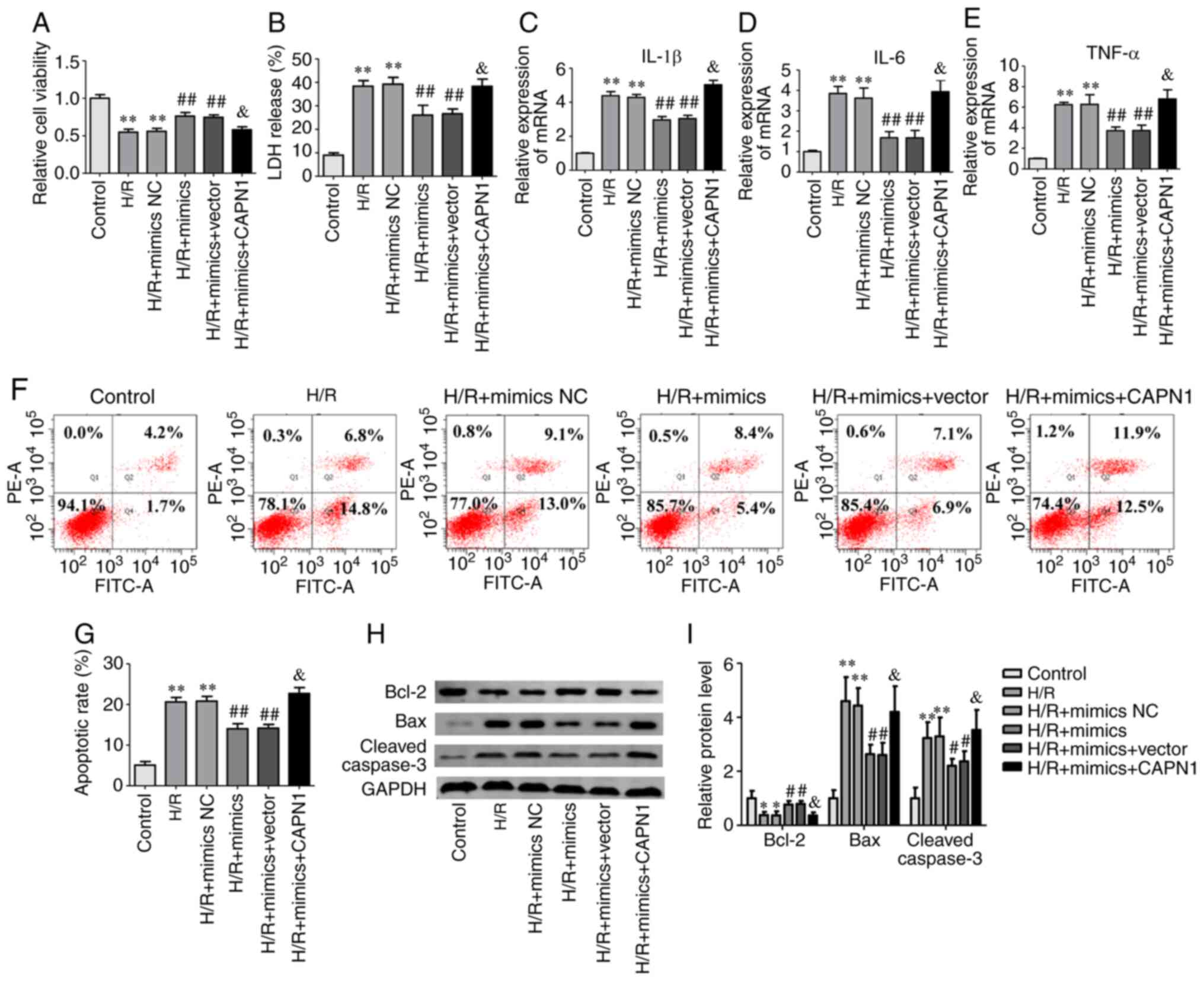 | Figure 5.CAPN1 overexpression abrogates the
effect of miR-140-5p on H/R-induced cell injury. The AML-12 cells
were transfected with miR-140-5p mimics and/or CAPN1 overexpression
plasmid in the H/R-induced cell injury model. (A) Relative cell
viability, (B) LDH release, (C-E) inflammatory cytokines IL-1β,
IL-6, and TNF-α, (F) cell apoptosis and (G) apoptotic rate
analysis, (H) western blot analysis and (I) quantification of the
protein levels of Bax, Bcl-2 and cleaved caspase-3. *P<0.05 and
**P<0.01 vs. the control group; #P<0.05 and
##P<0.01 vs. the H/R group; &P<0.05
vs. the H/R + mimics group. CAPN1, calpain 1; miR, microRNA; H/R,
hypoxia/reoxygenation; NC, negative control; LDH, lactate
dehydrogenase; IL, interleukin; TNF, tumor necrosis factor. |
Discussion
Liver I/R injury is an important pathogenesis of
liver transplantation, resection and hemorrhagic shock in clinical
practice, and is characterized by oxidative stress, inflammation
and apoptosis (2,3). Liver I/R injury can be divided into
two distinct pathophysiological processes: Ischemia and reperfusion
(3). Currently, effective treatment
strategies for hepatic I/R injury are limited due to the lack of
basic research that can be translated into clinical applications.
Therefore, further studies are needed to identify new targets for
alleviating hepatic I/R injury.
Emerging studies have revealed that miRNAs are
involved in the process of liver I/R injury. For example, Li et
al established that miR-142-3p was downregulated in the liver
tissues after I/R, while upregulated miR-142-3p ameliorated liver
injury by targeting myristoylated alanine-rich C-kinase substrate
(MARCKS) (7). Other miRNAs, such as
miR-125b, miR-128-3b and miR-450b-5p (5,6,8), are
reportedly involved in liver I/R. miR-140-5p has been investigated
extensively in tumor research, including glioma, breast and lung
cancer, and hepatocellular carcinoma (13,14,17,18).
miR-140-5p has also been extensively studied in non-tumor diseases.
Han et al revealed that miR-140-5p inhibited apoptosis in
brain I/R injury by negatively regulating the expression of the
Wnt/b-catenin signaling pathway (38). Wang et al revealed that
miR-140-5p attenuated the inflammatory response to brain injury
after intracerebral hemorrhage by targeting toll-like receptor 4
(TLR4) (20). Apoptosis and
inflammation play important roles in I/R injury and are often used
as indexes of damage evaluation (2,30).
Therefore, it was hypothesized that miR-140-5p may be involved in
liver I/R. In the present study, miR-140-5p was significantly
downregulated in both mouse liver tissues after I/R and AML12 cells
exposed to H/R. In H/R-induced AML12 cell injury, miR-140-5p
overexpression markedly increased cell viability, while LDH,
apoptosis, and inflammatory factors were decreased. However, cell
injury was aggravated after the use of miR-140-5p inhibitors. In
the mouse liver I/R injury model, miR-140-5p decreased the levels
of ALT and AST in the serum, the necrotic area of the liver, the
expression of inflammatory cytokines in liver tissue, and
TUNEL-positive hepatic cells, and reversed the changes in
apoptosis-related proteins caused by I/R. The results of in
vitro and in vivo experiments demonstrated the
protective effect of miR-140-5p in liver I/R.
CAPN1 belongs to the calpains family, and along with
other members, is responsible for limited proteolysis of a variety
of target substrates (39). Under
physiological conditions, calpains are involved in numerous
processes such as cytoskeleton remodeling, signal transduction
pathway regulation, platelet activation, cell differentiation, and
cell apoptosis (39–41). CAPN1 overactivation results in
unregulated proteolysis and aberrant activation of signaling
cascades, leading to cellular damage and ultimately cell death
(42). CAPN1 is activated during
heart and brain I/R injury due to increased intracellular calcium
concentration, destroying cell structure and increasing apoptosis,
reactive oxygen species, and inflammatory cytokines, while
inhibiting CAPN1 protects the heart and brain from I/R injury
(26–28). In liver I/R injury, CAPN1 activity
significantly increased, and CAPN1 inhibition resulted in a
significant decrease in liver apoptosis and necrosis (33). In the present study, it was revealed
that the expression of CAPN1 was significantly increased in the
liver tissues following I/R and in AML12 cells subjected to H/R,
and miR-140-5p overexpression inhibited the expression of CAPN1
in vitro and in vivo. The dual-luciferase reporter
assay further confirmed that miR-140-5p negatively regulated CAPN1.
CAPN1 overexpression inhibited the protective effects of miR-140-5p
on AML12 cell injury. The results of the present study indicated
that miR-140-5p decreased liver I/R injury by targeting CAPN1.
In conclusion, our present study demonstrated that
miR-140-5p alleviated liver I/R injury by negatively regulating the
expression of CAPN1 in mouse liver I/R injury and H/R-induced
injury in AML12 cells. These findings may contribute to further
understanding the mechanism of liver I/R injury and may provide
novel insights into liver I/R. miR-140-5p has been extensively
studied in tumor and non-tumor diseases and has exhibited favorable
results (13–24,38).
There is still a large gap in applying miR-140-5p research results
to benefit patients clinically. Further research is required to
clarify the role of miR-140-5p in liver I/R and other diseases in
the clinical setting and to determine how CAPN1 affects liver
injury.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81971881) and the
Medical Science and Technology Project of Henan Provincial Health
Commission (grant no. SB201901045).
Availability of data and materials
All data generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
QY, SC and HT performed the experiments. HY, JZ and
XS conducted the literature search and analyzed and interpreted the
data. SZ, WG and JL designed the study. QY and SC prepared and
wrote the study. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the First Affiliated Hospital of Zhengzhou
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
I/R
|
ischemia/reperfusion
|
|
H/R
|
hypoxia reoxygenation
|
|
CAPN1
|
calpain 1
|
|
RT-qPCR
|
reverse transcription
quantitative-polymerase chain reaction
|
|
3′-UTRs
|
3′-untranslated regions
|
|
NC
|
negative control
|
|
CCK-8
|
Cell counting Kit-8
|
|
LDH
|
lactate dehydrogenase
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
H&E
|
hematoxylin and eosin
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
dUTP nick end labeling
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
BCA
|
bicinchoninic acid
|
|
PVDF
|
polyvinylidene difluoride
|
References
|
1
|
Zhou J, Chen J, Wei Q, Saeb-Parsy K and Xu
X: The role of ischemia/reperfusion injury in early hepatic
allograft dysfunction. Liver Transpl. 26:1034–1048. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Konishi T and Lentsch AB: Hepatic
ischemia/reperfusion: Mechanisms of tissue injury, repair, and
regeneration. Gene Expr. 17:277–287. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elias-Miró M, Jiménez-Castro MB, Rodés J
and Peralta C: Current knowledge on oxidative stress in hepatic
ischemia/reperfusion. Free Radic Res. 47:555–568. 2013. View Article : Google Scholar
|
|
4
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang Z, Zheng D, Pu J, Dai J, Zhang Y,
Zhang W and Wu Z: MicroRNA-125b protects liver from
ischemia/reperfusion injury via inhibiting TRAF6 and NF-κB pathway.
Biosci Biotechnol Biochem. 83:829–835. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mou T, Luo Y, Huang Z, Zheng D, Pu X, Shen
A, Pu J, Li T, Dai J, Chen W and Wu Z: Inhibition of
microRNA-128-3p alleviates liver ischaemia-reperfusion injury in
mice through repressing the Rnd3/NF-κB axis. Innate Immun.
26:528–536. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Gao M, Xu LN, Yin LH, Qi Y and Peng
JY: MicroRNA-142-3p attenuates hepatic ischemia/reperfusion injury
via targeting of myristoylated alanine-rich C-kinase substrate.
Pharmacol Res. 156:1047832020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Z, Mou T, Luo Y, Pu X, Pu J, Wan L,
Gong J, Yang H, Liu Y, Li Z, et al: Inhibition of miR-450b-5p
ameliorates hepatic ischemia/reperfusion injury via targeting
CRYAB. Cell Death Dis. 11:4552020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chi X, Jiang Y, Chen Y, Yang F, Cai Q, Pan
F, Lv L and Zhang X: Suppression of microRNA-27a protects against
liver ischemia/reperfusion injury by targeting PPARgamma and
inhibiting endoplasmic reticulum stress. Mol Med Rep. 20:4003–4012.
2019.PubMed/NCBI
|
|
10
|
Wienholds E, Kloosterman WP, Miska E,
Alvarez-Saavedra E, Berezikov E, de Bruijn E, Horvitz HR, Kauppinen
S and Plasterk RH: MicroRNA expression in zebrafish embryonic
development. Science. 309:310–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tuddenham L, Wheeler G, Ntounia-Fousara S,
Waters J, Hajihosseini MK, Clark I and Dalmay T: The cartilage
specific microRNA-140 targets histone deacetylase 4 in mouse cells.
Febs Lett. 580:4214–4217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyaki S, Nakasa T, Otsuki S, Grogan SP,
Higashiyama R, Inoue A, Kato Y, Sato T, Lotz MK and Asahara H:
MicroRNA-140 is expressed in differentiated human articular
chondrocytes and modulates interleukin-1 responses. Arthritis
Rheum. 60:2723–2730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu D, Lv W, Zhou X, He Y, Yao H, Yu Y,
Zhang G and Zhang Q: Long non-coding RNA TMPO-AS1 promotes tumor
progression via sponging miR-140-5p in breast cancer. Exp Ther Med.
21:172021.PubMed/NCBI
|
|
14
|
Zhuo E, Cai C, Liu W, Li K and Zhao W:
Downregulated microRNA-140-5p expression regulates apoptosis,
migration and invasion of lung cancer cells by targeting zinc
finger protein 800. Oncol Lett. 20:3902020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mei J, Liu G, Wang W, Xiao P, Yang D, Bai
H and Li R: OIP5-AS1 modulates epigenetic regulator HDAC7 to
enhance non-small cell lung cancer metastasis via miR-140-5p. Oncol
Lett. 20:72020.PubMed/NCBI
|
|
16
|
Mao Z, Wang Z, Zhang S, Pu Y, Wang J,
Zhang T, Long Y, Liu Y, Ma Y and Zhu J: LRP4 promotes migration and
invasion of gastric cancer under the regulation of microRNA-140-5p.
Cancer Biomark. 29:245–253. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai RD, Zhang CC, Xie LL, Wang PC, Huang
CX, Chen JL and Lv HT: SNHG1 promotes malignant progression of
glioma by targeting miR-140-5p and regulating PI3K/AKT pathway.
Cancer Manag Res. 12:12011–12020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan L, Huang X, Chen J, Zhang K, Gu YH,
Sun J and Cui SY: Long noncoding RNA MALAT1 contributes to
sorafenib resistance by targeting miR-140-5p/Aurora-A signaling in
hepatocellular carcinoma. Mol Cancer Ther. 19:1197–1209. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Liu D, Xi Y and Li J, Liu B and Li
J: Upregulation of miRNA-140-5p inhibits inflammatory cytokines in
acute lung injury through the MyD88/NF-kappaB signaling pathway by
targeting TLR4. Exp Ther Med. 16:3913–3920. 2018.PubMed/NCBI
|
|
20
|
Wang S, Cui Y, Xu J and Gao H: miR-140-5p
Attenuates Neuroinflammation and Brain Injury in Rats Following
Intracerebral Hemorrhage by Targeting TLR4. Inflammation.
42:1869–1877. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Shen S, Li Z, Li W and Weng X:
MIR-140-5p affects chondrocyte proliferation, apoptosis, and
inflammation by targeting HMGB1 in osteoarthritis. Inflamm Res.
69:63–73. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao W, Fu Z, Zou Y, Wen D, Ma H, Zhou F,
Chen Y, Zhang M and Zhang W: MicroRNA-140-5p attenuated oxidative
stress in Cisplatin induced acute kidney injury by activating
Nrf2/ARE pathway through a Keap1-independent mechanism. Exp Cell
Res. 360:292–302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, Mao Z, Zhu J, Shen M and Chen F:
miR-140-5p inhibits oxidized low-density lipoprotein-induced
oxidative stress and cell apoptosis via targeting toll-like
receptor 4. Gene Ther. 2020. View Article : Google Scholar
|
|
24
|
Sun J, Tao S, Liu L, Guo D, Xia Z and
Huang M: miR-140-5p regulates angiogenesis following ischemic
stroke by targeting VEGFA. Mol Med Rep. 13:4499–4505. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Demarchi F and Schneider C: The calpain
system as a modulator of stress/damage response. Cell Cycle.
6:136–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu HT, Feng RQ, Tang JK, Zhou JJ, Gao F
and Ren J: CaMKII/calpain interaction mediates ischemia/reperfusion
injury in isolated rat hearts. Cell Death Dis. 11:3882020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yue RC, Lu SZ, Luo Y, Wang T, Liang H,
Zeng J, Liu J and Hu HX: Calpain silencing alleviates myocardial
ischemia-reperfusion injury through the NLRP3/ASC/Caspase-1 axis in
mice. Life Sci. 233:1166312019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao H, Xu M and Chu G: Association
between myocardial cell apoptosis and calpain-1/caspase-3
expression in rats with hypoxic-ischemic brain damage. Mol Med Rep.
15:2727–2731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. National Academies Press;
Washington, DC: 2010
|
|
30
|
Guo WZ, Fang HB, Cao SL, Chen SY, Li J,
Shi JH, Tang HW, Zhang Y, Wen PH, Zhang JK, et al:
Six-transmembrane epithelial antigen of the prostate 3 Deficiency
in hepatocytes protects the liver against ischemia-reperfusion
injury by suppressing transforming growth factor-β-activated kinase
1. Hepatology. 71:1037–1054. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yi Z, Deng M, Scott MJ, Fu G, Loughran PA,
Lei Z, Li S, Sun P, Yang C, Li W, et al: Immune-responsive gene
1/itaconate activates nuclear factor Erythroid 2-related factor 2
in hepatocytes to protect against liver ischemia-reperfusion
injury. Hepatology. 72:1394–1411. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kohli V, Madden JF, Bentley RC and Clavien
PA: Calpain mediates ischemic injury of the liver through
modulation of apoptosis and necrosis. Gastroenterology.
116:168–178. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li W and He F: Monocyte to macrophage
differentiation-associated (MMD) targeted by miR-140-5p regulates
tumor growth in non-small cell lung cancer. Biochem Biophys Res
Commun. 450:844–850. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kai Y, Peng W, Ling W, Jiebing H and Zhuan
B: Reciprocal effects between microRNA-140-5p and ADAM10 suppress
migration and invasion of human tongue cancer cells. Biochem
Biophys Res Commun. 448:308–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rothman AM, Arnold ND, Pickworth JA,
Iremonger J, Ciuclan L, Allen RM, Guth-Gundel S, Southwood M,
Morrell NW, Thomas M, et al: MicroRNA-140-5p and SMURF1 regulate
pulmonary arterial hypertension. J Clin Invest. 126:2495–2508.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Güllü G, Peker I, Haholu A, Eren F,
Küçükodaci Z, Güleç B, Baloglu H, Erzik C, Özer A and Akkiprik M:
Clinical significance of miR-140-5p and miR-193b expression in
patients with breast cancer and relationship to IGFBP5. Genet Mol
Biol. 38:21–29. 2015. View Article : Google Scholar
|
|
38
|
Han XR, Wen X, Wang YJ, Wang S, Shen M,
Zhang ZF, Fan SH, Shan Q, Wang L, Li MQ, et al: MicroRNA-140-5p
elevates cerebral protection of dexmedetomidine against
hypoxic-ischaemic brain damage via the Wnt/beta-catenin signalling
pathway. J Cell Mol Med. 22:3167–3182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sorimachi H, Hata S and Ono Y: Calpain
chronicle-an enzyme family under multidisciplinary
characterization. Proc Jpn Acad Ser B Phys Biol Sci. 87:287–327.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuchay SM and Chishti AH: Calpain-mediated
regulation of platelet signaling pathways. Curr Opin Hematol.
14:249–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kakurina GV, Kolegova ES, Shashova EE,
Cheremisina OV, Choynzonov EL and Kondakova IV: Relationship
between the mRNA expression levels of calpains 1/2 and proteins
involved in cytoskeleton remodeling. Acta Naturae. 12:110–113.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kling A, Jantos K, Mack H, Hornberger W,
Drescher K, Nimmrich V, Relo A, Wicke K, Hutchins CW, Lao Y, et al:
Discovery of novel and highly selective inhibitors of calpain for
the treatment of Alzheimer's disease:
2-(3-Phenyl-1H-pyrazol-1-yl)-nicotinamides. J Med Chem.
60:7123–7138. 2017. View Article : Google Scholar : PubMed/NCBI
|