Introduction
Acute kidney injury (AKI) is one of the most common
complications of sepsis and occurs in 40–50% of septic patients,
with a mortality rate of as high as 60% (1). However, the pathogenesis of
sepsis-induced AKI remains unclear. Autophagy has been reported to
serve a key role in sepsis-induced AKI and the inhibition of
autophagy results in the development of AKI during sepsis (2,3).
Previous studies (4,5) have confirmed that sepsis triggers
autophagy in multiple organs, including the kidney (6) and autophagic processes are involved in
the removal of damaged mitochondria and oxidative stress (7). However, excessive autophagy can cause
unwanted and deleterious cell death (8). Therefore, a moderate level of
autophagy is the key to reducing sepsis-induced AKI. Previous
studies have reported that miR-214 ameliorates
ischemia-reperfusion-induced AKI by inhibiting apoptosis (9) and miR-214 suppresses oxidative stress
in diabetic nephropathy via the reactive oxygen species
(ROS)/AKT/mTOR signaling pathway (10). The present study found that miR-214
can attenuate sepsis-induced myocardial dysfunction in mice by
inhibiting autophagy (11).
However, whether miR-214 can ameliorate sepsis-induced AKI remains
to be elucidated. In present study, it was hypothesized that
miR-214 attenuates CLP-induced AKI by reducing oxidative stress and
inhibiting autophagy through the regulation of the PTEN/AKT/mTOR
pathway.
Materials and methods
Animals
A total of 100 Kunming male mice (weight, 20.40±2.92
g; age, 6–8 weeks) supplied by the Medical Laboratory Animal Centre
of the Hebei Medical University (Shijiazhuang, China) were used in
the present study. All mice were acclimated to a 12-h light/dark
cycle at 24°C with 50% humidity and were given free access to food
and water at ≥1 week before the experiments. All the experimental
procedures were performed in strict accordance with the National
Institute of Health guidelines (NIH Publication No 85–23, revised
1996) and approval by the Institutional Animal Care and Use
Committees of the Cangzhou Central Hospital (approval no.
2017-020-01). All surgeries were performed under anesthesia and
every effort was made to minimize suffering.
Cecal ligation and puncture (CLP)
CLP was performed on mice to create a murine sepsis
model, as previously described (12). Following anesthetizing by isoflurane
inhalation (induced at 3% and maintained at 0.5%), a 1 cm midline
incision was cut. The exposed cecum (1 cm distance from the end)
was ligated with two punctures using a 23-gauge needle. The cecum
gently extruded a small amount of feces and was placed back in its
anatomical position. The abdominal wall was sutured in layers with
a 3-0 silk braid. Following the procedure, 1 ml of 0.9% saline was
injected subcutaneously. The mice were provided free access to only
water. Sham model mice were operated in the same manner as the CLP
model without CLP.
Experimental design
Mice (n=6 for sham surgery and CLP) were randomly
assigned to seven groups: Sham group, CLP group, adenovirus
(Ad)-green fluorescent protein (GFP) + CLP group, Ad-miR-214 + CLP
group, anti-miR-214 + CLP group, PTEN inhibitor + CLP group and
Ad-miR-214 + PTEN inhibitor + CLP group. The Sham group mice were
exposed to the same procedure but without ligation and puncture of
the cecum. Mice in the other groups received cecal ligation and
perforation. All mice were quickly anesthetized by isoflurane
inhalation to collect blood, urine and kidney samples 24 h after
the last treatment.
Adenovirus-mediated Ad-miR-214,
anti-miR-214, or Ad-GFP gene transfer in vivo and PTEN inhibitor
injection
Ad-miR-214, anti-miR-214, or Ad-GFP (Shanghai
GenePharma Co., Ltd.) was delivered into the abdominal cavity of
mice 4 days prior to CLP. Briefly, mice were anesthetized by
isoflurane inhalation. A catheter containing 200 µl of adenovirus
(2×1011 pfu, expressing miR-214, anti-miR-214, or
Ad-GFP) was administered to normal mice via intraperitoneal
injection. The PTEN inhibitor (VO-OHpic, intraperitoneal,
Sigma-Aldrich; Merck KGaA) was administered to CLP mice that had
received anti-miR-214 via intraperitoneal injection at a single
dosage of 10 µg/kg 30 min prior to the administration of
adenovirus.
Assessment of kidney function
Blood samples were harvested from mice heart,
followed by centrifugation (at room temperature for 15 min at 3,000
× g) for collecting serum. The serum levels of blood urea nitrogen
(BUN) and serum creatinine (Cr) were determined using a Hitachi
7600 automatic analyzer (Hitachi, Ltd.). ELISA was used to analyze
levels of kidney injury molecule-1 (KIM-1; cat. no. RKM100; R&D
Systems, Inc.) and neutrophil gelatinase-associated lipocalin
(NGAL; cat. no. DY3508; R&D Systems, Inc.) in urine
samples.
Assay for serum inflammation
cytokine
Serum TNF-α (cat. no. H052) and IL-6 (cat. no. H007)
were examined using commercial ELISA kits (Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
instructions.
Measurement of oxidative stress
markers
The corresponding assay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) was used to measure the
levels of malondialdehyde (MDA) and test the activity of superoxide
dismutase (SOD) in accordance with the manufacturer's
instructions.
Histology and tubular injury
score
All mice were subjected to kidney perfusion under
anesthesia 24 h after CLP. The kidney specimens were fixed in 4%
paraformaldehyde for 72 h at 4°C. The tissue samples were then
dehydrated in a graded series of ethanol solutions, embedded in
paraffin and cut into 4-µm sections. Sections (4 µm) were cut using
a microtome and the tissue sections were stained with hematoxylin
(5 min) and eosin (1 min) at room temperature for histological
examination. The slides were evaluated and graded using a
microscope (BX51, Olympus Corporation). Renal tissues with the
following histopathological changes were judged injured: Loss of
brush border, vacuolization, cast formation, tubular dilation and
disruption, cell lysis and cellular necrosis. Tissue damage was
checked in a blinded manner and scored by the percentage of damaged
tubules: 0, no damage; 1, 0–25; 2, 25–50; 3, 50–75; 4, >75%
(13).
Transmission electron microscopy
(TEM)
Fresh kidneys were washed in phosphate buffered
saline, and cut into 1 mm cubes and sequentially fixed in 2.5%
glutaraldehyde for 24 h at 4°C. The sections were immersed in 1%
osmium tetroxide for 2 h at 4°C, dehydrated in graded ethanol and
embedded in epoxy resin. Finally, the ultrathin sections (60 nm)
were doubled stained with uranyl acetate and lead citrate at 20°C
for 60 min. The observation was performed on a transmission
electron microscope (Tecnai; Hitachi, Ltd.) at 80 kV using Electron
Microscopy Film 4489 (ESTAR thick base; Kodak).
Immunohistochemistry (IHC)
Fresh kidney tissues were fixed in 4%
paraformaldehyde (for 72 h at 4°C) and embedded in paraffin.
Specimens were cut into 4 µm-thick sections and deparaffinized in
xylene. After tissue sections were washed with PBS, they were
boiled in 10 mM citrate buffer (pH 6.0) for 4 min for antigen
retrieval and then blocked with 10% goat serum in PBS at room
temperature for 1 h. The primary antibody (anti-LC3B; 1:400; cat.
no. 4412; Cell Signaling Technology, Inc.) was added in accordance
with the instructions and incubated at 4°C for 12 h. The secondary
antibody (goat antirabbit HRP; 1:2,000; cat. no. BS13278; Bioworld
Technology, Inc.) was added and incubated at room temperature for
10 min. DAB (100 µl) was added and counterstained for 5 min with
the staining observed under a light microscope (Model CX31-P;
Olympus Corporation). The intensity of positive staining, which
appeared brown, was determined using Image-Pro Plus 6.0 image
analysis software (Media Cybernetics, Inc.). The integral optical
density (IOD) was calculated to represent the intensity. IOD values
increased as protein expression increased.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from kidney tissue following
the induction of the model using TRIzol reagent (Thermo Fisher
Scientific, Inc.). According to the instructions of the TaqMan
reverse transcription kit (cat. no. N8080234; Invitrogen; Thermo
Fisher Scientific, Inc.), RNA was reverse transcribed into cDNA.
The following thermocycling conditions were used (miR-214): Initial
denaturation at 95°C for 5 min; followed by 40 cycles of
denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and
elongation at 72°C for 30 sec. RT-qPCR was performed using an ABI
Prism 7500 Sequence Detection System (PerkinElmer, Inc.) and a
standard SYBR Green PCR kit (Toyobo Life Science). U6 was used as
the internal control for miR-214. Primer sequences were as follows:
miR-214, forward, 5′-AGCATAATACAGCAGGCACAGAC-3′ and reverse,
5′-AAAGGTTGTTCTCCACTCTCTCAC-3′; U6, forward,
5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
5′-GGAACGCTTCACGAATTTG-3′. These experiments were replicated six
times. Results were analyzed using the 2−ΔΔCq method
(14). The mRNA expression levels
of LC3, p62, PTEN, AKT and mTOR were evaluated via RT-qPCR. With
β-actin as an internal reference of these genes, the
2−ΔΔCq was used to measure the relative expression of
target genes. The primer sequences shown in Table I were synthesized by Sangon Biotech
Co., Ltd.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Sequence |
|---|
| miR-214-3p | F:
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACTGCC-3′ |
|
| R:
5′-GCACAGCAGGCACAGACA-3′ |
| PTEN | F:
5′-CACAGAATTCCAGACATGACAGCCATCATC-3′ |
|
| R:
5′-GTGGATCCTCTAGGTTTATCCCTCTTG-3′ |
| mTOR | F:
5′-AACAACGGCTTTCCACCAGG-3′ |
|
| R:
5′-CACCCTAAGTGAGCCCTTGGA-3′ |
| AKT | F:
5′-CCACGCACACTCGGGCCG-3′ |
|
| R:
5′-CAATGCAGAGGGGTGCAGG-3′ |
| LC3 | F:
5′-CATGCCGTCCGAGAAGACCT-3′ |
|
| R:
5′-GATGAGCCGGACATCTTCCACT-3′ |
| p62 | F:
5′-TCCCTGTCAAGCAGTATCC-3′ |
|
| R:
5′-TCCTCCTTGGCTTTGTCTC-3′ |
| U6 | F:
5′-CTCGCTTCGGCAGCACA-3′ |
|
| R:
5′-AACGCTTCACGAATTTGCGT-3′ |
| β-actin | F:
5′-ACACTGTGCCCATCTACGAGG-3′ |
|
| R:
5′-AGGGGCCGGACTCGTCATACT-3′ |
Western blotting
Kidney tissue was mixed with RIPA lysate (Beyotime
Institute of Biotechnology) to make the homogenate and lysis was
stopped when no visible tissue was observed. The samples were
centrifuged at 13,000 × g for 10 min at −4°C and the supernatant
was recovered for the western blot analysis. In brief, protein
concentrations were determined using the micro BCA protein assay
kit (Thermo Fisher Scientific, Inc.). Protein samples (80 µg per
sample) were subjected to separate by reducing 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred
onto polyvinylidene difluoride membranes at 4°C overnight.
Separated proteins were transferred to PVDF membranes. Following
blocking in 5% skimmed milk at room temperature for 2 h, membranes
were incubated overnight (at 4°C) with primary antibodies. The
following primary antibodies (all Cell Signaling Technology, Inc.)
were used: Light chain 3B (1:1,000; cat. no. 4412), p62 (1:1,000;
cat. no. 4412), Anti-PTEN (1:1,000; cat. no. 9188),
anti-phosphorylated (p)-AKT (Ser473) (1:1,000; cat. no. 4060),
anti-AKT (1:1,000; cat. no. 9272), anti-p-mTOR (Ser 2448) (1:1,000;
cat. no. 5536), anti-mTOR (1:1,000; cat. no. 2972) and β-actin
(1:2,000; cat. no. 4970). After washing, the membranes were
incubated (at room temperature for 2 h) with the HRP-conjugated
anti-rabbit secondary antibodies (1:3,000; cat. no. A0208; Beyotime
Institute of Biotechnology). Protein bands were detected with
Immobilon Western (MilliporeSigma) and analyzed using Total-Lab
TL120 software (Nonlinear Dynamics, 2.01). The expression of
protein was normalized to β-actin.
Statistical analysis
Statistical analysis was performed using the
GraphPad Prism 9.0 (GraphPad Software, Inc.). All data were
presented as means ± standard deviation or medians (interquartile
ranges) for continuous variables, depending on their distributions.
Baseline characteristics and outcomes were compared using one-way
ANOVA followed by Tukey's post hoc test, or Kruskal-Wallis test
followed by Dunn's test as appropriate. P<0.05 was considered to
indicate a statistically significant difference.
Results
Time point 24 h after CLP
Previous studies have reported (6,15,16)
that biochemical (i.e., LC3 and p62) analysis reveals that
autophagy flux is suppressed with progression of sepsis following
6–8 h of CLP. In the present study, the number of autolysosomes in
the kidney of CLP-treated mice increased within 24 h following
surgery. In addition, the analysis of markers of kidney injury
showed that renal function was most seriously damaged 24 h after
CLP. Therefore, the time point 24 h after CLP was choose for the
following experiments.
Regulatory effect on miR-214 in kidney
tissues
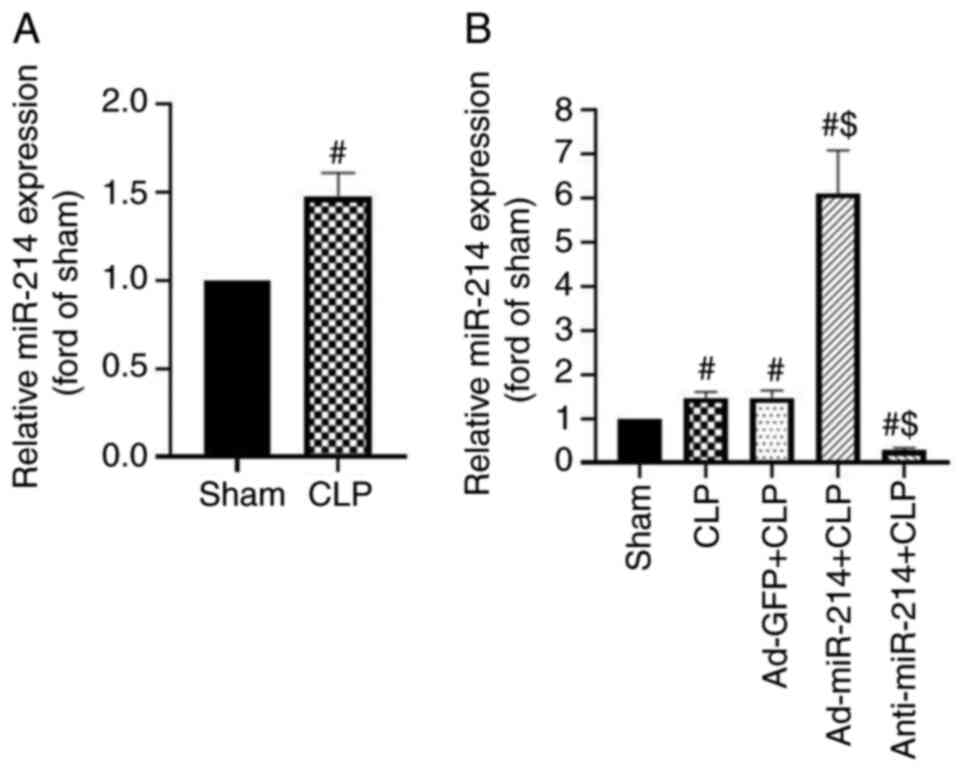
RT-qPCR analysis was used to detect miR-214
expression in CLP-treated mice. It was found that miR-214
expression was slightly upregulated in the kidney tissues following
CLP surgery 24 h, as compared with the sham group (1.47-fold,
P<0.01, Fig. 1A). The present
study examined the reactive increase in miR-214 expression during
sepsis as a compensatory protective mechanism. Therefore, it
evaluated the role of miR-214 in AKI during sepsis by regulating
its expression. As shown in Fig.
1B, Ad-miR-214 increased miR-214 expression by 4.13-fold 4 days
after intraperitoneally injecting 2×1011 pfu/mice
adenovirus, whereas anti-miR-214 decreased miR-214 expression by
81.16% in the kidney tissue, as compared with the sham group (both
P<0.01). By contrast, Ad-GFP as a control group did not affect
miR-214-3p expression, compared with the sham group (both
P>0.05).
Effect of miR-214 on renal dysfunction
in septic mice
All mice were sacrificed to collect blood, urine and
kidney samples 24 h after the CLP surgery. BUN and Cr are important
indicators of the severity of renal impairment (17). Furthermore, NGAL and KIM-1 have been
identified as specific biomarkers of kidney injury and their
increased expression is associated with early renal tubular injury
in AKI (17). As shown in Fig. 2A-D, the levels of BUN, Cr, KIM-1 and
NGAL were significantly increased following CLP surgery compared
with the sham group. However, Ad-miR-214 significantly decreased
BUN, Cr, KIM-1 and NGAL levels in comparison with the CLP group
(all P<0.01), whereas anti-miR-214 exhibited opposite effects in
these kidney function parameters. However, following pretreatment
with PTEN inhibitor, the protection effects of Ad-miR-214 were
enhanced. The results show that miR-214 attenuates kidney
dysfunction in septic mice.
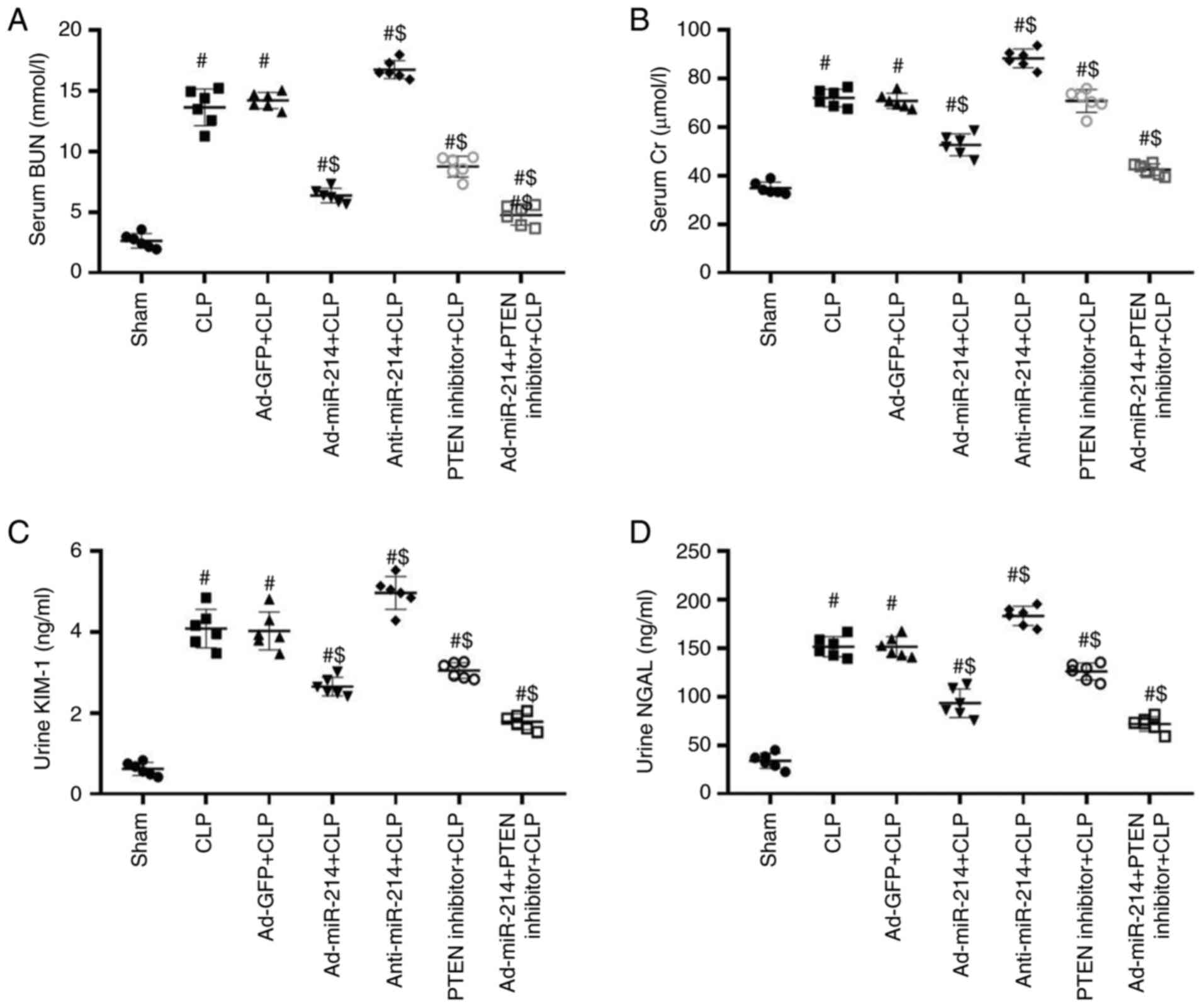 | Figure 2.miR-214 improves renal dysfunction in
CLP-induced AKI. (A) Serum BUN levels, (B) Cr levels, (C) Urine
KIM-1 levels, (D) Urine NGAL levels. n=6, #P<0.01 vs.
sham group; $P<0.01 vs. CLP group. miR, microRNA;
CLP, cecal ligation and puncture; AKI, acute kidney injury; BUN,
blood urea nitrogen; Cr, serum creatinine; KIM-1, kidney injury
molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; Ad,
adenovirus. |
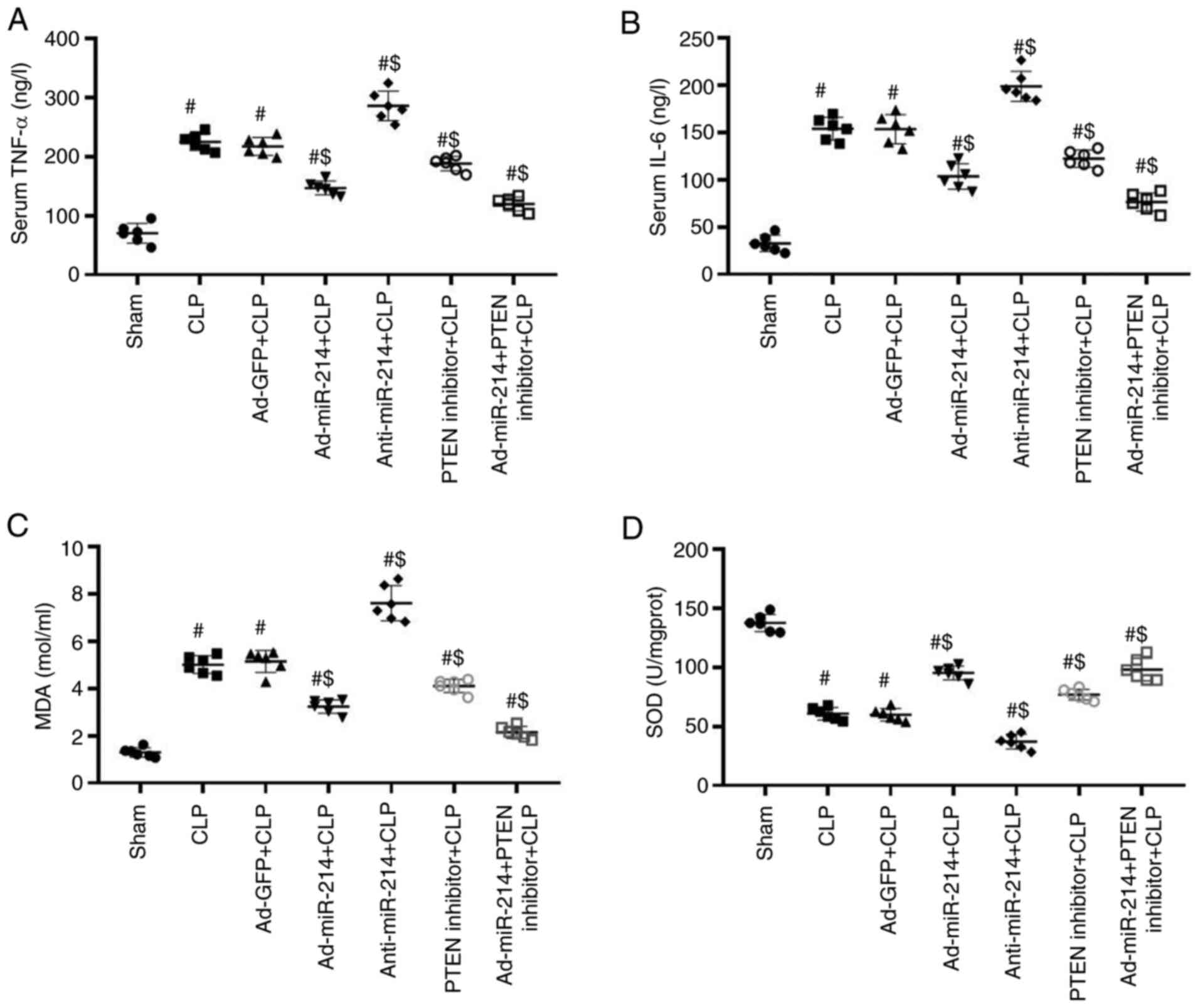
Effect of miR-214 on renal
inflammation and oxidative stress
As shown in Fig. 3A and
B, CLP significantly elevated the levels of TNF-α and IL-6,
whereas Ad-miR-214 significantly decreased the levels of these
markers. Compared with the Ad-GFP group, the levels of these
inflammatory cytokines were significantly increased in the
anti-miR-214 group, while can be reduced by Ad-miR-214. However,
following pretreatment with PTEN inhibitor, the protection effects
of Ad-miR-214 were enhanced. These results suggest that miR-214 can
reduce the levels of inflammatory cytokines.
As shown in Fig. 3C and
D, the CLP increased the levels of MDA and decreased the levels
of SOD in comparison with the CLP group (both P<0.01). However,
Ad-miR-214 significantly increased the levels of MDA and decreased
levels of SOD in comparison with the CLP group (all P<0.01),
whereas anti-miR-214 exhibited opposite effects in these oxidative
stress parameters (both P<0.01). However, following pretreatment
with PTEN inhibitor, the antioxidant effects of Ad-miR-214 were
enhanced.
Effect of miR-214 on renal
histopathological damage
As shown in Fig. 4,
there were substantial pathological changes in the CLP group, which
included edema of renal tubular epithelial cells (green arrow),
tubular necrosis (blue arrow), telangiectasia and severe
congestion/hemorrhage (red arrow). However, the CLP-induced kidney
damage was significantly improved by pretreatment with Ad-miR-214.
The damaging effect was enhanced by anti-miR-214.
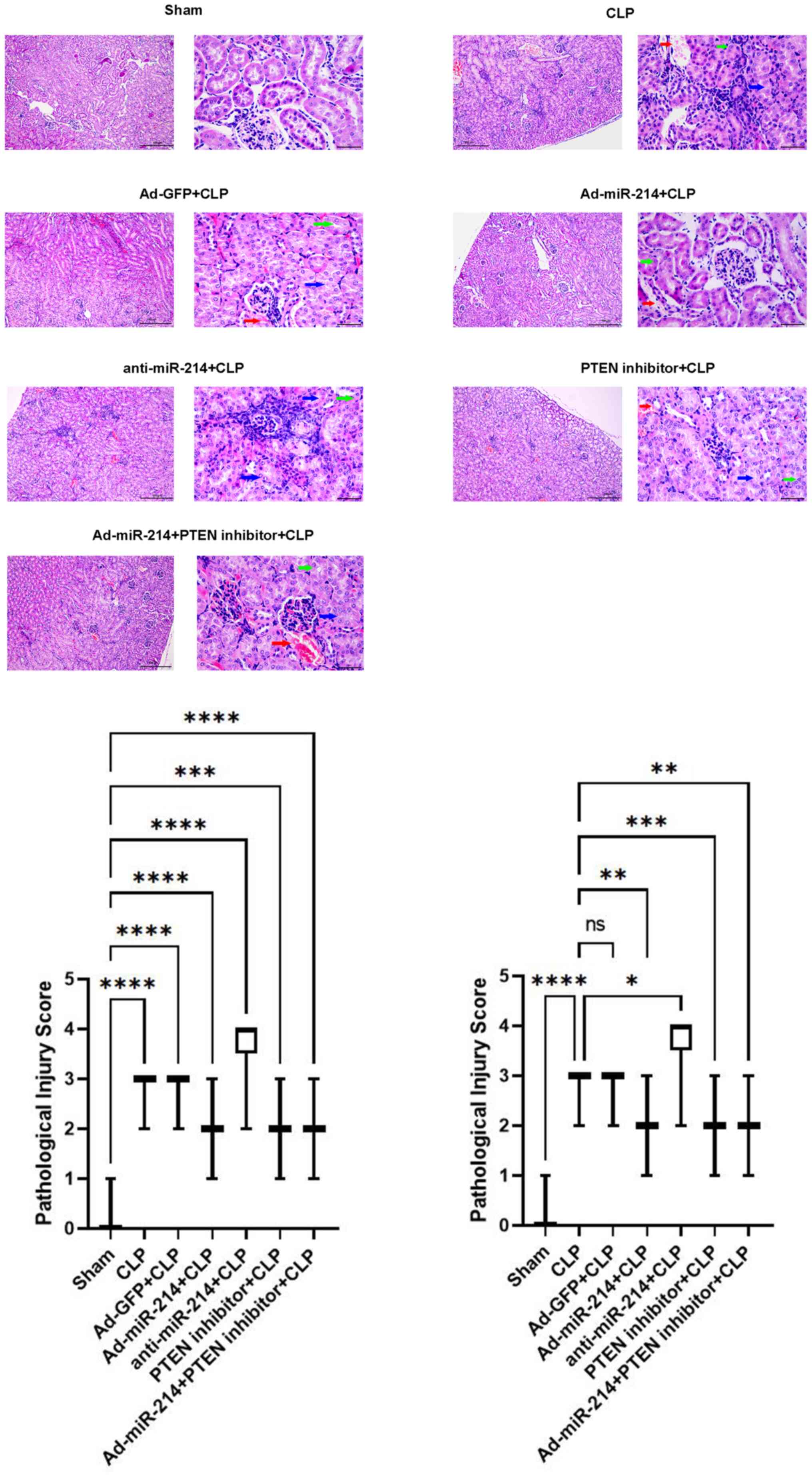 | Figure 4.miR-214 attenuates renal
histopathological damage. Histological changes in the kidney
tissues at 24 h post-CLP (hematoxylin-eosin). Green arrow indicates
edema of renal tubular epithelial cells; blue arrow indicates
tubular necrosis; red arrow indicates hemorrhage. Magnification,
×400, scale bar, 50 µm; ×100, scale bar, 100 µm. Semiquantitative
histopathological score of injury. n=6, (Kidney pathological images
of each mouse were randomly selected from 5 fields), *P<0.05,
**P<0.01, ***P<0.001, ****P<0.0001 vs. the sham group and
CLP group. miR, microRNA; CLP, cecal ligation and puncture; Ad,
adenovirus. |
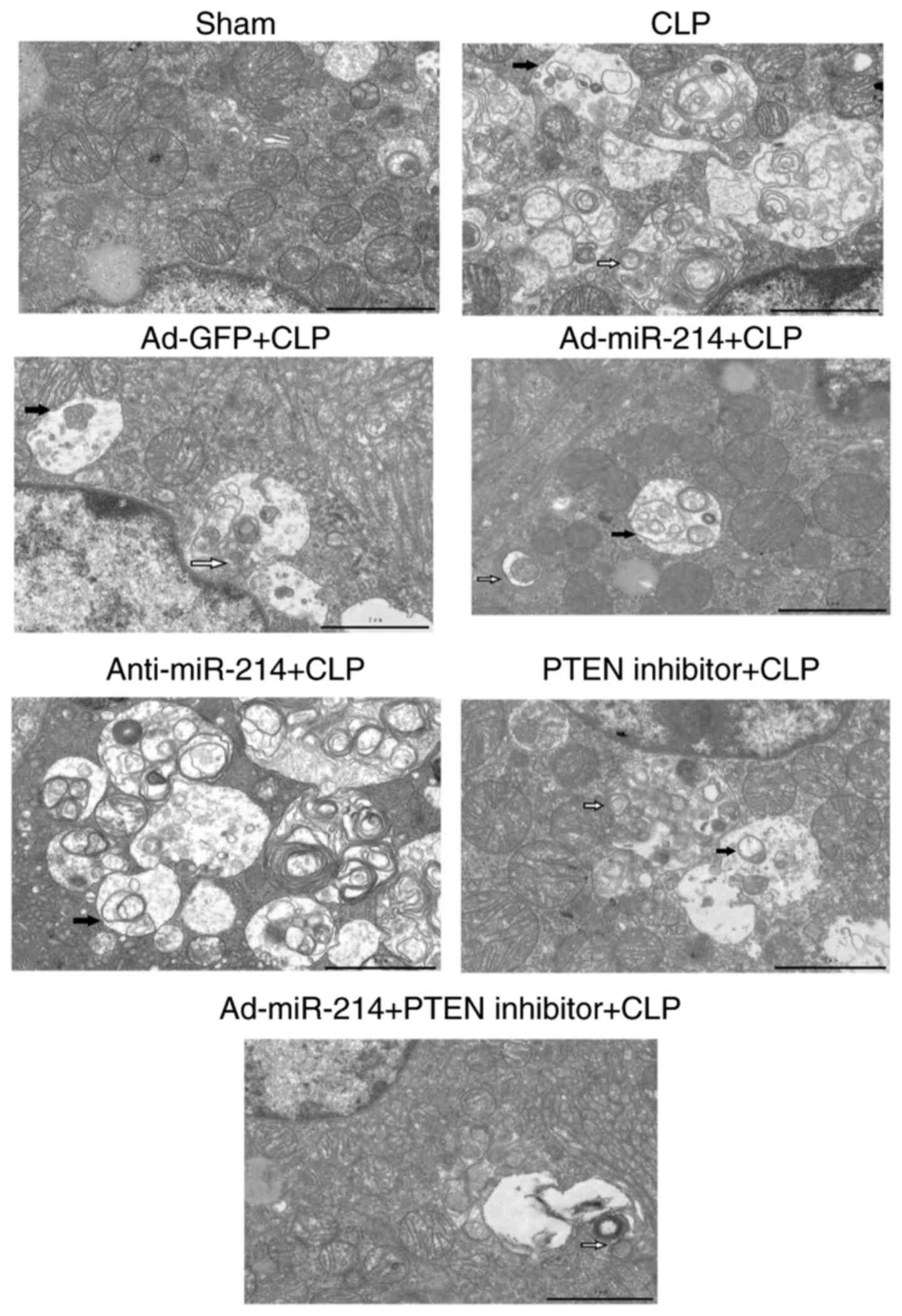
Effect of miR-214 on renal
ultrastructural changes
Fig. 5 shows that in
the sham group, cells had an intact nuclear morphology and cell
mitochondria exhibited normal morphology with clearly discernible
cristae. In the CLP group, mitochondria defects were observed, such
as swelling, disorganization, reduction or vanishing of the cristae
and increasing mitophagy (white arrow) and autolysosomes (black
arrow). Ad-miR-214 alleviated cell mitochondria injury and
mitophagy, which were induced by CLP. Anti-miR-214 aggravated cell
mitochondria injury and mitophagy, which were induced by CLP.
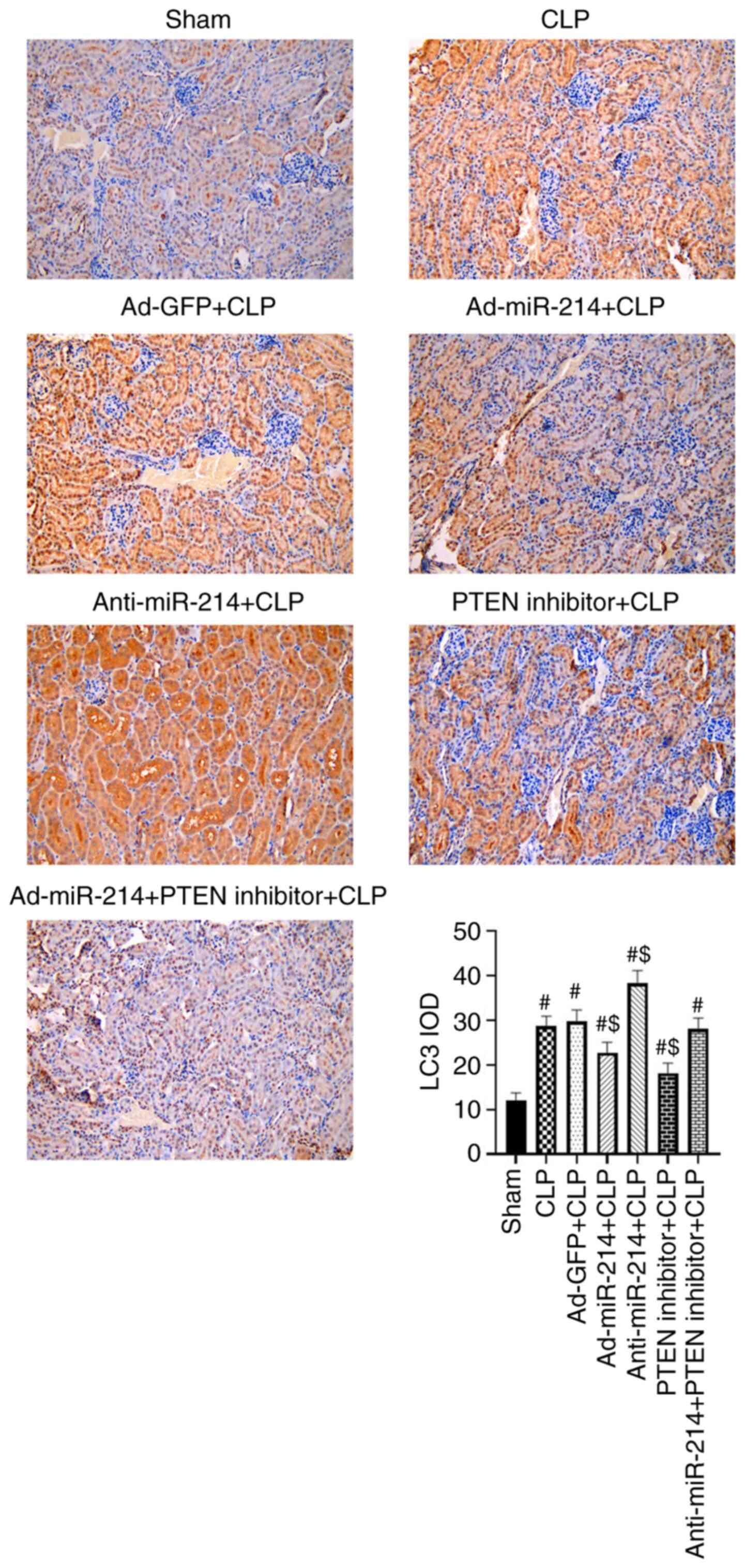
Effect of miR-214 on renal autophagy
in septic mice
The present study examined the changes in LC3 in
kidney tissues via IHC staining. As shown in Fig. 6, the LC3 intensity of positive
staining increased significantly in the CLP group compared with the
sham group (P<0.01) due to the activated autophagy. The
expression level of LC3 was lower in the Ad-miR-214 group and
higher in the anti-miR-214 group in comparison with the CLP group
(P<0.01). However, the administration of PTEN inhibitor enhanced
the inhibition of autophagy effect of Ad-miR-214.
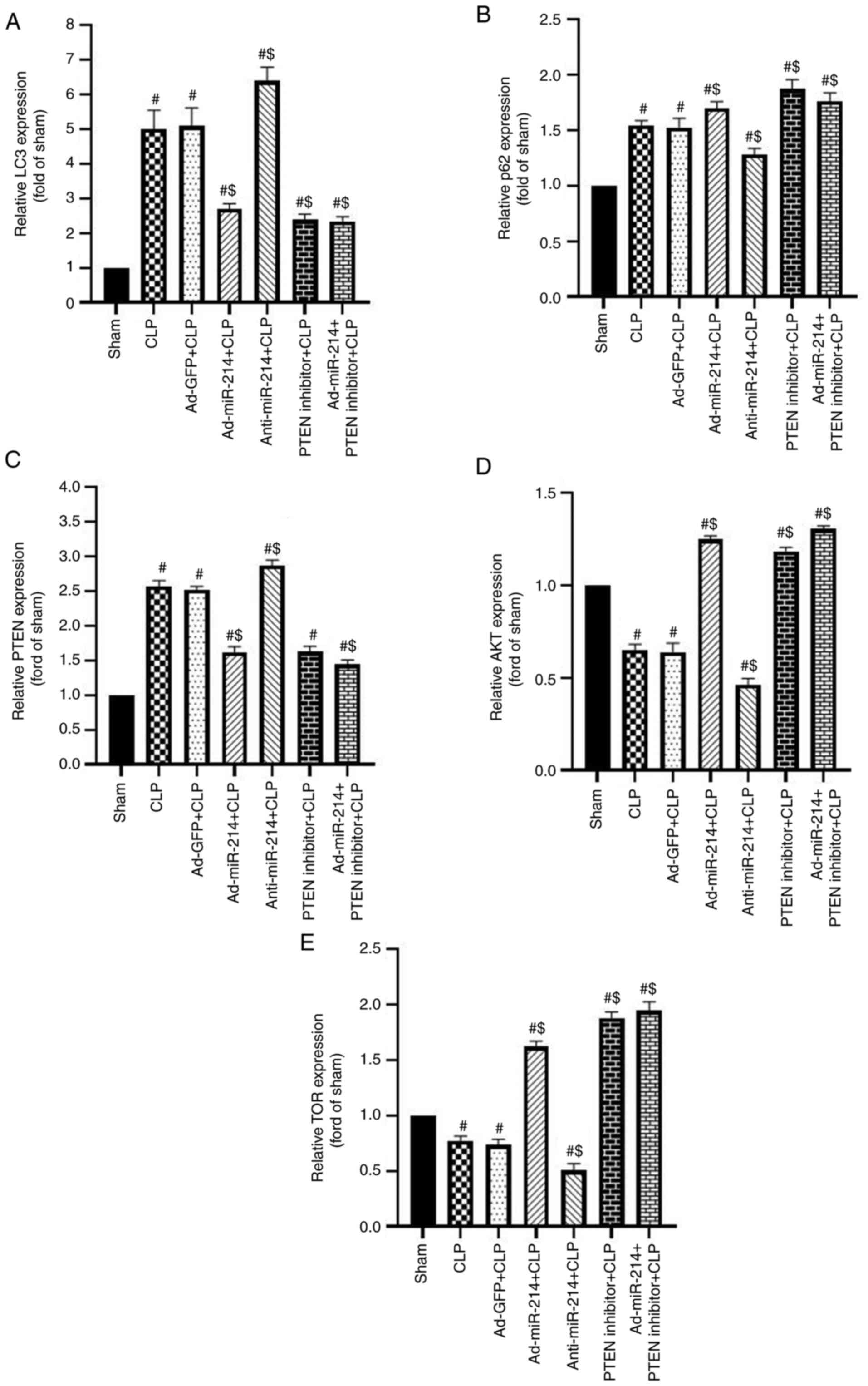
miR-214 activates the AKT/mTOR pathway
to inhibit autophagy by silencing PTEN in kidney tissues
The effect of CLP on autophagy in kidney tissues was
investigated by assessing the levels of LC3-II/I and p62. The
PTEN/AKT/mTOR signaling pathway serves an important role in
autophagy (18). To investigate the
effect of miR-214 on the PTEN-AKT/mTOR pathway, the protein levels
of LC3-II/I, p62, AKT, p-AKT, mTOR, p-mTOR and PTEN were analyzed
through western blotting and RT-qPCR analysis. As shown in Fig. 7A-C, modification in these proteins
rapidly occurs, with an increase in the rate of LC3-II/LC3-I, and
reduction in the levels of p62 (both P<0.01) in the CLP-induced
sepsis group. Compared with the CLP group, the rate of LC3-II/LC3-I
was significantly decreased, while the level of p62 (both
P<0.01) was significantly increased in the Ad-miR-214 group.
However, the inhibition of miR-214 displayed an opposite tendency
to the above indicators (both P<0.01). By contrast, the negative
control had no effect on the changes in the rate of LC3-II/LC3-I
and the levels of p62 in kidney tissues (both P>0.05). The two
indicators of LC3-II/LC3-I and p62 had no significant difference
among the Ad-miR-214 group, the PTEN inhibitor group and the
Ad-miR-214 + PTEN inhibitor group (all P>0.05). These results
showed that autophagy was induced by CLP, and the overexpression of
miR-214 could partially inhibit it.
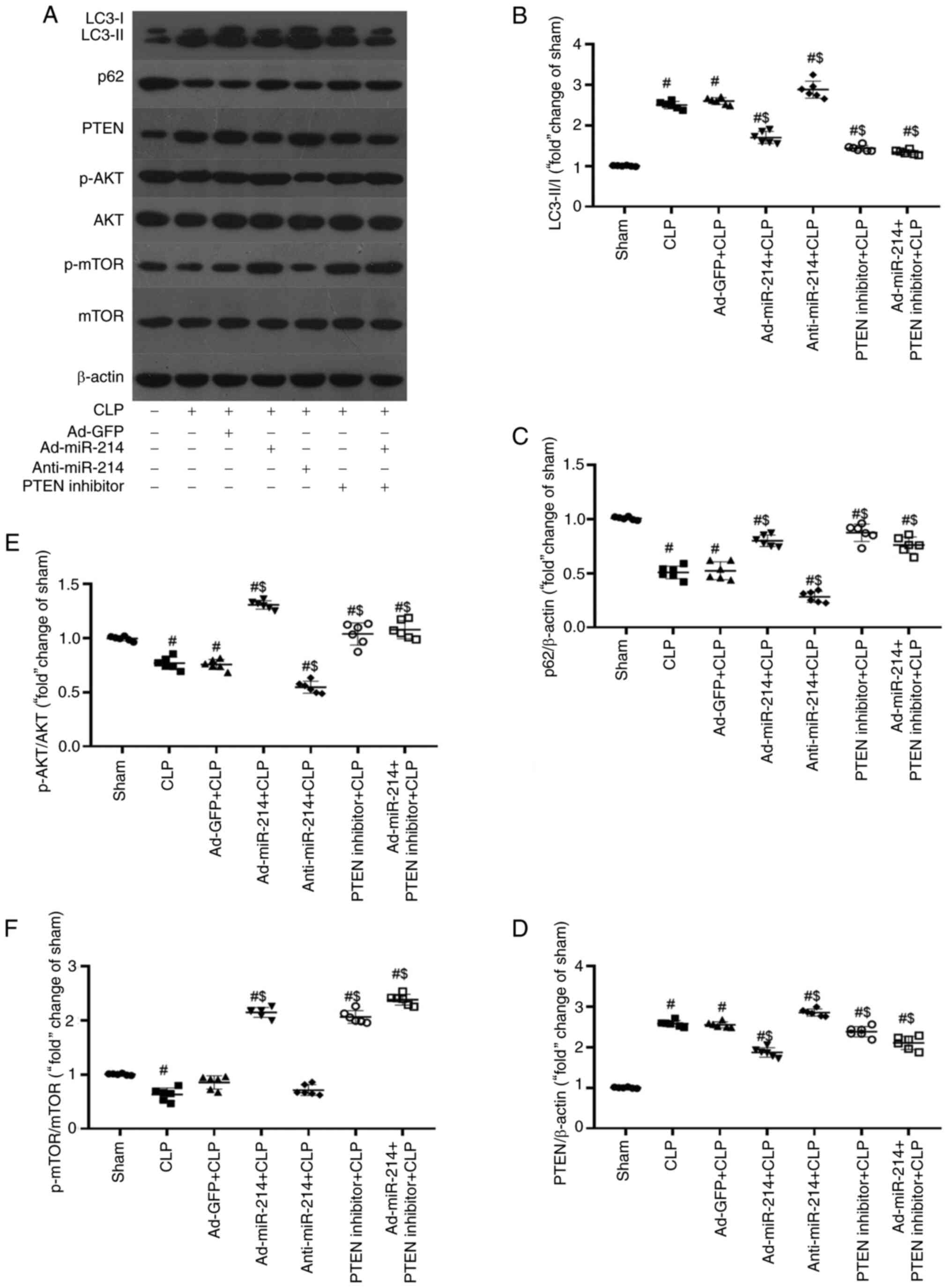 | Figure 7.miR-214 inhibits autophagy by
regulating the PTEN/AKT/mTOR pathway. miR-214 inhibits renal
autophagy and activates autophagy-related AKT/mTOR pathway by
silencing PTEN. (A) Representative image of immunoblotting of LC3,
p62, PTEN, p-AKT, AKT, p-mTOR, and mTOR with β-actin as loading
control. (B and C) Effect of miR-214 on renal autophagy, western
blot analyses two autophagy markers LC3-II/I and p62, along with
control protein β-actin. (D-F) Effect of miR-214 on the
autophagy-related PTEN/AKT/mTOR pathway. n=6, #P<0.01
vs. sham group; $P<0.01 vs. CLP group. miR, microRNA;
p-, phosphorylated; Ad, adenovirus CLP, cecal ligation and
puncture. |
As shown in Fig. 7A and
D-F, the expression level of PTEN (P<0.01) was increased,
and the expression levels of p-AKT (Ser473) and p-mTOR (Ser2448)
(P<0.01) were decreased by CLP. Compared with the CLP group, the
expression level of PTEN was reduced, while those of p-AKT and
p-mTOR (all P<0.01) were subsequently increased in the
Ad-miR-214 group. By contrast, the negative control had no effect
on the changes in the expression levels of PTEN, p-AKT and p-mTOR
in kidney tissues (all P>0.05). There was no significant
difference in the above indicators among the Ad-miR-214 group, the
PTEN inhibitor group and the Ad-miR-214 + PTEN inhibitor group (all
P>0.05). These findings suggests that CLP induces kidney tissue
autophagy by inhibiting the AKT/mTOR pathway. Ad-miR-214 activated
the AKT/mTOR pathway by silencing PTEN in kidney tissues. However,
compared with the CLP group, anti-miR-214 did not significantly
inhibited the expression of mTOR. Therefore, RT-qPCR was further
used to determine the expression of mRNA of genes related to the
PTEN/AKT/mTOR signaling pathway. According to the results of
RT-qPCR (Fig. 8), in comparison
with the Sham group, the mRNA expression levels of p62, LC3 and
PTEN were markedly increased, while the mRNA expression of AKT and
mTOR were decreased in the CLP group. Compared with the CLP group,
the Ad-miR-214, PTEN inhibitor and Ad-miR-214 + PTEN inhibitor
groups displayed decreased mRNA expression of LC3 and PTEN, but
increased mRNA expression of p62, AKT and mTOR (all P<0.05); in
the anti-miR-214 group, an opposite changing tendency was observed
(all P<0.05). There was no significant difference in the above
indicators among the Ad-miR-214 group, the PTEN inhibitor group and
the Ad-miR-214 + PTEN inhibitor group (all P>0.05). Thus, the
effect of p62 on autophagy may occur at the transcriptional level
rather than at the post-transcriptional level. The current results
demonstrated that miR-214 downregulated the expression of PTEN and
activated the AKT/mTOR signaling pathway.
Discussion
The present study found that excessive autophagy was
detrimental to renal function in septic mice. Previous studies have
shown that miR-214 is associated with increased proliferation,
metastasis, invasion and functions as an oncogene for cells and
tissues (19–22). Studies report that miR-214 serves a
protective role against AKI via attenuating apoptosis, oxidative
stress and downregulating inflammatory factors (21,23).
The present study further examined the mechanisms underlying the
protective effect of miR-214 on sepsis-induced AKI by focusing on
the potential involvement of miR-214 in the modulation of
autophagy.
The results showed that oxidative stress occurred in
the kidney following the administration of CLP surgery. Meanwhile,
the activation of autophagy occurs in the kidney. Elevated LC3 II/I
and the reduction of p62 in the kidney was observed following
treatment with CLP surgery. As expected, the overexpression of
miR-214 significantly attenuated kidney pathological injuries and
kidney dysfunction caused by sepsis. However, the inhibition of
miR-214 displayed an opposite tendency to renoprotection. In
addition, the protection effect of Ad-miR-214 was enhanced by PTEN
inhibitor.
In a septic kidney, excessive inflammation is
accompanied by massive increases in the production of ROS and a
large number of ROS triggers changes in mitochondrial structure and
impairs mitochondrial function, which causes the body to enter a
vicious cycle which aggravates kidney damage (24,25).
Therefore, oxidative stress may be one of the main pathological
mechanisms of sepsis-induced AKI. The present study provided
further evidence demonstrating excessive oxidative stress, which
was indicated by increased MDA production and reduced SOD activity
in the kidney tissues following CLP surgery. Moreover, it was found
that the overexpression of miR-214 could protect the kidney against
CLP-induced oxidative injury, which is involved in autophagic
inhibition. This is consistent with the previous studies that
miR-214 protects various cells and tissues against oxidative stress
(26,27).
Autophagy serves as a ‘double-edged sword’ in the
development of sepsis: Basal autophagy can exert protective effects
by removing toxic oxidative proteins, but excessive autophagy may
lead to autophagic cellular death under severe stress, such as ROS
eruption (28,29). It has been shown that autophagy is
activated initially in sepsis, followed by a subsequent phase of
dysfunction due to autophagic cell death (30), which aggravates sepsis-induced
oxidative injury. In the present study, PTEN inhibitor or
overexpression of miR-214 displayed antioxidative renoprotection in
CLP-treated mice. However, the inhibition of miR-214 displayed an
opposite tendency to antioxidative renoprotection. So excessive or
inadequate autophagy can cause kidney damage; both are maladaptive
and eventually provoke cell death. Hence, keeping moderate levels
of autophagy is the key to attenuate oxidative injury in septic
condition.
Accumulating evidence has indicated that miR-214
inhibits autophagy in various cells and tissues (31–33).
In the present study, the overexpression of miR-214 was found to
inhibit CLP-induced autophagy in kidney tissues, as indicated by
the changes in protein markers, such as LC3-II/I and p62.
Furthermore, the results demonstrated that overexpression of
miR-214 attenuated CLP-induced oxidative injury by inhibiting
excessive autophagy. The present study also investigated the
molecular mechanisms by which miR-214 modulates kidney autophagy in
sepsis-induced AKI. PTEN/AKT/mTOR is an important signaling pathway
that regulates kidney autophagy (34,35)
and which serves a key role in sepsis-induced AKI, and PTEN is a
negative inhibitor of the PI3K/AKT/mTOR signaling pathway. Previous
studies reported that miR-214 can regulate autophagy through the
PI3K/AKT/mTOR signaling pathway in various models (36–38).
Ma et al (39) found that
miR-214 can downregulate autophagy in diabetic kidneys. Therefore,
it was hypothesized that the effects of miR-214 on CLP-induced AKI
might be involved in the regulation of the PTEN/AKT/mTOR pathway.
Notably, the results of the present study showed that CLP surgery
significantly elevated the levels of PTEN but decreased the levels
of p-AKT and p-mTOR, indicating that CLP surgery inactivated the
AKT/mTOR pathway. In addition, the overexpression of miR-214
activated the AKT/mTOR pathway by the silencing of PTEN in
CLP-induced autophagy in kidney tissues. However, anti-miR-214 did
not significantly inhibited the expression of mTOR in western blot
analyses. Thus, RT-qPCR was further used to determine the
expression of mRNA of genes related to the PTEN/AKT/mTOR signaling
pathway. The present study identified that miR-214 downregulated
the expression of PTEN and activated the AKT/mTOR signaling pathway
to inhibit autophagy. However, further exhaustive studies should be
carried out to obtain additional details related to the mechanism
of kidney autophagy in septic condition.
To conclude, the results of the present study
suggested that miR-214 served a protective role against
sepsis-induced kidney injury by reducing oxidative stress and
inhibiting autophagy through regulation of the PTEN/AKT/mTOR
pathway. The present findings suggested that miR-214 may be a
potential therapeutic target for sepsis-induced AKI. However, the
present study used mice 6–8 week old, so further research on the
mechanism of miR-214 is needed in adult mice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS and SD contributed conception and design of the
study. ZS and YW conducted experiments. ZS and SD organized the
database. PZ and ZS performed the statistical analysis. ZS wrote
the manuscript. All authors contributed to manuscript revision,
read and approved the final version of the manuscript. ZS and SD
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study adhered to the Guide for the Care
and Use of Laboratory Animals published by the US National
Institutes of Health (NIH Publication no. 85-23, revised 1996) and
all experimental protocols were approved by the Animal
Experimentation Ethics Committee of Cangzhou Central Hospital
(approval no. 2017-020-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Plotnikov EY, Pevzner IB, Zorova LD,
Chernikov VP, Prusov AN, Kireev II, Silachev DN, Skulachev VP and
Zorov DB: Mitochondrial damage and mitochondria-targeted
antioxidant protection in LPS-induced acute kidney injury.
Antioxidants (Basel). 8:1762019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Wang L, Meng L, Cao G and Wu Y:
Sirtuin 6 overexpression relieves sepsis-induced acute kidney
injury by promoting autophagy. Cell Cycle. 18:425–436. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao W, Zhang L, Chen R, Lu H, Sui M, Zhu
Y and Zeng L: SIRT3 protects against acute kidney injury via
AMPK/mTOR-regulated autophagy. Front Physiol. 9:15262018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lelubre C and Vincent JL: Mechanisms and
treatment of organ failure in sepsis. Nat Rev Nephrol. 7:417–427.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Greco E, Lupia E, Bosco O, Vizio B and
Montrucchio G: Platelets and Multi-Organ failure in sepsis. Int J
Mol Sci. 18:22002017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu Y, Wang L, Meng L, Cao GK, Zhao YL and
Zhang Y: Biological effects of autophagy in mice with
sepsis-induced acute kidney injury. Exp Ther Med. 17:316–322.
2019.PubMed/NCBI
|
|
7
|
Yu T, Liu D, Gao M, Yang P, Zhang M, Song
F, Zhang X and Liu Y: Dexmedetomidine prevents septic myocardial
dysfunction in rats via activation of α7nAChR and PI3K/Akt-mediated
autophagy. Biomed Pharmacother. 120:1092312019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hotchkiss RS, Strasser A, McDunn JE and
Swanson PE: Cell death. N Engl J Med. 361:1570–1583. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu X, Li W and Li H: miR-214 ameliorates
acute kidney injury via targeting DKK3 and activating of
Wnt/β-catenin signaling pathway. Biol Res. 51:312018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang S, Fei X, Lu Y, Xu B, Ma Y and Wan H:
miRNA-214 suppresses oxidative stress in diabetic nephropathy via
the ROS/Akt/mTOR signaling pathway and uncoupling protein 2. Exp
Ther Med. 17:3530–3538. 2019.PubMed/NCBI
|
|
11
|
Sang Z, Zhang P, Wei Y and Dong S:
miR-214-3p Attenuates sepsis-induced myocardial dysfunction in mice
by inhibiting autophagy through PTEN/AKT/mTOR Pathway. Biomed Res
Int. 2020:14090382020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takahashi W, Watanabe E, Fujimura L,
Watanabe-Takano H, Yoshidome H, Swanson PE, Tokuhisa T, Oda S and
Hatano M: Kinetics and protective role of autophagy in a mouse
cecal ligation and puncture-induced sepsis. Crit Care. 17:R1602013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang C, Han H, Yan M, Zhu S, Liu J, Liu Z,
He L, Tan J, Liu Y, Liu H, et al: PINK1-PRKN/PARK2 pathway of
mitophagy is activated to protect against renal
ischemia-reperfusion injury. Autophagy. 14:880–897. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sunahara S, Watanabe E, Hatano M, Swanson
PE, Oami T, Fujimura L, Teratake Y, Shimazui T, Lee C and Oda S:
Influence of autophagy on acute kidney injury in a murine cecal
ligation and puncture sepsis model. Sci Rep. 8:10502018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou RX, Li XH, Qu Y, Li SP and Huang Q:
Role of p38 mitogen-activated protein kinase signaling pathway in
the hippocampal neurons autophagy of rats with sepsis. Sichuan Da
Xue Xue Bao Yi Xue Ban. 50:512–519. 2019.(In Chinese). PubMed/NCBI
|
|
17
|
Vaidya VS, Ozer JS, Dieterle F, Collings
FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder
DJ, et al: Kidney injury molecule-1 outperforms traditional
biomarkers of kidney injury in preclinical biomarker qualification
studies. Nat Biotechnol. 5:478–485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu X, Fan Q, Xu L, Li L, Yue Y, Xu Y, Su
Y, Zhang D and Wang L: Ursolic acid attenuates diabetic mesangial
cell injury through the up-regulation of autophagy via
miRNA-21/PTEN/Akt/mTOR suppression. PLoS One. 2:e01174002015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Chen W, Zhang H, Liu T and Zhao L:
miR-214 targets the PTEN-mediated PI3K/Akt signaling pathway and
regulates cell proliferation and apoptosis in ovarian cancer. Oncol
Lett. 14:5711–5718. 2017.PubMed/NCBI
|
|
20
|
Gao Y, Fang P, Li WJ, Zhang J, Wang GP,
Jiang DF and Chen FP: LncRNA NEAT1 sponges miR-214 to regulate M2
macrophage polarization by regulation of B7-H3 in multiple myeloma.
Mol Immunol. 117:20–28. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan Z, Zang B, Gong X, Ren J and Wang R:
MiR-214-3p exacerbates kidney damages and inflammation induced by
hyperlipidemic pancreatitis complicated with acute renal injury.
Life Sci. 241:1171182020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yahya SMM and Yahya SMM: The Effect of
miR-98 and miR-214 on apoptotic and angiogenic pathways in
hepatocellular carcinoma HepG2 cells. Indian J Clin Biochem.
35:353–358. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan Y, Ma Z, Zhu J, Zeng M, Liu H and Dong
Z: miR-214 represses mitofusin-2 to promote renal tubular apoptosis
in ischemic acute kidney injury. Am J Physiol Renal Physiol.
318:F878–F887. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lowes DA, Webster NR, Murphy MP and Galley
HF: Antioxidants that protect mitochondria reduce interleukin-6 and
oxidative stress, improve mitochondrial function, and reduce
biochemical markers of organ dysfunction in a rat model of acute
sepsis. Br J Anaesth. 110:472–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu H, Jin F, Liu D, Shu G, Wang X, Qi J,
Sun M, Yang P, Jiang S, Ying X and Du Y: ROS-responsive nano-drug
delivery system combining mitochondria-targeting ceria
nanoparticles with atorvastatin for acute kidney injury.
Theranostics. 10:2342–2357. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu XZ, Yang ZH, Zhang HJ, Zhu LL, Mao XL
and Yuan Y: MiR-214 protects MC3T3-E1 osteoblasts against
H2O2-induced apoptosis by suppressing oxidative stress and
targeting ATF4. Eur Rev Med Pharmacol Sci. 21:4762–4770.
2017.PubMed/NCBI
|
|
27
|
Wang Y, Zhao R, Liu D, Deng W, Xu G, Liu
W, Rong J, Long X, Ge J and Shi B: Exosomes derived from
miR-214-enriched bone marrow-derived mesenchymal stem cells
regulate oxidative damage in cardiac stem cells by targeting
CaMKII. Oxid Med Cell Longev. 2018:49712612018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Decuypere JP, Parys JB and Bultynck G:
Regulation of the autophagic bcl-2/beclin 1 interaction. Cells.
1:284–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lei S, Zhang Y, Su W, Zhou L, Xu J and Xia
ZY: Remifentanil attenuates lipopolysaccharide-induced oxidative
injury by downregulating PKCβ2 activation and inhibiting autophagy
in H9C2 cardiomyocytes. Life Sci. 213:109–115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ho J, Yu J, Wong SH, Zhang L, Liu X, Wong
WT, Leung CC, Choi G, Wang MH, Gin T, et al: Autophagy in sepsis:
Degradation into exhaustion? Autophagy. 12:1073–1082. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Li Q, Liu C, Gao S, Ping H, Wang
J and Wang P: MiR-214-3p attenuates cognition defects via the
inhibition of autophagy in SAMP8 mouse model of sporadic
Alzheimer's disease. Neurotoxicology. 56:139–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu X, Luo A, Liu Y, Wang S, Li Y, Shi W,
Liu Z and Qu X: MiR-214 increases the sensitivity of breast cancer
cells to tamoxifen and fulvestrant through inhibition of autophagy.
Mol cancer. 14:2082015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Wang WN, Xu SB, Wu H, Dai B, Jian
DD, Yang M, Wu YT, Feng Q, Zhu JH, et al: MicroRNA-214-3p: A link
between autophagy and endothelial cell dysfunction in
atherosclerosis. Acta Physiol (Oxf). Oct 14–2018.(Epub ahead of
print). doi: 10.1111/apha.12973. View Article : Google Scholar
|
|
34
|
Li XY, Wang SS, Han Z, Han F, Chang YP,
Yang Y, Xue M, Sun B and Chen LM: Triptolide restores autophagy to
alleviate diabetic renal fibrosis through the
miR-141-3p/PTEN/Akt/mTOR pathway. Mol Ther Nucleic Acids. 9:48–56.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song N, Zhang T, Xu X, Lu Z, Yu X, Fang Y,
Hu J, Jia P, Teng J and Ding X: miR-21 protects against
ischemia/reperfusion-induced acute kidney injury by preventing
epithelial cell apoptosis and inhibiting dendritic cell maturation.
Front Physiol. 9:7902018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu Q, Shang Y, Shen T, Liu F, Xu Y and
Wang H: Neuroprotection of miR-214 against isoflurane-induced
neurotoxicity involves the PTEN/PI3K/Akt pathway in human
neuroblastoma cell line SH-SY5Y. Arch Biochem Biophys.
678:1081812019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gong L, Xu H, Zhang X, Zhang T, Shi J and
Chang H: Oridonin relieves hypoxia-evoked apoptosis and autophagy
via modulating microRNA-214 in H9c2 cells. Artif Cells Nanomed
Biotechnol. 47:2585–2592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu M, Li Z, Liang B, Li L, Liu S, Tan W,
Long J, Tang F, Chu C and Yang J: Hydrogen sulfide ameliorates rat
myocardial fibrosis induced by thyroxine through PI3K/AKT signaling
pathway. Endocr J. 65:769–781. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma Z, Li L, Livingston MJ, Zhang D, Mi Q,
Zhang M, Ding HF, Huo Y, Mei C and Dong Z: p53/microRNA-214/ULK1
axis impairs renal tubular autophagy in diabetic kidney disease. J
Clin Invest. 130:5011–5026. 2020. View Article : Google Scholar : PubMed/NCBI
|