Introduction
Asthma is a common chronic inflammatory disorder
with heterogeneous pathophysiology, characterized by airway
inflammation, variable airflow obstruction and bronchial
hyperresponsiveness (1). Asthma is
thought to affect ~300 million individuals worldwide and the number
of patients with asthma is predicted to reach 400 million by 2025
(2,3). Although various compounds have been
reported to be effective in the treatment of asthma, inhaled
corticosteroids (ICS) remain an irreplaceable pharmacologic
treatment for asthma in clinical settings (2). However, some patients with refractory
asthma are resistant to corticosteroid treatment and long-term use
of ICS causes obvious side effects, such as obesity, diabetes,
hypertension and depression (4,5).
Therefore, it is important to identify new compounds and develop
novel medicines for treating asthma.
The asthmatic pathology is a T helper type 2
(Th2)-mediated immune response that is associated with the
production of interleukin (IL)-4, IL-5, IL-13 and immunoglobulin
(Ig)E, as well as pulmonary eosinophil infiltration, mast cell
activation and Th2 lymphocyte activation (6). In response to allergen stimulation,
injured airway epithelial cells generate various bioactive
molecules, such as C-C motif chemokine ligand (CCL)5, CCL2, CCL22
and IL-8, all of which promote inflammatory cell infiltration and
macrophage polarization (7). In
response to type 2 cytokines, recruited macrophages are polarized
to alternatively activated (M2) subtypes, which contribute to the
overactivation of Th2 cells, inflammatory deterioration and
progressive lung injury (8,9). Therefore, manipulating macrophage
polarization is a promising treatment strategy for asthma.
Traditional medicine is an important resource for
developing new medicines because plant-based natural compounds are
successfully used to treat numerous diseases (10). Sesquiterpene lactones are a group of
natural compounds with various biological activities, including
anti-tumor, anti-inflammatory and mycobacterial activities
(11–13). Isoalantolactone (IAL) is a
sesquiterpene lactone extracted from the roots of Inula
helenium L. and has been known to possess several bioactive
effects (14), including
antibacterial (15) and
anti-lipogenic effects (16) and
has proven efficacy in treating lung injuries (17) and pulmonary fibrosis (17). Recently, the anti-inflammatory
activity of IAL has been identified in lipopolysaccharide
(LPS)-induced acute lung injury and sepsis disorders by inhibition
of nuclear factor E2-related factor 2 (Nrf2), NF-κB and tumor
necrosis factor receptor associated factor 6 (TRAF6) ubiquitination
(18–20). However, it is still unknown whether
IAL has a pharmacological effect on asthmatic inflammation.
The present study determined the effect of IAL on
ovalbumin (OVA)-induced asthmatic inflammation. It was found that
treatment with IAL attenuated OVA-induced eosinophil infiltration,
IgE generation and the production of IL-4, IL-5, CCL17 and CCL22.
In addition, IAL alleviated OVA-induced asthmatic inflammation by
inhibiting alternatively activated macrophages, with a reduction in
STAT6 phosphorylation and the expression of peroxisome
proliferator-activated receptor (PPAR)-γ and Krüppel-like factor 4
(KLF4). The results indicated that IAL is a promising agent for the
treatment of asthma.
Materials and methods
Mice
A total of 60 Specific pathogen-free (SPF) female
BALB/c mice, 6–8 weeks of age, with an average weight of 20–25 g,
were purchased from Slac Laboratory Animal Corporation and housed
in SPF conditions (~19–27°C, 12-h light/dark cycle and ~40–70%
humidity). All experimental protocols described in the present
study were approved by the Institutional Animal Care and Use
Committee of Shanghai Pudong Hospital and the procedures were
approved by the Biological Research Ethics Committee of Shanghai
Pudong Hospital.
Compound and reagents
IAL (cat. no. C15H20O2; MW, 232.31; purity >98%)
was purchased from TargetMol; OVA (Grade V) was purchased from
Sigma-Aldrich (Merck KGaA); Dulbecco's modified Eagle's medium
(DMEM), antibiotics (10,000 units/ml penicillin and 10,000 µg/ml
streptomycin), 0.25% trypsin and phosphate buffered saline (PBS)
were purchased from Thermo Fisher Scientific, Inc.; Fetal bovine
serum (FBS) was purchased from EVERY GREEN (Zhejiang Tianhang
Biotechnology Co., Ltd.); and bicinchoninic acid (BCA) protein
assay kit was purchased from Beyotime Institute of Biotechnology.
The primary antibodies used in the present study were synthesized
by Shanghai HuaGen Biotech Co., Ltd.. Phosphorylated (p)-STAT6
(Tyr641; cat. no. 56554), total (t)-STAT6 (cat. no. 5397), PPAR-γ
(cat. no. 2443), KLF4 (cat. no. 12173) and β-actin (cat. no. 3700)
were purchased from Cell Signaling Technology, Inc. The peroxidase
AffiniPure goat anti-rabbit IgG (H + L; cat. no. 111-035-003) and
peroxidase AffiniPure goat anti-mouse IgG (H + L) (cat. no.
115-035-003) secondary antibodies were purchased from Jackson
ImmunoResearch Laboratories and diluted in EZ-Buffers (cat. no.
C520011-0100; Sangon Biotech Co., Ltd.). Other chemical reagents
were obtained from Sigma-Aldrich. Loading buffer was prepared by 1M
TrisCl (cat. no. A610195; Sangon Biotech Co., Ltd.), 10% SDS (cat.
no. B548118; Sangon Biotech Co., Ltd.), 7.5 mM bromophenol blue
(cat. no. 60503ES08; Shanghai Yeasen Biotechnology Co., Ltd.) 25%
glycerin (cat. no. A501745; Sangon Biotech Co., Ltd.) and 35.75 µM
β-mercaptoethanol (cat. no. 200-464-6; Sigma-Aldrich; Merck
KGaA).
OVA-induced asthma mouse model
Mice were randomly divided into three groups as
follows: PBS + vehicle group, OVA + vehicle group and OVA + IAL
group. IAL was dissolved in a vehicle comprising castor
oil:ethanol: Physiological saline at a 1:1:8 ratio. For the OVA +
vehicle group, mice were sensitized on days 0, 7 and 14 [40 µg OVA
and 40 mg Al(OH)3 in 0.2 ml PBS, intraperitoneal (i.p.)]
and challenged at days 25, 26 and 27 [10 µg OVA in 0.2 ml PBS,
intranasal (i.n.)]. For the OVA + IAL group, following
sensitization, mice were administered IAL (20 mg/kg, i.p.) once
daily from days 22 to 27, together with challenging at days 25, 26
and 27 (10 µg OVA in 0.2 ml PBA, i.n.). For the PBS + vehicle
group, mice were injected with PBS (0.2 ml, i.p.) on days 0, 7 and
14 and the same volume of vehicle as the mice in the OVA + IAL
group. Mice were anesthetized with sodium pentobarbital (50 mg/kg;
i.p.) to collect samples on day 28 and blood, bronchoalveolar
lavage fluid (BALF) and lung tissues were collected for further
analysis (20).
Analysis of protein concentration in
BALF
The BALF was centrifuged at 500 × g, 4°C following
collection. The supernatant was collected for analysis of protein
concentration using the BCA protein assay kit according the
manufacturer's protocols.
Lung histology
After fixing with 4% paraformaldehyde (Sangon
Biotech Co., Ltd.) overnight, the left lung was embedded in
paraffin and dehydrated using graded ethanol. Then the tissues were
cut into 5 µm thick sections for haematoxylin and eosin (H&E)
staining (Nanjing Jiancheng Bioengineering Institute). Images were
captured using a microscope (RX51; Olympus Corporation).
Preparation of bone marrow-derived
macrophages
Mice were sacrificed by cervical dislocation
following anesthesia as aforementioned. Mouse bone marrow-derived
macrophages (BMDMs) were isolated from BALB/c mice and incubated
with DMEM supplemented with 10% FBS, 1% penicillin/streptomycin at
37°C and 10 ng/ml macrophage colony-stimulating factor (M-CSF;
PeproTech, Inc.) for 6 days. BMDMs were incubated at 37°C, 5%
CO2 and stimulated with mouse 10 ng/ml IL-4 (mIL-4;
PeproTech, Inc.) and 0, 3, 10, or 30 µM IAL. Cells and culture
supernatants were collected for RNA and protein expression
analyses.
Western blotting
Western blotting was conducted as previously
described (9). Briefly, BMDMs were
plated in 6-well plates (1×106/well) and incubated
overnight at 37°C, 5% CO2. BMDMs were pre-treated with
IAL (0, 3, 10, or 30 µM) for 30 min and challenged with mIL-4 (10
ng/ml) for 30 min or 24 h, with dimethyl sulfoxide (DMSO) as a
solvent control. Cells were washed twice with PBS, harvested using
RIPA lysis buffer (Beyotime Institute of Biotechnology) and protein
concentration was determined using the BCA method. Subsequently,
the samples were loaded on 10% SDS/PAGE gels with 20 µg protein
loaded per lane, transferred to nitrocellulose filter membranes and
blocked with 5% milk at room temperature for 2 h. The membrane was
incubated with primary antibodies (1:1,000) overnight at 4°C. Blots
were then incubated with secondary antibodies (1:10,000) for 1 h at
room temperature. The antibody-specific proteins were visualized
using ECL western blotting substrate (Thermo Fisher Scientific,
Inc.) β-actin was used as loading control. Quantification of
western blots was performed using ImageJ software 1.4.3.67
(National Institute of Mental Health).
Isolation of total RNA and reverse
transcription-quantitative (RT-q) PCR
Total RNA was extracted from BMDMs and lung tissues
using TRIzol reagent (Thermo Fisher Scientific, Inc.). cDNA was
prepared using the ReverTra Ace RT kit (Toyobo Life Science)
according to the manufacture's protocol. Gene expression values
were quantified by PCR using StepOne Plus (Thermo Fisher
Scientific, Inc.). The following thermocycling conditions were used
for PCR: Initial denaturation at 94°C for 30 sec; 35 cycles of 94°C
for 30 sec, 55°C for 30 sec and 72°C for 2 min; and a final
extension at 72°C for 3 min and stored at 4°C. The primers were
synthesized by Huagene Biosciences. Relative mRNA levels were
normalized to GAPDH mRNA levels. The quantification of RT-qPCR was
based on the ΔΔCq (21). The
sequences of primers used for each gene were as follows: Arginase-1
(Arg-1) (forward, 5′-CAATGAAGAGCTGGCTGGTGT-3′, reverse,
5′-GTGTGAGCATCCACCCAAATG−3′), Ym-1 (forward,
5′-TACTCACTTCCACAGGAGCAGG-3′, reverse,
5′-CTCCAGTGTAGCCATCCTTAGG-3′), CCL17 (forward,
5′-CGAGAGTGCTGCCTGGATTACT-3′, reverse,
5′-GGTCTGCACAGATGAGCTTGCC-3′), CCL22 (forward,
5′-GTGGAAGACAGTATCTGCTGCC-3′ reverse, 5′-AGGCTTGCGGCAGGATTTTGAG-3′)
and GAPDH (forward, 5′-CATCACTGCCACCCAGAAGACTG-3′ reverse,
5′-ATGCCAGTGAGCTTCCCGTTCAG-3′).
Flow cytometry assay
After blocking Fc receptors with Fc blocking
anti-mouse CD16/32 antibody (BD Biosciences), cells were collected
from BALF and incubated with phycoerythrin-conjugated anti-Siglec-F
antibodies (BD Biosciences) and allophycocyanin-conjugated
anti-CD11c antibodies (BioLegend) at 4°C for 30 min. Samples were
analyzed using a flow cytometer (LSRFortessa; BD Biosciences) and
FlowJo v10 software (FlowJo LLC).
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of IL-4 (cat. no. M4000B), IL-5
(cat. no. M5000), CCL17 (cat. no. MCC170) and CCL22 (cat. no.
MCC220) were quantified using ELISA kits from R&D Systems, Inc.
and OVA-specific IgE (cat. no. 88-50460-88) was quantified using an
ELISA kit from Thermo Fisher Scientific, Inc., according to the
manufacturer's instructions.
Statistical analysis
GraphPad Prism 5 (GraphPad Software, Inc.) was used
to perform the data analysis. All data, which were obtained from at
least three independent experiments, were evaluated with one-way
ANOVA and Tukey's test was used to determine the difference between
two groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
IAL ameliorates pathological injury in
OVA-induced asthma
To investigate the effect of IAL on asthmatic
inflammation, an OVA-induced mouse asthma model (Fig. 1A) was established as shown in
Fig. 1, inflammatory cell
infiltration was induced in OVA-induced asthmatic inflammation.
However, IAL pretreatment significantly reduced cell infiltration
(Fig. 1B), indicating that IAL
relieved OVA-induced asthmatic inflammation. In addition, treatment
with IAL did not result in an increase in serum OVA-specific IgE,
whereas OVA treatment significantly increased the expression of
OVA-specific IgE (Fig. 1C).
Furthermore, IAL significantly reduced the protein concentration in
BALF caused by OVA treatment (Fig.
1D). These results indicated that IAL had an inhibitory effect
on asthmatic inflammation.
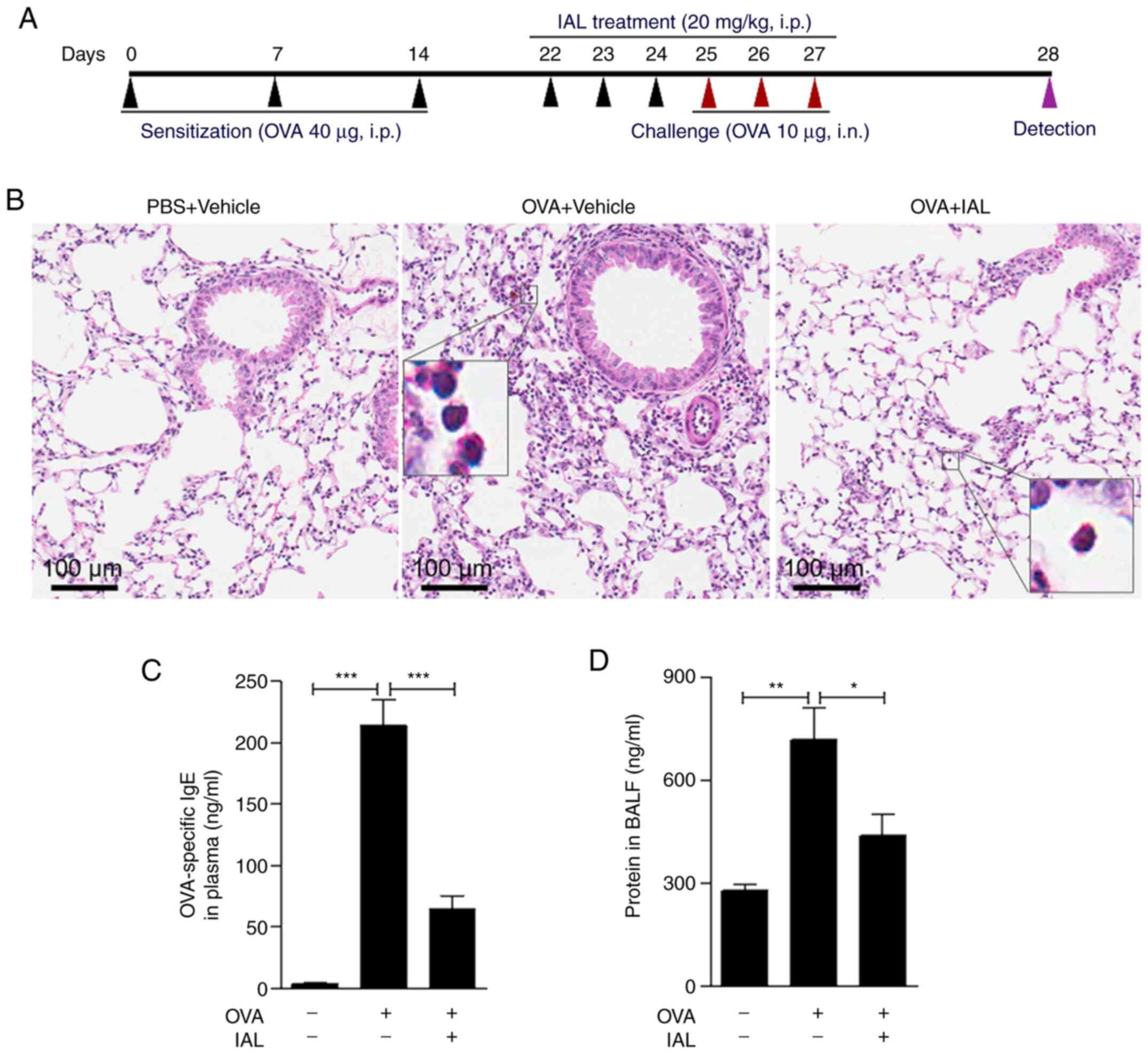 | Figure 1.IAL ameliorates pathologic injury in
OVA-induced asthma. (A) Experimental scheme of the OVA-induced
asthma mouse model and IAL treatment. Eight-week-old BALB/c mice
were i.p. injected with 40 µg OVA or PBS at days 0, 7 and 14 for
sensitization and then i.n. challenged with 10 µg OVA or PBS at
days 25, 26 and 27. Mice received IAL (20 mg/kg, i.p.) or vehicle
at days 22 to 27 and were killed at day 28 for further analysis.
(B) The lung lobes were excised and subject to hematoxylin and
eosin staining. Scale bars, 100 µm; magnification of inserts, ×400.
(C) The content of IgE protein in plasma and the (D) concentration
of total protein in BALF were measured. Values represent means ±
standard error of the mean, n=5 mice in each group, *P<0.05,
**P<0.01, ***P<0.001. IAL, isoalantolactone; OVA, ovalbumin;
i.p., intraperitoneal; i.n., intranasal; BALF, bronchoalveolar
lavage fluid. |
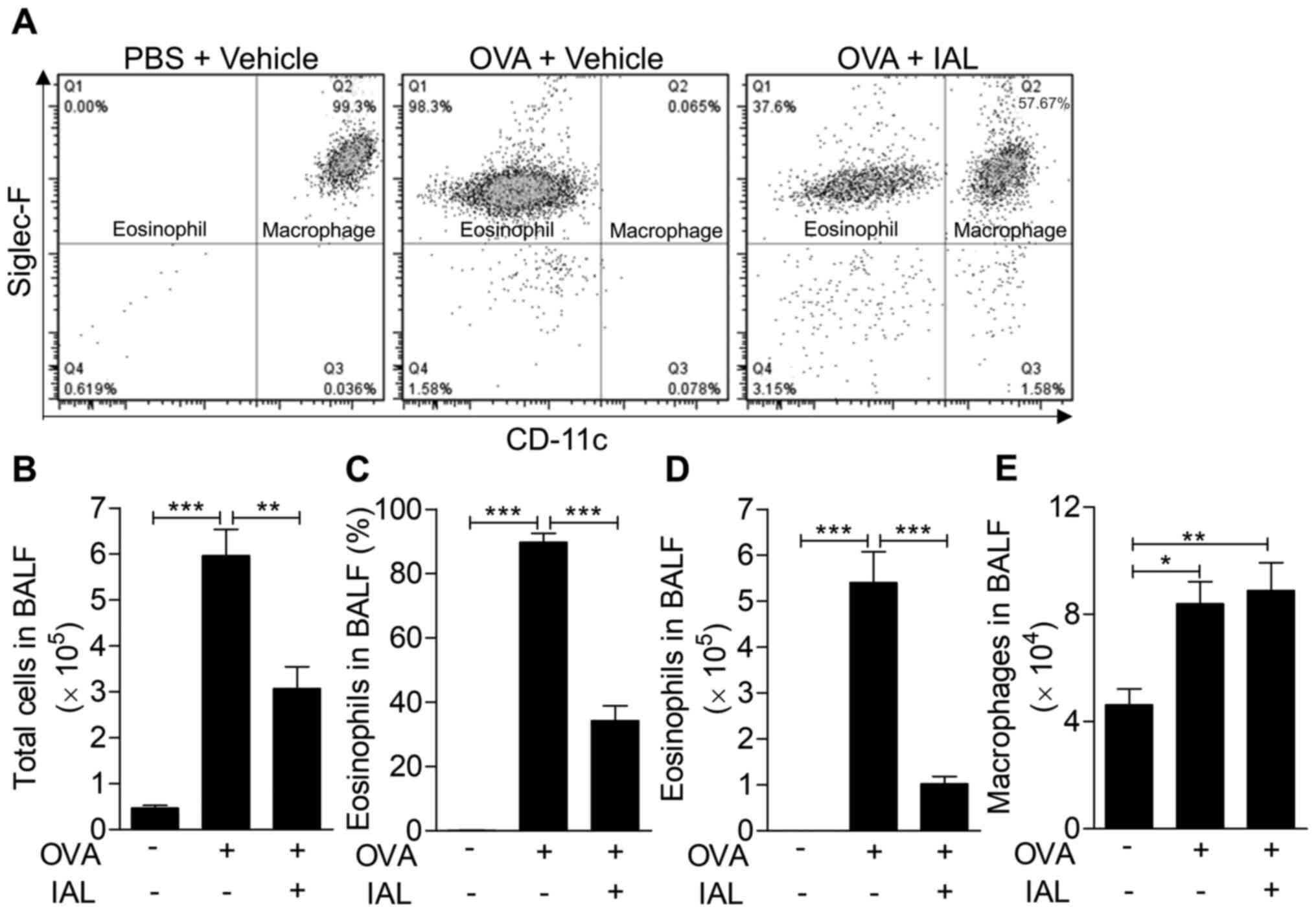
IAL reduces the permeability of lung
tissue and the infiltration of inflammatory cells
In the process of asthma, lung tissues are destroyed
by the infiltration of inflammatory mediators and inflammatory
cells, especially eosinophils, which are significantly increased in
BALF. Therefore, the number of eosinophils in BALF indicates the
severity of asthma (22). Thus, the
accumulation of eosinophils in BALF was measured to determine the
therapeutic effect of IAL on inflammatory cell infiltration. As
shown in Fig. 2, the proportion of
eosinophils increased significantly following OVA induction, while
it was significantly reduced following IAL treatment, from 98.3 to
37.6% (Fig. 2A). In addition, the
number of total cells in the BALF significantly increased following
OVA induction. Following IAL treatment, the total number of cells
in the BALF was significantly lower (Fig. 2B). Consistent with this, the
proportion of eosinophils and the number of eosinophils in BALF
were significantly decreased following IAL treatment (Fig. 2C and D). Furthermore, the absolute
number of macrophages in BALF was detected and it was found that
the total macrophage number in BALF was enhanced following
challenge with OVA. However, IAL treatment did not have an effect
on the OVA-induced number of macrophages (Fig. 2E). These results indicated that IAL
inhibited OVA-induced eosinophil accumulation.
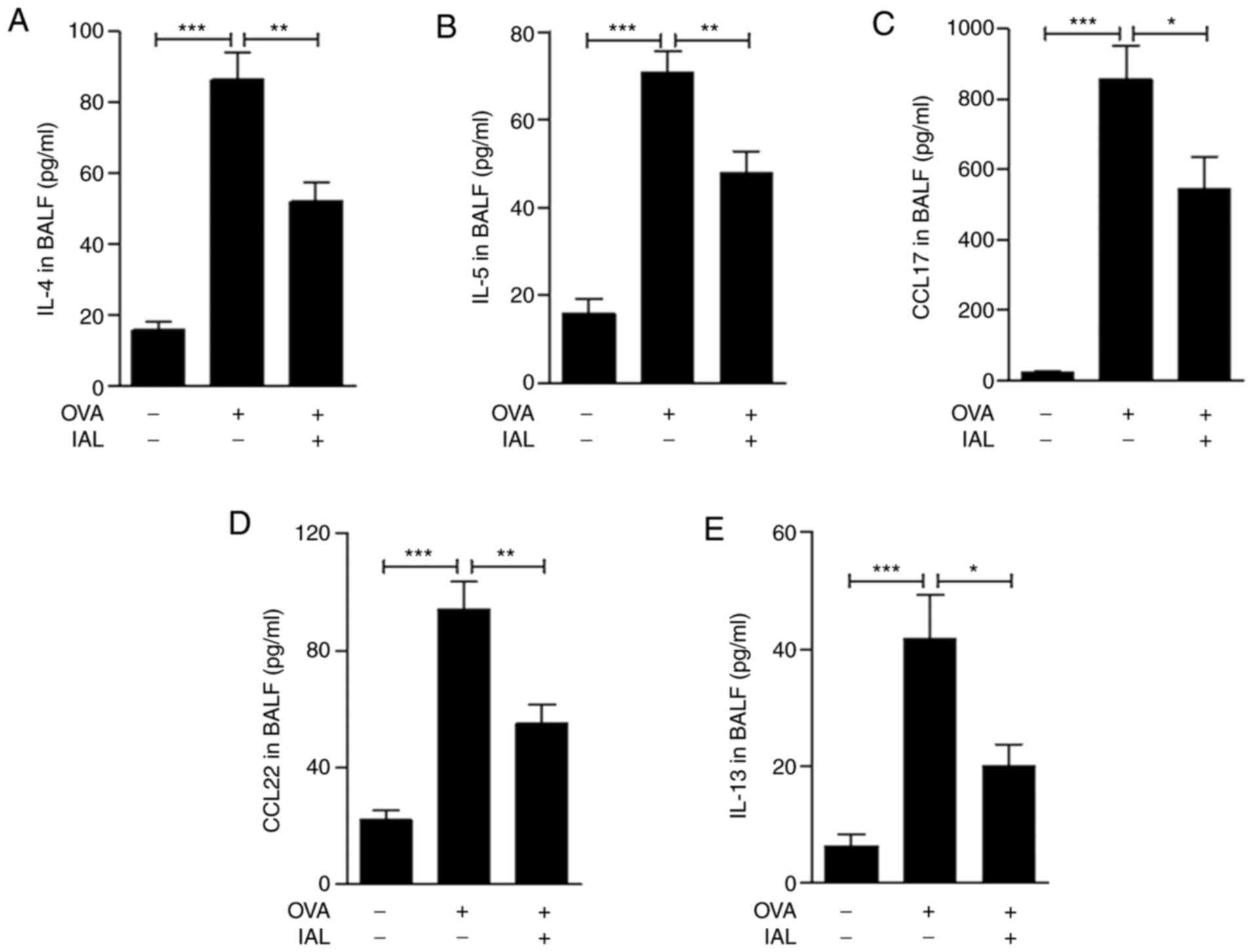
IAL inhibits OVA-induced generation of
type 2 cytokines
Asthma is generally associated with enhanced type 2
immune responses, including increased production of IL-4, IL-5,
IL-13, CCL17 and CCL22 (23–25).
Therefore, the generation of IL-4, IL-5, IL-13, CCL17 and CCL22 in
BALF was detected. The results demonstrated that compared to the
control group, the expression of IL-4, IL-5, IL-13, CCL17 and CCL22
was significantly increased following OVA treatment and was
significantly reduced by IAL treatment (Fig. 3). These results indicated that IAL
ameliorated OVA-induced asthmatic inflammation and Th2 cytokine
production.
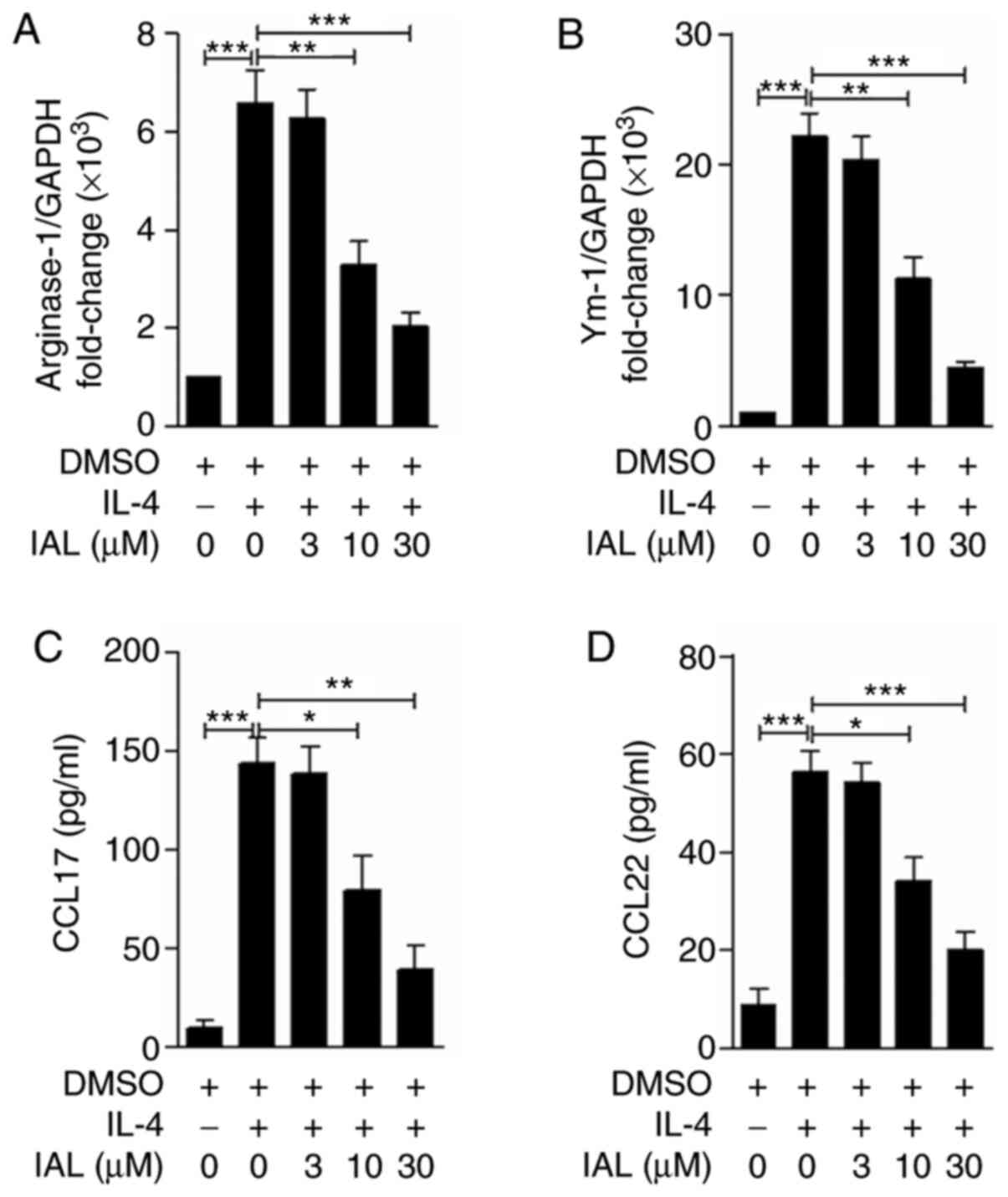
IAL inhibits alternatively activated
macrophages in vitro
It is well established that alternatively activated
M2 macrophages, induced by IL-4, serve an important role in
asthmatic inflammation (24). Arg-1
and Ym-1 are expressed by alternatively activated macrophages
during asthmatic inflammation (26)
and CCL17 and CCL22 are the main chemokines involved in eosinophil
recruitment (7). To determine the
effect of IAL on macrophage M2 polarization, BMDMs were pretreated
with IAL (0, 3, 10, or 30 µM) for 30 min and stimulated with IL-4
(10 ng/ml) for 24 h. The mRNA and protein expression levels of
CCL17 and CCL22 were detected. Although IL-4 treatment induced the
expression of Arg-1, Ym-1, CCL17 and CCL22 in BMDMs, they were
significantly decreased in a dose-dependent manner following
treatment with IAL (Fig. 4). These
results suggested that IAL inhibited IL-4-induced M2
macrophages.
IAL downregulates the phosphorylation
of STAT6 and the expression of PPAR-γ and KLF4 in BMDMs
To determine how IAL inhibits alternatively
activated macrophages, BMDMs were pre-treated with IAL (0, 3, 10,
or 30 µM) and BMDMs challenged with IL-4. p-STAT6 and PPAR-γ and
KLF4 were detected by western blotting. As shown in Fig. 5, the phosphorylation of STAT6 and
the expression of PPAR-γ and KLF4 were induced by IL-4 treatment,
while IAL inhibited their expression in a dose-dependent manner
(Fig. 5). Taken together, these
results suggested that IAL inhibited IL-4-mediated activation of
STAT6/PPAR-γ/KLF4.
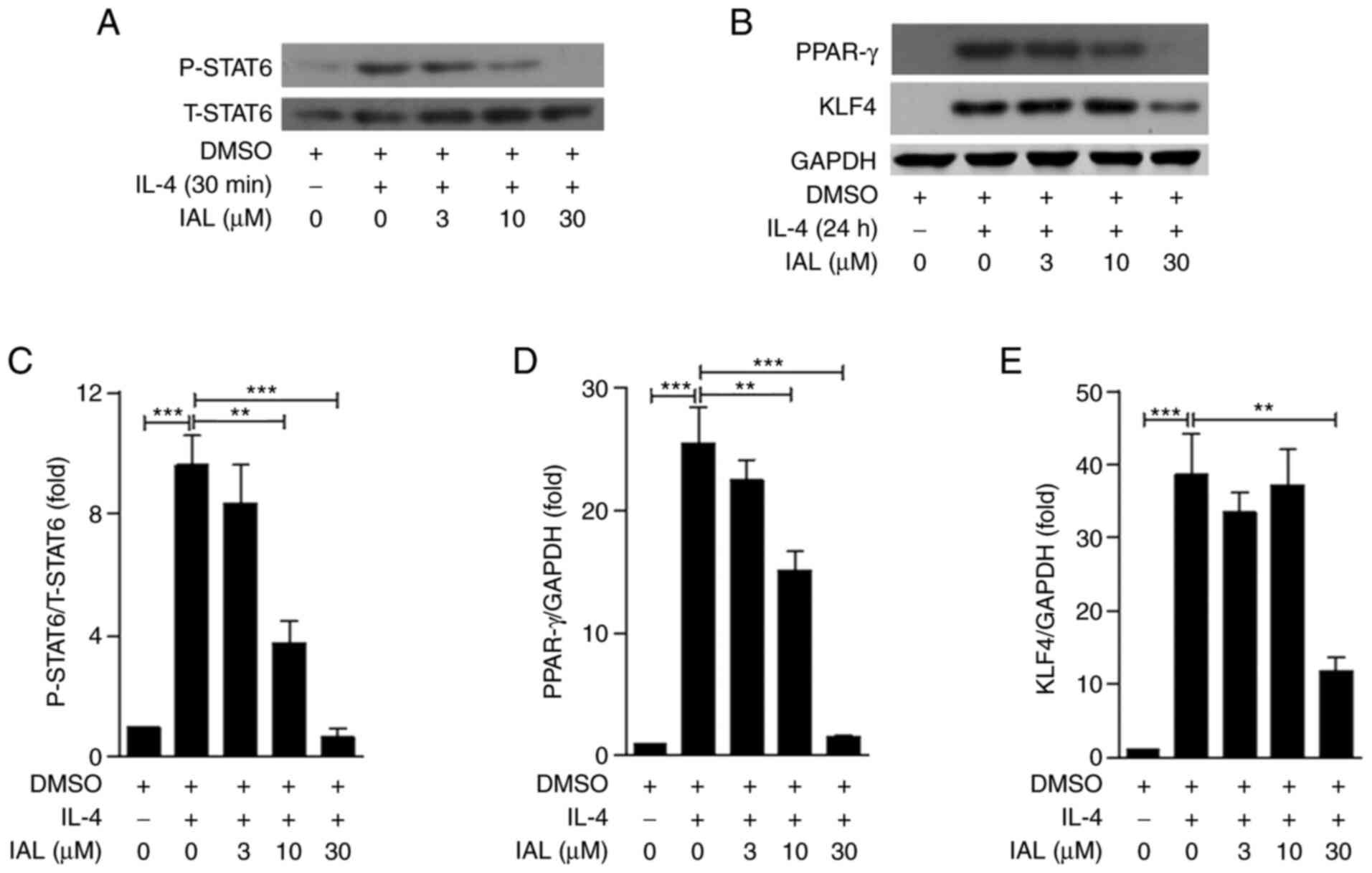 | Figure 5.IAL downregulates the phosphorylation
of STAT6 and the expression of PPAR-γ and KLF4 in BMDMs. Following
preincubating with IAL (0, 3, 10, or 30 µM) for 30 min, BMDMs were
challenged with IL-4 (10 ng/ml) for 30 min or 24 h. (A) The
expression of total and phosphorylated STAT6 in BMDMs following
challenge with IL-4 for 30 min and (B) the expression of PPAR-γ and
KLF4 in BMDMs following challenge with IL-4 for 24 h were measured
by using western blotting. Quantitative analyses of (C) P-STAT6,
(D) PPAR-γ and (E) KLF4 were normalized to T-STAT6 and GAPDH.
Values represent means ± standard error of the mean, n=3;
**P<0.01, ***P<0.001. IAL, isoalantolactone; BMDMs, bone
marrow-derived macrophages; PPAR-γ, peroxisome
proliferator-activated receptor γ; KLF4, Krüppel-like factor 4; p,
phosphorylated; t, total. |
Discussion
Asthma is a heterogeneous inflammatory pulmonary
disease that lacks effective medicines and causes serious issues to
patients throughout life (27).
Components of traditional medicines have been widely identified as
promising drugs for treating various diseases, including asthma
(28–30). In line with this, the effect of IAL
on inflammatory diseases, such as acute lung injury and sepsis, has
been reported (19,20). However, the effect of IAL on asthma
and the underlying mechanism remain unclear. The present study
investigated the anti-inflammatory effect of IAL on OVA-induced
asthma by inhibiting M2 macrophages. The results demonstrated that
IAL has an inhibitory effect on the STAT6/PPAR-γ/KLF4 pathway and
can therefore be considered as a potential therapeutic agent for
the treatment of asthma.
Macrophages, the most abundant immune cells in the
pulmonary microenvironment, serve a vital role in the pathology of
asthmatic inflammation (31).
Macrophages can be polarized to either classically activated (M1)
phenotypes, induced by interferon (IFN)-γ and LPS, or M2
phenotypes, induced by interleukin (IL)-4 and IL-13 (32). Upon exposure to pathogens, such as
peanuts, pollen, house dust mites and fungal spores, mononuclear
monocytes are recruited to the lung and then activated to different
subtypes by a variety of cytokines produced from injured epithelial
cells and innate immune cells (32). Increased M2 macrophage polarization
is considered a major phenomenon of asthmatic inflammation;
increased M2 macrophages promote Th2 polarization of T cells and
contribute to the aggravation of allergic asthma (27,28).
Studies have shown that various endogenous proteins participate in
the development of asthma by modulating M2 polarized macrophages.
For instance, NLRP3 aggravates the pathological process of asthma
by upregulating M2 macrophage polarization. The ATP/P2X7r axis also
accelerates the development of asthma by promoting M2 macrophage
polarization (33). Therefore,
modulation of macrophage polarization is a promising method for
treating asthma. Studies have investigated the inhibition of
macrophage M2 polarization as a potential target for asthma
treatment, such as treatment with mannose androgen and long non
coding (lnc)RNA PTPRE-AS1 (34–36).
It is worth noting that macrophage polarization is an important
target of various natural products. For example, smiglaside A can
effectively improve pulmonary inflammation by modulating macrophage
polarization (37). Peucedanum
japonicum extract attenuates allergic airway inflammation by
inhibiting Th2 cell activation and macrophage activation (38). In addition to asthma, M2 polarized
macrophages serve a critical role in several chronic diseases. For
example, a recent study suggests that M2 macrophages enhance RT
immunity in lung cancer (39). A
previous study has also shown that M2 polarized BMDMs secrete
exosomes containing miR-690 that, when administered to obese mice,
improved glucose-insulin homeostasis (40). The present study suggested that IAL
suppresses the M2 macrophage phenotype and reduces the expression
of Arginase-1, Ym-1, CCL7 and CCL22, which together contributes to
the alleviation of OVA-induced asthma in vitro and in
vivo. Therefore, IAL is a promising compound for the treatment
of asthmatic inflammation and a potential agent for alternatively
activated macrophage-related diseases, such as tumors, obesity and
diabetes.
The M2 phenotype of macrophages is regulated by
various signaling pathways, including the IL-4-mediated signaling
pathway, lncRNA-MM2P and VEGF signaling (41–43).
An improved understanding of the molecular mechanisms underlying
macrophage polarization is essential to understand the causal
relationship between allergen exposure and the development of
allergic diseases such as asthma. JAK1/STAT6 signaling, the main
downstream signal following IL-4 treatment, is regarded as a major
therapeutic target for various compounds, such as kaempferol,
emodin and chrysophanol (44–46).
PPAR-γ, a subgroup of ligand-activated nuclear receptors, is
induced by IL-4 and participates in the activation of M2
macrophages (47). The STAT6/PPAR-γ
signaling pathway is also an important target for the HuoXueTongFu
Formula (composed of six crude herbs: Chinese rhubarb, peach
kernel, Corydalis yanhusuo, radish seed, Glauber's salt and
safflower at a ratio of 5:5:5:5:5:3) to induce macrophage M2
polarization (48). KLF4 is a
downstream protein of PPAR-γ (49).
Through binding to the PPAR response element within the KLF4
promoter, PPAR-γ induces the expression of KLF4 and promotes M2
macrophage polarization (50).
Several studies have indicated that the KLF4 related pathway is
closely associated with the regulation of macrophage polarization
and is the target of various compounds, such as tanshinone IIA,
iron and Epigallocatechin-3-Gallate (51–53).
Collectively, the present study confirmed that the inhibition of
IAL in macrophage activation is associated with the reduction of
KLF4 expression by inhibiting the STAT6/PPAR-γ signaling
pathway.
The target signaling pathways of IAL have been
widely investigated in various diseases, such as pancreatic cancer,
osteoporosis and other metabolic bone diseases. IAL attenuates the
expression of p-STAT3 and STAT3, leading to apoptosis of prostate
cancer cells (54). The p38
MAPK/NF-κB signaling pathway can also be inhibited by IAL treatment
in MDA-MB-231 cells, as demonstrated by inhibition of migration and
invasion (55). The
anti-inflammatory effect of IAL on pulmonary disorders is modulated
by the inhibition of NF-κB or ubiquitination of TRAF6 (19,20).
The present study suggested that the alternative activation of
macrophages regulated by IAL was related to the STAT6/PPAR-γ/KLF4
pathway, which provides further information on the multiple
pharmacological functions of IAL.
The present study demonstrated that IAL has a
potential therapeutic effect on OVA-induced asthmatic inflammation
in mice via inhibition of M2 macrophage polarization and
attenuation of the STAT6/PPAR-γ/KLF4 signaling pathway. These
findings suggested that IAL is a candidate for the treatment of
asthma and other diseases associated with macrophage
polarization.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key
Discipline Construction Project of the Pudong Health Bureau of
Shanghai (grant no. PWZzk2017-30), Natural Science Foundation of
Shanghai (grant no. 20Z11901004) and the Discipline Construction
Promoting Project of Shanghai Pudong Hospital (grant nos.
Zdzk2020-11, Zdzk2020-21 and Zdzk2020-23).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YSh designed the project and drafted the manuscript.
YSo and XL performed the experiments and drafted the manuscript. FL
and HZ participated in the data analysis and revised the
manuscript. YSh and XL confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocols of the present study were
approved by the Institutional Animal Care and Use Committee of
Shanghai Pudong Hospital (Shanghai, China) and procedures were
approved by the Biological Research Ethics Committee of Shanghai
Pudong Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mims JW: Asthma: Definitions and
pathophysiology. Int Forum Allergy Rhinol. 5 (Suppl 1):S2–S6. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nanda A and Wasan AN: Asthma in adults.
Med Clin North Am. 104:95–108. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ober C and Yao TC: The genetics of asthma
and allergic disease: A 21st century perspective. Immunol Rev.
242:10–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma Y, Ge A, Zhu W, Liu YN, Ji NF, Zha WJ,
Zhang JX, Zeng XN and Huang M: Morin attenuates ovalbumin-induced
airway inflammation by modulating oxidative stress-responsive MAPK
signaling. Oxid Med Cell Longev. 2016:58436722016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lambrecht BN and Hammad H: The immunology
of asthma. Nat Immunol. 16:45–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Holgate ST: Innate and adaptive immune
responses in asthma. Nat Med. 18:673–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yi S, Zhai J, Niu R, Zhu G, Wang M, Liu J,
Huang H, Wang Y, Jing X, Kang L, et al: Eosinophil recruitment is
dynamically regulated by interplay among lung dendritic cell
subsets after allergen challenge. Nat Commun. 9:38792018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Draijer C and Peters-Golden M: Alveolar
macrophages in allergic asthma: The forgotten cell awakes. Curr
Allergy Asthma Rep. 17:122017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song Y, Wu Y, Li X, Shen Y, Ding Y, Zhu H,
Liu F, Yu K, Sun L and Qian F: Protostemonine attenuates
alternatively activated macrophage and DRA-induced asthmatic
inflammation. Biochem Pharmacol. 155:198–206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, He C, Tang Y, Liu W, Xu Y, Li Z,
Qin X and Jin S: Cremastra appendiculata (D.Don) Makino, a
potential anti-tumor traditional Chinese medicine: Review. J
Ethnopharmacol. 279:1143572021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lajter I, Vasas A, Béni Z, Forgo P, Binder
M, Bochkov V, Zupkó I, Krupitza G, Frisch R, Kopp B and Hohmann J:
Sesquiterpenes from Neurolaena lobata and their antiproliferative
and anti-inflammatory activities. J Nat Prod. 77:576–582. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Butturini E, Carcereri de Prati A, Boriero
D and Mariotto S: Natural sesquiterpene lactones enhance
chemosensitivity of tumor cells through redox regulation of STAT3
signaling. Oxid Med Cell Longev. 2019:45689642019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quintana J and Estévez F: Recent advances
on cytotoxic sesquiterpene lactones. Curr Pharm Des. 24:4355–4361.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu R, Peng Y, Wang M and Li X: Intestinal
absorption of isoalantolactone and alantolactone, two sesquiterpene
lactones from radix inulae, using caco-2 cells. Eur J Drug Metab
Pharmacokinet. 44:295–303. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu N, Lv Q, Sun X, Zhou Y, Guo Y, Qiu J,
Zhang P and Wang J: Isoalantolactone restores the sensitivity of
gram-negative enterobacteriaceae carrying MCR-1 to carbapenems. J
Cell Mol Med. 24:2475–2483. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung YS, Lee HS, Cho HR, Kim KJ, Kim JH,
Safe S and Lee SO: Dual targeting of Nur77 and AMPKα by
isoalantolactone inhibits adipogenesis in vitro and decreases body
fat mass in vivo. Int J Obes (Lond). 43:952–962. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Lu C, Liu S, Liu S, Su C, Xiao T, Bi
Z, Sheng P, Huang M, Liu X, et al: Synthesis and discovery of a
drug candidate for treatment of idiopathic pulmonary fibrosis
through inhibition of TGF-β1 pathway. Eur J Med Chem. 157:229–247.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan CB, Tian L, Yang B and Zhou HY:
Isoalantolactone protects LPS-induced acute lung injury through
Nrf2 activation. Microb Pathog. 123:213–218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He G, Zhang X, Chen Y, Chen J, Li L and
Xie Y: Isoalantolactone inhibits LPS-induced inflammation via NF-κB
inactivation in peritoneal macrophages and improves survival in
sepsis. Biomed Pharmacother. 90:598–607. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding YH, Song YD, Wu YX, He HQ, Yu TH, Hu
YD, Zhang DP, Jiang HC, Yu KK, Li XZ, et al: Isoalantolactone
suppresses LPS-induced inflammation by inhibiting TRAF6
ubiquitination and alleviates acute lung injury. Acta Pharmacol
Sin. 40:64–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duchene B, Caffry S, Kaminsky DA, Que LG,
Poynter ME and Dixon AE: Functional significance of 8-isoprostanes
in sinonasal disease and asthma. Respir Med. 185:1065062021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Noel JC and Berin MC: Role of innate
immunity and myeloid cells in susceptibility to allergic disease.
Ann NY Acad Sci. Jun 22–2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee HS, Park DE, Bae B, Oh K, Jung JW, Lee
DS, Kim IG, Cho SH and Kang HR: Tranglutaminase 2 contributes to
the asthmatic inflammation by modulating activation of alveolar
macrophages. Immun Inflamm Dis. May 4–2021.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watanabe S, Suzukawa M, Tashimo H, Ohshima
N, Asari I, Imoto S, Kobayashi N, Tohma S, Nagase T and Ohta K:
High serum cytokine levels may predict the responsiveness of
patients with severe asthma to benralizumab. J Asthma. Jul
1–2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Binnemars-Postma K, Bansal R, Storm G and
Prakash J: Targeting the Stat6 pathway in tumor-associated
macrophages reduces tumor growth and metastatic niche formation in
breast cancer. FASEB J. 32:969–978. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Papi A, Brightling C, Pedersen SE and
Reddel HK: Asthma. Lancet. 391:783–800. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu
L, Zhao M, Liu Q, Cheng Z, Zou J, et al: Naturally occurring
anti-cancer compounds: Shining from Chinese herbal medicine. Chin
Med. 14:482019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao Z, Li Q, Wu X, Zhao X, Zhao L and Tong
X: New insights into the mechanisms of Chinese herbal products on
diabetes: A focus on the ‘bacteria-mucosal
immunity-inflammation-diabetes’ axis. J Immunol Res.
2017:18130862017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu C, Liao JZ and Li PY: Traditional
Chinese herbal extracts inducing autophagy as a novel approach in
therapy of nonalcoholic fatty liver disease. World J Gastroenterol.
23:1964–1973. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fricker M and Gibson PG: Macrophage
dysfunction in the pathogenesis and treatment of asthma. Eur Respir
J. 50:17001962017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saradna A, Do DC, Kumar S, Fu QL and Gao
P: Macrophage polarization and allergic asthma. Transl Res.
191:1–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li R, Shang Y, Hu X, Yu Y, Zhou T, Xiong W
and Zou X: ATP/P2X7r axis mediates the pathological process of
allergic asthma by inducing M2 polarization of alveolar
macrophages. Exp Cell Res. 386:1117082020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Y, Do DC, Ishmael FT, Squadrito ML,
Tang HM, Tang HL, Hsu MH, Qiu L, Li C, Zhang Y, et al: Mannose
receptor modulates macrophage polarization and allergic
inflammation through miR-511-3p. J Allergy Clin Immunol.
141:350–364.e8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Becerra-Díaz M, Strickland AB, Keselman A
and Heller NM: Androgen and androgen receptor as enhancers of M2
macrophage polarization in allergic lung inflammation. J Immunol.
201:2923–2933. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han X, Huang S, Xue P, Fu J, Liu L, Zhang
C, Yang L, Xia L, Sun L, Huang SK and Zhou Y: LncRNA PTPRE-AS1
modulates M2 macrophage activation and inflammatory diseases by
epigenetic promotion of PTPRE. Sci Adv. 5:eaax92302019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Xu Y, Zhang P, Ruan W, Zhang L,
Yuan S, Pang T and Jia AQ: Smiglaside A ameliorates LPS-induced
acute lung injury by modulating macrophage polarization via
AMPK-PPARγ pathway. Biochem Pharmacol. 156:385–395. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chun JM, Lee AR, Kim HS, Lee AY, Gu GJ,
Moon BC and Kwon BI: Peucedanum japonicum extract attenuates
allergic airway inflammation by inhibiting Th2 cell activation and
production of pro-inflammatory mediators. J Ethnopharmacol.
211:78–88. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang F, Sang Y, Chen D, Wu X, Wang X,
Yang W and Chen Y: M2 macrophage-derived exosomal long non-coding
RNA AGAP2-AS1 enhances radiotherapy immunity in lung cancer by
reducing microRNA-296 and elevating NOTCH2. Cell Death Dis.
12:4672021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ying W, Gao H, Dos Reis FCG, Bandyopadhyay
G, Ofrecio JM, Luo Z, Ji Y, Jin Z, Ly C and Olefsky JM: MiR-690, an
exosomal-derived miRNA from M2-polarized macrophages, improves
insulin sensitivity in obese mice. Cell Metab. 33:781–790.e5. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chung S, Kim JY, Song MA, Park GY, Lee YG,
Karpurapu M, Englert JA, Ballinger MN, Pabla N, Chung HY and
Christman JW: FoxO1 is a critical regulator of M2-like macrophage
activation in allergic asthma. Allergy. 74:535–548. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cao J, Dong R, Jiang L, Gong Y, Yuan M,
You J, Meng W, Chen Z, Zhang N, Weng Q, et al: LncRNA-MM2P
identified as a modulator of macrophage M2 polarization. Cancer
Immunol Res. 7:292–305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wheeler KC, Jena MK, Pradhan BS, Nayak N,
Das S, Hsu CD, Wheeler DS, Chen K and Nayak NR: VEGF may contribute
to macrophage recruitment and M2 polarization in the decidua. PLoS
One. 13:e01910402018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cortes JR, Perez-G M, Rivas MD and
Zamorano J: Kaempferol inhibits IL-4-induced STAT6 activation by
specifically targeting JAK3. J Immunol. 179:3881–3887. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu T, Zhang W, Feng SJ and Yu HP: Emodin
suppresses LPS-induced inflammation in RAW264.7 cells through a
PPARγ-dependent pathway. Int Immunopharmacol. 34:16–24. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wen Q, Mei L, Ye S, Liu X, Xu Q, Miao J,
Du S, Chen D, Li C and Li H: Chrysophanol demonstrates
anti-inflammatory properties in LPS-primed RAW 264.7 macrophages
through activating PPAR-γ. Int Immunopharmacol. 56:90–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nelson VL, Nguyen HCB, Garcìa-Cañaveras
JC, Briggs ER, Ho WY, DiSpirito JR, Marinis JM, Hill DA and Lazar
MA: PPARγ is a nexus controlling alternative activation of
macrophages via glutamine metabolism. Genes Dev. 32:1035–1044.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao M, Bian YY, Yang LL, Chen YQ, Wang
YJ, Ma YT, Pei YQ, Li WL and Zeng L: HuoXueTongFu formula
alleviates intraperitoneal adhesion by regulating macrophage
polarization and the SOCS/JAK2/STAT/PPAR-γ signalling pathway.
Mediators Inflamm. 2019:17693742019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kapoor N, Niu J, Saad Y, Kumar S, Sirakova
T, Becerra E, Li X and Kolattukudy PE: Transcription factors STAT6
and KLF4 implement macrophage polarization via the dual catalytic
powers of MCPIP. J Immunol. 194:6011–6023. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li S, Zhou Q, He H, Zhao Y and Liu Z:
Peroxisome proliferator-activated receptor γ agonists induce cell
cycle arrest through transcriptional regulation of Kruppel-like
factor 4 (KLF4). J Biol Chem. 288:4076–4084. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen W, Li X, Guo S, Song N, Wang J, Jia L
and Zhu A: Tanshinone IIA harmonizes the crosstalk of autophagy and
polarization in macrophages via miR-375/KLF4 pathway to attenuate
atherosclerosis. Int Immunopharmacol. 70:486–497. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Almatroodi SA, Almatroudi A, Alsahli MA,
Aljasir MA, Syed MA and Rahmani AH: Epigallocatechin-3-Gallate
(EGCG), an active compound of green tea attenuates acute lung
injury regulating macrophage polarization and Krüpple-like-factor 4
(KLF4) expression. Molecules. 25:28532020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Handa P, Thomas S, Morgan-Stevenson V,
Maliken BD, Gochanour E, Boukhar S, Yeh MM and Kowdley KV: Iron
alters macrophage polarization status and leads to steatohepatitis
and fibrogenesis. J Leukoc Biol. 105:1015–1026. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen W, Li P, Liu Y, Yang Y, Ye X, Zhang F
and Huang H: Isoalantolactone induces apoptosis through
ROS-mediated ER stress and inhibition of STAT3 in prostate cancer
cells. J Exp Clin Cancer Res. 37:3092018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang J, Cui L, Feng L, Zhang Z, Song J,
Liu D and Jia X: Isoalantolactone inhibits the migration and
invasion of human breast cancer MDA-MB-231 cells via suppression of
the p38 MAPK/NF-κB signaling pathway. Oncol Rep. 36:1269–1276.
2016. View Article : Google Scholar : PubMed/NCBI
|