Introduction
Pirarubicin (THP), an analogue of doxorubicin, can
interfere with the synthesis of DNA and mRNA, block the cell into
G1 phase in cell proliferation cycle, interfere with tumor cell
division and inhibit tumor growth; thus it has strong anti-cancer
activity (1,2). Its chemical structure is a
tetrahydropyran group inserted into the OH group at the 4 position
of the amino sugar part of doxorubicin, which greatly reduces the
toxic and side effects of THP (3).
However, its cardiotoxicity cannot be ignored (4). At present, there is no completely
effective treatment for THP-induced cardiotoxicity and the approved
dexrazoxane is expensive (5).
Canagliflozin, a sodium-glucose co-transporter-2
(SGLT2) inhibitor, can reduce blood glucose by decomposing glucose
and excreting it through the kidney (6). In addition to blood glucose control,
canagliflozin also has cardiovascular protective effects, including
reducing cardiac preload, improving hemodynamics, reducing
inflammation and oxidative stress and improving cardiac energy
supply. Studies have shown that canagliflozin can alleviate the
cardiovascular symptoms of diabetic patients with or without
cardiovascular diseases and has prospects of broad application in
the cardiovascular field (7–9).
Superoxide dismutase (SOD) is an important
antioxidant enzyme, which is widely distributed in various
organisms. It is often used to measure the antioxidant capacity of
tissues or cells (10,11). NADPH oxidase (NOX) is the key enzyme
of redox signal and the main source of reactive oxygen species
(ROS) (12). NOX2 was mainly
expressed in the heart and increased when oxidative stress
increased (13).
The present study was only a preliminary study to
explore the cardiotoxic effect of THP and to understand the
corresponding protective effect of caglitazine. It aimed to provide
a theoretical basis for clinical prevention and treatment of
anthracycline cardiotoxicity and cardiovascular protective effect
of canagliflozin.
Materials and methods
Materials
Pirarubicin, purity ≥98%, was obtained from Shanghai
Aladdin Biochemical Technology Co., Ltd.. Canagliflozin was
obtained from Janssen-Cilag International NV. Brain natriuretic
peptide (BNP; cat. no. MB-1608A), creatine kinase MB (CK-MB; cat.
no. MB-6930A) and cardiac troponin T (cTnT; cat. no. MB-7278A) test
kits were purchased from Shanghai Meixuan Biological Science and
Technology Ltd. Malondialdehyde (MDA; cat. no. A003-1-2),
superoxide dismutase (SOD; cat. no. A001-3-2) and lactate
dehydrogenase (LDH; cat. no. A020-2-2) test kits were obtained from
Nanjing Jiancheng Bioengineering Institute. SGLT2 inhibitor
(SGLT2i) was purchased from MedChemExpress. The antibodies for SOD2
(1:3,000; cat. no. 13141T), pro/cleaved-caspase- (1:1,000; cat. no.
14220T/9664T), Bcl-2/Bax (1:1,000; cat. no. 4223T/2772T) were
obtained from Cell Signaling Technology, Inc. The antibody for NOX2
(1:1,000; cat. no. 19013-1-AP) was obtained from ProteinTech Group,
Inc.. All chemicals and reagents were analytical grade.
Animal model
The present study was performed according to the
Guide for the Care and Use of Laboratory Animals (14) and was approved by the Animal Ethics
Committee of the First Affiliated Hospital of Chongqing Medical
University (CMU; approval no. 20195101). A total of 40 Male Sprague
Dawley (SD) rats (180–200 g; age, 6 weeks) were obtained from the
CMU experimental animal center. SD rats were housed at 23±2°C with
humidity of 40–60% and a 12/12-h light/dark cycle. Rats were
randomly divided equally into 4 groups (n=10 in each group): normal
group (CON; normal-diet-fed rats), canagliflozin group
(canagliflozin-diet-fed rats, 60 mg•kg−1), THP group
(normal-diet-fed rats; 3 mg•kg−1 THP was injected via
caudal vein once a week) and canagliflozin + THP group
(canagliflozin-diet-fed rats, 60 mg•kg−1; 3
mg•kg−1 THP was injected via caudal vein once a week).
The food consumption and body weight was measured twice a week.
Electrocardiogram and Doppler
echocardiography
The experiment ended at week 8. The rats were
anesthetized with inhaled isoflurane (2%, maintenance dose was also
2%). Needle electrodes were inserted subcutaneously into the right
upper limb, right lower limb and left lower limb respectively. The
lead IV electrocardiography (ECG) was recorded by BL-420F
biological function measurement system (Chengdu Taimeng Technology
Company). The hair of the precordial region was removed and the
Doppler echocardiography was measured by Vivid E95 ultrasonic
diagnostic apparatus (General Electric Company).
Sample collection, preparation,
section staining and biochemical indexes
At the end of the 8th week, the rats were weighed
after fasting overnight and sacrificed by cervical dislocation
under anesthesia (inhalation of 2% isoflurane). Blood samples (1–2
ml per rat) were collected from abdominal aorta immediately after
sacrifice and centrifuged at 314 × g, 4°C for 30 min within 6 h.
The supernatant was frozen in a −80°C refrigerator and serum LDH,
BNP, CK-MB, cTn-T, SOD and MDA contents were determined as soon as
possible according to the operation procedure of the kit. Heart
samples were excised and weighed. The left ventricular part of the
heart was immersed in 10X its volume of 4% paraformaldehyde
solution and stored for 4 h in a refrigerator. The rest of the left
ventricular portion of the heart was stored in −80°C refrigerator
for follow-up experiments. The next day, the heart tissue was
dehydrated, dewaxed, embedded in paraffin and cut into 5 µm
sections. Hematoxylin and eosin staining was performed according to
the instructions of the kit (30°C, 30 min). TUNEL apoptosis
detection kit (green fluorescence) was purchased from Beyotime
Institute of Biotechnology. The paraffin section was dewaxed in
xylene, dehydrated with absolute alcohol, washed with distilled
water and then 20 µg/ml proteinase K without DNase added (37°C for
30 min), before washing with PBS for three times. TUNEL solution
(50 µl) was added to the target area and incubated at 37°C for 60
min. DAPI staining solution (100%; Beyotime Institute of
Biotechnology) was used to stain the nuclei (37°C, 3–5 min). After
washing with PBS 3 times, an anti-fluorescence quenching sealing
solution was used to seal the plates, which were observed under a
fluorescence microscope (magnification, ×200). A total of three
fields of view were observed. Apoptosis level=apoptotic cells/total
cells ×100%.
Cell culture and treatment
A total of 20 neonatal SD rats (male, 1–3 days, CMU
Experimental Animal Center) were anesthetized with ketamine (55
mg/kg) plus xylazine (15 mg/kg) and disinfected with 75% ethanol.
After the neonatal rats were sacrificed by cervical dislocation,
the ventricles were quickly separated under aseptic conditions. The
blood clots, blood vessels, fat and other tissues were washed 3
times in PBS buffer and then cut into sections with diameter <1
mm, digested by trypsin and II collagenase and then filtered,
centrifuged, resuspended and seeded. Finally, primary rat
cardiomyocytes were obtained by differential adhesion method
[following 1.5 h culture in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) with 10% FBS (PAN-Biotech GmbH) and penicillin/streptomycin
at 37.5°C with 5% CO2, the culture supernatant
containing cardiomyocytes was collected and re-seeded to obtain
primary cardiomyocytes]. The primary cardiomyocytes were divided
into four groups: Normal group (CON), canagliflozin group
(canagliflozin, 60 µm, 14 h), THP group (THP, 10 µm, 12 h), THP and
canagliflozin co culture group (canagliflozin, 60 µm, 14 h + THP,
10 µm, 12 h). In canagliflozin +THP group, the cells were pre
incubated with canagliflozin (60 µm) for 2 h and then co cultured
with THP (10 µm) for 12 h.
Western blotting
Heart tissue and primary rat cardiomyocytes was
lysed in radioimmunoprecipitation (RIPA) lysis buffer. BCA kit was
used to determine the protein concentration in the supernatant.
Then ~50 µg heart tissue lysate or 20 µg of cell lysate was used
for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12%
gel) and proteins were then transferred to an FL PVDF membrane (EMD
Millipore) at 4°C for 1.5 h. After blocking with 5% blocking
protein powder (room temperature), the first antibody was incubated
overnight at 4°C and the second antibody was incubated at room
temperature for 1.5 h. The western blotting results were analyzed
by BeyoECL Plus (Beyotime Institute of Biotechnology) in Image Lab
(version: 5.2.1; Bio-Rad Laboratories, Inc.). The specific protein
expression levels were normalized to GAPDH.
Statistical analysis
Data were presented as mean ± SD. The significance
of differences between groups were analyzed statistically using one
or two-way analysis of variance (ANOVA), followed by a Tukey's
multiple-comparison post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
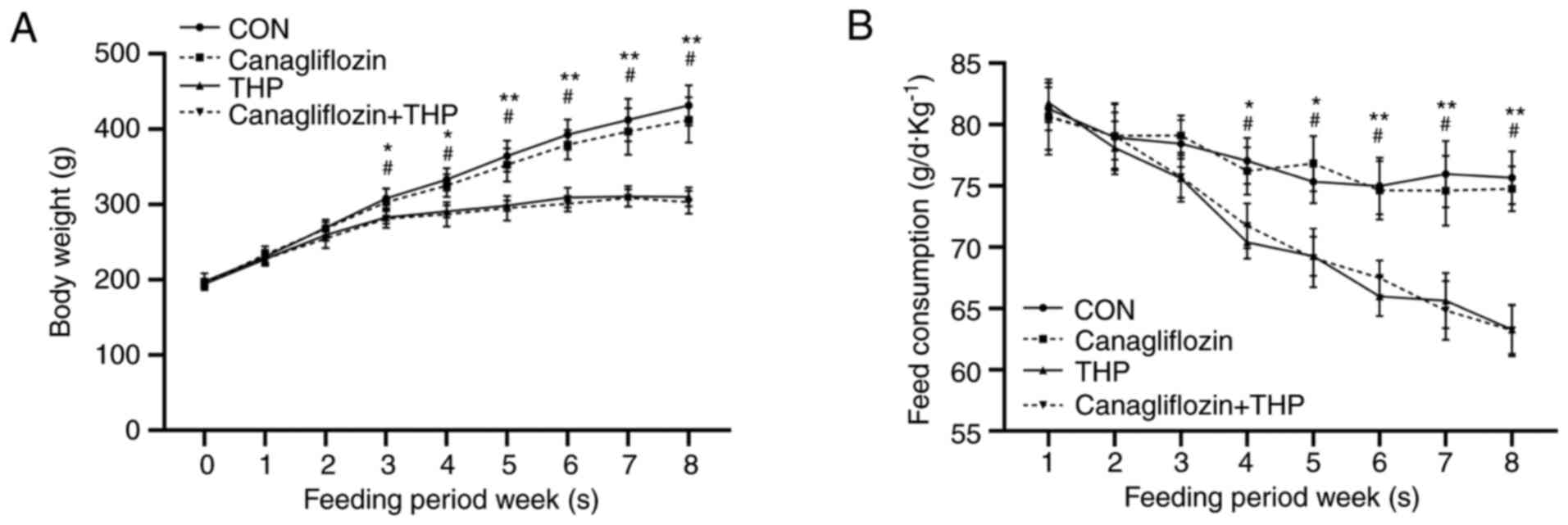
THP causes the decrease of body weight
and food intake, but canagliflozin has no effect
The body weight (Fig.
1A, P<0.05 vs. CON) and food intake (Fig. 1B, P<0.05 vs. CON) of THP rats
began to decrease in the third and fourth weeks, especially in the
fifth and sixth week (P<0.01 vs. CON). However, there was no
significant improvement in the above changes after treatment with
canagliflozin (Fig. 1, P>0.05
vs. THP).
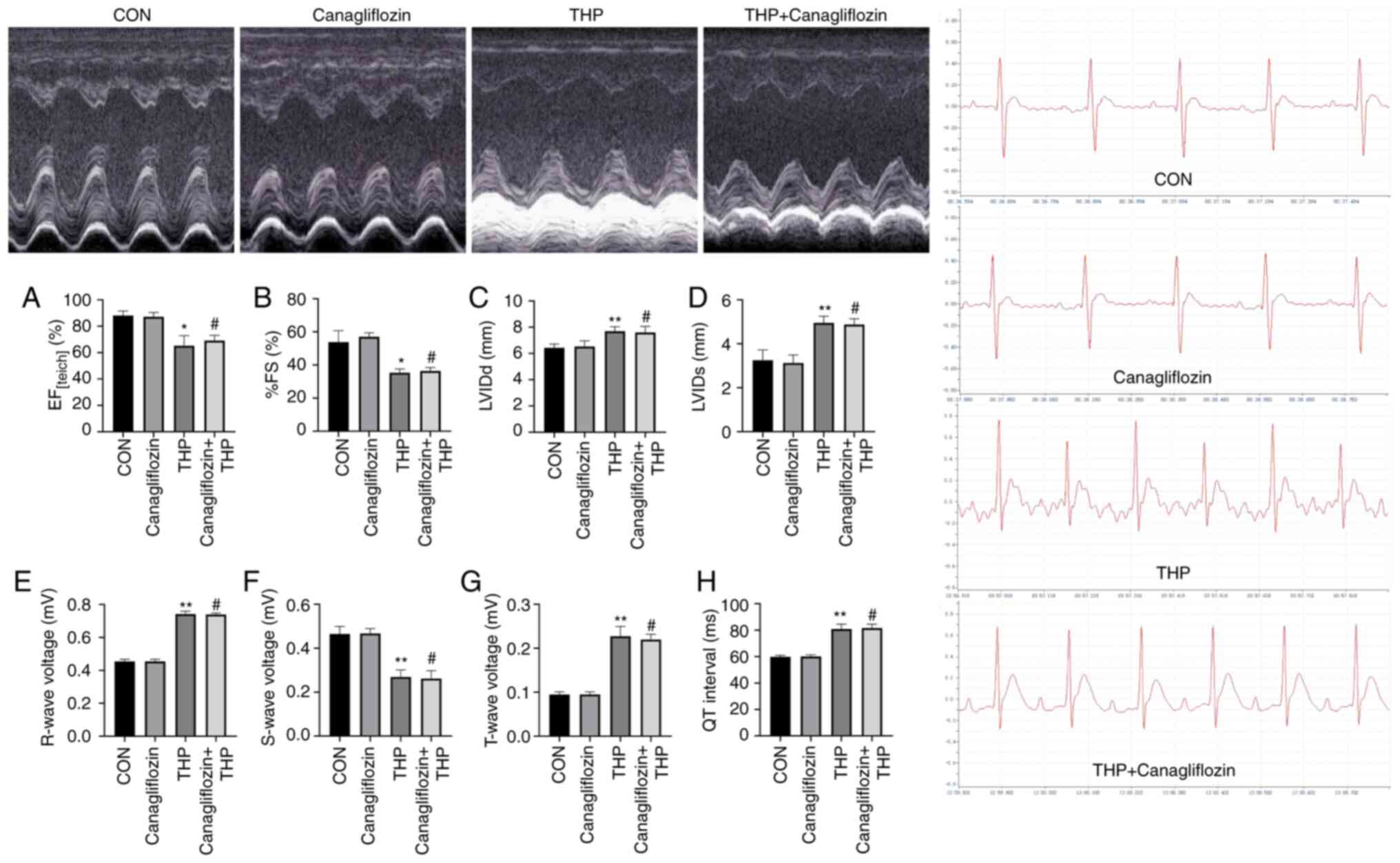
Canagliflozin does not improve the
THP-induced changes of ECG and echocardiography in rats
At 8 weeks after THP injection, a series of ECG and
echocardiographic (Fig. 2) changes
occurred in SD rats, including: Ejection fraction (Fig. 2A) and fractional shortening Fig. 2B) decreased, left ventricular
internal diameter end diastole (Fig.
2C) and left ventricular internal diameter end systole
(Fig. 2D) increased; R wave
(Fig. 2E) and T wave (Fig. 2F) increased; S wave (Fig. 2G) decreased; QT interval (Fig. 2H) was prolonged.
Following canagliflozin treatment, the above changes
were not significantly improved (Fig.
2A-H; P>0.05 vs. THP).
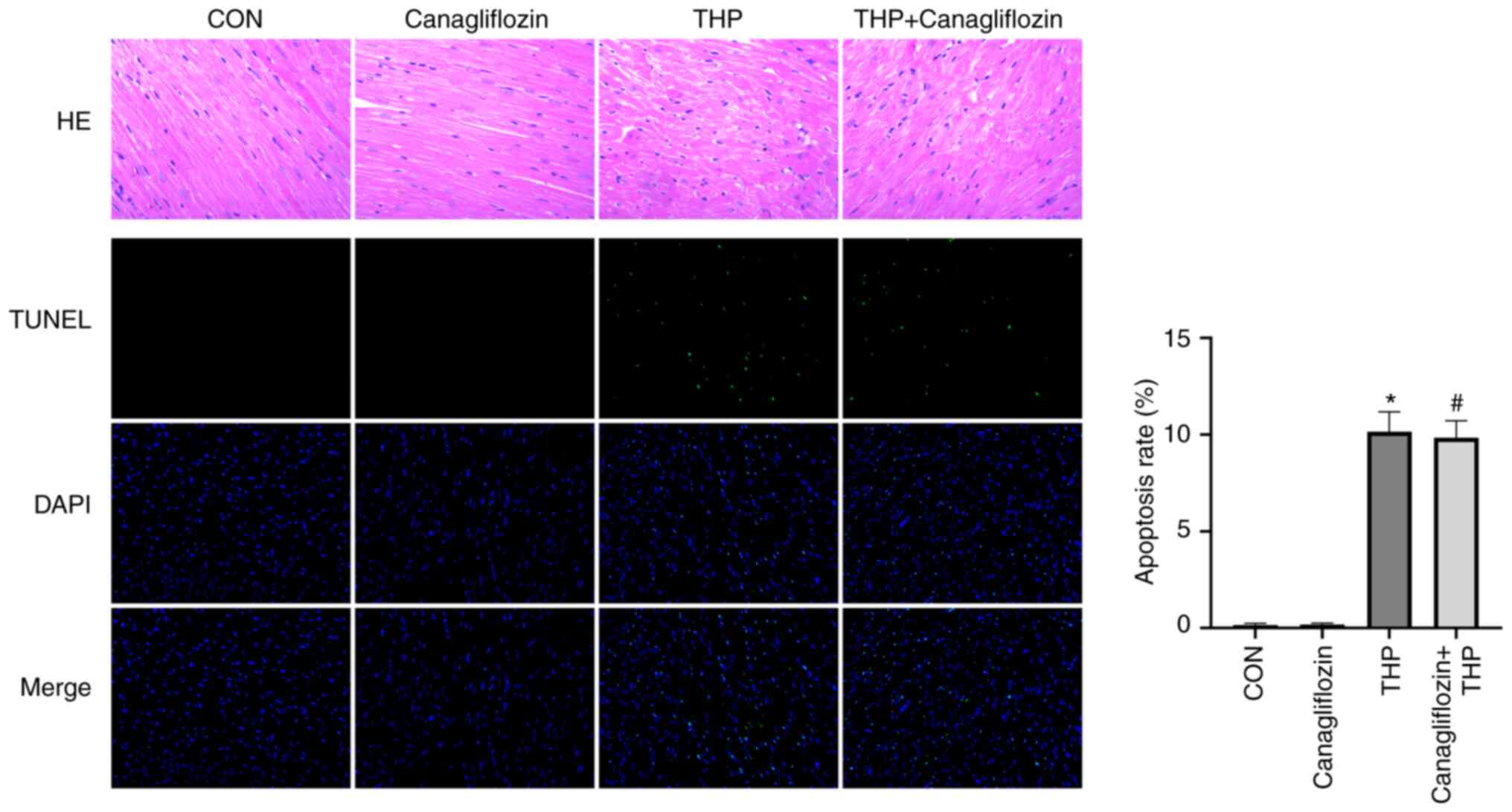
Canagliflozin has no significant
effect on THP-induced cardiac tissue changes and apoptosis in
rats
As shown in Fig. 3,
the arrangement of cardiomyocytes was disordered, the intercellular
space was enlarged and the cardiomyocytes were focal vacuolization
or steatosis in the rats injected with THP alone. Compared with THP
group, the treatment of canagliflozin showed no significant
improvement on cardiac tissue.
TUNEL staining (Fig.
3) showed that there was no cardiomyocyte apoptosis in CON and
canagliflozin group, but there was regional cardiomyocyte apoptosis
in THP injection group. The treatment of canagliflozin had no
effect on THP-induced cardiomyocyte apoptosis. The quantitative
results are shown in Fig. 3A.
The role of THP and canagliflozin in
blood and heart tissue biochemical indexes
The SD rats were sacrificed after 8 weeks. Blood and
heart tissue samples were collected and tested.
In blood, THP caused the decrease of SOD level
(Fig. 4A) and the increase of MDA
(Fig. 4B), LDH (Fig. 4C), CK-MB (Fig. 4D), cTnT (Fig. 4E) and BNP (Fig. 4F). However, the treatment of
canagliflozin did not effectively improve the above changes
(Fig. 4A-F; P>0.05 vs. THP).
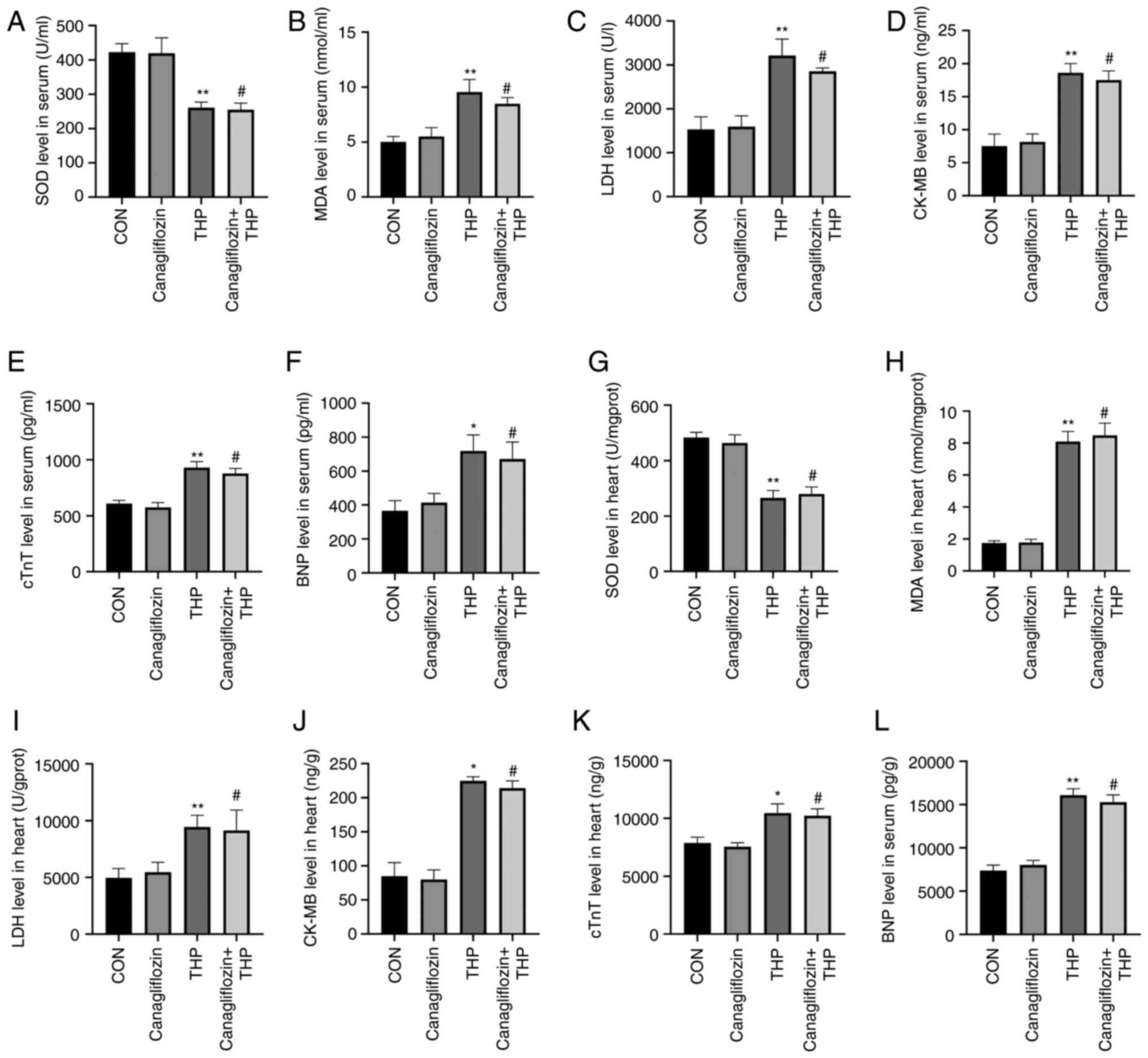 | Figure 4.Canagliflozin cannot effectively
improve the level of serum and heart tissue related biochemical
markers of THP-induced heart injury. (A) SOD, (B) MDA, (C) LDH, (D)
CK-MB, (E) cTnT and (F) BNP levels in serum. (G) SOD, (H) MDA, (I)
LDH, (J) CK-MB, (K) cTnT and (L) BNP levels in heart. All values
are the mean ± SD. *P<0.05 vs. CON; **P<0.01 vs. CON;
#P>0.05 vs. THP. THP, pirarubicin; SOD, superoxide
dismutase; MDA, malondialdehyde; LDH, lactate dehydrogenase; BNP,
brain natriuretic peptide; CK-MB, creatine kinase MB; cTnT, cardiac
troponin T; CON, normal group. |
The same was true of heart tissue, THP-induced the
decrease of SOD level (Fig. 4G) and
the increase of MDA (Fig. 4H), LDH
(Fig. 4I), CK-MB (Fig. 4J), cTnT (Fig. 4K) and BNP (Fig. 4L) in rat heart. However, the
treatment of canagliflozin does not effectively improve the above
changes (Fig. 4G-L, P>0.05 vs.
THP).
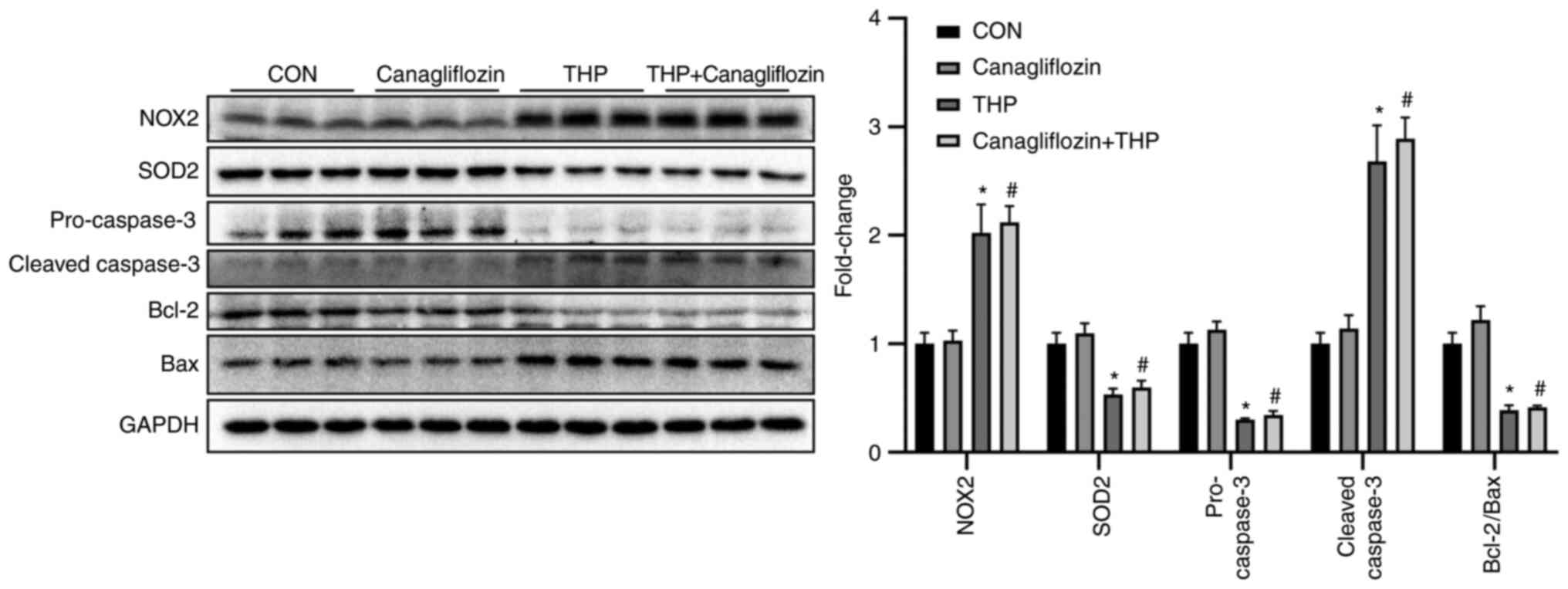
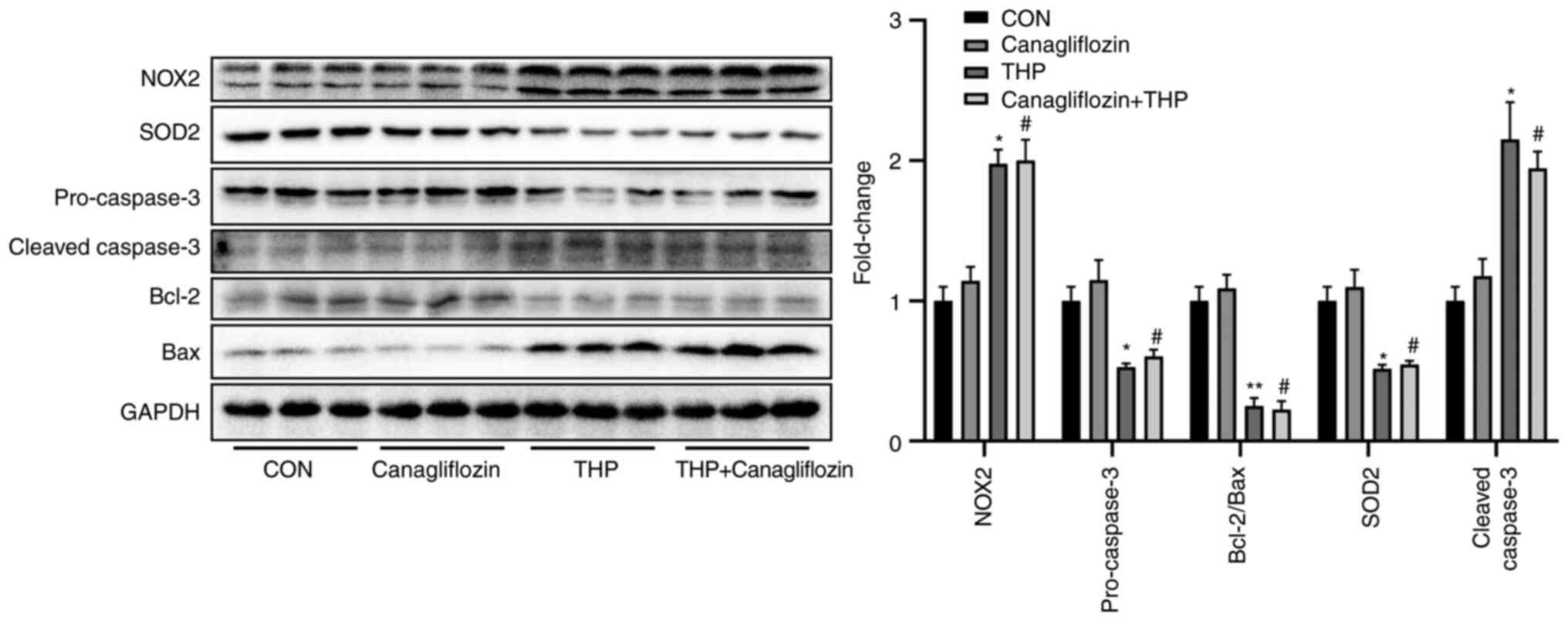
Effects of THP and canagliflozin on
the expression of related proteins in vivo
As shown in Fig. 5,
THP injection for 8 weeks led to the decrease of the protein
expression of SOD2, pro-caspase- and Bcl-2/Bax and the increase of
the protein expression of NOX2 and cleaved-caspase-, which
suggested that THP caused oxidative stress and increased apoptosis
in rat heart. However, treatment with canagliflozin does not
effectively improve the above changes (Fig. 5; P>0.05 vs. THP). Further
evidence was provided by quantitative analysis (Fig. 5).
Effects of THP and canagliflozin on
the expression of related proteins in vitro
The same applied in vitro. As shown in
Fig. 6: THP treatment of
cardiomyocytes led to the decrease of the protein expression of
SOD2, pro-caspase- and Bcl-2/Bax and the increase of the protein
expression of NOX2 and cleaved-caspase-, which suggested that THP
caused oxidative stress and increased apoptosis in rat
cardiomyocytes. However, the treatment of SGLT2i does not
effectively improve the above changes (Fig. 6; P>0.05 vs. THP). Further
evidence was provided by quantitative analysis (Fig. 6).
Discussion
In accordance with parts of the hypothesis of the
present study, the body weight and food intake of rats were
significantly decreased after intravenous injection of 10
mg•kg−1/day THP for 8 weeks. A series of cardiotoxic
manifestations were observed, including changes in echocardiography
and electrocardiogram outputs, increased LDH, CK-MB, cTnT and BNP
levels in serum and heart. Additionally, THP effectively induced
oxidative stress and apoptosis in the heart, reduced SOD activity
and increased MDA levels in serum and heart, leading to significant
changes in protein expression in the heart. However, against parts
of the hypothesis of the present study, adding canagliflozin (60
mg•kg−1/week) to rat diet did not improve these
THP-mediated conditions. In brief, the in vitro studies
failed. Western blotting data showed that THP still induced
oxidative stress and apoptosis in cardiomyocytes, but canagliflozin
could not improve this state and similarly no significant
differences was observed when compared with the THP group. These
results suggested that the cardioprotective effect of canagliflozin
may not function during THP-induced cardiotoxicity and myocardial
cell injury.
An important study outcome was that THP induced
cardiotoxicity in rats, which may have been caused by oxidative
stress and increased cardiomyocyte apoptosis. Currently, it is
generally accepted that anthracycline induced cardiotoxicity is
cumulative and dose-dependent (15). Reactive oxygen species (ROS),
oxidative stress induced by lipid peroxidation and cardiomyocyte
apoptosis all have dominant roles in anthracycline induced
cardiotoxicity (16). SOD is one
such important antioxidant enzymes in organisms (17), with the SOD2 protein expressed in
mitochondria (18). Previous
studies have shown that excessive consumption of mitochondrial SOD2
causes mitochondrial damage and apoptosis (18,19).
NADPH oxidase consumes oxygen and produces superoxide which is also
the main source of ROS in cardiovascular system (20). NOX2 is a classical representative
structural model of NADPH oxidase and is also the main form
expressed in cardiomyocytes (20).
NOX2, via its quinone structure, generates high ROS levels during
metabolism, leading to cardiomyocyte apoptosis and necrosis
(3,21,22).
In addition, THP also chelates iron ions and triggers oxygen free
radicals, resulting in lipid peroxidation of myocardial cell
membranes and mitochondrial DNA damage (23). Paglia and Radcliffe (24) reported that increased iron ion
levels enhances the sensitivity of cardiomyocytes to DOX, thereby
increasing ROS free radical production, leading to oxidative stress
and damage to myocardial tissue ultrastructures and cardiomyocytes.
THP also induced cardiomyocyte apoptosis, which was putatively
related to decreased Bcl-2/Bax ratios and caspase family activation
(25,26). The Bcl-2/Bax ratio is typically
reflective of the degree of apoptosis (27). When this ratio decreases,
permeability of the mitochondrial outer membrane changes, releasing
cytochrome c and apoptosis-inducing factors to the cytoplasm,
caspase cascade reaction and caspase-independent pathways are
involved in the occurrence of apoptosis (28–30).
Another unexpected outcome of the present study was
that canagliflozin, which is believed to have strong cardiovascular
protection potential (7,8,31), did
not exhibit corresponding cardiovascular protection in a
THP-induced cardiotoxicity model. A similar phenomenon was also
apparent in the in vitro studies. As previously mentioned,
the cardiotoxicity induced by THP is mainly due to the THP
accumulation in cardiomyocytes, concomitant with excessive ROS
production and eventual apoptosis (29,32).
Canagliflozin inhibits SGLT2, with studies showing that SGLT2 is
mainly distributed in the renal cortex and specifically binds to
the SGLT2 receptor at this location (32). In addition to blood glucose control,
the cardiovascular protective effect of canagliflozin are
attractive qualities with a broad application base (7,33).
Canagliflozin increases urinary sodium excretion, reduces water and
sodium retention, alleviates pre- and post-cardiac loads and exerts
cardiovascular protection (34).
THP-induced cardiotoxicity also causes hemodynamic changes to a
certain extent, but the condition is not caused by sodium and water
retention, but by direct damage to the heart (35). The present study hypothesized that
this is one of the main reasons why canagliflozin cannot exert its
effect. In addition, previous studies have shown that canagliflozin
reduces inflammation and oxidative stress in patients with T2DM and
atherosclerosis (36,37). The present study hypothesized that
this beneficial protective effect is closely related to weight loss
and hypoglycemic effect, but THP does not lead to abnormal increase
in blood glucose and blood lipid levels in rats, which may be
another important reason for the ineffectiveness of canagliflozin.
Increasing myocardial energy metabolism efficiency, inhibiting
Na+-H+ exchange protein activity, reducing
cytoplasmic Na+ and Ca2+ concentration and
increasing mitochondrial Ca2+ concentration may be
another way for canagliflozin to exert myocardial protective
effect, which has practical significance for THP-induced
cardiotoxicity (38,39). The present study hypothesized that
this effect may not be the main pharmacological action of
canagliflozin in protecting heart, but its effects on improving THP
cardiotoxicity are limited.
The present study showed THP-induced cardiomyocyte
injury in vivo and in vitro, possibly caused by
increased oxidative stress and apoptosis. It was only a preliminary
study and there are a number of deficiencies, including the lack of
positive control drugs. However, the authors of the present study
suggested that the cardiac toxicity model based on THP is a mature
model, which does not affect the experimental conclusion: In the
present study, it appeared that caglitazine did not improve the
cardiac toxicity induced by THP. Future studies are required to
analyze the potential cardioprotective effects of
canagliflozin.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 31501097), Chongqing
Science and health joint project (grant no. 2020FYYX101), China
Postdoctoral Science Foundation (grant no. 2019M652612) and the
Natural Science Foundation of Hubei Province, China (grant no.
2019CFB407).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to patent application
but are available from the corresponding author on reasonable
request.
Authors' contributions
HS, QZ, PP and RF conceptualized the study and
analyzed and interpreted data. YW, HY and HT analyzed and
interpreted data and revised the manuscript critically for
important intellectual content. DW designed the study and analyzed
the data. PP and RF drafted the manuscript. All authors confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Animal Ethics
Committee of the First Affiliated Hospital of Chongqing Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou J, Zhang X, Li M, Wu W, Sun X, Zhang
L and Gong T: Novel lipid hybrid albumin nanoparticle greatly
lowered toxicity of pirarubicin. Mol Pharm. 10:3832–3841. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng SE, Xiong S, Lin F, Qiao GL, Feng T,
Shen Z, Min DL, Zhang CL and Yao Y: Pirarubicin inhibits
multidrug-resistant osteosarcoma cell proliferation through
induction of G2/M phase cell cycle arrest. Acta Pharmacologica
Sinica. 33:832–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhai L, Guo C, Cao Y, Xiao J, Fu X, Huang
J, Huang H, Guan Z and Lin T: Long-term results of pirarubicin
versus doxorubicin in combination chemotherapy for aggressive
non-Hodgkin's lymphoma: Single center, 15-year experience. Int J
Hematol. 91:78–86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cong W, Liang Q, Li L, Shi J, Liu Q, Feng
Y, Wang Y and Luo G: Metabonomic study on the cumulative
cardiotoxicity of a pirarubicin liposome powder. Talanta. 89:91–98.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Getz KD, Sung L, Alonzo TA, Leger KJ,
Gerbing RB, Pollard JA, Cooper T, Kolb EA, Gamis AS, Ky B and
Aplenc R: Effect of dexrazoxane on left ventricular systolic
function and treatment outcomes in patients with acute myeloid
leukemia: A report from the children's oncology group. J Clin
Oncol. 38:2398–2406. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lamos EM, Younk LM and Davis SN:
Canagliflozin, an inhibitor of sodium-glucose cotransporter 2, for
the treatment of type 2 diabetes mellitus. Expert Opin Drug Metab
Toxicol. 9:763–775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neal B, Perkovic V and Matthews DR:
Canagliflozin and cardiovascular and renal events in type 2
diabetes. N Engl J Med. 377:20992017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Budoff MJ and Wilding JPH: Effects of
canagliflozin on cardiovascular risk factors in patients with type
2 diabetes mellitus. Int J Clin Pract. 71:e129482017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davies MJ, Merton K, Vijapurkar U, Yee J
and Qiu R: Efficacy and safety of canagliflozin in patients with
type 2 diabetes based on history of cardiovascular disease or
cardiovascular risk factors: A post hoc analysis of pooled data.
Cardiovasc Diabetol. 16:402017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ismy J, Sugandi S, Rachmadi D,
Hardjowijoto S and Mustafa A: The effect of exogenous superoxide
dismutase (SOD) on caspase-3 activation and apoptosis induction in
Pc-3 prostate cancer cells. Res Rep Urol. 12:503–508.
2020.PubMed/NCBI
|
|
11
|
Yabaji SM, Dhamija E, Mishra AK and
Srivastava KK: ESAT-6 regulates autophagous response through SOD-2
and as a result induces intracellular survival of mycobacterium
bovis BCG. Biochim Biophys Acta Proteins Proteom. 1868:1404702020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chocry M and Leloup L: The NADPH oxidase
family and its inhibitors. Antioxid Redox Signal. 33:332–353. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hegyi B, Borst JM, Bailey LRJ, Shen EY,
Lucena AJ, Navedo MF, Bossuyt J and Bers DM: Hyperglycemia
regulates cardiac K+ channels via O-GlcNAc-CaMKII and
NOX2-ROS-PKC pathways. Basic Res Cardiol. 115:712020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barthold SW, Bayne KA, Davis MA, Bayne K
and Davis M: Guide for the care and use of laboratory animals.
Publication. 327:963–965. 2011.
|
|
15
|
Armenian S and Bhatia S: Predicting and
preventing anthracycline-related cardiotoxicity. Am Soc Clin Oncol
Educ Book. 38:3–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lei X, Zhu SG, Das A, Chen Q, Durrant D,
Hobbs DC, Lesnefsky EJ and Kukreja RC: Dietary inorganic nitrate
alleviates doxorubicin cardiotoxicity: Mechanisms and implications.
Nitric Oxide. 26:274–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Brien KM, Dirmeier R, Engle M and Poyton
RO: Mitochondrial protein oxidation in yeast mutants lacking
manganese-(MnSOD) or copper- and zinc-containing superoxide
dismutase (CuZnSOD): Evidence that MnSOD and CuZnSOD have both
unique and overlapping functions in protecting mitochondrial
proteins from oxidative damage. J Biol Chem. 279:51817–15827. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen M, Du ZY, Zheng X, Li DL, Zhou RP and
Zhang K: Use of curcumin in diagnosis, prevention, and treatment of
Alzheimer's disease. Neural Regen Res. 13:742–752. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fabrizio P, Liou LL, Moy VN, Diaspro A,
Valentine JS, Gralla EB and Longo VD: SOD2 functions downstream of
Sch9 to extend longevity in yeast. Genetics. 163:35–46. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manuneedhi Cholan P, Cartland SP and
Kavurma MM: NADPH oxidases, angiogenesis, and peripheral artery
disease. Antioxidants (Basel). 6:562017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vavrova A, Jansova H, Mackova E, Machacek
M, Haskova P, Tichotova L, Sterba M and Simunek T: Catalytic
inhibitors of topoisomerase II differently modulate the toxicity of
anthracyclines in cardiac and cancer cells. PLoS One. 8:e766762013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Faulk A, Weissig V and Elbayoumi T:
Mitochondria-specific nano-emulsified therapy for myocardial
protection against doxorubicin-induced cardiotoxicity. Methods Mol
Biol. 991:99–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cascales A, Sánchez-Vega B, Navarro N,
Pastor-Quirante F, Corral J, Vicente V and de la Peña FA: Clinical
and genetic determinants of anthracycline-induced cardiac iron
accumulation. Int J Cardiol. 154:282–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paglia DE and Radcliffe RW: Anthracycline
cardiotoxicity in a black rhinoceros (Diceros bicornis): Evidence
for impaired antioxidant capacity compounded by iron overload. Vet
Pathol. 37:86–88. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu LS, Bai XQ, Gao Y, Wu Q, Ren Z, Li Q,
Pan LH, He NY, Peng J and Tang ZH: PCSK9 promotes oxLDL-induced
PC12 cell apoptosis through the Bcl-2/Bax-caspase 9/3 signaling
pathway. J Alzheimers Dis. 57:723–734. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu R, Tang S, Wang M, Xu X, Yao C and Wang
S: MicroRNA-497 induces apoptosis and suppresses proliferation via
the Bcl-2/Bax-caspase9-caspase3 pathway and cyclin D2 protein in
HUVECs. PLoS One. 11:e01670522016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM
and Zhou ZN: Intermittent hypoxia attenuates ischemia/reperfusion
induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax
expression. Cell Res. 13:385–391. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Finsterer J and Ohnsorge P: Influence of
mitochondrion-toxic agents on the cardiovascular system. Regul
Toxicol Pharmacol. 67:434–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pal MK, Jaiswar SP, Srivastav AK, Goyal S,
Dwivedi A, Verma A, Singh J, Pathak AK, Sankhwar PL and Ray RS:
Synergistic effect of piperine and paclitaxel on cell fate via
cyt-c, Bax/Bcl-2-caspase-3 pathway in ovarian adenocarcinomas
SKOV-3 cells. Eur J Pharmacol. 791:751–762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao H, Xu M and Chu G: Association
between myocardial cell apoptosis and calpain-1/caspase-3
expression in rats with hypoxic-ischemic brain damage. Mol Med Rep.
15:2727–2731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mahaffey KW, Neal B, Perkovic V, de Zeeuw
D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, et al:
Canagliflozin for primary and secondary prevention of
cardiovascular events: Results from the CANVAS program
(canagliflozin cardiovascular assessment study). Circulation.
137:323–334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghezzi C, Neal B, Perkovic V, de Zeeuw D,
Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T and Li Q:
Dapagliflozin binds specifically to sodium-glucose cotransporter 2
in the proximal renal tubule. J Am Soc Nephrol. 28:802–810. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Marx N and McGuire DK: Sodium-glucose
cotransporter-2 inhibition for the reduction of cardiovascular
events in high-risk patients with diabetes mellitus. Eur Heart J.
37:3192–3200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Verma S, McMurray JJV and Cherney DZI: The
metabolodiuretic promise of sodium-dependent glucose cotransporter
2 inhibition: The search for the sweet spot in heart failure. JAMA
Cardiol. 2:939–940. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Y, Ma XY, Zhang T, Qin M, Sun B, Li
Q, Hu DW and Ren LQ: Protective effects of apocynum venetum against
pirarubicin-induced cardiotoxicity. Am J Chin Med. 47:1075–1097.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gaal LV, Garvey T, Leiter L, Vijapurkar U,
List J, Cuddihy R, Ren J and Davies M: Effects of canagliflozin
versus glimepiride on inflammatory biomarkers and chemokines in
patients with type 2 diabetes mellitus. J Am Coll Cardiol. 69
(Suppl 11):S16722017. View Article : Google Scholar
|
|
37
|
Yaribeygi H, Atkin SL, Butler AE and
Sahebkar A: Sodium-glucose cotransporter inhibitors and oxidative
stress: An update. J Cell Physiol. 234:3231–3237. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Clancy CE, Chen-Izu Y, Bers DM,
Belardinelli L, Boyden PA, Csernoch L, Despa S, Fermini B, Hool LC,
Izu L, et al: Deranged sodium to sudden death. J Physiol.
593:1331–1345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kohlhaas M, Liu T, Knopp A, Zeller T, Ong
MF, Böhm M, O'Rourke B and Maack C: Elevated cytosolic
Na+ increases mitochondrial formation of reactive oxygen
species in failing cardiac myocytes. Circulation. 121:1606–1613.
2010. View Article : Google Scholar : PubMed/NCBI
|