Introduction
Osteoporosis is a metabolic bone disease
characterized by reduced bone tissue volume per unit volume and
degeneration of the bone tissue microstructure, resulting in
decreased bone strength, increased brittleness and the risk of
fracture (1). The morbidity of
osteoporosis increases with age in both males and postmenopausal
females (2), mainly due to advanced
age and estrogen deficiency (3),
affecting >50% of females >50 years of age (4). Other pathological factors are
associated with immune responses, including chronic inflammation
(5) or immune regulation (6), as well as an imbalance in osteoclast
or osteoblast differentiation (7).
Various efforts have been taken to improve the diagnosis and
treatment of osteoporosis; however, high recurrence rates, high
cost of treatment, poor patient compliance, poisonous side effects
of treatment drugs and malabsorption render the clinical efficacy
suboptimal (8). Therefore, there
are a number of challenges associated with the prevention and
treatment of osteoporosis. Accordingly, exploring the molecular
mechanisms underlying osteoporosis is necessary and meaningful to
identify a new target and treatment direction.
Long non-coding RNAs (lncRNAs) are a class of
non-coding RNAs without protein-coding function (9). They are typically 200–100,000
nucleotides in length and produced by RNA polymerase II (10). However, lncRNAs are involved in
multiple cellular processes and serve various roles in epigenetic,
transcriptional and post-transcriptional regulation of gene
expression (10–15). Certain studies have reported that
lncRNAs play important regulatory roles in the occurrence and
development of osteoporosis. For example, Zhang et al
(16) demonstrated that lncRNA
MSC-antisense (AS)1 promotes the osteogenic differentiation of bone
marrow-derived stromal cells (BMSCs) and alleviates the progression
of osteoporosis by sponging miR-140-5p to upregulate bone
morphogenic protein (BMP)2. Conversely, Wang et al (17) reported that lncRNA maternally
expressed 3 (MEG3) inhibits the osteogenic differentiation of bone
marrow mesenchymal stem cells from postmenopausal osteoporosis
(PMOP) by targeting the expression of microRNA (miRNA/miR)-133a-3p.
Ma et al (18) showed that
lncRNA neighboring enhancer of FOXA2 is downregulated in PMOP and
associated with the course of treatment and recurrence, which may
be involved in the inhibition of IL-6 secretion. lncRNA DANCR is
upregulated in blood mononuclear cells and promotes bone resorption
by releasing TNF-α and IL-6, resulting in osteoporosis (19). Downregulation of lncRNA ANCR
promotes osteoblast differentiation by targeting enhancer of zeste
homolog 2 and regulating runt-related transcription factor 2
(RUNX2) expression (20,21). These observations suggest that
lncRNAs are closely associated with the development of
osteoporosis. lncRNA growth arrest-specific 5 (GAS5) isolated at
the lymphoma-associated chromosomal locus (1q25) and exerts an
important regulatory role in tumorigenesis (22). Additionally, GAS5 negatively
regulates cell survival, participates in the development of bone
diseases and is upregulated in patients with osteoarthritis
(23). However, Feng et al
(24) reported that GAS5 is
downregulated in patients with osteoporosis, and GAS5
overexpression promotes the osteogenic differentiation of human
mesenchymal stem cells by regulating miR-498 to upregulate RUNX2
expression, which alleviates the development of osteoporosis. Thus,
the mechanisms of GAS5 in osteoporosis require further
exploration.
miRNAs are functional non-coding RNAs that can
recognize specific target genes by incomplete base pairing,
inhibiting the translation of these target genes (25). As potential therapeutic targets or
biomarkers, certain miRNAs have gained increasing attention in
osteoporosis, including miR-144-3p (26) and miR-132-3p (27). However, only one study has reported
the role of miR-10a-3p in osteoporosis. Kaempferol promotes BMSC
osteogenic differentiation and improves osteoporosis by
downregulating miR-10a-3p (28), a
topic that requires further exploration. Vascular endothelial
growth factor A (VEGFA), originally identified as an
endothelial-specific mitogen and permeability factor, serves a
critical role in promoting angiogenesis, which is essential and
dependent on bone formation (29,30).
Additionally, VEGFA is expressed during osteoblast differentiation,
and the exogenous addition of VEGFA can stimulate osteoblast-like
cell differentiation, which can attenuate osteoporosis (31). Angiogenesis plays a positive
regulatory role in attenuating osteoporosis by enhancing bone
formation (29). However, the
association between miR-10a-3p and VEGFA remains unclear, and the
effects of miR-10a-3p on VEGFA regulation in angiogenesis warrant
further exploration.
The present study investigated the effect of
knockdown or overexpression GAS5 or miR-10a-3p on angiogenesis of
osteoblasts. The present study aimed to verify whether GAS5
overexpression regulates angiogenesis via miR-10a-3p/VEGFA, which
may provide novel targets and pathways for the clinical treatment
of osteoporosis.
Materials and methods
Clinical sample collection
Blood samples were obtained (March 2019 to June
2019; Brain Hospital of Hunan Province, Changsha, China) from
median cubital vein of patients with osteoporosis (n=10; 6 females
and 4 males; age, 56–73 years) and healthy subjects (n=10; 5
females and 5 males; age, 57–72 years). The inclusion criteria were
as follows: Patients who were diagnosed with osteoporosis via X-ray
examination and without other diseases; and healthy subjects who
exhibited normal bone density via X-ray examination and without
other diseases. The basic information of the patients and healthy
controls is presented in Table I.
Patients with rheumatoid arthritis and other metabolic diseases
were excluded. The healthy subjects had no bone diseases and could
walk freely. The blood samples were centrifuged (1,000 × g, 5 min
at room temperature), serum was collected and was frozen and stored
at −80°C. The study was approved by the Brain Hospital of Hunan
Province (approval no. 2019058). Informed consent was obtained from
all the participants before sample collection.
 | Table I.Basic information of patients with
osteoporosis and healthy individuals in the present study. |
Table I.
Basic information of patients with
osteoporosis and healthy individuals in the present study.
| A, Patients with
osteoporosis |
|---|
|
|---|
| Patient number | Sex | Age, years |
|---|
| 1 | Female | 62 |
| 2 | Female | 56 |
| 3 | Male | 71 |
| 4 | Female | 64 |
| 5 | Male | 70 |
| 6 | Female | 66 |
| 7 | Female | 58 |
| 8 | Male | 67 |
| 9 | Female | 60 |
| 10 | Male | 73 |
|
| B, Healthy
individuals |
|
| Patient
number | Sex | Age,
years |
|
| 1 | Female | 59 |
| 2 | Male | 64 |
| 3 | Male | 69 |
| 4 | Female | 63 |
| 5 | Female | 57 |
| 6 | Male | 68 |
| 7 | Male | 62 |
| 8 | Female | 60 |
| 9 | Male | 72 |
| 10 | Female | 65 |
Detection of bone mineral density
(BMD)
The BMD of the patients with osteoporosis and
healthy subjects was determined via peripheral dual-energy X-ray
absorptiometry (PIXImus II; Lunar; GE Healthcare). They were
arranged in the prone position, and an image was acquired in 5 min.
The BMD (g/cm2) of the entire body was determined except
the head.
Cell culture
Human osteoblasts (hFOB1.19) and human umbilical
vein endothelial cells (HUVECs) were cultured in DMEM (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin,
100 µg/ml streptomycin and 0.5 µg/ml fungizone at 37°C in a
humidified 5% CO2 incubator.
Cell transfection
pcDNA-GAS5 or pcDNA-VEGFA (pcDNA; control), short
hairpin RNA (sh)GAS5 (shNC; control) were synthesized by
GeneCopoeia, Inc. miR-10a-3p mimics
(5′-CAAAUUCGGAUCUACAGGGUAUU-3′), miR-10a-3p inhibitor
(5′-CACAAAUUCGGAUCUACAGGGUA-3′), NC (mimics NC,
5′-UUCUCCGAACGUGUCACGUTT-3′); inhibitor NC,
(5′-CAGUACUUUUGUGUAGUACAA-3′) were purchased from Sangon Biotech
Co., Ltd. Briefly, 2Then, 1×104 hFOB1.19 cells were
transfected with pcDNA-GAS5 or pcDNA-VEGFA (0.5 µg), miR-10a-3p
mimics/inhibitor (50 nM) or mimics NC/inhibitor NC (50 nM) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h, during which time the medium was not
replaced. The expression of the relative genes in transfected cells
was subsequently determined via reverse transcription-quantitative
(RT-q)PCR.
RT-qPCR
Total RNA in the cells was extracted using
TRIzol® reagent (Takara Bio, Inc.). The RNA
concentration and purity were detected via spectrophotometry.
Subsequently, 1 µg total RNA was reverse transcribed into cDNA
using a PrimeScript™ RT Reagent kit with gDNA Eraser (Takara Bio,
Inc.) according to the manufacturer's protocol. qPCR was performed
according to the manufacturer's instructions of an SYBR Premix Ex
Taq II kit (Takara Bio, Inc.) using an ABI Prism 7500 HT Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The reaction procedures were as follows: Pre-denaturation at
94°C for 10 min, followed by 40 cycles of denaturation at 94°C for
30 sec, annealing at 60°C for 30 sec and extension for 60 sec at
72°C. The relative expression levels were calculated using the
2−ΔΔCq method (32) and
were normalized to GAPDH or U6. The primer sequences were as
follows: GAS5 forward, 5′-CTTGCCTGGACCAGCTTAAT-3′ and reverse,
5′-CAAGCCGACTCTCCATACCT-3′; miR-10a-3p forward,
5′-GCGCGCAAATTCGTATCTAGG-3′ and reverse,
5′-GTCGTATCCAGTGCAGGGTCC-3′; VEGFA forward,
5′-CCCGGGCCTCGGTTCCAG-3′ and reverse, 5′-GTCGTGGGTGCAGCCTGGG-3′;
GAPDH forward, 5′-GAGTCAACGGATTTGGTCGTT-3′ and reverse,
5′-TTGATTTTGGAGGGATCTCG-3′; and U6 forward,
5′-TGCGGGTGCTCGCTTCGGCAGC-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGT-3′.
ELISA
The cells were treated with radioimmunoprecipitation
assay (RIPA) lysis buffer (Beyotime Institute of Biotechnology).
VEGFA was examined using a Human VEGF ELISA kit (Abcam; cat. no.
ab222510) according to the manufacturer's protocols.
Western blot analysis
Total proteins in cells were extracted using RIPA
lysis buffer containing protease and phosphatase inhibitors
(Selleck Chemicals). Protein concentrations were determined using a
BCA kit. The protein samples (25 µg/lane) were separated via 12%
SDS-PAGE and transferred onto PVDF membranes (EMD Millipore). The
membranes were blocked in 5% BSA (Beijing Solarbio Science &
Technology Co., Ltd.; cat. no. SW3015) for 1 h at room temperature
and incubated with anti-VEGFA (Abcam; 1:1,000; cat. no. ab1316) and
anti-GAPDH (Abcam; 1:1,000; cat. no. ab8245) antibodies at 4°C
overnight. On the following day, the membranes were incubated with
HRP-conjugated secondary antibodies diluted at 1:3,000 (Abcam; cat.
no. ab97040) at room temperature for 2 h. The protein bands were
visualized using an Immobilon Western Chemilum HRP Substrate (EMD
Millipore, WBKLS0100) and analyzed using ImageJ software 1.52a
(National Institutes of Health).
Matrigel angiogenesis
Conditioned medium (CM) was collected from hFOB1.19
cells transfected with GAS5 or miR-10a-3p. Matrigel was slowly
melted at 4°C overnight, and 10 µl was added to each well of the
angiogenic microslide. After Matrigel solidification,
2×105 cells/ml of a HUVEC cell suspension was prepared.
Next, 50 µl cell suspension (~10,000 cells) was added to the
Matrigel and then blocked with a coverslip. After incubating with
100 µl/well of the indicated CM for 8 h at 37°C, tubular structures
were captured in five fields of view using a light inverted
microscope (cat. no. IX73; Olympus Corporation) at ×100
magnification and analyzed using ImageJ software 1.52a (National
Institutes of Health).
Dual-luciferase reporter assay
The interaction between GAS5 and miR-10a-3p, and
VEGFA and miR-10a-3p was predicted by StarBase V2.0 (starbase.sysu.edu.cn). To determine the relationship
between GAS5 and miR-10a-3p, as well as between miR-10a-3p and
VEGFA, wild-type (WT) or mutant (MUT) sequences of the
3′-untranslated region of GAS5 or VEGFA were inserted into pmirGLO
luciferase vectors to generate GAS5-WT, GAS5-MUT, VEGFA-WT and
VEGFA-MUT vectors (Promega Corporation). Osteoblasts were
co-transfected with the above plasmids and miR-10a-3p or mimics NC
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The transfected cells were washed with PBS, and
the luciferase activity was measured after 48 h using a
dual-luciferase assay system (Promega Corporation). The firefly
luciferase activity was normalized to Renilla luciferase
activity.
Statistical analysis
For statistical analyses, one-way ANOVA (multiple
comparisons) and Student's t-test (two comparisons) were performed
using SPSS software (v12.0; SPSS, Inc.). Tukey's post hoc test was
used for multiple comparisons. Correlation was assessed by
Pearson's coefficient. The results were presented as the mean ± SD
of three independent experiments. P<0.05 was considered to
indicate a statistically significant difference.
Results
lncRNA GAS5 is downregulated and
miR-10a-3p is upregulated in patients with osteoporosis
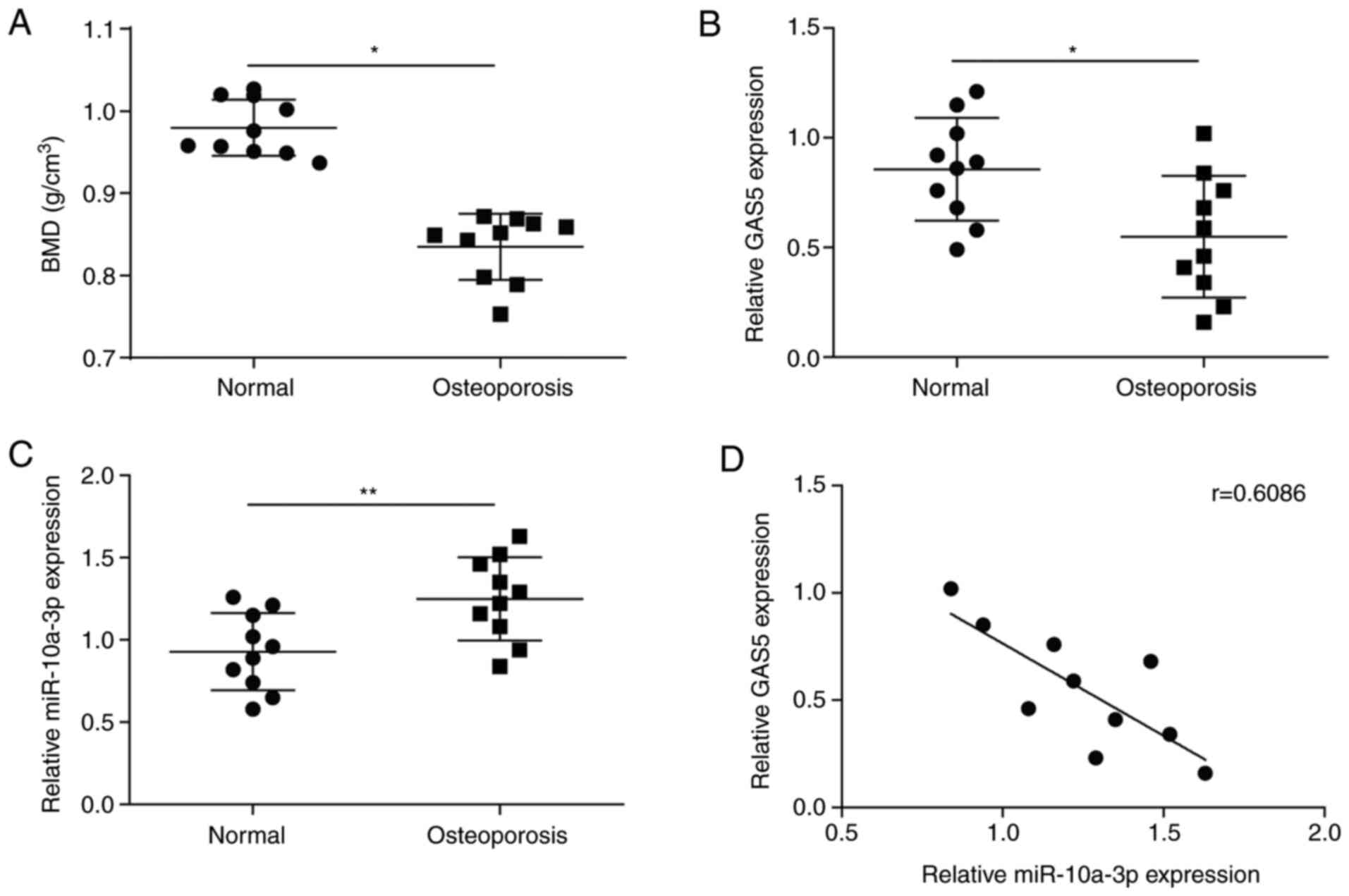
The bone mineral density of patients with
osteoporosis and healthy subjects was detected, revealing that
patients exhibited significantly reduced bone mineral density
compared with healthy subjects (Fig.
1A). Representative X-ray images of patients with osteoporosis
and healthy subjects are shown in Fig.
S1. Serum was collected from the blood samples of patients with
osteoporosis and healthy subjects to detect the levels of GAS5 and
miR-10a-3p via RT-qPCR. The expression of GAS5 was significantly
downregulated in patients compared with the healthy subjects
(Fig. 1B). Conversely, the levels
of miR-10a-3p were significantly increased in patients (Fig. 1C). Additionally, there was a
negative linear correlation between the expression of GAS5 and
miR-10a-3p (Fig. 1D). These results
indicated that GAS5 was downregulated and miR-10a-3p was
upregulated in osteoporosis.
Overexpression of lncRNA GAS5
effectively promotes angiogenesis
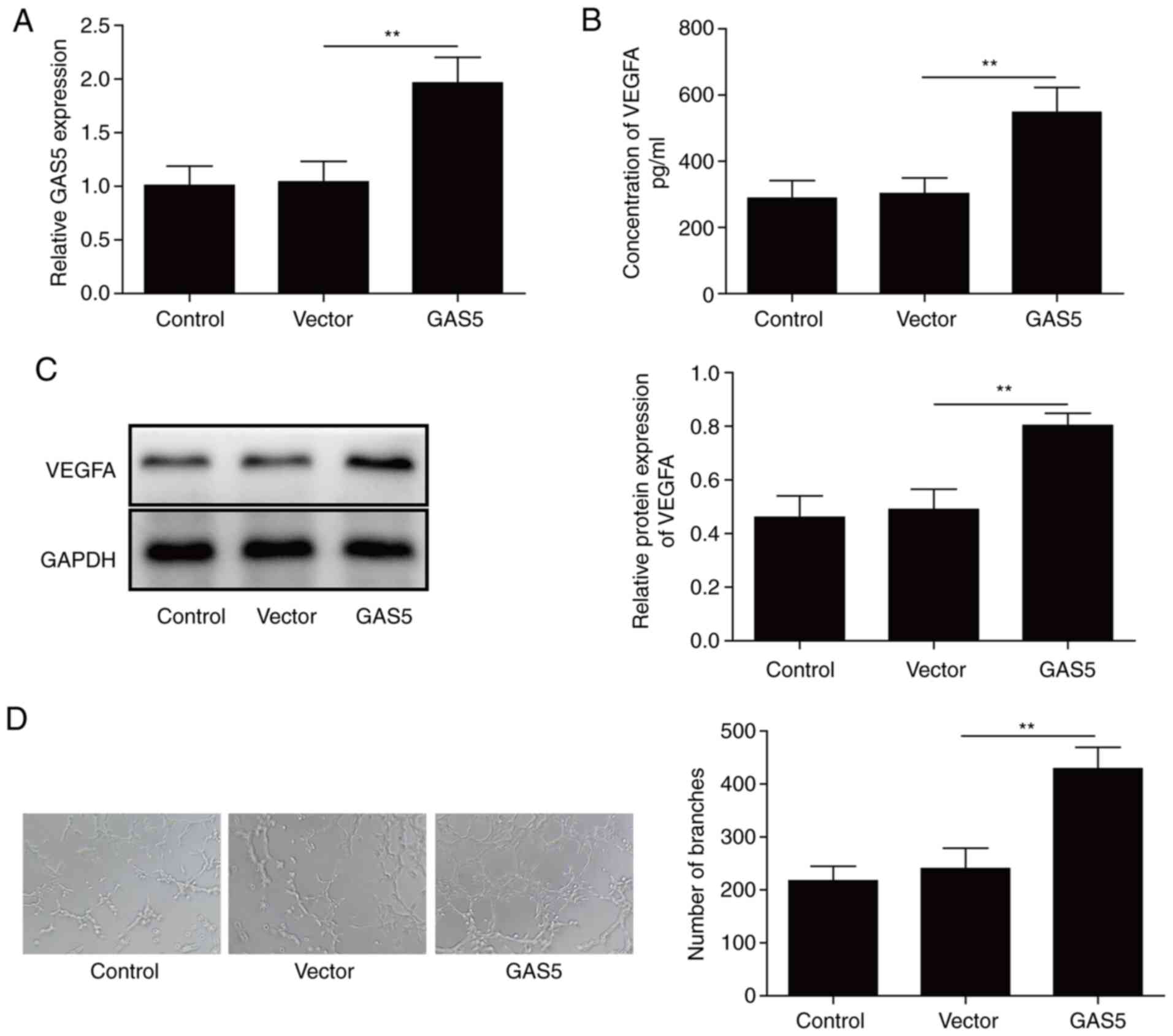
GAS5 was overexpressed in osteoblasts to investigate
the effects of GAS5. RT-qPCR analysis that GAS5 expression is
significantly increased in osteoblasts after GAS5 transfection
compared with in the untransfected and empty vector control groups
(Fig. 2A), indicating that GAS5 was
successfully transfected in osteoblasts. The levels of VEGFA were
detected via ELISA and western blotting. The levels of VEGFA were
significantly elevated in osteoblasts after GAS5 transfection
(Fig. 2B and C), indicating that
GAS5 induced the upregulation of VEGFA. Additionally, a Matrigel
angiogenesis assay revealed increased angiogenesis for HUVECs
treated with CM from the GAS5 group compared with the control and
empty vector groups (Fig. 2D),
implying that GAS5 induced angiogenesis via VEGFA. Thus, GAS5
overexpression promoted angiogenesis by increasing the levels of
VEGFA.
lncRNA GAS5 acts as a sponge of
miR-10a-3p
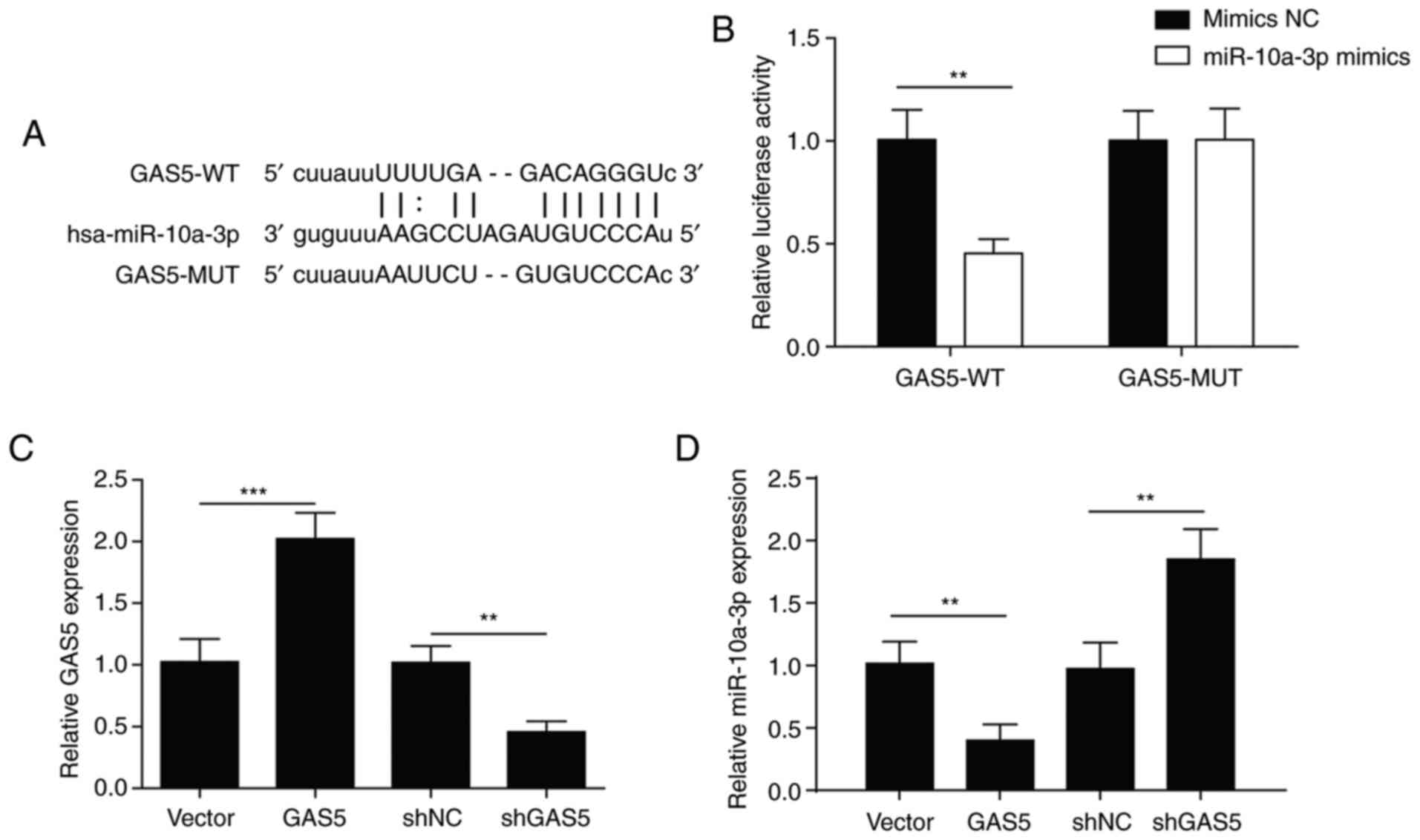
A negative correlation was observed between GAS5 and
miR-10a-3p, but the upstream or downstream relationship remains
unknown. Therefore, the binding site between miR-10a-3p and GAS5
was predicted using StarBase V2.0 (Fig.
3A). A dual-luciferase reporter assay showed that the
luciferase activity in the GAS5-WT group was significantly
decreased following co-transfection with miR-10a-3p compared with
mimics NC, indicating that GAS5 interacted with miR-10a-3p. When
GAS5 was mutated, luciferase activity was not notably affected by
transfection with miR-10a-3p compared with mimics NC (Fig. 3B). These results indicated an
association between GAS5 and miR-10a-3p.
The expression levels of GAS5 and miR-10a-3p were
detected in osteoblasts following transfection with GAS5 or shGAS5.
The expression of GAS5 was upregulated in osteoblasts after
transfection with GAS5 (Fig. 3C),
while the expression of miR-10a-3p was downregulated (Fig. 3D). Opposing effects were observed
after transfection with shGAS5 (Fig. 3C
and D). These results indicated that GAS5 targeted
miR-10a-3p.
VEGFA is a target gene of
miR-10a-3p
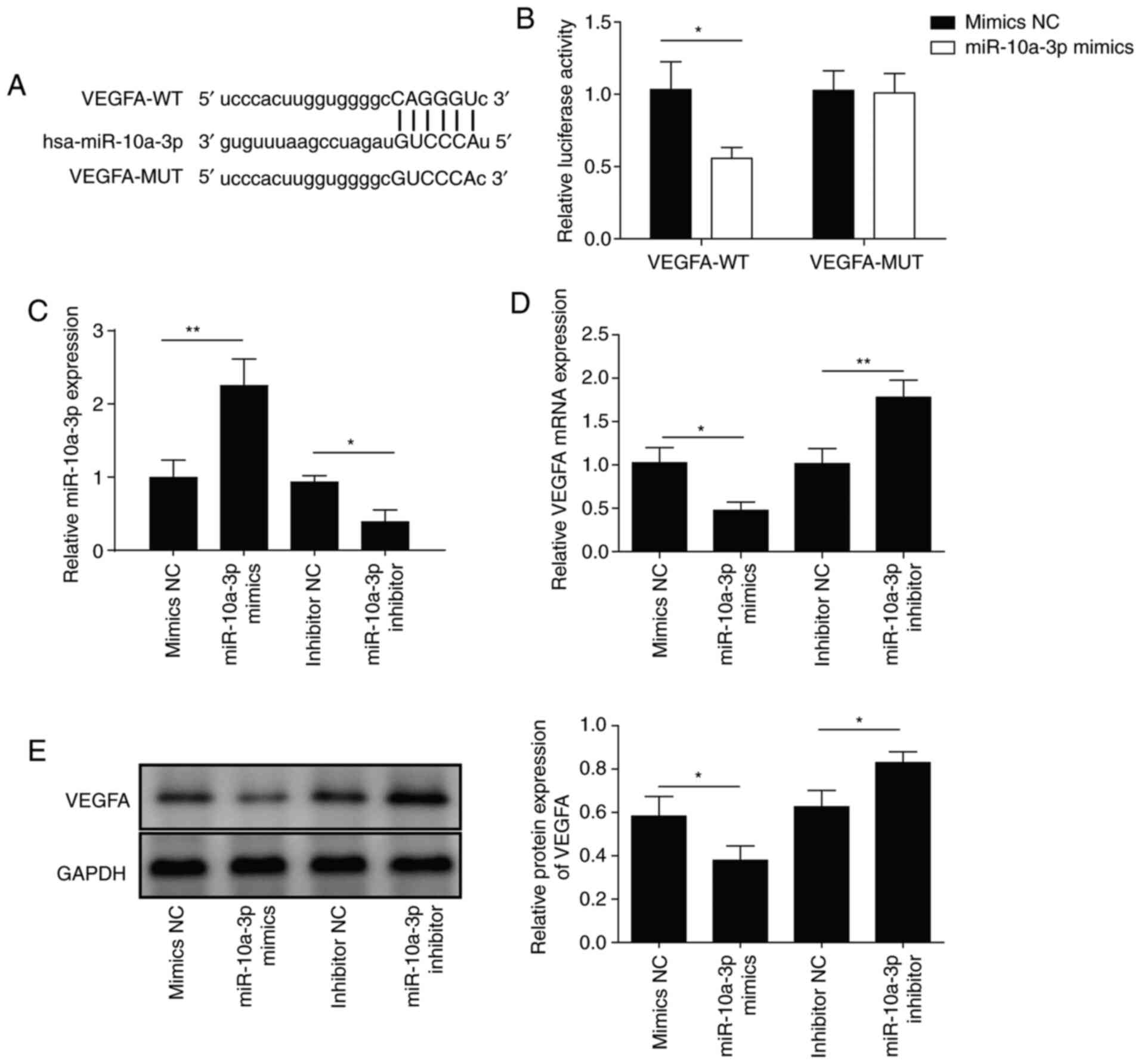
The above findings indicated that GAS5 induced
downregulation of miR-10a-3p. However, whether a targeting
relationship exists between miR-10a-3p and VEGFA remains unknown.
Therefore, a binding site between miR-10a-3p and VEGFA was
predicted by StarBase V2.0 (Fig.
4A). A dual-luciferase reporter assay showed that miR-10a-3p
bound to VEGFA in a targeted manner (Fig. 4B). After transfection with
miR-10a-3p mimics, miR-10a-3p was upregulated and VEGFA mRNA was
downregulated. When the miR-10a-3p inhibitor was transfected,
opposing effects were observed (Fig. 4C
and D). Similar findings were observed at the protein level
(Fig. 4E). These results suggested
that miR-10a-3p inhibited the expression of its target VEGFA.
lncRNA GAS5 promotes angiogenesis via
the miR-10a-3p/VEGFA axis
Next, the mechanism underlying the promotion of
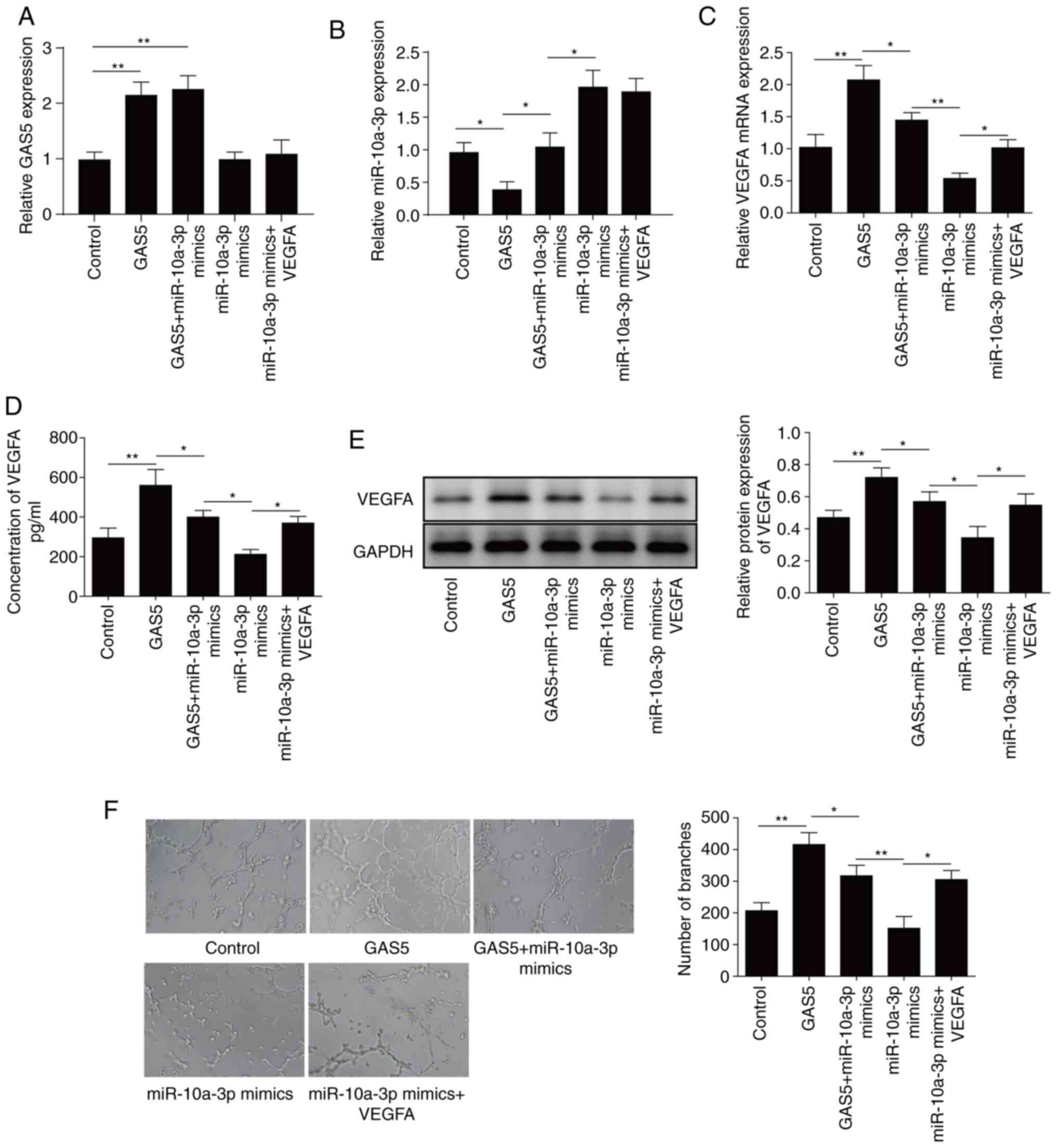
angiogenesis by GAS5 was explored. After transfection with GAS5 or
VEGFA overexpression vectors (Fig.
S2), the expression of GAS5 was significantly increased, while
miR-10a-3p was significantly downregulated (Fig. 5A and B), and the mRNA and protein
levels of VEGFA were increased (Fig.
5C-E). After transfection with miR-10a-3p mimics, miR-10a-3p
was significantly increased (Fig.
5B), and the mRNA and protein levels of VEGFA were decreased
(Fig. 5C-E). When GAS5 and
miR-10a-3p mimics were transfected simultaneously, their effects
were offset; however, the addition of VEGFA attenuated the
inhibition of VEGFA expression by miR-10a-3p mimics (Fig. 5C-E). These results demonstrated that
GAS5 regulated the expression of VEGFA by inhibiting
miR-10a-3p.
Moreover, GAS5 overexpression promoted angiogenesis,
whereas miR-10a-3p mimics exhibited an inhibitory effect. The
presence of miR-10a-3p suppressed angiogenesis after GAS5
overexpression. However, the overexpression of VEGFA reversed the
inhibition by miR-10a-3p mimics of angiogenesis (Fig. 5F), indicating that GAS5 promoted
angiogenesis by increasing the expression of VEGFA.
In summary, GAS5 was downregulated in patients with
osteoporosis, which induced upregulation of its target miR-10a-3p.
Subsequently, miR-10a-3p inhibited the expression of its target
VEGFA to suppress angiogenesis. Thus, the present study indicated
that GAS5 promoted angiogenesis via the miR-10a-3p/VEGFA axis in
osteoporosis.
Discussion
Osteoporosis is a severe bone disease, resulting in
decreased bone strength, and increased fragility and risk of
fracture, resulting in substantial harm to postmenopausal women and
older men (33). Anti-resorptive
agents (such as bisphosphonates and selective estrogen receptor
modulators) and anabolic drugs that stimulate bone formation
(including parathyroid hormone analogues and sclerostin inhibitors)
are current treatment strategies for osteoporosis (34). Previously, no study has compared the
anti-fracture efficacy of different bisphosphonates, but recent
evidence suggests that zoledronate treatment is more effective than
risedronate or alendronate (35).
Alendronate and calcium have been reported to exhibit higher
efficacy when combined with oral Chinese herbal medicines in the
treatment of senile osteoporosis (36). Despite their efficacy, long-term
adherence remains a challenge due to the severe side effects and
loss of potency (34).
A number of studies have investigated osteoporosis
at the genetic level, and it has been reported that lncRNAs serve
important roles in influencing osteoporosis. Examples of lncRNAs
that promote osteogenic differentiation and alleviate the
progression of osteoporosis include lncRNA MSC-AS1 (16) and GAS5 (23,24).
Conversely, lncRNA MEG3 (17),
lncRNA DANCR (19) and lncRNA-ANCR
(20,21) inhibit osteogenic differentiation,
resulting in osteoporosis. lncRNA GAS5 is reported to alleviate the
development of osteoporosis via the miR-498/RUNX2 (24) and miR-135a-5p/FOXO1 pathways
(37). However, treatments for
osteoporosis using lncRNA GAS5-targeted drugs have not been
reported. In the present study, it was demonstrated that GAS5
overexpression downregulated its target miR-10a-3p, which
subsequently induced the upregulation of VEGFA and promoted
angiogenesis, indicating potential for the treatment of
osteoporosis. The study described a novel mechanism and novel
targets with relevance for the treatment of osteoporosis.
miRNAs decrease the expression of their targets via
specific binding, thus serving regulatory roles in cells. miR-133a
promotes bone loss by altering the serum levels of
osteoclastogenesis-related factors, decreasing lumbar spine BMD and
altering bone histomorphology (38). miR-208a-3p, miR-155-5p and miR-637
were significantly upregulated in postmenopausal and premenopausal
patients with osteoporosis, suggesting their association with
disease pathogenesis (39).
miR-19b-3p promotes the proliferation and osteogenic
differentiation of BMSCs, revealing a role for miR-19b-3p in
postmenopausal osteoporosis (40).
However, no study has reported on the pro-angiogenic role of
miR-10a-3p in osteoporosis. In the present study, the
downregulation of miR-10a-3p by GAS5 overexpression increased the
levels of VEGFA. These data are the first to indicate potential
roles for GAS5 and miR-10a-3p in osteoporosis.
Several signaling pathways have been reported to be
involved in the occurrence and development of osteoporosis, such as
the JNK pathway (41),
Wnt/β-catenin pathway (42), Janus
kinase 2/STAT3 signaling pathway (43) and BMP6/Smad1/5/9 pathway (44), through which various proteins are
targeted to regulate cell proliferation and osteogenic
differentiation. VEGFA can stimulate the differentiation of
osteoblast-like cells (27) and
influence BMD in osteoporosis (45). In the present study, VEGFA was
upregulated via the inhibition of miR-10a-3p by GAS5
overexpression; subsequently, VEGFA promoted osteoblastic
angiogenesis. The present study further elucidated the role of
VEGFA in osteoporosis and described a novel miR-10a-3p/VEGFA
signaling pathway, providing new leads for understanding the
mechanisms underlying the occurrence and development of
osteoporosis.
The etiology of osteoporosis is complex, involving
endocrine, immune, lifestyle, environmental and nutritional factors
(46). Numerous studies have
focused on the genetic level and have described several molecular
signaling pathways. Therefore, investigating these molecular
mechanisms may provide improved understanding of osteoporosis
pathogenesis and potential therapeutic interventions. It was found
that GAS5 overexpression upregulated the level of VEGFA by
inhibiting miR-10a-3p, thus promoting angiogenesis. The present
study is the first to describe the targeted relationship between
GAS5, miR-10a-3p and VEGFA, increasing knowledge concerning this
signaling pathway and providing a possible therapeutic target for
osteoporosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW conceived and designed the study. WW and YFL
performed experiments and acquired data. WW and YL analyzed the
data. QL assisted in data acquisition and interpretation and
revised the manuscript. WW and YL prepared the manuscript. WW and
YL confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Brain Hospital of
Hunan Province (approval no. 2019058). Informed consent was
obtained from all participants prior to sample collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
lncRNA
|
long non-coding RNA
|
|
GAS5
|
growth arrest-specific 5
|
|
VEGFA
|
vascular endothelial growth factor
A
|
|
PMOP
|
postmenopausal osteoporosis
|
|
miRNA/miR
|
microRNA
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
CM
|
conditioned medium
|
References
|
1
|
Ahlborg HG, Rosengren BE, Järvinen TL,
Rogmark C, Nilsson JA, Sernbo I and Karlsson MK: Prevalence of
osteoporosis and incidence of hip fracture in women-secular trends
over 30 years. BMC Musculoskelet Disord. 11:482010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baccaro LF, Conde DM, Costa-Paiva L and
Pinto-Neto AM: The epidemiology and management of postmenopausal
osteoporosis: A viewpoint from Brazil. Clin Interv Aging.
10:583–591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Syed FA and Ng AC: The pathophysiology of
the aging skeleton. Curr Osteoporos Rep. 8:235–240. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xue F, Wagman RB, Yue S, Smith S, Arora T,
Curtis JR, Ehrenstein V, Sørensen HT, Tell G, Kieler H, et al:
Incidence rate of potential osteonecrosis of the jaw among women
with postmenopausal osteoporosis treated with prolia or
bisphosphonates: Abstract number 348. Arthritis Rheum. 67:492–495.
2015.
|
|
5
|
Montalcini T, Romeo S, Ferro Y, Migliaccio
V, Gazzaruso C and Pujia A: Osteoporosis in chronic inflammatory
disease: The role of malnutrition. Endocrine. 43:59–64. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao R: Immune regulation of osteoclast
function in postmenopausal osteoporosis: A critical
interdisciplinary perspective. Int J Med Sci. 9:825–832. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zaidi M: Skeletal remodeling in health and
disease. Nat Med. 13:791–801. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beaupre LA, Majumdar SR, Dieleman S, Au A
and Morrish DW: Diagnosis and treatment of osteoporosis before and
after admission to long-term care institutions. Osteoporos Int.
23:573–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu Y, An JJ, Tabys D, Xie YD, Zhao TY, Ren
HW and Liu N: Effect of lactoferrin on the expression profiles of
long non-coding RNA during osteogenic differentiation of bone
marrow mesenchymal stem cells. Int J Mol Sci. 20:48342019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoon JH, Abdelmohsen K and Gorospe M:
Posttranscriptional gene regulation by long noncoding RNA. J Mol
Biol. 425:3723–3730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jarroux J, Morillon A and Pinskaya M:
History, discovery, and classification of lncRNAs. Adv Exp Med
Biol. 1008:1–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao J: The functional role of long
non-coding RNAs and epigenetics. Biol Proced Online. 16:112014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin D, Wu X, Yu H, Jiang L, Zhou P, Yao X,
Meng J, Wang L, Zhang M and Zhang Y: Systematic analysis of
lncRNAs, mRNAs, circRNAs and miRNAs in patients with postmenopausal
osteoporosis. Am J Transl Res. 10:1498–1510. 2018.PubMed/NCBI
|
|
16
|
Zhang N, Hu X, He S, Ding W, Wang F, Zhao
Y and Huang Z: lncRNA MSC-AS1 promotes osteogenic differentiation
and alleviates osteoporosis through sponging microRNA-140-5p to
upregulate BMP2. Biochem Biophys Res Commun. 519:790–796. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Li Y and Zhang Y, Ma L, Lin L,
Meng J, Jiang L, Wang L, Zhou P and Zhang Y: lncRNA MEG3 inhibited
osteogenic differentiation of bone marrow mesenchymal stem cells
from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed
Pharmacother. 89:1178–1186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma X, Guo Z, Gao W, Wang J, Liu Y, Gao F,
Sun S, Zhou X, Yang Z and Zheng W: lncRNA-NEF is downregulated in
postmenopausal osteoporosis and is related to course of treatment
and recurrence. J Int Med Res. 47:3299–3306. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tong X, Gu PC, Xu SZ and Lin XJ: Long
non-coding RNA-DANCR in human circulating monocytes: A potential
biomarker associated with postmenopausal osteoporosis. Biosci
Biotechnol Biochem. 79:732–737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu L and Xu PC: Downregulated lncRNA-ANCR
promotes osteoblast differentiation by targeting EZH2 and
regulating Runx2 expression. Biochem Biophys Res Commun.
432:612–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai N, Li C and Wang F: Silencing of
lncRNA-ANCR promotes the osteogenesis of osteoblast cells in
postmenopausal osteoporosis via targeting EZH2 and RUNX2. Yonsei
Med J. 60:751–759. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamura Y, Takahashi N, Kakegawa E,
Yoshida K, Ito Y, Kayano H, Niitsu N, Jinnai I and Bessho M: The
GAS5 (growth arrest-specific transcript 5) gene fuses to BCL6 as a
result of t(1;3)(q25;q27) in a patient with B-cell lymphoma. Cancer
Genet Cytogenet. 182:144–149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song J, Ahn C, Chun CH and Jin EJ: A long
non-coding RNA, GAS5, plays a critical role in the regulation of
miR-21 during osteoarthritis. J Orthop Res. 32:1628–1635. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng J, Wang JX and Li CH: lncRNA GAS5
overexpression alleviates the development of osteoporosis through
promoting osteogenic differentiation of MSCs via targeting
microRNA-498 to regulate RUNX2. Eur Rev Med Pharmacol Sci.
23:7757–7765. 2019.PubMed/NCBI
|
|
25
|
Saliminejad K, Khorram Khorshid HR,
Soleymani Fard S and Ghaffari SH: An overview of microRNAs:
Biology, functions, therapeutics, and analysis methods. J Cell
Physiol. 234:5451–5465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang C, He H, Wang L, Jiang Y and Xu Y:
Reduced miR-144-3p expression in serum and bone mediates
osteoporosis pathogenesis by targeting RANK. Biochem Cell Biol.
96:627–635. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu Z, Zhang L, Wang H, Wang Y, Tan Y, Dang
L, Wang K, Sun Z, Li G, Cao X, et al: Targeted silencing of
miRNA-132-3p expression rescues disuse osteopenia by promoting
mesenchymal stem cell osteogenic differentiation and osteogenesis
in mice. Stem Cell Res Ther. 11:582020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Yi X, Tu S, Cheng C and Luo J:
Kaempferol promotes BMSC osteogenic differentiation and improves
osteoporosis by downregulating miR-10a-3p and upregulating CXCL12.
Mol Cell Endocrinol. 520:1110742021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu R, Lv WC, Xu Y, Gong MY, Chen XJ, Jiang
N, Xu Y, Yao QQ, Di L, Lu T, et al: Endothelial ZEB1 promotes
angiogenesis-dependent bone formation and reverses osteoporosis.
Nat Commun. 11:4602020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang M, Li CJ, Sun X, Guo Q, Xiao Y, Su T,
Tu ML, Peng H, Lu Q, Liu Q, et al: miR-497~195 cluster regulates
angiogenesis during coupling with osteogenesis by maintaining
endothelial Notch and HIF-1α activity. Nat Commun. 8:160032017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deckers MM, Karperien M, van der Bent C,
Yamashita T, Papapoulos SE and Löwik CW: Expression of vascular
endothelial growth factors and their receptors during osteoblast
differentiation. Endocrinology. 141:1667–1674. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ensrud KE and Crandall CJ: Osteoporosis.
Ann Intern Med. 167:ITC17–ITC32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li H, Xiao Z, Quarles LD and Li W:
Osteoporosis: Mechanism, molecular target, and current status on
drug development. Curr Med Chem. 28:1489–1507. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Compston J: Practical guidance for the use
of bisphosphonates in osteoporosis. Bone. 25:1153302020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang H, Mo S, Yang L, Wang P, Sun K, Xiong
Y, Liu H, Liu X, Wu Z, Ou L, et al: Effectiveness associated with
different therapies for senile osteoporosis: A network
meta-analysis. J Tradit Chin Med. 40:17–27. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Zhao D, Zhu Y, Dong Y and Liu Y:
Long non-coding RNA GAS5 promotes osteogenic differentiation of
bone marrow mesenchymal stem cells by regulating the
miR-135a-5p/FOXO1 pathway. Mol Cell Endocrinol. 496:1105342019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Z, Zhang W and Huang Y: miRNA-133a is
involved in the regulation of postmenopausal osteoporosis through
promoting osteoclast differentiation. Acta Biochim Biophys Sin
(Shanghai). 50:273–280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ismail SM, El Boghdady NA, Hamoud HS and
Shabayek MI: Evaluation of circulating miRNA-208a-3p, miRNA-155-5p
and miRNA-637 as potential non-invasive biomarkers and the possible
mechanistic insights into pre- and postmenopausal osteoporotic
females. Arch Biochem Biophys. 684:1083312020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiaoling G, Shuaibin L and Kailu L:
MicroRNA-19b-3p promotes cell proliferation and osteogenic
differentiation of BMSCs by interacting with lncRNA H19. BMC Med
Genet. 21:112020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Meng YC, Lin T, Jiang H, Zhang Z, Shu L,
Yin J, Ma X, Wang C, Gao R and Zhou XH: miR-122 exerts inhibitory
effects on osteoblast proliferation/differentiation in osteoporosis
by activating the PCP4-mediated JNK pathway. Mol Ther Nucleic
Acids. 20:345–358. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu TJ and Guo JL: Overexpression of
microRNA-141 inhibits osteoporosis in the jawbones of
ovariectomized rats by regulating the Wnt/β-catenin pathway. Arch
Oral Biol. 113:1047132020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fu Y, Xu Y, Chen S, Ouyang Y and Sun G:
miR-151a-3p promotes postmenopausal osteoporosis by targeting SOCS5
and activating JAK2/STAT3 signaling. Rejuvenation Res. 23:313–323.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang T, Zhang C, Wu C, Liu J, Yu H, Zhou
X, Zhang J, Wang X, He S, Xu X, et al: miR-765 inhibits the
osteogenic differentiation of human bone marrow mesenchymal stem
cells by targeting BMP6 via regulating the BMP6/Smad1/5/9 signaling
pathway. Stem Cell Res Ther. 11:622020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee HS and Park T: Nuclear receptor and
VEGF pathways for gene-blood lead interactions, on bone mineral
density, in Korean smokers. PLoS One. 13:e01933232018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lane NE: Epidemiology, etiology, and
diagnosis of osteoporosis. Am J Obstet Gynecol. 194 (Suppl
2):S3–S11. 2006. View Article : Google Scholar : PubMed/NCBI
|