Introduction
Gastric cancer (GC) is the third most common cause
of cancer-related mortality worldwide. After surgical removal of
cancer tissue, the five-year survival rate of patients with stage
II GC is still <35% (1).
Previous studies have shown that risk factors for GC include
chronic infection of Helicobacter pylori, age, radiation
exposure, low socioeconomic status and low levels of physical
activity (2,3). The morbidity of GC progressively
increases with age, with the median age at diagnosis being 70
years; however, at present, ~10% of GC cases are diagnosed at the
age of 45 or younger (2). Thus, it
is of great importance to determine the potential underlying
mechanisms of GC progression.
Homeobox B13 (HOXB13) is an important member of the
HOX family, which encode nuclear transcription factors that drive
cell differentiation during normal development and tumorigenesis
(4). HOXB13 has been reported to
act as a tumor suppressor gene in a number of types of human
cancer, including colon cancer (5),
prostate cancer (6) and renal cell
carcinoma (7). A previous study
demonstrated that HOXB13 expression was significantly downregulated
in primary GC tissues compared with the corresponding non-malignant
gastric tissues, and HOXB13 expression was positively correlated
with the 5-year overall survival rate (8). This finding suggested that HOXB13 may
also act as a tumor suppressor gene in GC progression and may have
potential as a promising biomarker for the diagnosis and prognosis
of GC. Although studies have shown that the mechanism of HOXB13 in
GC may be related to its own methylation and regulation of the
IGF-1R/PI3K/AKT/mTOR pathway (8,9), the
specific underlying mechanism of the effects of HOXB13 in GC
remains to be further elucidated. Thus, the present study aimed to
investigate the potential underlying mechanism of HOXB13 in GC
progression.
TEA domain (TEAD)1 and TEAD4 are key effectors of
the Hippo signaling pathway and have been reported to exert
oncogenic roles in gastric tumorigenesis (10). Notably, the Hippo signaling pathway
was previously found to serve a regulatory role in GC tumorigenesis
(11). Vestigial-like family member
4 (VGLL4) was discovered to act as a tumor suppressor in GC by
directly competing with Yes1-associated transcriptional regulator
(YAP) to bind to TEADs, thereby suppressing the tumor-promoting
effect of the TEAD family in GC (12). Hence, the present study explored
whether the HOXB13-mediated regulation of GC cell progression was
related to TEAD4 and VGLL4.
The present study aimed to determine the effects of
HOXB13 on the proliferation, migration, invasion and apoptosis of
GC cells and to investigate the underlying mechanism to provide a
novel insight into the current understanding of the pathogenesis of
GC.
Materials and methods
Bioinformatics Methods
The interaction between HOXB13 and TEAD4 was
predicted using the Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING) database (https://string-db.org/cgi/input.pl). HOXB13 was
identified as a transcription factor of VGLL4 using the JASPAR
database (http://jaspar.genereg.net).
Cell lines and culture
Human GC cell lines, including MKN-45, MKN-74, AGS
and HGC-27, and normal gastric GES-1 cells were obtained from the
American Type Culture Collection. Cells were cultured in DMEM/F12
1:1 medium (HyClone; Cytiva) supplemented with 10% FBS (HyClone;
Cytiva) and maintained at 37°C with 5% CO2.
Cell transfection
HOXB13 expression in GC cell lines was examined
using the Cancer Cell Line Encyclopedia (CCLE; http://portals.broadinstitute.org/ccle).
HOXB13 overexpression (Ov-HOXB13), TEAD4 overexpression (Ov-TEAD4)
and pcDNA3.1 negative control (Ov-NC) plasmids were purchased from
Shanghai GenePharma Co., Ltd. Briefly, 2×104 HGC-27
cells/well were transfected with 100 pmol pcDNA3.1 vectors using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Subsequently, cells were transfected with either small interfering
RNA (siRNA) targeting VGLL4 (si-VGLL4-1, 5′-AGGACCTAGACTGTGACAA-3′;
si-VGLL4-2, 5′-TCGCACTGACCAAGAACAG-3′) or si-NC
(5′-CUAGAACUGGACAACGACA-3′) according to the manufacturer's
protocol. All the siRNAs were used at a final concentration of 10
nM. After transfection at 37°C for 6 h, the medium was replaced
with complete medium. Then, following incubation at 37°C for 36 h,
the transfection efficiencies of the overexpression plasmids and
siRNAs were verified using reverse transcription-quantitative PCR
(RT-qPCR) and western blotting, and the most effective siRNA was
used for the subsequent functional experiments.
Cell viability assay
Viability of HGC-27 cells was determined using a
Cell Counting Kit-8 (CCK-8) assay (Beyotime Institute of
Biotechnology). Briefly, 2×103 cells/well were seeded
into 96-well plates and cultured at 37°C for 24 h. After the
indicated transfections, 10 µl CCK-8 reagent was added to each well
and incubated at 37°C for an additional 4 h. The absorbance was
measured at a wavelength of 450 nm using an automated microplate
reader (Beckman DU6400 spectrophotometer; Beckman Coulter,
Inc.).
Colony formation assay
Following transfection, mixed 1×103
HGC-27 cells with 1 ml 0.3% soft agar and inoculated them into
6-well plates pre-plated with 1.5 ml 0.6% soft agar and cultured at
37°C for 2 weeks with complete medium in a 5% CO2
incubator. After colony formation, colonies were fixed with 4%
paraformaldehyde (Biosharp Life Sciences) for 20 min and stained
with 1% crystal violet (Nanjing Biotech Co., Ltd.) for 30 min at
room temperature. Colonies were counted using an inverted light
microscope (Olympus Corporation).
Wound healing assay
HGC-27 were seeded (1×105 cells/well)
into 6-well plates and cultured at 37°C overnight. After reaching
80–90% confluence, the cells were transiently transfected for 6 h,
and then cultured in complete medium at 37°C for an additional 18
h. An artificial wound was made by scratching the cell monolayer
with a sterile pipette tip. Cells were washed with phosphate buffer
solution (PBS) to remove the debris, and the media was replaced
with serum-free DMEM/F12 1:1 medium. The plates and the wound
closure were visualized at 0 and 24 h using an inverted light
microscope (magnification, ×100; Olympus Corporation). The ratio of
the wound area to the initial wound area was calculated using
ImageJ 1.46r (National Institutes of Health).
Transwell assay
The Transwell membranes were precoated with
Matrigel, and the substrate membrane was air-dried at room
temperature for 3 h and then hydrated with DMEM. Following
transfection, 4×104 HGC-27 cells/well were seeded into
the upper chambers, in serum-free medium. A total of 500 µl
complete medium supplemented with 10% FBS was added into the lower
chambers as a chemoattractant. Following 24 h of incubation at
37°C, the cells were fixed with 4% paraformaldehyde for 10 min and
stained with 1% crystal violet for 20 min at room temperature. The
invading cells were counted using an inverted light microscope
(Olympus Corporation; magnification, ×200) in five pre-determined
fields of view.
TUNEL staining
Following transfection, HGC-27 cells
(2×106 cells/well in 6-well plates) were fixed with 4%
paraformaldehyde at room temperature for 30 min, washed with PBS
and incubated with 0.3% Triton-X 100 for 5 min to permeabilize the
cell membrane. Apoptosis was analyzed using a TUNEL Apoptosis
Detection kit (Beyotime Institute of Biotechnology) according to
the manufacturer's protocol. Briefly, cells were incubated with 50
µl TUNEL reaction buffer for 1 h at 37°C in the dark. The nuclei
were counterstained with DAPI for 5 min at room temperature in the
dark and the slides were then mounted with anti-fade mounting
medium. The levels of apoptosis were estimated as the ratio of the
number of TUNEL-positive cells to the total number of DAPI-positive
cells using a fluorescence microscope (magnification, ×200; Olympus
Corporation).
Dual luciferase reporter gene
assay
Luciferase reporter plasmids (Promega Corporation)
were constructed with wild-type (WT) and mutant-type (MUT)
3′-untranslated region sequences. The MUT sequences included
MUT-VGLL4-SITE1 (S1; −1,061 to −1,052) and MUT-VGLL4-SITE2 (S2;
−1,876 to −1,867). Firefly luciferase was used as the primary
reporter to monitor the binding of the protein to the cloned target
sequences. Renilla luciferase was used as the control
reporter for normalization. The luciferase reporter plasmids and
regulating factors were co-transfected into HGC-27 cells using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). All plasmids were used at a concentration of 50
ng per well in the 12-well plates. After transfection at 37°C for 6
h, fresh complete medium was replaced, and luciferase activities of
different groups were detected until 48 h after transfection. The
relative luciferase activities were measuring using a Dual
Luciferase Reporter assay system (Promega Corporation).
Chromatin immunoprecipitation (ChIP)
assay
The binding of HOXB13 to the VGLL4 promoter was
validated using a ChIP assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. Briefly,
formaldehyde was added at a final concentration of 1% to the
cultured HGC-27 cells and incubated at room temperature for 10 min
to cross-link the protein and DNA; then three sets of 20-sec pulses
were used to obtain chromatin fragments. An anti-HOXB13 antibody
(1:20; cat. no. ab201682; Abcam) was used to generate the
immunoprecipitations and an anti-IgG antibody (1:40; cat. no.
sc-2025; Santa Cruz Biotechnology, Inc.) was used as the blank
control group to exclude the influence of other factors in the ChIP
assay. The recovered DNA fragments were evaluated using RT-qPCR.
The primer sequences were as follows: VGLL4, forward
5′-AACTGCAACCTCTCGCACTG-3′ and reverse
5′-GCTCGGGCTCCTTGTAATTCT-3′.
RT-qPCR
Total RNA was extracted from 1×105 cells
using TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Total RNA was reverse
transcribed into cDNA using PrimeScript™ RT reagent Kit (Takara
Bio, Inc.). qPCR was subsequently performed in a 96-well optical
plate using Thunderbird SYBR qPCR mix (Toyobo Life Science) and an
ABI Prism 7500 Real-Time PCR Detection System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95°C for
1 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min.
The relative expression levels of HOXB13, TEAD4 and VGLL4 were
calculated using the 2−ΔΔCq method (13) and normalized to GAPDH. The primer
sequences were as follows: VGLL4, forward
5′-GGCAGCATTTGCAGACTCCAG-3′ and reverse
5′-GGTGATGAACTTGTTAGCCGC-3′; TEAD4, forward
5′-CTGGACAAGCCCATCGACAA-3′ and reverse 5′-CAGCTCGTTCCGACCATACA-3′;
and VGLL4, forward 5′-TTTGTGAAGTTGAAGAGCCAGG-3′ and reverse
5′-GCAGCTTCGCCTTCGT-3′. All experiments were performed in
triplicate.
Western blotting
Total protein was extracted from cells
(2×106) using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Total protein concentration was determined using
the BCA method and 20 µg protein/lane was separated by 10%
SDS-PAGE. The separated proteins were transferred onto PVDF
membranes and blocked with 5% skimmed milk for 2 h at room
temperature. The membranes were then incubated with following
primary antibodies overnight at 4°C: Anti-HOXB13 (1:1,000; cat. no.
ab201682; Abcam), anti-proliferating cell nuclear antigen (PCNA;
1:1,000; cat. no. ab92552; Abcam), anti-Ki-67 (1:1,000; cat. no.
ab92742; Abcam), anti-MMP2 (1:1,000; cat. no. ab92536; Abcam),
anti-MMP9 (1:1,000; cat. no. ab76003; Abcam), anti-issue inhibitor
of metalloproteinases 1 (TIMP-1; 1:1,000; cat. no. ab211926;
Abcam), anti-Bcl-2 (1:1,000; cat. no. ab182858; Abcam), anti-Bax
(1:1,000; cat. no. ab32503; Abcam), anti-cleaved caspase-3 (1:500;
cat. no. ab32042; Abcam), anti-caspase-3 (1:1,000; cat. no.
ab32351; Abcam), anti-TEAD4 (1:1,000; cat. no. ab155244; Abcam),
anti-cellular communication network factor 2 (CCN2; 1:1,000; cat.
no. ab209780; Abcam), anti-cysteine rich angiogenic inducer 61
(Cyr61; 1:1,000; cat. no. ab230947; Abcam), anti-amphiregulin
(AREG; 1:1,000; cat. no. ab180722; Abcam) and anti-GAPDH (1:2,000;
cat. no. ab181602; Abcam). Following the primary antibody
incubation, the membranes were washed three times with TBS +0.05%
Tween-20 and incubated with a goat anti-rabbit HRP-conjugated
secondary antibody (1:1,000; cat. no. ab6721; Abcam) at room
temperature for 2 h. Protein bands were visualized using a Pierce
ECL Plus Western Blotting (Pierce; Thermo Fisher Scientific, Inc.)
and analyzed using ImageJ (version 1.8; National Institutes of
Health).
Statistical analysis
All experiments were repeated three times. All data
are reported as the mean ± standard deviation and analyzed with
GraphPad Prism 8.0 software (GraphPad Software, Inc.). One-way
ANOVA followed by Tukey's post-hoc test was used to compare
differences among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
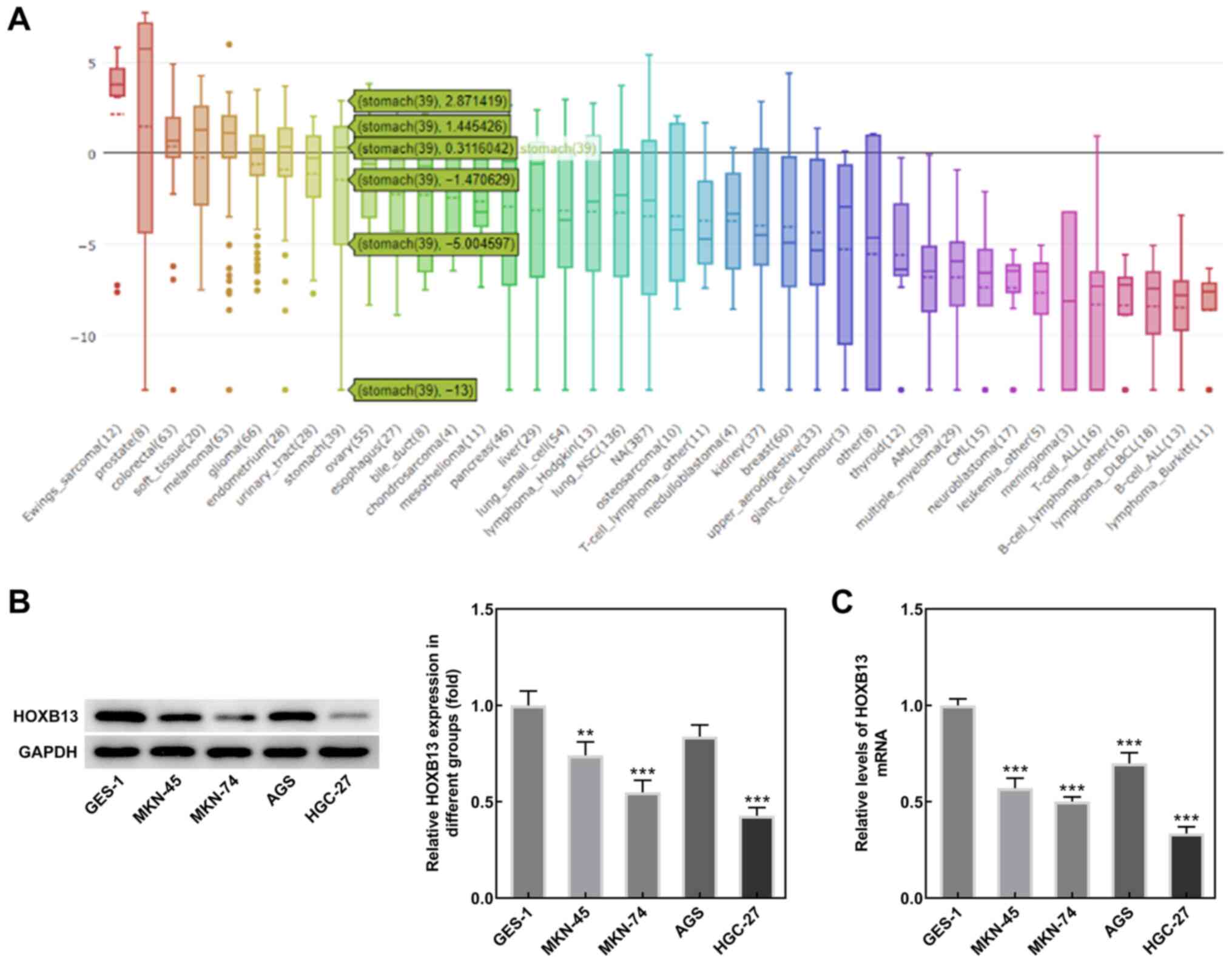
HOXB13 expression levels are notably
lower in GC tissues and cell lines
Analysis of the CCLE database revealed that HOXB13
expression was lower in GC tissues compared with normal gastric
tissues (Fig. 1A). Results from
western blotting and RT-qPCR analyses demonstrated that HOXB13
protein and mRNA expression levels, respectively, were also
significantly lower in MKN-45, MKN-74 and HGC-27 cells compared
with GES-1 cells, while HOXB13 was only significantly reduced in
AGS cells at the mRNA level, and the expression levels were lowest
in HGC-27 cells (Fig. 1B and C).
Therefore, the HGC-27 cell line was selected for use in subsequent
experiments. These results suggested that HOXB13 may be involved in
GC progression.
Overexpression of HOXB13 inhibits the
proliferation, migration and invasion of HGC-27 GC cells
To investigate the role of HOXB13 in the progression
of GC, HGC-27 cells were successfully transfected with Ov-HOXB13
plasmid, as demonstrated by RT-qPCR (Fig. 2A). Ov-HOXB13 transfection notably
inhibited the proliferative ability of HGC-27 cells compared with
the Ov-NC group (Fig. 2B and C).
Ov-HOXB13 also significantly downregulated the expression levels of
the proliferation-related markers, PCNA and Ki-67, compared with
the Ov-NC group (Fig. 2D).
Moreover, the results from the wound healing and Transwell assays
showed that the overexpression of HOXB13 significantly decreased
the migratory and invasive abilities of HGC-27 cells (Fig. 2E and F, respectively), and
downregulated the protein expression levels of the migration- and
invasion-related proteins, TIMP-1, MMP2 and MMP9 (Fig. 2G). These results suggested that
Ov-HOXB13 may inhibit the proliferation, migration and invasion of
HGC-27 cells.
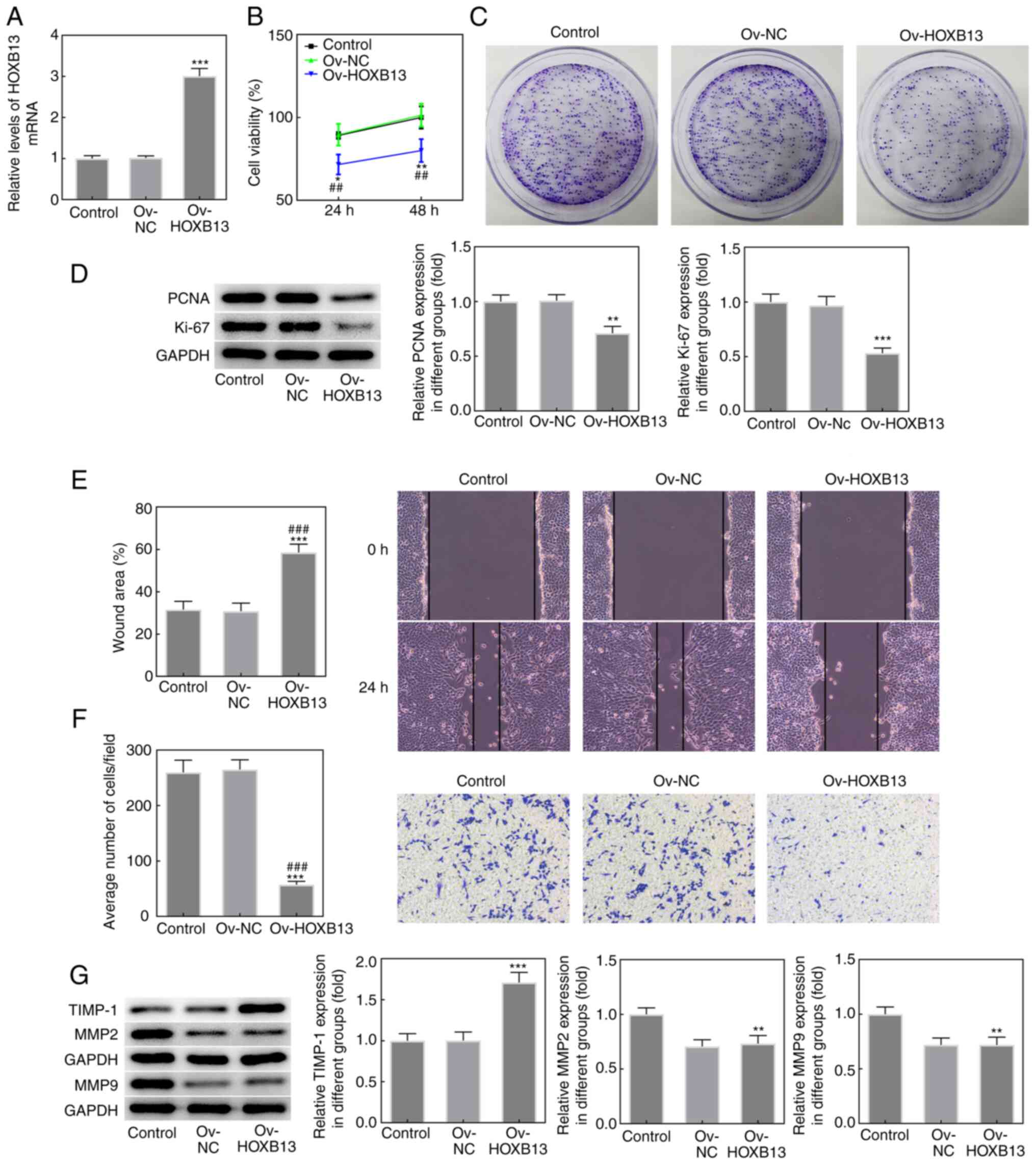 | Figure 2.Overexpression of HOXB13 inhibits the
proliferation, migration and invasion of HGC-27 gastric cancer
cells. (A) Expression levels of HOXB13 in HGC-27 cells following
transfection with Ov-HOXB13 plasmid were analyzed using reverse
transcription-quantitative PCR. (B) Cell viability was detected
using a Cell Counting Kit-8 assay. (C) Cell proliferation was
analyzed using a colony formation assay. (D) Protein expression
levels of PCNA and Ki-67 were determined by western blotting. (E)
Cell migration was measured using a wound healing assay. (F) Cell
invasion was analyzed using a Transwell assay. (G) Protein
expression levels of TIMP-1, MMP2 and MMP9 were determined by
western blotting. *P<0.05, **P<0.01, ***P<0.001 vs. Ov-NC;
##P<0.01, ###P<0.001 vs. Control.
HOXB13, homeobox B13; NC, negative control; Ov, overexpression;
PCNA, proliferating cell nuclear antigen; TIMP-1, tissue inhibitor
of metalloproteinases 1. |
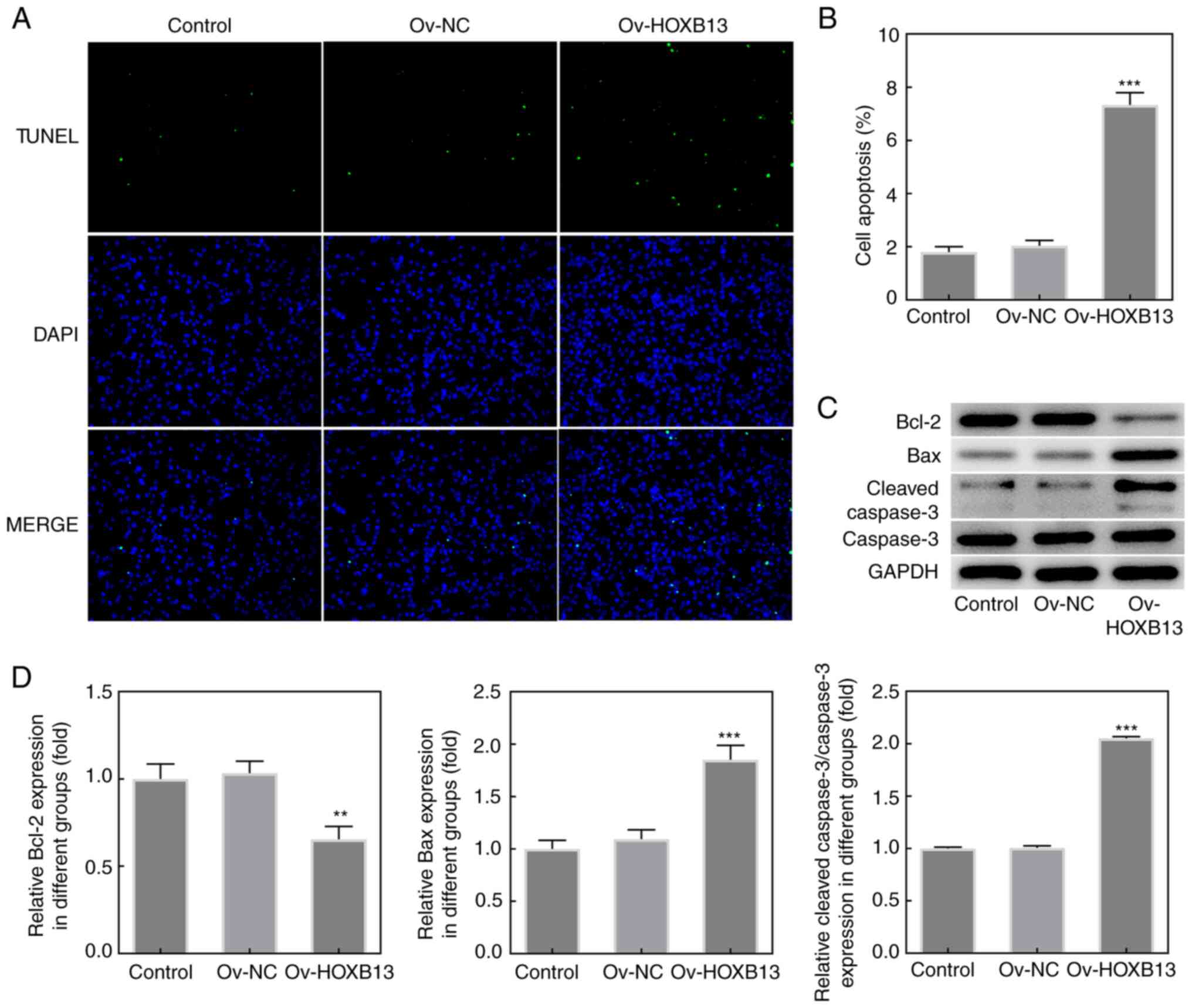
Overexpression of HOXB13 promotes the
apoptosis of HGC-27 GC cells
To further investigate the effects of HOXB13 in GC
progression, cell apoptosis was detected using a TUNEL assay
following the overexpression of HOXB13 in HGC-27 cells. As shown in
Fig. 3A and B, the cell apoptotic
rate was notably increased following the overexpression of HOXB13.
In addition, the expression levels of the anti-apoptotic protein,
Bcl-2, were downregulated following the overexpression of HOXB13,
whereas the expression levels of the proapoptotic proteins, Bax and
cleaved caspase-3, were upregulated following the overexpression of
HOXB13 (Fig. 3C and D). These
results indicated that Ov-HOXB13 may promote the apoptosis of
HGC-27 cells.
Overexpression of HOXB13 downregulates
TEAD4 expression in HGC-27 GC cells
To determine the mechanism underlying the role of
HOXB13 in the development of GC, the interaction between HOXB13 and
TEAD4 was predicted using the STRING database (Fig. 4A). Results from western blotting
analysis and RT-qPCR revealed that the protein and mRNA expression
levels of TEAD4 were higher in GC cell lines compared with GES-1
cells (Fig. 4B and C,
respectively). Notably, Ov-HOXB13 transfection significantly
downregulated the expression of TEAD4 (Fig. 4D and E), suggesting that the
overexpression of HOXB13 may downregulate TEAD4 expression in
HGC-27 cells.
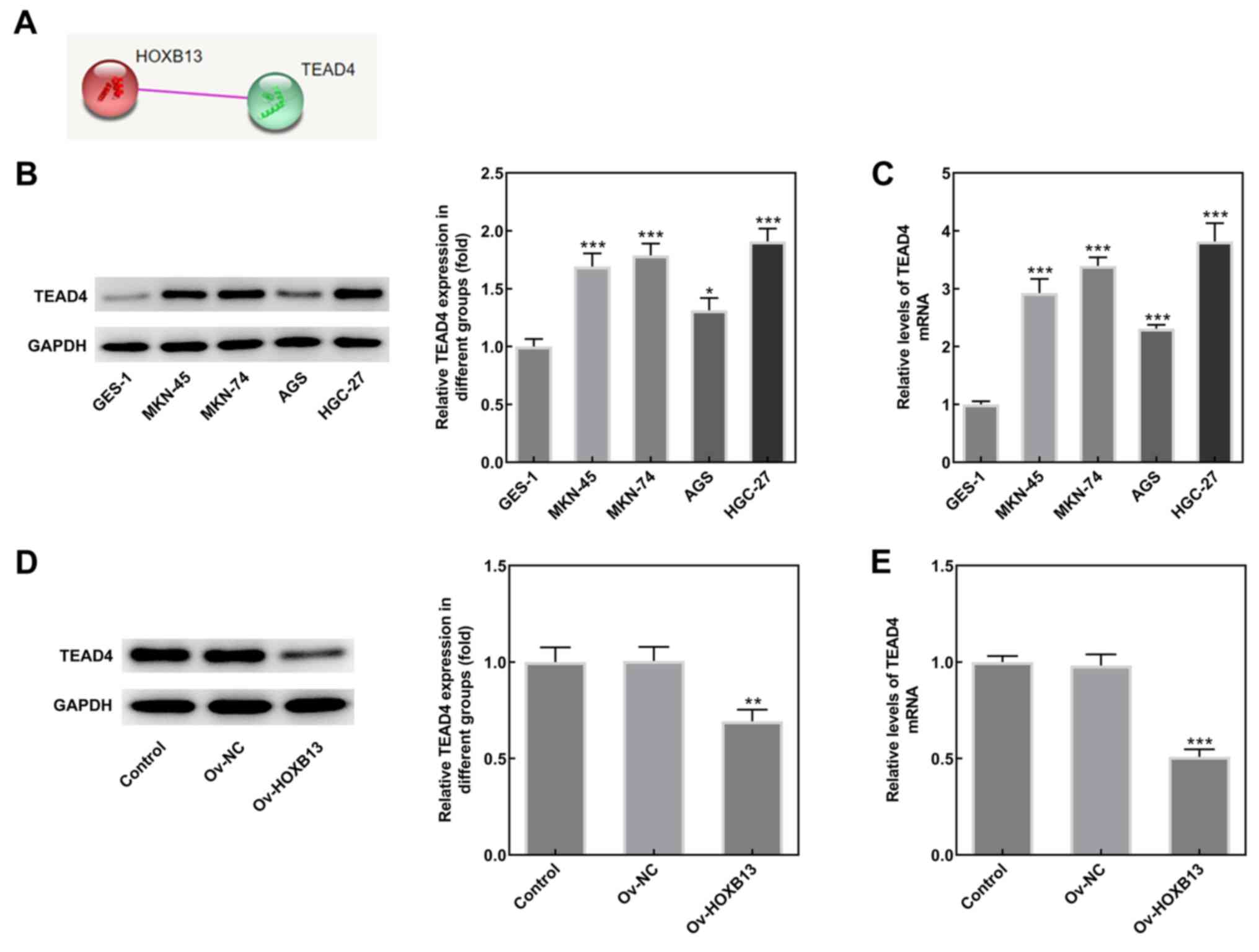 | Figure 4.Overexpression of HOXB13 downregulates
TEAD4 expression in HGC-27 GC cells. (A) Interaction between HOXB13
and TEAD4 was predicted using the Search Tool for the Retrieval of
Interacting Genes/Proteins database. (B) TEAD4 protein and (C) mRNA
expression levels in several human GC cell lines (MKN-45, MKN-74,
AGS and HGC-27) and GES-1 cells were determined by western blotting
and RT-qPCR, respectively. *P<0.05, ***P<0.001 vs. GES-1. (D)
TEAD4 protein and (E) mRNA expression levels in different groups
(Control, Ov-NC and Ov-HOXB13) were determined by western blotting
and RT-qPCR, respectively. **P<0.01, ***P<0.001 vs. Ov-NC.
GC, gastric cancer; HOXB13, homeobox B13; NC, negative control; Ov,
overexpression; TEAD4, TEA domain 4; RT-qPCR, reverse
transcription-quantitative PCR. |
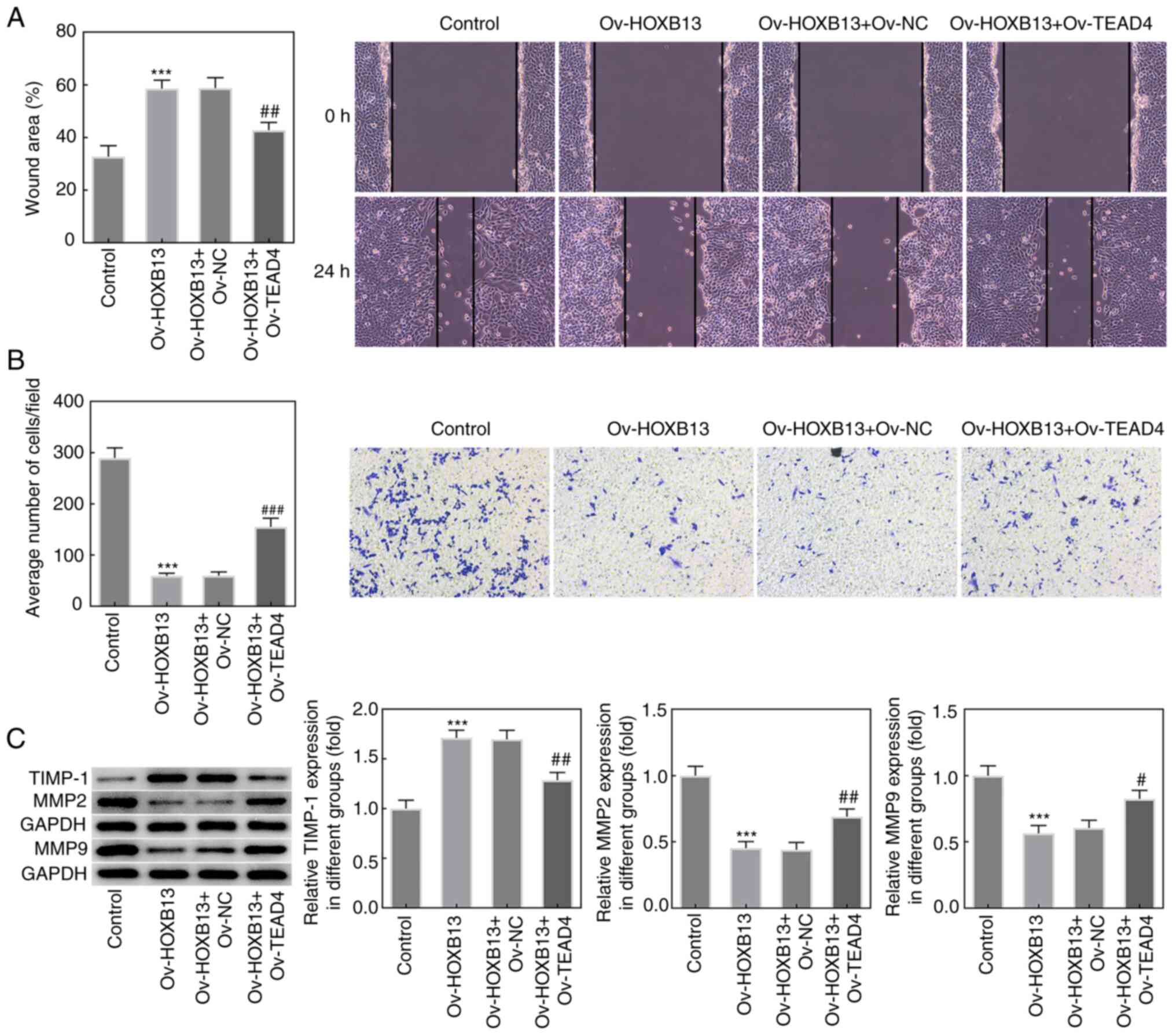
Overexpression of TEAD4 reverses the
effects of Ov-HOXB13 on the proliferation, migration, invasion and
apoptosis of HGC-27 GC cells
To confirm whether TEAD4 mediated the effects of
HOXB13 in GC progression, an Ov-TEAD4 plasmid was constructed, and
the expression of TEAD4 was notably upregulated following
transfection compared with the Ov-NC group (Fig. 5A). Additionally, as shown in
Fig. 5B and C, the results from the
western blotting and RT-qPCR analyses, respectively, demonstrated
that co-transfection with the Ov-HOXB13 and Ov-TEAD4 plasmids
upregulated TEAD4 expression levels in HGC-27 cells compared with
co-transfection with the Ov-HOXB13 + Ov-NC plasmids group. In
addition, the results of the CCK-8 and colony formation assays
demonstrated that the overexpression of TEAD4 partially reversed
the effects of the Ov-HOXB13 plasmid on the proliferation of HGC-27
cells (Fig. 5D and E,
respectively). Similarly, the expression levels of PCNA and Ki-67
in HGC-27 cells co-transfected with Ov-HOXB13 and Ov-TEAD4 plasmids
were markedly upregulated compared with the Ov-HOXB13 + Ov-NC group
(Fig. 5F).
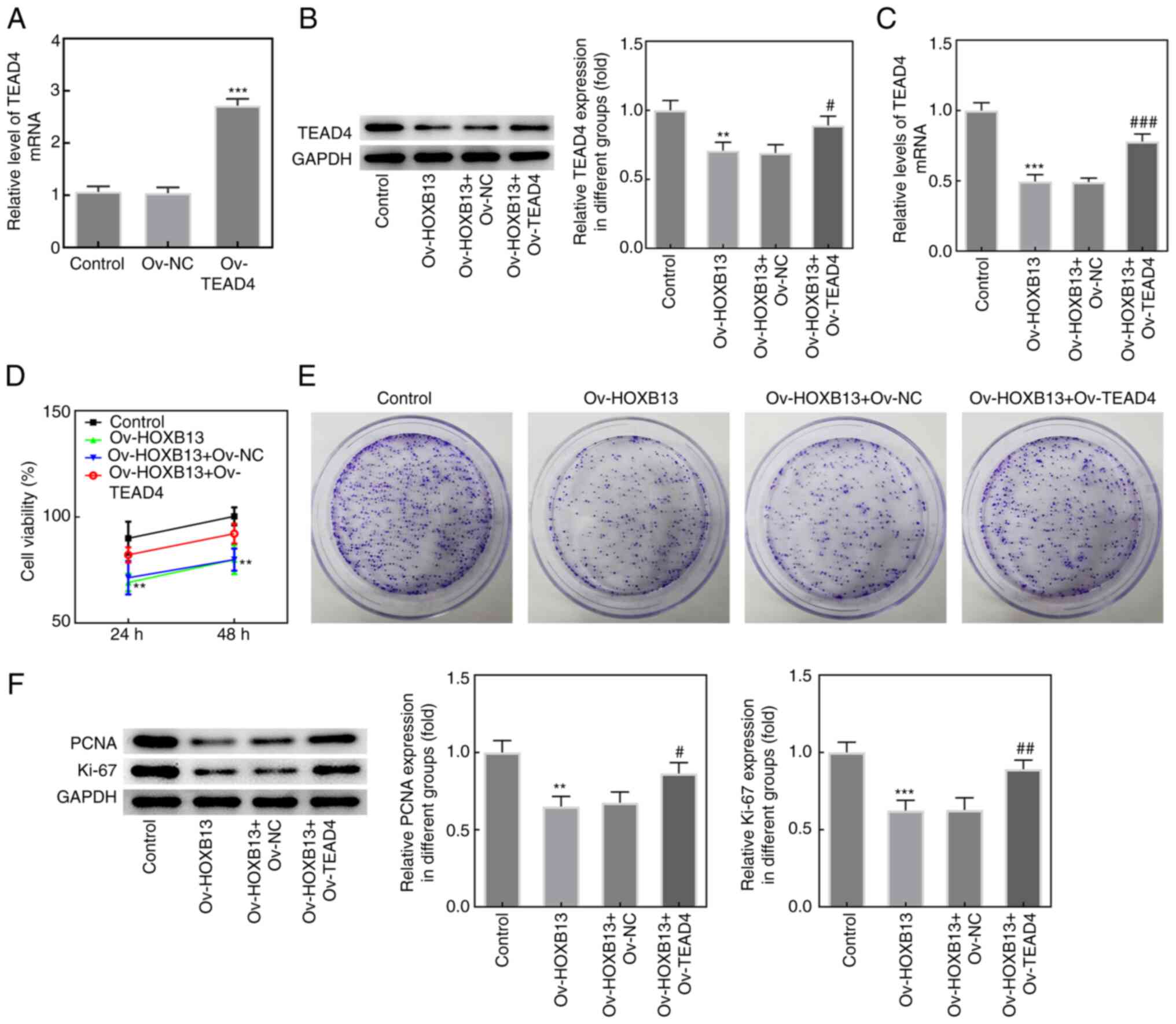 | Figure 5.TEAD4 overexpression reduces the
effects of HOXB13 on HGC-27 cell proliferation. (A) TEAD4
expression was evaluated with RT-qPCR after transfection with
Ov-TEAD4 plasmid. ***P<0.001 vs. Ov-NC. (B) TEAD4 protein and
(C) mRNA expression levels in HGC-27 cells were determined by
western blotting and RT-qPCR, respectively. (D) Cell viability was
detected by Cell Counting Kit-8 assay. (E) Cell proliferation was
analyzed using colony formation assay. (F) The protein expression
levels of PCNA and Ki-67 were determined by western blotting.
**P<0.01, ***P<0.001 vs. Control; #P<0.05,
##P<0.01, ###P<0.001 vs. Ov-HOXB13 +
Ov-NC. HOXB13, homeobox B13; NC, negative control; Ov,
overexpression; PCNA, proliferating cell nuclear antigen; RT-qPCR,
reverse transcription-quantitative PCR; TEAD4, TEA domain 4. |
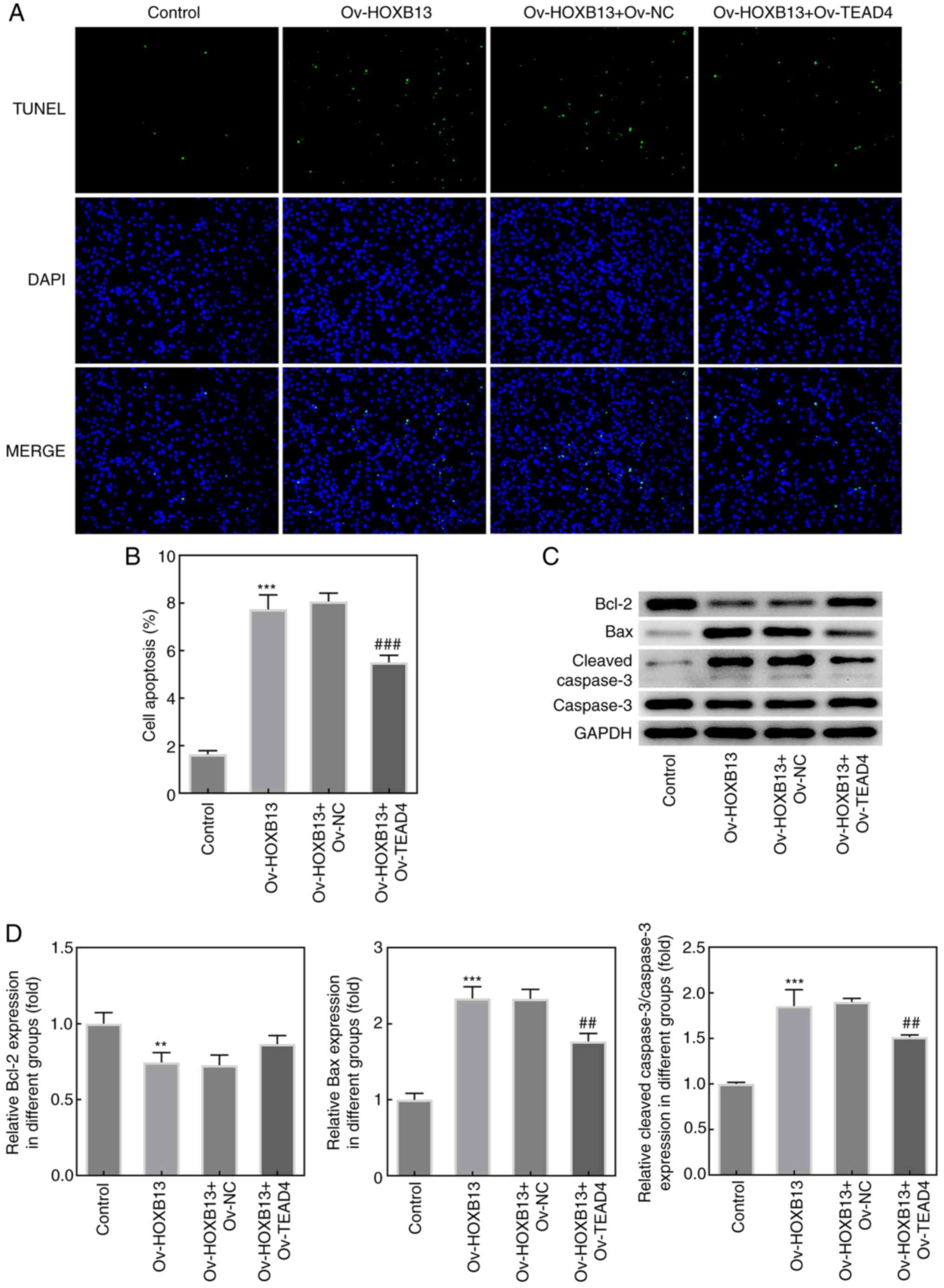
Moreover, the results from wound healing and
Transwell assays demonstrated that the overexpression of TEAD4
blocked the suppressive effect of Ov-HOXB13 on the migration and
invasion of HGC-27 cells (Fig. 6A and
B, respectively), and the expression of TIMP-1, MMP2 and MMP9
(Fig. 6C). Notably, as shown in
Fig. 7A and B, the apoptotic rate
of HGC-27 cells co-transfected with Ov-HOXB13 and Ov-TEAD4 plasmids
was decreased compared with the Ov-HOXB13 + Ov-NC group.
Overexpression of TEAD4 reversed the effects of Ov-HOXB13 on the
proliferation, migration, invasion and apoptosis of HGC-27 GC
cells. Transfection with the Ov-TEAD4 plasmid also downregulated
the expression levels of Bax and cleaved caspase-3 compared with
the Ov-HOXB13 + Ov-NC group, while the upregulation of Bcl-2 was
not significant (Fig. 7C and D).
These results suggested that the overexpression of TEAD4 may
reverse the effects of Ov-HOXB13 on the proliferation, migration,
invasion and apoptosis of HGC-27 cells.
HOXB13 inhibits the involvement of
TEAD4 in the Hippo signaling pathway by regulating VGLL4
expression
To investigate the underlying mechanisms associated
with the interaction between HOXB13 and TEAD4, two VGLL4 siRNAs
were constructed and transfected into HGC-27 cells (Fig. 8A). As si-VGLL4-1 was found to
downregulate the expression of VGLL4 to the greatest extent, it was
selected for use in subsequent experiments. As shown in Fig. 8B and C, the knockdown of VGLL4
markedly upregulated TEAD4 protein and mRNA expression levels,
respectively, compared with the Ov-HOXB13 + si-NC group. The JASPAR
database was used to predict that HOXB13 is a potential
transcription factor that can bind to the promoter of VGLL4. As
exhibited in Fig. 8D, two putative
binding sites (S1/S2) of HOXB13 were identified in the VGLL4
promoter. Luciferase reporter plasmid containing WT-VGLL4-S1 or
WT-VGLL4-S2 sites were activated following HOXB13 overexpression
(Fig. 8E). Additionally, notable
enrichment of VGLL4 promoter sequences (S1/S2) were obtained
through ChIP using the anti-HOXB13 antibody, whereas no significant
enrichment was obtained using the control IgG (Fig. 8F). Finally, the results from the
western blotting analysis revealed that Ov-HOXB13 significantly
downregulated the expression levels of downstream effectors of the
Hippo signaling pathway, including CCN2, Cyr61 and AREG, whereas
the knockdown of VGLL4 exerted the opposite effects on the
expression of these proteins compared with the Ov-HOXB13 + si-NC
group (Fig. 8G). These results
suggested that HOXB13 may inhibit the involvement of TEAD4 in the
Hippo signaling pathway by regulating VGLL4 expression.
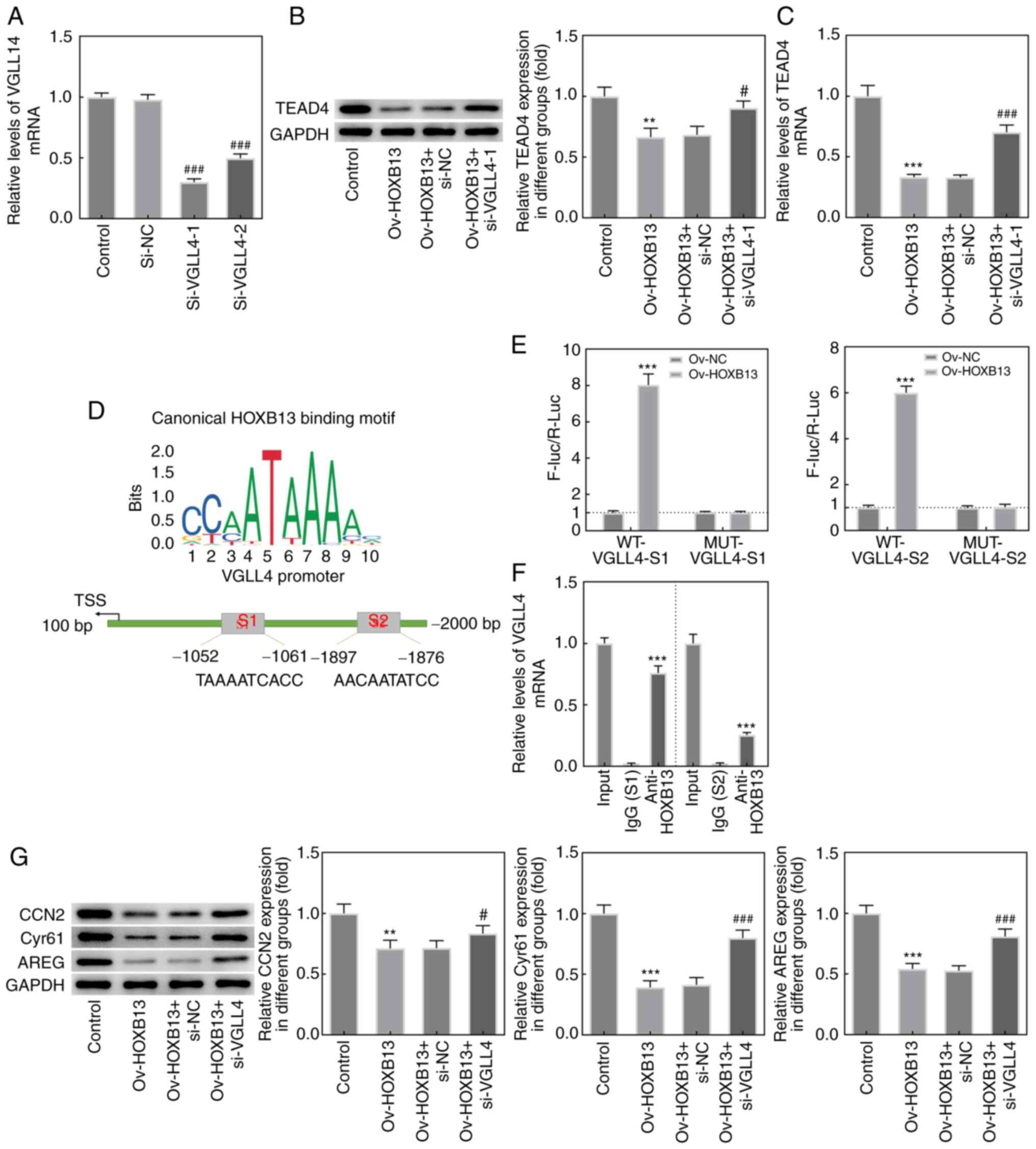 | Figure 8.HOXB13 inhibits the involvement of
TEAD4 in Hippo signaling pathway by regulating VGLL4 expression.
(A) Relative mRNA expression levels of VGLL4 in transfected HGC-27
cells were examined by RT-qPCR. ###P<0.001 vs. si-NC.
(B) Protein and (C) mRNA expression levels of TEAD4 in HGC-27 cells
were determined by western blot assay and RT-qPCR, respectively.
**P<0.01, ***P<0.001 vs. Control; #P<0.05,
###P<0.001 vs. Ov-HOXB13 + si-NC. (D) The binding of
HOXB13 to VGLL4 promoter regions (S1/S2). (E) The interaction
between HOXB13 and VGLL4 was determined by luciferase reporter gene
assay. ***P<0.001 vs. Ov-NC. (F) The direct binding of HOXB13
and VGLL4 promoter was confirmed using chromatin
immunoprecipitation. ***P<0.001 vs. IgG. (G) Protein expression
levels of downstream effectors of the Hippo signaling pathway,
including CCN2, Cyr61 and AREG, were determined with western blot
analysis and semi-quantified. **P<0.01, ***P<0.001 vs.
Control; #P<0.05, ###P<0.001 vs.
Ov-HOXB13 + si-NC. AREG, amphiregulin; CCN2, cellular communication
network factor 2; Cyr61, cysteine rich angiogenic inducer 61;
HOXB13, homeobox B13; F-luc/R-Luc, Firefly
luciferase/Renilla luciferase; MUT, mutant-type; NC,
negative control; Ov, overexpression; si, small interfering RNA;
TEAD4, TEA domain 4; TSS, transcription start site; VGLL4,
vestigial-like family member 4; WT, wild-type; RT-qPCR, reverse
transcription-quantitative PCR; S1, site 1; S2, site 2. |
Discussion
GC is one of the most common types of malignancy
worldwide and is a leading cause of cancer-related mortality
(14,15). GC is a global health burden;
therefore, it is urgent to determine the potential underlying
mechanisms involved in its onset and development to identify
promising biomarkers for the diagnosis and treatment of GC. The
results of the present study revealed that Ov-HOXB13 suppressed the
proliferation, migration and invasion of HGC-27 GC cells; thus, the
study subsequently investigated the underlying molecular mechanism
of HOXB13 in GC.
Proliferation and metastasis are hallmarks of the
malignant biological behavior of GC, and inhibiting these processes
has been suggested to be crucial for improving the biomedical
treatment of GC worldwide (16,17).
HOXB13 was reported to act as a tumor suppressor in numerous types
of human cancer, including GC (8,18). Our
previous study found that HOXB13 promoted the proliferation,
migration and invasion of glioblastoma by transcriptional
upregulation of the long non-coding RNA (lncRNA) homeobox cluster C
antisense RNA 3 (19). In the
present study, HOXB13 expression was discovered to be downregulated
in various cancer cell lines following analysis using the CCLE
database. In addition, HOXB13 expression was downregulated in human
GC cell lines compared with normal gastric cells. Moreover, the
overexpression of HOXB13 inhibited the proliferation, migration and
invasion of HGC-27 cells, and promoted cell apoptosis. Thus, it was
suggested that HOXB13 may serve an anticarcinogenic role in GC
progression. Subsequently, further experiments were performed to
investigate the detailed mechanism underlying the role of HOXB13 in
GC. Notably, HOXB13 was found to interact with TEAD4, as
demonstrated by analysis using the STRING database. The expression
levels of TEAD4 were upregulated in human GC cell lines compared
with normal gastric cells. In addition, the overexpression of
HOXB13 downregulated the expression levels of TEAD4 in HGC-27
cells. Lim et al (20)
demonstrated that the expression levels of TEAD4 were upregulated
in GC cells, which is consistent with the findings of the present
study. Notably, Ov-TEAD4 reversed the suppressive effects of
Ov-HOXB13 on the proliferation, migration and invasion, and the
promoting effect on the apoptosis of HGC-27 cells in the present
study. In a previous study, TEAD4 was reported to contribute to GC
progression by regulating the expression of the lncRNA motor neuron
and pancreas homeobox 1 antisense RNA 1 (21). Thus, it was hypothesized that TEAD4
may mediate the effects of HOXB13 in GC progression.
Based on the discovery of genome-wide integrated
expression transcriptome and immune antibody proteomics, the VGLL4
gene is on chromosome 3p25.3~3p25.2 and includes 14 exons. Wide
expression of VGLL4 genes was detected in human tissues (22). VGLL4 was described as a tumor
suppressor in numerous types of cancers, such as lung, breast and
colorectal (23–25). A previous study has proposed that
VGLL4 inhibits epithelial-mesenchymal transition in part through
suppressing Wnt/β-catenin signaling pathway in gastric cancer
(26). It was previously reported
that VGLL4 acted as a tumor suppressor in GC by directly competing
with YAP for the binding to TEADs, thereby suppressing the
tumor-promoting effect of the TEAD family in GC (12). The findings of the present study
demonstrated that the knockdown of VGLL4 significantly upregulated
TEAD4 expression levels. In addition, as a transcription factor of
VGLL4, HOXB13 was found to interact with VGLL4 and upregulate its
expression. Jiao et al (25)
reported that VGLL4, which was previously identified as a YAP
antagonist, targeted the TEAD4-transcription factor 4 complex and
negatively regulated TEAD4 transactivation to inhibit colorectal
carcinoma tumor growth. YAP is the effector protein of the Hippo
pathway (27). As a tumor
suppressor pathway, the Hippo signaling pathway is involved in
multiple cellular processes driving cell proliferation and
differentiation, and its dysregulation was found to contribute to
the tumorigenesis of multiple cancer types (28). The results of the present study
revealed that the knockdown of VGLL4 reversed the suppressive
effects of HOXB13 on the expression levels of downstream effectors
of the Hippo signaling pathway, including CCN2, Cyr61 and AREG,
suggesting that HOXB13 may inhibit the involvement of TEAD4 in the
Hippo signaling pathway by regulating VGLL4 expression.
In conclusion, the findings of the present study
suggested that HOXB13 may suppress the proliferation, migration and
invasion, and promote the apoptosis of GC cells through the
transcriptional activation of VGLL4 to inhibit the involvement of
TEAD4 in the Hippo signaling pathway. These results may provide
evidence for a new regulatory mechanism involving HOXB13 in GC,
suggesting a theoretical basis for the development of novel
targeted therapies. The lack of experimental verification of the
direct interaction mechanism between VGLL4 and TEAD4 is a potential
limitation of the present study and an aim of future studies.
Additionally, the potential use of GSEA to assess more collectively
through bioinformatics analysis the association between HOXB13
expression and Hippo signaling pathways will be also performed in
future studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HG designed the study and wrote the manuscript. HG,
GL, JH, JL, DW and SZ performed the experiments and analyzed the
data. XX conceived and supervised the study and co-wrote the
manuscript. XX and HG confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Machlowska J, Baj J, Sitarz M, Maciejewski
R and Sitarz R: Gastric cancer: Epidemiology, risk factors,
classification, genomic characteristics and treatment strategies.
Int J Mol Sci. 21:40122020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thrift AP and El-Serag HB: Burden of
gastric cancer. Clin Gastroenterol Hepatol. 18:534–542. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
VanOpstall C, Perike S, Brechka H, Gillard
M, Lamperis S, Zhu B, Brown R, Bhanvadia R and Griend DJV:
MEIS-mediated suppression of human prostate cancer growth and
metastasis through HOXB13-dependent regulation of proteoglycans.
Elife. 9:e536002020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie B, Bai B, Xu Y, Liu Y, Lv Y, Gao X, Wu
F, Fang Z, Lou Y, Pan H and Han W: Tumor-suppressive function and
mechanism of HOXB13 in right-sided colon cancer. Signal Transduct
Target Ther. 4:512019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Wang R, Wu Z and Bai P: Circular
RNA ITCH suppressed prostate cancer progression by increasing
HOXB13 expression via spongy miR-17-5p. Cancer Cell Int.
19:3282019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okuda H, Toyota M, Ishida W, Furihata M,
Tsuchiya M, Kamada M, Tokino T and Shuin T: Epigenetic inactivation
of the candidate tumor suppressor gene HOXB13 in human renal cell
carcinoma. Oncogene. 25:1733–1742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sui BQ, Zhang CD, Liu JC, Wang L and Dai
DQ: HOXB13 expression and promoter methylation as a candidate
biomarker in gastric cancer. Oncol Lett. 15:8833–8840.
2018.PubMed/NCBI
|
|
9
|
Guo C, Chu H, Gong Z, Zhang B, Li C, Chen
J and Huang L: HOXB13 promotes gastric cancer cell migration and
invasion via IGF-1R upregulation and subsequent activation of
PI3K/AKT/mTOR signaling pathway. Life Sci. 278:1195222021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y, Huang T, Zhang J, Wong CC, Zhang
B, Dong Y, Wu F, Tong JHM, Wu WKK, Cheng ASL, et al: TEAD1/4 exerts
oncogenic role and is negatively regulated by miR-4269 in gastric
tumorigenesis. Oncogene. 36:6518–6530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han Y: Analysis of the role of the Hippo
pathway in cancer. J Transl Med. 17:1162019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiao S, Wang H, Shi Z, Dong A, Zhang W,
Song X, He F, Wang Y, Zhang Z, Wang W, et al: A peptide mimicking
VGLL4 function acts as a YAP antagonist therapy against gastric
cancer. Cancer Cell. 25:166–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Machlowska J, Maciejewski R and Sitarz R:
The pattern of signatures in gastric cancer prognosis. Int J Mol
Sci. 19:16582018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Q, Tian S, Liu ZR and Dong WG:
Knockdown of RIPK2 inhibits proliferation and migration, and
induces apoptosis via the NF-k B signaling pathway in gastric
cancer. Front Genet. 12:6274642021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng MM, Li BX, Yang L and Guan QL: CBX2
depletion inhibits the proliferation, invasion and migration of
gastric cancer cells by inactivating the YAP/β-catenin pathway. Mol
Med Rep. 23:1372021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cannon-Albright LA, Stevens J, Teerlink CC
and Agarwal N: The HOXB13 p.Gly84Glu variant observed in an
extended five generation high-risk prostate cancer pedigree
supports risk association for multiple cancer sites. Cancer
Epidemiol. 69:1018342020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Sun Y, Xu TY, Qian K, Huang B,
Zhang K, Song Z, Qian T, Shi J and Li L: HOXB13 promotes
proliferation, migration, and invasion of glioblastoma through
transcriptional upregulation of lncRNA HOXC-AS3. J Cell Biochem.
120:15527–15537. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lim B, Kim HJ, Heo H, Huh N, Baek SJ, Kim
JH, Bae DH, Seo EH, Lee SI, Song KS, et al: Epigenetic silencing of
miR-1271 enhances MEK1 and TEAD4 expression in gastric cancer.
Cancer Med. 7:3411–3424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shuai Y, Ma Z, Liu W, Yu T, Yan C, Jiang
H, Tian S, Xu T and Shu Y: TEAD4 modulated LncRNA MNX1-AS1
contributes to gastric cancer progression partly through
suppressing BTG2 and activating BCL2. Mol Cancer. 19:62020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fagerberg L, Hallstrom BM, Oksvold P,
Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S,
Danielsson A, Edlund K, et al: Analysis of the human
tissue-specific expression by genome-wide integration of
transcriptomics and antibody-based proteomics. Mol Cell Proteomics.
13:397–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang WJ, Gao YJ, Li PX, Shi Z, Guo T, Li
F, Han X, Feng Y, Zheng C, Wang Z, et al: VGLL4 functions as a new
tumor suppressor in lung cancer by negatively regulating the
YAP-TEAD transcriptional complex. Cell Res. 24:331–343. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Shen H, Withers H, Yang N, Denson
KE, Mussell AL, Truskinovsky A, Fan Q, Gelman IH, Frangou C and
Zhang J: VGLL4 selectively represses YAP-dependent gene induction
and tumorigenic phenotypes in breast cancer. Sci Rep. 7:61902017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiao S, Li C, Hao Q, Miao H, Zhang L, Li L
and Zhou Z: VGLL4 targets a TCF4-TEAD4 complex to coregulate Wnt
and Hippo signalling in colorectal cancer. Nat Commun. 8:140582017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Wang Z, Zhang W, Qian K, Liao G, Xu
W and Zhang S: VGLL4 inhibits EMT in part through suppressing
Wnt/β-catenin signaling pathway in gastric cancer. Med Oncol.
32:832015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sugihara T, Isomoto H, Gores G and Smoot
R: YAP and the Hippo pathway in cholangiocarcinoma. J
Gastroenterol. 54:485–491. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stanger BZ: Quit your YAPing: A new target
for cancer therapy. Genes Dev. 26:1263–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|