Introduction
Sepsis is a severe life-threatening organ
dysfunction condition caused by a dysregulated host response to
infection (1). Sepsis, with
accompanying systemic inflammatory response syndrome, affects
multiple systems and organs and culminates in multiple organ
dysfunction syndrome (2).
Myocardial injury is a major and serious complication of sepsis;
sepsis-induced myocardial injury has a high incidence rate and is
observed in 40–50% of patients with sepsis (3). Moreover, studies have reported that
sepsis-induced cardiac dysfunction may be as high as 70% (4) and is a major predictor of mortality
from sepsis (5). However, current
treatments for myocardial injury following sepsis are not ideal, as
mechanisms underpinning sepsis-induced myocardial injury are not
fully understood.
Inflammasomes are multi-protein cellular complexes
that activate inflammatory cascades (6). Currently, the NOD-like receptor 3
(NLRP3) inflammasome is the most characterized and widely described
inflammasome complex and comprises NLRP3, the adaptor protein, ASC
(also known as PYCARD) and caspase-1 (7). Increasingly, evidence has indicated
that activated NLRP3 triggers caspase-1, which then triggers mature
interleukin-1β (IL-1β) production and secretion (8,9). IL-1β
is a vital cytokine that mediates the secretion of large quantities
of other inflammatory mediators and proinflammatory cytokines that
damage multiple organs (10,11).
Thus, the NLRP3/caspase-1/IL-1β axis exerts significant
proinflammatory effects. At the molecular level, the NLRP3
inflammasome is activated during sepsis, with high expression of
the NLRP3/caspase-1/IL-1β signaling pathway (12). Although some studies have reported
NLRP3 is essential for bacterial clearance with protective roles in
infectious diseases (13), it is
currently unclear whether NLRP3 inflammasome activation exerts
protective or detrimental roles during sepsis-induced myocardial
injury. Targeting anti-inflammatory mediators is a promising
therapeutic strategy for preventing and treating sepsis-induced
organ dysfunction, however, little success has been achieved as the
biological process is highly complex. Several studies have reported
that NLRP3 inhibitors specifically target in vivo NLRP3
activation (14–16). Thus, it is conceivable to develop
novel drugs to treat or prevent septic organ injury that not only
target inflammatory mediators, but also NLRP3, thereby eliminating
the main inflammatory response activator (17).
Ulinastatin, also called urinary trypsin inhibitor
(UTI), is a broad-spectrum protease inhibitor derived from human
urine that inhibits multiple endogenous proteases (18). It exhibits a protective effect via
anti-inflammatory and anti-oxidative stress responses (19). Previous studies have shown that UTI
exerts organ-protective effects during sepsis (20,21).
However, whether UTI suppresses NLRP3 inflammasome activation,
downregulates the expression of related mediators, and elicits
protective effects toward myocardial injury induced by sepsis
remains largely unknown.
In the present study, animal studies were conducted
to investigate NLRP3 inflammasome mechanisms in sepsis-induced
myocardial injury and evaluate the effects of UTI on injured
myocardium in septic rats.
Materials and methods
Animals
A total of 75 male Wistar rats weighing 180–200 g
(age, 7–9 weeks old) were purchased from the Laboratory Animal
Center of Dalian Medical University (Dalian, China) and housed
under controlled temperatures (20–25°C) and humidity (2–30%) under
a 12 h light/dark cycle. Rats were allowed access to food and water
ad libitum. This research was approved by the Ethics
Committee of Dalian Medical University (approval no. L20160097) and
was performed in accordance with the National Institute of Health
guidelines for ethical animal research (22).
Study design and UTI use
Rats (n=75) were randomly divided into five groups,
with 15 rats per group: i) Control; ii) sham-operation; iii) cecal
ligation and puncture (CLP); iv) CLP plus low-dose UTI (5,000
U/kg); and v) CLP plus high-dose UTI (20,000 U/kg). UTI was
provided by Guangdong Techpool Bio-Pharma Co. Ltd.
CLP
CLP procedures were performed according to a
previous study by Rittirsch et al (23) to generate a sepsis model. Animals
were anesthetized with an intraperitoneal injection of 1%
pentobarbital sodium (50 mg/kg; Shanghai Ling Feng Chemical Reagent
Co., Ltd.) before a midline laparotomy. Abdominal fur was removed,
the area disinfected and a sterile towel was spread over the area.
A 1-cm midline incision along the abdomen midline was performed,
the cecum was located and carefully isolated to avoid vascular
injury. Approximately 1/3 of the entire cecum was ligated with a
No. 4 suture on the distal side of the ileocecal valve. Then, the
distal cecum was punctured twice with a sterile 18-gauge needle and
a small amount of intestinal contents was squeezed out (24). After the cecum was placed back into
the abdominal cavity, the abdominal wall incision was sutured layer
by layer. Rats were resuscitated with a saline solution
administered by subcutaneous injection (3 ml/100 g bodyweight).
Sham-operation animals were treated the same way, but no cecal
ligation or puncture was performed. Control group animals were not
surgically processed, but were resuscitated with subcutaneous
saline. Animal spontaneous activity, reaction to exogenous stimuli
and posture were observed. Clinical severity scores were calculated
every 6 h to evaluate the clinical state and sepsis severity was
determined by two experienced observers blinded to treatments. All
assessment scores were collected for analysis (25).
Survival observations
Animals were observed for 48 h after surgery, with
survival status recorded and survival rate comparisons made between
different groups. Rats were sacrificed if they demonstrated any of
the following characteristics: Loss of spontaneous respiratory
movement and heartbeat for a period of 2 min, a moribund state
and/or hypothermia (rectal temperature <32°C).
Administration of UTI and specimen
retention
After surgery, at 0, 6, 12, 18 and 24 h, rats
assigned to low- and high-dose UTI groups were treated with 5,000
and 20,000 U/kg (intraperitoneal injection) UTI, respectively.
Control, sham-operation and CLP groups were injected with the same
volume of normal saline. At 48 h post-surgery and before animals
were sacrificed, blood from the angular vein was collected into
sodium citrate tubes. At the study end, surviving rats in each
group were humanely euthanized by cervical dislocation. Tissues
from the left ventricle were harvested, with samples placed in
buffered formalin, liquid nitrogen and frozen at −80°C. A study
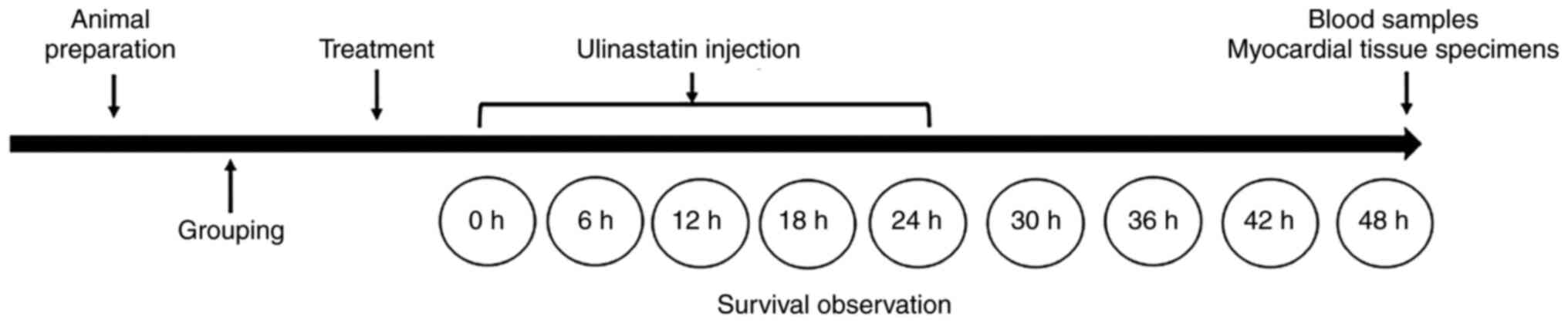
flowchart is shown in Fig. 1.
Inflammatory markers and myocardial
injury measurement
Blood samples from time points were immediately
centrifuged at 3,000 × g for 15 min at room temperature. The serum
was removed and stored at −80°C until enzyme-linked immunosorbent
assays (ELISA) were performed. Then, tumor necrosis factor-α
(TNF-α), IL-1β, cardiac troponin I (cTnI) and B-type natriuretic
peptide (BNP) serum levels were measured by commercial ELISA kits
as per the manufacturer's instructions. cTnI serum levels were
expressed as µg/ml, whereas BNP, TNF-α and IL-1β were expressed as
pg/ml. ELISA kits for TNF-α (cat. no. ab236712) and IL-1β (cat. no.
ab255730) quantification were obtained from Abcam, cTnI ELISA kits
(cat. no. BPE30309) and BNP ELISA kits (cat. no. BPE30445) were
purchased from Shanghai Lengton Bioscience Co., Ltd.
Myocardial histology
For hematoxylin and eosin (H&E) staining, left
ventricle apical tissue was collected and immediately washed twice
in phosphate-buffered saline (PBS, pH 7.4) to remove blood. The
tissue was fixed in 10% neutral formalin for 72 h at 25°C. Then,
samples were successively dehydrated (successive immersion in 75,
85, 90 and 95% alcohol, anhydrous acetic acid, anhydrous ethanol
and anhydrous ethanol for 30 min each) and paraffin embedded.
Tissue sections (4 µm) were then fixed in ethanol as follows:
Immersed in 70, 80 and 90% ethanol for 4–5 sec, and immersed in
anhydrous ethanol for 5 min at room temperature. The sections were
stained with hematoxylin for 5 min and eosin solution for 3 min at
room temperature, followed by dehydration with graded alcohol and
clearing in xylene. Stained sections were then analyzed and imaged
using light microscopy (magnification, ×400; Olympus
Corporation).
Transmission electron microscopy
Left ventricle apical tissues were fixed in cold
2.5% glutaraldehyde for 12 h, post-fixed in 1% osmium tetroxide for
1 h at room temperature, dehydrated at room temperature and
embedded in Epon for 12 h at 45°C. Ultrathin sections (60–80 nm)
were randomly cut and stained with lead citrate for 10 min at room
temperature and uranyl acetate for 30 min at room temperature.
Transmission electron microscopy (JEM-2000EX; JEOL, Ltd.) was used
to examine myocardium structures between ×10,000 and ×30,000
magnification. Five electron micrographs were randomly and
sequentially obtained from one section per animal.
Reverse transcription-quantitative
(RT-q)PCR
NLRP3 and caspase-1 mRNA expression levels in the
left ventricular myocardium were determined via RT-qPCR. Total mRNA
was extracted from tissue using RNAiso Plus (Takara Biotechnology
Co., Ltd.) and reverse transcribed to cDNA using a PrimeScript™ RT
reagent kit (Takara Biotechnology Co., Ltd.) with gDNA Eraser
according to the manufacturer's instructions. RT-qPCR was conducted
using SYBR® Premix Ex Taq™ II kit (Takara Biotechnology
Co., Ltd.) following the manufacturer's protocols. The RT-qPCR
procedure was conducted as follows: One cycle of 95°C for 30 sec;
40 cycles of 95°C for 5 sec and 60°C for 30 sec. All samples were
analyzed in triplicate. Relative expression was calculated using
the 2−ΔΔCq method with GAPDH as an endogenous reference
(26). All RT-qPCR primer sequences
are shown in Table I.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Genes | Forward primers
(5′→3′) | Reverse primers
(5′→3′) |
|---|
| NLRP3 |
CAGCGATCAACAGGCGAGAC |
AGAGATATCCCAGCAAACCTATCCA |
| Caspase-1 |
ACTCGTACACGTCTTGCCCTCA |
CTGGGCAGGCAGCAAATTC |
| GADPH |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
Western blotting
NLRP3 and caspase-1 protein expression levels in the
left ventricular myocardium were determined by western blotting.
Tissues were homogenized in lysis reagent (Sigma-Aldrich; Merck
KGaA) supplemented with phenylmethylsulfonyl fluoride (Beyotime
Institute of Biotechnology), and protein concentrations were
determined using a bicinchoninic acid assay kit (Sangon Biotech
Co., Ltd.). Total protein was separated via 8/10% polyacrylamide
gels, and subsequently transferred to polyvinylidene difluoride
membranes. Primary antibodies against β-actin (1:1,000; cat. no.
KC5A08; Kangchen BioTech Co., Ltd.), anti-caspase-1 (1:1,000; cat.
no. SC-56036; Santa Cruz Biotechnology, Inc.), and anti-NLRP3
(1:1,000; cat. no. 19771-1-AP; ProteinTech Group, Inc.). Blots were
blocked in 5% milk for 2 h at 37°C, incubated overnight at 4°C with
primary antibodies and washed in PBS with 0.05% Tween-20 for 20
min. Followed by incubation with a goat anti-rabbit secondary
antibody (1:1,000; ProteinTech Group, Inc.) at room temperature for
2 h. Protein bands were visualized using enhanced chemiluminescence
(Applygen Technologies, Inc.) and semi-quantified using ImageJ
software (version 1.8.0; National Institutes of Health).
Statistical analysis
All experiments were repeated three times. All
statistical analyses were performed using SPSS 20.0 (IBM Corp.).
Values are expressed as the mean ± standard deviation. Data were
analyzed using a two-tailed unpaired Student's t-test to compare
two groups. Comparisons among multiple groups were conducted using
one-way ANOVA with post hoc Bonferroni test. Survival rates over
time were conducted using Kaplan-Meier and log-rank analyses.
P<0.05 was considered to indicate a statistically significant
difference.
Results
UTI enhances survival in a rat sepsis
model
Kaplan-Meier survival curves and clinical severity
score curves were used to determine the protective effects of UTI
on survival rates and sepsis-related symptom alleviation.
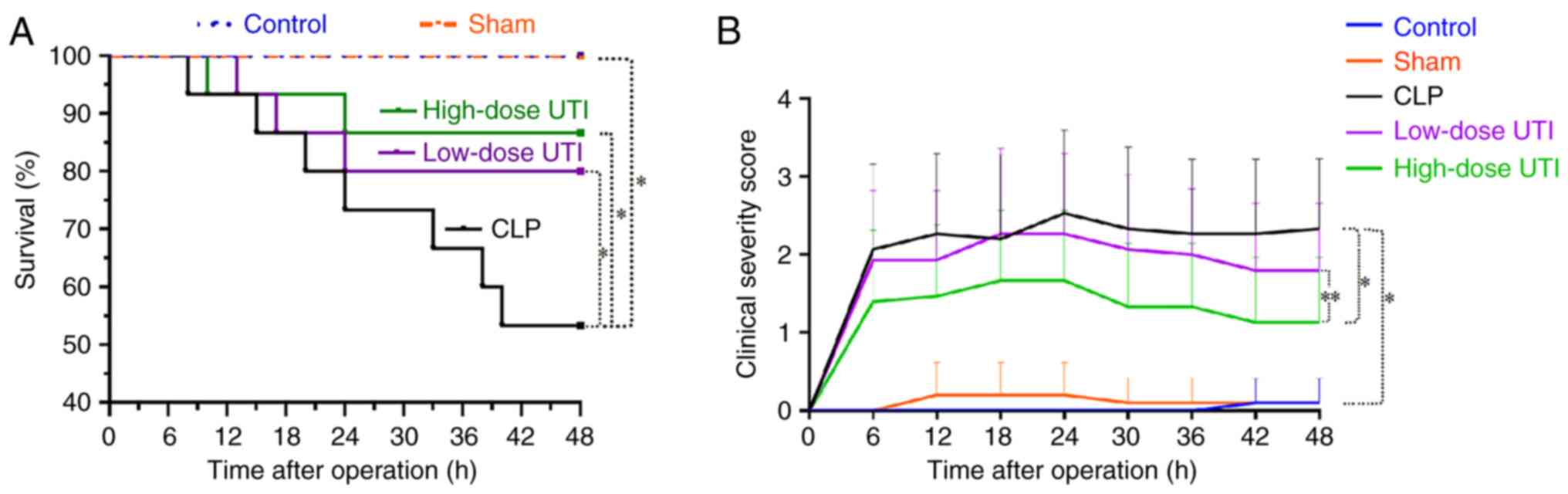
Kaplan-Meier survival curves at 48 h are shown for control, sham,
CLP, low- and high-dose UTI animals (Fig. 2A). Control and sham animals appeared
normal with all surviving; blue and orange curves for both
corresponded to a 100% survival rate. However, the CLP group showed
a steep decline in survival after surgery; most animals died from
septic shock within 10–40 h. The survival rate of CLP, low- and
high-dose UTI rats were 53.3, 80.0 and 86.7%, respectively. The UTI
groups exhibited significantly improved survival rates compared
with the CLP group (P<0.01; Fig.
2A). Clinical severity score curves revealed a delayed response
to CLP operation stimuli and peaked at 24 h. Scores in the CLP
group were significantly higher than both UTI treatment groups;
clinical severity scores in the low-dose UTI group were slightly
higher than those in the high-dose UTI group (P<0.05; Fig. 2B). These findings suggested that UTI
potentially alleviated sepsis-related symptoms and reduced
mortality rate in a septic rat model.
UTI alleviates myocardial tissue
damage
Using light microscopy, H&E myocardial stained
sections were used to investigate myocardial tissue damage and
inflammatory cell infiltration, whereas ultra-structural and
mitochondrial changes were examined using transmission electron
microscopy.
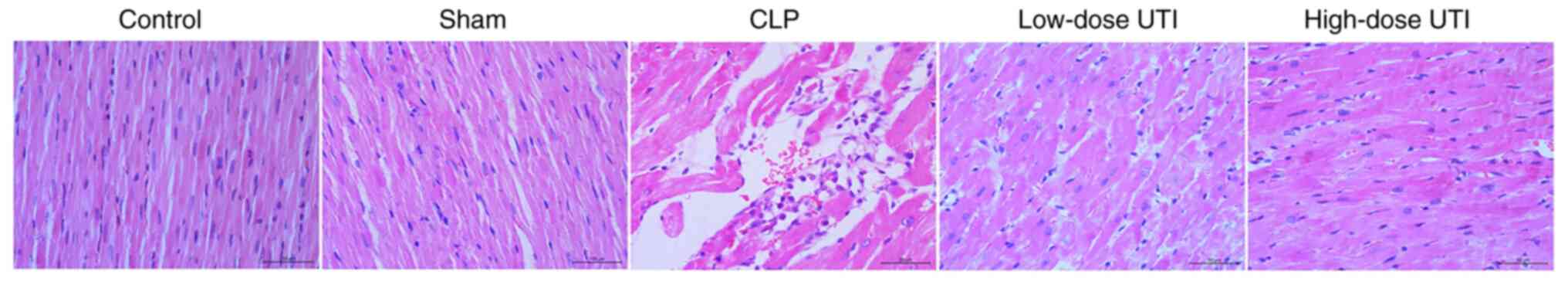
In the control group, myocardial cell morphology was
intact with a regular distribution and normal mitochondrial
morphology. Sham-group observations were similar. By contrast, the
CLP group displayed a scattered coagulated myocardial cell
necrosis, massive neutrophil infiltration between cardiomyocytes,
and blurred nuclear and organizational structures. Pathological
changes in the low- and high-dose UTI groups appeared less severe
than CLP animals. By contrast, cell morphology in the high-dose UTI
group had significantly improved, and only slightly improved in the
low-dose UTI group (Fig. 3).
Myocardial morphological structures and
mitochondrial crista were ordered and clear in control and sham
groups. However, in the CLP group, mitochondria were significantly
swollen, myocardial fibers were disordered, broken and dissolved.
By contrast, in UTI groups, especially the high-dose group, heart
morphological structures were improved and mitochondrial crista was
clearer (Fig. 4). These structural
changes indicated that UTI potentially exerted cardioprotective
effects by suppressing cardiac inflammation in CLP-treated
rats.
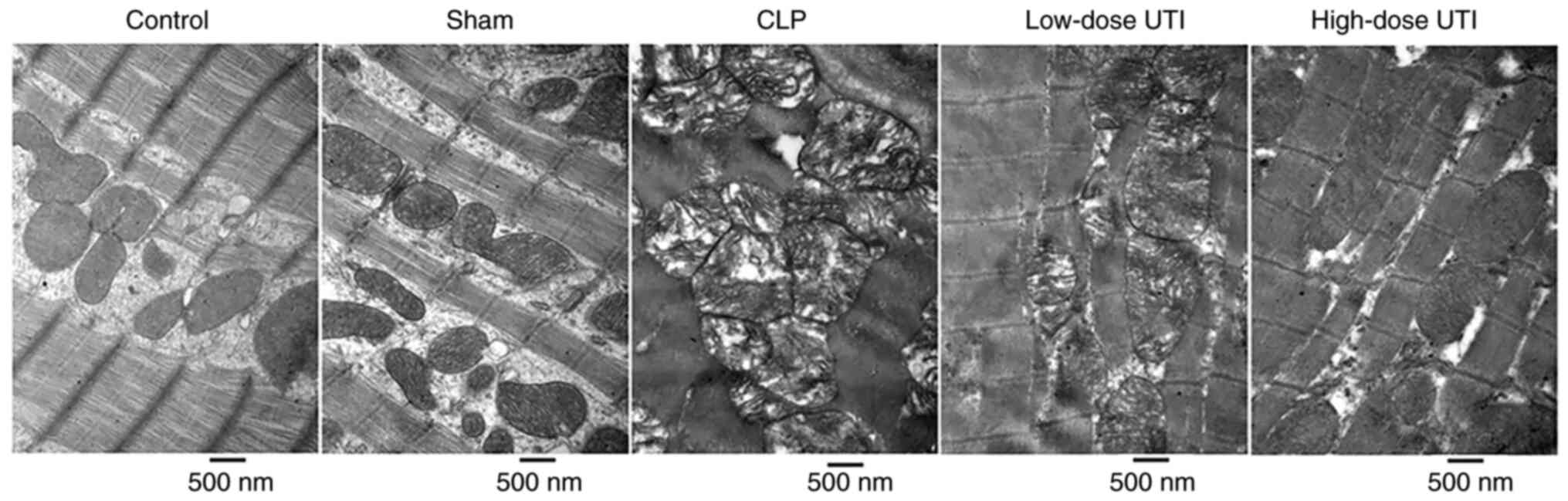 | Figure 4.Myocardial tissue observations under
a transmission electron microscope. Control and sham groups showed
regularly arranged myocardial tissue without swollen mitochondria.
Mitochondrial membrane structures were clear. In the CLP group,
myocardial fibers were irregularly arranged or disrupted, with
myocardial cell swelling. Most mitochondria were severely swollen,
broken and dissolved. Vacuolization was also visible in
mitochondria. In the low-dose UTI group, the myocardial structures
were complete and mitochondrial membrane structures had partially
disappeared. Only light vacuolization was observed in some
mitochondria. In the high-dose UTI group, myocardial structures had
improved, mitochondria were slightly swollen and membrane
structures were clear. Magnification, ×30,000. UTI, ulinastatin;
CLP, cecal ligation and puncture. |
UTI suppresses inflammatory mediator
release and attenuates myocardial injury
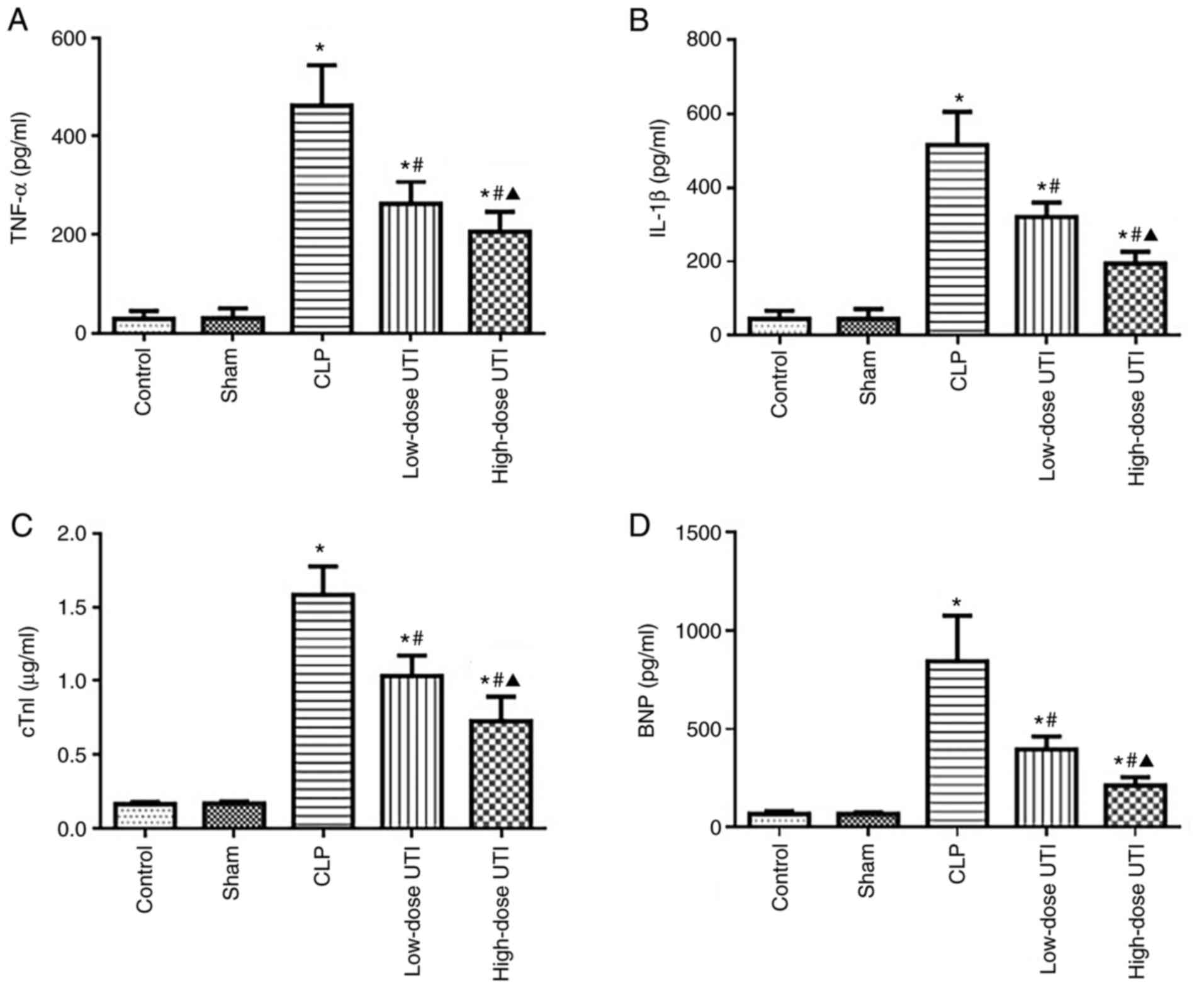
In response to 48 h post-surgery sepsis mediated
inflammatory marker level increases in serum, significant increases
in TNF-α and IL-1β serum levels were observed in the CLP group,
moderate increases in the low-dose UTI group and mild increases in
the high-dose UTI group compared with the control and sham groups
(P<0.01). No statistically significant differences were found
between control and sham groups (P>0.05). Compared with the CLP
group, TNF-α and IL-1β serum levels were significantly decreased in
the low- and high-dose UTI groups (P<0.01), and significantly
decreased in the high-dose group compared with the low-dose group
(P<0.05; Fig. 5A and B). Based
on these comparisons, it was concluded UTI treatments inhibited
inflammatory responses during sepsis, particularly at the higher
dose.
cTnI and BNP serum levels are indicators of
myocardial injury, therefore, to test the effects of UTI on
sepsis-induced myocardial injury, the serum levels of these
myocardial injury markers were examined at 48 h post-surgery.
Significant increases in cTnI and BNP serum levels were observed in
the CLP group, moderate increases in the low-dose UTI group and
mild increases in the high-dose UTI group compared with the control
and sham groups (P<0.01). No statistically significant
differences were observed between control and sham groups
(P>0.05). Compared with the CLP group, cTnI and BNP serum levels
were significantly decreased in the low- and high-dose UTI groups
(P<0.01), while in the high-dose group levels were significantly
decreased compared with the low-dose group (P<0.05; Fig. 5C and D). Taken together, these
observations demonstrated that UTI exerted protective roles against
sepsis-induced myocardial injury.
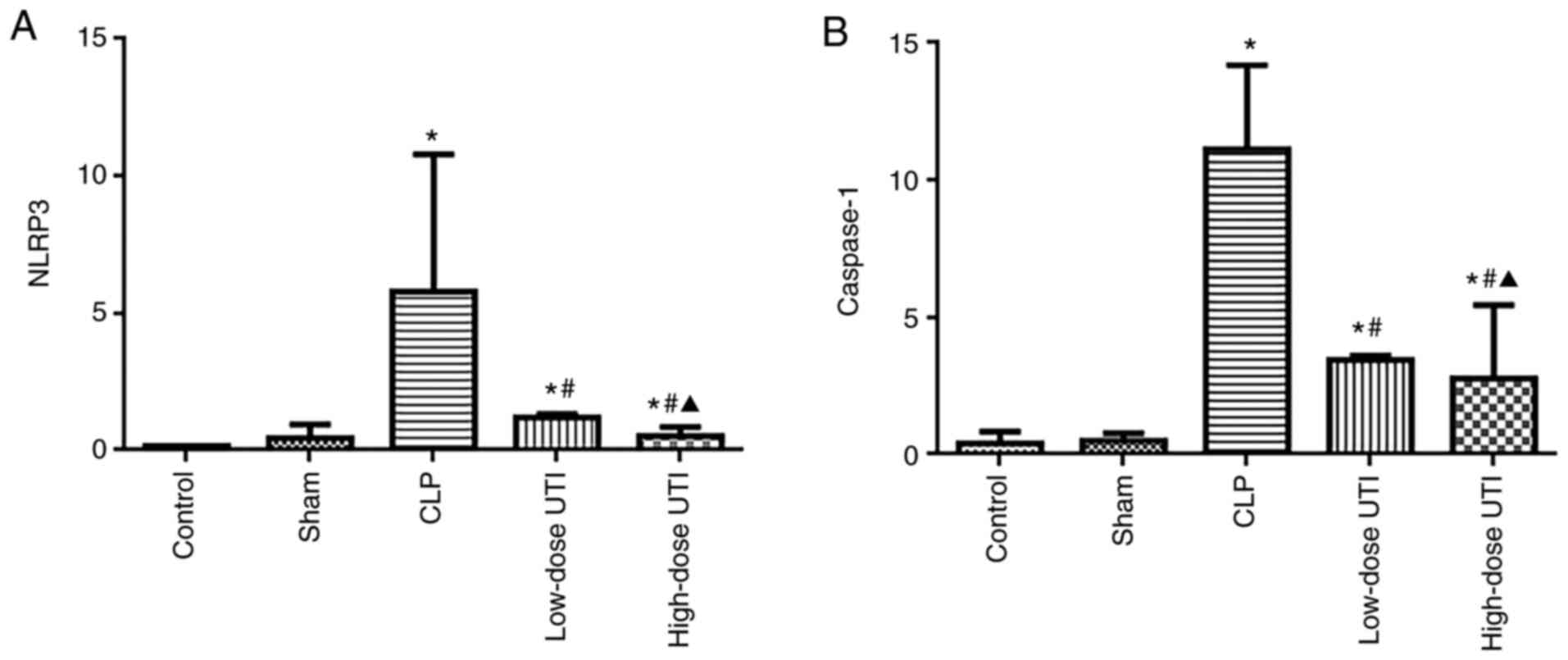
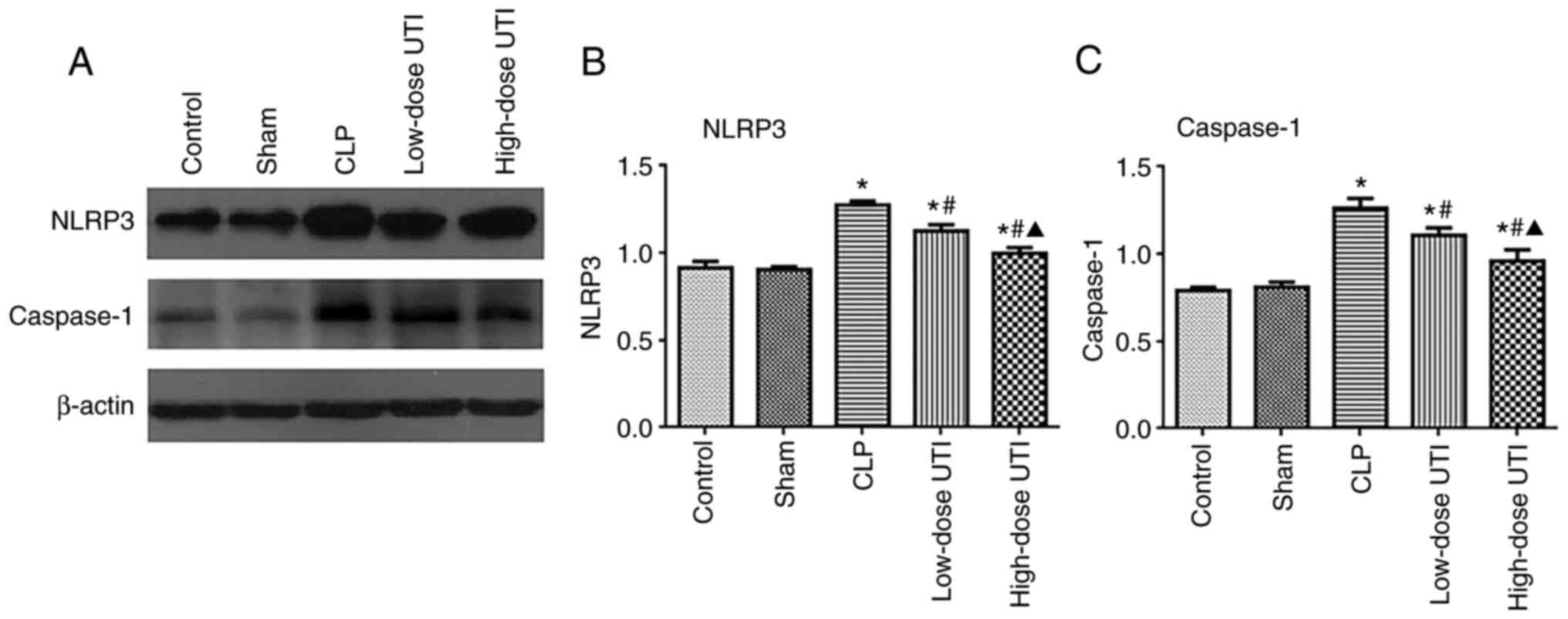
UTI inhibits NLRP3 and caspase-1
expression in myocardial tissue
NLRP3 inflammasome activation is reflected by the
enhanced expression of NLRP3 and caspase-1 (27). Therefore, to verify NLRP3 and
caspase-1 inhibition by UTI, mRNA and protein levels were analyzed
in all groups. No significant expression differences were observed
between control and sham groups (P>0.05), however the CLP group
had higher NLRP3 and caspase-1 expression levels than the other
groups (P<0.01), whereas the high-dose UTI group had lower NLRP3
and caspase-1 mRNA expression than the low-dose UTI group
(P<0.05; Fig. 6A and B). NLRP3
and caspase-1 protein expression levels gradually decreased as the
UTI concentration increased (P<0.01; Fig. 7A-C). Thus, UTI significantly
inhibited NLRP3 and caspase-1 protein expression.
Discussion
In the present study, animal models, biochemical
assays, histopathological examinations, and mRNA and protein
expression analyses were used to elucidate the molecular mechanisms
underpinning sepsis-induced myocardial dysfunction, and to
investigate the cardioprotective effects of UTI. Firstly, TNF-α,
IL-1β, cTnI and BNP levels were significantly increased and
secondly, myocardial tissue pathological alterations were induced,
indicating a sepsis-induced myocardial injury model was
successfully established.
UTI is widely used to treat acute pancreatitis and
pancreatic injury (28–30). In addition to its protease
inhibition activities, previous studies have also shown UTI
exhibits organ-protective effects (31,32),
with these effects related to anti-inflammatory and anti-oxidative
stress responses. Due to the essential role of anti-inflammatory
responses in sepsis-related organ injury pathogenesis, these
mechanisms present novel potential strategies for organ dysfunction
prognoses and sepsis treatments (21,33).
Masuda et al (34) reported
that UTI reduced the degree of myocardial injury during sepsis due
to its anti-inflammatory and anti-oxidative stress responses.
Inflammatory mediator levels produced during sepsis
serve as markers to assess the severity and therapeutic responses
to infections (35,36). TNF-α and IL-1β are major
inflammatory cytokines produced during sepsis (37,38)
and are often used to assess the extent of inflammation (39). TNF-α and IL-1β both have roles in
sepsis-induced myocardial injury (40). In the present study, it was observed
that myocardial injury was linked to the release of TNF-α and
IL-1β, whereas UTI decreased these levels, which was similar to
results reported in previous studies (41–43).
The cTnI marker is a sensitive indicator of
myocardial injury and is mainly released by cardiac myocytes
(44). Similarly, BNP is a
well-recognized biomarker of myocardial dysfunction and has been
used to assess sepsis-induced myocardial dysfunction (45). The findings observed in the current
study showed that cTnI and BNP serum levels were significantly
elevated in the CLP group, but significantly lower in the UTI
groups. These biomarker alterations suggested sepsis exerted
adverse effects on the myocardium, whereas UTI provided protection
against myocardial injury via anti-inflammatory effects in this rat
model.
The animal studies in the present study were
performed to verify NLRP3 inflammasome mechanisms during septic
myocardial injury; elevated NLRP3 and caspase-1 expression levels
in the myocardium were synchronous with TNF-α and IL-1β serum
expression levels. Accumulating evidence has indicated that the
NLRP3 inflammasome has a role during cardiac dysfunction (46). The NLRP3 inflammasome is composed of
NLRP3, ASC and caspase-1, with endogenous cytokines, including
TNF-α, activating the inflammasome via the NF-κB pathway (12). This activation releases IL-1β and
promotes inflammatory responses, thereby inducing myocardial injury
(41). The present study
demonstrated that the NLRP3 inflammasome activation (measured as
NLRP3 and caspase-1 activation) was significantly increased in the
CLP group compared with control and sham groups, therefore
indicating that myocardial injury is related to NLRP3 inflammasome
activation.
The effects of UTI on injured myocardium in septic
rats was also evaluated in the current study. It was found that
various UTI doses reduced NLRP3 and caspase-1 protein expression
during myocardial injury induced by CLP, suggesting that UTI
suppressed NLRP3 inflammasome activation. Furthermore, when
compared with low-dose UTI, the high-dose group displayed an
improved outcome as it inhibited the NLRP3 inflammasome to a higher
degree. Additionally, a similar trend was observed for IL-1β. A
previous study also confirmed that NLRP3 inflammasome activation
can mediate IL-1β maturation via caspase-1 (47). Taken together, these findings
suggested that the protective effects of UTI on the myocardium may
be mediated by the downregulation of the NLRP3/caspase-1/IL-1β
pathway.
The results of the present study were consistent
with previous studies showing that UTI was associated with a
reduced risk of mortality in sepsis (48,49).
However, high-dose UTI did not show advantages in terms of fatality
rates; as the study observation period was relatively short, the
humane end points were set at 48 h, therefore if observation times
were extended, the results might not have been the same.
Additionally, it was observed that clinical severity scores in the
low-dose UTI group were significantly higher than the high-dose
group; there were statistically significant differences with
respect to biomarkers, histopathological examinations, and gene and
protein expression levels. Therefore, these findings provided
crucial data on the effectiveness of UTI intervention for
sepsis-induced myocardial injury treatment.
There are several limitations of the present study.
Firstly, the percent survival rate was expressed by Kaplan-Meier
survival curve analysis, the sample size of 15 rats per group was
relatively small. Secondly, this study mainly focused on the
microstructure and molecular biology of sepsis-induced myocardial
injury, and echocardiography for the evaluation of septic
myocardial injury was not used. Thirdly, two doses of UTI (5,000
U/kg as low-dose UTI and 20,000 U/kg as high-dose UTI) were used to
observe the effects on animals, and dose-response curves were not
determined to test the effects of UTI on sepsis-induced myocardial
injury.
In conclusion, it was demonstrated that UTI
potentially exerted beneficial effects by inhibiting inflammatory
cytokine release and downregulating NLRP3 inflammasome activation.
Similarly, high-dose UTI significantly preserved the myocardium.
This study has provided a scientific basis that suggests that UTI
may serve as a novel therapeutic strategy targeting the
NLRP3/caspase-1/IL-1β signaling pathway during sepsis-induced
myocardial injury. Further clinical studies are required to
determine its clinical efficacy.
Acknowledgements
The authors are grateful to Dr Dongmei Hu (School of
Statistics, Dalian Medical University, Dalian, China) for her help
with the statistical analysis.
Funding
This work was supported by the Natural Science
Foundation of Liaoning Province (grant no. 20180551029), the
Scientific Research Project of Liaoning Provincial Department of
Education (grant no. LZ2020036) and the National Natural Science
Foundation of China (grant no. 81803896).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JQ and YZ designed the study and performed
experiments. YZ drafted the manuscript. XX and XG collected and
analyzed experimental data. JQ and YZ confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This research was approved by the Ethics Committee
of Dalian Medical University (approval no. L20160097).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen J: The immunopathogenesis of sepsis.
Nature. 420:885–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Turner A, Tsamitros M and Bellomo R:
Myocardial cell injury in septic shock. Crit Care Med.
27:1775–1780. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beesley SJ, Weber G, Sarge T, Nikravan S,
Grissom CK, Lanspa MJ, Shahul S and Brown SM: Septic
cardiomyopathy. Crit Care Med. 46:625–634. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blanco J, Muriel-Bombín A, Sagredo V,
Taboada F, Gandía F, Tamayo L, Collado J, García-Labattut A,
Carriedo D, Valledor M, et al: Incidence, organ dysfunction and
mortality in severe sepsis: A Spanish multicentre study. Crit Care.
12:R1582008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duewell P, Kono H, Rayner KJ and Latz E:
NLRP3 inflammasomes are required for atherogenesis and activated by
cholesterol crystals. Nature. 464:1357–1361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baroja-Mazo A, Martín-Sánchez F, Gomez AI,
Martínez CM, Amores-Iniesta1 J, Compan V, Barberà-Cremades M, Yagüe
J, Ruiz-Ortiz E, Antón J, et al: The NLRP3 inflammasome is released
as a particulate danger signal that amplifies the inflammatory
response. Nat Immunol. 15:738–748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Franklin BS, Bossaller L, De Nardo D,
Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR,
Al-Amoudi A, et al: The adaptor ASC has extracellular and
‘prionoid’ activities that propagate inflammation. Nat Immunol.
15:727–737. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amin J, Boche D and Rakic S: What do we
know about the inflammasome in humans? Brain Pathol. 27:192–204.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sutterwala FS, Haasken S and Cassel SL:
Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci.
1319:82–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Danielski LG, Giustina AD, Bonfante S,
Barichello T and Petronilho F: The NLRP3 inflammasome and its role
in sepsis development. Inflammation. 43:24–31. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong Y, Lu Y, Yang X, Tang Y, Zhao K,
Yuan C and Zhong X: The roles of NLRP3 inflammasome in bacterial
infection. Mol Immunol. 122:80–88. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang H, He H, Chen Y, Huang W, Cheng J,
Ye J, Wang A, Tao J, Wang C, Liu Q, et al: Identification of a
selective and direct NLRP3 inhibitor to treat inflammatory
disorders. J Exp Med. 214:3219–3238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Y, Jiang H, Chen Y, Wang X, Yang Y,
Tao J, Deng X, Liang G, Zhang H, Jiang W and Zhou R: Tranilast
directly targets NLRP3 to treat inflammasome-driven diseases. EMBO
Mol Med. 10:e86892018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang P, Tsuchiya K, Kinoshita T,
Kushiyama H, Suidasari S, Hatakeyama M, Imura H, Kato N and Takashi
S: Vitamin B6 prevents IL-1β protein production by inhibiting NLRP3
inflammasome activation. J Biol Chem. 291:24517–24527. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li PL: Cardiovascular pathobiology of
inflammasomes: Inflammatory machinery and beyond. Antioxid Redox
Signal. 22:1079–1083. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Umeadi C, Kandeel F and Al-Abdullah IH:
Ulinastatin is a novel protease inhibitor and neutral protease
activator. Transplant Proc. 40:387–389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu CE, Zhang MY, Zou CW and Guo L:
Evaluation of the pharmacological function of ulinastatin in
experimental animals. Molecules. 17:9070–9080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Q, Yan Q and Chen S: Use of ulinastatin
was associated with reduced mortality in critically ill patients
with sepsis. J Thorac Dis. 11:1911–1918. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Q, Yan Q and Chen S: Ulinastatin is
effective in reducing mortality for critically ill patients with
sepsis: A causal mediation analysis. Sci Rep. 8:143602018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals, 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
23
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Otero-Antón E, González-Quintela A,
López-Soto A, López-Ben S, Llovo J and Pérez LF: Cecal ligation and
puncture as a model of sepsis in the rat: Influence of the puncture
size on mortality, bacteremia, endotoxemia and tumor necrosis
factor alpha levels. Eur Surg Res. 33:77–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gonnert FA, Recknagel P, Seidel M, Jbeily
N, Dahlke K, Bockmeyer CL, Winning J, Lösche W, Claus RA and Bauer
M: Characteristics of clinical sepsis reflected in a reliable and
reproducible rodent sepsis model. J Surg Res. 170:e123–e134. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paik S, Kim JK, Silwal P, Sasakawa C and
Jo EK: An update on the regulatory mechanisms of NLRP3 inflammasome
activation. Cell Mol Immunol. 18:1141–1160. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Uemura K, Murakami Y, Hayashidani Y and
Sueda T, Hashimoto Y, Ohge H and Sueda T: Randomized clinical trial
to assess the efficacy of ulinastatin for postoperative
pancreatitis following pancreaticoduodenectomy. J Surg Oncol.
98:309–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao C, Huan J, Li W and Tang J: Protective
effects of ulinastatin on pancreatic and renal damage in rats
following early scald injury. Burns. 35:547–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng C, Yang H, Huang S and Li T: Early
local drug therapy for pancreatic contusion and laceration.
Pancreatology. 19:285–289. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Atal SS and Atal S: Ulinastatin-a newer
potential therapeutic option for multiple organ dysfunction
syndrome. J Basic Clin Physiol Pharmacol. 27:91–99. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Zhou J and Bai S: Combination of
glutamine and ulinastatin treatments greatly improves sepsis
outcomes. J Inflamm Res. 13:109–115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karnad DR, Bhadade R, Verma PK, Moulick
ND, Daga MK, Chafekar ND and Iyer S: Intravenous administration of
ulinastatin (human urinary trypsin inhibitor) in severe sepsis: A
multicenter randomized controlled study. Intensive Care Med.
40:830–838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Masuda T, Sato K, Noda C, Noda C, Ikeda
KM, Matsunaga A, Ogura MN, Shimizu K, Nagasawa H, Matsuyama N and
Izumi T: Protective effect of urinary trypsin inhibitor on
myocardial mitochondria during hemorrhagic shock and reperfusion.
Crit Care Med. 31:1987–1992. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bozza FA, Salluh JI, Japiassu AM, Soares
M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC and Bozza PT:
Cytokine profiles as markers of disease severity in sepsis: A
multiplex analysis. Crit Care. 11:R492007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Van der Poll T, Van de Veerdonk FL,
Scicluna BP and Netea MG: The immunopathology of sepsis and
potential therapeutic targets. Nat Rev Immunol. 17:407–420. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cannon JG, Tompkins RG, Gelfand JA, Michie
HR, Stanford GG, van der Meer JW, Endres S, Lonnemann G, Corsetti
J, Chernow B, et al: Circulating interleukin-1 and tumor necrosis
factor in septic shock and experimental endotoxin fever. J Infect
Dis. 161:79–84. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Girardin E, Grau GE, Dayer JM,
Roux-Lombard P and Lambert PH: Tumor necrosis factor and
interleukin-1 in the serum of children with severe infectious
purpura. N Engl J Med. 319:397–400. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hedayat M, Mahmoudi MJ, Rose NR and Rezaei
N: Proinflammatory cytokines in heart failure: Double-edged swords.
Heart Fail Rev. 15:543–562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hunter JD and Doddi M: Sepsis and the
heart. Br J Anaesth. 104:3–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kumar A, Thota V, Dee L, Olson J, Uretz E
and Parrillo JE: Tumor necrosis factor alpha and interleukin 1beta
are responsible for in vitro myocardial cell depression induced by
human septic shock serum. J Exp Med. 183:949–958. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cao YZ, Tu YY, Chen X and Liu MH:
Protective effect of Ulinastatin against murine models of sepsis:
Inhibition of TNF-α and IL-6 and augmentation of IL-10 and IL-13.
Exp Toxicol Pathol. 64:543–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu CL, Li H, Xia JM, Li X, Zeng X, Liao
XX, Zhan H, Jing XL and Dai G: Ulinastatin improved cardiac
dysfunction after cardiac arrest in New Zealand rabbits. Am J Emerg
Med. 31:768–774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fromm RE Jr: Cardiac troponins in the
intensive care unit: Common causes of increased levels and
interpretation. Crit Care Med. 35:584–588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Charpentier J, Luyt CE, Fulla Y,
Vinsonneau C, Cariou A, Grabar S, Dhainaut JF, Mira JP and Chiche
JD: Brain natriuretic peptide: A marker of myocardial dysfunction
and prognosis during severe sepsis. Crit Care Med. 32:660–665.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W
and Tang Q: STING-IRF3 contributes to lipopolysaccharide-induced
cardiac dysfunction, inflammation, apoptosis and pyroptosis by
activating NLRP3. Redox Biol. 24:1012152019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
He Y, Hara H and Núñez G: Mechanism and
regulation of NLRP3 inflammasome activation. Trends Biochem Sci.
41:1012–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Linder A and Russell JA: An exciting
candidate therapy for sepsis: Ulinastatin, a urinary protease
inhibitor. Intensive Care Med. 40:1164–1167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang N, Lui X, Zheng X, Cao H, Wei G, Zhu
Y, Fan S, Zhou H and Zheng J: Ulinastatin is a novel candidate drug
for sepsis and secondary acute lung injury, evidence from an
optimized CLP rat model. Int Immunopharmacol. 17:799–807. 2013.
View Article : Google Scholar : PubMed/NCBI
|