Introduction
Gastrointestinal (GI) symptoms without any organic
changes are called functional gastrointestinal disorders (FGID).
The most common of these disorders is irritable bowel syndrome
(IBS) (1). The pathophysiology
behind FGID is unknown, but visceral hypersensitivity,
psychological factors, low-grade inflammation, alterations in gut
microbiota composition, or hormonal profile have been discussed
(2).
IBS symptoms are frequently experienced during food
intake, and as such, dietary interventions are usually prescribed
to improve the symptoms (3). Also,
patients with IBS have been found to have altered expression of
endocrine cells in the GI tract and different levels of circulating
hormones (4–6).
Dietary changes may influence the production of gut
hormones since the production is predominantly influenced by food
ingestion and food nutrient content (7). Hormones such as C-peptide, gastric
inhibitory peptide (GIP), glucagon, glucagon-like peptide-1
(GLP-1), and insulin are key hormones in regulation of glucose
homeostasis. These hormones control energy and glucose metabolism
by acting on the function of the digestive system in glucose
regulation, motility, and pancreatic function (8,9).
Leptin controls appetite and food intake, thereby regulating energy
intake (10). Thus, the improvement
of IBS symptoms with dietary changes may possibly be linked to the
effect of changes in gut hormones (11).
The first line of dietary advice is the National
Institute for Health and Care Excellence (NICE) guidelines, which
recommend regular meal patterns and decreased intake of mineral
water, caffeine, fat, and spicy foods (12), or the low FODMAP diet, which
advocates exclusion of fermentable oligo-, di- and monosaccharides
and polyols (13). These diets have
an effect in 20–50% of IBS patients (14).
Recently, a diet with starch and sucrose reduction
(SSRD) has been shown to markedly reduce the GI symptoms in IBS
patients with a response rate of 74% (15,16).
The reduction in GI symptoms correlated with the reduction in
intake of carbohydrates, disaccharides, starch, sucrose, and sugar
(17). We found decreased levels of
C-peptide, insulin, GIP, and leptin after introduction of the SSRD,
but the hormonal changes only correlated with the decrease of
carbohydrate intake and weight, not with the decrease of GI
symptoms (18). Circulating levels
of inflammatory factors were not affected by the diet (17). Although the dietary changes led to
corresponding changes in feces microbiota, this was not correlated
with the changes in GI symptoms (unpublished data).
The mechanisms behind the effect in IBS of SSRD is
not determined, but three different theories are plausible
explanations to GI symptoms after intake of starch and sucrose,
i.e., sucrase-isomaltase (SI) deficiency, overloading of the
physiological absorptive system, or food-induced dysfunction of the
nervous system (Table I).
 | Table I.Possible explanations for
gastrointestinal symptoms after starch and sucrose intake. |
Table I.
Possible explanations for
gastrointestinal symptoms after starch and sucrose intake.
| Etiology | Possible
mechanisms | Symptoms |
|---|
| Sucrase-isomaltase
deficiency | Unabsorbed
carbohydrates lead to fermentation, gas production, water diffusion
and distention | Abdominal bloating,
flatulence, pain and diarrhea |
| Fructose
intolerance | Unabsorbed
carbohydrates lead to fermentation, gas production, water diffusion
and distention | Abdominal bloating,
flatulence, pain and diarrhea |
| Endocrine and
metabolic effects in the tissue and nerves | Hyperinsulinemia,
increased release of GIP and GLP-1, dyslipidemia, oxidative stress,
advanced glycemic end products and other metabolic changes affect
the central and peripheral nerves with altered motility and
perception | Abdominal bloating,
pain, constipation and diarrhea |
Genetic variants of sucrase-isomaltase
deficiency
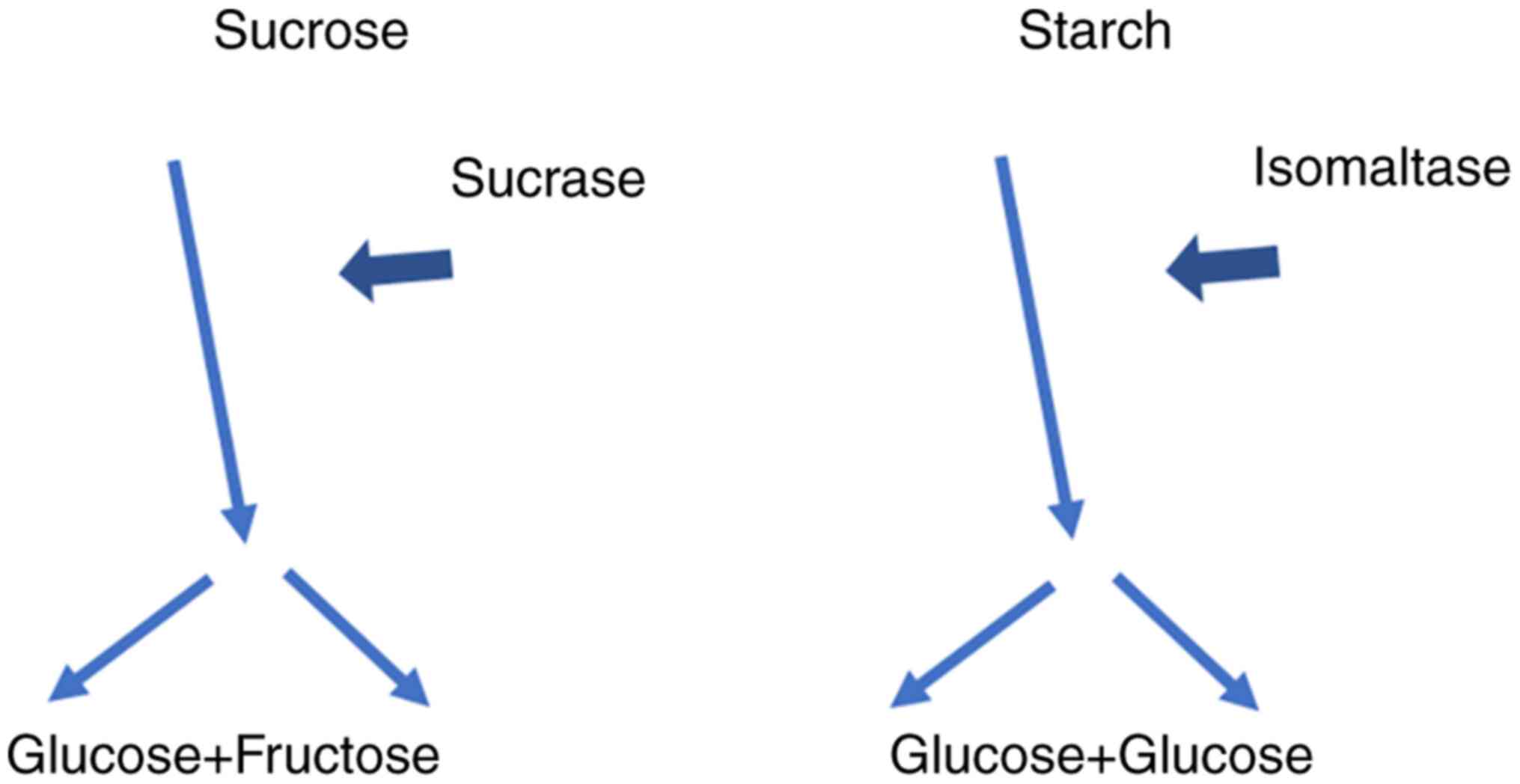
Sucrose, or saccharose, consists of one glucose and
one fructose molecule. The bond between these two molecules is
broken by the membrane-bound enzyme SI. The same enzyme also
hydrolyzes the glucose molecules in the short oligosaccharides and
starch (Fig. 1). This α-glucosidase
enzyme consists of two enzyme domains of the glycoside hydrolase
family GH31, one serving as isomaltase, the other as sucrase
(19). Severe congenital
sucrase-isomaltase deficiency (CSID) is an autosomal recessive
unusual condition with mutations of the SI gene on chromosome
3q25-26. Lack of the SI enzyme leads to impaired ability to
hydrolyze sucrose, maltose, short glucose oligomers, and branched
dextrins. More than 25 different mutations have been described
(20). CSID was unknown among the
indigenous people of Greenland as long as they lived on fish. After
the introduction of the Western diet with a high proportion of
sugar and starch in the latter half of the last century, the
prevalence of CSID in Greenland has risen to between 5 and 10%
(21). The prevalence of CSID
varies but has been described as 5–10% in Greenland, 3–7% in Canada
and 3% in Alaska. The prevalence in North America and Europe varies
between 1/500 and 1/2,000 (22).
Functional variants appear to be more common in the
population than CSID and can lead to similar maldigestion (22). These functional variants of this
mutation have previously been described in high prevalence in IBS
(23,24). In addition to the degree of enzyme
deficiency, the type of dietary intake also influences the
efficiency of hydrolysis, since naturally phytochemicals can
inhibit the enzyme activity (25).
Undigested sugars are fermented by the gut
microbiota with ensuing gas production and water diffusion, which
lead to symptoms in the form of abdominal tension, abdominal pain,
and diarrhea (14). The classic
CSID manifests itself during infancy when one begins to introduce
fruits and juices into the diet and leads to severe diarrhea, poor
weight gain, irritability, and diaper rash. The treatment mainly
consists of avoiding starch and sucrose, which reverses the
symptoms. Milder forms of mutations can present clinically later in
life with the same symptoms as in other carbohydrate intolerances,
especially diarrhea, and can be misdiagnosed as IBS in adults
(26).
Monosaccharide absorption in the small
intestine
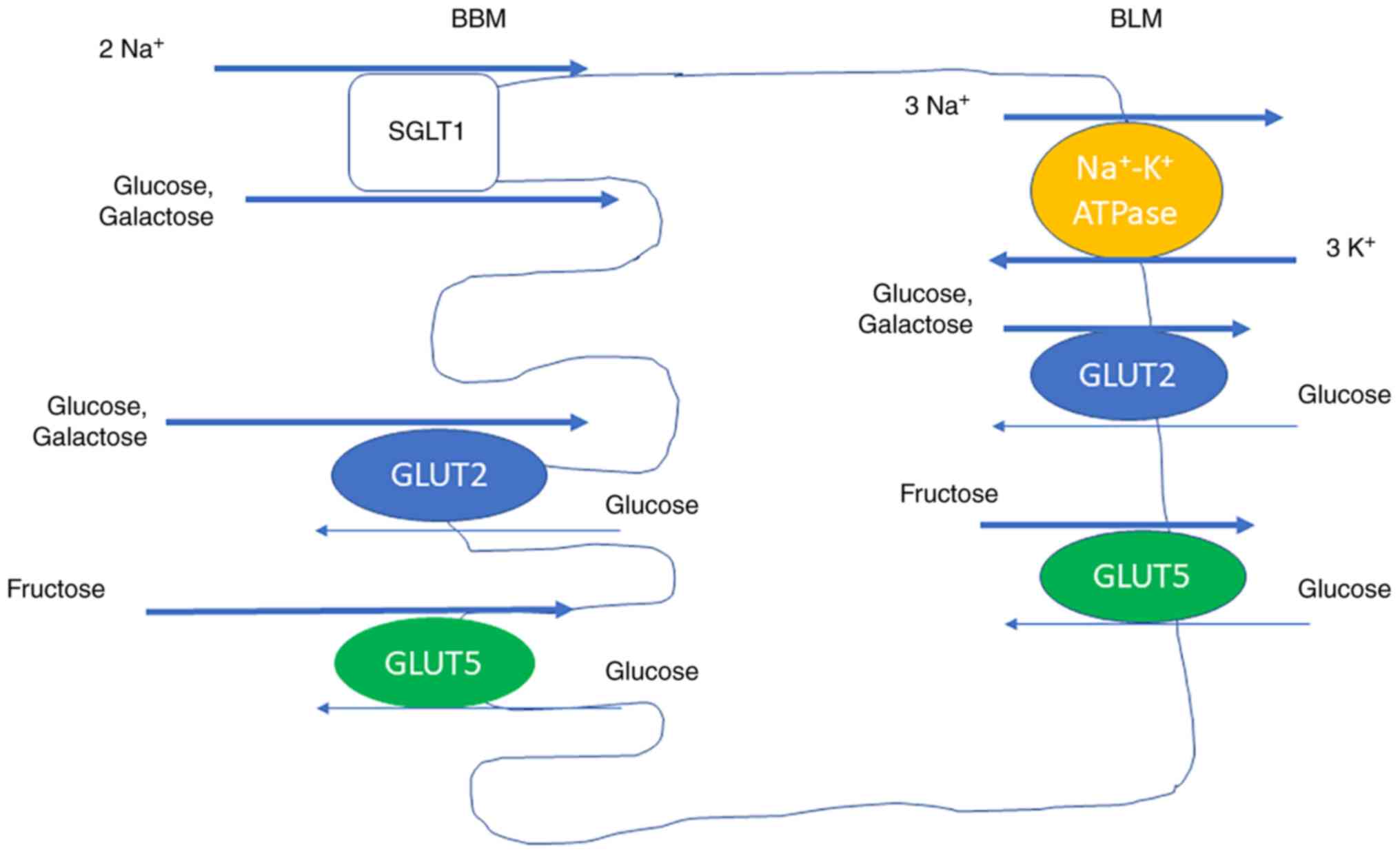
Glucose, galactose, and fructose are the most common
monosaccharides. The absorption of monosaccharides at the luminal
brush border membrane (BBM) and transportation out of the cells at
the basolateral membrane (BLM) are mediated by transport molecules.
Glucose and galactose are absorbed at the BBM by sodium-glucose
cotransporter 1 (SGLT1), driven by the Na+ gradient, and
by glucose transporter 2 (GLUT2), driven by diffusion. SGLT1 is the
rate limiting transporter for glucose absorption (27). GLUT2 is mainly expressed in the BBM
during presence of high luminal glucose concentrations, and not
between the meals, when the glucose concentration is low. Glucose
and galactose leave the cell at the BLM by GLUT2. Fructose is
transported by GLUT5 both at the BBM and the BLM (Fig. 2) (28).
The absorptive capacity varies in nonspecific ways,
e.g., depending on the absorptive surface, number of enterocytes,
and their degree of differentiation. This slow adaption has been
observed in response to changed nutritional supply, during
diabetes, and after surgery (29,30).
Specific adaptions of monosaccharides are rapid and may occur
within minutes or hours but may also develop during days. They
include changes in the amount of transport molecules and are due to
transcriptional and/or posttranscriptional regulations. They have
been described following diurnal rhythm, after up-take of
carbohydrate-rich meals, and in response to carbohydrate content,
hormone levels, and neuronal activation (28). The capacity for glucose absorption
peaks in the late light/early dark phase in rats during free access
to food (31). This is consistent
with the peaking of SGLT1 mRNA expression (32) and sucrase activity at the same time
point (33). This may be considered
physiologically since 90% of the ingestion in rodents are during
the night (32). High glucose
concentration in the small intestine leads to short-term
upregulation of SGLT1 in human and rodents. Both glucagon, GLP,
insulin, EGF, and prostaglandins upregulate SGLT1, whereas
cholecystokinin (CCK) and leptin downregulate the transport
molecule (28).
Several mechanisms are involved in the long-term
regulation of SGLT1 and include both a direct effect of glucose,
galactose, and fructose and an indirect effect through taste
reception and hormonal changes. The effect is mainly exerted
through translational and posttranslational levels, but also on
mRNA level (34,35).
The expression of GLUT2 is coordinated with the
expression of SGLT1 regarding diurnal rhythm, glucose concentration
in the small intestine, and hormonal effects (32). Specific food, such as strawberries
and blueberries containing flavonoids and other phenolic compounds,
downregulate both the transport and mRNA levels of SGLT1 and GLUT2
(36). The expression of GLUT5 is
coordinated with the expression of SGLT1 and GLUT2 regarding
diurnal rhythm and fructose intake (32). A great portion of the absorbed
fructose is converted in the enterocytes to glucose through
gluconeogenesis.
Gastrointestinal effects of monosaccharide
absorption
Malfunction of monosaccharide
transporters
Glucose-galactose malabsorption (GGM) is a rare
congenital autosomal recessive disorder with severe diarrhea in
newborn, depending on defect Na+-glucose cotransport in
the small intestine (37). The
therapy is removal of glucose and galactose from the diet.
Malabsorption of glucose and galactose may also depend on mutations
in gene encoding neurogen-3 and leads to depletion of endocrine
cells and cause general monosaccharide malabsorption (38). Fanconi-Bickel syndrome is another
rare congenital disease with impaired absorption of glucose and
galactose (39).
The dramatic increase of nutrients and beverages
which are enriched with sucrose has led to higher amount of
fructose in the food (40). In
humans, the capacity of fructose absorption is much smaller than
the glucose absorption (41). In a
population of healthy adults, an intestinal load of 25 g fructose
was only absorbed completely by one-half of the individuals
(42). Isolated fructose
intolerance due to defects in GLUT5, or overloading of the
physiological capacity of fructose absorption, may be one reason to
symptoms of abdominal pain, cramps, and diarrhea after ingestion of
high amounts of fructose. This condition may be mistaken as FGID
(43).
Effects of transport molecules on
hormone secretion and local inflammation
Since SGLT1 is involved in membrane polarization,
this includes activation of several Ca+ channels,
activation of PKCβII, and phosphorylation of myosin II and MAP
kinases, among several other proteins (28). An increased SGLT1-mediated glucose
transport may protect from lipopolysaccharide (LPS)-induced
apoptosis (44). Further, SGLT1
influences the effects of cytotoxic drugs in the small intestine
and seem to have the same protective effect regarding these drugs
as concerning LPS (44,45).
High luminal glucose concentrations involve SGLT1
and GLUT2 in the glucose-dependent stimulation of GLP-1 and GIP
secretion, which also involves sweet taste receptors. A high
intracellular glucose level increases carbohydrate metabolism,
which lead to openings of Ca+ channels and exocytosis of
vesicles containing GLP-1 and GIP (27), hormones with effects on GI motility
(9).
Effect of sugar-rich diets on the
development of polyneuropathy
The pathophysiology behind polyneuropathy is
multifactorial and involves hyperglycemia and metabolic
disturbances. The mechanisms involve oxidative stress, accumulation
of advanced glycation end products, P13K/Akt signaling pathways,
and chronic inflammation (46–49).
Animal models with diets high of fat and/or sugar have been
acknowledged for decades to study metabolic effects and their
complications. A diet for 4 weeks with high sucrose intake to rats
showed a progressive increase in blood glucose levels, insulin
levels, and Homeostatic Model Assessment for Insulin Resistance
(HOMA-IR) (50). In similarity, a
high fat-sucrose diet for 3 months led to increased fasting glucose
levels after 25 days, although the levels were not defined as
hyperglycemia. In parallel, the insulin levels, free fatty acid
levels, and HOMA-IR were also elevated (51). Increased mechanical pain sensitivity
was found ~1 month later (51),
which indicates that the neuropathy may occur after chronic
exposure to hyperinsulinemia, dyslipidemia, and impaired glucose
tolerance, as has been suggested in humans (52–54).
Histopathological examinations revealed myelin breakdown, axonal
degeneration, and small fiber neuropathy in the periphery. Also,
the spinal dorsal column was affected with loss of inter-fiber
matrix and dramatic reduction of myelin and axonal degeneration
(51). A cafeteria diet (CAD)
including cakes, biscuits, meat pies, and potato chips for 3 months
to rats led to increased body weight and increased glucose and
insulin levels consistent with prediabetes. Although the nerve
conduction tests were normal, a superexcitability was found in the
tibial motor nerve (55).
Excitability parameters are electrophysiological measure of axonal
membrane and ion channels, and the finding of superexcitability
indicates an early functional change in the peripheral axon
(56). The superexcitability
correlated with weight, insulin levels, HOMA-IR, and leptin levels
(55), which suggest that metabolic
changes may drive early axonal dysfunction.
In line with these animal studies, changes in motor
excitability have been demonstrated in human prediabetes (57) and found prior to overt
polyneuropathy (58). Improvement
of the metabolic control leads to regression of painful neuropathy
(59).
Discussion
During the last decades, the sugar intake has been
markedly increased (40). The
higher luminal sugar concentrations, the more upregulation of the
enzymatic and absorptive systems, with higher glucose absorption
consequently (28). Accordingly,
the increased sugar consumption has several effects on the
homeostasis and thereby on health (60). Decreased intake of starch and
sucrose does not only influence the GI symptoms (17), it also leads to weight reductions
and a less risk of metabolic syndromes (61). Since reduction of carbohydrates
correlated with the reduction of GI symptoms (17), there must be a direct effect of the
diet on the bowel. Although no changes in circulatory inflammatory
factors were found (17), local
inflammation in the GI tract may occur (62).
Elevated levels of C-peptide and hyperinsulinemia
have been described in IBS (63,64).
Numerous patients with IBS also suffer from metabolic syndromes
(63,65). The lower values of C-peptide,
insulin, GIP, and leptin and reduced weight after introduction of
the SSRD may have several effects in the body (28). A healthier diet should probably
improve not only the GI symptoms but also the general health status
(66). The animal models described
above stress the metabolic changes following high sugar intake with
ensuing painful neuropathy (50,51,55).
Also, human studies stress the impact of hyperglycemia,
hyperinsulinemia, and metabolic syndrome on neuropathy (52–54).
As described in several publications, IBS is in most cases
characterized by central and/or peripheral hypersensitivity
(67). This hypersensitivity may
explain the experience of pain in the absence of organic changes
(1). Neural function tests are
seldom performed in IBS patients, but dietary trials including
neural function tests are warranted in IBS. The effect of SSRD also
on the experience of extra-intestinal symptoms support the role of
a general hypersensitivity in IBS, possible to reverse by a diet
with reduced content of starch and sucrose (16).
There is a close relation between luminal and
intracellular glucose concentrations, expression of glucose
transporters, and the release of gut hormones (28,34,35).
The endocrine changes observed in IBS may be secondary to a diet
with high contents of sugar (16,18)
and represent markers of disturbed metabolism (18,28)
and does not necessarily mean that the symptoms are related to the
endocrine differences (3,6,11). The
balance between different hormonal systems may be of greater
importance than the effect of single hormones. Further, local
factors due to impaired metabolic control such as oxidative stress,
accumulation of advanced glycation end products, and chronic
low-grade inflammation may be of importance for pain perception and
experience of different symptoms (46–49).
In the SSRD study, there was a correlation both
between carbohydrates, disaccharides, starch, sucrose, and total
sugar with the total burden of GI symptoms (17). Thus, not only fructose intolerance
could explain the improvement after the diet. The GI symptoms may
depend on SI deficiency in some cases and fructose intolerance in
other cases. In the SSRD intervention, some IBS patients with
functional variants did not have any effect of the diet, and some
patients without variants had a prompt effect of the diet (data not
shown). Although a few of the patients with functional variants of
SI did not have any effect of SSRD, this may depend on poor
compliance to the diet, and does not prove that the genetics is of
no importance. The marked effect of SSRD in IBS patients without
any functional variants point to several different mechanisms. An
overloading of glucose has not been described in the literature,
but fructose intolerance could be one possible hypothesis.
In addition to SGLT1, GLUT2, and GLUT5, several
other monosaccharide transporters exist in the GI tract. The
regulation of these transporters is extremely complex, and a number
of more in-depth studies are needed in vitro as well as
in vivo, to understand the interaction between food load,
absorption, and GI symptoms. The overconsumption of
carbohydrate-rich food in the society requires that we learn more
about these mechanisms to improve health and diminish the risk of
metabolic syndrome and GI symptoms (68).
Acknowledgements
Not applicable.
Funding
The study was funded by the Development Foundation
of Region Skåne (grant nos. REGSKANE-818781 and
2018-Projekt0024).
Availability of data and materials
Not applicable.
Authors' contributions
BO has reviewed the literature, written the
manuscript and created the figures. The author has read and
approved the final manuscript. Data sharing is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
Lacy BE, Mearin F, Chang L, Chey WD, Lembo
AJ, Simren M and Spiller R: Bowel Disorders. Gastroenterology.
150:P1393–P1407.E5. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Black CJ and Ford AC: Global burden of
irritable bowel syndrome: Trends, predictions and risk factors. Nat
Rev Gastroenterol Hepatol. 17:473–486. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Salhy M, Ostgaard H, Gundersen D,
Hatlebakk JG and Hausken T: The role of diet in the pathogenesis
and management of irritable bowel syndrome (review). Int J Mol Med.
29:723–731. 2012.PubMed/NCBI
|
|
4
|
Besterman HS, Sarson DL, Rambaud JC,
Stewart JS, Guerin S and Bloom SR: Gut hormone responses in the
irritable bowel syndrome. Digestion. 21:219–224. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Semnani S, Roshandel G, Keshtkar A, Najafi
L, Amiriani T, Farajollahi M, Moradi A and Joshaghani H: Serum
leptin levels and irritable bowel syndrome: A new hypothesis. J
Clin Gastroenterol. 43:826–830. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El-Salhy M, Hatlebakk JG, Gilja OH and
Hausken T: Irritable bowel syndrome: Recent developments in
diagnosis, pathophysiology, and treatment. Expert Rev Gastroenterol
Hepatol. 8:435–443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cummings DE and Overduin J:
Gastrointestinal regulation of food intake. J Clin Invest.
117:13–23. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wahren J, Ekberg K, Johansson J,
Henriksson M, Pramanik A, Johansson BL, Rigler R and Jörnvall H:
Role of C-peptide in human physiology. Am J Physiol Metab.
278:E759–E768. 2000.PubMed/NCBI
|
|
9
|
Drucker DJ: The role of gut hormones in
glucose homeostasis. J Clin Invest. 117:24–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al-Suhaimi EA and Shehzad A: Leptin,
resistin and visfatin: The missing link between endocrine metabolic
disorders and immunity. Eur J Med Res. 18:122013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Salhy M, Gilja OH, Gundersen D,
Hatlebakk JG and Hausken T: Interaction between ingested nutrients
and gut endocrine cells in patients with irritable bowel syndrome
(review). Int J Mol Med. 34:363–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McKenzie YA, Bowyer RK, Leach H, Gulia P,
Horobin J, O'Sullivan NA, Pettitt C, Reeves LB, Seamark L, Williams
M, et al: British dietetic association systematic review and
evidence-based practice guidelines for the dietary management of
irritable bowel syndrome in adults (2016 update). J Hum Nutr Diet.
29:549–575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Algera J, Colomier E and Simrén M: The
dietary management of patients with irritable bowel syndrome: A
narrative review of the existing and emerging evidence. Nutrients.
11:21622019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mitchell H, Porter J, Gibson PR, Barrett J
and Garg M: Review article: Implementation of a diet low in FODMAPs
for patients with irritable bowel syndrome-directions for future
research. Aliment Pharmacol Ther. 49:124–139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nilholm C, Larsson E, Roth B, Gustafsson R
and Ohlsson B: Irregular dietary habits with a high intake of
cereals and sweets are associated with more severe gastrointestinal
symptoms in IBS patients. Nutrients. 11:12792019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nilholm C, Roth B and Ohlsson B: A dietary
intervention with reduction of starch and sucrose leads to reduced
gastrointestinal and extra-intestinal symptoms in IBS patients.
Nutrients. 11:16622019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nilholm C, Larsson E, Sonestedt E, Roth B
and Ohlsson B: Assessment of a 4-week starch- and sucrose-reduced
diet and its effects on gastrointestinal symptoms and inflammatory
parameters among patients with irritable bowel syndrome. Nutrients.
13:4162021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saidi K, Nilholm C, Roth B and Ohlsson B:
A carbohydrate-restricted diet for patients with irritable bowel
syndrome lowers serum C-peptide, insulin, and leptin without any
correlation with symptom reduction. Nutr Res. 86:23–36. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rose DR, Chaudet MM and Jones K:
Structural studies of the intestinal α-glucosidases,
maltase-glucoamylase and sucrase-isomaltase. J Pediatr
Gastroenterol Nutr. 66 (Suppl 3):S11–S13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Treem WR: Congenital sucrase-isomaltase
deficiency. J Pediatr Gastroenterol Nutr. 21:1–14. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gudmand-Høyer E, Fenger HJ, Kern-Hansen P
and Madsen PR: Sucrase deficiency in Greenland. Incidence and
genetic aspects. Scand J Gastroentreol. 22:24–28. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Treem WR: Clinical aspects and treatment
of congenital sucrase-isomaltase deficiency. J Pediatr
Gastroenterol Nutr. 55 (Suppl 2):S7–S13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garcia-Etxebarria K, Zheng T, Bonfiglio F,
Bujanda L, Dlugosz A, Lindberg G, Schmidt PT, Karling P, Ohlsson B,
Simren M, et al: Increased prevalence of rare sucrase-isomaltase
pathogenic variants in irritable bowel syndrome patients. Clin
Gastroenterol Hepatol. 16:1673–1676. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Henström M, Diekmann L, Bonfiglio F,
Hadizadeh F, Kuech EM, Von Köckritz-Blickwede M, Thingholm LB,
Zheng T, Assadi G, Dierks C, et al: Functional variants in the
sucrase-isomaltase gene associate with increased risk of irritable
bowel syndrome. Gut. 67:263–270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tundis R, Loizzo MR and Menichini F:
Natural products as alpha-amylase and alpha-glucosidase inhibitors
and their hypoglycaemic potential in the treatment of diabetes: An
update. Mini Rev Med Chem. 10:315–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SB, Calmet FH, Garrido J,
Garcia-Buitrago MT and Moshiree B: Sucrase-isomaltase deficiency as
a potential masquerader in irritable bowel syndrome. Dig Dis Sci.
65:534–540. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gorboulev V, Schürmann A, Vallon V, Kipp
H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard
R, et al: Na(+)-D-glucose cotransporter SGLT1 is pivotal for
intestinal glucose absorption and glucose-dependent incretin
secretion. Diabetes. 61:187–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koepsell H: Glucose transporters in the
small intestine in health and disease. Pflugers Arch.
472:1207–1248. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smith MW, Peacock MA and James PS:
Galactose increases microvillus development in mouse jejunal
enterocytes. Comp Biochem Physiol A Comp Physiol. 100:489–493.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lorenz-Meyer H, Thiel F, Menge H,
Gottesbüren H and Riecken EO: Structural and functional studies on
the transformation of the intestinal mucosa in rats with
experimental diabetes. Res Exp Med (Berl). 170:89–99. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stevenson NR, Ferrigni F, Parnicky K, Day
S and Fierstein JS: Effect of changes in feeding schedule on the
diurnal rhythms and daily activity levels of intestinal brush
border enzymes and transport systems. Biochim Biophys Acta.
406:131–145. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iwashina I, Mochizuki K, Inamochi Y and
Goda T: Clock genes regulate the feeding schedule-dependent diurnal
rhythm changes in hexose transporter gene expressions through the
binding of BMAL1 to the promoter/enhancer and transcribed regions.
J Nutr Biochem. 22:334–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hara E and Saito M: Diurnal change in
digestion and absorption of sucrose in vivo in rats. J Nutr Sci
Vitaminol (Tokyo). 35:667–671. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miyamoto K, Hase K, Takagi T, Fujii T,
Taketani Y, Minami H, Oka T and Nakabou Y: Differential responses
of intestinal glucose transporter mRNA transcripts to levels of
dietary sugars. Biochem J. 295:211–215. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Margolskee RF, Dyer J, Kokrashvili Z,
Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B
and Shirazi-Beechey SP: T1R3 and gustducin in gut sense sugars to
regulate expression of Na+-glucose cotransporter 1. Proc
Natl Acad Sci USA. 104:15075–15080. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pereira DF, Cazarolli LH, Lavado C,
Mengatto V, Figueiredo MS, Guedes A, Pizzolatti MG and Silva FR:
Effects of flavonoids on α-glucosidase activity: Potential targets
for glucose homeostasis. Nutrition. 27:1161–1167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lindquist B and Meeuwisse GW: Chronic
diarrhoea caused by monosaccharide malabsorption. Acta Paediatr.
51:674–685. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang J, Cortina G, Wu SV, Tran R, Cho JH,
Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, et al: Mutant
neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med.
355:270–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Santer R, Schneppenheim R, Suter D, Schaub
J and Steinmann B: Fanconi-Bickel syndrome-the original patient and
his natural history, historical steps leading to the primary
defect, and a review of the literature. Eur J Pediatr. 157:783–797.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsan L, Décarie-Spain L, Noble EE and
Kanoski SE: Western diet consumption during development: setting
the stage for neurocognitive dysfunction. Front Neurosci.
15:6323122021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Douard V and Ferraris RP: Regulation of
the fructose transporter GLUT5 in health and disease. Am J Physiol
Endocrinol Metab. 295:E227–E237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Skoog SM and Bharucha AE: Dietary fructose
and gastrointestinal symptoms: A review. Am J Gastroenterol.
99:2046–2050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wasserman D, Hoekstra JH, Tolia V, Taylor
CJ, Kirschner BS, Takeda J, Bell GI, Taub R and Rand EB: Molecular
analysis of the fructose transporter gene (GLUT5) in isolated
fructose malabsorption. J Clin Invest. 98:2398–2402. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu LC, Turner JR and Buret AG: LPS/CD14
activation triggers SGLT-1-mediated glucose uptake and cell rescue
in intestinal epithelial cells via early apoptotic signals upstream
of caspase-3. Exp Cell Res. 312:3276–3286. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ikari A, Nagatani Y, Tsukimoto M, Harada
H, Miwa M and Takagi K: Sodium-dependent glucose transporter
reduces peroxynitrite and cell injury caused by cisplatin in renal
tubular epithelial cells. Biochim Biophys Acta. 1717:109–117. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vincent AM, Hayes JM, McLean LL,
Vivekanandan-Giri A, Pennathur S and Feldman EL:
Dyslipidemia-induced neuropathy in mice: The role of oxLDL/LOX-1.
Diabetes. 58:2376–2385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim B and Feldman EL: Insulin resistance
in the nervous system. Trends Endocrinol Metabol. 23:133–141. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Callaghan B and Feldman E: The metabolic
syndrome and neuropathy: Therapeutic challenges and opportunities.
Ann Neurol. 74:397–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Preguiça I, Alves A, Nunes S, Gomes P,
Fernandes R, Viana SD and Reis F: Diet-induced rodent models of
diabetic peripheral neuropathy, retinopathy and nephropathy.
Nutrients. 12:2502020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pranprawit A, Wolber FM, Heyes JA, Molan
AL and Kruger MC: Short-term and long-term effects of excessive
consumption of saturated fats and/or sucrose on metabolic variables
in sprague dawley rats: A pilot study. J Sci Food Agric.
93:3191–3197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xie F, Fu H, Hou JF, Jiao K, Costigan M
and Chen J: High energy diets-induced metabolic and prediabetic
painful polyneuropathy in rats. PLoS One. 8:e574272013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Smith AG, Rose K and Singleton JR:
Idiopathic neuropathy patients are at high risk for metabolic
syndrome. J Neurol Sci. 273:25–28. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Calcutt NA, Cooper ME, Kern TS and Schmidt
AM: Therapies for hyperglycaemia-induced diabetic complications:
From animal models to clinical trials. Nat Rev Drug Discov.
8:417–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Smith AG: Impaired glucose tolerance and
metabolic syndrome in idiopathic neuropathy. J Peripher Nerv Syst.
17 (Suppl 2):S15–S21. 2012. View Article : Google Scholar
|
|
55
|
Hossain MJ, Kendig MD, Wild BM, Issar T,
Krishnan AV, Morris MJ and Arnold R: Evidence of altered peripheral
nerve function in a rodent model of diet-induced prediabetes.
Biomedicines. 8:3132020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bostock H, Cirkurel K and Burke D:
Threshold tracking techniques in the study of human peripheral
nerve. Muscle Nerve. 21:137–158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lin YC, Lin CS, Chang TS, Lee JE, Tani J,
Chen HJ and Sung JY: Early sensory neurophysiological changes in
prediabetes. J Diabetes Investig. 11:458–465. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sung JY, Tani J, Chang TS and Lin CS:
Uncovering sensory axonal dysfunction in asymptomatic type 2
diabetic neuropathy. PLoS One. 12:e1712232017. View Article : Google Scholar
|
|
59
|
Callaghan BC, Cheng HT, Stables CL, Smith
SL and Feldman EL: Diabetic neuropathy: Clinical manifestations and
current treatments. Lancet Neurol. 11:521–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ramne S, Drake I, Ericson U, Nilsson J,
Orho-Melander M, Engström G and Sonestedt E: Identification of
inflammatory and disease-associated plasma proteins that associate
with intake of added sugar and sugar-sweetened beverages and their
role in type 2 diabetes risk. Nutrients. 12:31292020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Darwiche G, Höglund P, Roth B, Larsson E,
Sjöberg T, Wohlfart B, Steen S and Ohlsson B: An okinawan-based
nordic diet improves anthropometry, metabolic control, and
health-related quality of life in scandinavian patients with type 2
diabetes: A pilot trial. Food Nutr Res. 60:325942016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Della Corte KW, Perrar I, Penczynski KJ,
Schwingshackl L, Herder C and Buyken AE: Effect of dietary sugar
intake on biomarkers of subclinical inflammation: A systematic
review and meta-analysis of intervention studies. Nutrients.
10:6062018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Eriksson EM, Andrén KI, Eriksson HT and
Kurlberg GK: Irritable bowel syndrome subtypes differ in body
awareness, psychological symptoms and biochemical stress markers.
World J Gastroenterol. 14:4889–4896. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mazur M, Furgała A, Jabłoński K, Mach T
and Thor P: Autonomic nervous system activity in
constipation-predominant irritable bowel syndrome patients. Med Sci
Monit. 18:CR493–CR499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gulcan E, Taser F, Toker A, Korkmaz U and
Alcelik A: Increased frequency of prediabetes in patients with
irritable bowel syndrome. Am J Med Sci. 338:116–119. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Roberts CK, Hevener AL and Barnard RJ:
Metabolic syndrome and insulin resistance: Underlying causes and
modification by exercise training. Compr Physiol. 3:1–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Camilleri M and Boeckxstaens G: Dietary
and pharmacological treatment of abdominal pain in IBS. Gut.
66:966–974. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Grundy SM, Cleeman JI, Daniels SR, Donato
KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith
SC Jr, et al: Diagnosis and management of the metabolic syndrome:
An American heart association/national heart, lung, and blood
institute scientific statement. Circulation. 112:2735–2752. 2005.
View Article : Google Scholar : PubMed/NCBI
|