Introduction
Vascular calcification refers to the abnormal
ectopic calcification resulting from the deposition of calcium or
phosphates in soft tissues (1).
Vascular calcification has been identified as a major risk factor
for cardiovascular disease (2).
Cardiovascular disease is responsible for >50% of the total
number of mortalities of patients with chronic kidney disease (CKD)
(3). Therefore, the high incidence,
rapid progression and irreversibility of vascular smooth muscle
cell (VSMC) calcification has attracted attention.
The mechanism of vascular calcification is similar
to that of bone and cartilage ossification (4). Possible mechanisms leading to vascular
calcification in patients with CKD may involve VSMC injury, VSMC
phenotypic transition from the original myoblast to an
osteoblast/chondrocyte-like phenotype and the inhibition of
vascular calcification accompanied by an increase of
pro-calcification factors (5). The
central event of vascular calcification is the phenotypic
transition of VSMCs, from the original myoblast type to the
osteogenesis/chondrocyte-like phenotype, followed by ectopic
osteogenesis (6). Phosphate has an
important role in this process by increasing the expression of
Runt-related transcription factor 2 (Runx2) and other proteins,
such as alkaline phosphatase (ALP) and bone morphogenic proteins
(7–9).
Intermedin (IMD), also known as adrenomedullon 2,
belongs to the calcitonin gene-related peptide family. It is a
cardiovascular polypeptide and brain-gut peptide composed of 47
amino acids (10,11). IMD mRNA is expressed in various
tissues, including the kidney, pituitary gland, hypothalamus, and
stomach (12). In total, three
isoforms have been identified (IMD1-47, IMD8-47 and IMD1-53) that
are cleaved from the prepropeptide and have different biological
activities (10,13). For example, IMD1-53 attenuates VSMC
calcification (14,15), however, the mechanism has not been
elucidated. In addition, the administration of IMD1-47 leads to
vasodilation and marked hypotension through calcitonin-related
receptor complexes, and increases coronary blood flow and cardiac
function by releasing NO (16).
However, the effects of IMD1-47 on the calcification of rat
cardiovascular VSMCs, to the best of our knowledge, has not been
studied previously. Therefore, in the present study, the effect of
IMD1-47, an important isoform of IMD, was investigated on the
calcification of rat cardiovascular VSMCs induced by exposure to
high phosphate (HP). Furthermore, the mechanism of action was
examined by investigating the involvement of the Wnt/β-catenin
pathway.
Materials and methods
VSMC culture and HP treatment
Cardiovascular VSMCs were isolated from the thoracic
aorta of Sprague Dawley rats (7–8 weeks old, 220–240 g) using a
previously described method (17,18). A
total of 6 male rats were used. All of the rats were housed in the
animal center of Shanghai Fourth Rehabilitation Hospital at
23–25°C, relative humidity (50–70%) conditions and 12 h light/12 h
dark cycle, and allowed to free access to food and water. The
thoracic aorta was dissected into roughly 5 mm2
sections, and digested with collagenase (3 mg/ml) and elastase (1
mg/ml) for 4 h at 37°C in DMEM (Gibco; Thermo Fisher Scientific,
Inc.). Staining of α-smooth muscle actin was used to confirm the
purity of the VSMCs (data not shown), as described by Wang et
al (19). The VSMCs were then
cultured in DMEM containing 10% FBS (Beyotime Institute of
Biotechnology) and 1% penicillin/streptomycin. All cells were
cultured for 5–7 passages before use in experiments. When cells
reached a confluency of 80–90%, the cells were treated with fresh
DMEM medium with or without 10 mmol/l β-sodium glycerophosphate
(Cayman Chemical Company) for 8 days. Alizarin red staining was
used to confirm the efficiency of HP to induce VSMC calcification
(data not shown). The optical density value of the alizarin red
exacted from cells was measured with a spectrophotometery at 570 nm
to determine the calcification. The detailed method was described
by Zhu et al (20).
Experimental design
To investigate the effects of IMD1-47 on HP-induced
calcification in VSMCs, VSMCs were randomly assigned into six
groups: Control, HP alone (hereafter HP group); HP with IMD1-47
treatment (0.1, 0.5 or 1 nM IMD1-47; hereafter HP+IMD1-47 groups);
and IMD1-47 treatment alone (hereafter IMD1-47 group). Similar
concentrations of IMD was used as reported in a previous study
(14). IMD1-47 was purchased from
Phoenix Europe GmbH. Cells in the control group were incubated with
complete DMEM/10% FBS media, while cells in the HP group were
incubated with DMEM supplemented with 10 mmol/l β-sodium
glycerophosphate and 10% FBS for 8 days. The three HP+IMD1-47
groups were incubated with 0.1 (low dose), 0.5 (medium dose) or 1
(high dose) nM IMD1-47 in HP medium for 8 days. The IMD1-47 group
was incubated with 0.5 nM IMD1-47 in DMEM with 10% FBS.
To confirm the efficiency of the β-catenin small
interfering (si)RNA, the cells were divided into three groups:
Control, scrambled siRNA and β-catenin siRNA. The expression of
active β-catenin was determined using western blotting. The
β-catenin siRNA sequence was 5′-CAGGGGGUUGUGGUUAAGCUCUU-3′ and the
scramble siRNA sequence was 5′-TTCTCCGAACGTGTCACGT-3′. Then, to
investigate the effect of β-catenin silencing on VSMC
calcification, the cells were divided into five groups: Control,
HP, IMD1-47, scrambled siRNA and β-catenin siRNA. For siRNA
transfection, 60 pmol siRNA was transfected into VSMCs with
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). 6 h later, the culture medium was replaced with fresh DMEM
with 10% FBS. After 48 h, the VSMCs in the control group received
no treatment, and the VSMCs in the other groups received HP
treatment for 8 days; the VSMCs in the IMD1-47, scrambled siRNA and
β-catenin siRNA groups were also treated with 0.5 nM IMD1-47 for 8
days.
Determination of cellular calcium
content using fluorescence
VSMCs (2.5×104 cells/cm2) were
cultured on coverslips and received HP or IMD1-47 treatments for 8
days. At the time of examination, after 8 days of treatment, the
VSMCs were placed in PBS with 10 µmol/l Fluo-3 AM (Sigma-Aldrich;
Merck KGaA) in a dark room at 37°C for 40 min. The stained cells
were then visualized using laser scanning confocal microscopy
(magnification, ×200) and the cellular calcium content was
quantitatively analyzed using Leica LAS AF Lite software v2.6.3
(Leica Microsystems GmbH). The change in the relative fluorescence
intensity of Fluo-3 indicated the change in cellular calcium
content.
Detection of cellular calcium content
using colorimetry
To detect cellular calcium content using a
colorimetric method, VSMCs (in 6-well plates) were washed twice
with PBS and then incubated with 0.6 M HCl at 37°C for 24 h for
decalcification. The supernatant was discarded and the cells were
washed with PBS three times. The cells were then incubated with 0.1
M NaOH and 0.1% SDS for 30 min to lyse the cells. A Calcium
Quantitative Detection kit (Roche Diagnostics) was used to
determine the cellular calcium content according to the
manufacturer's protocol and the bicinchoninic acid method was used
to determine the cellular protein concentration for normalization.
The final calcium content was calculated as µg/mg protein.
Measurement of IMD levels in the cell
culture medium
To examine the relationships between HP and IMD, the
levels of IMD in the cell culture medium was measured after cells
were treated with HP using the radioimmunoassay method. Briefly,
after cells were treated with normal culture media or the cell
culture media supplemented with 10 mmol/l β-sodium glycerophosphate
for 8 days, the cell culture media was collected and centrifuged at
1,600 × g for 15 min at 4°C. The supernatant was loaded onto a
Sep-Pak C18 cartridge (Waters Corporation) equilibrated with 0.5
mmol/l acetic acid. After elution with 50% CH3CN
containing 0.1% trifluoroacetic acid, the sample was lyophilized
and the residue was dissolved in radioimmunoassay buffer, and
analyzed using an IMD radioimmunoassay kit (Phoenix
Pharmaceuticals, Inc.), according to the manufacturer protocol.
Reverse transcription-quantitative
(RT-q) PCR assay for osteoprotegerin (OPG), Runx2, osteopontin
(OPN) and β-catenin
The mRNA levels of OPG, Runx2, OPN and β-catenin
were determined by RT-qPCR. After cells were treated with HP or
IMD1-47 as aforementioned, total RNA was isolated using TRIzol
reagent (Thermo Fisher Scientific, Inc.). In total, 2 µg of total
RNA was reverse transcribed using the Verso™ cDNA kit (ABgene;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. qPCR was performed using SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd.) in a Thermal Cycler Dice (Takara
Biotechnology Co., Ltd.), following the manufacturer's
instructions. The qPCR reactions were conducted using the following
reaction conditions: 95°C for 15 sec, followed by 35 cycles of 95°C
for 5 sec and 60°C for 30 sec. The primer sequences are listed in
Table I. Each experiment was
repeated three times. Relative mRNA expression levels of OPG,
Runx2, OPN and β-catenin were normalized to GAPDH as an internal
control and calculated using the 2−ΔΔCq method (21).
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Sense primer
(5′-3′) | Antisense primer
(5′-3′) |
|---|
| OPG |
ATCATTGAATGGACAACCCAGG |
TGCGTGGCTTCTCTGTTTCC |
| Runx2 |
CCGTCCATCCACTCTACCAC |
ATGAAATGCTTGGGAACTGC |
| OPN |
GTTGGTGGAGGATGTCTG |
TACTTGGAAGGGTCTGTG |
| β-catenin |
GCCAAGTGGGTGGTATAGAG |
CTGGGTATCCTGATGTGC |
| GAPDH |
AACGGATTTGGTCGTATTG |
GGAAGATGGTGATGGGATT |
Determination of ALP activity
To determine the activity of ALP, after VSMCs were
treated with HP or IMD1-47 for 8 days (in 6-well plates), they were
rinsed with PBS three times, and then 500 µl of 0.1% Triton X-100
was added to each well and incubated at 4°C overnight. The cells
were then disrupted by repeated shaking and centrifuged at 18,000 ×
g for 3 min at 4°C to collect the supernatant. Finally, the ALP
activity in the supernatant was measured following a
chemiluminescence method, which was described previously (22).
Western blotting
First of all, the proteins were extracted from VSMCs
using lysis buffer (Beyotime Institue of Biotechnology) containing
50 mM HEPES, 150 mM NaCl, 10% (vol/vol) glycerol, 1 mM EDTA (pH
8.0), 1% (vol/vol) NP-40, 1 mM DTT, 1 mM PMSF, and protease and
phosphatase inhibitors (Beyotime Institue of Biotechnology, pH
7.4). The protein concentration was determined using a BCA kit
(Beyotime Institue of Biotechnology). To measure protein expression
by western blotting, 25 µg of total cellular protein was separated
using 10% polyacrylamide gels. The proteins were then transferred
to polyvinylidene fluoride membranes and blocked in TBS/0.1%
Tween-20 (TBST) containing 5% milk at room temperature for 1 h.
Membranes were then rinsed with TBST and shaken for 5–10 min. The
membranes were incubated with the following primary antibodies
overnight at 4°C: OPG (0.2 µg/ml; cat. no. O1139; Sigma-Aldrich;
Merck KGaA), Runx2 (5 µg/ml; cat. no. SAB1412247; Sigma-Aldrich;
Merck KGaA), OPN (1:1,000; cat. no. SAB1306579; Sigma-Aldrich;
Merck KGaA), β-actin (1 µg/ml; cat. no. A1978; Sigma-Aldrich; Merck
KGaA), Wnt1 (1:1,000; cat. no. SAB2102711; Sigma-Aldrich; Merck
KGaA), Wnt3a (1 µg/ml; cat. no. SAB1400757; Sigma-Aldrich; Merck
KGaA) and active-β-catenin (1:1,000; cat. no. 05-665;
Sigma-Aldrich; Merck KGaA). The following day, the membranes were
rinsed with TBST three times and incubated with goat anti-rat IgG
antibody, HRP conjugate (cat. no. AP136P, 1:5,000, Sigma-Aldrich;
Merck KGaA) for 2 h at 25°C. The membranes were rinsed three times
in TBST. Protein bands were visualized using an
Electro-Chemi-Luminescence Substrate kit (Rahn AG) and quantified
using the Quantity One software 4.6.2 (Bio-Rad Laboratories,
Inc.).
Statistical analysis
SPSS 13.0 statistical software for Windows (SPSS,
Inc.) was used to analyze all the data using one-way ANOVA followed
by the Student-Newman-Keuls post hoc test. Data are expressed as
the mean ± SEM. P<0.05 was considered to indicate a
statistically significant difference. Experiments were repeated
three times.
Results
Effects of HP and IMD1-47 on VSMC
calcification
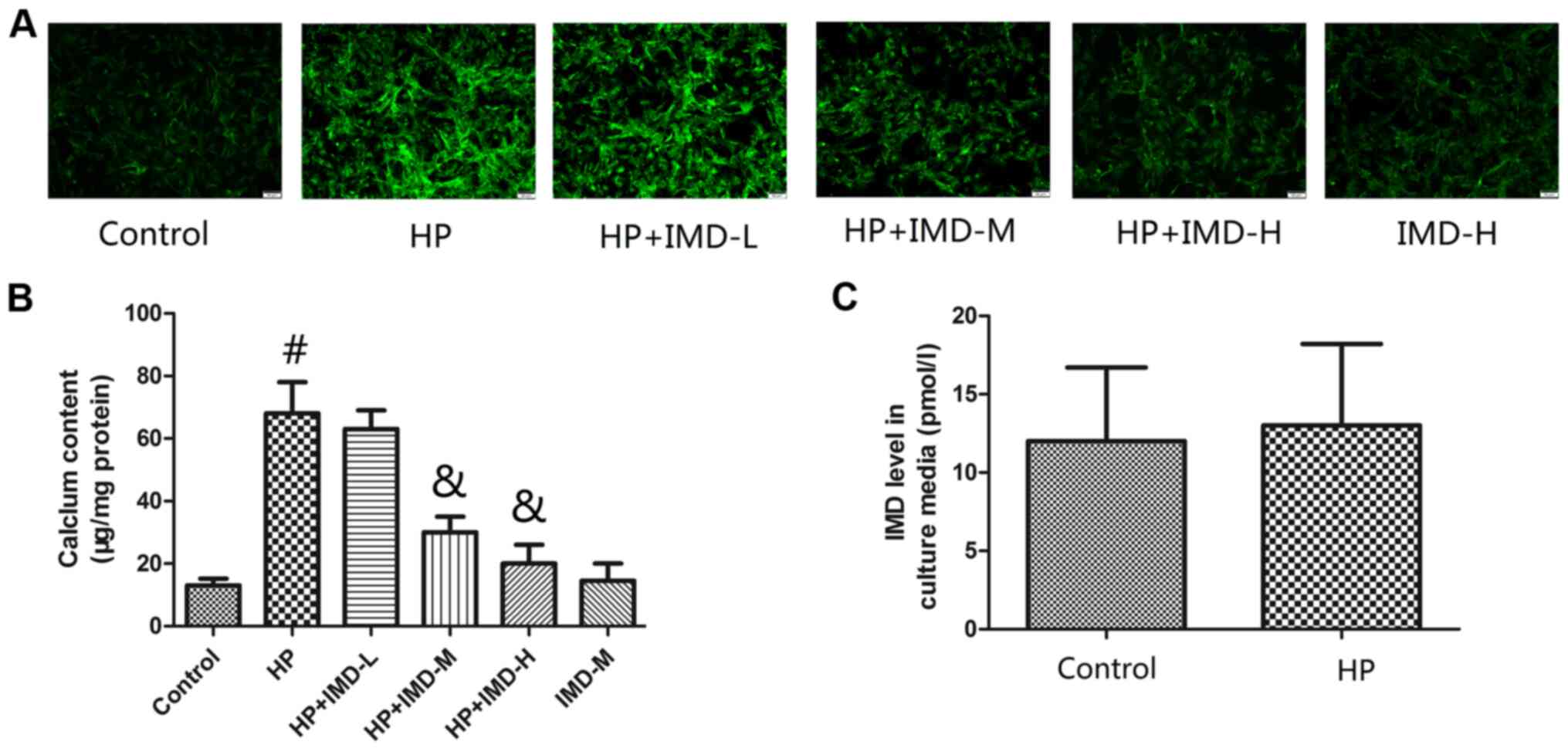
The cellular calcium content in VSMCs was determined
using fluorescence (Fig. 1A) and
colorimetric (Fig. 1B) methods. HP
treatment significantly increased the cellular calcium content of
VSMCs compared with untreated control group (P<0.05). Treatment
with the low dose of IMD1-47 did not significantly change the
cellular calcium content, however, exposure to the medium and high
IMD1-47 doses significantly decreased the cellular calcium content
of VSMCs compared with the HP alone group (P<0.05). Treatment
with the medium IMD1-47 dose alone did not significantly change the
cellular calcium content of the VSMCs, compared with the untreated
control group. Of note, treatment of VSMCs with HP alone did not
affect the endogenous levels of IMD in the cell culture medium
compared with the control group (P>0.05).
Effects of HP and IMD1-47 on
osteogenic proteins and mRNAs in VSMCs
The expression levels of osteogenic proteins OPG,
Runx2 and OPN were determined using western blotting.
Representative western blotting results are shown in Fig. 2A and quantification of the fold
increases in OPG, Runx2 and OPN protein expression levels compared
with the control group are shown in Fig. 2B-D. The relative mRNA expression
levels of OPG, Runx2 and OPN are shown in Fig. 2E. HP significantly enhanced the
protein and mRNA expression levels of OPG, Runx2 and OPN compared
with the control (P<0.05). Although the low IMD1-47 dose had no
significant effect on the expression of either of these factors,
the medium and high doses of IMD1-47 significantly decreased the
protein and mRNA expression levels of OPG, Runx2 and OPN compared
with the HP alone group (P<0.05). Treatment with the medium
IMD1-47 dose alone (without HP) did not significantly change the
protein or mRNA expression levels of OPG, Runx2 or OPN compared
with the control (P>0.05).
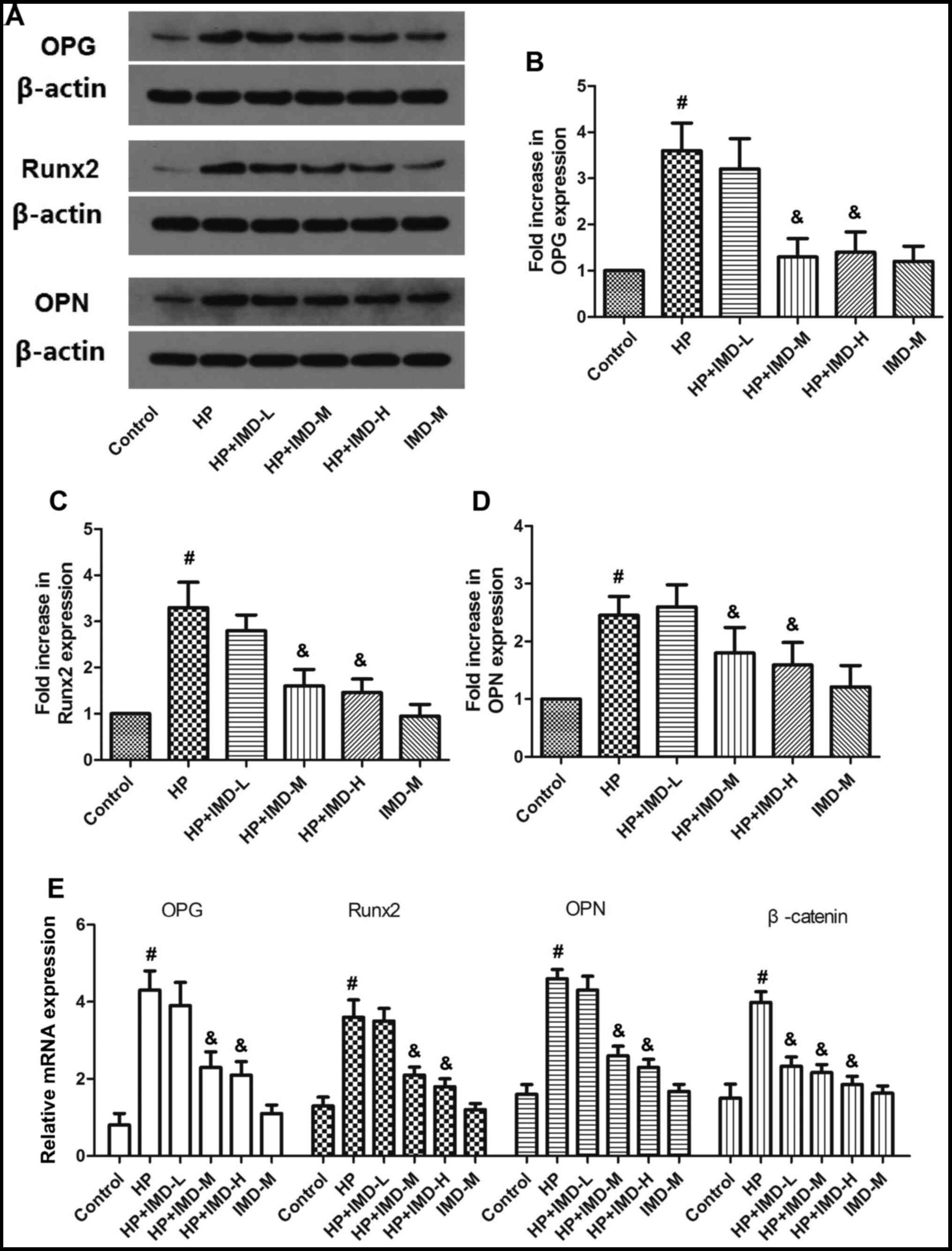 | Figure 2.Effects of HP and IMD on osteogenic
protein and mRNA expression in vascular smooth muscle cells. (A)
Representative images from western blot analysis for the protein
expression levels of osteogenic proteins. (B) Quantification of
western blot results for OPG, (C) Runx2 and (D) OPN. (E) Relative
mRNA expression levels of OPG, Runx2 and OPN were detected by
reverse transcription-quantitative PCR. Data are expressed as the
mean ± SEM. n=8. #P<0.05 vs. control;
&P<0.05 vs. HP. HP, high phosphate; IMD,
intermedin1-47; L, low dose; M, medium c dose; H, high dose; Runx2,
Runt-related transcription factor 2; OPG, osteoprotegerin; OPN,
osteopontin. |
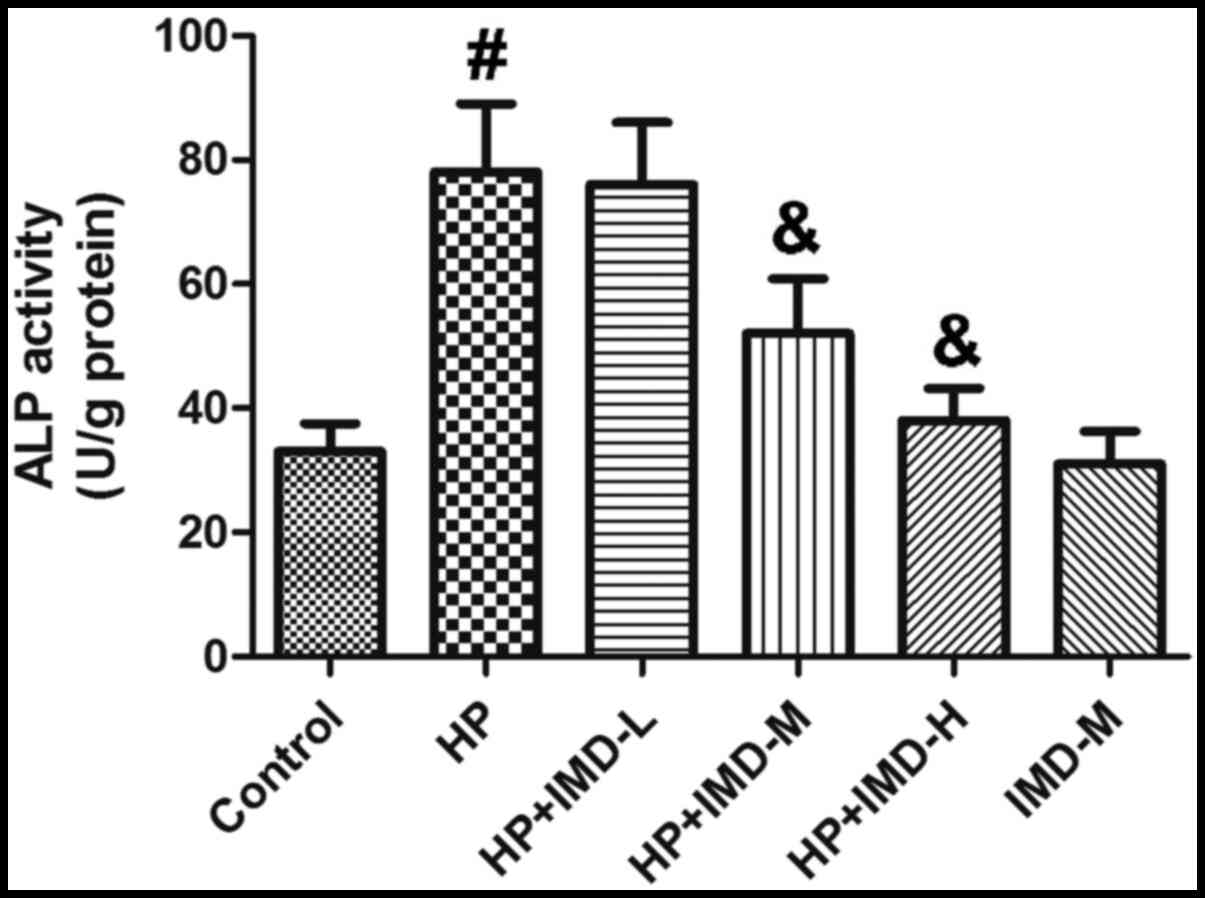
Effects of HP and IMD1-47 on ALP
activity in VSMCs
The effects of HP and IMD1-47 on ALP activity are
shown in Fig. 3. HP treatment
significantly increased ALP activity compared with the control
(P<0.05). The low dose of IMD1-47 had no significant effect on
ALP activity, however, the medium and high IMD1-47 doses
significantly decreased ALP activity compared with the HP alone
group (P<0.05). Treatment with the medium IMD1-47 dose (without
HP) did not significantly change the ALP activity compared with the
control (P>0.05).
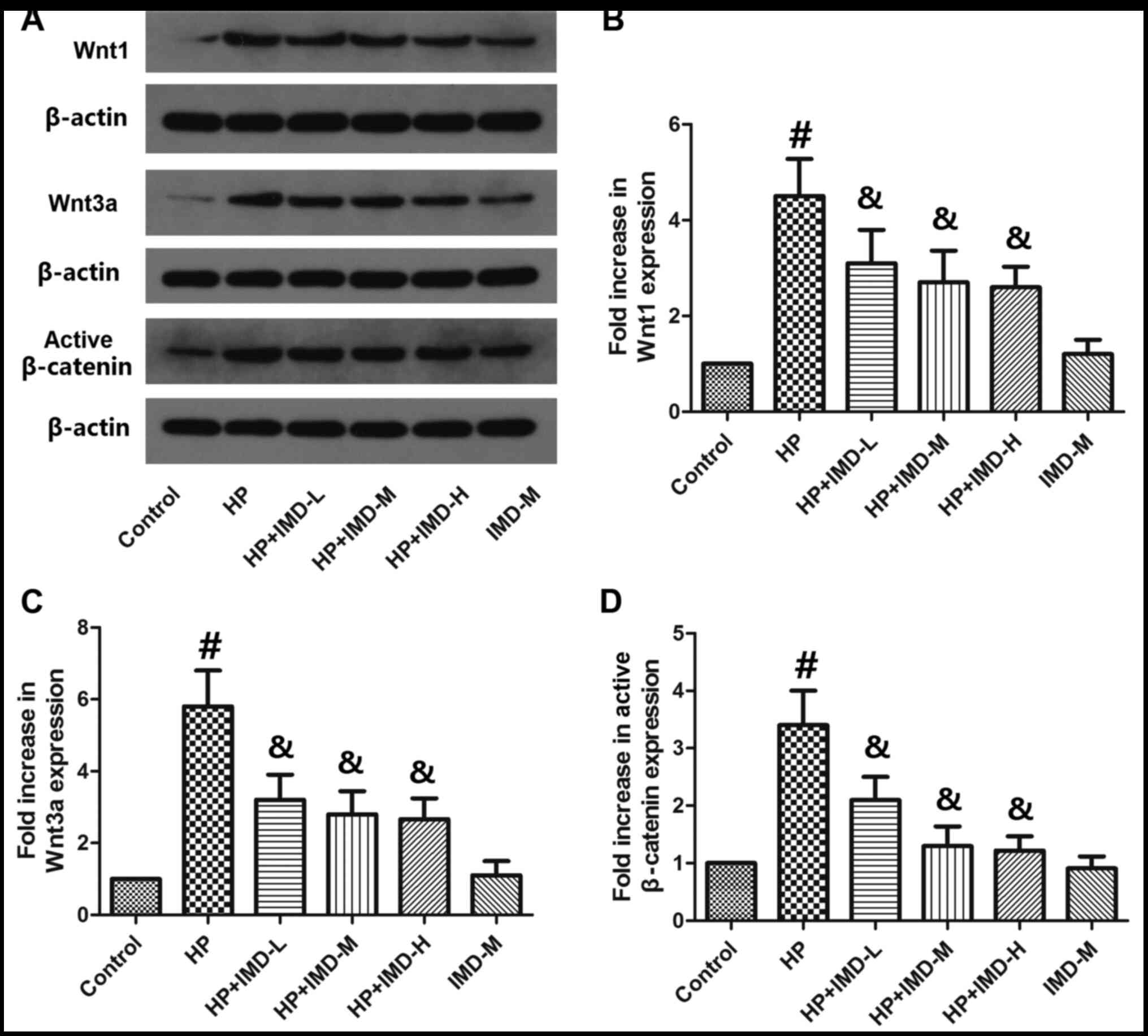
Effects of HP and IMD1-47 on the
Wnt/β-catenin pathway
Wnt1, Wnt3a and active β-catenin protein expression
levels were determined by western blotting (Fig. 4), with the aim to investigate the
mechanism driving the effects of HP and IMD1-47. HP treatment
significantly increased the protein levels of Wnt1 and Wnt3a
(P<0.05; Fig. 4B and C). The low
dosage of IMD1-47 significantly decreased Wnt1 and Wnt3a expression
compared with the HP alone group. Furthermore, HP treatment
significantly enhanced the protein expression levels of active
β-catenin compared with the control (P<0.05; Fig. 4D). All IMD1-47 doses tested
significantly decreased the protein expression levels of active
β-catenin compared with the HP alone group. Treatment with the
medium IMD1-47 dose alone (without HP) did not significantly change
the protein levels of Wnt1, Wnt3a or active β-catenin compared with
the control group (P>0.05).
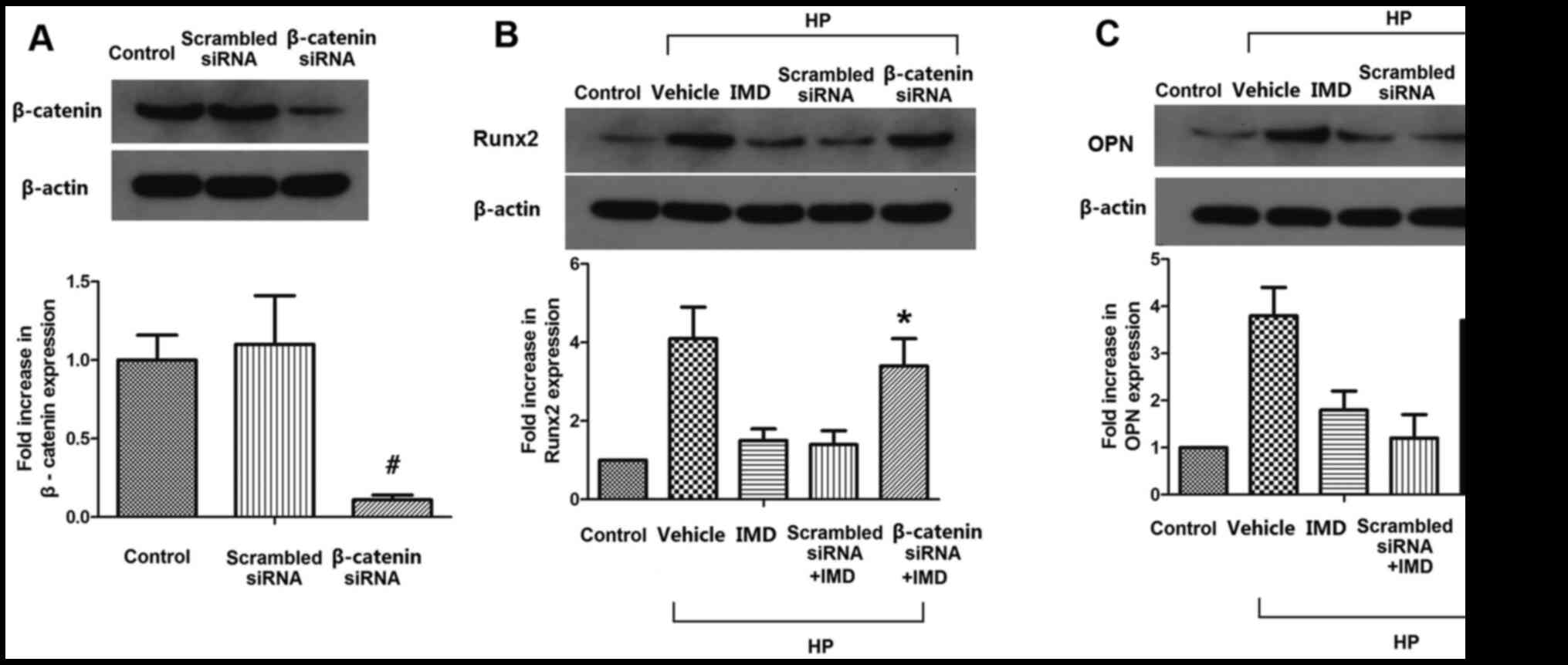
β-catenin silencing abolishes the
effects of IMD1-47 on Runx2 and OPN in VSMCs
The efficiency of the β-catenin siRNA was confirmed
by western blotting. Transfection of the cells with scrambled siRNA
did not significantly alter the protein expression levels of
β-catenin; however, transfection with the β-catenin-specific siRNA
significantly reduced the active β-catenin protein expression
levels (Fig. 5A). Next, the protein
expression levels of the osteogenic proteins Runx2 and OPN were
determined in VSMCs in the control, vehicle, IMD1-47, scrambled
siRNA and β-catenin siRNA treatment groups (Fig. 5B and C). Except for the control
group, VSMCs in all groups received HP treatment, and VSMCs in the
IMD1-47, scrambled siRNA and β-catenin siRNA groups also received
the medium IMD1-47 dose. The results demonstrated that the
scrambled siRNA did not significantly change the levels of Runx2
and OPN compared with IMD1-47 treatment, however, β-catenin siRNA
significantly increased the levels of Runx2 and OPN expression
compared with the IMD1-47 treatment group (P<0.05; Fig. 5B and C).
β-catenin silencing abolishes the
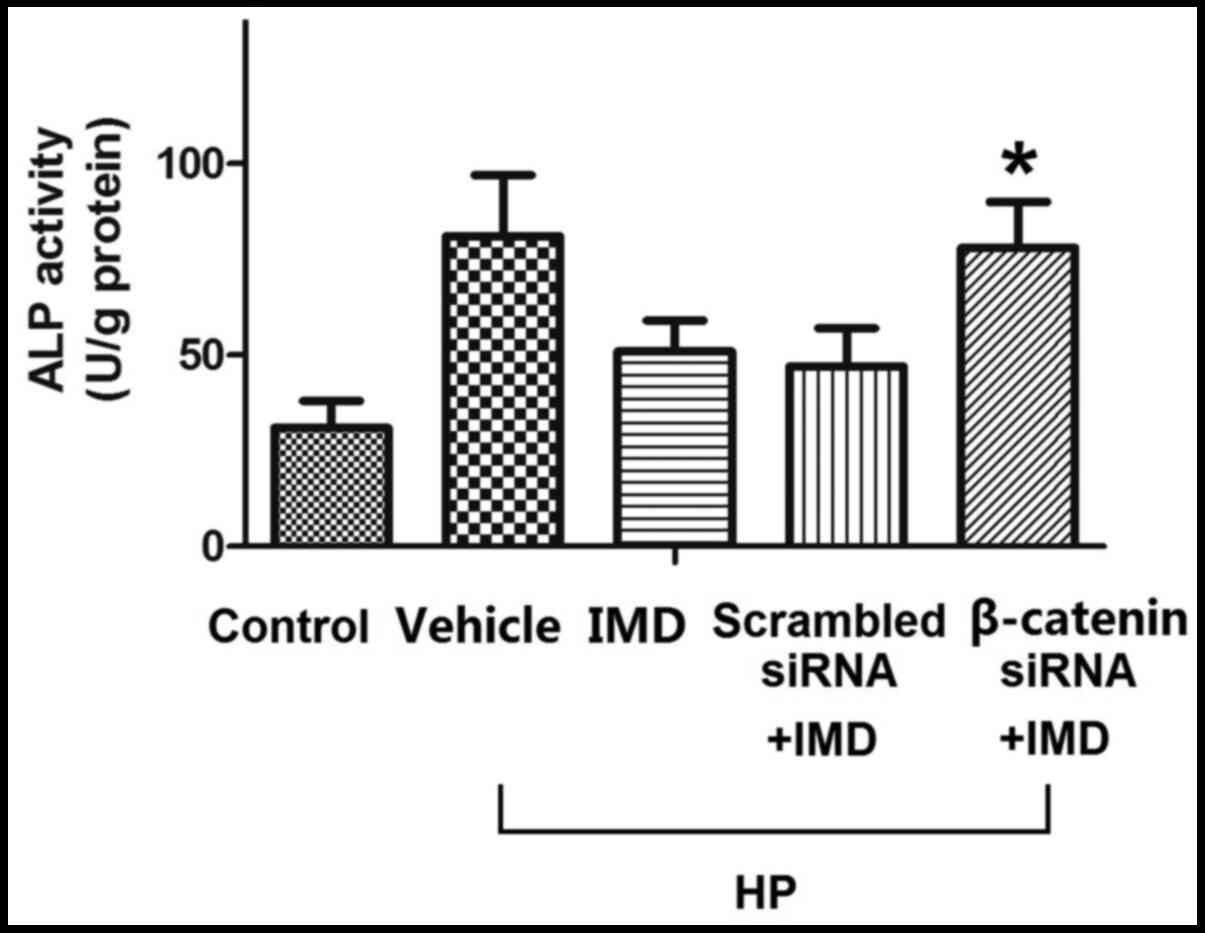
effect of IMD1-47 on ALP activity in VSMCs
ALP activity was measured in VSMCs in the control,
vehicle, IMD1-47, scrambled siRNA and β-catenin siRNA treatment
groups. Transfection with scrambled siRNA had no significant effect
on ALP activity, however, β-catenin siRNA significantly increased
the ALP activity compared with the IMD1-47 treatment group
(P<0.05; Fig. 6).
Discussion
VSMCs are the major cellular components of the
vascular membrane. As the osteogenic-like phenotypic transition of
VSMCs is the structural basis of vascular calcification and a
central event in cardiovascular calcification, VSMCs have been
intensely studied in recent years (18,23).
In the present study, 10 mM β-sodium glycerophosphate was used to
stimulate osteoblast-like differentiation and calcification in rat
VSMCs. These VSMCs were then treated with three different doses of
IMD1-47 and the effects of HP and IMD1-47 on VSMC calcification,
the expression of osteogenic markers (OPG, Runx2 and OPN) and ALP
activity were investigated. HP treatment significantly enhanced the
cellular calcium content of VSMCs, the expression of osteogenic
markers and ALP activity, while IMD1-47 significantly reversed
these effects in a dose-dependent manner. Treatment with IMD1-47
alone did not affect the cellular calcium content of VSMCs, the
expression of osteogenic markers or ALP activity compared with the
control untreated cells. To characterize the underlying mechanism,
the protein expression levels of Wnt1, Wnt3a and active β-catenin
were measured, and the results demonstrated that HP significantly
enhanced the expression of Wnt1, Wnt3a and active β-catenin.
IMD1-47 significantly reversed the effect of HP on the expression
of these factors. Notably, treatment with IMD1-47 alone did not
affect the expression of Wnt1, Wnt3a or active β-catenin. When
VSMCs were transfected with β-catenin siRNA, the protein levels of
Runx2 and OPN were significantly increased compared with the
IMD1-47 group, indicating a role for the Wnt/β-catenin pathway in
the effects of IMD1-47 on the expression of osteogenic markers.
The process of vascular calcification is similar to
that of bone and cartilage formation (24). Mineralized vesicles, containing
hydroxyapatite and bone matrix proteins, including OPG and OPN, are
associated with the mineralization of bone and cartilage (25). The phenotype of VSMCs in the blood
vessel changes significantly during the vascular calcification
process (23). When calcification
occurs, VSMCs migrate and actively proliferate, producing a large
amount of extracellular matrix (23). Furthermore, some
atherosclerosis-related factors confer osteoblast-like
characteristics on VSMCs (26,27).
Runx2, a key transcription factor in vascular calcification, is
also expressed in mineralized VSMCs (28). These previous studies have shown
that the osteoblast-like phenotypic transition of VSMCs is closely
related to vascular calcification. The results of the present study
demonstrated that HP treatment significantly increased the cellular
calcium content of VSMCs, indicating that HP effectively induced
VSMC calcification. Both medium and high IMD1-47 doses
significantly decreased the cellular calcium content of VSMCs,
suggesting an effect of IMD1-47 on VSMC calcification. It is also
possible that HP may have affected the endogenous levels of IMD1-47
in VSMCs, thereby inducing VSMC calcification. To explore this
hypothesis, the IMD levels in the cell culture media were measured
after treatment of the VSMCs with HP. The results showed that the
levels of IMD were not affected by HP treatment, indicating that HP
treatment alone did not regulate the endogenous expression of
IMD.
Changes in the expression levels of the osteogenic
markers OPG, Runx2 and OPN were assessed in VSMCs. HP treatment
significantly enhanced the protein and RNA expression levels of
OPG, Runx2 and OPN compared with untreated control cells. Both
medium and high IMD1-47 doses inhibited the HP-induced increases in
the expression of OPG, Runx2 and OPN. Runx2 is a specific factor in
the phenotypic transition of VSMC to an osteoblast-like cell
phenotype and is a member of the core binding factor family
(29). It was reported that a
deficiency in Runx2 significantly decreased the activation of NF-κB
and the formation of osteoclast-like cells (30). Runx2 is also expressed in calcified
VSMCs, not only in osteoblasts (31). Runx2 is important for the regulation
of the expression of ALP and is a molecular marker of osteogenic
transition (32). The regulation of
Runx2 by IMD1-47 indicated that IMD1-47 may directly regulate the
core binding factor family and interfere with the osteoblast-like
phenotypic transition of VSMCs.
ALP is an early marker of osteoblast formation and
is secreted into the extracellular matrix during
osteogenic/chondrogenic bone mineralization (33). High concentrations of ALP promote
the accumulation of phosphate, making it insoluble, which results
in phosphate crystallization and mineralization (34). ALP is expressed at low levels in
normal VSMCs, however, it is highly expressed in calcified blood
vessels. Levamisole, a specific ALP inhibitor, has been reported to
inhibit VSMC calcification in a dose-dependent manner (35). In the present study, ALP activity in
VSMCs was significantly enhanced by treatment with HP for 8 days.
With increasing IMD1-47 concentrations, ALP activity was found to
decrease compared with the HP group. However, treatment with
IMD1-47 alone did not affect the ALP activity, indicating that
IMD1-47 effectively inhibited HP-induced osteoblast formation and
mineralization.
The Wnt/β-catenin signaling pathway in VSMCs has an
important role in arterial calcification (36). In β-catenin knock-out mice, the bone
formation process is significantly inhibited, and bone marrow
mesenchymal progenitor cells are prevented from differentiating
into osteoblasts (37).
Furthermore, activation of the Wnt/β-catenin pathway was reported
to promote the expression of bone formation-related factors, such
as Runx2, OPG, osteoblast-specific gene, bone morphogenic protein,
cyclin Dl and matrix metalloproteinase 7 (38). Phenotypically transformed VSMCs
acquire the characteristics of osteoblast-like cells, such as ALP
expression on the plasma membrane, and secretion of type I collagen
and OPN, which are regulated by the Wnt/β-catenin pathway (39). It has also been reported that
Wnt/β-catenin is activated at the site of cardiovascular
calcification (40–42). The treatment of diabetic mice with
the Wnt signaling pathway inhibitor DKK1 inhibited aortic
mineralization and early bone formation-related factor activation
(43). In addition, the degree of
aortic calcification and sclerosis can be reduced by inhibiting the
Wnt/β-catenin signaling pathway. For instance, when the
Wnt/β-catenin pathway was inhibited, the expression of bone
formation-associated factors collagen type 1 α 1 chain, Runx2 and
NADPH oxidase 1 were decreased (42). In arterial calcification caused by
both diabetes and chronic renal failure, the expression of Wnt3a
and Wnt7a in the arterial wall was significantly increased
(43). Similar with previous
studies (40–43), the present study found that HP
significantly increased the protein expression levels of Wnt1,
Wnt3a and active β-catenin, while IMD1-47 significantly decreased
their levels compared with the HP group. When cells were treated
with IMD1-47 and β-catenin siRNA, osteogenic protein levels in
VSMCs were significantly increased compared with the group treated
with IMD1-47 alone. Similarly, β-catenin siRNA significantly
increased the activity of ALP compared with the IMD1-47 treatment
group alone. These results indicated that HP treatment may induce
VSMC calcification through the activation of Wnt1/Wnt3a and
β-catenin. By contrast, IMD1-47 may inhibit VSMC calcification by
suppressing the activation of the Wnt/β-catenin pathway.
In conclusion, the present study indicated that
IMD1-47 inhibited VSMC calcification induced by high levels of
phosphate by suppressing the Wnt/β-catenin signaling pathway. The
present study adds a new dimension to the current understanding of
the biological effects of IMD1-47. The present study revealed the
protective effect and mechanism of IMD1-47 on VSMC calcification,
and provided an experimental basis for its potential use in the
clinic.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ designed the study, conducted some of the
experiments and prepared the manuscript. NT performed the
experiments. JZ collected and analyzed the data, and interpreted
the results. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Welfare
and Ethics Committee of Shanghai XuHui Central Hospital (approval
no. XHCH-2018-032).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kendrick J and Chonchol M: The role of
phosphorus in the development and progression of vascular
calcification. Am J Kidney Dis. 58:826–834. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pun PH, Smarz TR, Honeycutt EF, Shaw LK,
Al-Khatib SM and Middleton JP: Chronic kidney disease is associated
with increased risk of sudden cardiac death among patients with
coronary artery disease. Kidney Int. 76:652–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qunibi WY: Consequences of
hyperphosphatemia in patients with end-stage renal disease (ESRD).
Kidney Int Suppl. 90:S8–S12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moe SM and Chen NX: Pathophysiology of
vascular calcification in chronic kidney disease. Circ Res.
95:560–567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ossareh S: Vascular calcification in
chronic kidney disease: Mechanisms and clinical implications. Iran
J Kidney Dis. 5:285–299. 2011.PubMed/NCBI
|
|
6
|
Neven E, De Schutter TM, De Broe ME and
D'Haese PC: Cell biological and physicochemical aspects of arterial
calcification. Kidney Int. 79:1166–1177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Persy V and D'Haese P: Vascular
calcification and bone disease: The calcification paradox. Trends
Mol Med. 15:405–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gutierrez OM, Mannstadt M, Isakova T,
Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner
H and Wolf M: Fibroblast growth factor 23 and mortality among
patients undergoing hemodialysis. N Engl J Med. 359:584–592. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lomashvili KA, Monier-Faugere MC, Wang X,
Malluche HH and O'Neill WC: Effect of bisphosphonates on vascular
calcification and bone metabolism in experimental renal failure.
Kidney Int. 75:617–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roh J, Chang CL, Bhalla A, Klein C and Hsu
SY: Intermedin is a calcitonin/calcitonin gene-related peptide
family peptide acting through the calcitonin receptor-like
receptor/receptor activity-modifying protein receptor complexes. J
Biol Chem. 279:7264–7274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takei Y, Inoue K, Ogoshi M, Kawahara T,
Bannai H and Miyano S: Identification of novel adrenomedullin in
mammals: A potent cardiovascular and renal regulator. FEBS Lett.
556:53–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong Y, Hay DL, Quirion R and Poyner DR:
The pharmacology of adrenomedullin 2/intermedin. Br J Pharmacol.
166:110–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao L, Peng DQ, Zhang J, Song JQ, Teng X,
Yu YR, Tang CS and Qi YF: Extracellular signal-regulated kinase 1/2
activation is involved in intermedin1-53 attenuating myocardial
oxidative stress injury induced by ischemia/reperfusion. Peptides.
33:329–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang JR, Duan XH, Zhang BH, Teng X, Zhou
YB, Liu Y, Yu YR, Zhu Y, Tang CS and Qi YF: Intermedin1-53
attenuates vascular smooth muscle cell calcification by inhibiting
endoplasmic reticulum stress via cyclic adenosine
monophosphate/protein kinase A pathway. Exp Biol Med (Maywood).
238:1136–1146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang JR, Guo J, Wang Y, Hou YL, Lu WW,
Zhang JS, Yu YR, Xu MJ, Liu XY, Wang XJ, et al: Intermedin1-53
attenuates vascular calcification in rats with chronic kidney
disease by upregulation of α-Klotho. Kidney Int. 89:586–600. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grossini E, Molinari C, Mary DA, Uberti F,
Caimmi PP and Vacca G: Intracoronary intermedin 1–47 augments
cardiac perfusion and function in anesthetized pigs: Role of
calcitonin receptors and beta-adrenoreceptor-mediated nitric oxide
release. J Appl Physiol (1985). 107:1037–1050. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Golovina VA and Blaustein MP: Preparation
of primary cultured mesenteric artery smooth muscle cells for
fluorescent imaging and physiological studies. Nat Protoc.
1:2681–2687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Willems BA, Furmanik M, Caron MM, Chatrou
ML, Kusters DH, Welting TJ, Stock M, Rafael MS, Viegas CS, Simes
DC, et al: Ucma/GRP inhibits phosphate-induced vascular smooth
muscle cell calcification via SMAD-dependent BMP signalling. Sci
Rep. 8:49612018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Li J, Liu J, Xu M, Tong X and Wang
J: Chlorogenic acid prevents isoproterenol-induced DNA damage in
vascular smooth muscle cells. Mol Med Rep. 14:4063–4068. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu D, Mackenzie NC, Millán JL,
Farquharson C and MacRae VE: The appearance and modulation of
osteocyte marker expression during calcification of vascular smooth
muscle cells. PLoS One. 6:e195952011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu H, Zhang W, Qiao Y, Jiang X, Liu X and
Ding C: Antibacterial activity and increased bone marrow stem cell
functions of Zn-incorporated TiO2 coatings on titanium. Acta
Biomater. 8:904–915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leopold JA: Vascular calcification:
Mechanisms of vascular smooth muscle cell calcification. Trends
Cardiovasc Med. 25:267–274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moe SM, O'Neill KD, Duan D, Ahmed S, Chen
NX, Leapman SB, Fineberg N and Kopecky K: Medial artery
calcification in ESRD patients is associated with deposition of
bone matrix proteins. Kidney Int. 61:638–647. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wallin R, Wajih N, Greenwood GT and Sane
DC: Arterial calcification: A review of mechanisms, animal models,
and the prospects for therapy. Med Res Rev. 21:274–301. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakagawa Y, Ikeda K, Akakabe Y, Koide M,
Uraoka M, Yutaka KT, Kurimoto-Nakano R, Takahashi T, Matoba S,
Yamada H, et al: Paracrine osteogenic signals via bone
morphogenetic protein-2 accelerate the atherosclerotic intimal
calcification in vivo. Arterioscler Thromb Vasc Biol. 30:1908–1915.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fakhry M, Roszkowska M, Briolay A,
Bougault C, Guignandon A, Diaz-Hernandez JI, Diaz-Hernandez M,
Pikula S, Buchet R, Hamade E, et al: TNAP stimulates vascular
smooth muscle cell trans-differentiation into chondrocytes through
calcium deposition and BMP-2 activation: Possible implication in
atherosclerotic plaque stability. Biochim Biophys Acta Mol Basis
Dis. 1863:643–653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giachelli CM: Vascular calcification
mechanisms. J Am Soc Nephrol. 15:2959–2964. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Engelse MA, Neele JM, Bronckers AL,
Pannekoek H and de Vries CJ: Vascular calcification: Expression
patterns of the osteoblast-specific gene core binding factor
alpha-1 and the protective factor matrix gla protein in human
atherogenesis. Cardiovasc Res. 52:281–289. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun Y, Byon CH, Yuan K, Chen J, Mao X,
Heath JM, Javed A, Zhang K, Anderson PG and Chen Y: Smooth muscle
cell-specific runx2 deficiency inhibits vascular calcification.
Circ Res. 111:543–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tyson KL, Reynolds JL, McNair R, Zhang Q,
Weissberg PL and Shanahan CM: Osteo/chondrocytic transcription
factors and their target genes exhibit distinct patterns of
expression in human arterial calcification. Arterioscler Thromb
Vasc Biol. 23:489–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Y, Hu Y, Yang L, Zhou J, Tang Y,
Zheng L and Qin P: Runx2 alleviates high glucose-suppressed
osteogenic differentiation via PI3K/AKT/GSK3β/β-catenin pathway.
Cell Biol Int. 41:822–832. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Endres M, Hutmacher DW, Salgado AJ, Kaps
C, Ringe J, Reis RL, Sittinger M, Brandwood A and Schantz JT:
Osteogenic induction of human bone marrow-derived mesenchymal
progenitor cells in novel synthetic polymer-hydrogel matrices.
Tissue Eng. 9:689–702. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoshi K, Ejiri S and Ozawa H:
Localizational alterations of calcium, phosphorus, and
calcification-related organics such as proteoglycans and alkaline
phosphatase during bone calcification. J Bone Miner Res.
16:289–298. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shioi A, Nishizawa Y, Jono S, Koyama H,
Hosoi M and Morii H: Beta-glycerophosphate accelerates
calcification in cultured bovine vascular smooth muscle cells.
Arterioscler Thromb Vasc Biol. 15:2003–2009. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rong S, Zhao X, Jin X, Zhang Z, Chen L,
Zhu Y and Yuan W: Vascular calcification in chronic kidney disease
is induced by bone morphogenetic protein-2 via a mechanism
involving the Wnt/β-catenin pathway. Cell Physiol Biochem.
34:2049–2060. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kramer I, Halleux C, Keller H, Pegurri M,
Gooi JH, Weber PB, Feng JQ, Bonewald LF and Kneissel M: Osteocyte
Wnt/beta-catenin signaling is required for normal bone homeostasis.
Mol Cell Biol. 30:3071–3085. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meng J, Ma X, Wang N, Jia M, Bi L, Wang Y,
Li M, Zhang H, Xue X, Hou Z, et al: Activation of GLP-1 receptor
promotes bone marrow stromal cell osteogenic differentiation
through β-catenin. Stem Cell Reports. 6:579–591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xavier JR, Thakur T, Desai P, Jaiswal MK,
Sears N, Cosgriff-Hernandez E, Kaunas R and Gaharwar AK: Bioactive
nanoengineered hydrogels for bone tissue engineering: A
growth-factor-free approach. ACS Nano. 9:3109–3118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rajamannan NM, Subramaniam M, Caira F,
Stock SR and Spelsberg TC: Atorvastatin inhibits
hypercholesterolemia- induced calcification in the aortic valves
via the Lrp5 receptor pathway. Circulation. 112:5243062005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shao JS, Cheng SL, Pingsterhaus JM,
Charlton-Kachigian N, Loewy AP and Towler DA: Msx2 promotes
cardiovascular calcification by activating paracrine Wnt signals. J
Clin Invest. 115:1210–1220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng SL, Shao JS, Halstead LR,
Distelhorst K, Sierra O and Towler DA: Activation of vascular
smooth muscle parathyroid hormone receptor inhibits
Wnt/beta-catenin signaling and aortic fibrosis in diabetic
arteriosclerosis. Circ Res. 107:271–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J,
Behrmann A, Cheng SL and Towler DA: Aortic Msx2-Wnt calcification
cascade is regulated by TNF-alpha-dependent signals in diabetic
Ldlr-/-mice. Arterioscler Thromb Vasc Biol. 27:2589–2596. 2007.
View Article : Google Scholar : PubMed/NCBI
|