Introduction
Colon carcinoma is a common digestive systemic
sarcoma, with increasing incidence each year. According to the
global cancer statistics in 2018, colon carcinoma has become the
fourth leading cause of tumor-related deaths (1). Despite apparent curative surgery and
chemotherapy, the mortality of colon carcinoma remains high,
leading to poor five-year survival rates (2–5).
Although dietary habits and living environments are improving, the
onset age is shifting towards the younger population (6). Hence, patients succumb to colon
carcinoma due to the rapid progression of this disease and its
frequent recurrent metastasis. However, the pathological mechanism
of colon cancer remains unclear. Thus, it is urgent to discover a
pathogenic target gene that facilitates this disease.
Multiple factors, including oncogenes and tumor
suppressors, are involved in tumorigenesis and metastasis. Among
which, the irregular function of microRNAs (miRs/miRNAs) has been
demonstrated to play an important role in cancer pathogenesis
(7–9). miRNAs are highly conserved non-coding
RNA molecules consisting of 20–24 nucleotides. miRNAs affect mRNA
degradation or expression by completely or incompletely binding to
the 3′-untranslated region (3′-UTR) of target genes. In addition,
various miRNAs have been reported to modulate colon cancer
progression (10–12). For example, miR-487b-3p has been
found to inhibit colon cancer progression through directly
targeting metabotropic glutamate receptor 3 (GRM3), thus indicating
that the miR-487b-3p/GRM3/TGF-β signaling axis is an important
regulator of colon cancer tumorigenesis (13). Meanwhile, miR-524-5p was also
reported to repress angiogenesis in colon cancer cells via
targeting serine/threonine-protein kinase WNK1 (14). Of note, hsa-miR-15a-5p has been
identified to be downregulated in colon cancer (15). However, its biological role and
potential mechanism in colon cancer remains to be elucidated.
The present study aimed to identify the key
functions of has-miR-15a-5p in colon cell carcinoma and
demonstrated that hsa-miR-15a-5p targeted G1/S-specific cyclin-D1
(CCND1) and functioned as a tumor suppressor of colon cancer
progression in vitro, as evidenced by the reduced
proliferation, migration and invasion in colon cancer cells with
overexpressed hsa-miR-15a-5p. The present findings provided a novel
mechanism via which hsa-miR-15a-5p regulated colon cancer
progression, thus hsa-miR-15a-5p could be a promising target for
colon cancer diagnosis and therapy.
Materials and methods
Tissue samples
A total of 30 colon cancer and paired adjacent
normal tissues (>5 cm from cancer tissue) were obtained from
patients (18 male patients and 12 female patients) undergoing
surgery between March 2017 and December 2018 at Jiangsu Cancer
Hospital (Nanjing, China). The patients were aged between 42 and 65
years. Among them, the median age of male patients was 53.4 years
(range, 42–63 years), while the median age of female patients was
51.3 years (range, 45–65 years). The inclusion criteria were as
follows: None of the patients received antitumor therapy, such as
radiotherapy or chemotherapy, before surgery and final diagnosis
was confirmed by routine pathological examination. The exclusion
criteria were as follows: Patients who received pre-operative
radiotherapy or chemotherapy. All specimens were frozen and
conserved in liquid nitrogen at −80°C. Written informed consent was
obtained from all patients before surgery. The present study was
approved by the Ethics Committee of the Affiliated Cancer Hospital
of Nanjing Medical University and Jiangsu Cancer Hospital and
Jiangsu Institute of Cancer Research [(2016) approval no. 231].
Cell culture and transfection
Human colon cancer cell lines, SW480 and HCT116, the
human colorectal cancer cell line, HT29, and human normal colonic
epithelial cells, NCM460, were obtained from the American Type
Culture Collection. Human colon cancer cell lines, SW480 and
HCT116, were derived from patients with colon cancer. The human
colorectal cancer cell line, HT29, was derived from patients with
colorectal adenocarcinoma and HT29 cell line was authenticated by
STR profiling before distribution. All cells were incubated in DMEM
(cat. no. 319-005-CL; Wisent, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 µg/ml streptomycin in a humid atmosphere at 37°C in 5%
CO2. miRNA mimics (hsa-miR-15a-5p), miRNA inhibitors
(hsa-miR-15a-5p), negative control mimics (NC mimics) and miRNA
negative control inhibitors (NC inhibitors) were purchased from
Guangzhou RiboBio Co., Ltd. Among them, non-targeting NC mimics and
NC inhibitors were used as the controls. HT29 cell lines were
seeded on 6-well plates (1×105 cells/well) one day prior
to transfection. The synthetic oligonucleotides or plasmids were
used at 50 nmol/ml for transfection. Transfection was performed
with Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C according to the manufacturer's
instructions. The cells were harvested 24–48 (for miRNA and mRNA
expression) or 48–72 h (for protein expression) after transfection
for functional assays or RNA/protein extraction. The sequences were
as follows: hsa-miR-15a-5p mimics, 5′-UAGCAGAUCCCAAUGGUCGGUG-3′;
hsa-miR-15a-5p inhibitor, 5′-CACAAACCAUUAUGUGCUGCUA-3′; mimic NC,
5′-AAUUCGUAGCUUGCAUGCAAGC-3′ and inhibitor NC,
5′-CAGUACUUUGUGUAGUACAA-3′.
Overexpression of CCND1 in HT29
cells
The CCND1 cDNA sequence was cloned into pcDNA 3.1
vector to upregulate its expression. Plasmids (pcDNA3.1-CCND1 and
pcDNA 3.1) were purchased from OBiO Technology (Shanghai) Corp.,
Ltd. For plasmid transfection, HT29 cells were seeded into 6-well
plates (1×105 cells/well). Cells were cultured to 80%
confluence, and either pcDNA3.1 vector (2.5 µg) or pcDNA3.1-CCND1
(2.5 µg) was transfected using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C according to the
manufacturer's instructions. Cells were cultured in DMEM for 6 h at
37°C. Subsequent experiments were performed 48 h post-transfection.
pcDNA 3.1 vector (NC) was used as a negative control.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Cells were harvested and total RNA was extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA concentration was quantified using a
NanoDrop™ spectrophotometer (NanoDrop Technologies; Thermo Fisher
Scientific, Inc.). RNA (1 µg) was reverse-transcribed at 37°C for
15 min and 85°C for 5 sec using the PrimeScript Reverse
Transcriptase system (Takara Biotechnology Co., Ltd.). The qPCR
amplification was performed using SYBR Green Master Mix (Takara
Biotechnology Co., Ltd.) under the following conditions: Initial
denaturation at 95°C for 10 min; followed by 40 cycles at 94°C for
15 sec, 55°C for 30 sec and 70°C for 30 sec. The 2−DDCq
method was employed to calculate the relative expression levels and
U6 was taken as an endogenous control to normalize miR-15a-5p
expression (16). The following
primers were used for PCR: miR-15a-5p forward (F),
5′-TAGCAGCACATAATGGTTTGTG-3′ and reverse (R),
5′-GAACATGTCTGCGTATCTCAC-3′; CCND1 F, 5′-GGCGGAGGAGAACAAACA-3′ and
R, 5′-ATGCAGGGCGGATTGGAAA-3′; β-actin F, 5′-CCTCGCCTTTGCCGATCC-3′
and R, 5′-GGATCTTCATGAGGTAGTCAGTC-3′; and U6 F,
5′-CTCGCTTCGGCAGCAC-3′ and R, 5′-ACGCTTCACGAATTTGCGT-3′.
Western blot assay
Total protein from HT-29 cells was extracted using a
RIPA kit (Beyotime Institute of Biotechnology) with the addition of
1% proteinase inhibitor. Protein concentrations were detected via
the BCA method (Beyotime Institute of Biotechnology). After 5 min
heating at 95°C, proteins from each sample were separated by 10%
SDS-PAGE (Nanjing KeyGen Biotech Co., Ltd.) using 20 µg per lane,
then transferred onto a 0.22 µm PVDF membrane (Beyotime Institute
of Biotechnology). The proteins were blocked with 5% non-fat milk
at room temperature for 2 h, then protein bands were incubated with
primary antibodies overnight at 4°C. Next, the protein bands were
washed with TBST (TBS buffer with 0.1% Tween 20) three times and
incubated with a horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary IgG (1:5,000; cat. no. ab6721; Abcam) for 1h
at room temperature. The protein bands were washed with TBST an
additional four times and visualized using an ECL kit (Bio-Rad
Laboratories, Inc. and observed using Image Lab software 4.0
(Bio-Rad Laboratories, Inc.). Primary antibodies against CCND1
(cat. no. ab40754, 1:5,000) and β-actin (cat. no. ab8227, 1:5,000)
were purchased from Abcam. β-actin was used as an endogenous
control to normalize CCND1 expression.
5-Ethynyl-2′-deoxyuridine (EdU)
assay
The cellular proliferation rate was measured using
an EdU assay. A total of 1×105 cells/well were plated
into 24-well plates and left to adhere overnight. Following
transfection, the cells were incubated with 100 µl/well EdU
(Nanjing KeyGen Biotech Co., Ltd.) for 2 h, fixed with 4%
paraformaldehyde for 30 min at room temperature and incubated with
DAPI solution to counterstain cell nuclei for 15–30 min at room
temperature. Proliferative cells were determined under a
fluorescence microscope (Leica Microsystems GmbH) at 400×
magnification.
Cell Counting Kit (CCK)-8 assay
A CCK-8 assay was performed to assess cell
viability. HT29 cells were seeded into a 96-well plate at a density
of 1×105 cells/well. The detection was performed at 0,
24, 48 and 72 h. After adding 10 µl CCK-8 reagent (Nanjing
Jiancheng Bioengineering Institute) per well and incubating for 2
h, the absorbance at 450 nm was measured using a Multiscan FC plate
reader and analyzed with SkanIt for Multiscan FC 3.1 software
(Thermo Fisher Scientific, Inc.).
Wound scratch assay
The HT29 cells (1×105 cells/well)
cultured in a 6-well plate were grown to form a monolayer.
Meanwhile, in order to inhibit cell proliferation, the monolayer of
cells was treated with 5 µM mitomycin-C (Sigma-Aldrich; Merck KGaA)
for 2 h. Following incubation for 48 h at 37°C to 90% confluence,
cells were scratched using a 100-µl pipette tip. The cells were
cultured in 10% FBS-free DMEM at 37°C and washed twice using PBS.
Images were captured at 0 and 48 h after scratching using a light
microscope at ×100 magnification (Olympus Corporation). The wound
zone distances were measured using ImageJ 1.51 software (National
Institutes of Health).
Transwell assay
Cell migration and invasion assays were performed
using 24-well Transwell chambers (Nanjing KeyGen Biotech Co.,
Ltd.). For the invasion assay, the Transwell chambers were
precoated with Matrigel (Nanjing KeyGen Biotech Co., Ltd.) for 6 h
at 37°C. A total of 1×106 cells in 100 µl serum-free
DMEM were plated in the upper chamber and 500 µl medium
supplemented with 10% FBS was used in the bottom chambers as
chemoattractant. The cells were incubated at 37°C and 5%
CO2 for 24 h. The migrated and invaded cells on the
reverse side of the chamber inserts were fixed with 4%
polyoxymethylene (Sigma-Aldrich; Merck KGaA) for 30 min at 25°C and
stained with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA) for 15
min at 25°C. The number of cells was measured in five randomly
selected fields of view using a light microscope (IX73, Olympus
Corporation; magnification, ×200).
Dual-luciferase reporter assay
The target gene of hsa-miR-15a-5p was analyzed using
bioinformatics to verify whether CCND1 was the direct target gene
of hsa-miR-15a-5p.
The amplified 3′-UTR fragments of CCND1 were cloned
into the pmirGLO luciferase reporter vector (Promega Corporation).
The HT29 cells were seeded in 24-well plates at a density of
1×105 cells/well with DMEM supplemented with 10% FBS.
Subsequently, cells were transfected with 50 nM hsa-miR-15a-5p
mimics (5′-UAGCAGAUCCCAAUGGUCGGUG-3′), 100 nM mimic negative
control (5′-AAUUCGUAGCUUGCAUGCAAGC-3′) and 0.5 µg pmirGLO
luciferase reporter vector containing the wild type (WT) or mutant
(Mut) 3′-UTR sequences of CCND1. Transfection were performed using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) at
37°C according to the manufacturer's instructions. At 48 h
post-transfection, the luciferase activity was assessed by
dual-luciferase reporter assay system (Promega Corporation).
Firefly luciferase activities were normalized to Renilla luciferase
activities.
Statistical analysis
All data were analyzed using SPSS 19.0 statistical
software (IBM Corp.). Data are expressed as the mean ± SD (n=3).
Differences between the paired tissue samples from patients in
Fig. 1A were analyzed using a
paired Student's t-test, while comparisons between two groups were
determined using an unpaired Student's t-test for unpaired samples.
Comparisons among multiple groups were analyzed by one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
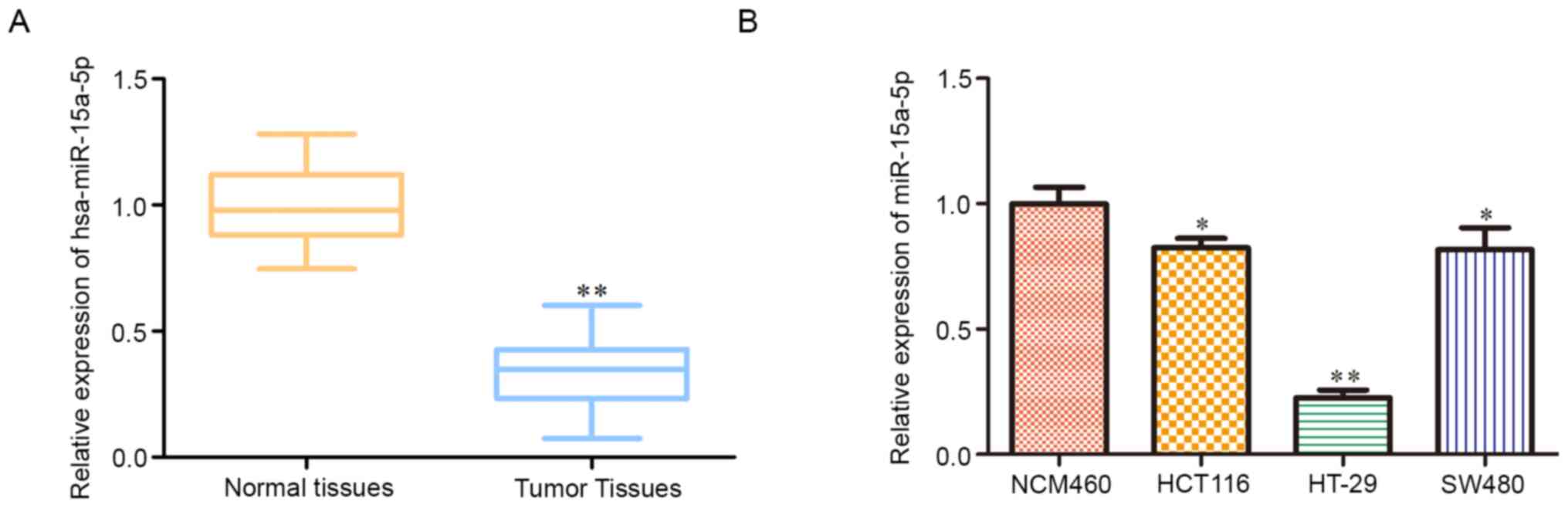
hsa-miR-15a-5p is decreased in colon
tumor tissues and cell lines
RT-qPCR analysis was performed to detect
hsa-miR-15a-5p expression levels in colon carcinoma. The results
showed that hsa-miR-15a-5p expression was significantly decreased
in colon carcinoma tissues compared with adjacent normal tissues
(Fig. 1A). Likewise, hsa-miR-15a-5p
expression levels were significantly downregulated in human colon
carcinoma cell lines (SW480 and HCT116) and human colorectal cancer
cell line (HT29) compared with the human normal colonic epithelial
cell line NCM460, especially in HT29 cells (Fig. 1B). Hence, HT29 cells were chosen for
subsequent experiments.
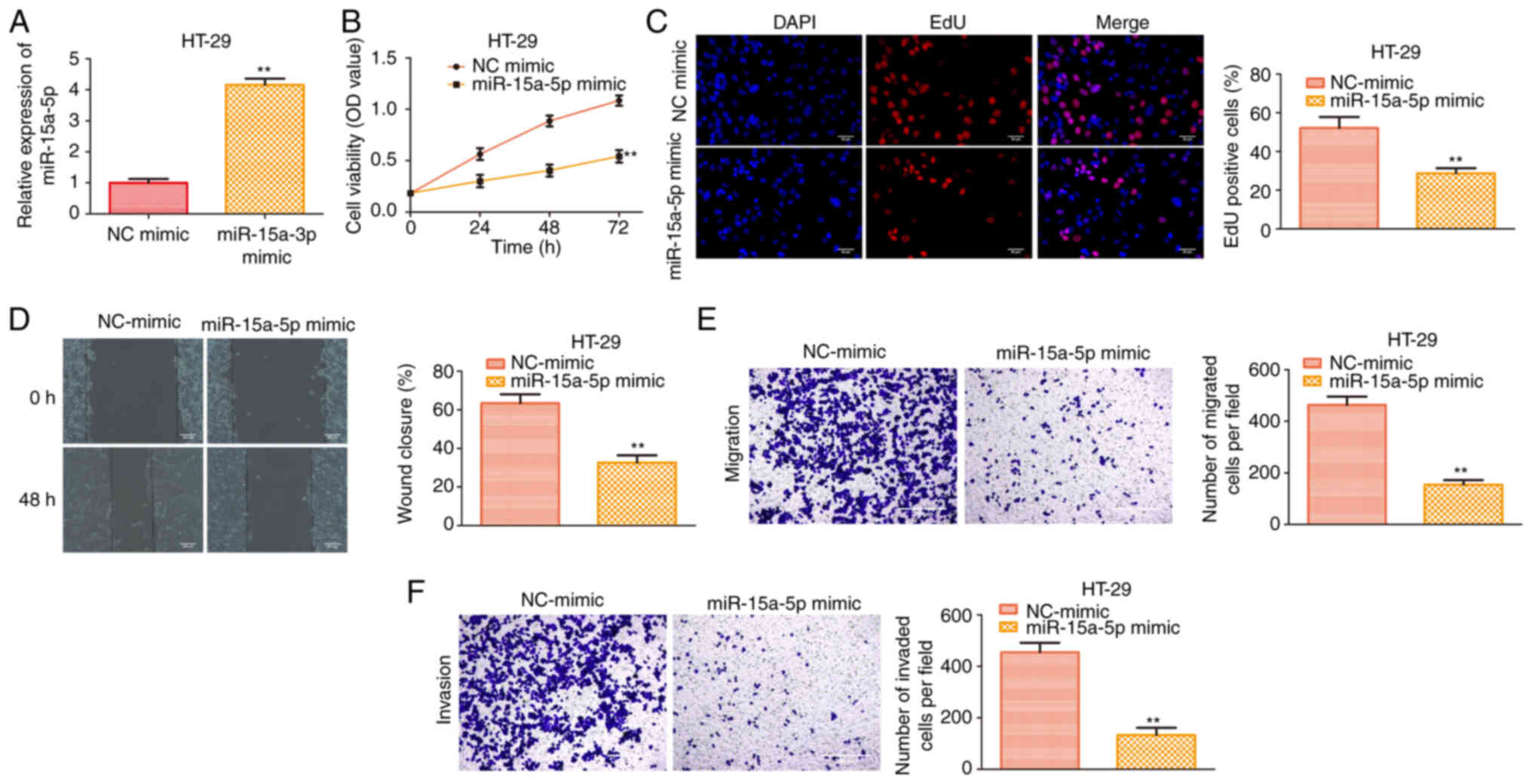
hsa-miR-15a-5p overexpression inhibits
proliferation, migration and invasion of colon cancer cells
To further investigate the role of hsa-miR-15a-5p in
the regulation of colon cancer, hsa-miR-15a-5p was artificially
overexpressed in HT29 cells. As shown in Fig. 2A, the results showed that
hsa-miR-15a-5p expression was increased following transfection with
the hsa-miR-15a-5p mimic. CCK-8 and EdU incorporation assays
revealed that hsa-miR-15a-5p overexpression significantly inhibited
the cell viability and proliferative activity of HT29 cells
(Fig. 2B and C). In line with the
aforementioned results, hsa-miR-15a-5p repressed cell migration and
invasion of HT29 cells (Fig. 2D-F).
These findings suggested that the proliferative, migratory and
invasive abilities of HT29 cells were reduced by hsa-miR-15a-5p
overexpression.
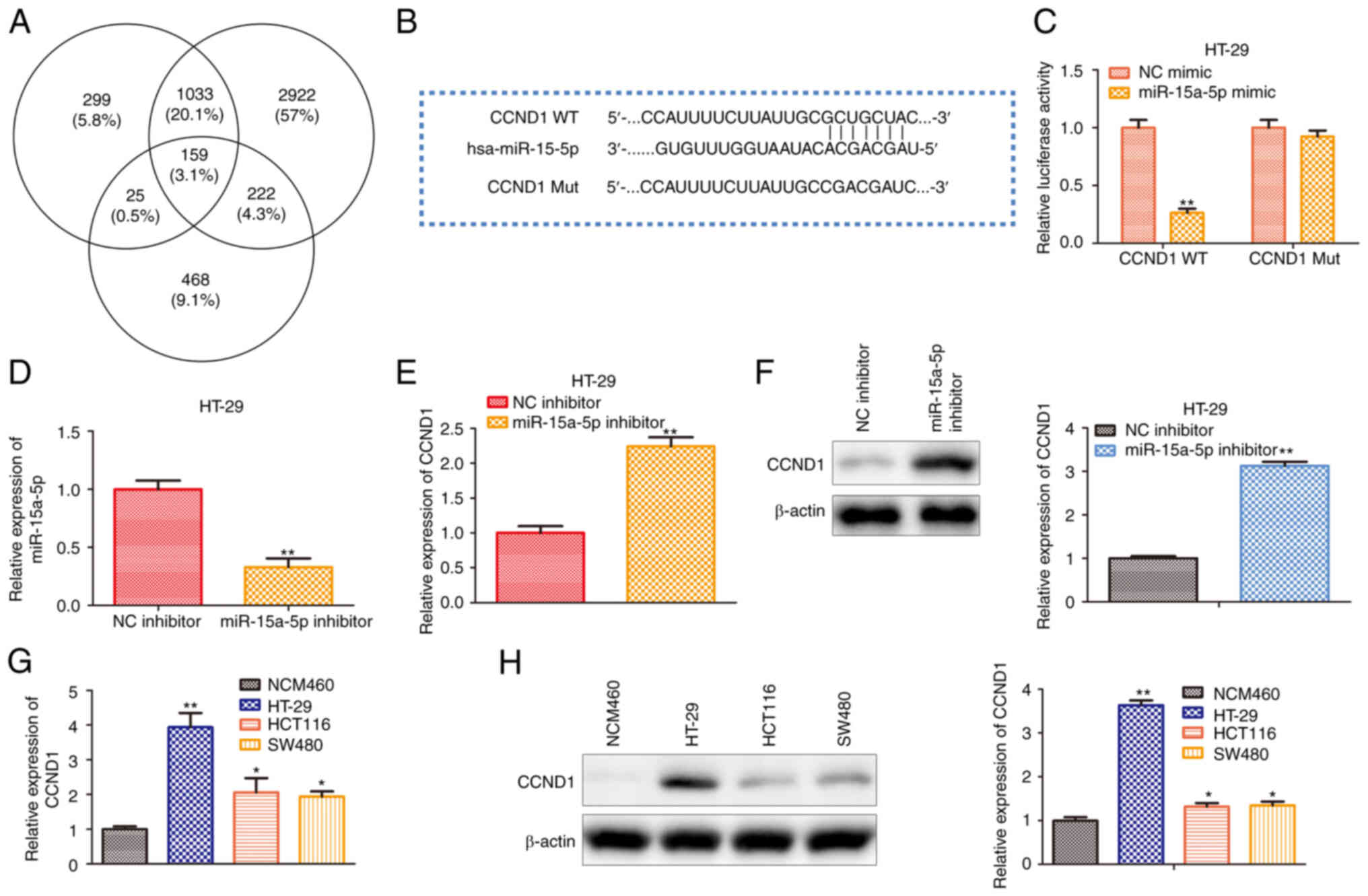
hsa-miR-15a-5p directly targets the
CCND1 gene
To investigate the underlying mechanism of
hsa-miR-15a-5p in regulating colon cancer progression in
vitro, the present study first predicted the target gene of
hsa-miR-15a-5p using multiple bioinformatics analyses. ENCORI
(starbase.sysu.edu.cn/), TargetScan
(targetscan.org/mamm_31/) and miRWalk
(mirwalk.umm.uni-heidelberg.de/) were used to predict
and screen the putative target of has-miR-15a-5p. As shown in
Fig. 3A, 159 potential targets were
clustered, and CCND1 was considered as the potential target since
it is a well-known cancer-associated gene (17). To verify whether hsa-miR-15a-5p
binds to the CCND1 gene, the present study designed luciferase
reporter plasmids containing WT CCND1 3′-UTR or hsa-miR-15a-5p
binding site mutated at the CCND1 3′-UTR (Fig. 3B). As presented in Fig. 3C, hsa-miR-15a-5p overexpression
decreased the luciferase activity of WT CCND1 3′-UTR, whereas a
mutation at the hsa-miR-15a-5p binding site in the 3′-UTR of CCND1
almost completely abolished the repressive effects of
hsa-miR-15a-5p overexpression on luciferase activity. Moreover, the
expression levels of hsa-miR-15a-5p in HT29 cells transfected with
hsa-miR-15a-5p inhibitor were assessed. It was found that the
expression of hsa-miR-15a-5p was reduced (Fig. 3D). Meanwhile, RT-qPCR and western
blotting indicated that hsa-miR-15a-5p repressed CCND1 expression
at both transcriptional and translational levels (Fig. 3E and F). In addition, to estimate
the role of CCND1 in colon cancer, the present study examined the
expression levels of CCND1 in human normal colonic epithelial cell
line NCM460, human colon carcinoma cell lines (SW480 and HCT116)
and human colon adenocarcinoma cell line (HT29) by RT-qPCR and
western blotting. Compared with the NCM460 cell line, the mRNA and
protein expression levels of CCND1 in colon cancer cell lines were
significantly increased, especially in the HT29 cell line (Fig. 3G and H).
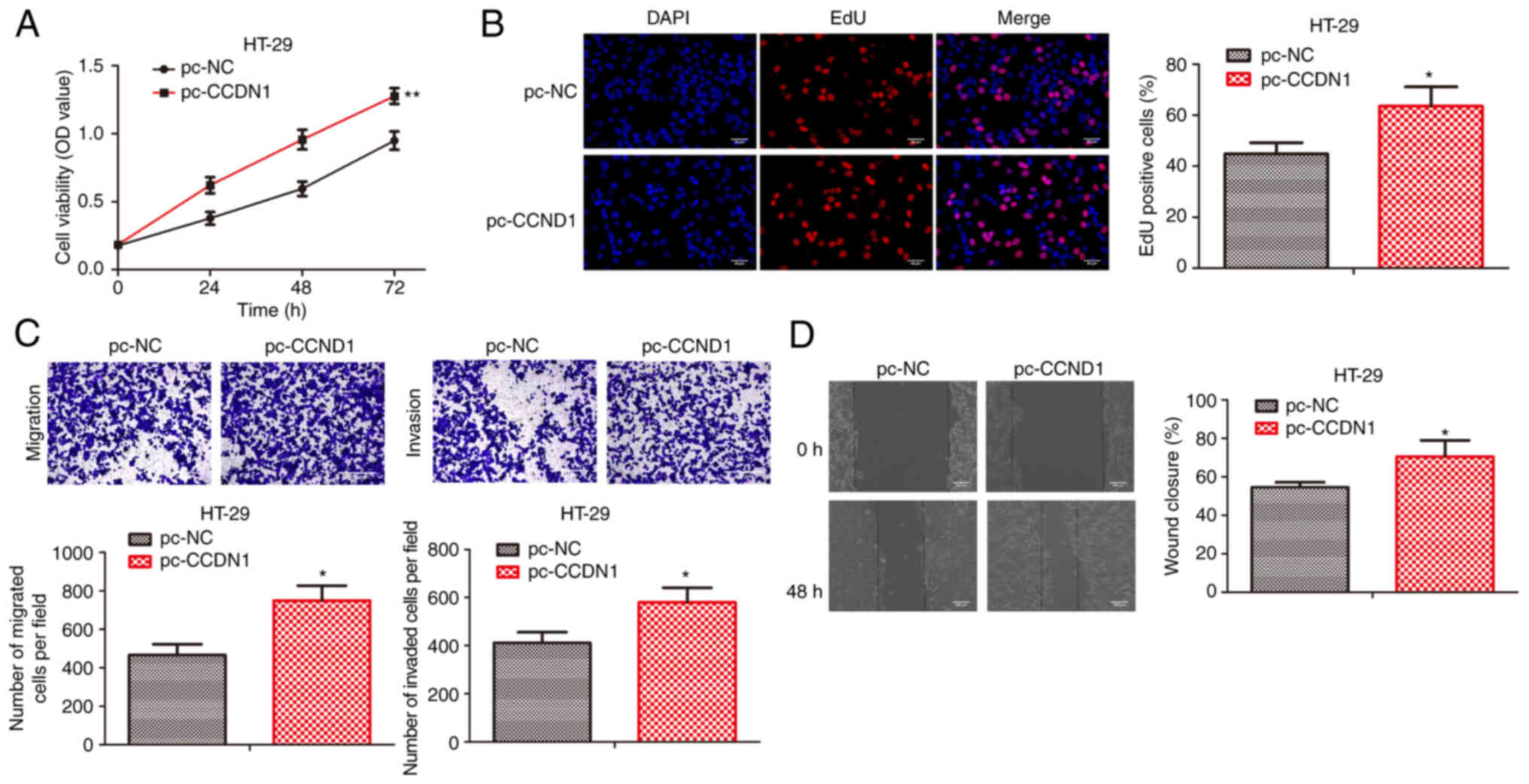
CCDN1 overexpression promotes colon
cancer progression
To investigate the function of CCDN1 in colon cancer
progression, pcDNA-control and pcDNA-CCDN1 were transfected into
HT29 cells. CCK-8 and EdU incorporation assays revealed that CCDN1
overexpression significantly promoted the cell viability and
proliferative activity of HT29 cells (Fig. 4A and B). In line with the
aforementioned results, CCDN1 overexpression promoted cell
migration and invasion of HT29 cells (Fig. 4C and D). Therefore, CCDN1
overexpression could promote colon cancer progression.
hsa-miR-15a-5p inhibits oncogenic
progression of colon cancer via targeting the CCND1 gene
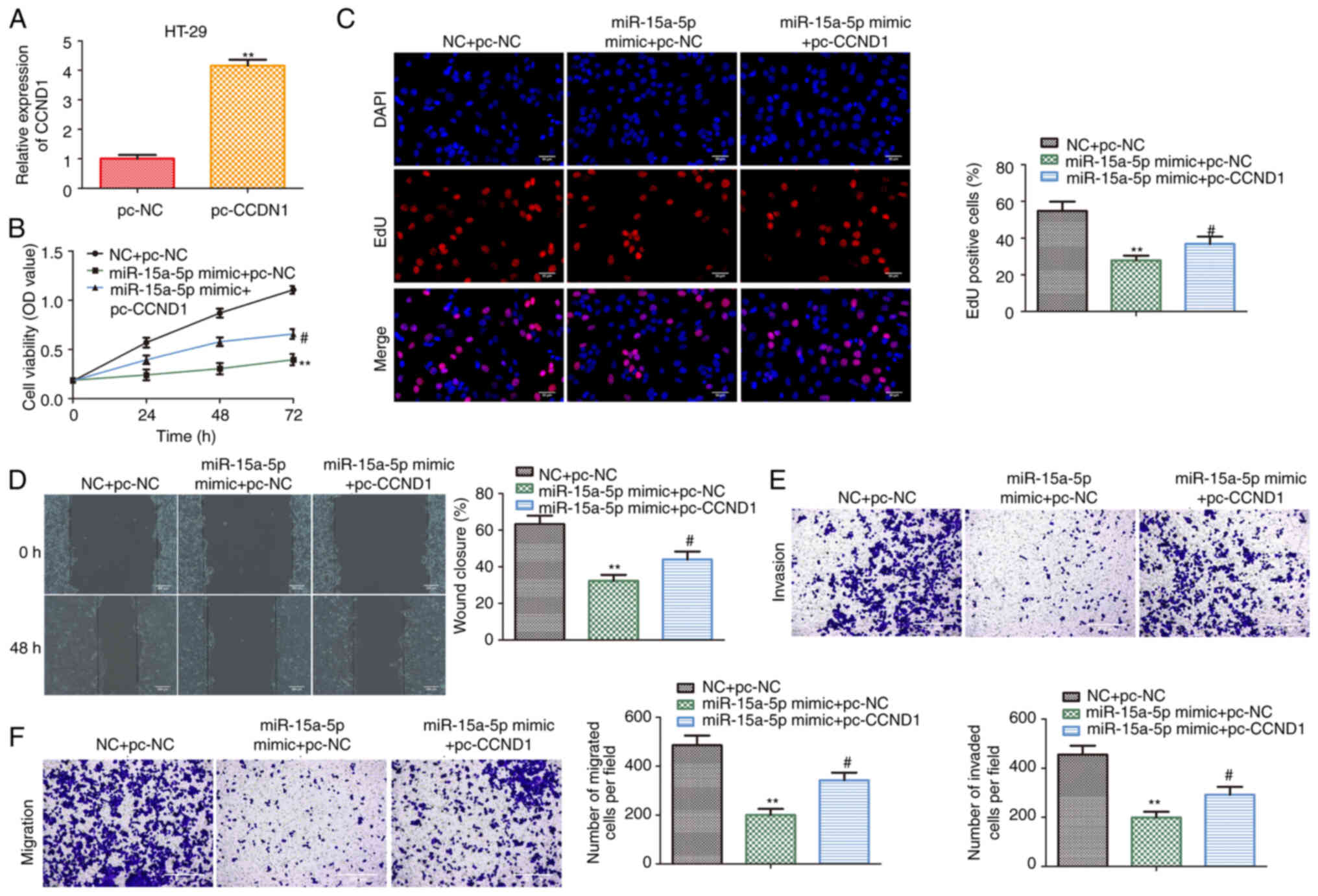
To confirm whether the effects of hsa-miR-15a-5p on
colon cancer cells were mediated by CCND1, CCND1 was overexpressed
in HT29 cells transfected with hsa-miR-15a-5p mimic. As shown in
Fig. 5A, the expression of CCND1
was increased following transfection with pcDNA-CCND1 compared with
pcDNA-control in HT29 cells. CCK-8 and EdU assays indicated that
the proliferative capacity of HT29 cells was inhibited by
transfection with a hsa-miR-15a-5p mimic, while this was promoted
following CCND1 overexpression (Fig. 5B
and C). Similar to the results of cell proliferation assays,
CCND1 overexpression partially abolished the inhibitory effects of
hsa-miR-15a-5p on HT29 cell migration and invasion (Fig. 5D-F). These results suggested that
CCND1 functions as a mediatory factor in relaying hsa-miR-15a-5p
signaling to colon cancer progression in vitro.
Discussion
Colon carcinoma is the third most common malignancy
and the fourth leading cause of cancer mortality worldwide
(1). It has been well-established
that aging, the environment, a high-fat diet and heredity factors
are key risk factors for colon carcinoma (2–4). The
majority of patients suffer from poor prognosis due to the high
recurrence of colon cancer (18).
Hence, understanding the underlying molecular mechanism of colon
cancer is of importance.
In the past decades, numerous studies have reported
that miRNAs act as regulatory factors in cancer progression and
metastasis via targeting crucial genes (19,20).
Identification of miRNAs with key roles in targeting and regulating
genes in signaling and metabolic pathways is a novel approach for
cancer treatment (21). Of note,
hsa-miR-15a-5p is reported to be closely associated with colorectal
cancer survival (15). miR-15a-5p
is part of the miR-15/16 cluster on chromosome 13q14.3, which acts
as a tumor suppressor of chronic lymphocytic lymphoma by promoting
apoptosis via targeting BCL2 (22).
The present study found that hsa-miR-15a-5p expression was
significantly downregulated in colon tumor tissues and cell lines.
To further investigate the effects of hsa-miR-15a-5p on colon
cancer, hsa-miR-15a-5p was overexpressed in HT29 cells via
hsa-miR-15a-5p mimic transfection. The present study found a
negative regulatory effect of hsa-miR-15a-5p overexpression on
colon cancer cell proliferation, migration and invasion. Therefore,
similar to its function in chronic lymphocytic lymphoma,
hsa-miR-15a-5p acts as a tumor suppressor in colon cancer.
Multiple bioinformatics analyses predicted CCND1 was
a potential target of hsa-miR-15a-5p. CCND1 has emerged as a key
regulator of cell cycle progression by interacting with CDKs during
the G1 to S-phase transition (17).
Dysregulation of the cell cycle is the most typical cause of
carcinomas, while CCND1 expression is upregulated in various
cancers, including non-small cell lung cancer, pancreatic ductal
adenocarcinoma and ovarian cancer, resulting in abnormalities of
cell proliferation (23–25). More importantly, it has also been
found to induce malignant phenotypes by promoting cell migration
and metastasis (26,27). The present study demonstrated that
CCND1 was the direct target of hsa-miR-15a-5p using a
dual-luciferase reporter assay. In addition, CCND1 expression was
upregulated, and negatively regulated by hsa-miR-15a-5p in colon
cancer cells, which was confirmed by RT-qPCR and western blot
analyses. Consistently, CCND1 was correspondingly highly expressed
in colon cancer tissues and cell lines. To further determine the
role of CCND1 in colon cancer, CCND1 was artificially overexpressed
in HT29 cells, and it was found that CCND1 overexpression partially
hindered the repressive effects of hsa-miR-15a-5p on the
proliferation, migration and invasion of colon cancer cells.
In summary, the present study found that
hsa-miR-15a-5p expression was significantly decreased in clinical
colon tumor samples and cell lines. The present study provided
evidence that hsa-miR-15a-5p may act as a tumor suppressor of colon
cancer by targeting CCND1. These findings provided an innovative
target and a potential for the treatment and prognosis of colon
cancer.
Acknowledgements
Not applicable.
Funding
This work was supported by the Hospital-level topic
of Jiangsu Cancer Hospital (grant no. ZM202011) and The Sixth Batch
of National Heritage Studios of Traditional Chinese Medicine
Experts [Education and Development of Traditional Chinese Medicine;
grant no. (2017) 29].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL, ZZ, XZ and YB conceived and designed the study
and methodology. ZL, ZZ, YaW, YiW, WL and ZW performed the
experiments and collected the data. ZL, ZZ and YaW analyzed and
interpreted the data. ZL, XZ and YB confirm the authenticity of all
the raw data. ZL and ZZ drafted the manuscript. XZ and YB revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Affiliated Cancer Hospital of Nanjing Medical
University and Jiangsu Cancer Hospital and Jiangsu Institute of
Cancer Research [(2016) approval no. 231]. All participants
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miRNA/miR
|
microRNA
|
|
CCND1
|
G1/S-specific cyclin-D1
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
CCK-8
|
Cell Counting Kit-8
|
|
EdU
|
5-ethynyl-2′-deoxyuridine
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Itatani Y, Kawada K, Fujishita T, Kakizaki
F, Hirai H, Matsumoto T, Iwamoto M, Inamoto S, Hatano E, Hasegawa
S, et al: Loss of SMAD4 from colorectal cancer cells promotes CCL15
expression to recruit CCR1+ myeloid cells and facilitate
liver metastasis. Gastroenterology. 145:1064–1075.e11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aghakhani A, Hamkar R, Ramezani A,
Bidari-Zerehpoosh F, Sabeti S, Ghavami N, Banifazl M, Rashidi N and
Eslamifar A: Lack of human papillomavirus DNA in colon
adenocarcinama and adenoma. J Cancer Res Ther. 10:531–534.
2014.PubMed/NCBI
|
|
4
|
Zhang Y, Lin C, Liao G, Liu S, Ding J,
Tang F, Wang Z, Liang X, Li B, Wei Y, et al: MicroRNA-506
suppresses tumor proliferation and metastasis in colon cancer by
directly targeting the oncogene EZH2. Oncotarget. 6:32586–32601.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen L, Wang J, Fu L, Zhang B, Zhang H and
Ye B: Prognostic significance of metastasis associated in colon
cancer 1 (MACC1) expression in patients with gallbladder cancer. J
Cancer Res Ther. 10:1052–1056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Manjelievskaia J, Brown D, McGlynn KA,
Anderson W, Shriver CD and Zhu K: Chemotherapy use and survival
among young and middle-aged patients with colon cancer. JAMA Surg.
152:452–459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nicoloso MS, Spizzo R, Shimizu M, Rossi S
and Calin GA: MicroRNAs-the micro steering wheel of tumour
metastases. Nature reviews. Cancer. 9:293–302. 2009.PubMed/NCBI
|
|
8
|
Schetter AJ, Leung SY, Sohn JJ, Zanetti
KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, et
al: MicroRNA expression profiles associated with prognosis and
therapeutic outcome in colon adenocarcinoma. JAMA. 299:425–436.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwarzenbach H: Clinical relevance of
circulating, cell-free and exosomal microRNAs in plasma and serum
of breast cancer patients. Oncol Res Treat. 40:423–429. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alvarez-Díaz S, Valle N, Ferrer-Mayorga G,
Lombardía L, Herrera M, Domínguez O, Segura MF, Bonilla F, Hernando
E and Muñoz A: MicroRNA-22 is induced by vitamin D and contributes
to its antiproliferative, antimigratory and gene regulatory effects
in colon cancer cells. Hum Mol Genet. 21:2157–2165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai SD, Chen JS, Xi ZW, Zhang LJ, Niu ML
and Gao ZY: MicroRNA-144 inhibits migration and proliferation in
rectal cancer by downregulating ROCK-1. Mol Med Rep. 12:7396–7402.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cottonham CL, Kaneko S and Xu L: miR-21
and miR-31 converge on TIAM1 to regulate migration and invasion of
colon carcinoma cells. J Biol Chem. 285:35293–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yi H, Geng L, Black A, Talmon G, Berim L
and Wang J: The miR-487b-3p/GRM3/TGFβ signaling axis is an
important regulator of colon cancer tumorigenesis. Oncogene.
36:3477–3489. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Li Z, Zhu Y, Li Z, Yao L, Zhang L,
Yuan L, Shang Y, Liu J and Li C: miR-524-5p inhibits angiogenesis
through targeting WNK1 in colon cancer cells. Am J Physiol
Gastrointest Liver Physiol. 318:827–839. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mullany LE, Herrick JS, Sakoda LC,
Samowitz W, Stevens JR, Wolff RK and Slattery ML: miRNA involvement
in cell cycle regulation in colorectal cancer cases. Genes Cancer.
9:53–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang L, Guo R, Yuan Z, Shi H and Zhang D:
LncRNA HOTAIR regulates CCND1 and CCND2 expression by sponging
miR-206 in ovarian cancer. Cell Physiol Biochem. 49:1289–1303.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Douaiher J, Ravipati A, Grams B, Chowdhury
S, Alatise O and Are C: Colorectal cancer-global burden, trends,
and geographical variations. J Surg Oncol. 115:619–630. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan Y, Zhang J, Fu H and Shen L: miR-144
functions as a tumor suppressor in breast cancer through inhibiting
ZEB1/2-mediated epithelial mesenchymal transition process. Onco
Targets Ther. 9:6247–6255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Q, Xia HW, Ge XJ, Zhang YC, Tang QL
and Bi F: Serum miR-19a predicts resistance to FOLFOX chemotherapy
in advanced colorectal cancer cases. Asian Pac J Cancer Prev.
14:7421–7426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ritchie W, Rasko JE and Flamant S:
MicroRNA target prediction and validation. Adv Exp Med Biolgy.
774:39–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aqeilan RI, Calin GA and Croce CM: miR-15a
and miR-16-1 in cancer: Discovery, function and future
perspectives. Cell Death Differ. 17:215–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Orhan C, Bulut P, Dalay N, Ersen E and
Buyru N: Downregulation of TCEAL7 expression induces CCND1
expression in non-small cell lung cancer. Mol Biol Rep.
46:5251–5256. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen G, Hu M, Qu X, Wang K and Qu Y:
MicroRNA-584 directly targets CCND1 and inhibits cell proliferation
and invasion in pancreatic cancer. Mol Med Rep. 19:719–726.
2019.PubMed/NCBI
|
|
25
|
Dai J, Wei RJ, Li R, Feng JB, Yu YL and
Liu PS: A study of CCND1 with epithelial ovarian cancer cell
proliferation and apoptosis. Eur Rev Med Pharmacol Sci.
20:4230–4235. 2016.PubMed/NCBI
|
|
26
|
Hao CY, Zhao S, Zhang LL and Liu D: SNHG16
promotes the progression of osteoarthritis through activating
microRNA-93-5p/CCND1 axis. Eur Rev Med Pharmacol Sci. 23:9222–9229.
2019.PubMed/NCBI
|
|
27
|
Nie M, Wang Y, Yu Z, Li X, Deng Y, Wang Y,
Yang D, Li Q, Zeng X, Ju J, et al: AURKB promotes gastric cancer
progression via activation of CCND1 expression. Aging (Albany NY).
12:1304–1321. 2020. View Article : Google Scholar : PubMed/NCBI
|