Introduction
Diabetes is a global epidemic that affects 300
million individuals worldwide and is one of the primary threats to
human health, with a markedly increasing incidence (1). Hyperglycemia is a feature of diabetes
mellitus and can cause numerous complications, including heart
disease, cataracts, kidney disease and nerve damage (1,2). The
primary causes of all forms of diabetes include the loss of insulin
or insufficient quantities of insulin production/secretion from
pancreatic β-cells in the islets of Langerhans (3).
The pancreas is the second largest gland in the body
and is composed of endocrine and exocrine components (3). Exocrine islets form an important
digestive system that produces digestive enzymes, including
chymotrypsin, amylase and lipase, which are used to digest
proteins, and degrade carbohydrates and fats. Endocrine islets are
the source of several hormones, including insulin and glucagon
(4,5). Insulin, which is secreted by β cells
in the pancreas, is the most important metabolic hormone for
controlling blood glucose. Loss of islet β cells may lead to
diabetes and require therapeutic intervention.
Regenerative medicine is considered as a valuable
therapeutic approach that has gained increasing acceptance
worldwide. Devising potential strategies to develop
insulin-producing β-cells might result in profound effects for the
treatment of diabetes. Previous studies in fish (6), mice (7) and frogs (8) have demonstrated the differentiation
potential of multipotent stem cells (SCs) (5). Controlled differentiation of SCs,
including embryonic (9), pancreatic
(10) and bone marrow mesenchymal
(11) SCs, into functional
insulin-producing cells (IPCs) fulfills the requirement of
transplantable β cells. However, the protocol for the controlled
differentiation of SCs is not completely understood.
Pancreas-derived mesenchymal stromal cells (PMSCs)
are one of the types of mesenchymal cells isolated from pancreatic
tissues. Compared with other cells types, PMSCs are highly enriched
during vascular development, chemotaxis and wound healing (12). PMSCs also possess the ability to
decrease hyperglycemia in type 1 diabetes model mice after
intrapancreatic injection (12).
PMSCs are a potential therapeutic agent for regenerative medicine
applications (13). Recently, Lee
et al (13) reported a
two-step induced protocol to transform porcine-derived mesenchymal
cells into insulin-producing cells; although the function of
induced cells was estimated, the biological characterization and
morphological alterations of the induced cells were not
described.
In the present study, Bama miniature pig-derived
mesenchymal stem cells (MSCs) were selected as the experimental
cell source for a number of reasons: i) Swine are an appropriate
animal model for humans, as they possess a high degree of
similarity in tissue structure and genetics with humans (14); ii) MSCs with high proliferative
capacity can be transformed into various cell types and display a
vital role in regenerative medicine (15); and iii) SCs derived from the
pancreas are more easily differentiated into functional β cells
compared with other cells (3). The
present study described a novel protocol for generating pancreatic
islet β cells that may serve as a model to facilitate the study of
the mechanisms controlling islet cell neogenesis and may aid in the
identification of pharmaceutical targets to treat insulin-dependent
diabetes.
Materials and methods
PMSC isolation, culture and
purification
All animal procedures were approved by the
Institutional Animal Care and Use Committee of Chinese Academy of
Agricultural Sciences (approval no. IAS 2018–19, Beijing, China).
The 2–3-month-old Wuzhishan miniature pig (Sus scrofa; also
known as Bama miniature pigs) was provided by the Animal Husbandry
Experimental Base, Institute of Animal Sciences, Chinese Academy of
Agricultural Sciences (16). The
animals were sacrificed through overdose of ketamine (100 mg/kg;
cat. no. 087K1253; Sigma-Aldrich; Merck KGaA) and xylazine (25
mg/kg; cat, no. KH070901; Hengrui Co.) (16). The pancreas was removed from each
miniature pig embryo (n=3). After thoroughly washing with PBS
buffer, tissue sections were dissected into 1-mm3 thick
segments and isolated via collagenase digestion under sterile
conditions. Tissues were digested with 5.5 mg/ml collagenase P
(Roche Diagnostics) for 60 min at 37°C with gentle agitation every
10 min. Following digestion, cells were neutralized with RPMI-1640
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.). The cell suspension was
filtered through a 74-µm mesh sieve and then centrifuged at 225 × g
for 15 min at room temperature. Cell pellets were resuspended with
RPMI-1640 supplemented with 10% FBS and 1% penicillin-streptomycin
(Gibco; Thermo Fisher Scientific, Inc.). Cells (1.5×106)
were seeded into plates and incubated at 37°C with 5%
CO2. Following incubation for 3–4 h, 104
non-adherent cells were removed and reseeded in a new plate. Cells
were incubated with RPMI-1640 medium supplemented with 20 ng/ml
human-derived fibroblast growth factor (bFGF; PeproTech, Inc.) and
20 ng/ml human-derived epidermal growth factor (EGF; PeproTech,
Inc.) at 37°C. After 12 h, 100s of cells started growing with a
spindle shape. When the cells reached 80–90% adherence, they were
digested with 0.125% trypsin and considered as passage 1. Passage
4, passage 13 and passage 18 cells were obtained by the same
method. PMSCs were purified by the different adhesion time method
(17). According to the preliminary
experiment, PMSCs displayed a shorter adhesion time compared with
other cells types, thus PMSCs could be purified after the third
passage. In addition, PMSCs should be mesenchymal cell specific
marker Vimentin positive and display fibroblast-like morphology;
these were checked to assess the purity of isolated PMSCs.
Contaminating cells, such as blood and duct cells, displayed a
different adherence time and could be excluded by passaging.
Growth kinetics
Cells from passage 3, 8 and 16 were seeded
(1×104 cell/well) into 24-well plates. The cell
suspension was absorbed and dropped onto the edge of the cell
counting plate, and the mean value of four large squares was
calculated. Cell counting was repeated three times to obtain a mean
value for each large square. The cell count (cells/ml) was
calculated as follows: The mean cell number ×104. Cells
were counted every day for 7 days. The population doubling time
(PDT) was calculated according to the following formula:
PDT=(t-t0) xlog2/(log Nt-log N0),
where t0 is the starting time, t is the termination
time, N0 is the initial cell number and Nt is the
ultimate cell number.
Colony-formation assay
Cells from passage 3, 8 and 14 were seeded
(1×104 cell/well) into 24-well plates and cultured for 4
days. Then, the cells were fixed with 4% paraformaldehyde for 30
min at room temperature and stained with GIMSA (cat. no. C0133,
Beyotime Institute of Biotechnology) for 30 min at room
temperature. Subsequently, the number of colony-forming units was
counted to calculate the colony-forming rate (%) according to the
following formula: (Number of colony-forming units/number of seeded
cells) ×100. The experiment was repeated three times to calculate
the mean value. The light microscope was used for images at ×4
magnification.
Immunofluorescence staining
Purified PMSCs were seeded into 6-well plates. At
70% confluence, cells were washed three times with PBS, fixed with
4% paraformaldehyde for 30 min at room temperature and
permeabilized with 0.25% Triton X-100 (Sigma-Aldrich; Merck KGaA)
for 20 min in room temperature. Following blocking with 10% normal
goat serum (1:10; OriGene Technologies, Inc.) for 30 min at room
temperature, cells were incubated for 1 h at 37°C in 3% BSA
(Sigma-Aldrich; Merck KGaA) supplemented with the following primary
antibodies: Anti-pancreatic and duodenal homeobox 1 (Pdx1; 1:200;
cat. no. ab47267; Abcam), anti-Vimentin (1:200; cat. no. ab8978;
Abcam), anti-neurogenin 3 (Ngn3; 1:200; cat. no. bs-0922R; Beijing
Biosynthesis Biotechnology Co., Ltd.), anti-Nestin (1:200; cat. no.
ab221660; Abcam) and anti-CD45 (1:200; cat. no. ab10558; Abcam).
Following washing three times with PBS, cells were incubated with a
FITC-conjugated rabbit anti-goat secondary antibody (1:200; cat.
no. ZF-0314; OriGene Technologies, Inc.) or anti-mouse Alexa Fluor
secondary antibody (1:200; cat. no. sc-516608; Santa Cruz
Biotechnology, Inc) at room temperature in the dark for 1 h. Cells
were washed three times in the dark and then incubated with 1 µg/ml
DAPI (Sigma-Aldrich; Merck KGaA) for 15 min in room temperature.
Stained cells were visualized and counted in eight randomly
selected non-overlapping fields of view using a TE-2000-E inverted
fluorescence microscope (Nikon Corporation). For the control group,
PBS was used in place of primary antibodies. Samples were assessed
in triplicate.
Flow cytometry
Differentiated cell clusters or PMSCs both at a
number of 106 were dispersed into single-cell
suspensions by incubation with trypsin for 1 min at 37°C. Cells
were fixed with 4% paraformaldehyde for 30 min at room temperature,
washed with PBS and blocked with 1% normal goat serum for 20 min at
room temperature. PMSCs were incubated with anti-CD73 (1:200; cat.
no. ab175396; Abcam), anti-CD90 (1:200; cat. no. ab222781; Abcam)
and anti-CD34 (1:200; cat. no. 81289; Abcam) primary antibodies for
biological characterization at 4°C overnight. IPCs and PMSCs were
resuspended in PBS with anti-insulin (1:200; cat. no. ab181547;
Abcam) and incubated at 4°C overnight. Following washing twice with
PBS, cells were incubated at room temperature with FITC-conjugated
goat anti-rabbit IgG (1:200; cat. no. bs-40295G-HRP; BIOSS) and
goat anti-mouse Alexa Fluor IgG (1:200; cat. no. bs-40296-HRP;
BIOSS) secondary antibodies for 2 h. For the control group, cells
were only incubated with the secondary antibody and were not
incubated with specific primary antibodies. Following washing,
cells were analyzed using a Beckman Cytomics FC500 flow cytometer
(Beckman Coulter, Inc.) and Flow software (Beckman CXP 2.1; Beckman
Coulter, Inc.). Samples were assessed in triplicate. The assay was
conducted in 60-mm dishes.
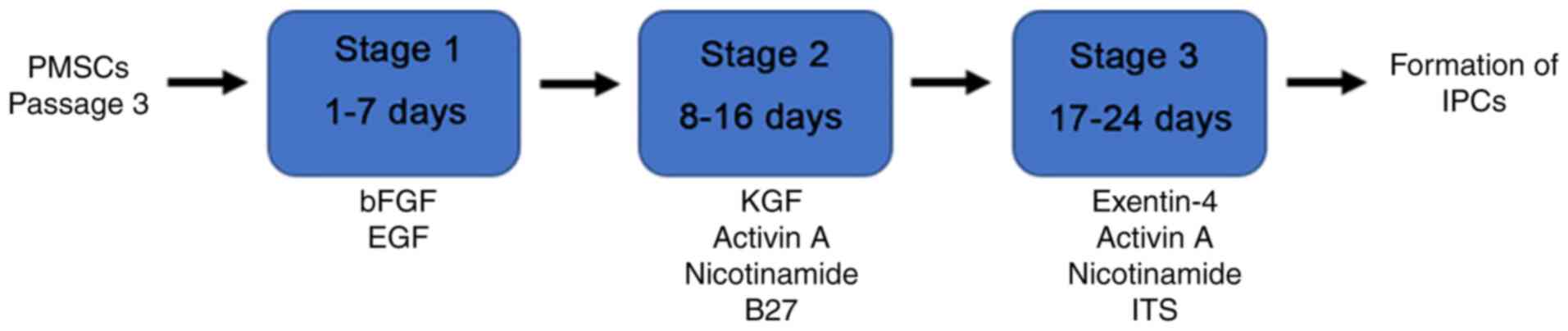
Transdifferentiation of PMSCs into
IPCs in vitro
At passage 3, PMSCs were transdifferentiated into
IPCs in vitro according to the protocol presented in
Fig. 1. Firstly, PMSCs were
incubated in medium supplemented with 20 ng/ml bFGF and 20 ng/ml
EGF for 7 days. Subsequently, cells were transferred into medium
containing 10 ng/ml keratinocyte growth factor (KGF; PeproTech,
Inc.), 10 ng/ml Activin A (PeproTech, Inc.), 10 mmol/l nicotinamide
(PeproTech, Inc.) and 2% B27 supplement (PeproTech, Inc.) for 9
days. Cells were transferred into medium supplemented with 10 ng/ml
Exendin-4 (PeproTech, Inc.), 10 ng/ml Activin A, 10 mmol/l
nicotinamide (Gibco; Thermo Fisher Scientific, Inc.) and 1 g/l
insulin-transferrin-selenium (ITS; Sigma-Aldrich; Merck KGaA) in
stage 3. All culture mediums were replaced every 2–3 days. The
control group was cultured in medium without any supplementation.
Cells were observed using an inverted light microscope every
day.
Dithizone (DTZ) staining
Following induction, cells were washed with PBS,
fixed with 4% paraformaldehyde at room temperature for 15 min and
stained with DTZ (Sigma-Aldrich; Merck KGaA) staining solution for
30 min at room temperature. Stained cells were observed using a
light microscope. The staining protocol was based on a study
conducted by Shiroi et al (18). Samples were assessed in triplicate.
The assay was conducted in 60-mm dishes.
Immunofluorescence staining assays of
IPCs markers
According to the aforementioned protocol,
immunofluorescence staining was performed using primary antibodies
targeted against the following: Insulin (1:200; cat. no. ab9823;
Abcam), glucagon (1:200; cat. no. ab189279; Abcam), C-peptide
(1:200; cat. no. ab82696; Abcam) and NK6 homeobox 1 (Nkx6.1; 1:200;
cat. no. ab251565; Abcam). The nuclear was stained with DAPI (cat.
no. C1002; Beyotime Institute of Biotechnology) for 20 min at room
temperature. Samples were assessed in triplicate. The assay was
conducted in 60-mm dishes.
Glucose-stimulated insulin secretion
(GSIS)
Following washing with PBS, differentiated IPCs were
pre-cultured in low-glucose (2 mM) buffer for 1 h to remove
residual insulin. Cell clusters were washed twice with PBS and then
incubated in low-glucose (5 mM) buffer for 30 min at 37°C. The
supernatant was collected. Subsequently, cell clusters were washed
twice in PBS and then incubated in high-glucose (25 mM) buffer for
30 min at 37°C. The supernatant was collected via centrifugation at
339 × g for 10 min at room temperature. Cell clusters were
dispersed into single cells using trypsin and the cell number was
counted. Undifferentiated cell samples were used as controls.
Supernatant samples containing secreted insulin were processed
using the mouse insulin ELISA kit (cat. no. 0740; Pro Lab Marketing
Pvt. Ltd.), which displays 0.019 ng/ml super sensitivity. The
protocol was modified from the method described by Pagliuca et
al (19) and Bai et al
(20). Samples were assessed in
triplicate. The assay was conducted in 60-mm dishes. The experiment
was repeated three times.
Statistical analysis
Comparisons among multiple groups were analyzed
using one-way ANOVA followed by the Tukey-Kramer post hoc test in
GraphPad Prism 8 (GraphPad Software, Inc.). Comparisons between two
groups were analyzed using an unpaired Student's t-test.
Data are presented as the mean ± SD. P<0.05 was considered to
indicate a statistically significant difference.
Results
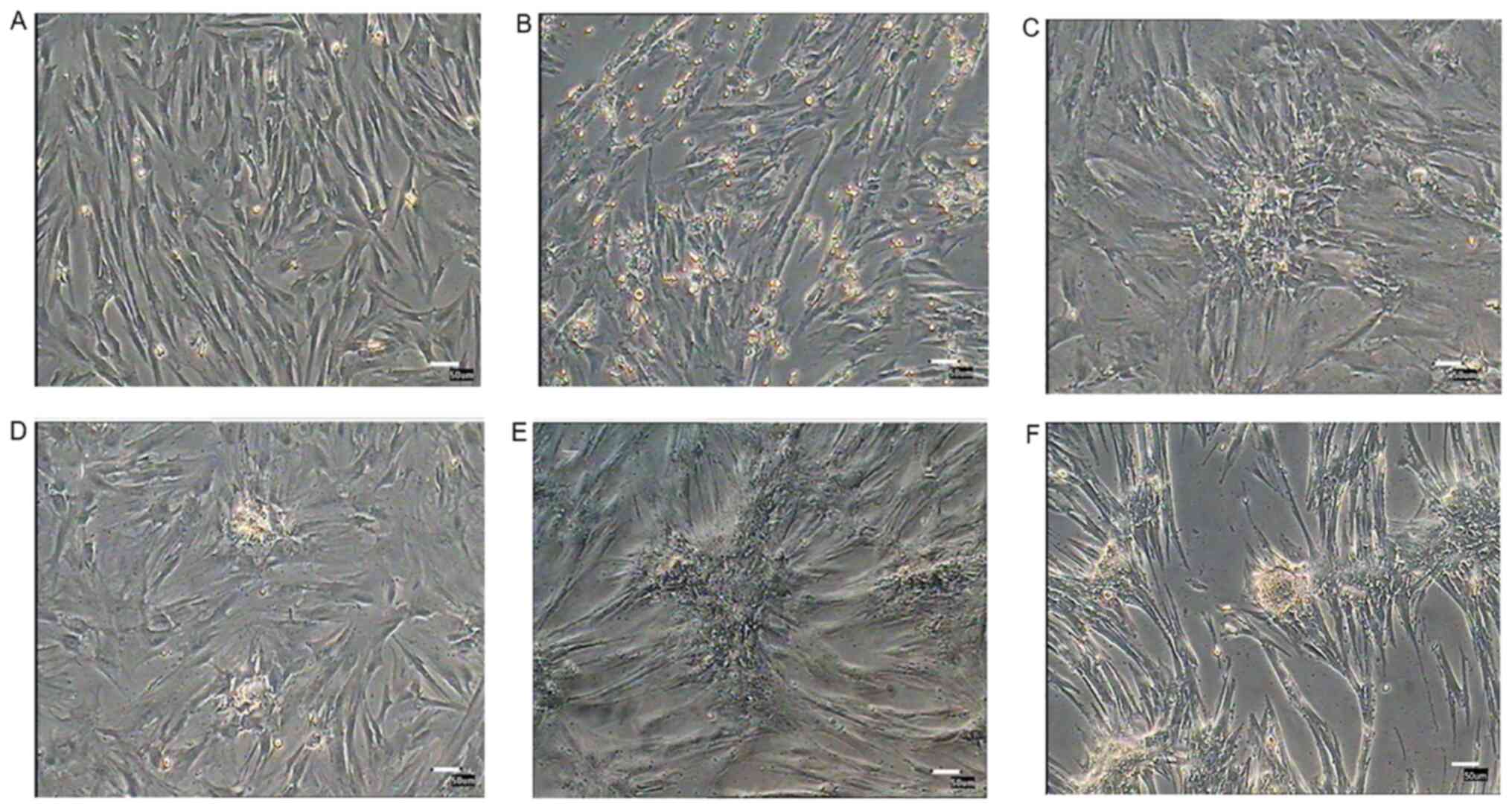
Morphological observation of
PMSCs
Following culture for 12 h, only a small number of
cells were attached to the plate. Adherent cells gradually
proliferated. In primary cultures, PMSCs were mixed with epithelial
cells and other different types of cells. At passage 4, PMSCs were
purified and displayed fibroblast-like morphology, with long
spindle-like and polygon-like cell features. At passage 18, PMSCs
rapidly expanded and then cell proliferation slowed down, resulting
in flattened cells with a lack of three-dimensional structure that
displayed signs of senescence (Fig.
2A).
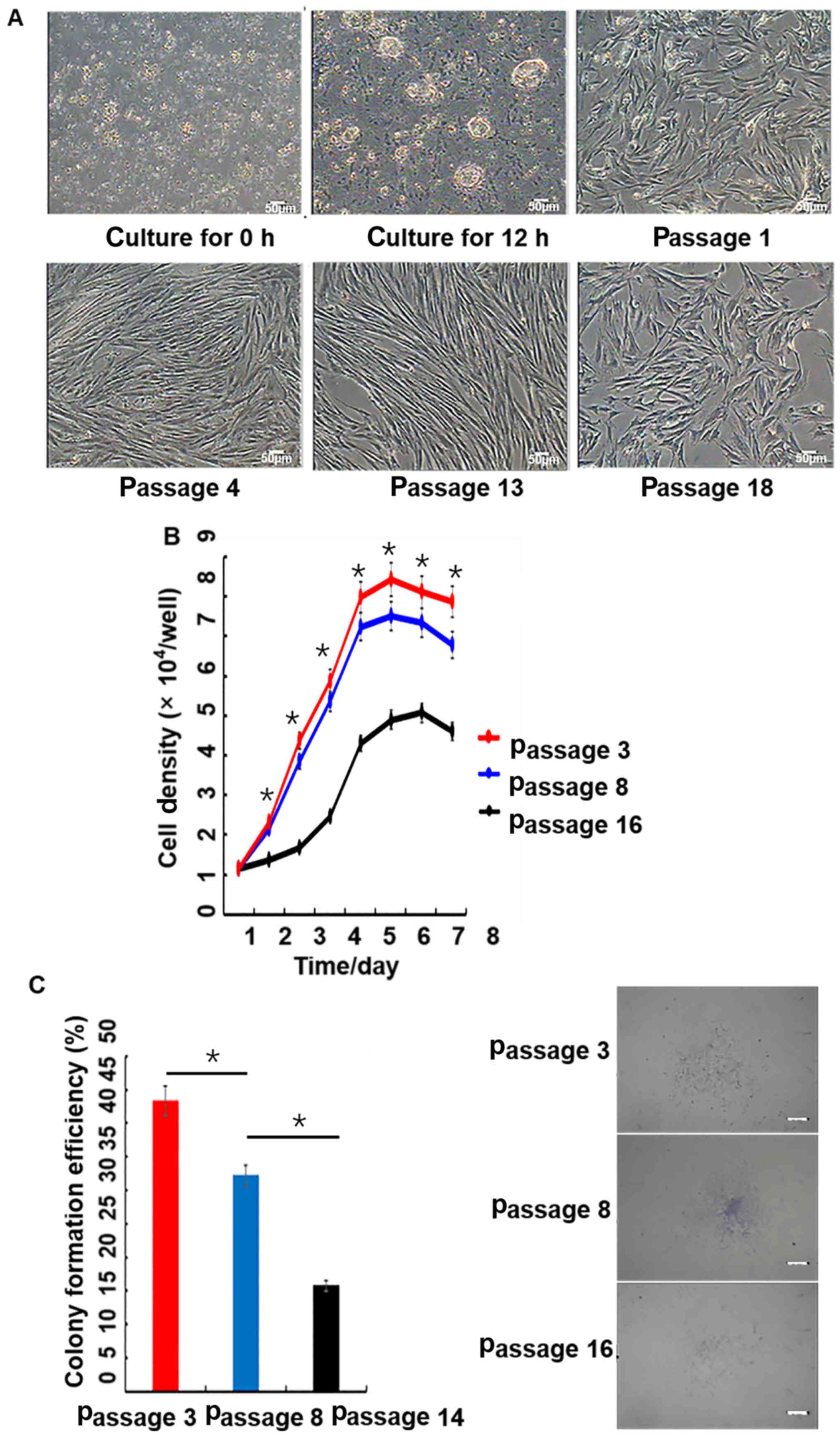 | Figure 2.Morphology, growth curves and
colony-forming efficiency of porcine PMSCs in vitro. (A)
Morphology of cells at 0 and 12 h, as well as passages 1, 4, 13 and
18; scale bar, 50 µm. (B) Growth curves of PMSCs. The growth curves
of passage 3, 8 and 16 PMSCs were all sigmoidal. The growth rate of
passage 16 cells was significantly slower than younger passage
cells. The growth curves consisted of latent, logarithmic and
plateau phases. The population doubling time, which was calculated
from the growth curve, was ~48 h. Passage 3 has a significant
difference with passage 16. (C) Colony-formation assay. Colony
formation rates of PMSCs at passage 3, 8 and 14. Scale bar, 50 µm.
*P<0.05. PMSC, pancreas-derived mesenchymal stromal cell. |
Growth kinetics
The growth curve of PMSCs displayed a typical ‘S’
shape, cells underwent latency, logarithmic and plateau phases
(Fig. 2B). However, passage 16
cells proliferated significantly slower compared with passage 3 and
8 cells. Based on the growth curve, the PDT of PMSCs was estimated
to be ~48 h.
Colony-forming assay
At passage 3, 8 and 14, the colony-forming ability
of PMSCs was detected by microscopy. Colony-forming efficiencies
were 43.57, 32.48 and 16.00% at passage 3, 8 and 14, respectively
(Fig. 2C).
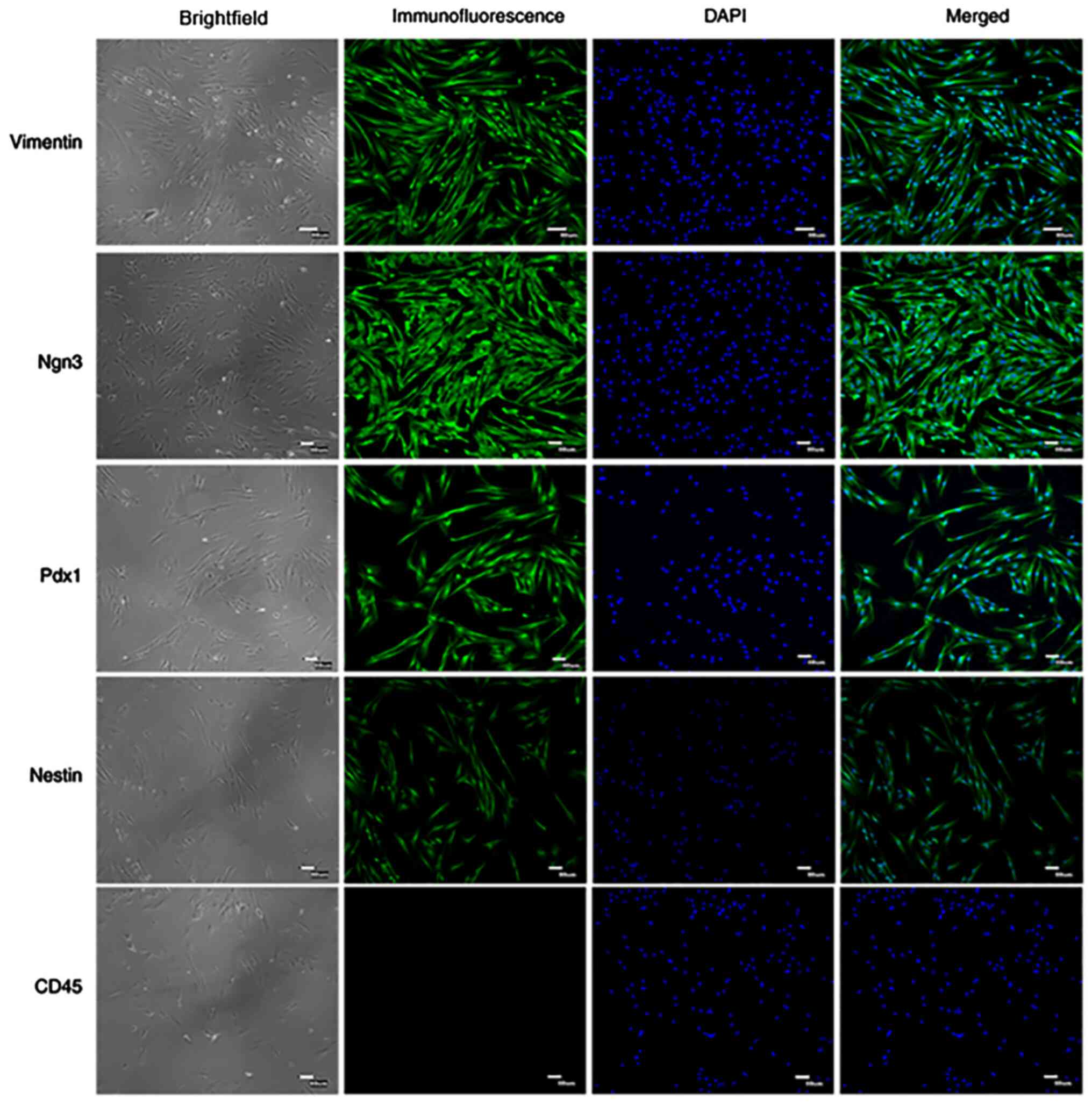
Immunofluorescence staining detection
of PMSC surface antigens markers
Specific surface markers, including Vimentin,
Nestin, Ngn3, Pdx1 and CD45, were detected by immunofluorescence
staining in passage 4 cells (Fig.
3). The results demonstrated that PMSCs positively expressed
Vimentin, Nestin, Ngn3 and Pdx1, but did not express CD45. Vimentin
is an important marker of mesenchymal cells (17) and the results demonstrated that
Vimentin was highly expressed in PMSCs.
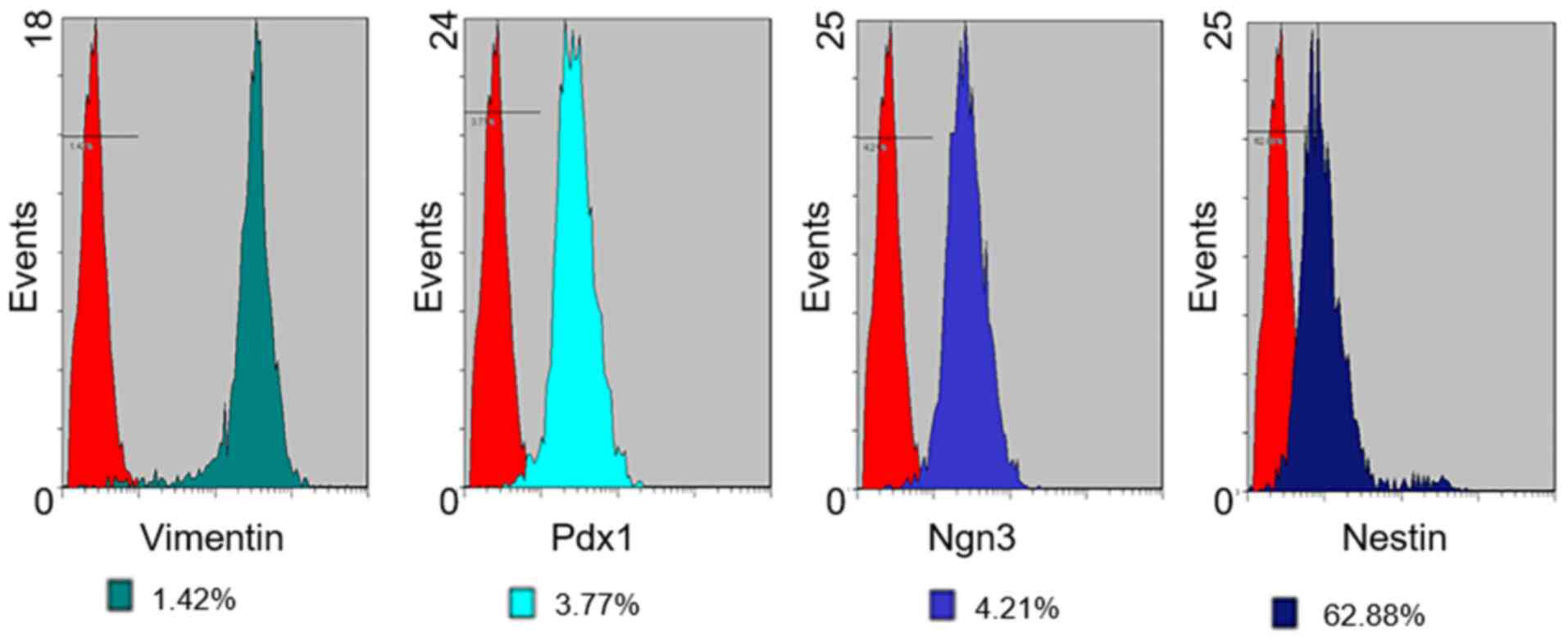
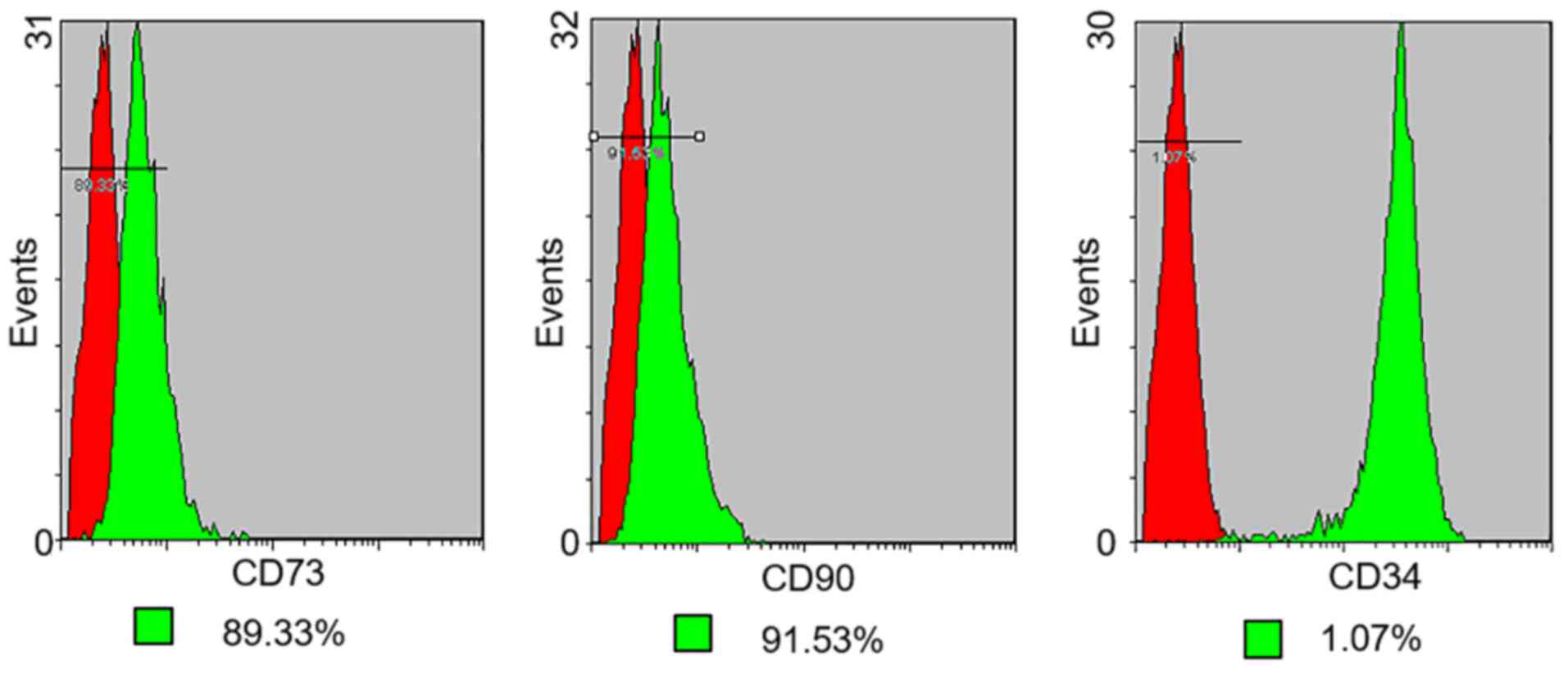
Flow cytometry
The expression of cell surface antigens by PMSCs in
early passage cells (up to passage 3) was analyzed by flow
cytometry (Figs. 4 and 5). Cultured PMSCs were composed of a
single phenotypic population. The positive rates of Vimentin, Pdx1,
Ngn3, Nestin, CD73, CD90 and CD34 were 98.58, 96.23, 95.79, 37.12,
89.33, 91.53 and 1.07%, respectively, which were calculated by 1
minus the rate of control rate.
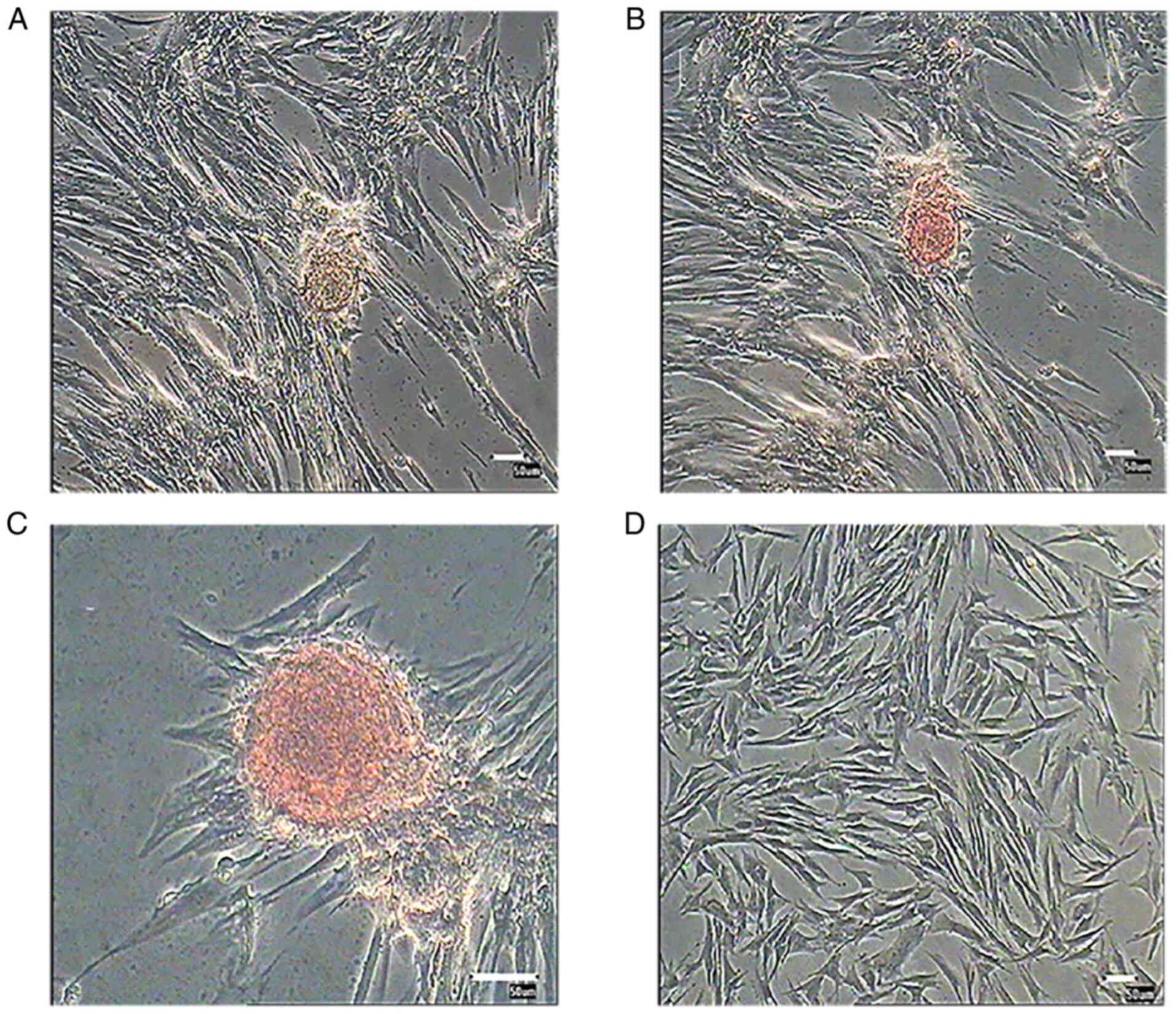
Morphological alterations and DTZ
staining of PMSC-derived IPCs
PMSCs at passage 3 were cultured in differentiation
medium for 3 weeks. Induced cells were observed daily for
morphological alterations. After 12 days, a number of small cell
clusters formed and the number of cell clusters increased as cells
differentiated (Fig. 6). After 3
weeks induction, cell clusters were stained with DTZ (Fig. 7) and showed a scarlet color.
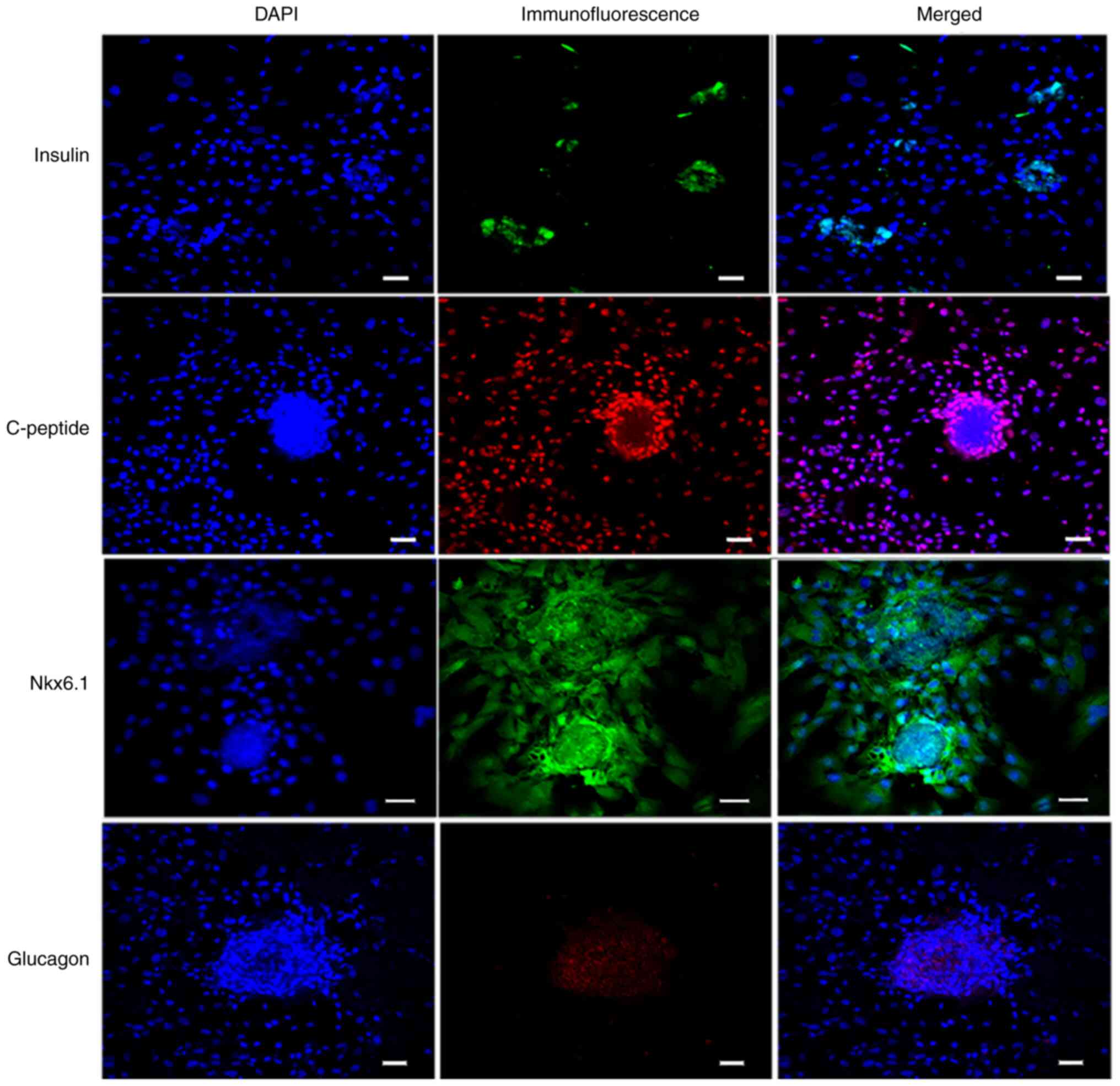
Immunofluorescence staining assays of
IPC markers
Differentiated cells were analyzed by performing an
immunofluorescence staining assay (Fig.
8). The differentiated islet-like cell clusters expressed
insulin and Nkx6.1, whereas the undifferentiated PMSCs around the
cell clusters did not express insulin and Nkx6.1. Glucagon staining
suggested that the differentiated cell clusters were monohormonal
cells. IPCs were positive for insulin and C-peptide, which are
stoichiometric byproducts of proinsulin processing (20). The results suggested that PMSCs were
differentiated into IPCs using the optimized three-step culture
procedure.
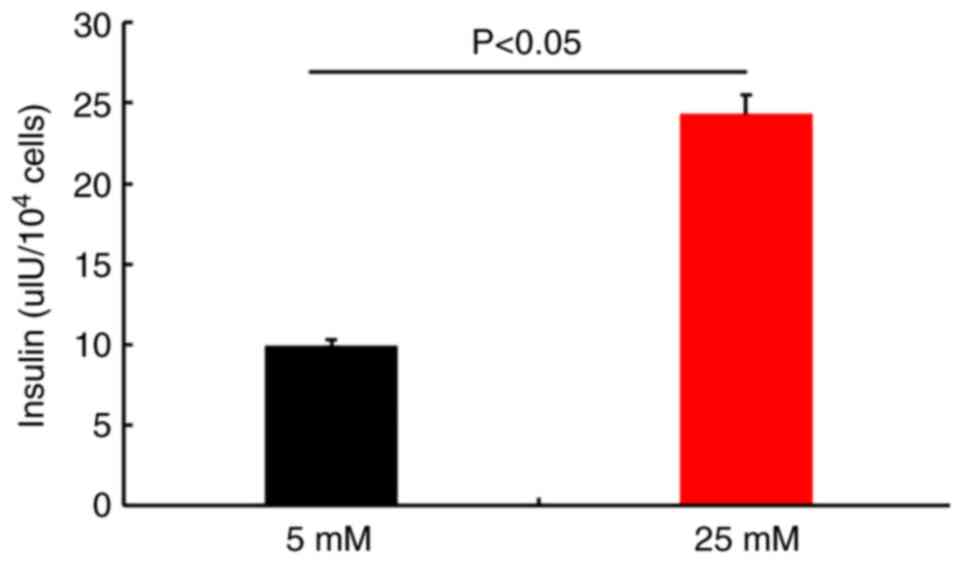
GSIS
Insulin secretion was evaluated by performing
ELISAs. The results demonstrated that newly generated IPCs secreted
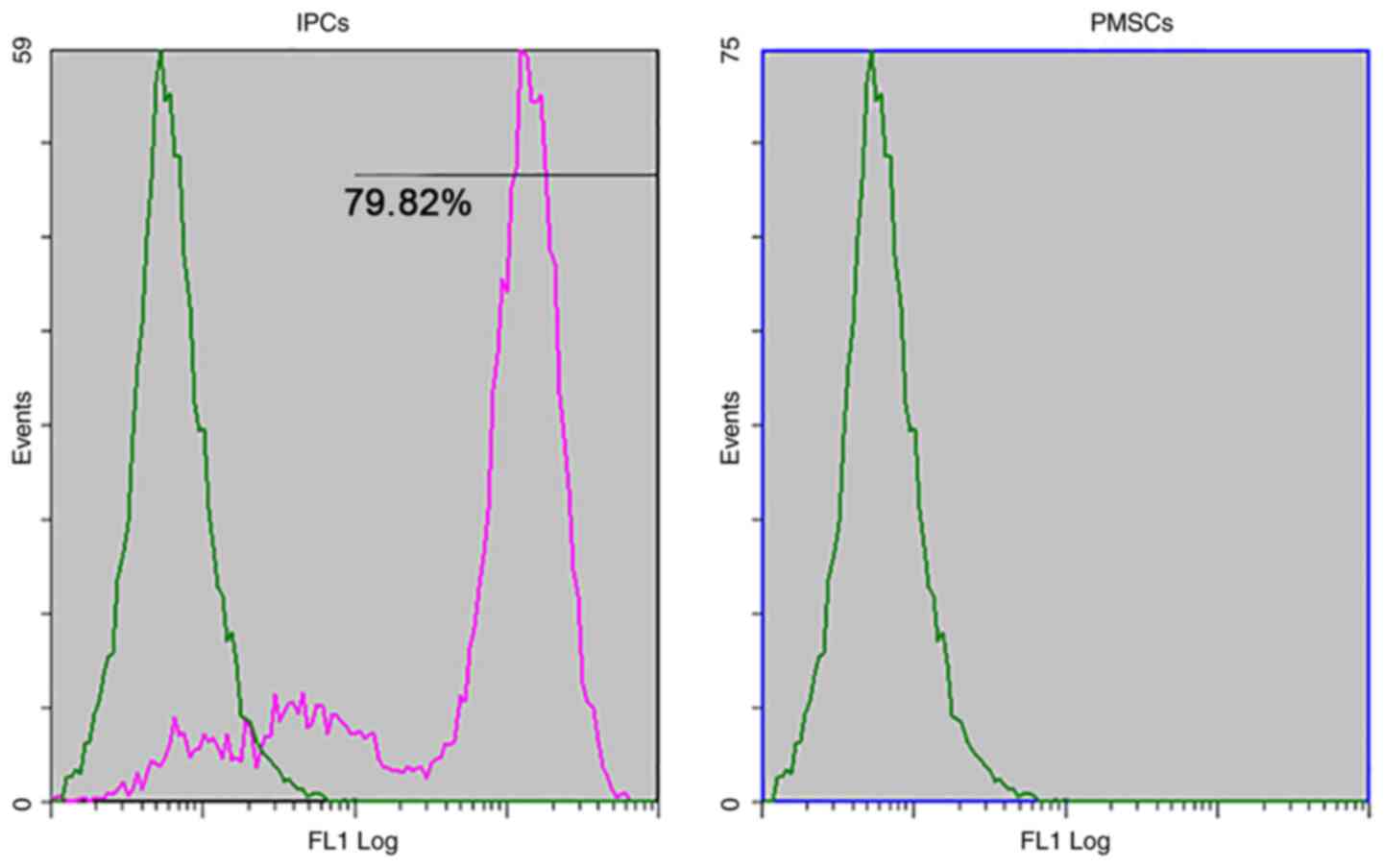
insulin in response to glucose stimulation (Fig. 9). The differentiation rate of PMSCs
was determined by flow cytometry. The results demonstrated that
79.82±1.36% of the total number of differentiated cells were
insulin-positive (Fig. 10), which
suggested that PMSCs had differentiated into functional IPCs.
Discussion
SCs have multiple differentiation potentials and
display self-renewal capacities; under certain conditions, SCs
significantly proliferate and differentiate into specific lineages
(21). MSCs derived from the
mesoderm can also differentiate into multiple mesodermal and
nonmesodermal cell lineages in vitro (21,22).
Owing to their high proliferative ability, lack of adverse
influence from allogeneic antigens and autoantigens, and teratoma
formation abilities, MSCs are a promising source for clinical
research (23). The differentiation
of MSCs into IPCs from bone marrow- (24–26),
umbilical cord blood- (27,28), adipose- (29,30)
and placenta-derived (31) MSCs has
been described.
The present study described an optimized three-step
induction protocol, which was used to obtain IPCs from porcine
PMSCs. Increasing evidence has demonstrated that SCs present in
pancreatic ducts, islets and acini display a specific potential for
differentiation (13). Compared
with the two-step induction protocol (13), the clear morphology of islet-like
cells was a notable benefit observed with the three-step protocol
used in the present study. In addition, pancreatic SCs are derived
from pancreas ductal tissues and display a cobblestone like
morphology, whereas PMSCs are derived from acinar tissue and
display a fibroblast-like morphology (20). PMSCs can be isolated using a simpler
protocol and display a higher proliferation ability than pancreas
stem cells (21). The present study
induced PMSC transformation into IPCs using a three-step protocol.
Firstly, cell growth in a favorable microenvironment was sustained
by adding bFGF and EGF, which promoted cell development. Secondly,
Activin A, B27 KGF and nicotinamide were used to stimulate PMSC
neural and entoderm differentiation. Finally, ITS, Activin A,
nicotinamide and exendin-4 were added to the culture medium to
sustain entoderm differentiation and to stimulate cells into an
islet-like morphology. In addition, compared with other adult SCs,
PMSCs are more likely to form IPCs (21), which suggested that they may serve
as an ideal candidate for diabetes therapy.
The present study demonstrated the successful
expansion of porcine PMSCs in vitro and the differentiation
into IPC clusters after 3 weeks induction; PMSCs aggregated and
formed islet-like cell clusters that were DTZ-positive and
expressed specific markers of β cells. The GSIS experiment results
indicated that the differentiated cells secreted insulin in
response to glucose alterations. The weak expression of insulin
indicated that the protocol requires further revision. Thus, the
function of induced IPCs with the three-step protocol requires
additional investigation in vivo using animal models and
other MSC types, such as bone marrow, adipose and other cells in
vitro.
The results of the present study indicated that
PMSCs displayed a strong self-renewal capacity and expressed the
surface markers of MSCs. The present study also provided evidence
for the generation of functional IPCs from PMSCs using an optimized
three-step protocol. However, the present study only investigated
the function of IPCs in vitro and lacked investigation using
an in vivo animal model. In addition, the three-step
protocol required multiple factors and lasted for 3 weeks; thus,
attempts should be made to simplify and optimize the protocol
further. The application of the three-step protocol in other cells
types, such as MSCs, should also be investigated in the future as
it may be of great significance for the treatment of diabetes.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Science Foundation of China (grant nos. 31201765, 31272403 and
31472064) and the earmarked fund for Modern Agri-industry
Technology Research System (grant no. nycytx-40-01).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ and QW performed the experiments, contributed to
data analysis, drafted the manuscript and were responsible for the
authenticity of data. HJ and HL revised the draft manuscript in the
Results and Discussion sections and also acquire the raw data. QY
and JY performed the data analysis. WG provided the final version
publication of the manuscript, designed the project and also
interpretated the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the
Institutional Animal Care and Use Committee of Chinese Academy of
Agricultural Sciences (approval no. IAS 2018-19).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guariguata L, Whiting DR, Hambleton I,
Beagley J, Linnenkamp U and Shaw JE: Global estimates of diabetes
prevalence for 2013 and projections for 2035. Diabetes Res Clin
Pract. 103:137–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Paoli M and Werstuck GH: Role of
estrogen in type 1 and type 2 diabetes mellitus: A review of
clinical and preclinical data. Can J Diabetes. 44:448–452. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Q and Melton DA: Pancreas
regeneration. Nature. 557:351–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan FC and Brissova M: Pancreas
development in humans. Curr Opin Endocrinol Diabetes Obes.
21:77–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bakhti M, Böttcher A and Lickert H:
Modelling the endocrine pancreas in health and disease. Nat Rev
Endocrinol. 15:155–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fathi E, Farahzadi R and Sheikhzadeh N:
Immunophenotypic characterization, multi-lineage differentiation
and aging of zebrafish heart and liver tissue-derived mesenchymal
stem cells as a novel approach in stem cell-based therapy. Tissue
Cell. 57:15–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arnold K, Sarkar A, Yram MA, Polo JM,
Bronson R, Sengupta S, Seandel M, Geijsen N and Hochedlinger K:
Sox2(+) adult stem and progenitor cells are important for tissue
regeneration and survival of mice. Cell Stem Cell. 9:317–329. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gross JB and Hanken J: Use of fluorescent
dextran conjugates as a long-term marker of osteogenic neural crest
in frogs. Dev Dyn. 230:100–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu F, Wei R, Yang J, Liu J, Yang K, Wang
H, Mu Y and Hong T: FoxO1 inhibition promotes differentiation of
human embryonic stem cells into insulin producing cells. Exp Cell
Res. 362:227–234. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan J, Liu L, Li B, Xie Q, Sun J, Pu H and
Zhang L: Pancreatic stem cells differentiate into insulin-secreting
cells on fibroblast-modified PLGA membranes. Mater Sci Eng C Mater
Biol Appl. 97:593–601. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daryabor G, Shiri EH and Kamali-Sarvestani
E: A simple method for the generation of insulin producing cells
from bone marrow mesenchymal stem cells. In Vitro Cell Dev Biol
Anim. 55:462–471. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cooper TT, Sherman SE, Bell GI, Ma J,
Kuljanin M, Jose SE, Lajoie GA and Hess DA: Characterization of a
Vimentin high/Nestinhigh proteome and tissue
regenerative secretome generated by human pancreas-derived
mesenchymal stromal cells. Stem Cells. 38:666–682. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee S, Moon S, Oh JY, Seo EH, Kim YH, Jun
E, Shim IK and Kim SC: Enhanced insulin production and
reprogramming efficiency of mesenchymal stem cells derived from
porcine pancreas using suitable induction medium.
Xenotransplantation. 26:e124512019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iqbal MA, Hong K, Kim JH and Choi Y:
Severe combined immunodeficiency pig as an emerging animal model
for human diseases and regenerative medicines. BMB Rep. 52:625–634.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu X, Liu G, Halim A, Ju Y, Luo Q and Song
AG: Mesenchymal stem cell migration and tissue repair. Cells.
8:7842019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu J, Yu L, Guo J, Xiang J, Zheng Z, Gao
D, Shi B, Hao H, Jiao D, Zhong L, et al: Generation of pig induced
pluripotent stem cells using an extended pluripotent stem cell
culture system. Stem Cell Res Ther. 10:1932019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kleeblatt J, Schubert JK and Zimmermann R:
Detection of gaseous compounds by needle trap sampling and direct
thermal-desorption photoionization mass spectrometry: Concept and
demonstrative application to breath gas analysis. Anal Chem.
87:1773–1781. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shiroi A, Yoshikawa M, Yokota H, Fukui H,
Ishizaka S, Tatsumi K and Takahashi Y: Identification of
insulin-producing cells derived from embryonic stem cells by
zinc-chelating dithizone. Stem Cells. 20:284–292. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pagliuca FW, Millman JR, Gürtler M, Segel
M, Van Dervort A, Ryu JH, Peterson QP, Greiner D and Melton DA:
Generation of functional human pancreatic β cells in vitro. Cell.
159:428–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai C, Gao Y, Zhang X, Yang W and Guan W:
MicroRNA-34c acts as a bidirectional switch in the maturation of
insulin-producing cells derived from mesenchymal stem cells.
Oncotarget. 8:106844–106857. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu M and Han ZC: Mesenchymal stem cells:
biology and clinical potential in type 1 diabetes therapy. J Cell
Mol Med. 12:1155–1168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S, Zhu Z, Wang Y, Liu S, Zhao C,
Guan W and Zhao Y: Therapeutic potential of Bama miniature pig
adipose stem cells induced hepatocytes in a mouse model with acute
liver failure. Cytotechnology. 70:1131–1141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Venkatesh K and Sen D: Mesenchymal stem
cells as a source of dopaminergic neurons: A potential cell based
therapy for parkinson's disease. Curr Stem Cell Res Ther.
12:326–347. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gabr MM, Zakaria MM, Refaie AF, Khater SM,
Ashamallah SA, Ismail AM, El-Halawani SM and Ghoneim MA:
Differentiation of human bone marrow-derived mesenchymal stem cells
into insulin-producing cells: Evidence for further maturation in
vivo. Biomed Res Int. 2015:5758372015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Huang H, Liu X, Xia H and Li M: In
vitro generation of insulin-producing cells from the neonatal rat
bone marrow mesenchymal stem cells. Xi Bao Yu Fen Zi Mian Yi Xue Za
Zhi. 31:346–349. 2015.(In Chinese). PubMed/NCBI
|
|
26
|
Zhang S, Zhao C, Liu S, Wang Y, Zhao Y,
Guan W and Zhu Z: Characteristics and multi-lineage differentiation
of bone marrow mesenchymal stem cells derived from the Tibetan
mastiff. Mol Med Rep. 18:2097–2109. 2018.PubMed/NCBI
|
|
27
|
Van Pham P, Thi-My Nguyen P, Thai-Quynh
Nguyen A, Minh Pham V, Nguyen-Tu Bui A, Thi-Tung Dang L, Gia Nguyen
K and Kim Phan N: Improved differentiation of umbilical cord
blood-derived mesenchymal stem cells into insulin-producing cells
by PDX-1 mRNA transfection. Differentiation. 87:200–208. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsai PJ, Wang HS, Shyr YM, Weng ZC, Tai
LC, Shyu JF and Chen TH: Transplantation of insulin-producing cells
from umbilical cord mesenchymal stem cells for the treatment of
streptozotocin-induced diabetic rats. J Biomed Sci. 19:472012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou H, Yang J, Xin T, Li D, Guo J, Hu S,
Zhou S, Zhang T, Zhang Y, Han T and Chen Y: Exendin-4 protects
adipose-derived mesenchymal stem cells from apoptosis induced by
hydrogen peroxide through the PI3K/Akt-Sfrp2 pathways. Free Radic
Biol Med. 77:363–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karaoz E, Okcu A, Ünal ZS, Subasi C,
Saglam O and Duruksu G: Adipose tissue-derived mesenchymal stromal
cells efficiently differentiate into insulin-producing cells in
pancreatic islet microenvironment both in vitro and in vivo.
Cytotherapy. 15:557–570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kadam S, Muthyala S, Nair P and Bhonde R:
Human placenta-derived mesenchymal stem cells and islet-like cell
clusters generated from these cells as a novel source for stem cell
therapy in diabetes. Rev Diabet Stud. 7:168–182. 2010. View Article : Google Scholar : PubMed/NCBI
|