Introduction
Cervical cancer is a common gynecological malignant
tumor with a high mortality rate. There are 530,000 newly diagnosed
cases of cervical cancer worldwide and >270,000 deaths each year
(1). The diagnosis of early and
mid-stage cervical cancer is increasingly accurate, and patient
prognosis has improved (2,3). However, cervical cancer remains the
leading cause of morbidity and mortality among women worldwide.
Previous studies have demonstrated that non-coding
RNA serves an important role in physiological functions (4,5), such
as metabolism, differentiation and apoptosis of various types of
cell (6). Certain studies have
suggested that the expression of microRNA (miRNA or miR) is
associated with the occurrence and development of diverse types of
cancer, such as breast cancer and thyroid cancer (7,8). Long
non-coding RNA (lncRNA) sponges miRNA and decreases miRNA levels,
thereby regulating downstream target genes (9). This lncRNA/miRNA/mRNA axis has been
implicated in the regulation of biological processes in cancer
cells, such as osteosarcoma (10,11).
A previous study indicated that the expression of
MIR9-3 host gene (HG) is a biomarker of the development of cervical
cancer (12). Additionally,
MIR9-3HG is upregulated in head and neck squamous cell carcinoma
tissue (13). However, whether
MIR9-3HG affects the proliferation and metastasis of cervical
cancer cells is unclear.
The present study investigated the expression of
MIR9-3HG and its potential regulatory role in the proliferation of
cervical cancer cells in vitro. Cancer cell apoptosis was
detected via flow cytometry. The potential molecular mechanism of
action of MIR9-3HG was further investigated by screening
bioinformatics databases, then validated via in vitro and
in vivo experiments. The aim of the study was to provide
insight into the role of MIR9-3HG in the proliferation and
apoptosis of cervical cancer cells, as well as the underlying
molecular mechanism.
Materials and methods
Cell culture and treatment
Cervical cancer cell lines (cervical squamous cell
carcinoma, C33A; human papillomavirus-related cervical squamous
cell carcinoma, SiHa; human papillomavirus-related endocervical
adenocarcinoma, HeLa; human papillomavirus-related cervical
squamous cell carcinoma, CaSki; and human cervical epithelial
immortalized cells, H8 (used as normal control group.) were
obtained from the Chinese Academy of Sciences (Shanghai, China).
All cell lines were cultured with RPMI-1640 medium (HyClone;
Cytiva) supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
with 5% CO2. MIR9-3HG and EP300 knockdown and ER300
overexpression were performed in C33A and SiHa cells using
plasmids. The miR-498 inhibitor and mimic were purchased from
Shanghai GeneChem Co., Ltd. Polybrene was obtained from Shanghai
GeneChem Co., Ltd. and used to enhance transfection efficacy.
Lentivirus, plasmids and miR-mimic or miR-inhibitor
transfection. MIR9-3HG and EP300 knockdown lentiviral vectors, as
well as EP300 overexpression pcDNA3.1 plasmids were obtained from
Shanghai GeneChem Co., Ltd. A total of 3×106 293T cells
were seeded into a 10-cm cell culture dish and cultured overnight
at 37°C with 5% CO2. The lentivirus was packaged and
prepared by transfecting vectors into 293T cells (American Type
Culture Collection). The ratio of the lentiviral plasmid:packaging
vector was 4:3:1.
The lentivirus was collected, then transduced into
C33A or SiHa cells (lentivirus titer 2×10 TU/ml) using polybrene
(Beijing Solarbio Science & Technology Co., Ltd.). The miR-498
inhibitor (120 nM; 5′-CUUUUUGCGGGGGACCGAACUUU-3′), miR-498 mimic
(40 nM; 5′-GAAAAACGCCCCCUGGCUUGAAA-3′) and their negative controls
(NC; random sequence anti-miR molecule and scrambled miRNA,
respectively) were transfected into C33A or SiHa cells using
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.) at
room temperature for 48 h.
Cell Counting Kit (CCK)-8 assay
CCK-8 assay was performed to evaluate the cell
viability. C33A and SiHa cells (2×104 cells/ml) were
plated into four 96-well plates and cultured at 37°C. After 24, 48
or 72 h transfection, CCK-8 reagent (10 µl) (Dojindo Molecular
Technologies, Inc.) was added into culture medium and added into
the 96-well plates. The cells were incubated for 1 h. After
incubation, absorbance at 450 nm was measured using a
spectrophotometer (Thermo Fisher Scientific, Inc.).
5-Ethynyl-2′-deoxyuridine (Edu)
assay
The proliferation of C33A and SiHa cells was
evaluated via EdU assay (Yusheng Company; http://www.yeasen.com/search?q=EdU), according to the
manufacturer's instructions. The nuclei were stained with DAPI for
5 min at room temperature in the dark according to manufacturer′
protocol (Sigma-Aldrich; Merck KGaA).
Apoptosis assay
Apoptosis rates were determined using commercial
kits (Beyotime Institute of Biotechnology). Cells were washed in
PBS to remove FBS, then incubated with Annexin-V and PI for 40 min
at 4°C in the dark. The apoptosis rate was detected via flow
cytometry (Attune NxT; Thermo Fisher Scientific, Inc.). The ratio
of early and late stage apoptosis was expressed as Q2+Q3. The
apoptosis levels were analyzed with FlowJo 10 software (Becton,
Dickinson and Company).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA of C33A and SiHa cells or nude mice
tissues was collected using TRIzol® at room temperature
(Thermo Fisher Scientific, Inc.). RNA was reverse transcribed into
cDNA using an RT kit (Takara Bio, Inc.) at 42°C for 60 min,
followed by transcription at 70°C for 5 min. Amplification of cDNA
was performed using the ABI 7500 system (Thermo Fisher Scientific,
Inc.). For the detection for miR-498 levels, miRNA was extracted
using Rapid Extraction kit for miRNA (HaiGene; http://www.haigene.cn/) and then reverse transcribed
using TaqMan miRNA RT kit. The quantification for miR-498 was
performed using TaqMan miRNA qPCR kit (HaiGene; http://www.haigene.cn/) and U6 was used as the
internal reference. The qPCR thermocycling conditions were as
follows: Initial denaturation at 95°C for 5 min, followed by 35 of
cycles of denaturation at 95°C for 30 sec, annealing at 56.2°C for
30 sec and extension at 72°C for 30 sec; followed by a final
extension at 72°C for 7 min. The results were analyzed via the
2−∆∆Cq method (14). The
primers used were as follows: MIR9-3HG forward,
5′-CAGATGTTCGGTCCCCACTC-3′ and reverse, 5′-TCGGCCTCCTTTGCTTAGAC-3′;
miR-498 forward, 5′-GGTTTGAAGCCAGGCGGTTTC-3′ and reverse,
5′-CAGTGCAGGGTCCGAGGTAT-3′; cysteine and histidine rich domain
containing 1 (CHORDC1) forward, 5′-CATGCACCACTACACTCAGC-3′ and
reverse, 5′-GCCTCCTTGCTGACTGATTC-3′; eukaryotic translation
initiation factor 3 subunit J (EIF3J) forward,
5′-CGGAGCAGCAGGAAATCTCT-3′ and reverse, 5′-GCAGGTTTTGTTGCGAAGTG-3′;
EP300 forward, 5′-AAAAATAAGAGCAGCCTGAG-3′ and reverse,
5′-AGACCTCTTTATGCTTCTTCC-3′; lectin, mannose binding 1 (LMAN1)
forward, 5′-GCACAAGGGCATTTTGGA-3′ and reverse,
5′-GAAAGGACATCATGGTCATCTG-3′; GRIP and coiled-coil
domain-containing protein 2 (GCC2) forward,
5′-AGCTTCAGAAAACCATGCAAGAA-3′ and reverse,
5′-GCTCAGCTTGTACTCAGGGC-3′; DEAH-box helicase 35 (DHX35) forward,
5′-TACACCTACACCGGCAAAAGC-3′ and reverse,
5′-AAAACAGCTCCGCATCAACCT-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′
and U6 forward, 5′-CGGGTTTGTTTTGCATTTGT-3′ and reverse,
5′-AGTCCCAGCATGAACAGCTT-3′. The detection of expression of MIR9-3HG
in the cytoplasm and nucleus was performed using commercial kits
Cytoplasmic & Nuclear RNA Purification Kit (50) (cat. no.
21000; Norgen Biotek Corp.) according to the manufacturer's
instructions.
Western blotting
Total protein of C33A cells, SiHa cells or tumor
tissue of nude mice was collected using RIPA buffer (Beyotime
Institute of Biotechnology). Protein concentration was determined
using the BCA method (Beyotime Institute of Biotechnology).
Proteins (50 µg) of each lane were separated on 10% SDS-PAGE gels
(Beyotime Institute of Biotechnology), then transferred to PVDF
membranes (EMD Millipore). Following blocking with 5% skimmed milk
(Thermo Fisher Scientific, Inc.) for 2 h at 4°C, the membranes were
incubated with primary antibodies (all Abcam) at 4°C overnight. The
primary antibodies used were specific for EP300 (cat. no. ab10485;
1:5,000), Ki-67 (cat. no. ab92742; 1:5,000) and GAPDH (cat. no.
ab9485; 1:2,500). After incubation, the membranes were washed in
PBST containing 0.05% Tween-20, then incubated with secondary
antibodies (goat anti Rabbit IgG; cat. no. ab216777; 1:10,000).
Protein bands were visualized using enhanced chemiluminescence
reagent (Pierce; Thermo Fisher Scientific, Inc.) and then analyzed
using ImageJ software 1.46r (National Institutes of Health).
RNA immunoprecipitation assay
(RIP)
RIP was performed using commercial kits (EZ-Magna
RIP™ RNA-Binding Protein Immunoprecipitation kit; EMD Millipore),
according to the manufacturer's instructions. pSL-MS2-12X (Addgene,
Inc.), together with pcDNA3.1 or pcDNA3.1- MIR9-3HG (Genomeditech),
was used to construct pcDNA3.1-MS2-12X (2 µg) or pcDNA3.1-
MIR9-3HG-MS2-12X vectors, which were then co-transfected into C33A
or SiHa cells with pMS2-GFP (Addgene, Inc.) using Lipofectamine
3000 (Thermo Fisher Scientific, Inc.) at room temperature. After 48
h, the transfected cells were collected for RIP. Briefly, cells
were lysed in Complete RIP lysis buffer containing RIP Lysis
Buffer, Protease Inhibitor Cocktail and RNase Inhibitor (Guangzhou
Sai Cheng Biotechnology Co., Ltd.) for 5 min. Magnetic beads were
resuspended in RIP Wash Buffer and mixed with anti-GFP or IgG (5
µg; GFP, cat. no. 11814460001; IgG, cat. no. 06681743001; Roche
Diagnostics). Following incubation at room temperature for 30 min,
the RIP Buffer was added to the magnetic bead and antibody mixture,
followed by the cell lysate. Following incubation overnight at 4°C,
the precipitate was collected and resuspended in Proteinase K
Buffer (150 µl). The miRNA enriched by IP was reverse-transcribed
to cDNA for RT-qPCR analysis, as described previously.
RNA pull-down assay
Biotin-labeled RNA probes [lncRNA full-length or
mutant (Mut)] were obtained through in vitro transcription.
The pSP72 plasmid (Addgene, Inc.) containing full-length wild-type
(WT) or Mut MIR-3HG was digested using a single enzyme to obtain a
linear template, which was then labeled using Biotin RNA Labeling
Mix (Roche Diagnostics). The biotin-labeled RNA (3 µg) was added to
cell lysate prepared using 1 ml TRIzol (Takara Bio, Inc.), then
incubated at room temperature for 1 h. The RNA mixture (100 µg) was
then mixed with magnetic beads labeled by streptavidin according to
manufacturer's protocol for 1 h (Dyna beads M-280 Streptavidin;
Thermo Fisher Scientific, Inc.). After 60 min, the magnetic beads
were deposited with a magnetic field holder and then the
supernatant was discarded Then, the beads were washed three times
with lysis buffer. The beads were re-suspended in Elution Buffer
(15 µl) at 70°C. The magnetic beads were deposited with a magnetic
field holder to collect the supernatant for RT-qPCR analysis of
miR-498 expression levels as described previously.
Bioinformatic prediction
The expression of MIR9-3HG in cervical tumor and
normal tissue was analyzed using the Gene Expression Profiling
Interactive Analysis (GEPIA) database (cervical tumor number =306;
normal number =13; gepia.cancer-pku.cn/). The targeting miRNAs were
predicted using the PITA (genie.weizmann.ac.il/pubs/mir07/mir07_dyn_data.html),
DIANA-microT (diana.cslab.ece.ntua.gr/microT) and miRNAMAP
(mirnamap.mbc.nctu.edu.tw) databases. The
association of miR-498 or EP300 with MIR9-3HG was predicted through
Starbase v2.0 (http://starbase.sysu.edu.cn/).
Dual luciferase assay
Luciferase assays were performed using Pierce™
Renilla-Firefly Luciferase Dual Assay kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Transient plasmid transfection was performed when the cells reached
70–80% confluence. The miR-498 mimic was co-transfected into C33A
or SiHa cells together with MIR9-3HG-WT-1, MIR9-3HG-WT-2,
MIR9-3HG-Mut-1, MIR9-3HG-Mut-2, MIR9-3HG-WT-1-WT-2,
MIR9-3HG-WT-1-Mut-2, MIR9-3HG-WT-2-Mut-1 or MIR9-3HG-Mut-1-Mut-2
luciferase reporter plasmid (Promega Corporation) using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) at
37°C for 48 h. After discarding medium, the cells were washed with
PBS 2–3 times. Then, 100 µl lysis buffer was added to each well and
incubated in an orbital shaker in a cold room for ~20 min to fully
lyse the cells. Luciferase activity was analyzed using a
luminometer fluorescence detector (Turner BioSystems; Thermo Fisher
Scientific, Inc.). Renilla luciferase activity was used as
internal reference.
Animal experiments
A total of 18 female nude mice (age, 4–6 weeks;
weight, 16–18 g) were obtained from the Shanghai Experimental
Animal Center, Chinese Academy of Sciences (Shanghai, China). The
cage tools, feed, padding and drinking water used were sterilized
by high pressure steam and handled by professionals. The room had a
12-h light/dark cycle, a temperature of 25–27°C and a relative
humidity of 40–45%. The mice had free access to food and water.
C33A cells were re-suspended with PBS
(5×106 cells/ml). C33A cells (0.2 ml) in each
experimental group (n=3 mice) were injected subcutaneously into in
the right armpit of nude mice. Vernier calipers were used to
measure tumor volume every three days for three weeks. After three
weeks, mice were sacrificed by cervical dislocation. Death was
indicated by cessation of breathing, muscle relaxation and lack of
nerve reflex. The tumor tissue was weighed using electronic scales.
The experiment lasted for 21 days and there was no mouse death
during experiment. The animal health and behavior were monitored
every day. The experiments were approved by the Ethics Committee of
the First Affiliated Hospital of Zhejiang Chinese Medical
University.
Statistical analysis
All data were analyzed using GraphPad Prism 7.0
software (GraphPad Software, Inc.). Data are presented as the mean
± SD (n=3). The comparison between groups was performed by one-way
ANOVA, followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
MIR9-3HG knockdown inhibits
proliferation and promotes apoptosis of cervical cancer cells
The data obtained from GEPIA database (which
contains information from The Cancer Genome Atlas database) showed
that the expression of MIR9-3HG in cervical tumor tissue was
significantly higher than that in adjacent normal tissue (4-fold
difference; Fig. 1A). The
expression of MIR9-3HG in cell lines was determined using RT-qPCR.
The expression of MIR9-3HG in cervical cell lines was higher than
that in cervical epithelial cells (2.9-fold difference; Fig. 1B). Additionally, the expression
levels of MIR9-3HG in C33A and SiHa cells appeared to be higher
compared with those in HeLa or CaSki cells. Therefore, C33A and
SiHa cells were selected for subsequent experiments and MIR9-HG was
silenced in both cell lines. The expression of MIR9-3HG decreased
2.8-fold following knockdown (Fig.
1C). CCK-8 assays were then performed to detect cell viability.
Viability of C33A and SiHa cells was inhibited following knockdown
of MIR9-3HG (Fig. 1D). Next, cell
proliferation was determined via EdU assay. The proliferation of
C33A and SiHa cells was inhibited following MIR9-3HG knockdown
(Fig. 1E). Flow cytometry was used
to measure the apoptosis rates in these cells. The apoptosis rates
of C33A and SiHa cells following MIR9-3HG knockdown were
significantly increased (4.2-fold; Fig.
1F and G).
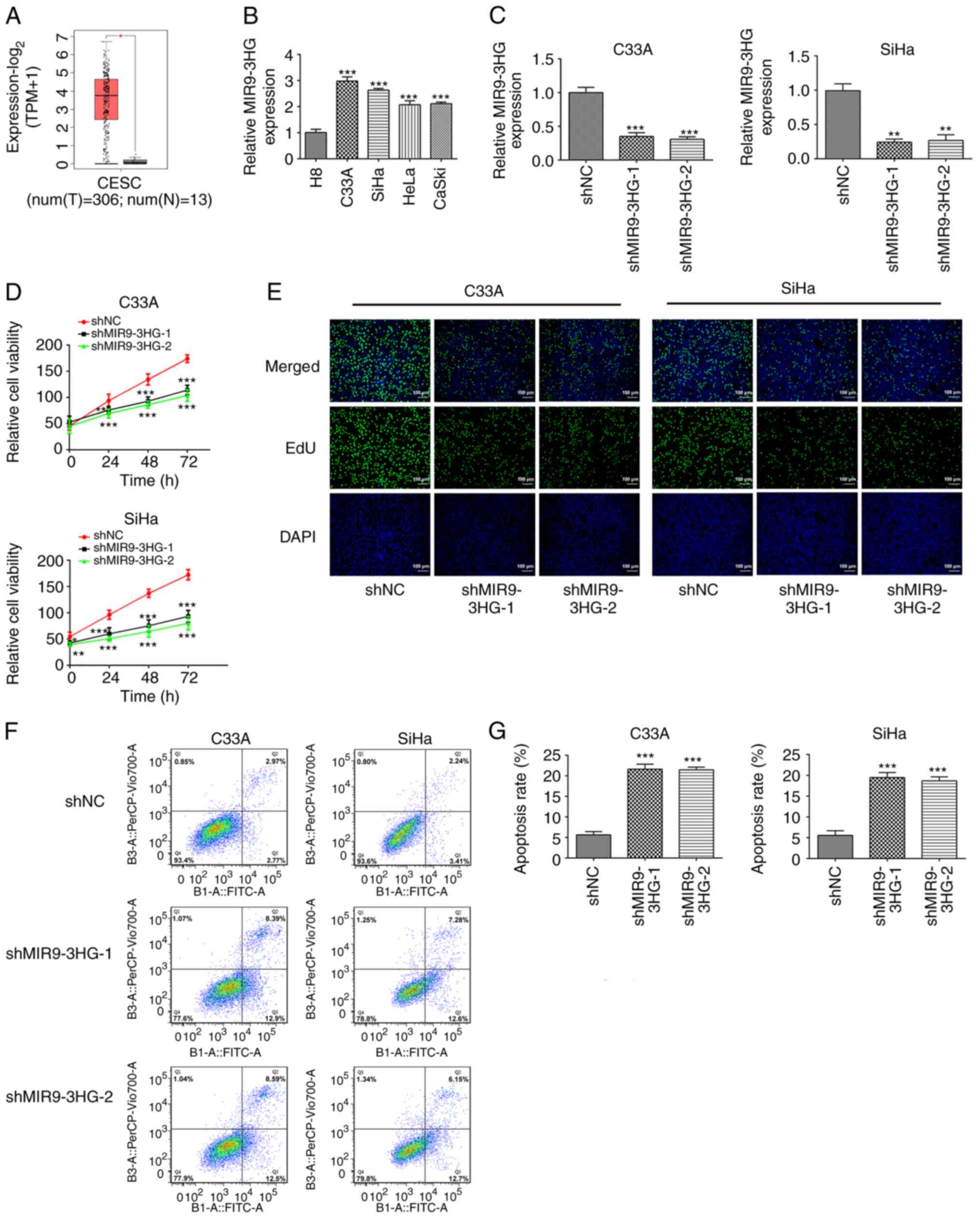 | Figure 1.Knockdown of MIR9-3HG suppresses
proliferation and promotes apoptosis of cervical cancer cells. (A)
Expression levels of MIR9-3HG in cervical cancer and normal tissue
from The Cancer Genome Atlas database. *P<0.05. (B) RT-qPCR was
performed to detect the expression levels of MIR9-3HG in cervical
cancer cell lines. ***P<0.001 vs. H8. (C) RT-qPCR was performed
to detect the expression levels of MIR9-3HG in cells of knockdown
groups. **P<0.01, ***P<0.001 vs. shNC. Cell (D) viability,
(E) proliferation and (F and G) apoptosis were detected via Cell
Counting Kit-8 and EdU assays and flow cytometry, respectively.
Magnification, ×100. Data are presented as the mean ± SD.
*P<0.05, **P<0.01, ***P<0.001 vs. shNC. MIR9-3HG, MIR9-3
host gene; RT-q, reverse transcription-quantitative; shNC, short
hairpin negative control; EdU, 5-ethynyl-2′-deoxyuridine; T, tumor;
N, normal; CESC, cervical squamous cell carcinoma and endocervical
adenocarcinoma. |
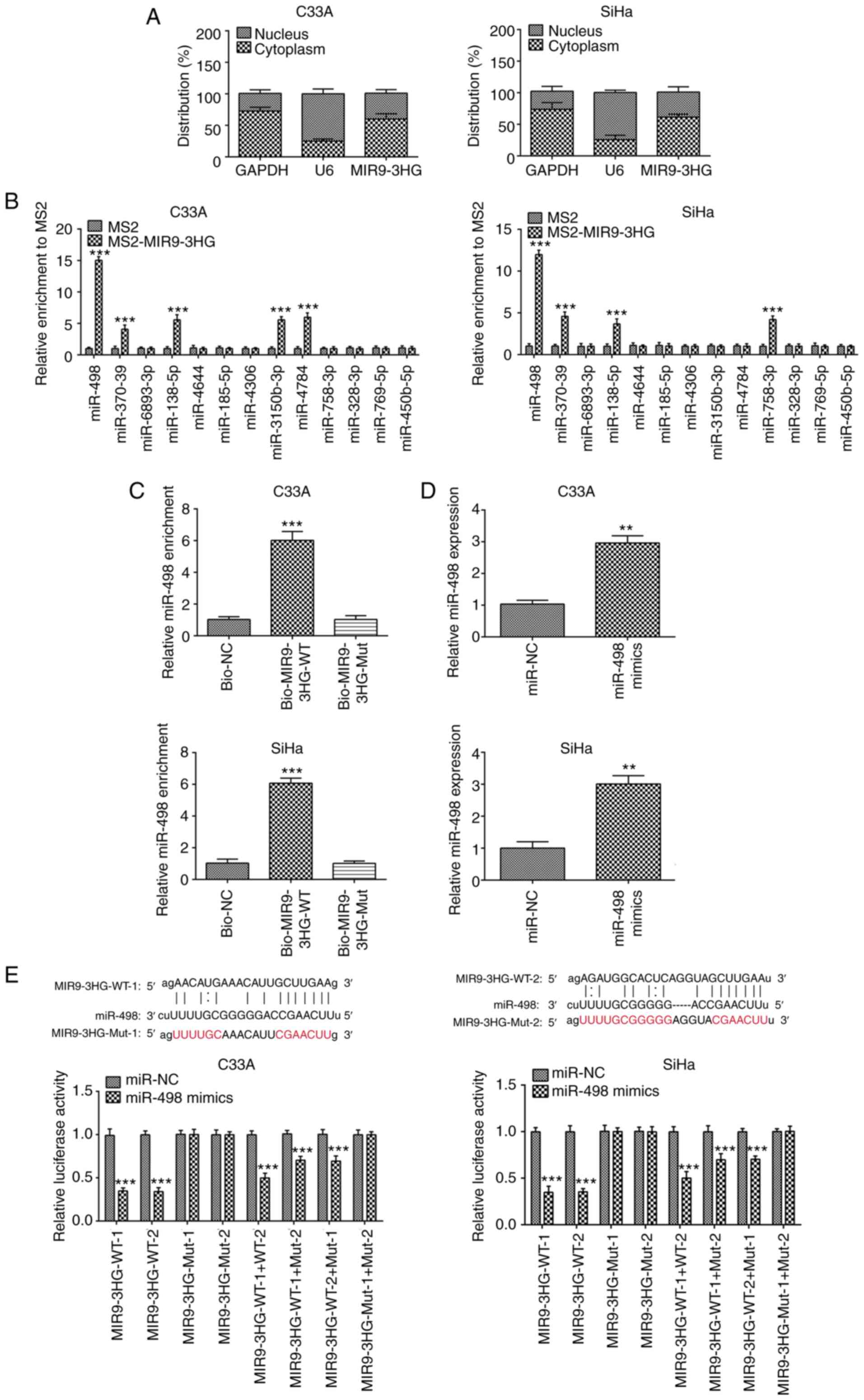
MIR9-3HG interacts with miR-498
In order to investigate the molecular mechanism of
MIR9-3HG in the pathogenesis of cervical cancer, the distribution
of MIR9-3HG was measured in the cytoplasm and nucleus of C33A and
SiHa cells. MIR9-3HG was primarily distributed in the cytoplasm of
C33A and SiHa cells (Fig. 2A).
Using Starbase, MIR9-3HG was demonstrated to interact with multiple
miRNAs. RIP assay was then performed to confirm the interaction
between MIR9-3HG and these miRNAs. MIR9-3HG overexpression resulted
in greater miR-498 enrichment compared with the control (Fig. 2B), indicating an interaction between
MIR9-3HG and miR-498. This was also confirmed via RNA pull-down
assay. The enrichment in miR-498 was decreased 5.9-fold in C33A or
SiHa cells with MIR9-3HG-Mut, compared with the Biotin-labeled
MIR9-3HG WT group (Fig. 2C).
miR-498 was then overexpressed in C33A
and SiHa cells
The expression of miR-498 was increased in the
overexpression group, compared with NC (2.9-fold difference,
Fig. 2D). In order to verify the
interaction between MIR9-3HG and miR-498, luciferase reporter assay
was performed. A significant decrease in luciferase activity was
observed following overexpression of miR-498 in C33A and SiHa cells
(Fig. 2E).
miR-498 inhibits the expression of
EP300
In order to investigate the role of miR-498 in the
development of cervical cancer at the molecular level, potential
interactions between miR-498 and target proteins were identified
using databases (Fig. 3A). A total
of six proteins were consistently identified in the PITA
(genie.weizmann.ac.il/pubs/mir07/mir07_dyn_data.html),
DIANA-microT (diana.cslab.ece.ntua.gr/microT) and miRNAMAP
(mirnamap.mbc.nctu.edu.tw) databases:
CHORDC1, EIF3J, EP300, LMAN1, GCC2 and DHX35.
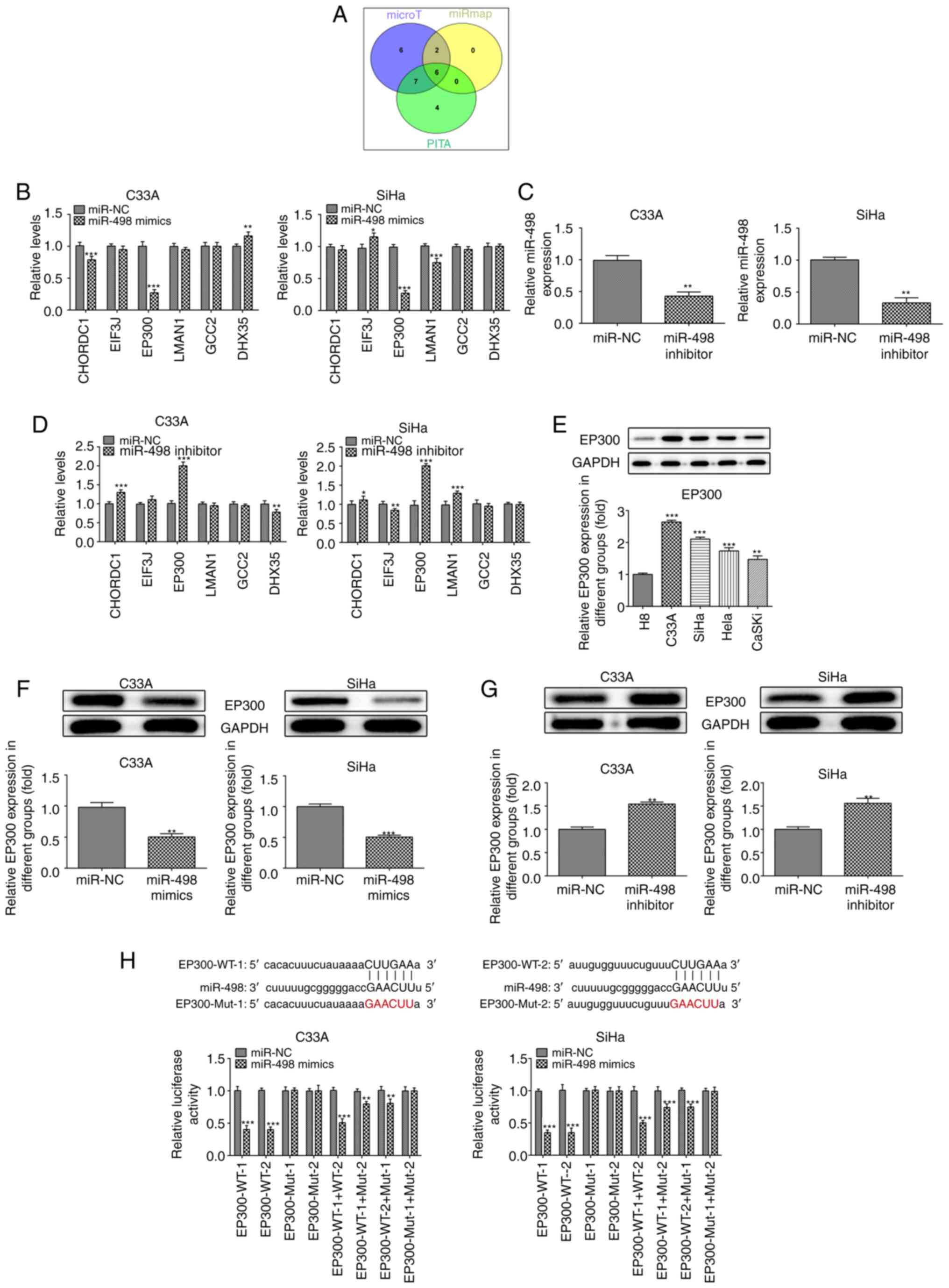 | Figure 3.miR-498 targets and inhibits
expression of EP300. (A) Target proteins of miR498 were predicted
using PITA, DIANA-microT and miRNAMAP databases. (B) mRNA levels of
intersection proteins predicted by databases were detected by
RT-qPCR in C33A and SiHa cells following miR-498 mimic
transfection. *P<0.05, **P<0.01, ***P<0.001 vs. miR-NC.
(C) miR-498 inhibitor significantly decreased the levels of
miR-498, as detected by RT-qPCR. **P<0.01 vs. miR-NC. (D)
miR-498 inhibitor significantly affected the mRNA levels of
CHORDC1, EP300 and DHX35, predicted by databases. *P<0.05,
**P<0.01, ***P<0.001 vs. miR-NC. (E) Protein levels of EP300
was determined via western blotting in cervical cancer cell lines.
**P<0.01, ***P<0.001 vs. H8. (F) miR-498 overexpression
significantly decreased the protein levels of EP300 in C33A and
SiHa cells. **P<0.01, ***P<0.001 vs. miR-NC. (G) miR-498
silencing significantly increased the protein levels of EP300 in
C33A and SiHa cells. **P<0.01 vs. miR-NC. (H) Luciferase
reporter assays were performed to validate the target association
between EP300 and miR-498. Data are presented as the mean ± SD.
**P<0.01, ***P<0.001 vs. miR-NC. miR, microRNA; NC, negative
control; RT-q, reverse transcription-quantitative; CHORDC1,
cysteine and histidine rich domain containing 1; DHX35, DEAH-box
helicase 35; EIF3J, eukaryotic translation initiation factor 3
subunit J; LMAN1, lectin, mannose binding 1; GCC2, GRIP and
coiled-coil domain-containing protein; WT, wild-type; Mut,
mutant. |
The expression of EP300 was significantly decreased
in C33A and SiHa cervical cancer cells following miR-498
overexpression (Fig. 3B).
Subsequently, miR-498 inhibitor was used to knockdown the
expression of miR-498 in C33A and SiHa cells. The expression of
miR-498 decreased 2.2-fold following transfection with the
inhibitor (Fig. 3C). The expression
levels of EP300 and other genes were also detected using RT-qPCR;
results showed that the expression of EP300 was increased 2.1-fold
following transfection with the miR-498 inhibitor (Fig. 3D). Western blotting was performed to
detect the protein levels of EP300 in cervical cancer cell lines.
Higher expression levels of EP300 were found in C33A, SiHa, HeLa
and CaSki cells, compared with H8 cells (Fig. 3E and F). Furthermore, C33A and SiHa
cells expressed relatively higher levels of EP300 compared with
HeLa and CaSKi. Additionally, the protein levels of EP300 were
significantly inhibited (2-fold) following overexpression of
miR-498 in C33A and SiHa cells. Moreover, expression of EP300 was
increased following transfection with miR-498 inhibitor (Fig. 3G). These results demonstrated that
miR-498 regulated EP300 expression levels. Luciferase reporter
assay also showed that luciferase activity decreased following
overexpression of miR-498 (Fig.
3H). This suggested that the upregulation of EP300 in C33A and
SiHa cells resulted in low miR-498 levels, potentially by reversing
the inhibition of EP mRNA translation or the induction of
degradation via targeting the 3′untranslated region (UTR) of EP
mRNA.
Overexpression of EP300 promotes the
proliferation of cervical cancer cells
EP300 was overexpressed in C33A cells. RT-qPCR and
western blotting indicated that the mRNA and protein levels of
EP300 in C33A cells increased following EP300 overexpression,
compared with the control group (1.9-fold; Fig. 4A and B). Subsequently, miR-498
inhibitor and PC-EP300 plasmids were transfected into C33A cells,
followed by MIR9-3HG silencing for 24, 48 or 72 h, and cell
viability was measured via CCK-8 assay. The viability of C33A cells
increased following the inhibition of miR-498 (Fig. 4C). Similarly, the viability of C33A
cells was also increased following EP300 overexpression.
Additionally, in an EdU assay, the proliferation of C33A cells
increased following transfection with the miR-498 inhibitor or
EP300 overexpression (Fig. 4D).
Thus, miR-498 inhibition EP300 overexpression produced similar
effects. Moreover, the inhibition of miR-498 decreased the
apoptosis rate of C33A cells; however, EP300 knockdown abolished
this effect (Fig. 4E and F).
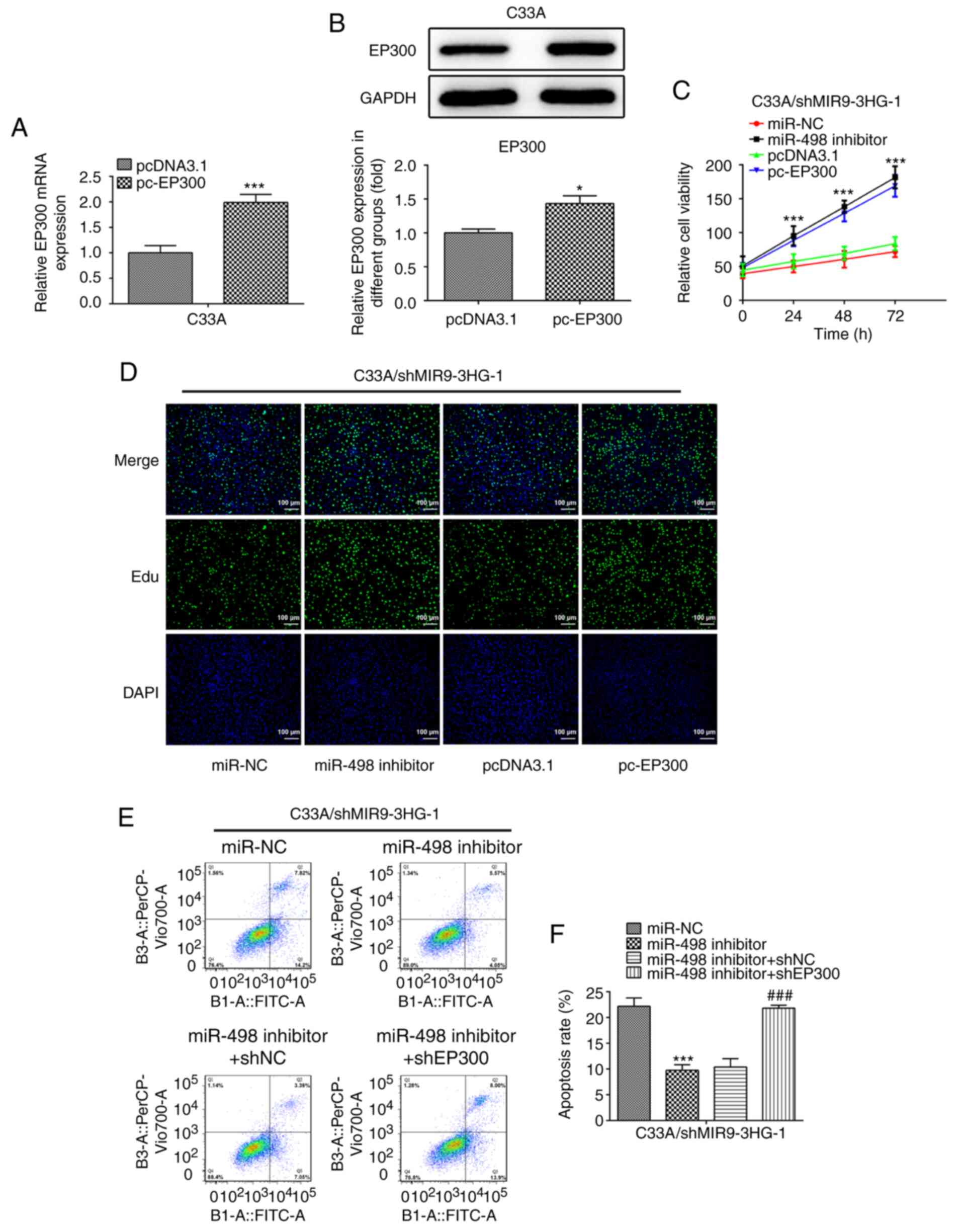 | Figure 4.Overexpression of EP300 enhances
proliferation and suppresses apoptosis of cervical cancer cells.
PC-EP300 upregulated (A) mRNA and (B) protein levels of EP300, as
detected via reverse transcription-quantitative PCR and western
blotting, respectively. *P<0.05, ***P<0.001 vs. pcDNA3.1. (C)
Viability, (D) proliferation and (E and F) apoptosis were
determined via Cell Counting Kit-8 and EdU and flow cytometry
assay, respectively, following miR-498 inhibition or EP300
overexpression in C33A cells with MIR9-3HG silencing.
Magnification, ×100. Data are presented as the mean ± SD.
***P<0.001 vs. miR-NC or pcDNA3.1. ###P<0.001
miR-498 inhibitor + shNC. EdU, 5-ethynyl-2′-deoxyuridine; miR,
microRNA; MIR9-3HG, MIR0-3 host gene; NC, negative control; sh,
short hairpin. |
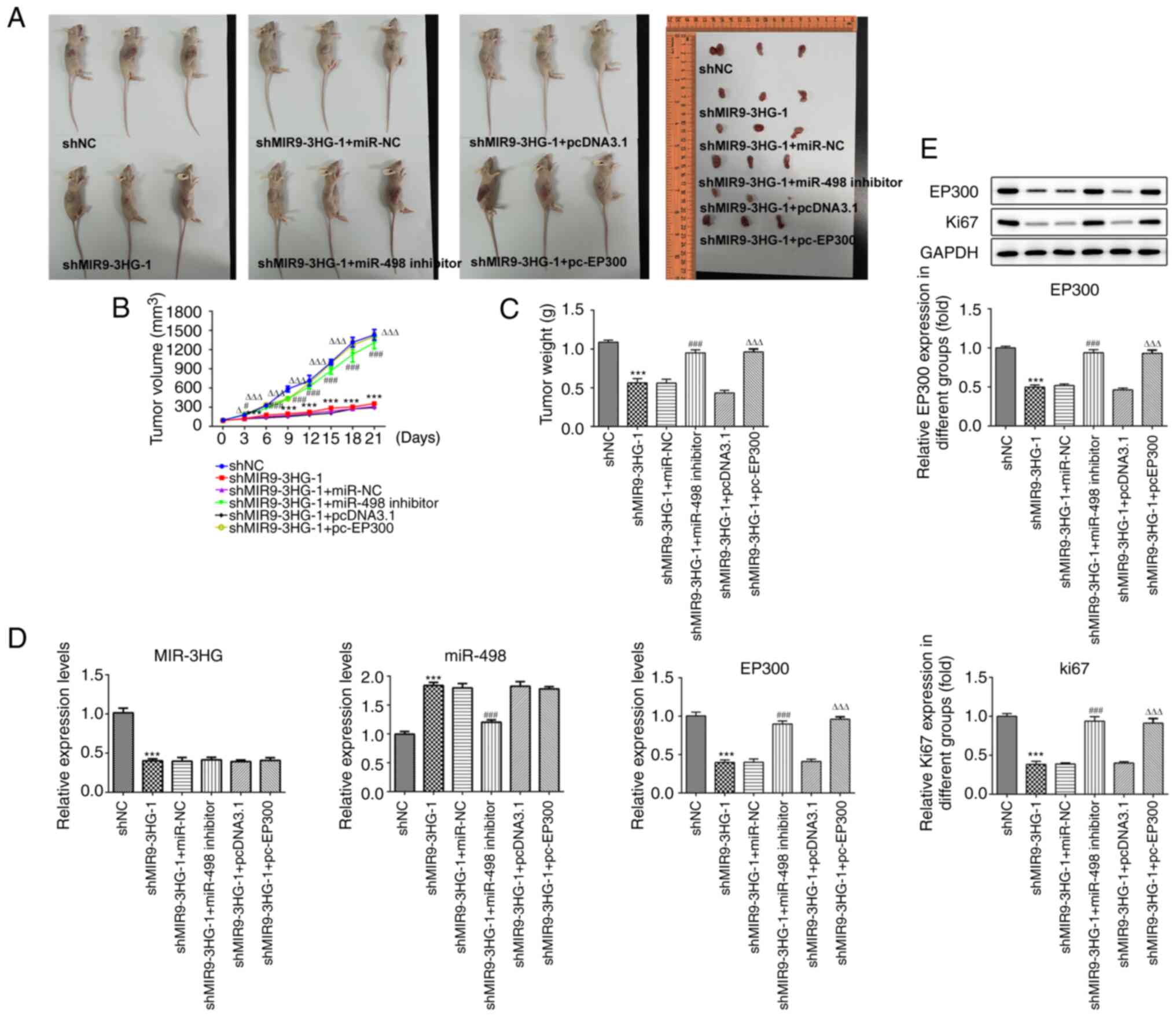
Knockdown of MIR9-3HG inhibits
proliferation of tumors in vivo
In order to validate the mechanism of action
MIR9-3HG in cervical cancer in vivo, C33A cells with
shMIR9-3HG alone or in combination with miR-498 or PC-EP300
transfection were subcutaneously injected into nude mice. The
results indicated that MIR9-3HG knockdown inhibited the
proliferation of C33A cells in vivo (Fig. 5A-C). Tumor volume and weight also
decreased following MIR9-3HG knockdown. The maximum tumor diameter
and volume were 1.480 cm and 1,513.405 mm3,
respectively. Furthermore, the inhibition of miR-498 rescued the
inhibition of tumor growth in vivo. Tumor volume and weight
were also rescued following miR-498 inhibition or EP300
overexpression. Next, RT-qPCR was performed to measure the
expression levels of miR-498 and EP300. The results showed that
expression of miR-498 increased following MIR9-3HG knockdown,
whereas EP300 overexpression did not significantly alter the levels
of miR-498 (Fig. 5D and E), further
highlighting EP300 as a downstream molecular effector of miR-498.
Furthermore, MIR9-3HG silencing decreased the mRNA and protein
levels of EP300, but this effect was reversed following
co-transfection with miR-498 inhibitor.
The levels of Ki-67 in tumors were also determined
via western blot analysis. Expression levels of Ki-67 were
decreased following MIR9-3HG knockdown, which was reversed
following inhibition of miR-498 or overexpression of EP300
(Fig. 5E). This suggested that Ki67
expression was regulated via the MIR9-3HG/miR-498/EP300 axis.
Discussion
Cervical cancer is one of the most common types of
gynecological malignancy and one of the leading causes of mortality
in women with cancer (15). Studies
have shown that cervical cancer cells invade and metastasize in the
body, leading to poor prognosis and high mortality (16,17).
Although surgery, chemotherapy and radiation therapy can treat
>90% of women with early-stage cervical cancer, recurrent and
metastatic disease remains the leading cause of cancer-related
mortality (18). Therefore,
understanding the molecular mechanism underlying the development
and occurrence of cervical cancer may provide insight into novel
diagnostic criteria and treatment options.
Previous studies have suggested that ncRNA serves a
role in the development of cervical cancer (19,20).
High levels of MIR9-3HG can induce the development of cervical
cancer and are associated with poor prognosis (12). In the present study, MIR9-3HG
expression was detected both in the nucleus and cytoplasm of
cervical cancer cells. lncRNA expression in the cytoplasm has been
implicated in the regulation of mRNA stability and the modulation
of translation (21). Another study
suggested that lncRNA expression in the nucleus can sponge target
miRNA, while cytoplasmic expression can inhibit gene expression via
locus-specific histone methylation (22). Therefore, cytoplasmic expression of
MIR9-3HG may account for its effect on proliferation and apoptosis
in C33A or SiHa cells.
A previous study indicated that expression of
MIR9-3HG also promotes the development of head and neck squamous
cell carcinoma (13). In the
present study, knockdown of MIR9-3HG inhibited the proliferation of
cervical cancer cells. Furthermore, silencing MIR9-3HG enhanced
apoptosis in these cells. Cell viability increased in a
time-dependent manner in the MIR9-3HG silencing group, suggesting
that the decrease in the number of cells may be due to inhibition
of proliferation and induction of apoptosis. miR-498 affects cell
cycle and alters the expression levels of cell cycle-associated
proteins, such as cyclin D1 and CDK4 (23–25).
Thus, the effect of suppression of MIR9-3HG silencing on cell
proliferation may be due to cell cycle regulation via miR-498. This
may also be due to apoptosis induction by MIR9-3HG silencing.
Certain studies have showed that miR-498 affects cell apoptosis,
potentially via regulating mitochondria-mediated
apoptosis-associated proteins, such as caspase-3, Bax and Bcl-2
(26,27). Based on the present results and
previous literature, it was hypothesized that the regulation of
apoptosis via MIR9-3HG/miR-498 in cervical cancer cells may be
mediated by affecting the mitochondria-mediated apoptosis
pathway.
The expression of miR-498 is associated with the
development of multiple types of cancer, such as cervical cancer
and lung cancer (28,29), which affects the proliferation,
migration and invasion of cancer cells by regulating the expression
levels of certain proteins, such as PTEN and zinc finger E-box
binding homeobox 1 (25,30,31). A
previous study demonstrated that circular protein arginine
methyltransferase 5 regulates the proliferation of non-small cell
lung cancer by sponging miR-498 (32). The present study revealed that
miR-498 regulated the expression of EP300 by binding to the 3′UTR
of EP300 mRNA. Moreover, EP300 mediated the influence of miR-498
inhibitor on promoting proliferation and suppressing apoptosis in
C33A cells. High levels of EP300 are associated with the
development of several types of cancer (33,34).
For example, EP300 enhances the proliferation of breast cancer
cells (35). In the present study,
a potential targeting association was predicted by searching the
PITA, DIANA-microT and miRNAMAP databases, which suggested that
miR-498 regulated the expression of EP300. In addition, inhibition
of miR-498 abolished the inhibition of proliferation of cervical
cancer cells and increased apoptosis mediated by MIR9-3HG
knockdown. Moreover, the increased proliferation and decreased
apoptosis mediated by the miR498 inhibitor were also reversed
following EP300 silencing. Collectively, the present study revealed
the regulatory role of the MIR9-3HG/miR-498/EP300 axis on
proliferation and apoptosis in C33A cells. Experiments using
tumor-bearing mice suggested that the MIR9-3HG/miR-498/EP300 axis
served a regulatory role in tumor growth.
The EP300 protein, which is regulated by miRNAs,
such as miR-106b and miR25, is a histone acetyltransferase that
regulates transcription via chromatin remodeling and serves an
important regulatory role in cell proliferation and apoptosis
(33,36,37).
Moreover, cells express lower levels of Bcl-2 protein following
EP300 knockdown, indicating potential involvement of the intrinsic
apoptosis pathway (33). The
mechanism by which the 3HG/miR-498/EP300 axis regulates apoptosis
is still unknown. Further research is required to determine how the
MIR9-3HG/miR-498/EP300 axis affects apoptosis in cervical cancer
and its potential as a diagnostic markers. In summary, MIR9-3HG may
promote proliferation and suppress apoptosis of cervical cancer
cells by regulating the expression levels of miR-498 and EP300.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author or first author
on reasonable request.
Authors' contributions
FL, YL and PY made substantial contributions to the
conception and design of the study, acquired, analyzed and
interpreted the data, and drafted and revised the manuscript for
important intellectual content. FL and PY were responsible for
confirming the authenticity of the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The experiments were approved by the Ethics
Committee of the First Affiliated Hospital of Zhejiang Chinese
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bouvard V, Zaitchouk T, Vacher M, Duthu A,
Canivet M, Choisy-Rossi C, Nieruchalski M and May E: Tissue and
cell-specific expression of the p53-target genes: Bax, fas, mdm2
and waf1/p21, before and following ionising irradiation in mice.
Oncogene. 19:649–660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang L, Yi K, Wang H, Zhao Y and Xi M:
Comprehensive analysis of lncRNAs microarray profile and
mRNA-lncRNA co-expression in oncogenic HPV-positive cervical cancer
cell lines. Oncotarget. 7:49917–49929. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naga Ch P, Gurram L, Chopra S and
Mahantshetty U: The management of locally advanced cervical cancer.
Curr Opin Oncol. 30:323–329. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klingenberg M, Matsuda A, Diederichs S and
Patel T: Non-coding RNA in hepatocellular carcinoma: Mechanisms,
biomarkers and therapeutic targets. J Hepatol. 67:603–618. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang K, Han Y, Hu Z, Zhang Z, Shao S, Yao
Q, Zheng L, Wang J, Han X, Zhang Y, et al: SCARNA10, a
nuclear-retained long non-coding RNA, promotes liver fibrosis and
serves as a potential biomarker. Theranostics. 9:3622–3638. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lang HL, Hu GW, Zhang B, Kuang W, Chen Y,
Wu L and Xu GH: Glioma cells enhance angiogenesis and inhibit
endothelial cell apoptosis through the release of exosomes that
contain long non-coding RNA CCAT2. Oncol Rep. 38:785–798. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sandiford OA, Moore CA, Du J, Boulad M,
Gergues M, Eltouky H and Rameshwar P: Human Aging and Cancer: Role
of miRNA in Tumor Microenvironment. Adv Exp Med Biol. 1056:137–152.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Roosbroeck K and Calin GA: Cancer
Hallmarks and MicroRNAs: The Therapeutic Connection. Adv Cancer
Res. 135:119–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
López-Urrutia E, Bustamante Montes LP,
Ladrón de Guevara Cervantes D, Pérez-Plasencia C and Campos-Parra
AD: Crosstalk Between Long Non-coding RNAs, Micro-RNAs and mRNAs:
Deciphering Molecular Mechanisms of Master Regulators in Cancer.
Front Oncol. 9:6692019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang JY, Yang Y, Ma Y, Wang F, Xue A, Zhu
J, Yang H, Chen Q, Chen M, Ye L, et al: Potential regulatory role
of lncRNA-miRNA-mRNA axis in osteosarcoma. Biomed Pharmacother.
121:1096272020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang B, Yu L, Han N, Hu Z, Wang S, Ding L
and Jiang J: LINC01116 targets miR-520a-3p and affects IL6R to
promote the proliferation and migration of osteosarcoma cells
through the Jak-stat signaling pathway. Biomed Pharmacother.
107:270–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu WJ, Shen Y, Sui J, Li CY, Yang S, Xu
SY, Zhang M, Yin LH, Pu YP and Liang GY: Integrated analysis of
long non coding RNA competing interactions revealed potential
biomarkers in cervical cancer: Based on a public database. Mol Med
Rep. 17:7845–7858. 2018.PubMed/NCBI
|
|
13
|
Hu Y, Guo G, Li J, Chen J and Tan P:
Screening key lncRNAs with diagnostic and prognostic value for head
and neck squamous cell carcinoma based on machine learning and
mRNA-lncRNA co-expression network analysis. Cancer Biomark.
27:195–206. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee YY, Choi CH, Kim TJ, Lee JW, Kim BG,
Lee JH and Bae DS: A comparison of pure adenocarcinoma and squamous
cell carcinoma of the cervix after radical hysterectomy in stage
IB-IIA. Gynecol Oncol. 120:439–443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JY, Kim DY, Kim JH, Kim YM, Kim YT
and Nam JH: Outcomes after radical hysterectomy in patients with
early-stage adenocarcinoma of uterine cervix. Br J Cancer.
102:1692–1698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta S, Kumar P and Das BC: HPV:
Molecular pathways and targets. Curr Probl Cancer. 42:161–174.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang HW, Xie H, Ma X, Zhao F and Gao Y:
Upregulation of lncRNA PANDAR predicts poor prognosis and promotes
cell proliferation in cervical cancer. Eur Rev Med Pharmacol Sci.
21:4529–4535. 2017.PubMed/NCBI
|
|
20
|
Zhao LP, Li RH, Han DM, Zhang XQ, Nian GX,
Wu MX, Feng Y, Zhang L and Sun ZG: Independent prognostic Factor of
low-expressed lncRNA ZNF667-AS1 for cervical cancer and inhibitory
function on the proliferation of cervical cancer. Eur Rev Med
Pharmacol Sci. 21:5353–5360. 2017.PubMed/NCBI
|
|
21
|
Yao RW, Wang Y and Chen LL: Cellular
functions of long noncoding RNAs. Nat Cell Biol. 21:542–551. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Katsushima K, Natsume A, Ohka F, Shinjo K,
Hatanaka A, Ichimura N, Sato S, Takahashi S, Kimura H, Totoki Y, et
al: Targeting the Notch-regulated non-coding RNA TUG1 for glioma
treatment. Nat Commun. 7:136162016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li W and Jiang H: Up-regulation of miR-498
inhibits cell proliferation, invasion and migration of
hepatocellular carcinoma by targeting FOXO3. Clin Res Hepatol
Gastroenterol. 44:29–37. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu M, Liu B, Xiong H, Wu F, Hu C and Liu
P: Trans−3,5,4´-trimethoxystilbene reduced gefitinib
resistance in NSCLCs via suppressing MAPK/Akt/Bcl-2 pathway by
upregulation of miR-345 and miR-498. J Cell Mol Med. 23:2431–2441.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Xu X, Ge G, Zang X, Shao M, Zou
S, Zhang Y, Mao Z, Zhang J, Mao F, et al: miR 498 inhibits the
growth and metastasis of liver cancer by targeting ZEB2. Oncol Rep.
41:1638–1648. 2019.PubMed/NCBI
|
|
26
|
Chai Q, Zheng M, Wang L, Wei M, Yin Y, Ma
F, Li X, Zhang H and Liu G: Circ_0068655 Promotes Cardiomyocyte
Apoptosis via miR-498/PAWR Axis. Tissue Eng Regen Med. 17:659–670.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li G, Tan W, Fang Y, Wu X, Zhou W, Zhang
C, Zhang Y, Liu Y, Jiu G and Liu D: circFADS2 protects LPS-treated
chondrocytes from apoptosis acting as an interceptor of
miR-498/mTOR cross-talking. Aging (Albany NY). 11:3348–3361. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rong X, Gao W, Yang X and Guo J:
Downregulation of hsa_circ_0007534 restricts the proliferation and
invasion of cervical cancer through regulating miR-498/BMI-1
signaling. Life Sci. 235:1167852019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao F, Han Y, Liu Z, Zhao Z, Li Z and Jia
K: circFADS2 regulates lung cancer cells proliferation and invasion
via acting as a sponge of miR-498. Biosci Rep. 38:382018.
View Article : Google Scholar
|
|
30
|
Duan XM, Liu XN, Li YX, Cao YQ, Silayiding
A, Zhang RK and Wang JP: MicroRNA-498 promotes proliferation,
migration, and invasion of prostate cancer cells and decreases
radiation sensitivity by targeting PTEN. Kaohsiung J Med Sci.
35:659–671. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu R, Liu F, Li L, Sun M and Chen K:
miR-498 regulated FOXO3 expression and inhibited the proliferation
of human ovarian cancer cells. Biomed Pharmacother. 72:52–57. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Li Y, He H and Wang F: Circular
RNA circ-PRMT5 facilitates non-small cell lung cancer proliferation
through upregulating EZH2 via sponging miR-377/382/498. Gene.
720:1440992019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Asaduzzaman M, Constantinou S, Min H,
Gallon J, Lin ML, Singh P, Raguz S, Ali S, Shousha S, Coombes RC,
et al: Tumour suppressor EP300, a modulator of paclitaxel
resistance and stemness, is downregulated in metaplastic breast
cancer. Breast Cancer Res Treat. 163:461–474. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Attar N and Kurdistani SK: Exploitation of
EP300 and CREBBP Lysine Acetyltransferases by Cancer. Cold Spring
Harb Perspect Med. 7:72017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sobczak M, Pitt AR, Spickett CM and
Robaszkiewicz A: PARP1 Co-Regulates EP300-BRG1-Dependent
Transcription of Genes Involved in Breast Cancer Cell Proliferation
and DNA Repair. Cancers (Basel). 11:112019. View Article : Google Scholar
|
|
36
|
Grunstein M: Histone acetylation in
chromatin structure and transcription. Nature. 389:349–352. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bemanian V, Noone JC, Sauer T, Touma J,
Vetvik K, Søderberg-Naucler C, Lindstrøm JC, Bukholm IR, Kristensen
VN and Geisler J: Somatic EP300-G211S mutations are associated with
overall somatic mutational patterns and breast cancer specific
survival in triple-negative breast cancer. Breast Cancer Res Treat.
172:339–351. 2018. View Article : Google Scholar : PubMed/NCBI
|