Introduction
Stroke remains the second major cause of mortality
worldwide and is the leading cause of death in China (1). According to a Global Burden of Disease
study, there were 5.5 million deaths and 116.4 million disability
adjusted life years due to stroke in 2017 (2). Although rapid progress has been
achieved in the understanding of the pathogenesis of ischemic
stroke, there is currently no effective therapeutic approach for
nerve repair (3). Accumulating
evidence has shown that cerebral ischemia can stimulate the
activation of quiescent neural stem cells (NSCs) in the
subventricular zone (SVZ) into transient amplifying progenitors,
which become neuroblasts after several divisions (4,5). The
neuroblasts migrate into the damaged cortex and differentiate into
mature neurons to promote the recovery of the neurological function
(6). The growth peak of NSCs in the
adult brain after ischemia is 7–10 days (7). However, this internal response cannot
functionally compensate for the ischemic damage, and the migrating
cells do not differentiate into mature neurons in the cortex
(8,9). Thus, it is critical to search for
therapeutic approaches and specific targets to augment the
differentiation of endogenous NSCs into neurons.
Neuroinflammation participates in the
pathophysiological progress of secondary brain injury after
ischemic stroke by increasing pro-inflammatory cytokines and
neuronal apoptosis (10). A
previous study has shown that the levels of inflammatory cytokines
gradually increase within 0–24 h of cerebral ischemia reperfusion
in rats, and reach a peak at 24 h (11). The interaction between
neuroinflammation and subsequent neurogenesis remains unknown, and
thus, has gained significant interest in recent years. Moreover,
further understanding of neuroinflammation and its association with
neurogenesis could provide a novel approach for brain repair.
Previous studies have reported that ephrin (Eph)
receptors can regulate the proliferation of stem cells and
progenitor cells in the central nervous system (CNS) (12). The Eph receptor family is the
largest known receptor tyrosine kinase family. The members of this
family bind their ligand ephrins to initiate bidirectional
signaling and regulate different physiological activities, which
have become popular research subjects internationally (13). Previous studies have shown that
ephrinB/EphB signaling served an early role in the regulation of
stem cell behavior. For instance, ephrinB3/EPH receptor B3 (EphB3)
signaling exhibited an inhibitory effect on NSC proliferation in
the developing SVZ (14). In the
adult CNS, overexpression of ephrinB2 or EphB2 promotes NSC
proliferation and represses neuroblast migration (15). However, research regarding EphB4
focuses on tumor cells. Restoration of EphB4 promotes tumor cell
proliferation, migration and angiogenesis (16,17).
Moreover, a previous study revealed that EphB4 regulated the
self-renewal, proliferation and neuronal differentiation of human
embryonic NSCs in vitro (18).
The present study aimed to investigate the role of
EphB4 in the neurogenesis and neuroinflammation of ischemic stroke
in vivo.
Materials and methods
Animals and middle cerebral artery
occlusion (MCAO) model
The animal experiment was approved by the Animal
Care and Use Committee of Inner Mongolia Baogang Hospital (approval
no. BG201802034YY). A total of 120 male C57BL/6J and
EphB4+/+ C57BL/6J mice (age, 6–8 weeks;
weight, 21–23 g), bred by Cyagen Biosciences, Inc., were maintained
on a 12-h light/dark cycle under specific pathogen-free conditions
(25°C; 50–70% humidity) with free access to food and water.
Transient MCAO was performed as previously reported
(19), with a slight modification.
The mice were randomly divided into four groups (n=5/group): Sham,
EphB4+/+ Sham group, MCAO group and
EphB4+/+ MCAO group. Mice were anaesthetized
with isoflurane (3% induction; 1–1.5% maintenance) in a mixture of
75% N2O and 25% O2 and the rectal temperature
was maintained at 37.0±0.5°C. Then, the right common carotid artery
and the right external carotid artery (ECA) were exposed. The ECA
was then dissected distally, ligated and coagulated. The right MCA
was occluded with a heparinized intraluminal filament (0.26 mm;
rounded tip). After 30 min of ischemia, the intraluminal filament
was withdrawn to accomplish reperfusion. The sham group underwent
the same procedure without insertion of the intraluminal filament.
After reperfusion for 1, 3, 7 and 14 days, the mice were sacrificed
with 120 mg/kg pentobarbital sodium via intraperitoneal injection.
Death was ascertained based on pupil dilation and an inability to
palpate the carotid pulse. Brain tissues were collected after the
mice were sacrificed. Due to severe injuries, eight mice with rapid
weight loss, loss of appetite or abnormal nervous system responses
were euthanized with 2% pentobarbital sodium (120 mg/kg body
weight).
2,3,5-triphenyltetrazolium chloride
(TTC) staining
TTC staining was used to evaluate the infarct
volume. Briefly, coronal brain slices (2 mm) were cut and immersed
in 2% TTC solution for 30 min at 37°C, followed by fixation in 4%
paraformaldehyde overnight. The sections were photographed with a
digital camera (Canon IXUS175; Canon, Inc.). The percentage of
infarct area was calculated using Image J software 5.0 (National
Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cerebral cortex tissues
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
cDNA was synthesized according to the M-MLV conserved transcriptase
instructions (Takara Biotechnology Co., Ltd.) using a PrimeScript™
RT reagent kit (Takara Bio, Inc.). RT was performed at 37°C for 15
min, 85°C for 5 sec, then 4°C for cooling. RT-qPCR was performed
using a TB Green® Premix Ex Taq™ II kit (Takara Bio,
Inc.). The thermocycling conditions were as follows: 20 sec at
95°C, followed by 40 cycles of 15 sec at 95°C and 60 sec at 60°C,
and 60°C for 1 min. The data were normalized to the control and
analyzed using the 2−ΔΔCq method (20). The primers used were as follows:
EphB4 forward (F), 5′-CCCATTTGAGCCTGTCAATGTC-3′ and reverse (R),
5′-TCAAGCTGCTGGGTGAGGA-3′; ephrinB2 F, 5′-TATGCAGAACTGCGATTTCCAA-3′
and R, 5′-TGGGTATAGTACCAGTCCTTGTC-3′; and GAPDH F,
5′-CATGAGAAGTATGACAACAGCCT-3′ and R, 5′-AGTCCTTCCACGATACCAAAGT-3′.
GAPDH was used as an internal reference.
Immunofluorescence analysis
Immunofluorescence staining was used to visualize
nerve cells. Bromodeoxyuridine (Brdu; 50 mg/kg; Sigma-Aldrich;
Merck KGaA) was injected intraperitoneally into all mice twice
daily for 4 consecutive days after MCAO. After being fixed with 4%
paraformaldehyde at 37°C for 24 h, brain tissue was embedded in
paraffin and sectioned at a thickness of 5 µm. For Brdu staining,
the sections were pretreated with 50% formamide, 280 mM NaCl and 30
mM sodium citrate at 65°C for 2 h, incubated in 2 M HCl at 37°C for
30 min to denature the DNA and rinsed in boric acid (0.1 M; pH 8.5)
at room temperature for 10 min. After blocking the sections with 5%
donkey serum (Jackson ImmunoResearch Europe) for 2 h at room
temperature, they were incubated for 48 h at 4°C with the following
primary antibodies: Anti-EphB4 (Santa Cruz Biotechnology, Inc.;
cat. no. sc-365510; 1:50), anti-Brdu (Abcam; cat. no. ab152095;
1:100), anti-Ki67 (Abcam; cat. no. ab15580; 1:200), anti-Nestin
(Abcam; cat. no. ab105389; 1:250), anti-Sox2 (Abcam; cat. no.
ab79351; 1:250), anti-doublecortin (DCX; Santa Cruz Biotechnology,
Inc.; cat. no. sc-271390; 1:100) and anti-neuronal nuclei (NeuN;
Abcam; cat. no. ab104224; 1:200). Subsequently, the sections were
incubated with Alexa Fluor 488- or 594-conjugated secondary
antibodies (Thermo Fisher Scientific, Inc.; cat. nos. A48269 and
A11016; both 1:2,000) for 2 h at room temperature. The fluorescent
images of cells were captured with a fluorescence microscope system
(Nikon Eclipse 80i; Nikon Corporation; magnification, ×200). In
total, three areas of interest were selected around the margin of
infarct area, and an average of the number of cells in the
peri-infarct cortex for each section was obtained (21).
Western blotting
Total proteins were extracted from the infarct side
of cerebral cortex with RIPA lysis buffer (Beyotime Institute of
Biotechnology). BCA Protein Assay reagent (Beyotime Institute of
Biotechnology) was applied to analyze proteins concentration.
Proteins (50 µg/lane) were separated by 10% SDS-PAGE and
transferred onto a nitrocellulose membrane. Subsequently, the
membranes were blocked with 5% non-fat milk powder for 2 h at room
temperature and incubated with primary antibodies, including
anti-EphB4 (Abcam; cat. no. ab254300; 1:1,000), anti-ephrinB2
(Abcam; cat. no. ab75868; 1:500), anti-phosphorylated (p)-ABL
proto-oncogene 1, non-receptor tyrosine kinase (ABL1; Cell
Signaling Technology, Inc.; cat. no. 2864; 1:1,000), anti-ABL1
(Cell Signaling Technology, Inc.; cat. no. 2862; 1:1,000),
anti-Cyclin D1 (Abcam; cat. no. ab134175; 1:2,000), anti-CDK4
(Abcam; cat. no. ab108355; 1:1,000) and β-actin (Abcam; cat. no.
ab8227; 1:1,000) at 4°C overnight, followed by the horseradish
peroxidase-labeled secondary antibodies (Abcam; cat. nos. ab205718
and ab205719; both 1:2,000) at 37°C for 1 h. The immunoreactive
proteins were visualized using an ECL kit (MilliporeSigma) and the
data were analyzed using ImageJ software (version 1.8.0; National
Institutes of Health). The expression values were normalized
against β-actin.
ELISA
The productions of IL-6, IL-1β, TNF-α, IL-10 and
monocyte chemoattractant protein (MCP)-1 in the tissues and plasma
of mice were measured using the corresponding mouse ELISA kits
(cat. nos. RAB0314, RAB0274, RAB0477 and RAB0245, RAB0055,
respectively; all Sigma-Aldrich; Merck KGaA) following the
manufacturer's instructions.
Statistical analysis
The data are presented as the mean ± SD of five
independent experiments. Statistical analyses were performed with
SPSS 17.0 (SPSS, Inc.). One-way ANOVA followed by Tukey's post-hoc
test was carried out to analyze multiple comparison, and unpaired
Student's t-test was used to detect differences between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
EphB4 is increased in the cerebral
cortex of MCAO model mice
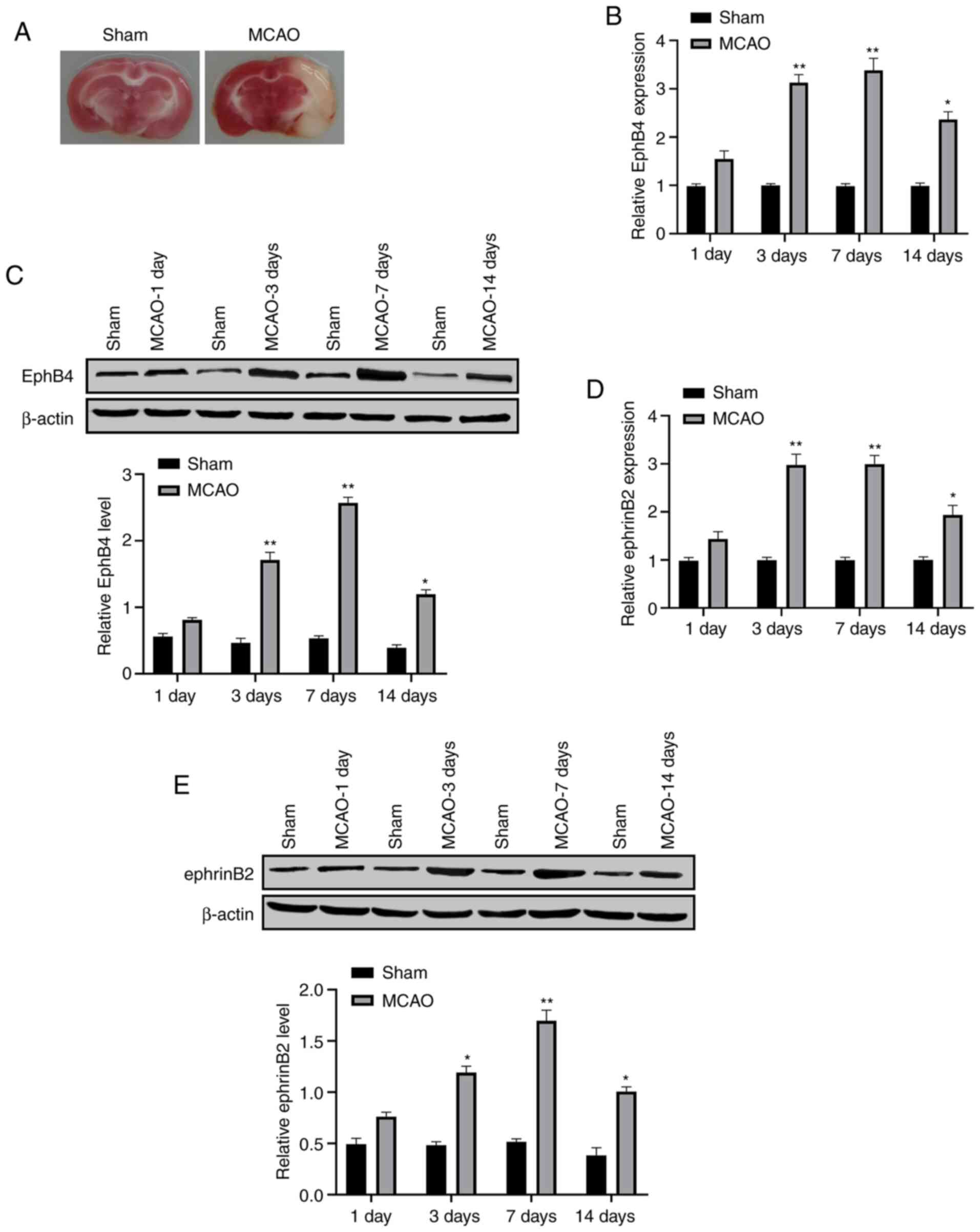
To evaluate the association of EphB4 with ischemic
stroke, the expression levels of EphB4 and ephrinB2 in the cortex
of MCAO model mice, followed by a reperfusion for 1, 3, 7 and 14
days, were detected. The TTC results confirmed the successful
ischemia induction (Fig. 1A). The
results from RT-qPCR assay demonstrated that the expression levels
of EphB4 and ephrinB2 were increased after MCAO and peaked at 7
days (Fig. 1B and D), which was
also confirmed by the results of western blotting assay (Fig. 1C and E).
EphB4 expression in nerve cells of the
cerebral ischemic cortex
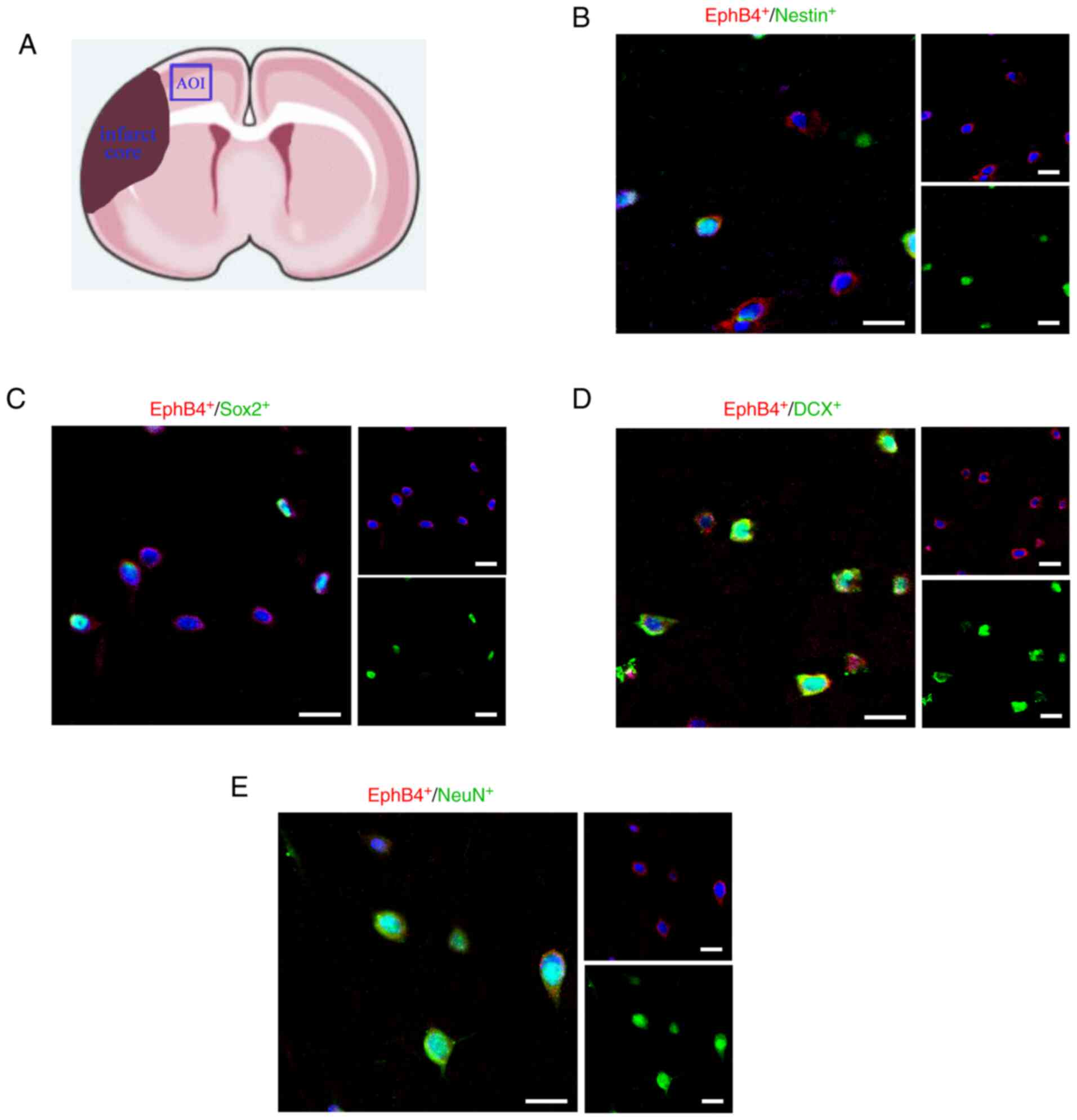
As reported in previous studies, in the cerebral
cortex, NSCs express Nestin, the generated neuronal progenitors
express Sox2, neuroblasts express DCX and the mature neurons
express NeuN (22,23). In total, three areas of interest
were selected around the margin of infarct area, and an average of
the number of cells in the peri-infarct cortex for each section was
calculated (Fig. 2A). The current
immunofluorescence results identified that EphB4 was expressed by
Nestin+, Sox2+, DCX+ and
NeuN+ cells in the peri-infarct cortex of MCAO model
mice (Fig. 2B-E). Moreover, EphB4
appeared to be expressed in NSCs (Nestin+) and persisted
as these cells became neuronal progenitors (Sox2+),
neuroblasts (DCX+) and eventually mature neurons
(NeuN+). These results indicated the potential
relationship between the EphB4 and neurogenesis.
Overexpression of EphB4 promotes NSC
proliferation in the cerebral cortex of MCAO model mice
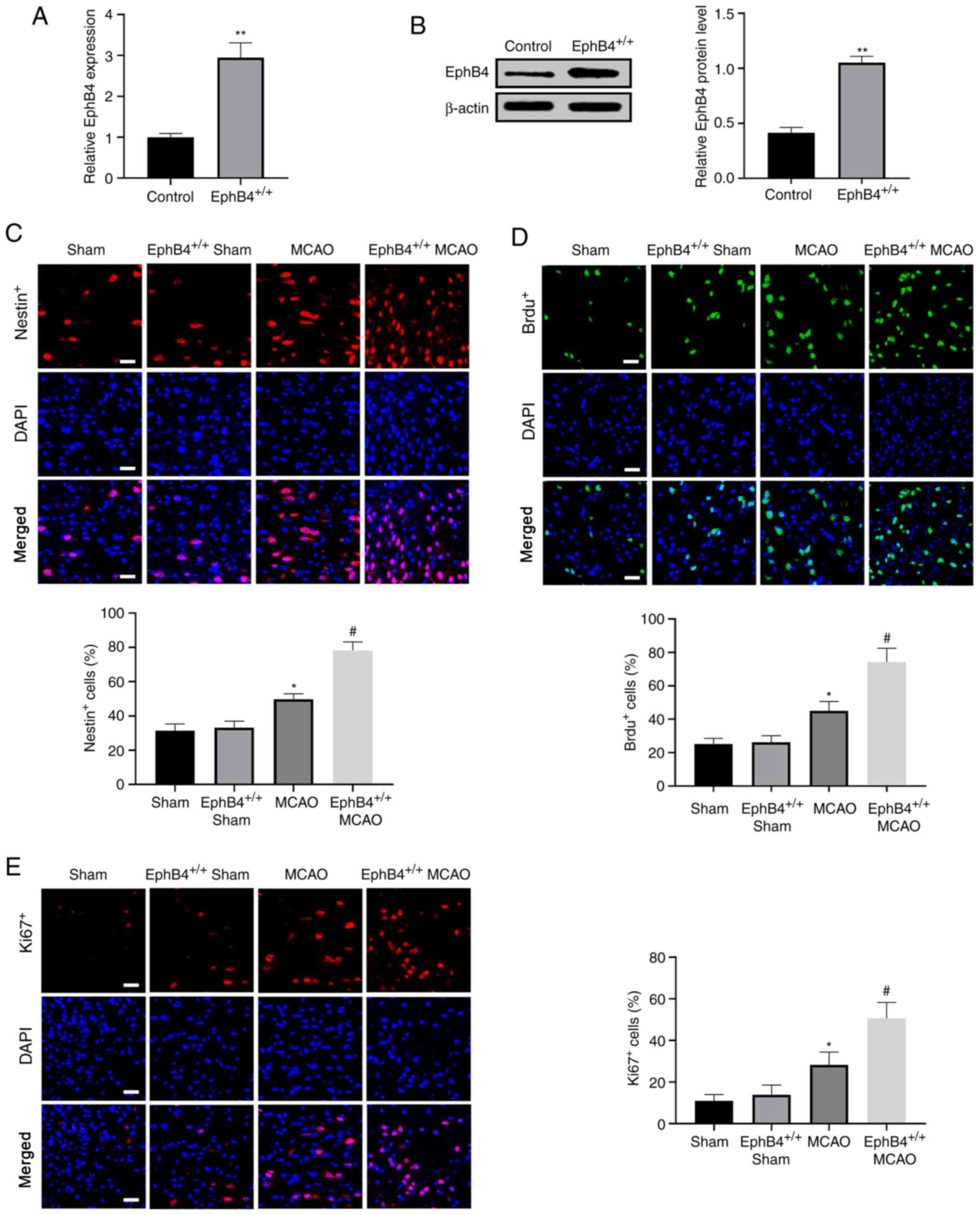
To confirm whether EphB4 modulated the proliferation
of nerve cells, NSCs labelled with Nestin+, and newborn
cells labeled with Brdu+ or Ki67+ were
observed in the peri-infarct cortex 7 days after MCAO using an
immunofluorescence assay. EphB4 expression was measured via RT-qPCR
and western blotting in EphB4+/+ mice prior
to MCAO. The results demonstrated that EphB4 was significantly
elevated in EphB4+/+ mice compared with
wild-type mice (Fig. 3A and B).
Immunofluorescence results revealed that there were no differences
in the number of positive cells (Nestin+,
Brdu+ and Ki67+) between sham mice and the
EphB4+/+ sham mice, while the number of these
positive cells was significantly increased in MCAO mice vs.
sham-operated mice. Overexpression of EphB4 further enhanced the
number of these positive cells in MCAO model mice (Fig. 3C-E). Overall, these data indicated
that overexpression of EphB4 promoted NSC proliferation after focal
cerebral ischemia in vivo.
Overexpression of EphB4 promotes NSC
differentiation in the cerebral cortex of MCAO model mice
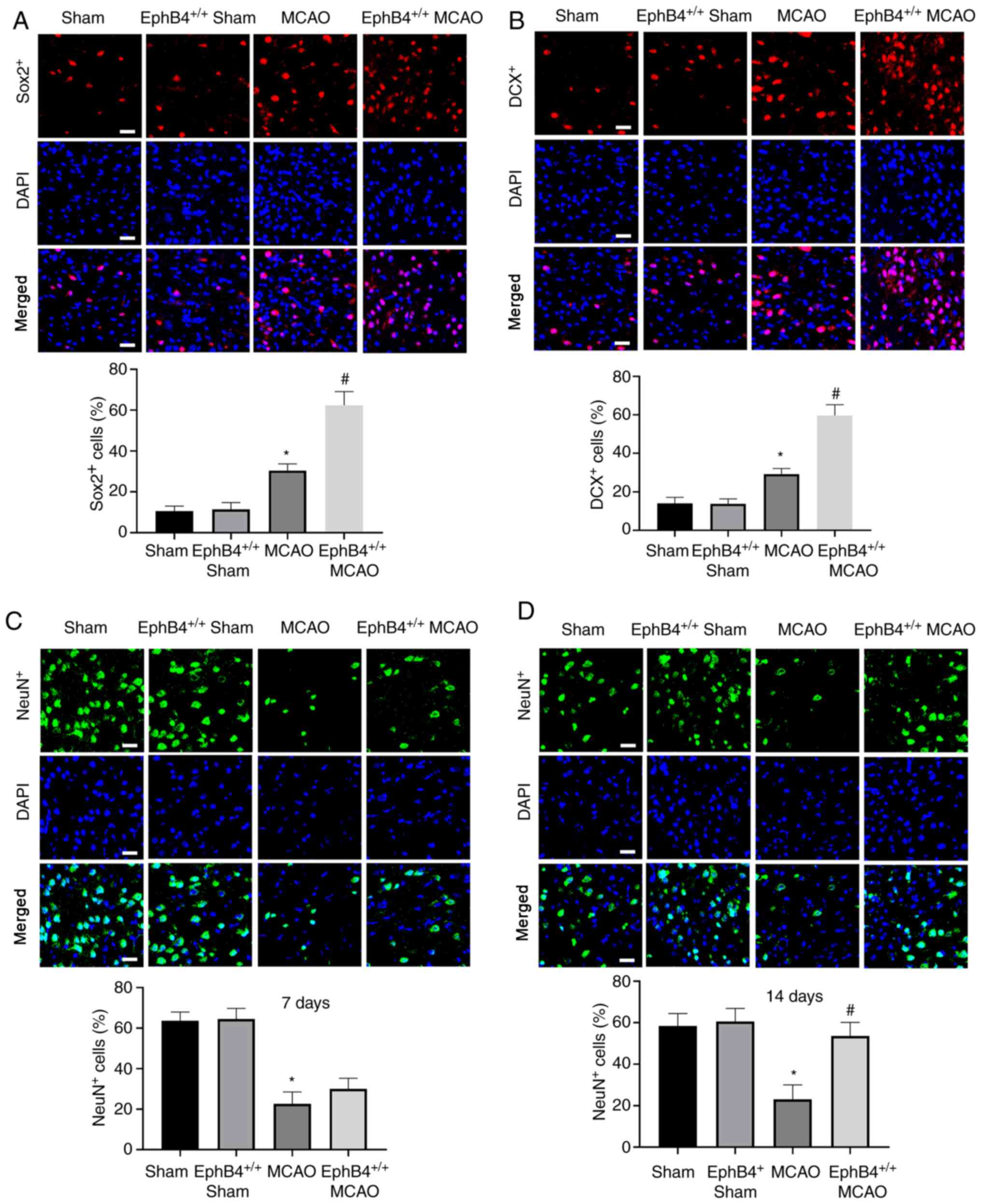
Sox2 is an important molecular marker of adult NSCs
(24). DCX is a
microtubule-associated protein expressed in immature neurons, which
is essential for neuronal migration and differentiation (25). Thus, Sox2+,
DCX+ and NeuN+ expression in the peri-infarct
cortex 7 days after ischemia reperfusion was detected. As show in
Fig. 4A and B, Sox2+ and
DCX+ cells were significantly increased at 7 days in
MCAO model mice compared with sham-operated mice. EphB4
overexpression further increased the number of Sox2+ and
DCX+ cells compared with the MCAO model mice. However,
NeuN+ cells exhibited no significant difference between
MCAO and EphB4+/+ MCAO groups at 7 days
(Fig. 4C). However, at 14 days, the
number of NeuN+ cells was significantly increased in the
EphB4+/+ MCAO model mice compared with the
MCAO model mice (Fig. 4D),
suggesting that restoration of EphB4 promoted NSC differentiation
after focal cerebral ischemia in vivo.
Overexpression of EphB4 attenuates the
inflammatory response in MCAO model mice
To investigate the effect of EphB4 on the
inflammation injury in ischemic stroke model mice, a series of
inflammatory cytokines, including IL-1β, IL-6, TNF-α, IL-10 and
MCP-1, in the tissues of cerebral cortex and the plasma of mice
were evaluated. The results demonstrated that the levels of IL-1β,
IL-6, TNF-α and MCP-1 were quickly increased in the tissues of MCAO
group compared with sham operation group. However, the
stroke-evoked enhancement of cytokine levels was markedly inhibited
in EphB4+/+ mice after MCAO (Fig. 5A-D). Interestingly, the level of
IL-10, generally regarded as an anti-inflammatory cytokine
(26), was significantly decreased
after stroke; however, the low level of IL-10 was significantly
increased in the EphB4+/+ MCAO group
(Fig. 5E). Consistent with the
aforementioned results, the levels of IL-1β, IL-6, TNF-α and MCP-1
were quickly inhibited in the plasma of
EphB4+/+ MCAO model mice, which were enhanced
by MCAO operation (Fig. 5F-I).
However, the level of IL-10 was significantly decreased in MCAO
mice vs. sham-operated mice, and EphB4+/+
increased the low level of IL-10 in MCAO model mice (Fig. 5J). These results suggest that
overexpression of EphB4 attenuated the MCAO-induced inflammatory
response.
 | Figure 5.Overexpression of EphB4 inhibits the
inflammatory cytokine levels MCAO model mice. The levels of (A)
IL-1β, (B) IL-6, (C) TNF-α, (D) MCP-1 and (E) IL-10 in the cortex
around the infarcted zone were detected via ELISA at 1 day after
MCAO. The levels of (F) IL-1β, (G) IL-6, (H) TNF-α, (I) MCP-1 and
(J) IL-10 in the plasma of MCAO model mice were detected via ELISA
at 1 day. n=5 for each group. Data are presented as mean ± SD.
*P<0.05, **P<0.01, ***P<0.001 vs. Sham;
#P<0.05, ##P<0.01 vs. MCAO. EphB4, EPH
receptor B4; MCAO, middle cerebral artery occlusion; MCP-1,
monocyte chemoattractant protein 1. |
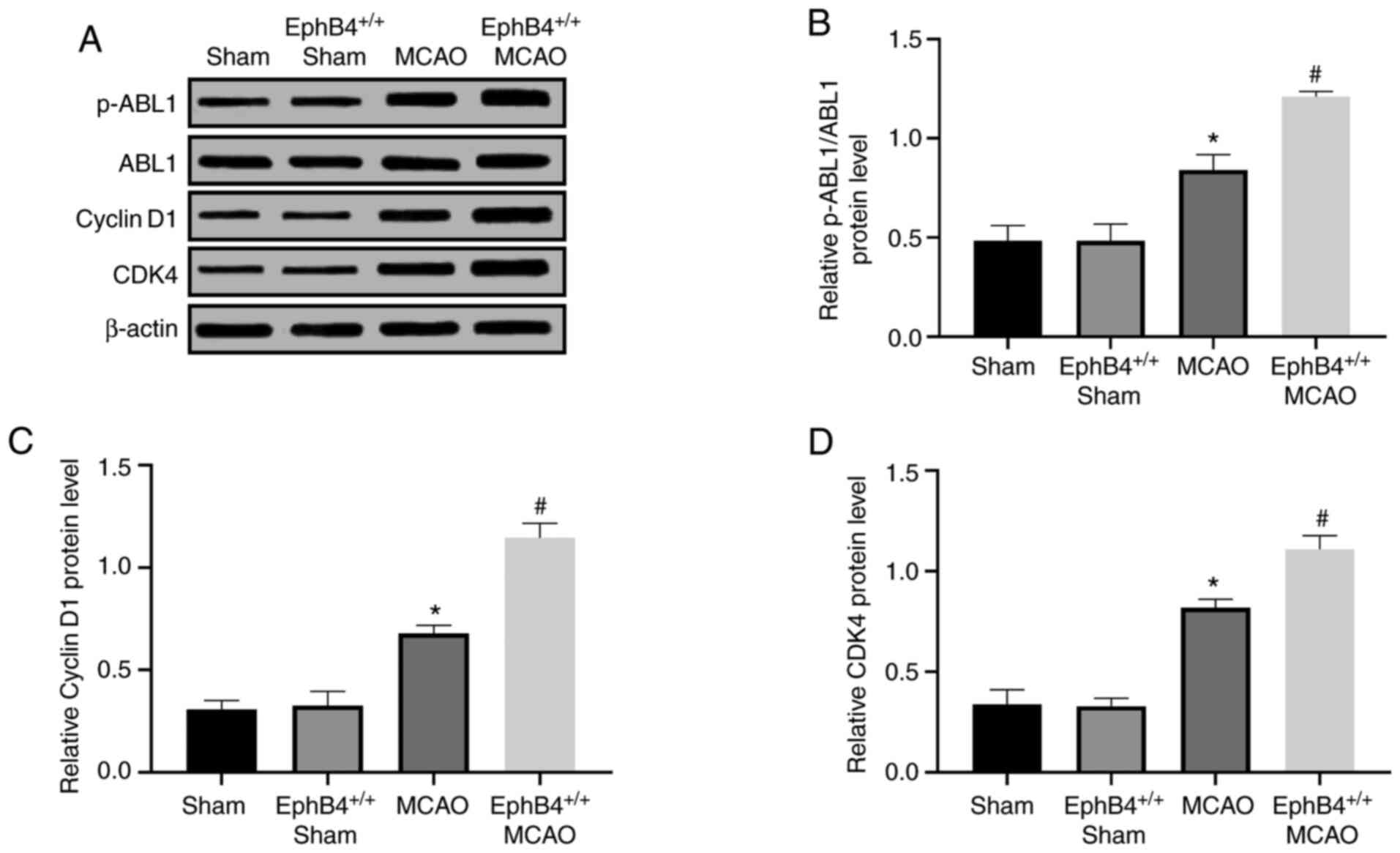
EphB4 activates the ABL1/Cyclin D1
signaling pathway in the cerebral cortex of MCAO model mice
To assess the potential mechanism of EphB4 in
neurogenesis, the related proteins of the ABL1/Cyclin D1 signaling
pathway were examined. As shown in Fig.
6A-D, the expression levels of p-ABL1, Cyclin D1 and CDK4
showed a significant increase in the MCAO-treated ischemia mice
compared with the sham-operated group. Moreover, compared with the
MCAO-treated ischemic mice, the abovementioned protein expression
levels were significantly increased in the
EphB4+/+ MCAO model mice at 7 days after
ischemia (Fig. 6B-D). These results
indicated that overexpression of EphB4 activated the ABL1/Cyclin D1
signaling pathway, thereby promoting cell proliferation and
differentiation in mice after ischemia stroke.
Discussion
Ischemic strokes result in numerous deaths and leave
survivors with significant disability, despite various reasonable
treatments (27). Neurogenesis has
been proven to be a primary neurovascular response during stroke
recovery (28,29). In the present study, it was found
that overexpression of EphB4 promoted the proliferation and
differentiation of NSCs in the cerebral cortex by modulating the
ABL1/Cyclin D1 signaling pathway after cerebral ischemia,
suggesting that EphB4 may be an effective strategic target for
improving neurological dysfunction in ischemic stroke.
EphB4 tyrosine kinase receptor and its ephrinB2
ligand have been identified as important regulators of tumor cell
proliferation, migration and differentiation (30–32).
For instance, EphB4 was highly expressed in hepatocellular
carcinoma and knockdown of EphB4 inhibited cell proliferation and
migration and tumor growth (33).
Kadife et al (34)
discovered that a high expression level of EphB4 facilitated
colorectal cancer cell migration and tumor growth. Interestingly,
EphB4 was reported to be upregulated after stroke, and was
suggested to be involved in neoangiogenesis and neuronal survival
(35,36), which was consistent with the present
findings that EphB4 was significantly increased in mice after MCAO.
A previous study also revealed that EphB4 was expressed in NSCs and
instructed NSC neuronal differentiation in the subgranular zone
(37). The present study
demonstrated that EphB4 was expressed in NSCs and may participate
in the differentiation of NSC in the cortex following MCAO.
Brdu is usually employed to label cells in the cell
cycle of S phase (38). Ki67 is a
nuclear protein associated with ribosomal RNA transcription that
acts as a marker for cell proliferation (39). A previous study showed that
Nestin+ cells can be observed extensively in restricted
regions where they may serve as a niche of stem/progenitor cells
with the capacity for proliferation and differentiation in the
adult mouse brain (40). Therefore,
in the present study, the increased levels of Nestin+,
Ki67+ and Brdu+ cells in the peri-infarct
cortex of the ischemia-treated mice indicated the endogenous NSC
response following MCAO. In the EphB4 overexpression group, the
increase of Nestin+, Ki67+ and
Brdu+ cells was further enhanced. These results
indicated that EphB4 overexpression promotes cell proliferation
post-ischemia.
Sox2, a member of the Sox family of transcription
factors, is highly expressed during the development of embryonic
stem cells and adult NSCs, and is considered to be the key to the
proliferation and differentiation of NSCs (41). DCX, a protein facilitating
microtubule polymerization, is expressed in migrating neuroblasts
and immature neurons, and can be classified as a marker for adult
neurogenesis (42). NeuN is a
neuronal protein and a neuron marker utilized to specifically label
mature neurons (43). Hence, in the
current study, the increased number of Sox2+,
DCX+ and NeuN+ cells in the cortex of the
EphB4+/+ MCAO model mice indicated that EphB4
may participate in promoting NSC differentiation into neurons
post-ischemia. The upregulation of these neural cells is considered
to contribute to functional recovery of brain.
The downstream factor of the ephrinB2/EphB4
signaling pathway, ABL1, binds to EphB4 in an activity-dependent
manner (16). Previous findings
have shown that ABL1 served an important role in the stimulation of
neurogenesis (44). Moreover, ABL1
could mediate the signaling between EphB4 and Cyclin D1/CDK4 to
regulate proliferation in NSCs (18). The current results revealed that
overexpression of EphB4 increased the protein expression levels of
p-ABL1, Cyclin D1 and CDK4, suggesting that EphB4 may promote
neurogenesis after ischemic stroke via the ABL1/Cyclin D1 signaling
pathway in vivo.
There were certain limitations in the present study.
Firstly, the effect of EphB4 on neurogenesis was not assessed in
vitro. Secondly, the level of EphB4 in patients with ischemic
stroke was not examined and the association between EphB4 levels
and lesion volume requires further study. The underlying mechanism
of EphB4 on neurogenesis and neuroinflammation should also be
investigated both in vitro and in vivo.
In conclusion, the present study demonstrated that
restoration of EphB4 promoted cell proliferation and
differentiation at 7 days after MCAO, and facilitated NSC
directional differentiation into neurons at 14 days after MCAO.
However, EphB4 upregulation inhibited neuroinflammation at 1 day
after MCAO. Moreover, it was found that overexpression of EphB4
promoted the activation of the ABL1/Cyclin D1 signaling pathway.
These findings suggested that EphB4 may be a valuable therapeutic
target for ischemic stroke.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW guaranteed the integrity of the entire study,
performed the experiments and wrote the manuscript. ZZ, SF and XiaL
constructed the MCAO model and collected brain tissues. ZZ, XinL
and SW performed the data analysis and statistical analysis. LY
designed the study, supervised the research and participated in
reviewing the manuscript. All authors read and approved the final
manuscript. JW and LY confirmed the authenticity of all the raw
data.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Inner Mongolia Baogang Hospital (approval no. BG201802034YY).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feigin VL, Forouzanfar MH, Krishnamurthi
R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L,
Truelsen T, et al: Global and regional burden of stroke during
1990–2010: Findings from the global burden of disease study 2010.
Lancet. 383:245–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krishnamurthi RV, Ikeda T and Feigin VL:
Global, regional and country-specific burden of ischaemic stroke,
intracerebral haemorrhage and subarachnoid haemorrhage: A
systematic analysis of the global burden of disease study 2017.
Neuroepidemiology. 54:171–179. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pires Monteiro S, Voogd E, Muzzi L, De
Vecchis G, Mossink B, Levers M, Hassink G, Van Putten M, Le Feber
J, Hofmeijer J and Frega M: Neuroprotective effect of hypoxic
preconditioning and neuronal activation in a in vitro human model
of the ischemic penumbra. J Neural Eng. 18:0360162021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Doetsch F, Caillé I, Lim DA,
Garcia-Verdugo JM and Alvarez-Buylla A: Subventricular zone
astrocytes are neural stem cells in the adult mammalian brain.
Cell. 97:703–716. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bond AM, Ming GL and Song H: Adult
mammalian neural stem cells and neurogenesis: Five decades later.
Cell Stem Cell. 17:385–395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan YP, Sailor KA, Lang BT, Park SW,
Vemuganti R and Dempsey RJ: Monocyte chemoattractant protein-1
plays a critical role in neuroblast migration after focal cerebral
ischemia. J Cereb Blood Flow Metab. 27:1213–1224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen L, Qiu R, Li L, He D, Lv H, Wu X and
Gu N: The role of exogenous neural stem cells transplantation in
cerebral ischemic stroke. J Biomed Nanotechnol. 10:3219–3230. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamashita T, Ninomiya M, Hernandez Acosta
P, García-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T,
Hirota Y, Kawase T, et al: Subventricular zone-derived neuroblasts
migrate and differentiate into mature neurons in the post-stroke
adult striatum. J Neurosci. 26:6627–6636. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaneko N and Sawamoto K: Adult
neurogenesis and its alteration under pathological conditions.
Neurosci Res. 63:155–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong X, Gao J, Zhang CY, Hayworth C, Frank
M and Wang Z: Neutrophil membrane-derived nanovesicles alleviate
inflammation to protect mouse brain injury from ischemic stroke.
ACS Nano. 13:1272–1283. 2019.PubMed/NCBI
|
|
11
|
Yang S, Wang H, Yang Y, Wang R, Wang Y, Wu
C and Du G: Baicalein administered in the subacute phase
ameliorates ischemia-reperfusion-induced brain injury by reducing
neuroinflammation and neuronal damage. Biomed Pharmacother.
117:1091022019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Retraction: Roles of Eph/ephrin
bidirectional signaling during injury and recovery of the central
nervous system. Neural Regen Res. 13:21402018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knöll B and Drescher U: Ephrin-As as
receptors in topographic projections. Trends Neurosci. 25:145–149.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
del Valle K, Theus MH, Bethea JR, Liebl DJ
and Ricard J: Neural progenitors proliferation is inhibited by
EphB3 in the developing subventricular zone. Int J Dev Neurosci.
29:9–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conover JC, Doetsch F, Garcia-Verdugo JM,
Gale NW, Yancopoulos GD and Alvarez-Buylla A: Disruption of
Eph/ephrin signaling affects migration and proliferation in the
adult subventricular zone. Nat Neurosci. 3:1091–1097. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noren NK, Foos G, Hauser CA and Pasquale
EB: The EphB4 receptor suppresses breast cancer cell tumorigenicity
through an Abl-Crk pathway. Nat Cell Biol. 8:815–825. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferguson BD, Liu R, Rolle CE, Tan YH,
Krasnoperov V, Kanteti R, Tretiakova MS, Cervantes GM, Hasina R,
Hseu RD, et al: The EphB4 receptor tyrosine kinase promotes lung
cancer growth: A potential novel therapeutic target. PLoS One.
8:e676682013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu T, Zeng X, Sun F, Hou H, Guan Y, Guo
D, Ai H, Wang W and Zhang G: EphB4 regulates self-renewal,
proliferation and neuronal differentiation of human embryonic
neural stem cells in vitro. Cell Physiol Biochem. 41:819–834. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen ST, Hsu CY, Hogan EL, Maricq H and
Balentine JD: A model of focal ischemic stroke in the rat:
Reproducible extensive cortical infarction. Stroke. 17:738–743.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao RQ, Zhang L, Wang W and Li L: Cornel
iridoid glycoside promotes neurogenesis and angiogenesis and
improves neurological function after focal cerebral ischemia in
rats. Brain Res Bull. 79:69–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
López-Juárez A, Remaud S, Hassani Z,
Jolivet P, Pierre Simons J, Sontag T, Yoshikawa K, Price J,
Morvan-Dubois G and Demeneix BA: Thyroid hormone signaling acts as
a neurogenic switch by repressing Sox2 in the adult neural stem
cell niche. Cell Stem Cell. 10:531–543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moraga A, Pradillo JM, Garcia-Culebras A,
Palma-Tortosa S, Ballesteros I, Hernández-Jiménez M, Moro MA and
Lizasoain I: Aging increases microglial proliferation, delays cell
migration, and decreases cortical neurogenesis after focal cerebral
ischemia. J Neuroinflammation. 12:872015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marei HE, Shouman Z, Althani A, Afifi N, A
AE, Lashen S, Hasan A, Caceci T, Rizzi R, Cenciarelli C and
Casalbore P: Differentiation of human olfactory bulb-derived neural
stem cells toward oligodendrocyte. J Cell Physiol. 233:1321–1329.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dayer AG, Cleaver KM, Abouantoun T and
Cameron HA: New GABAergic interneurons in the adult neocortex and
striatum are generated from different precursors. J Cell Biol.
168:415–427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carlson KN, Pavan-Guimaraes J, Verhagen
JC, Chlebeck P, Verhoven B, Jennings H, Najmabadi F, Liu Y,
Burlingham W, Capitini CM and Al-Adra D: IL-10 and TGF-β cytokines
decrease immune activation during normothermic ex vivo machine
perfusion of the rat liver. Liver Transpl. Jun 12–2021.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Onwuekwe I and Ezeala-Adikaibe B: Ischemic
stroke and neuroprotection. Ann Med Health Sci Res. 2:186–190.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Delavaran H, Sjunnesson H, Arvidsson A,
Lindvall O, Norrving B, van Westen D, Kokaia Z and Lindgren A:
Proximity of brain infarcts to regions of endogenous neurogenesis
and involvement of striatum in ischaemic stroke. Eur J Neurol.
20:473–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohira K, Furuta T, Hioki H, Nakamura KC,
Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, et
al: Ischemia-induced neurogenesis of neocortical layer 1 progenitor
cells. Nat Neurosci. 13:173–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wan X, Saban DV, Kim SN, Weng Y, Dammann
P, Keyvani K, Sure U and Zhu Y: PDCD10-deficiency promotes
malignant behaviors and tumor growth via triggering EphB4 kinase
activity in glioblastoma. Front Oncol. 10:13772020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang Y, Lei Y, Huang S, Li Z and Chen X,
Luo H, Cheng C, Chen J, Zou X and Chen X: Pristimerin exacerbates
cellular injury in conditionally reprogrammed patient-derived lung
adenocarcinoma cells by aggravating mitochondrial impairment and
endoplasmic reticulum stress through EphB4/CDC42/N-WASP signaling.
Oxid Med Cell Longev. 2020:74098532020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhatia S, Bukkapatnam S, Van Court B, Phan
A, Oweida A, Gadwa J, Mueller AC, Piper M, Darragh L, Nguyen D, et
al: The effects of ephrinB2 signaling on proliferation and invasion
in glioblastoma multiforme. Mol Carcinog. 59:1064–1075. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu M, Gong Z, Wu Q, Su Q, Yang T, Yu R,
Xu R and Zhang Y: Homoharringtonine suppresses tumor proliferation
and migration by regulating EphB4-mediated β-catenin loss in
hepatocellular carcinoma. Cell Death Dis. 11:6322020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kadife E, Ware TMB, Luwor RB, Chan STF,
Nurgali K and Senior PV: Effects of EphB4 receptor expression on
colorectal cancer cells, tumor growth, vascularization and
composition. Acta Oncol. 57:1043–1056. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoon Y, Voloudakis G, Doran N, Zhang E,
Dimovasili C, Chen L, Shao Z, Darmanis S, Tang C, Tang J, et al:
PS1 FAD mutants decrease ephrinB2-regulated angiogenic functions,
ischemia-induced brain neovascularization and neuronal survival.
Mol Psychiatry. Jun 15–2020.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ghori A, Freimann FB, Nieminen-Kelhä M,
Kremenetskaia I, Gertz K, Endres M and Vajkoczy P: EphrinB2
activation enhances vascular repair mechanisms and reduces brain
swelling after mild cerebral ischemia. Arterioscler Thromb Vasc
Biol. 37:867–878. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ashton RS, Conway A, Pangarkar C, Bergen
J, Lim KI, Shah P, Bissell M and Schaffer DV: Astrocytes regulate
adult hippocampal neurogenesis through ephrin-B signaling. Nat
Neurosci. 15:1399–1406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kempermann G, Gast D, Kronenberg G,
Yamaguchi M and Gage FH: Early determination and long-term
persistence of adult-generated new neurons in the hippocampus of
mice. Development. 130:391–399. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schaefer BM, Wallich R, Schmolke K, Fink
W, Bechtel M, Reinartz J and Kramer MD: Immunohistochemical and
molecular characterization of cultured keratinocytes after
dispase-mediated detachment from the growth substratum. Exp
Dermatol. 9:58–64. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Namiki J, Suzuki S, Masuda T, Ishihama Y
and Okano H: Nestin protein is phosphorylated in adult neural
stem/progenitor cells and not endothelial progenitor cells. Stem
Cells Int. 2012:4301382012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ferri AL, Cavallaro M, Braida D, Di
Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala
M, DeBiasi S and Nicolis SK: Sox2 deficiency causes
neurodegeneration and impaired neurogenesis in the adult mouse
brain. Development. 131:3805–3819. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brown JP, Couillard-Després S, Cooper-Kuhn
CM, Winkler J, Aigner L and Kuhn HG: Transient expression of
doublecortin during adult neurogenesis. J Comp Neurol. 467:1–10.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dachet F, Bagla S, Keren-Aviram G, Morton
A, Balan K, Saadat L, Valyi-Nagy T, Kupsky W, Song F, Dratz E and
Loeb JA: Predicting novel histopathological microlesions in human
epileptic brain through transcriptional clustering. Brain.
138:356–370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schlatterer SD, Suh HS, Conejero-Goldberg
C, Chen S, Acker CM, Lee SC and Davies P: Neuronal c-Abl activation
leads to induction of cell cycle and interferon signaling pathways.
J Neuroinflammation. 9:2082012. View Article : Google Scholar : PubMed/NCBI
|