Introduction
B19 virus (B19V), known as a human parvovirus, is a
widespread DNA virus that may be found in patients across a wide
age range (0–94 years); the immunity to B19V was reported to be
~78% in individuals >50 years old in various countries between
1992 and 1998 (1). The B19V genome
encodes a non-structural protein (NS1), two capsid proteins (VP1
and VP2) and two smaller proteins, 7.5 and 11 kDa in weight
(1). Of the B19V capsid proteins,
~95% are VP2, and the remaining 5% are VP1. The 227 amino acids at
the N-terminal region of B19V–VP1u are known as the B19V–VP1-unique
region (VP1u), which shares the same amino acid sequence with
B19V–VP1 (2). B19V-NS1 is known to
possess enzymatic activities, including helicase and ATPase
activities, and DNA-binding activities that regulate viral
transcription and replication (3,4).
B19V-NS1 also increases the expression of IL-6 and TNF-α in host
cells and induces cell apoptosis (5,6). The
major capsid protein of B19V, VP2, can bind P antigen (globoside)
on the cell surface for viral attachment, and the minor capsid
protein VP1 is associated with the induction of immune responses
during infection (7–9).
B19V infection has been linked to a range of
clinical manifestations, such as myocarditis (10,11),
hepatitis (12), nephrotic syndrome
(13), thyroid disease (14), nerve damage (15), several types of cancer (16) and autoimmune diseases (17). To determine the association between
B19V infection and cardiac disorders, the study of the presence of
the B19V genome in cardiac tissues has attracted increased
attention. Indeed, various studies have reported the presence of
the B19V genome in endomyocardial tissues (18,19).
Similar results were also obtained from the myocardium of patients
with dilated cardiomyopathy (DCM) (20,21),
fulminant myocarditis, sudden heart failure or chronic inflammatory
cardiomyopathy (22,23).
B19V–VP1u has been reported to significantly
increase TNF-α expression in H9c2 cells (24). Accumulating evidence has indicated
that immunization of BALB/c mice with B19V–VP1u proteins can cause
DCM (11). Moreover, the
administration of B19V–VP1u in naïve mice can elicit
ultrastructural changes of the heart and significantly increase the
levels of various markers of cardiac injury, such as lactate
dehydrogenase, creatine kinase isoenzyme and α-hydroxybutyric acid
dehydrogenase (25). Significantly
aggravated inflammation, including elevated MMP-9 activity and
levels of inflammatory cytokines (IL-1β, IL-6 and TNF-α), has been
detected in the left ventricles of the hearts of NZB/W F1 mice
receiving B19V–VP1u IgG (26).
Similar findings were also observed in patients with systemic lupus
erythematosus with DCM, who possessed antibodies against B19V–VP1u,
indicating an association between B19V–VP1u and DCM (27). These studies strongly suggest a
pathological role of B19V–V1u in cardiomyopathy. Notably, our
previous studies revealed the differential effects of truncated
B19V–VP1u fragments on autoimmunity, particularly anti-phospholipid
syndrome (28,29). However, the antigenicity of
B19V–VP1u in causing cardiac damage remains unknown. Therefore, the
aim of the present study was to further elucidate the roles of
different B19V–VP1u fragments in inducing cardiac injury.
Materials and methods
Human parvovirus B19V proteins
Human B19V recombinant proteins were prepared as
described in our previous publications (28,29).
The B19V-NS1 protein was purified using the Profinia protein
purification system (Bio-Rad Laboratories, Inc.) (28). The DNA sequences encoding B19-VP1,
VP1u and truncated B19V–VP1uA (residues 1–60), B (residues 61–129),
C (residues 130–195) and D (residues 196–227) were ligated into a
pET-32a vector-Novagene (MilliporeSigma). These recombinant
parvovirus B19 proteins were further induced with 1 mM IPTG for 3 h
at 37°C and purified with a PureProteome™ Nickel Magnetic Beads
system (MilliporeSigma). The purified proteins were further
analyzed using high performance liquid chromatography (Waters HPLC
600 System; Waters Corporation). Briefly, 400 µl (0.2 mg/ml)
purified proteins were injected into a 13-µm column
(Superdex® 75 10/300 GL; MilliporeSigma) and eluted with
elution buffer (25 mM Tris-HCL, pH 8.0; 150 mM NaCl) at a flow rate
of 0.5 ml/min. The purity of the purified proteins ranged between
98.1 and 99.3%, and the endotoxin levels of purified recombinant
proteins were all under the limits of detection (0.25 endotoxin
units/ml).
Ethics and animal
Animal experiments were performed in accordance with
the principles of replacement, refinement and reduction and were
approved by the Institutional Animal Care and Use Committee (IACUC)
of Chung Shan Medical University, Taichung (approval no. 1676;
approved on December 2015). A total of 32 BALB/c BYJNarl female
mice (age, 7 weeks; weight, 19–21 g) were obtained from the
National Laboratory Animal Center and administrated under the IACUC
at Chung Shan Medical University (Taichung, Taiwan). All animals
were maintained in an air-conditioned room with a 12-h light-dark
cycle at 22°C, and the relative humidity in the room was 55%.
Animals were allowed free access to water and standard laboratory
chow (Lab Diet 5001; PMI Nutrition International Inc.).
After 1 week of acclimation, the 8-week-old animals
were randomly divided into eight groups (n=4 per group) as follows:
i) Control group (PBS); ii) B19V–VP1 group; iii) B19V-NS1 group;
iv) B19V–VP1u group; v) VP1u-A group; vi) VP1u-B group; vii) VP1u-C
group; and viii) VP1u-D group. As described in previous reports
(30,31) and in our previous publication
(29), the initial and booster
injections (2–3 times) are necessary to obtain optimal antibody
responses. A total of 20 µg purified recombinant proteins or PBS
was mixed 1:1 (v/v) with Freund's complete adjuvant
(MillporeSigma), and subcutaneously injected into mice of each
group on day 1. The mice were then boosted with 20 µg recombinant
proteins or PBS mixed 1:1 (v/v) with Freund's incomplete adjuvant
(MillporeSigma) twice times each on days 14 and 28. All animals
were fasted for 12 h before sacrifice with CO2 when they
reached 16-weeks of age. A displacement rate of 20% of the chamber
volume with CO2 per min was used to euthanize the mice
(performed in April 2018). The blood from the heart and the left
ventricle tissues of mice were harvested (at 10 a.m.) and stored at
−80°C until required for subsequent analysis. To confirm the
induction of immunity by B19V–V1u and truncated B19V–VP1u
recombinant proteins, absorption experiments were performed using a
competitive (comp) ELISA (29) to
verify the specificity of the antibodies in naïve mice receiving
B19V–VP1u or truncated B19V–VP1u recombinant proteins (Fig. S1). Whole blood samples were
obtained from the hearts of mice immunized with B19-VP1u or
truncated B19V–VP1u recombinant proteins as aforementioned and were
centrifuged at 1,500 × g for 10 min at room temperature to obtain
the heart sera. For the absorption experiments (comp ELISA), the
sera were pre-incubated with 500 µM VP1u or truncated VP1u proteins
for 1 h at 37°C. Subsequently, all anti-sera (non-comp or comp
groups) against 2 µg B19-VP1u or truncated B19V–VP1u recombinant
proteins were immobilized on the surface of each sample well of
96-well plates. After incubation at room temperature for 120 min,
the liquid from each sample well was removed, and the wells were
washed with PBS and subsequently incubated with horseradish
peroxidase (HRP)-conjugated rabbit anti-mouse IgG (cat. no. A9044;
MilliporeSigma) at a dilution of 1:1,000. After incubation at room
temperature for 60 min, the liquid was removed from each sample
well and the wells were washed with PBS. The color reaction was
performed using 1 mg/ml substrate
2,2′azino-di-(3-ethylbenzthiazolin-6-sulphonic acid)
(MilliporeSigma) in the presence of 0.005%
H2O2 at room temperature for 15 min. A cutoff
value was determined using sera from CTL mice, and the value was
regarded as positive. Notably, the non-‘comp’ groups had enhanced
absorbance as inoculation raised an immune response, whereas the
‘comp’ groups had reduced absorbance as antibodies were bound to
the proteins from the initial absorption experiment (Fig. S1).
Gel zymography
MMP-9 and MMP-2 activities were measured as
described previously (32). Protein
concentrations were determined using a Bradford assay (Bio-Rad
Laboratories, Inc.) and quantified using a U3000 spectrophotometer
(Hitachi Ltd.). A total of 25 µg left ventricular tissue of mice
from each experimental group was resolved on an 8% SDS-gel, via
SDS-PAGE, that contained 0.1% gelatin. MMP-9 and MMP-2 activities
were detected by staining the gel with 0.5% coomassie brilliant
blue R-250 for 1 h at room temperature after soaking in reaction
solution (40 mM Tris-HCl, 10 mM CaCl2 and 0.02%
NaN3) for 18 h. A gel densitometry system (Alpha-Imager
2200; ProteinSimple) was used to quantify the relative MMP
levels.
Protein preparation and
immunoblotting
Protein concentration was determined by a modified
Bradford's assay using a U3000 spectrophotometer at 595 nm with BSA
(MilliporeSigma) as the standard. A total of 15 µg left ventricular
tissue from each animal was resected and homogenized in 600 µl
lysis buffer (PRO-PREP™; Intron Biotechnology, Inc.) with a tissue
homogenizer (Polytron RT 3000; Brinkmann Instruments, Inc.). The
homogenized tissues were then placed on ice for 30 min, and the
supernatants of tissue lysates were harvested by centrifuging at
4°C at 18,928 × g for 15 min. For immunoblotting, extracted
proteins (25 µg/lane) were separated using 12 or 15% SDS-gels, and
transferred to nitrocellulose membranes (Bio-Rad Laboratories,
Inc.). After blocking in 5% non-fat dry milk for 1 h at 25°C,
membranes were incubated with antibodies against IL-6 (1:500; cat.
no. sc-1265), IL-1β (1:500; cat. no. sc-7884), ERK (1:1,000; cat.
no. sc-514302), P38 (1:1,000; cat. no. sc-7972), phosphorylated
(p)-P38 (1:1,000; cat. no. sc-7973), atrial natriuretic peptide
(ANP; 1:500; cat. no. sc-20158) (all from Santa Cruz Biotechnology,
Inc.), heart-type fatty acid-binding protein (H-FABP; 1:500; cat.
no. ab16915), creatine kinase-MB (CK-MB; 1:500; cat. no. ab71722
(both from Abcam), p-ERK (1:1,000; cat. no. 05-797R;
MilliporeSigma) and α-tubulin (1:500; cat. no. PA5-16891; Thermo
Fisher Scientific, Inc.) for 3 h at 25°C. The membranes were
subsequently incubated with HRP-conjugated secondary antibodies
(1:5,000; cat. nos. sc-2004 or sc-2005; Santa Cruz Biotechnology,
Inc.) at 25°C for 1.5 h. Signals were visualized using Immobilon
Western Chemiluminescent HRP Substrate (MilliporeSigma), and
densitometry analysis was performed using an imaging analyzer (GE
ImageQuant TL 8.1; GE Healthcare Life Sciences).
H&E staining
For H&E staining, the left ventricle tissues of
mice were immersed in 10% formalin at 25°C for 24 h and embedded in
paraffin. The tissue blocks were sliced into 4-mm sections,
deparaffinized and dehydrated. Next, the slides were stained with
hematoxylin and eosin after processing at 25°C for 5 min with 100,
95 and 75% ethanol. Each slide was subsequently immersed in 85%
ethanol and 100% ethanol twice (20 min each immersion). Finally,
the slides were immersed in xylene twice for 2 min.
Photomicrographs were observed using a light microscope (Zeiss
Axiophot microscope; Zeiss AG) at ×200 and ×400 magnifications.
Mouse cytokine immunoassay
The serum IL-1β, IL-6, TNF-α and IFN-γ levels of
mice from each experimental group were detected with Bio-Plex mouse
cytokine 8-plex assay kit, according to manufacturer's instructions
(cat. no. Z6000004CF; Bio-Rad Laboratories, Inc.) and Bio-Plex
manager software version 3.0 using five parametric curve fitting
(Bio-Rad Laboratories, Inc.).
Statistical analysis
GraphPad Prism 5 software (GraphPad Software, Inc.)
was used to calculate the significant differences among groups
using a one-way ANOVA followed by Tukey's multiple-comparisons
test. All data are presented as the mean ± SEM, and were verified
in ≥3 independent experiments. P<0.05 was considered to indicate
a statistically significant difference.
Results
Effects of different recombinant B19V
proteins on inducing markers of cardiac inflammation in naïve
mice
To determine the influence of different B19V
proteins on increasing cardiac inflammation, MMP-9 and MMP-2
activities were detected in the left ventricles of mice treated
with PBS (Control), or recombinant B19V–VP1, B19V-NS1 or B19V–VP1u
proteins. A significantly higher MMP-9/MMP-2 ratio was observed in
left ventricular tissues of the mice from the B19V-NS1 and B19-VP1u
groups compared with the control group (Fig. 1A). The levels of the inflammatory
cytokines, IL-6 and IL-1β, were also detected. Significantly higher
amounts of IL-6 were observed in the left ventricular tissues of
the mice from the B19V-NS1 and B19-VP1u groups compared with the
control group (Fig. 1B). Moreover,
significantly increased IL-1β levels were detected in left
ventricular tissues of mice from the B19V–VP1, B19V-NS1 and
B19-VP1u groups compared with the control group (Fig. 1B). In addition, significantly
elevated p-ERK/ERK and p-P38/P38 ratios were observed in left
ventricular tissues of the mice from the B19V–VP1, B19V-NS1 and
B19-VP1u groups compared with the controls (Fig. 2).
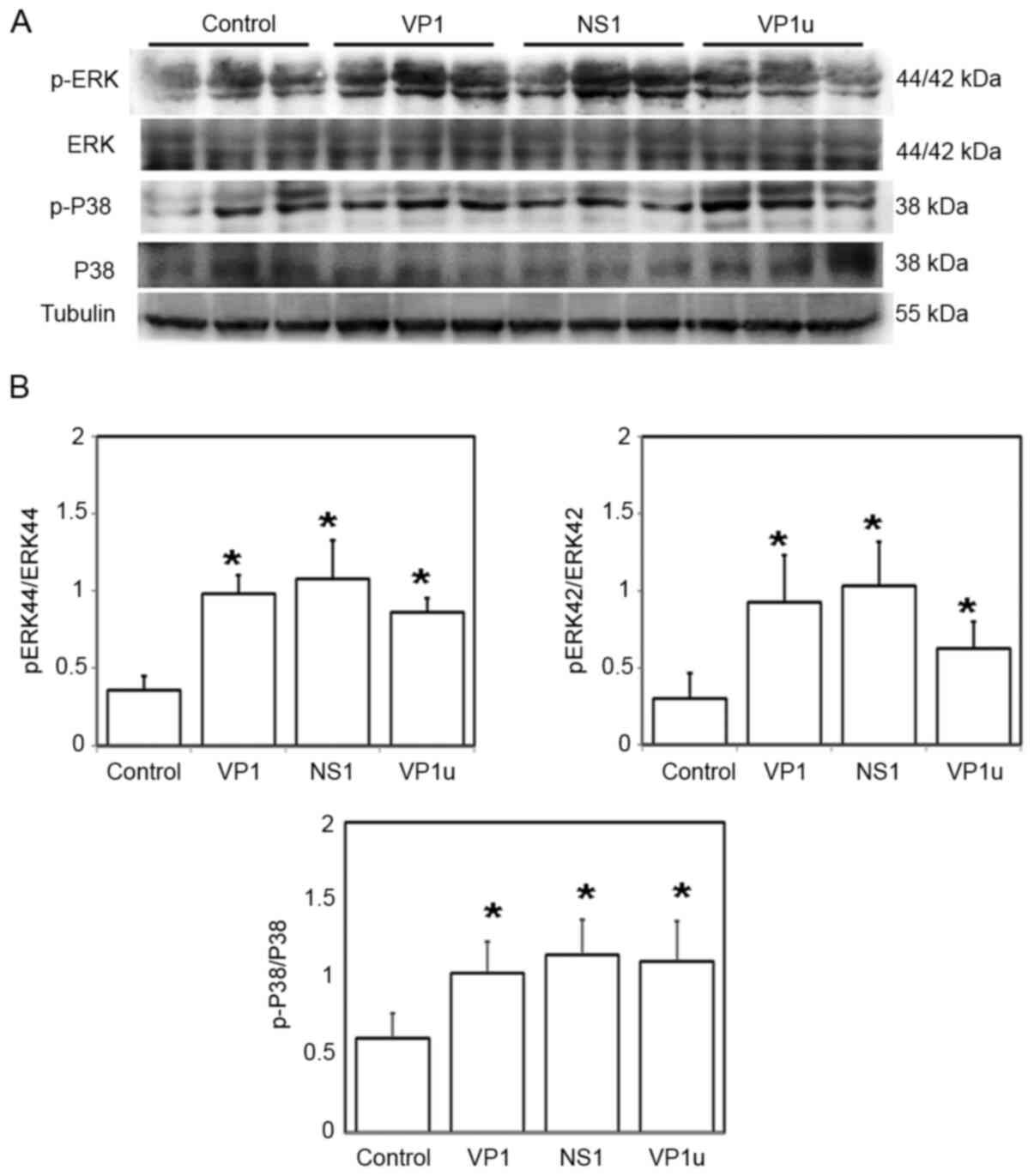 | Figure 2.Effects of B19V-NS1, VP1 and VP1u on
the phosphorylation of ERK and P38. (A) Protein expression levels
of ERK, p-ERK, P38, and p-P38 in the left ventricle tissues of the
mice receiving different treatments (PBS, VP1, NS1 or VP1u). (B)
Relative levels of p-ERK and p-P38 are based on total protein
expression. Similar results were obtained in three-repeated
experiments. *P<0.05 vs. Control (mice treated with PBS). n=4
for each group. B19V, B19 virus; B19V-NS1, B19V-non-structural
protein 1; VP1u, VP1-unique region; p-, phosphorylated. |
Effects of different recombinant B19V
proteins on inducing cardiac injury indicators in naïve mice
To understand the influence of different B19V
proteins on increasing cardiac injury in naïve mice, various
indicators of myocardial injury, including ANP, H-FABP and CK-MB,
were measured via immunoblotting. Significantly increased ANP
expression was detected in the left ventricular tissues of mice
from the B19V-NS1 and B19V–VP1u groups compared with the control
group (Fig. 3). Moreover,
significantly elevated H-FABP and CK-MB expression was observed in
left ventricular tissues of mice from the B19V–VP1, B19V-NS1 and
B19V–VP1u groups compared with the control group (Fig. 3). Accordingly, infiltration of
lymphocytes was observed in the left ventricles of mice injected
with B19V–VP1, B19V-NS1 and B19V–VP1u proteins (Fig. 4).
 | Figure 3.Effects of B19V-NS1, VP1 and VP1u on
the expression levels of ANP, H-FABP and CK-MB. (A) Protein
expression levels of ANP, H-FABP and CK-MB in the left ventricle
tissues of the mice receiving different treatments (PBS, VP1, NS1
or VP1u). (B) Relative levels of ANP, H-FABP and CK-MB are based on
tubulin expression. Similar results were obtained in three-repeated
experiments. *P<0.05 vs. Control (mice treated with PBS). n=4
for each group. B19V, B19 virus; B19V-NS1, B19V-non-structural
protein 1; VP1u, VP1-unique region; ANP, atrial natriuretic
peptide; H-FABP, heart-type fatty acid-binding protein; CK-MB,
creatine kinase isoenzyme-MB. |
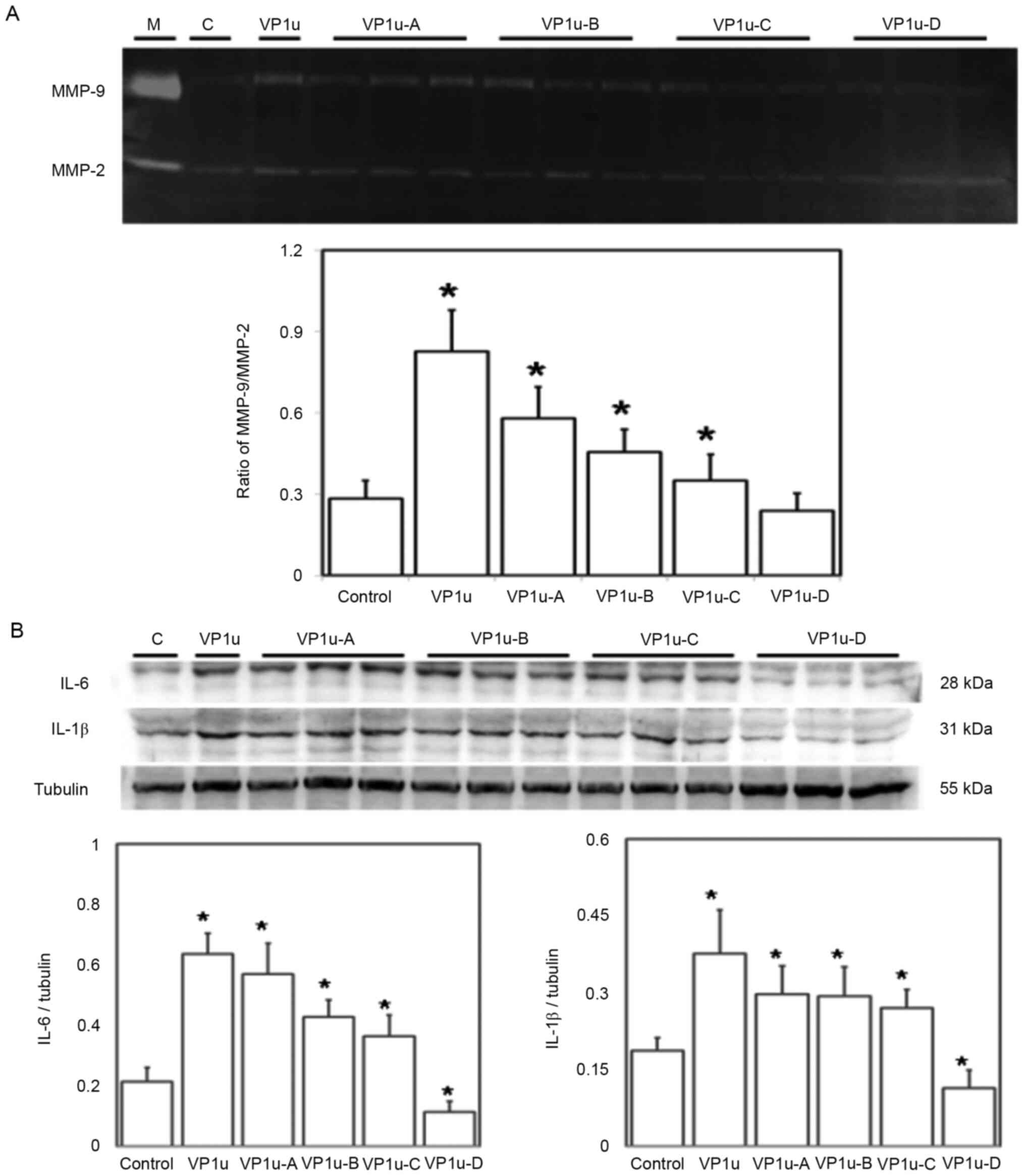
Effects of different regions of
recombinant B19V–VP1u proteins on inducing increases in the levels
of cardiac inflammatory markers in naïve mice
To further verify the effects of different regions
of B19V–VP1u on cardiac inflammation, the MMP-9 and MMP-2
activities, as well as IL-6 and IL-1β levels were detected in the
left ventricles of mice treated with PBS, or recombinant B19V–VP1u,
B19V–VP1u-A, B19V–VP1u-B, B19V–VP1u-C or B19V–VP1u-D proteins. A
significantly higher MMP-9/MMP-2 ratio was detected in left
ventricular tissues of mice from the B19V–VP1u, B19V–VP1u-A,
B19V–VP1u-B and B19V–VP1u-C groups compared with the controls
(Fig. 5A). Moreover, significantly
higher levels of IL-6 and IL-1β were detected in the left
ventricular tissues of the mice from the B19V–VP1u, B19V–VP1u-A,
B19V–VP1u-B and B19V–VP1u-C groups compared with the controls
(Fig. 5B). Conversely,
significantly lower IL-6 and IL-1β levels were observed in the left
ventricular tissues of mice from the B19V–VP1u-D group compared
with the controls (Fig. 5B).
Moreover, significantly increased ratios of p-ERK/ERK and p-P38/P38
were observed in the left ventricular tissues of mice from the
B19V–VP1u, B19V–VP1u-A, B19V–VP1u-B and B19V–VP1u-C groups compared
with the controls (Fig. 6).
Effects of different regions of
recombinant B19V–VP1u on indicators of cardiac injury in naïve
mice
To investigate the effects of different regions of
B19V proteins on increasing cardiac injury in naïve mice, the
expression levels of ANP, H-FABP and CK-MB proteins were detected
via immunoblotting. Significantly higher expression levels of ANP,
H-FABP and CK-MB were observed in the left ventricles of mice
injected with B19V–VP1u, B19V–VP1u-A and B19V–VP1u-B proteins
compared with the controls (Fig.
7). Accordingly, increased infiltration of lymphocytes was
observed in the left ventricles of mice injected with B19V–VP1u,
B19V–VP1u-A, B19V–VP1u-B and B19V–VP1u-C (Fig. 8). In addition, significantly higher
serum IL-1β, IL-6, TNF-α and IFN-γ levels were detected in mice
injected with B19V–VP1u and B19V–VP1u-A, and only higher serum
IL-1β and IL-6 levels were detected in mice injected with
B19V–VP1u-B proteins compared with the controls (Fig. 9).
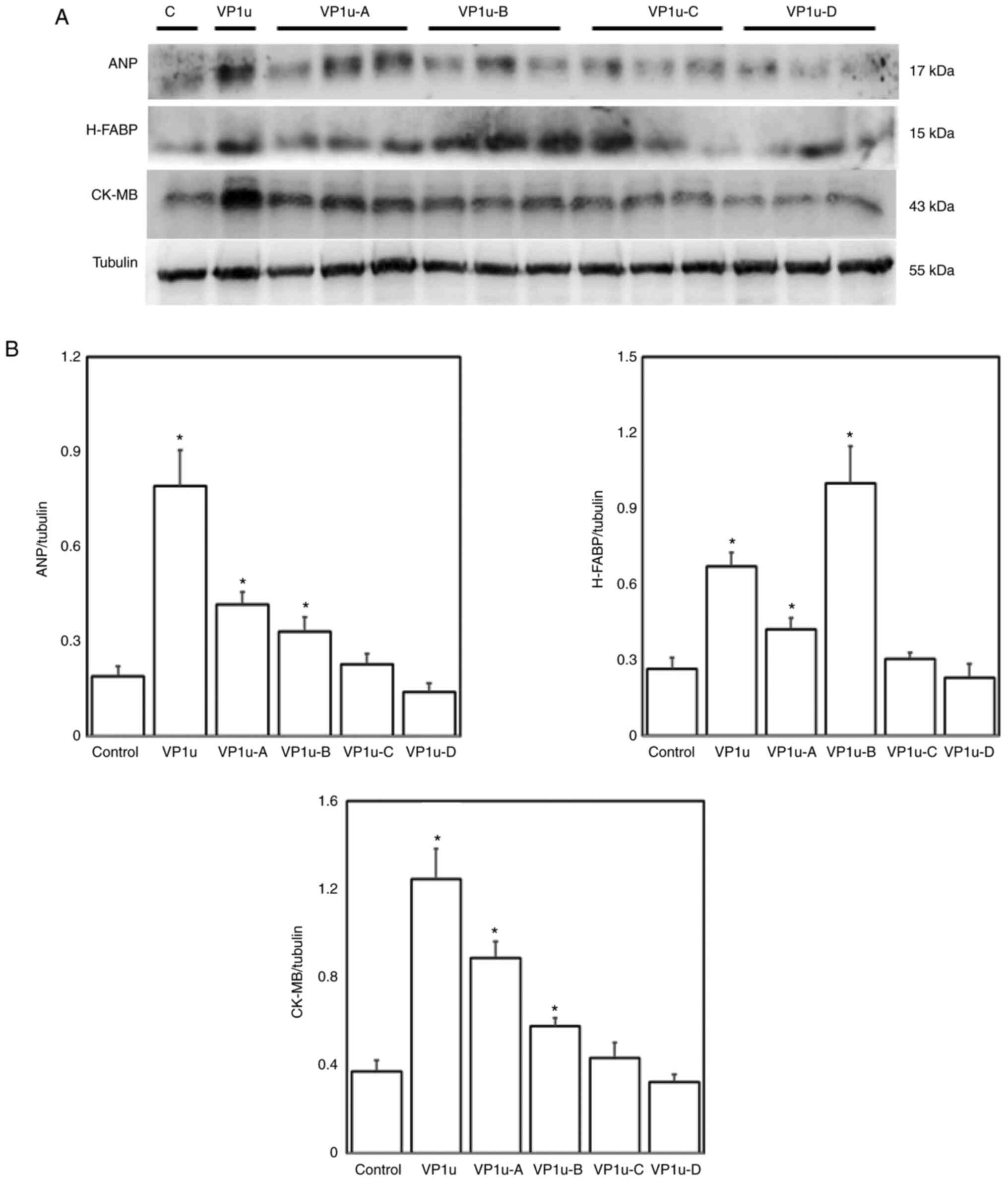 | Figure 7.Effects of B19V–VP1u and its
truncated fragments on expression levels of ANP, H-FABP and CK-MB.
(A) Protein expression levels of ANP, H-FABP and CK-MB in the left
ventricle tissues of the mice receiving different treatments (PBS,
VP1u, VP1u-A, VP1u-B, VP1u-C or VP1u-D). C indicates control mice
treated with PBS. (B) Relative levels of ANP, H-FABP and CK-MB are
based on tubulin expression. Similar results were obtained in
three-repeated experiments. *P<0.05 vs. Control (mice treated
with PBS). n=4 for each group. B19V, B19 virus; VP1u, VP1-unique
region; ANP, atrial natriuretic peptide; H-FABP, heart-type fatty
acid-binding protein; CK-MB, creatine kinase isoenzyme-MB. |
 | Figure 8.H&E staining of the left
ventricles in naïve mice treated with PBS (control), B19-VP1u,
VP1u-A, VP1u-B, VP1u-C or VP1u-D. Lymphocyte infiltration was
indicated by an arrow in the left ventricle tissues of naïve mice
receiving different treatments (PBS, VP1u, B19-VP1u-A, B19-VP1u-B,
B19-VP1u-C or B19-VP1u-D). The images in the left panel and right
panel were magnified by ×200 and ×400, respectively. Scale bar, 10
µm. B19V, B19 virus; VP1u, VP1-unique region. |
Discussion
Both B19V-NS1 and B19V–VP1u proteins have been
linked to various heart disorders such as inflammatory
cardiomyopathy and DCM (11,24–25).
Notably, significantly increased cardiac inflammation was detected
in NZB/W F1 mice receiving B19V–VP1u IgG (26). In addition, among patients with
systemic lupus erythematous, a potential association between
B19V–VP1u and DCM was also reported (27). Although these findings revealed an
association between B19V–VP1u and cardiac disorders, the roles of
the antigenicity and functional motifs of B19V–VP1u in the
induction of cardiac injury remain unknown. The present study
identified a significantly increased MMP-9/MMP-2 ratio and elevated
IL-6, IL-1β, ANP, H-FABP and CK-MB levels in the left ventricles of
mice treated with B19V-NS1 and B19V–VP1u. Moreover, significantly
increased MMP-9/MMP-2 ratios and IL-6 and IL-1β levels were
detected in the left ventricles of mice treated with B19V–VP1u-A,
B19V–VP1u-B and B19V–VP1u-C. Significantly higher levels of ANP,
H-FABP and CK-MB proteins were also observed in the left ventricles
of mice treated with B19V–VP1u, B19V–VP1u-A and B19V–VP1u-B,
accompanied by significantly higher serum IL-1β, IL-6, TNF-α and
IFN-γ levels. Conversely, significantly lower levels of IL-6 and
IL-1β were observed in the left ventricles of mice treated with
B19V–VP1u-D. These results are similar to previous findings, which
revealed that B19V–VP1u could significantly increase cardiac injury
(11,25) and the first to show the differential
effects of recombinant B19V–VP1u fragments on inducing the
expression of cardiac injury markers.
MMPs, metal-bound enzymes that require zinc and
calcium for their enzymatic activity and stability, are pivotal
mediators involved in the remodeling of the extracellular matrix
(33). Numerous studies have
reported the essential roles of MMPs in organ development and
subsequent tissue remodeling during inflammation and injury
(34). MMPs, specifically MMP-9,
also participate in the remodeling processes that occur following
various cardiac injuries, such as myocarditis, atherosclerosis,
heart failure, myocardial infarction and DCM (35). Indeed, MMP-9 has been shown to be
involved in the inflammation and cardiac remodeling of Chagas
disease, and the ratio of MMP-9/MMP-2 is considered as a potential
biomarker in delineating the risk of cardiovascular injury
(36).
ANP is expressed in the atria and ventricular
myocardium, and its major physiological function is to reduce
cardiac output, as well as to lower blood volume and systemic blood
pressure (37). ANP is directly
associated with increased stress caused by stretching, and is
considered as a marker of congestive heart failure in both animal
models and humans (38). ANP is
known as a biochemical marker of cardiac hypertrophy, which
ultimately leads to ventricular dilatation and heart failure
(39). FABPs are expressed in
various cells and tissues, such as cardiomyocytes, red skeletal
muscle, kidney, hepatocytes, small intestine and adipocytes, and
are associated with fatty acid metabolism (40). H-FABP is representative of its
family in cardiac and red skeletal muscle, and is recognized as a
biomarker of heart failure, chronic obstructive pulmonary disease
and long-term postischemic prognosis (41,42).
Although CK-MB is not heart-specific, CK-MB is still used in the
diagnosis of acute myocardial infarction (43,44).
To the best of our knowledge, the present study was the first to
reveal that B19V–VP1u-A and B19V–VP1u-B increased ANP, H-FABP and
CK-MB expression levels in the left ventricles of naïve mice
compared with the controls, whereas no differences in ANP, H-FABP
and CK-MB expression levels were observed in the mice treated with
B19V–VP1u-C and B19V–VP1u-D. These findings suggest that the
N-terminal residues (residues 1–129) of B19V–VP1u exert a notable
effect on the induction of the expression of various cardiac injury
markers in the left ventricle.
Various cytokines serve essential roles in the
pathogenesis of myocarditis and cardiomyopathy (45,46).
Elevated plasma TNF-α, IL-6 and IL-1β levels have been reported in
patients suffering from acute myocardial infarction (47,48).
Evidence from both human and animal models has shown that
proinflammatory cytokines, such as IL-1β, IL-2, IL-6 and TNF-α, are
involved in myocarditis, cardiac depression and heart failure
(49,50). The present study reported similar
results, that B19V–VP1u, B19V–VP1u-A and B19V–VP1u-B increased the
levels of IL-1β and IL-6 in the left ventricle of naïve mice.
Moreover, significantly increased levels of phosphorylation of P38
and ERK were also detected, which was consistent with previous
findings showing that both ERK and P38 signaling are involved in
the induction of IL-1β and IL-6 (51). These results demonstrated that the
129 N-terminal amino acids of B19V–VP1u (B19V–VP1u-A and
B19V–VP1u-B) served key roles in inducing IL-1β and IL-6 levels via
the ERK and P38 signaling pathways.
Previous studies have shown that the neutralizing
epitopes of B19V are located within the N-terminal 100 amino acids
of B19-VP1u, which contributes to B19V-induced immune responses
(52,53). The N-terminal amino acids from
residues 5–80 of B19-VP1u are essential for cellular binding and
B19V uptake (54), which indicates
an important role of the N-terminal region of B19V–VP1u in immune
regulation following B19V infection. However, little is known
regarding the functional motifs in the C-terminal domain of
B19V–VP1u. To the best of our knowledge, the present study was the
first to show that IL-1β and IL-6 levels were significantly lower
in the left ventricles of mice treated with B19V–VP1u-D (amino
acids 196–227) compared with mice from the control group. This
finding prompts a rational assumption that a negative regulatory
motif on IL-1β and IL-6 may be located within the C-terminal region
of B19V–VP1u. However, further studies are required to verify the
precise location of the motif and determine its function.
There were certain limitations to the present study.
Firstly, evidence has revealed that engagement of B19V–VP2 and
globoside (P-antigen), and the following interaction of B19V–VP1
and α5β1 integrin are essential for B19 infection of erythroid
cells and their progenitors (55).
However, to the best of our knowledge, no study has investigated
the potential receptor for B19V–VP1u and its truncated fragments on
cardiac cells. Therefore, further research is required to verify
the possible receptors on cardiac cells, as well as their
downstream interactions. Second, the present study lacked the
assessment of cardiac function using echocardiography or
electrocardiography, which may provide further understanding of the
physiological role of truncated B19V–VP1u fragments on cardiac
injuries. Third, the underlying mechanism and related signaling
molecules involved in truncated B19V–VP1u fragment-induced cardiac
injury remain unknown, as do the mechanism via which B19V DNA
entering cardiomyocytes. These issues require further
investigations to elucidate the differential effects and mechanisms
of truncated B19V–V1u fragments on cardiac injury.
In conclusion, the present study identified the
differential effects of truncated B19V–VP1u fragments and its
ability to induce an increase in the levels of cardiac inflammatory
and injury markers in naïve mice. The 129 N-terminal residues of
B19V–VP1u, including B19V–VP1u-A and B19V–VP1u-B, predominantly
induced the expression of inflammatory proteins, such as MMP-9,
cardiac IL-1β and IL-6, and increased the serum levels of IL-1β,
IL-6, TNF-α and IFN-γ, as well as cardiac injury markers, such as
ANP, H-FABP and CK-MB. Conversely, the C-terminal residues (amino
acids 196–227) attenuated the levels of proinflammatory cytokines,
including IL-1β and IL-6, but did not affect MMP-9 activity or the
induction of cardiac injury markers. Taken together, these findings
may provide further information and improve the current
understanding of the regulatory roles of B19V–VP1u in inducing
cardiac injury.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by Chung Shan Medical
University and Chi-Mei Medical Center cooperative project
CSMU-CMMC-108-01 (grant no. CMCSMU10801) and in part by the
Ministry of Science and Technology (grant nos. MOST
106-2314-B040-023, 107-2314-B040-004 and 109-2314-B040-021),
Taiwan. The funders had no role in study design, data collection
and analysis, decision to publish, or preparation of the
manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KCH was involved in the study conception and design,
and analysis of data. ZYH and JLY performed experiments and
analysis of data. TCH was involved in the study conception and
design, drafting and revising of the manuscript, performing
experiments, analysis of data, and study supervision. BST was
involved in the study conception and design, drafting and revising
of the manuscript, and analysis of data. TCH and BST confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Animal experiments were performed in accordance with
the principles of replacement, refinement and reduction and were
approved by the Institutional Animal Care and Use Committee (IACUC)
of Chung Shan Medical University, Taichung (approval no. 1676;
approved in December 2015).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kelly HA, Siebert D, Hammond R, Leydon J,
Kiely P and Maskill W: The age-specific prevalence of human
parvovirus immunity in Victoria, Australia compared with other
parts of the world. Epidemiol Infect. 124:449–457. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cotmore SF, McKie VC, Anderson LJ, Astell
CR and Tattersall P: Identification of the major structural and
nonstructural proteins encoded by human parvovirus B19 and mapping
of their genes by procaryotic expression of isolated genomic
fragments. J Virol. 60:548–557. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nüesch JPF, Corbau R, Tattersall P and
Rommelaere J: Biochemical activities of minute virus of mice
nonstructural protein NS1 Are modulated in vitro by the
phosphorylation state of the polypeptide. J Virol. 72:8002–8012.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tewary SK, Zhao H, Deng X, Qiu J and Tang
L: The human parvovirus B19 non-structural protein 1 N-terminal
domain specifically binds to the origin of replication in the viral
DNA. Virology. 449:297–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moffatt S, Yaegashi N, Tada K, Tanaka N
and Sugamura K: Human parvovirus B19 nonstructural (NS1) protein
induces apoptosis in erythroid lineage cells. J Virol.
72:3018–3028. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu Y, Ishii KK, Munakata Y, Saitoh T, Kaku
M and Sasaki T: Regulation of tumor necrosis factor alpha promoter
by human parvovirus B19 NS1 through activation of AP-1 and AP-2. J
Virol. 76:5395–5403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weigel-Kelley KA, Yoder MC and Srivastava
A: Recombinant human parvovirus B19 vectors: Erythrocyte P antigen
is necessary but not sufficient for successful transduction of
human hematopoietic cells. J Virol. 75:4110–4116. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurtzman GJ, Cohen BJ, Field AM, Oseas R,
Blaese RM and Young NS: Immune response to B19 parvovirus and an
antibody defect in persistent viral infection. J Clin Invest.
84:1114–1123. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anderson S, Momoeda M, Kawase M, Kajigaya
S and Young NS: Peptides derived from the unique region of B19
parvovirus minor capsid protein elicit neutralizing antibodies in
rabbits. Virology. 206:626–632. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mahrholdt H, Wagner A, Deluigi CC, Kispert
E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT,
et al: Presentation, patterns of myocardial damage, and clinical
course of viral myocarditis. Circulation. 114:1581–1590. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bogomolovas J, Šimoliūnas E, Rinkūnaitė I,
Smalinskaitė L, Podkopajev A, Bironaitė D, Weis CA, Marx A,
Bukelskienė V, Gretz N, et al: A novel murine model of parvovirus
associated dilated cardiomyopathy induced by immunization with
VP1-unique region of parvovirus B19. Biomed Res Int.
2016:16271842016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hatakka A, Klein J, He R, Piper J, Tam E
and Walkty A: Acute hepatitis as a manifestation of parvovirus B19
infection. J Clin Microbiol. 49:3422–3424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Komatsuda A, Ohtani H, Nimura T, Yamaguchi
A, Wakui H, Imai H and Miura AB: Endocapillary proliferative
glomerulonephritis in a patient with parvovirus B19 infection. Am J
Kidney Dis. 36:851–854. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferrari SM, Fallahi P, Antonelli A and
Benvenga S: Environmental issues in thyroid diseases. Front
Endocrinol (Lausanne). 8:502017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bozzola E, Krzysztofiak A and Cortis E:
Neurological impairment and arthritis in an immunocompetent child
with human parvovirus B19 chronic infection. Infez Med. 18:187–190.
2010.PubMed/NCBI
|
|
16
|
Hobbs JA and Adamson-Small LA: Parvovirus
and thyroid cancer. Semin Oncol. 42:304–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Page C, François C, Goëb V and Duverlie G:
Human parvovirus B19 and autoimmune diseases. Review of the
literature and pathophysiological hypotheses. J Clin Virol.
72:69–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pankuweit S, Moll R, Baandrup U, Portig I,
Hufnagel G and Maisch B: Prevalence of the parvovirus B19 genome in
endomyocardial biopsy specimens. Hum Pathol. 34:497–503. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pankuweit S, Lamparter S, Schoppet M and
Maisch B: Parvovirus B19 genome in endomyocardial biopsy specimen.
Circulation. 109:e1792004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tschöpe C, Bock CT, Kasner M, Noutsias M,
Westermann D, Schwimmbeck PL, Pauschinger M, Poller WC, Kühl U,
Kandolf R and Schultheiss HP: High prevalence of cardiac parvovirus
B19 infection in patients with isolated left ventricular diastolic
dysfunction. Circulation. 111:8792005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kühl U, Lassner D, Pauschinger M, Gross
UM, Seeberg B, Noutsias M, Poller W and Schultheiss HP: Prevalence
of erythrovirus genotypes in the myocardium of patients with
dilated cardiomyopathy. J Med Virol. 80:1243–1251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bültmann BD, Klingel K, Sotlar K, Bock CT,
Baba HA, Sauter M and Kandolf R: Fatal parvovirus B19-associated
myocarditis clinically mimicking ischemic heart disease: An
endothelial cell-mediated disease. Hum Pathol. 34:92–95. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duechting A, Tschöpe C, Kaiser H,
Lamkemeyer T, Tanaka N, Aberle S, Lang F, Torresi J, Kandolf R and
Bock CT: Human parvovirus B19 NS1 protein modulates inflammatory
signaling by activation of STAT3/PIAS3 in human endothelial cells.
J Virol. 82:7942–7952. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hii HP, Chiu CC, Lin DW, Shi YF, Hsu TC
and Tzang BS: Selective activation of inflammation factors by human
parvovirus B19 and human bocavirus VP1 unique region on H9c2
cardiomyocyte. Mol Med Rep. 18:4072–4078. 2018.PubMed/NCBI
|
|
25
|
Nie X, Zhang G, Xu D, Sun X, Li Z, Li X,
Zhang X, He F and Li Y: The VP1-unique region of parvovirus B19
induces myocardial injury in mice. Scand J Infect Dis. 42:121–128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tzang BS, Lin TM, Tsai CC, Hsu JD, Yang LC
and Hsu TC: Increased cardiac injury in NZB/W F1 mice received
antibody against human parvovirus B19 VP1 unique region protein.
Mol Immunol. 48:1518–1524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen DY, Chen YM, Tzang BS, Lan JL and Hsu
TC: Th17-related cytokines in systemic lupus erythematosus patients
with dilated cardiomyopathies: A possible linkage to parvovirus B19
infection. PLoS One. 9:e1138892014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsai CC, Chiu CC, Hsu JD, Hsu HS, Tzang BS
and Hsu TC: Human parvovirus B19 NS1 protein aggravates liver
injury in NZB/W F1 mice. PLoS One. 8:e597242013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin CY, Chiu CC, Cheng J, Lin CY, Shi YF,
Tsai CC, Tzang BS and Hsu TC: Antigenicity analysis of human
parvovirus B19-VP1u protein in the induction of anti-phospholipid
syndrome. Virulence. 9:208–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakashima I, Ota F, Kobayashi T, Kato O
and Kato N: The effect of antigen doses and time intervals between
antigen injections on secondary, tertiary and quaternary antibody
responses. Establishment of hyperimmunization with bovine serum
albumin in mice treated with capsular polysaccharide of Klebsiella
pneumoniae. Immunology. 26:443–454. 1974.PubMed/NCBI
|
|
31
|
Castiglione F, Mantile F, De Berardinis P
and Prisco A: How the interval between prime and boost injection
affects the immune response in a computational model of the immune
system. Comput Math Methods Med. 2012:8423292012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu TC, Chiu CC, Chang SC, Chan HC, Shi
YF, Chen TY and Tzang BS: Human parvovirus B19 VP1u Protein as
inflammatory mediators induces liver injury in naïve mice.
Virulence. 7:110–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu P, Sun M and Sader S: Matrix
metalloproteinases in cardiovascular disease. Can J Cardiol. 22
(Suppl B):25B–30B. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J and Khalil RA: Matrix
metalloproteinase inhibitors as investigational and therapeutic
tools in unrestrained tissue remodeling and pathological disorders.
Prog Mol Biol Transl Sci. 148:355–420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kampoli AM, Tousoulis D, Papageorgiou N,
Antoniades C, Androulakis E, Tsiamis E, Latsios G and Stefanadis C:
Matrix metalloproteinases in acute coronary syndromes: Current
perspectives. Curr Top Med Chem. 12:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fares RC, Gomes Jde A, Garzoni LR, Waghabi
MC, Saraiva RM, Medeiros NI, Oliveira-Prado R, Sangenis LH,
Chambela Mda C, de Araújo FF, et al: Matrix metalloproteinases 2
and 9 are differentially expressed in patients with indeterminate
and cardiac clinical forms of Chagas disease. Infect Immun.
81:3600–3608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Edwards BS, Zimmerman RS and Burnett JC
Jr: Atrial natriuretic factor: Physiologic actions and implications
in congestive heart failure. Cardiovasc Drugs Ther. 1:89–100. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Michel JB, Arnal JF and Corvol P: Atrial
natriuretic factor as a marker in congestive heart failure. Horm
Res. 34:166–168. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rohini A, Agrawal N, Koyani CN and Singh
R: Molecular targets and regulators of cardiac hypertrophy.
Pharmacol Res. 61:269–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vork MM, Glatz JF and van Der Vusse GJ: On
the mechanism of long chain fatty acid transport in cardiomyocytes
as facilitated by cytoplasmic fatty acid-binding protein. J Theor
Biol. 160:207–222. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Crisman TS, Claffey KP, Saouaf R, Hanspal
J and Brecher P: Measurement of rat heart fatty acid binding
protein by ELISA. Tissue distribution, developmental changes and
subcellular distribution. J Mol Cell Cardiol. 19:423–431. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sato Y, Kita T, Takatsu Y and Kimura T:
Biochemical markers of myocyte injury in heart failure. Heart.
90:1110–1113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mair J: Progress in myocardial damage
detection: New biochemical markers for clinicians. Crit Rev Clin
Lab Sci. 34:1–66. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Aydin S, Ugur K, Aydin S, Sahin İ and
Yardim M: Biomarkers in acute myocardial infarction: Current
perspectives. Vasc Health Risk Manag. 15:1–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Levine B, Kalman J, Mayer L, Fillit HM and
Packer M: Elevated circulating levels of tumor necrosis factor in
severe chronic heart failure. N Engl J Med. 323:236–241. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Matsumori A: Molecular and immune
mechanisms in the pathogenesis of cardiomyopathy-role of viruses,
cytokines, and nitric oxide. Jpn Circ J. 61:275–291. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Miyao Y, Yasue H, Ogawa H, Misumi I,
Masuda T, Sakamoto T and Morita E: Elevated plasma interleukin-6
levels in patients with acute myocardial infarction. Am Heart J.
126:1299–1304. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Neumann FJ, Ott I, Gawaz M, Richardt G,
Holzapfel H, Jochum M and Schömig A: Cardiac release of cytokines
and inflammatory responses in acute myocardial infarction.
Circulation. 92:748–755. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dinarello CA: The biological properties of
interleukin-1. Eur Cytokine Netw. 5:517–531. 1994.PubMed/NCBI
|
|
50
|
Blum A and Miller H: Role of cytokines in
heart failure. Am Heart J. 35:181–186. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Carter AB, Monick MM and Hunninghake GW:
Both Erk and p38 kinases are necessary for cytokine gene
transcription. Am J Respir Cell Mol Biol. 20:751–758. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Saikawa T, Anderson S, Momoeda M, Kajigaya
S and Young NS: Neutralizing linear epitopes of B19 parvovirus
cluster in the VP1 unique and VP1-VP2 junction regions. J Virol.
67:3004–3009. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zuffi E, Manaresi E, Gallinella G,
Gentilomi GA, Venturoli S, Zerbini M and Musiani M: Identification
of an immunodominant peptide in the parvovirus B19 VP1 unique
region able to elicit a long-lasting immune response in humans.
Viral Immunol. 14:151–158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Leisi R, Di Tommaso C, Kempf C and Ros C:
The receptor-binding domain in the VP1u region of parvovirus B19.
Viruses. 8:612016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qiu J, Söderlund-Venermo M and Young NS:
Human parvoviruses. Clin Microbiol Rev. 30:43–113. 2017. View Article : Google Scholar : PubMed/NCBI
|