Introduction
The lungs are one of the most vulnerable organs in
the human body, and alveolar macrophages are the major inflammatory
cells involved in the maintenance of the lung's defense against
foreign pathogens (1). Phagocytosis
caused by these macrophages and the accompanied release of
cytokines constitute the host cellular defense (2). Acute lung injury (ALI) is responsible
for the high morbidity of critically ill patients (3). By damaging the alveolar epithelium,
ALI can trigger the inflammatory response in the defensive system
of the lung (4). Despite the
complex mechanism that contributes to the occurrence of ALI, the
damage to the epithelial cells can speed up the development of this
disease (5,6). To date, specific pharmacological drugs
have not been used in clinical practice for the treatment of ALI
(7). Therefore, the severity of ALI
and the potential targets or drugs that can treat this disease
should be examined.
Phillygenin (PHI;
4-[(3S,3aR,6R,6aR)-6(3,4-dimethoxyphenyl)-1,3,3a,4,6,6a-hexahydrofuro[3,4-c]
furan-3yl]-2-methoxyphenol) (Fig.
1A) is a lignan component extracted from Forsythiae Fructus
(8), and it has been reported to
exert anti-inflammatory effects (9). The Toll-like receptor
(TLR)4/MyD88/NF-κB signaling pathway, which can be activated by
PHI, can inhibit lipopolysaccharide (LPS)-induced inflammation and
further alleviate liver fibrosis (8). Furthermore, PHI has been reported to
have significant antitumor effects in human esophageal and
pancreatic cancer types (10,11).
A search of the SwissTargetPrediction website
suggested that PHI can bind to and regulate the expression of MMP8.
Previous studies have indicated that MMP8 and MMP9 expression
levels are elevated in pediatric patients with ALI (11,12).
Decreased MMP8 activity and aberrantly increased MMP9 activity were
noted in patients with ALI with prolonged disease progression
(13). MMP8 serves an important
role in inflammation and degradation of tight junction proteins
(14). Increased expression of MMP8
is associated with the early inflammation stage of spinal cord
injury (SCI) and addition of a specific MMP8 inhibitor can markedly
alleviate the inflammatory response and cellular damage of SCI
(14). The use of MMP8 inhibitors
on astrocytes can suppress the levels of inducible nitric oxide
synthase, TNF-α, IL-1β, IL-6 and TLR2, and is accompanied by the
activation of peroxisome proliferator-activated receptor γ (PPARγ)
and the inhibition of NF-κB activity, which is induced by
lipoteichoic acid (15). The
notable therapeutic potential of curcumin in the treatment of
neonatal ALI is mediated by activating PPARγ/heme oxygenase-1
signaling in an animal model (16).
This finding provided evidence that activation of PPARγ may be a
novel therapeutic strategy for the treatment of ALI. Therefore, the
present study aimed to investigate whether PHI can inhibit the
induction of inflammation and apoptosis of pulmonary epithelial
cells by activating PPARγ signaling.
Materials and methods
Sample collection
The clinical study protocol was approved by
Gaolangang Hospital of Zhuhai People's Hospital (approval no.
2020-012; Zhuhai, China) between 2019/09 and 2020/02. Signed
informed consent forms were obtained from the children's family for
the collection and use of the specimens. Briefly, patients who were
admitted to the pediatric intensive care unit of Gaolangang
Hospital of Zhuhai People's Hospital were enrolled. Inclusion
criteria were pediatric patients [age, 2–10 years; 28 (46.7%)
females and 32 (53.3%) males] who were intubated and mechanically
ventilated with a ratio of partial pressure of arterial oxygen
(PaO2) to the fraction of inspired oxygen
(FiO2) of ≤300 (adjusted to 253 in Salt Lake City due to
altitude), bilateral pulmonary infiltrates and no clinical evidence
of left atrial hypertension. Patients were excluded if they were
<2 years of age; had respiratory failure from cardiac disease;
had hypoxemia without bilateral infiltrates; had received a bone
marrow or lung transplant; were supported on extracorporeal
membrane oxygenation; had a non-pulmonary condition that could be
exacerbated by the prone position; had participated in other
clinical trials within the preceding 30 days; or if there was a
decision to limit life support. Blood samples were collected within
the first 24 h of diagnosis. The serum samples of pediatric
patients with ALI (n=30) and normal subjects (n=30) were collected
for further analysis following submission of the informed consent
form.
Cell lines and treatment
The pulmonary epithelial cell line (BEAS-2B) was
obtained from the American Type Culture Collection. The cells were
maintained in RPMI-1640 medium (Thermo Fisher Scientific, Inc.)
containing 10% FBS (Thermo Fisher Scientific, Inc.) in an incubator
(Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2.
LPS was purchased from Sigma-Aldrich; Merck KGaA. For LPS
induction, BEAS-2B cells were plated into 6-well plates and
cultured for 48 h. LPS (100 ng/ml) or saline solution was added and
the cells were incubated for 24 h to induce inflammatory response
and apoptosis. PHI (purity 99%) was purchased from
Chroma-Biotechnology Co., Ltd. (https://chroma-biotech.company.lookchem.cn/). PHI was
used at the concentrations of 12.5, 25 and 50 µg/ml. This compound
was incubated with the cells 24 h following LPS induction (8).
Cell transfection
The MMP8-overexpression plasmid vector
(pcDNA3.1-MMP8) and empty vector (pcDNA3.1-NC) plasmid were
purchased from Shanghai GenePharma Co., Ltd. In accordance with the
manufacturer's instructions, following LPS treatment, 1 µg plasmids
were transfected into BEAS-2B cells using Lipofectamine®
3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Following incubation for 6 h, the medium was replaced with
RPMI-1640 medium with 10% FBS (Thermo Fisher Scientific, Inc).
After 48 h of incubation, the cells were collected and the
transfection efficiency was analyzed by reverse
transcription-quantitative PCR (RT-qPCR).
MTT assay
Cells were seeded in 96-well plates at a density of
3,000/well and were subsequently treated by different
concentrations of PHI for 24 h. A total of 10 µl MTT solution was
added to the medium and the cells were cultured for an additional 4
h. Dimethyl sulfoxide was added into each well to dissolve the
formazan particles. The absorbance was measured at 450 nm using a
microplate reader (Molecular Devices, LLC).
TUNEL assay
BEAS-2B cells treated with PHI (12.5, 25 or 50
µg/ml; 24 h; 37°C) and LPS were fixed with 4% paraformaldehyde at
25°C for 15 min. Then, a TUNEL kit (Roche Applied Science) was used
to detect apoptotic cells according to the manufacturer's protocol.
Subsequently, cell nuclei were counterstained with 0.2 µg/ml DAPI
at room temperature for 15 min and mounted with glycerol gelatin
(Sigma-Aldrich; Merck KGaA). An Olympus fluorescence microscope
(magnification, ×200) was used to acquire the images in ≥3 randomly
selected fields of view.
ELISA
BEAS-2B cells were plated in 96-well plates
(8×104 cells/ml) and incubated with LPS in the presence
or absence of PHI for 24 h. The concentration levels of IL-1β, IL-6
and TNF-α in the cell supernatant were measured by ELISA kits
according to the manufacturer's recommendations. The following
ELISA kits were purchased from Beyotime Institute of Biotechnology,
IL-1β ELISA kit (cat. no. PI305), IL-6 ELISA kit (cat. no. PI330)
and TNF-α ELISA kit (cat. no. PT518). The absorbance at 450 nm was
detected by a microplate reader.
RT-qPCR
Total RNA in the cells and the serum of pediatric
patients with ALI was extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's protocol. Subsequently, the extracts were dissolved
and the final RNA purity was measured by the Nucleic Acid/Protein
Analyzer (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was
synthesized using 5X All-In-One Master Mix (Invitrogen; Thermo
Fisher Scientific, Inc.) in accordance with the manufacturer's
protocols. RT-qPCR reactions were performed using SYBR-Green Master
Mix (Invitrogen; Thermo Fisher Scientific, Inc.). The reaction
conditions were as follows: Initial denaturation at 95°C for 10
min, followed by 40 cycles at 95°C for 15 sec and 60°C for 30 sec.
The mRNA levels of the target genes were normalized to the levels
of the GAPDH gene. The 2−ΔΔCq (17) method was used for analysis of the
data. The sequence of primer pairs were as follows: MMP8 forward
(F), 5′-AAGCCATTGATGCAGCTGTTT-3′ and reverse (R),
5′-AAACAGCTGCATCAATGGCTT-3′; and GAPDH F, 5′-CTGAGTACGTCGTGGAGTC-3′
and R, 5′-TGATGATCTTGAGGCTGTTGTC-3′.
Western blot analysis
BEAS-2B cells were lysed in RIPA lysis buffer
(Beyotime Institute of Biotechnology) and the protein content was
estimated using a BCA assay. The samples (30 µg) were subsequently
loaded on SDS-PAGE gels (10, 12 or 15%), which were prepared
previously. The gels were then transferred to nitrocellulose
membranes. The membranes were first blocked using fat-free milk
(5%) in TBS for 2 h at room temperature and then the membranes were
incubated at 4°C for 24 h with primary antibodies against the
following: Cox-2 (1:1,000; cat. no. sc-376861), Bcl-2 (1:1,000;
cat. no. sc-7382), Bax (1:1,000; cat. no. sc-7480), caspase3
(1:1,000; cat. no. sc-7272), caspase9 (1:1,000; cat. no. sc-56076),
MMP8 (1:1,000; cat. no. sc-137044), PPARγ (1:1,000; cat. no.
sc-7273), GAPDH (1:2,000; cat. no. sc-47724; all purchased from
Santa Cruz Biotechnology, Inc.), iNOS (1:1,000; cat. no. 13120),
cleaved (c)-caspase3 (1:1,000; cat. no. 9661) and c-caspase9
(1:1,000; cat. no. 9509; all purchased from Cell Signaling
Technology, Inc.). Following which, the membranes were incubated
with HRP-conjugated secondary antibody (1:1,000; cat. nos. 7074 and
7076; Cell Signaling Technology, Inc.) for 50 min at 25°C.
TBS with Tween-20 (0.2%) was used to wash the
membranes to remove non-specific binding of the antibodies and the
ECL luminescence agent (Santa Cruz Biotechnology, Inc.) was added.
The images were captured in a Bio-Rad chemiluminescence imager
(Bio-Rad Laboratories, Inc.). The expression levels of the proteins
were analyzed using ImageJ software version 1.4 (National
Institutes of Health) and normalized to those of the control.
Statistical analysis
Data analysis was performed with SPSS version 23.0
(IBM Corp.) and GraphPad (version 5.0; GraphPad Software, Inc.).
All experiments were repeated three times and the data are
expressed as the mean ± standard deviation. Two group comparisons
were performed using an unpaired Student's t-test was used for the
comparison between two group of samples and statistical differences
between groups were analyzed using one-way ANOVA followed by a
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
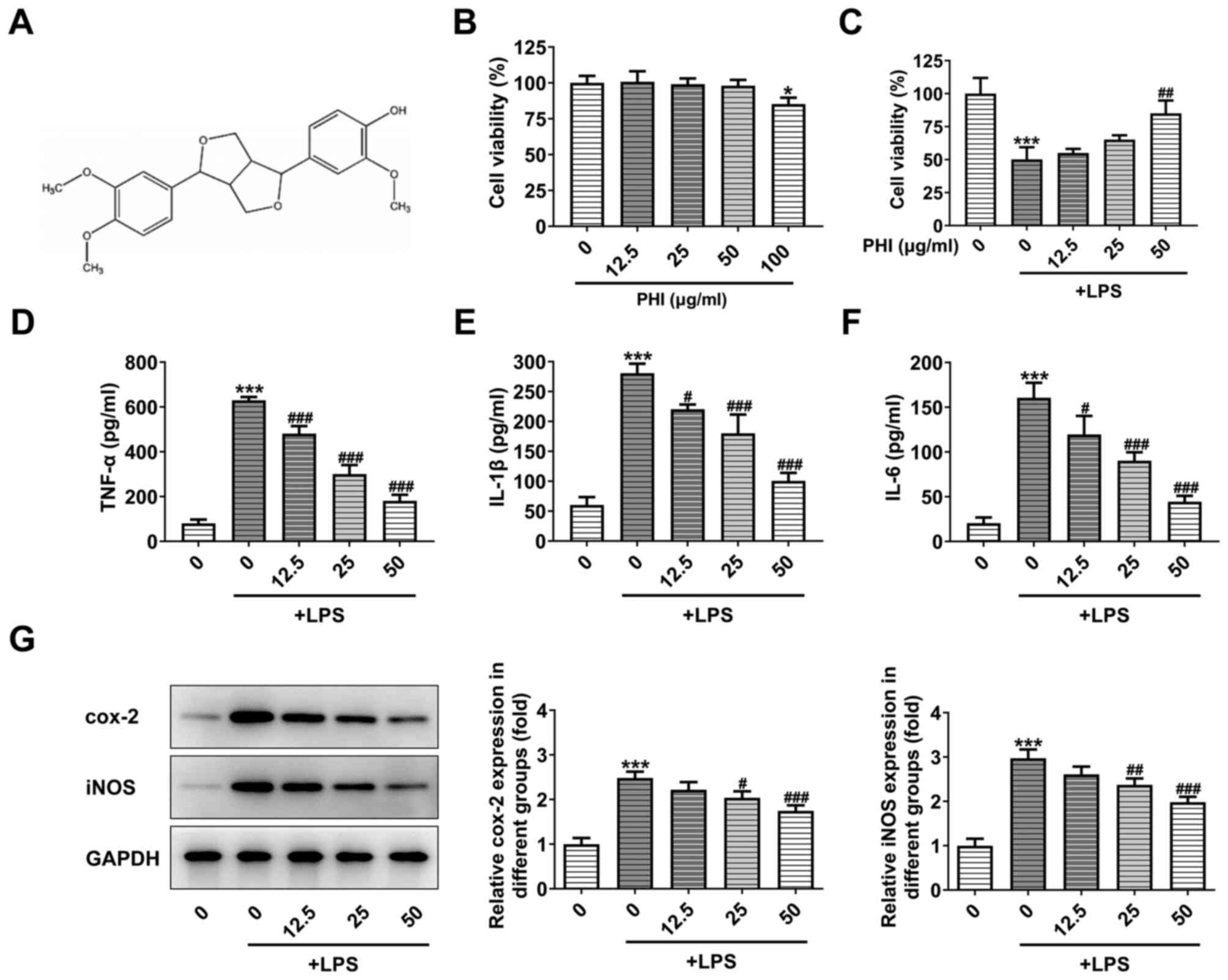
PHI treatment suppresses the
inflammatory response in LPS-induced BEAS-2B cells
To determine the effects of PHI on pulmonary
epithelial cells, BEAS-2B cell viability was detected. Treatment of
BEAS-2B cells with PHI (12.5, 25 and 50 µg/ml) indicated no
apparent difference in cell viability, while significant
differences were noted at 100 µg/ml PHI treatment (Fig. 1B). The cell viability of BEAS-2B
cells treated with LPS was markedly decreased. This effect was
partially reversed by PHI treatment at 12.5, 25 and 50 µg/ml
(Fig. 1C). Therefore, the following
concentrations of PHI were selected for subsequent studies: 12.5,
25 and 50 µg/ml. ELISA and western blot analysis indicated that the
levels of inflammatory cytokines and inflammation-associated
proteins were elevated following LPS induction, which could be
gradually reversed by PHI treatment at increasing concentrations
(Fig. 1D-G). Therefore, these
results indicated that PHI treatment suppressed the inflammatory
response in LPS-induced BEAS-2B cells.
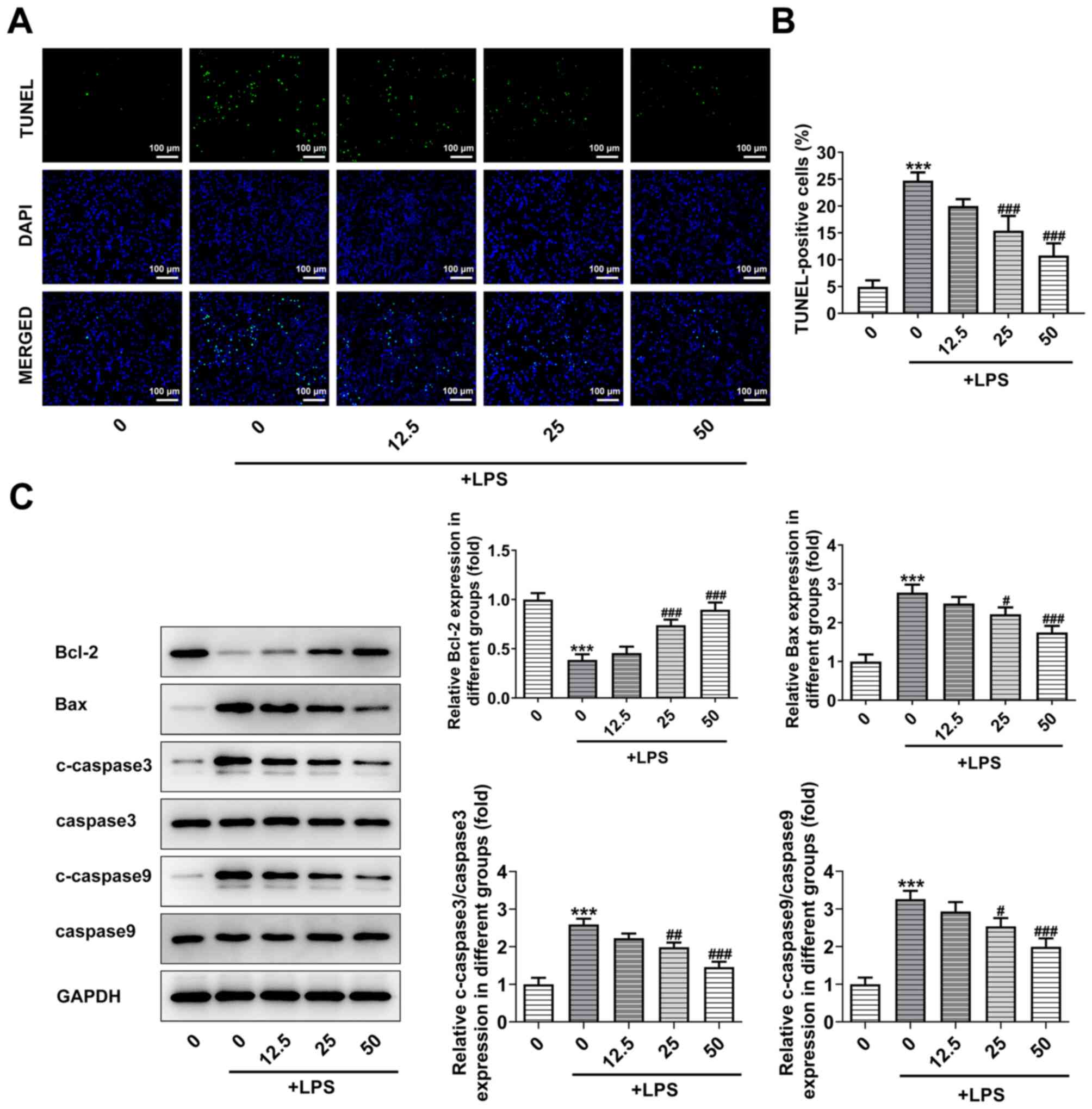
PHI treatment suppresses the induction
of BEAS-2B cell apoptosis
Induction of cell apoptosis plays a critical role in
the development of ALI (18). The
apoptotic effects of PHI on BEAS-2B cells were confirmed by TUNEL
assay and western blot analysis. As shown in Fig. 2A and B, the induction of cell
apoptosis was increased by LPS, while increasing doses of PHI
alleviated the effect of LPS on BEAS-2B cell apoptosis. In
addition, the expression levels of the pro-apoptotic proteins were
increased by LPS, while they were decreased by PHI. The opposite
finding was noted for the anti-apoptotic protein Bcl-2, which
demonstrated decreased expression following treatment with LPS and
increased expression by PHI in a dose-dependent manner (Fig. 2C). Taken together, the data revealed
that PHI treatment suppressed the induction of BEAS-2B cell
apoptosis.
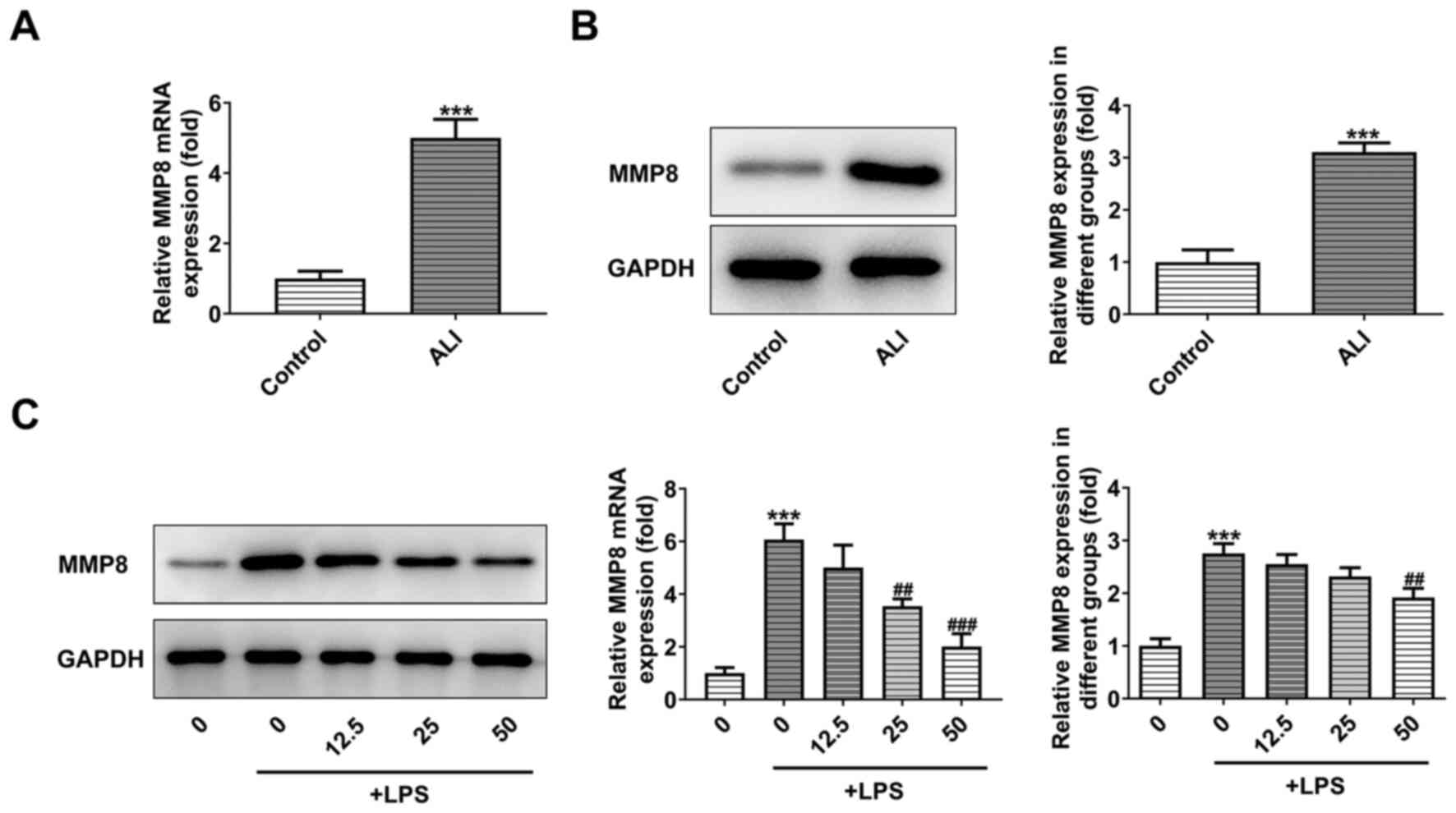
PHI treatment decreases the expression
levels of MMP8 in LPS-induced BEAS-2B cells
Serum samples of normal subjects and pediatric
patients with ALI were collected and assessed by RT-qPCR and
western blot analyses. As demonstrated in Fig. 3A and B, the expression levels of
MMP8 were significantly increased in patients with ALI. Following
the addition of increasing doses of PHI in LPS-induced BEAS-2B
cells, western blot analysis demonstrated that the expression
levels of MMP8 were gradually decreased (Fig. 3C). Therefore, PHI treatment
decreased the expression levels of MMP8 in LPS-induced BEAS-2B
cells.
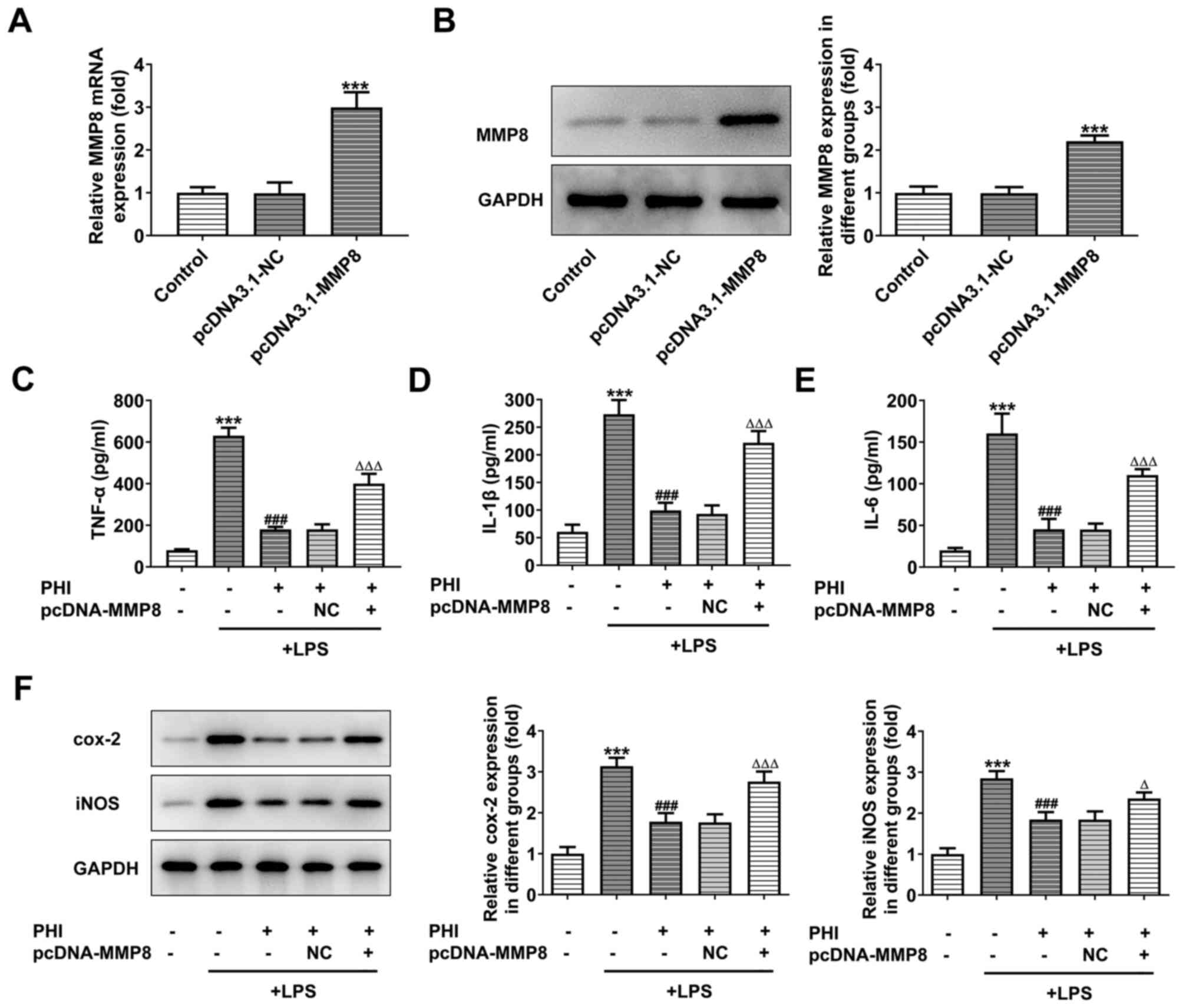
PHI treatment suppresses induction of
inflammation in LPS-treated BEAS-2B cells by downregulating MMP8
expression
To directly assess the effects of MMP8 on
LPS-induced BEAS-2B cells, which were treated with PHI, 50 µg/ml
PHI was selected for subsequent experiments and MMP8-overexpression
models were established. RT-qPCR and western blot analyses
indicated significantly higher levels of MMP8 in the pcDNA3.1-MMP8
group compared with those of the control group (Fig. 4A and B). The expression levels of
the inflammatory factors, which were inhibited by PHI in
LPS-induced BEAS-2B cells, were significantly elevated following
transfection of pcDNA3.1-MMP8 into the cells (Fig. 4C-E). Moreover, the
inflammation-associated proteins exhibited decreased expression
following PHI treatment in LPS-induced BEAS-2B cells, which could
be partially reversed by MMP8 overexpression (Fig. 4F). These results indicated that PHI
treatment suppressed the inflammation of LPS-induced BEAS-2B
cells.
PHI treatment activates PPARγ
signaling by downregulating MMP8 expression
The induction of apoptosis of LPS-treated BEAS-2B
cells was detected by TUNEL staining. PHI decreased the number of
apoptotic cells in LPS-induced BEAS-2B cells, while pcDNA3.1-MMP8
increased it, indicating that PHI treatment suppressed the
induction of apoptosis in LPS-treated BEAS-2B cells (Fig. 5A and B). Moreover, the changes in
the protein levels of the anti-apoptotic and pro-apoptotic proteins
further demonstrated that MMP8 overexpression partially reduced the
inhibitory effects of PHI on cell apoptosis (Fig. 5C). The results from the western blot
analysis indicated that PPARγ expression was significantly
decreased following LPS treatment of the cells, while additional
PHI treatment rescued its expression (Fig. 5D). This change was partially
reversed by MMP8 overexpression (Fig.
5D). Therefore, these results suggested that PHI treatment
activated PPARγ signaling by downregulating MMP8 expression.
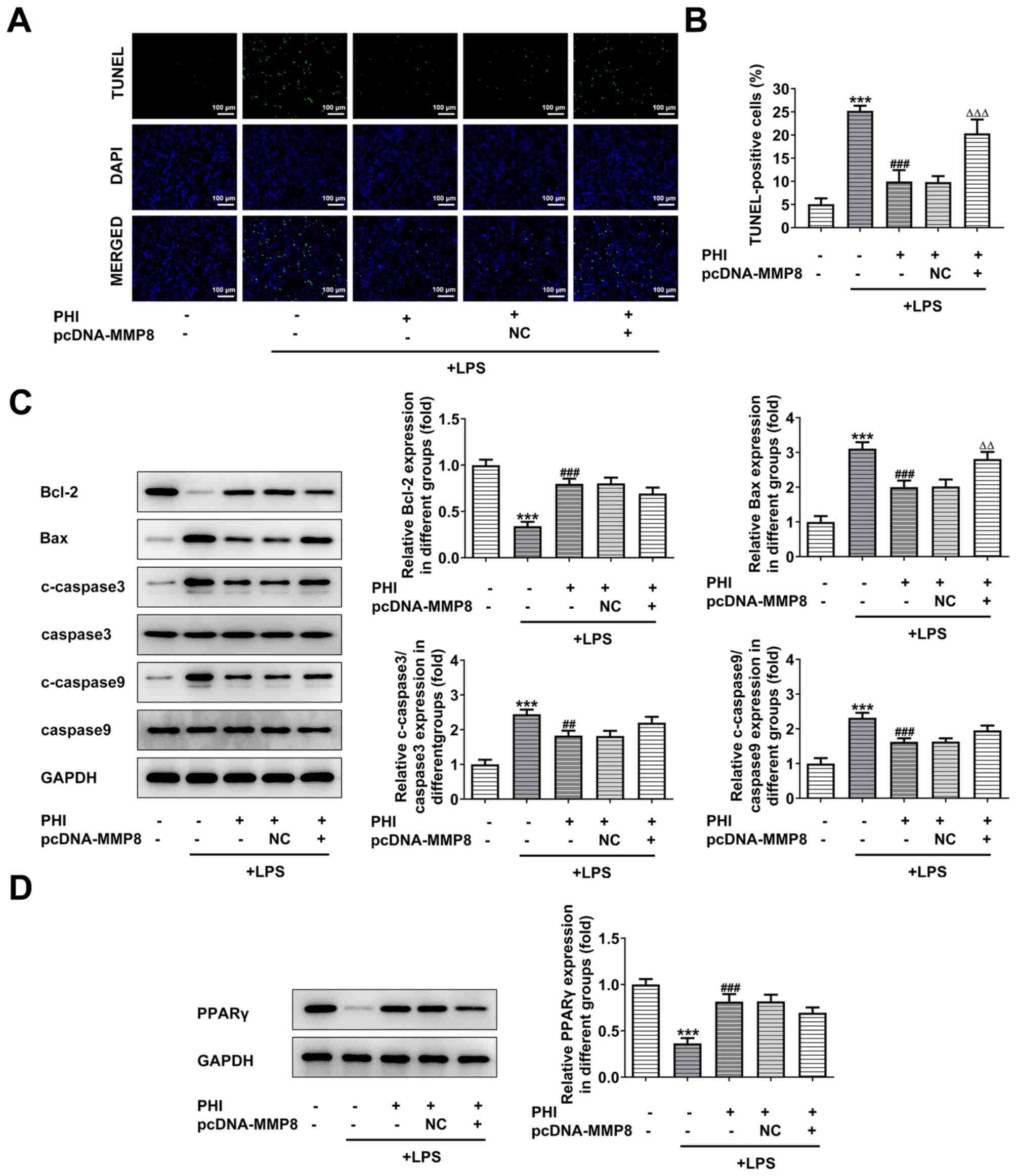 | Figure 5.PHI treatment activates PPARγ
signaling by downregulating MMP8 expression. (A and B) Following
MMP8 overexpression, the induction of cell apoptosis of LPS-treated
BEAS-2B cells, which were pretreated with PHI, was assessed using a
TUNEL assay (magnification, ×200; scale bar, 100 µm). ***P<0.001
vs. control; ###P<0.001 vs. LPS group;
∆∆∆P<0.001 vs. LPS + PHI group. (C) Western blotting
was used to determine the expression levels of the
apoptosis-associated proteins in LPS-induced BEAS-2B cells treated
with PHI and transfected with pcDNA3.1-MMP8. ***P<0.001 vs.
control; ##P<0.01, ###P<0.001 vs. LPS
group; ∆∆P<0.01 vs. LPS + PHI group. (D) Western
blotting was used to determine the expression levels of PPARγ in
LPS-induced BEAS-2B cells treated with PHI and transfected with
pcDNA3.1-MMP8. ***P<0.001 vs. control; ###P<0.001
vs. LPS group. PHI, phillygenin; PPARγ, peroxisome
proliferator-activated receptor γ; LPS, lipopolysaccharide; NC,
negative control; c-, cleaved. |
Discussion
Forsythiae Fructus is used as a single herb or added
in compound prescriptions in Asia (19). Dong et al (20) was the first to report the use of
this type of dried fruit as lianqiao (Forsythia). It has
been used for the treatment of infectious diseases, including acute
nephritis, since it exhibits heat-clearing and detoxification
effects (21). The Forsythia
Fructus extract further exerts anti-inflammatory, antibacterial,
antiviral and antioxidant effects (22,23).
PHI is the extract from Forsythia Fructus, which demonstrates
antitumor, antioxidant and hepatoprotective effects in vivo
and in vitro (24,25). A previous study has observed the
anti-inflammatory activity of PHI in mouse lymphocytes (9). Moreover, Forsythia Fructus could
enhance the defense mechanism in rats with LPS-induced liver injury
(26). Concurrently, the present
study indicated the importance of PHI in alleviating inflammation
and reducing apoptosis in LPS-induced BEAS-2B cells.
The generation of ROS and the reduction in MMP
expression levels have been reported to be important events in
triggering apoptosis. PHI was previously found to increase ROS and
decrease MMP levels in promyelocytic leukemia HL-60 cells,
indicating its effects on the suppression of cell apoptosis
(27). A previous study reported
the effects of MMP8 deletion on the improvement of septic patient
survival and the suppression of the inflammatory response in a
murine model (28). MMP8 inhibitors
have also been considered key regulators in mitigating myocardial
injury (29). In the present study,
MMP8 was found to be highly expressed in the serum of pediatric
patients with ALI, whereas PHI treatment decreased the levels of
MMP8 expression. Further experiments confirmed the regulatory role
of PHI in the expression of MMP8, and it was concluded that PHI
alleviated inflammation and reduced apoptosis in LPS-induced
BEAS-2B cells by downregulating MMP8 expression levels.
PPARs are ligand-activated transcription factors
that exert anti-inflammatory effects on certain diseases, including
brain injury (30,31). Furthermore, PPARs also regulate the
transcription of genes with critical roles in various cellular
processes (32,33). These transcription factors belong to
the superfamily of nuclear receptors and they are present in a
variety of tissues for cellular homeostasis. As PPARs are involved
in the development of numerous diseases, such as brain and
peripheral inflammation and cancer, and they are considered to be
potential molecular targets (30,34,35).
Previously, it was shown that PPARγ plays an important role in the
regulation of gene expression, such as that of lipoprotein(a), IL-1
and TNF-α (36,37). The activation of PPARγ can also
regulate different cellular processes, including cell
proliferation, metabolism and inflammation (38). In the present study, PPARγ signaling
was inhibited by LPS induction in BEAS-2B cells, whereas PHI
treatment led to an increase in the expression of PPARγ in
LPS-induced BEAS-2B cells. However, MMP8 overexpression partially
alleviated the effects of PHI on activating the PPARγ signaling
pathway.
In conclusion, the present study demonstrated that
PHI inhibited inflammation and apoptosis of pulmonary epithelial
cells by activating PPARγ signaling via downregulation of MMP8
expression, which may guide future studies on PHI and provide a
theoretical basis for the therapeutic potential of PHI. However,
there are numerous limitations of the present study. After
validating the effect of PHI on LPS-induced inflammation and
apoptosis in lung epithelial cells in vitro, it would
improve the results of the present study if experiments and
comparison tests with positive control tests had been performed in
animal models.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PY and YL collaborated on the manuscript, including
designing the research, collecting and analyzing the experiments
and data, and writing the manuscript. PY and YL confirmed the
authenticity of all the raw data. Both authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The clinical study protocol was approved by
Gaolangang Hospital of Zhuhai People's Hospital (Zhuhai, China;
approval no. 2020-012). Signed informed consent forms were obtained
from the children's guardians for the collection and use of the
specimens.
Patient consent for participation
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moreira Lopes TC, Mosser DM and Gonçalves
R: Macrophage polarization in intestinal inflammation and gut
homeostasis. Inflamm Res. 69:1163–1172. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miyajima A: Cytokines and their functions.
Nihon Rinsho. 63 (Suppl 4):S173–S177. 2005.(In Japanese).
PubMed/NCBI
|
|
3
|
Fidalgo P, Ahmed M, Meyer SR, Lien D,
Weinkauf J, Cardoso FS, Jackson K and Bagshaw SM: Incidence and
outcomes of acute kidney injury following orthotopic lung
transplantation: A population-based cohort study. Nephrol Dial
Transplant. 29:1702–1709. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin W and Dong C: IL-17 cytokines in
immunity and inflammation. Emerg Microbes Infect. 2:e602013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomashefski JF Jr: Pulmonary pathology of
acute respiratory distress syndrome. Clin Chest Med. 21:435–466.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen H, Bai C and Wang X: The value of the
lipopolysaccharide-induced acute lung injury model in respiratory
medicine. Expert Rev Respir Med. 4:773–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Huang J, Foley NM, Xu Y, Li YP, Pan
J, Redmond HP, Wang JH and Wang J: B7H3 ameliorates LPS-induced
acute lung injury via attenuation of neutrophil migration and
infiltration. Sci Rep. 6:312842016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu N, Wang C, Dai X, Zhou M, Gong L, Yu L,
Peng C and Li Y: Phillygenin inhibits LPS-induced activation and
inflammation of LX2 cells by TLR4/MyD88/NF-κB signaling pathway. J
Ethnopharmacol. 248:1123612020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du B, Zhang L, Sun Y, Zhang G, Yao J,
Jiang M, Pan L and Sun C: Phillygenin exhibits anti-inflammatory
activity through modulating multiple cellular behaviors of mouse
lymphocytes. Immunopharmacol Immunotoxicol. 41:76–85. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li H, Chen M, Yang Z, Wang Q, Wang J, Jin
D, Yang X, Chen F, Zhou X and Luo K: Phillygenin, a MELK Inhibitor,
inhibits cell survival and Epithelial-Mesenchymal transition in
pancreatic cancer cells. Onco Targets Ther. 13:2833–2842. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He J, Wei W, Yang Q and Wang Y:
Phillygenin exerts in vitro and in vivo antitumor effects in
drug-resistant human esophageal cancer cells by inducing
mitochondrial-mediated apoptosis, ROS generation, and inhibition of
the nuclear factor kappa B NF-kappaB signalling pathway. Med Sci
Monit. 25:739–745. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zinter MS, Delucchi KL, Kong MY, Orwoll
BE, Spicer AS, Lim MJ, Alkhouli MF, Ratiu AE, McKenzie AV,
McQuillen PS, et al: Early plasma matrix metalloproteinase
profiles. A novel pathway in pediatric acute respiratory distress
syndrome. Am J Respir Crit Care Med. 199:181–189. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kong MY, Gaggar A, Li Y, Winkler M,
Blalock JE and Clancy JP: Matrix metalloproteinase activity in
pediatric acute lung injury. Int J Med Sci. 6:9–17. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar H, Jo MJ, Choi H, Muttigi MS, Shon
S, Kim BJ, Lee SH and Han IB: Matrix metalloproteinase-8 inhibition
prevents disruption of blood-spinal cord barrier and attenuates
inflammation in rat model of spinal cord injury. Mol Neurobiol.
55:2577–2590. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee EJ, Park JS, Lee YY, Kim DY, Kang JL
and Kim HS: Anti-inflammatory and anti-oxidant mechanisms of an
MMP-8 inhibitor in lipoteichoic acid-stimulated rat primary
astrocytes: Involvement of NF-κB, Nrf2, and PPAR-γ signaling
pathways. J Neuroinflammation. 15:3262018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng K, Yang A, Hu X, Zhu D and Liu K:
Curcumin attenuates pulmonary inflammation in lipopolysaccharide
induced acute lung injury in neonatal rat model by activating
peroxisome proliferator-activated receptor γ (PPARγ) Pathway. Med
Sci Monit. 24:1178–1184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen C, Zhang Z, Tan F, Meng F, Lai L, Chi
X and Zhu Q: Stabilizing mast cells improves acute lung injury
after orthotopic liver transplantation via promotion of apoptosis
in polymorphonuclear neutrophils. Am J Physiol Lung Cell Mol
Physiol. 320:L266–L275. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou M, Yu S, Hong B, Li J, Han H and Qie
G: Antibiotics control in aquaculture requires more than
antibiotic-free feeds: A tilapia farming case. Environ Pollut.
268:1158542021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong Z, Lu X, Tong X, Dong Y, Tang L and
Liu M: Forsythiae fructus: A review on its phytochemistry, quality
control, pharmacology and pharmacokinetics. Molecules. 22:14662017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia J, Zhang F, Li Z, Qin X and Zhang L:
Comparison of fruits of forsythia suspensa at two different
maturation stages by NMR-based metabolomics. Molecules.
20:10065–10081. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao J, Shao SY, Zhang X, Yuan X, Feng ZM,
Jiang JS, Yang YN and Zhang PC: Two new lignans from the fruits of
Forsythia suspensa. J Asian Nat Prod Res. 22:418–424. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ko HC, Wei BL and Chiou WF: Dual
regulatory effect of plant extracts of Forsythia suspense on RANTES
and MCP-1 secretion in influenza A virus-infected human bronchial
epithelial cells. J Ethnopharmacol. 102:418–423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo N, Gai QY, Jiao J, Wang W, Zu YG and
Fu YJ: Antibacterial activity of fructus forsythia essential oil
and the application of EO-Loaded nanoparticles to food-borne
pathogens. Foods. 5:732016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song W, Wu J, Yu L and Peng Z: Evaluation
of the pharmacokinetics and hepatoprotective effects of phillygenin
in mouse. Biomed Res Int. 2018:79643182018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hao Y, Li D and Piao X and Piao X:
Forsythia suspensa extract alleviates hypersensitivity induced by
soybean beta-conglycinin in weaned piglets. J Ethnopharmacol.
128:412–418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duan D, Zhang B, Yao J, Liu Y and Fang J:
Shikonin targets cytosolic thioredoxin reductase to induce
ROS-mediated apoptosis in human promyelocytic leukemia HL-60 cells.
Free Radic Biol Med. 70:182–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Solan PD, Dunsmore KE, Denenberg AG, Odoms
K, Zingarelli B and Wong HR: A novel role for matrix
metalloproteinase-8 in sepsis. Crit Care Med. 40:379–387. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou X, Lu J, Chen D, Wang W, Cai Q, Li T
and Zhang J: Matrix metalloproteinase-8 inhibitors mitigate
sepsis-induced myocardial injury in rats. Chin Med J (Engl).
127:1530–1535. 2014.PubMed/NCBI
|
|
30
|
Villapol S: Roles of peroxisome
proliferator-activated receptor gamma on brain and peripheral
inflammation. Cell Mol Neurobiol. 38:121–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ju Z, Su M, Hong J, Kim E and Jung JH:
Anti-inflammatory effects of an optimized PPAR-γ agonist via NF-κB
pathway inhibition. Bioorg Chem. 96:1036112020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yonutas HM and Sullivan PG: Targeting PPAR
isoforms following CNS injury. Curr Drug Targets. 14:733–742. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kapadia R, Yi JH and Vemuganti R:
Mechanisms of anti-inflammatory and neuroprotective actions of
PPAR-gamma agonists. Front Biosci. 13:1813–1826. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
El Dairi R, Huuskonen P, Pasanen M and
Rysä J: Peroxisome proliferator activated receptor gamma
(PPAR-gamma) ligand pioglitazone regulated gene networks in term
human primary trophoblast cells. Reprod Toxicol. 81:99–107. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yousefnia S, Momenzadeh S, Seyed Forootan
F, Ghaedi K and Nasr Esfahani MH: The influence of peroxisome
proliferator-activated receptor γ (PPARγ) ligands on cancer cell
tumorigenicity. Gene. 649:14–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Janani C and Ranjitha Kumari BD: PPAR
gamma gene-a review. Diabetes Metab Syndr. 9:46–50. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hashemzadeh AA, Nasoohi N, Raygan F,
Aghadavod E, Akbari E, Taghizadeh M, Memarzadeh MR and Asemi Z:
Flaxseed Oil Supplementation improve gene expression levels of
PPAR-γ, LP(a), IL-1 and TNF-α in type 2 diabetic patients with
coronary heart disease. Lipids. 52:907–915. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gurley C, Nichols J, Liu S, Phulwani NK,
Esen N and Kielian T: Microglia and astrocyte activation by
toll-like receptor ligands: Modulation by PPAR-gamma Agonists. PPAR
Res. 2008:4531202008. View Article : Google Scholar : PubMed/NCBI
|