Introduction
Gastritis refers to inflammation caused by damage to
the gastric epithelium (1–3). The symptoms of gastritis include
epigastric pain, nausea, vomiting, belching and weight loss
(4). Acute gastritis is usually
caused by excessive alcohol consumption, long-term use of
non-steroidal anti-inflammatory drugs (NSAIDs) and stress (5,6). This
stimulation increases gastric acid secretion and damages the
gastric mucosa (7,8). The secretory pathways of gastric acid
include activation of histamine-induced histamine H2 receptor
(H2R), cholinergic receptor muscarinic 3 (CHRM3) and
cholecystokinin 2 receptor (CCK2R) activation by gastrin (9–13).
Thus, activated H2R, M3R and CCK2R increase the concentration of
cyclic adenosine monophosphate (cAMP) in parietal cells and
activate H+/K+ ATPase. This increases the
concentration of gastric acid, which damages the gastric mucosa
(14,15).
Gastric juice contains hydrochloric acid, a highly
corrosive acid (16), and the high
concentration of acid secreted from the gastric mucosa is fatal to
cells (17). However, it usually
has a useful action in the stomach, killing the ingested bacteria
and promoting the formation of pepsin (18). Furthermore, it prevents damage to
and contact with the stomach wall through a series of complex and
complementary physical and chemical processes (19).
Collectively, this process of structural and
functional protection of the stomach against the destruction of
autologous acids and pepsin, reflux bile and pancreatic enzymes, as
well as ingested abrasives or toxic substances, is called the
gastric mucosal barrier (20,21).
On the gastric mucosa, epithelial cells form a strong barrier
against penetration by the lumen content, including hydrogen ions
(22). Cells have tight junctions,
hydrophobic and abundant lipids, and a mucosal surface that removes
acids and secretes bicarbonate and mucus (23). When acid and pepsin diffuse back
into the tissues, the secretion of acid and pepsin is further
stimulated, leading to decreased mucosal blood flow and gastric
motility (24,25). Subsequently, as the acid and pepsin
diffuse back into the tissues, the secretion of acid and pepsin is
further stimulated and mucosal blood flow and gastric motility
decrease (26,27). Acid also damages connective tissue
and submucosal capillaries, causing local mucosal bleeding and
microulcers (28). Severe and
long-term exposure to acids may lead to significant gastric
ulceration (29). However,
elimination of the causative agent or adequate and immediate
treatment may quickly restore mucosal integrity (30,31).
The treatment of gastritis and gastric ulcers
involves inhibition of the offensive factors through the use of
antacids, anticholinergic drugs, H2R antagonists, anti-gastrin
drugs, hydrogen pump inhibitors, mucosal protective agents and
inhibitors of gastric secretions, such as prostaglandin-related
drugs and anti-pepsins (32,33).
Drugs that induce defensive factors and suppress the central
nervous system to reduce anxiety and stress have also been used
(34). However, these drugs to
treat gastritis and gastric ulcer have various side effects,
including arrhythmia, erectile dysfunction, gynecomastia and
recurrence, and it is necessary to develop novel drugs or health
supplements that are safer and more effective in the treatment of
gastritis (35,36).
Polydeoxyribonucleotides (PDRNs) extracted from
salmon sperm are known to help in the regeneration of tissues in
burns and wounds (37). A mixture
of DNA fragments with a composition that is the most similar to
human DNA promotes tissue regeneration and repair after surgery
(38–42). PDRN is an adenosine A2A receptor
agonist that enhances the expression of vascular endothelial growth
factor and promotes wound healing through angiogenesis in diabetic
foot ulcers (43,44). It also stimulates the conversion of
growth factors and extracellular matrix in damaged cells or tissues
through the purine adenosine A2A receptor (42). PDRN activates A2A receptors that
promote wound healing by preventing pro-inflammatory cytokines and
releasing pro-fibrotic cytokines (45,46).
It exerts anti-inflammatory effects by inhibiting the production of
pro-inflammatory cytokines such as interleukin-6 and tumour
necrosis factor-α and upregulating the production of
anti-inflammatory cytokines (45,47,48).
The present study investigated the protective
effects of f-PDRN extracted from salmon milt against
HCl-EtOH-induced gastric mucosal damage in rats. In addition, the
regulatory effects of f-PDRN on the expression of molecules
involved in the gastric acid secretion and inflammation pathways
were investigated in gastric parietal cells.
Materials and methods
Materials
The f-PDRN PRF002 (PharmaResearch) used in the
present study is a fragmented DNA polymer extracted from the testes
of adult salmon (Oncorhynchus keta, Salmonidae) that
returned to their breeding grounds in Namdaecheon (Gangwon, Korea)
(49). PRF002, with a molecular
weight of 50–1,500 kDa, was dissolved in 0.9% NaCl solution,
resulting in a DNA concentration of 75% or higher. Stillen Tab
(Dong-A ST Co.) is a nonsteroidal anti-inflammatory analgesic that
improves gastric mucosal lesions (haemorrhage, erythema and oedema)
and acute and chronic gastritis (50).
Construction of rat models of gastric
mucosal damage
A total of 60 male Hsd:Sprague Dawley®
(SD) rats (age, 7 weeks; bodyweight, 200±10 g) were purchased from
Koatech (49). The animals were
housed one per cage and allowed free access to tap water and food
that contained 0.44% sodium and 22.5% protein. Acclimatized to a
colony room with controlled ambient temperature (24±1°C), humidity
(50±10%) and 12-h light-dark cycles. All experiments involving
laboratory animals were performed in accordance with the guidelines
of the Institutional Animal Care and Use Committee of the KNOTUS
(Incheon, South Korea). The rats were anaesthetized with a mixture
of Zoletil (30 mg/kg) and Rompun (10 mg/kg) solution (3:1 ratio, 1
ml/kg, i.p.). Euthanasia was performed by introducing a flow rate
of 5 l/min of 100% carbon dioxide into a bedding-free cage
initially containing room air with the lid closed at a rate
sufficient to induce rapid anaesthesia for a 10 l volumetric
chamber, with death occurring within 2.5 min. The experimental
design was approved by the KNOTUS Management and Use Committee (no.
19-KE-262). The SD rats were divided into six groups consisting of
10 rats/group as follows: A normal control group and alcoholic
gastric irritation test control groups. The alcoholic gastric
irritation test control groups included the vehicle group, low-dose
PRF002 concentration (26 mg/kg/day) group, medium-dose PRF002
concentration (52 mg/kg/day) group, high-dose PRF002 concentration
(78 mg/kg/day) group and a positive control group (Stillen, a
non-steroidal anti-inflammatory drug; 150 mg/kg/day) (51). The f-PDRN dose selection in this
study was based on a dose of 52 mg/kg/day, which was previously
used for the treatment of arthritis (52). The vehicle group was treated with
0.9% NaCl and the alcoholic gastric irritation model group was
treated with 0.9% NaCl and 40% ethanol. The low-, medium-,
high-PRF002 and Stillen groups were administered the indicated
doses of PRF002 and Stillen by oral gavage and 40% ethanol for 7
days to irritate the stomach. All dietary intake was stopped 24 h
prior to the experiment to empty the stomach (53).
Analysis of the damaged area of the
gastric mucosa
HCl (150 mM) was added to 70% ethanol and 1 ml of
the mixture was orally administered to each of the rats by oral
gavage. The rats were sacrificed 1 h later, blood was collected and
the stomach was excised. The excised stomach was incised along the
greater curvature and washed in saline, and images were acquired
using a digital camera (Coolpix P5100; Nikon Corporation). Areas of
damage to the gastric mucosa were quantitated using ImageJ software
(version 1.4.3; National Institutes of Health). Measurements of the
damaged area and the total area of the stomach were used to
calculate the damaged area (%) as follows: Damaged area
(%)=(damaged area/total area) ×100.
Measurement of the volume of gastric
juice secretion
The excised gastric pylorus was slightly incised and
gastric juice was collected in a 15-ml tube. The collected gastric
juice was centrifuged at 2,400 × g at 4°C for 20 min and the
supernatant constituted the total amount of gastric fluid (ml).
Analysis of the acidity and pH of the
gastric juice
To the supernatant (1 ml) of the separated gastric
juice, 50 µl of 0.5% dimethylaminobenzene alcohol and 50 µl 1%
phenolphthalein alcohol solution was added. When the gastric juice
turned red, a 0.1N NaOH solution was added. The total acidity was
calculated as follows and expressed in mEq/l: Acidity=(volume of
NaOH × normality of NaOH ×100)/0.1 (mEq-1/100 g).
Separation and culture of gastric
parietal cells
After sacrificing the rats by CO2 gas
asphyxiation, the gastric tissue was separated and washed with PBS.
HEPES (20 mM) and cimetidine (5M) were added to DMEM (HyClone;
Cytiva) containing 1 mg/ml type 4 collagenase (Worthington
Biochemical Corporation) and 1 mg/ml BSA (Thermo Fisher Scientific,
Inc.) and stored at 37°C for 30 min. The cell suspension was
filtered through a nylon mesh (0.2 mm) and centrifuged at 1,000 × g
at 4°C for 15 min. The separated cells were filtered through a
40-µm cell strainer (BD Biosciences) and centrifuged for 10 min at
1,350 × g at 4°C. To the separated cells, complete DMEM/F-12
(Gibco-BRL; Thermo Fisher Scientific, Inc.), 20 mM HEPES, 0.2% BSA,
10 mM glucose, 1 insulin-transferrin-selenium-A (Gibco; Thermo
Fisher Scientific, Inc.), 1 mM glutamine, 100 U/ml
penicillin/streptomycin, 400 µg/ml gentamicin sulfate and 15 mg/ml
geneticin (pH 7.4) were added, followed by centrifugation for 10
min at 1,350 × g at 4°C. The collagenase-isolated cells were placed
in a Matrigel® Matrix plate (Corning, Inc.) and
incubated at 37°C with 5% CO2.
Measurement of cAMP levels in gastric
parietal cells
After stabilising the cells cultured in the
Matrigel® Matrix plate for 24 h, the growth media (DMEM
containing 1 mg/ml glucose and 50 µg/ml gentamycin) was removed and
the cells were centrifuged for 10 min at 1,350 × g at 4°C. The
supernatant was used to quantitate cAMP levels using a cAMP direct
immunoassay kit (Biovision) according to the manufacturer's
protocol. Measurements were performed using an ELISA plate reader
(Molecular Devices, LLC).
Reverse transcription-quantitative PCR
(RT-qPCR)
mRNA from each sample of the stomach wall was
extracted using an RNeasy Mini Kit (Qiagen GmbH) and
reverse-transcribed using a High-Capacity cDNA Reverse
Transcription Kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.; cat. no. 4374966) according to the manufacturer's
instructions. The cDNA (1 µg) was amplified using the following rat
primers: H2R sense, 5′-ACCAGCCTGGATGTCATGCT-3′ and antisense,
5′-CCTGTCAAGGCTGATCATGAAG-3′; H+/K+ ATPase
sense, 5′-CCTCACACAGAGGAGACTA-3′ and antisense,
5′-TGCCCAGTGTCCGGGTTCCA-3′; CCK2R sense, 5′-ACGTGGCGGCTTCCAA-3′ and
antisense, 5′-CCAGGCCCCAGTGCTCTGATGGTGG-3′; M3R sense,
5′-ACGCTCGCCAGGATGAAGT-3′ and antisense, 5′-GGCTTGGCTTCCAGCTCTT-3′;
mucin (MUC)5AC sense, 5′-GGCCAATGCGGCACTTGTACCAAT−3′ and antisense,
5′-GTCATCTGGACAGAAGCAGCCCTC−3′; matrix metalloproteinase-3 (MMP-3)
sense, 5′-CCTGCTTTGTCCTTTGATGC-3′ and antisense,
5′-TGAGTCAATCCCTGGAAAGTC−3′; MMP-9 sense, 5′-CATTCGCGTGGATAAGGA−3′
and antisense, 5′-ACAAGAAAGGACGGTGGG−3′; and GAPDH sense,
5′-TGATTCTACCCACGGCAAGTT−3′ and antisense,
5′-TGATGGGTTTCCCATTGATGA−3′. TOPreal™ qPCR 2X PreMIX (Enzynomics)
was used according to the manufacturer's protocol. qPCR was
performed using the following thermocycling conditions: Initial
denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for
5 sec and 60°C for 30 sec. Reactions were performed on a CFX96™
Real-Time System (Bio-Rad Laboratories, Inc.) with no template
control reactions for each primer set (Fig. S1) (54). The experiments were repeated three
times.
Determination of histamine, cAMP,
myeloperoxidase (MPO) and prostaglandin E2 (PGE2) levels
Whole blood samples were collected in Vacutainers
(BD Biosciences) with a clotting activator. After centrifugation at
2,000 × g for 10 min at 17°C, the supernatants were collected and
the histamine concentration in blood was analysed in the plasma.
Damaged gastric tissue samples were homogenized in 1 ml phosphate
buffer, followed by centrifugation of samples. Subsequently, PGE2
and MPO activity were analysed. Histamine (cat. no. ab213975), MPO
(cat. no. ab155458), cAMP (cat. no. ab133051) and prostaglandin E2
(cat. no. ab133021) ELISA kits (Abcam) were used to measure
histamine, MPO, cAMP and PGE2 levels, respectively, in the
supernatant according to the manufacturer's protocols using a
microplate reader.
Histological analysis
Paraffin was removed from the paraffin-embedded
gastric tissue sections and the tissues were stained according to a
previously published protocol (55). Gastric tissue sections were fixed in
10% phosphate-buffered formaldehyde, embedded in paraffin and
processed for histological analysis. Sections were cut to 5-µm
thickness, mounted on slides and stained with H&E according to
standard procedures.
Statistical analyses
All statistical data were derived from five
repetitions. The significance of differences among the experimental
groups was tested by one-way ANOVA using Prism 7.04 (Graph Pad
Software, Inc.) and a post-hoc Tukey's test was performed.
P<0.05 was considered to indicate a statistically significant
difference.
Results
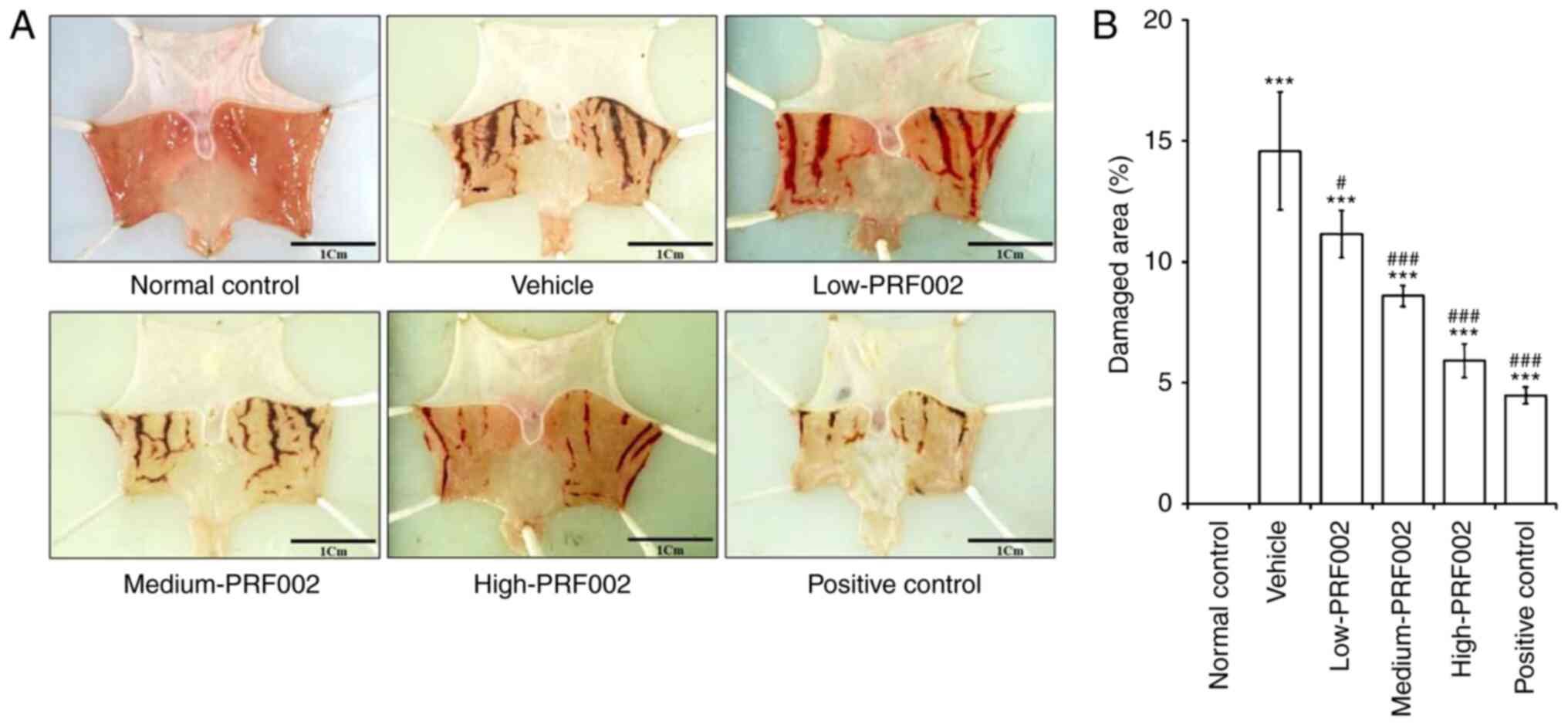
Protective effects of f-PDRN against
HCl/EtOH-induced gastric mucosa damage in rats
The protective efficacy of f-PDRN against gastric
mucosa damage was investigated using a rat model of
HCl/EtOH-induced gastric mucosal injury. The experimental groups
were as follows: Normal control, vehicle (negative control),
low-PRF002 (26 mg/kg/day) administration group, medium-PRF002 (52
mg/kg/day) administration group, high-PRF002 (78 mg/kg/day)
administration group and Stillen (positive control). In the
positive control group, Stillen, a novel drug, was used to decrease
acute and chronic gastritis and gastric mucosal lesions. Each
substance was administered orally at the indicated doses. On the
day of the autopsy, the stomach was removed, images of the gastric
mucosa from each experimental group were acquired with a digital
camera and the damaged area was quantified using Image J software.
The results revealed that gastric mucosal injury in the medium- and
high-PRF002-administered groups was markedly lower than that in the
low-PRF002 group. Comparison and analysis of the sites of gastric
mucosal injury revealed that the area of damage in all gastric
mucosal injury groups was markedly higher than that in the normal
control group. In addition, it was confirmed that in the f-PDRN
oral administration groups, the area of gastric mucosal injury
decreased in a dose-dependent manner (Fig. 1A and B).
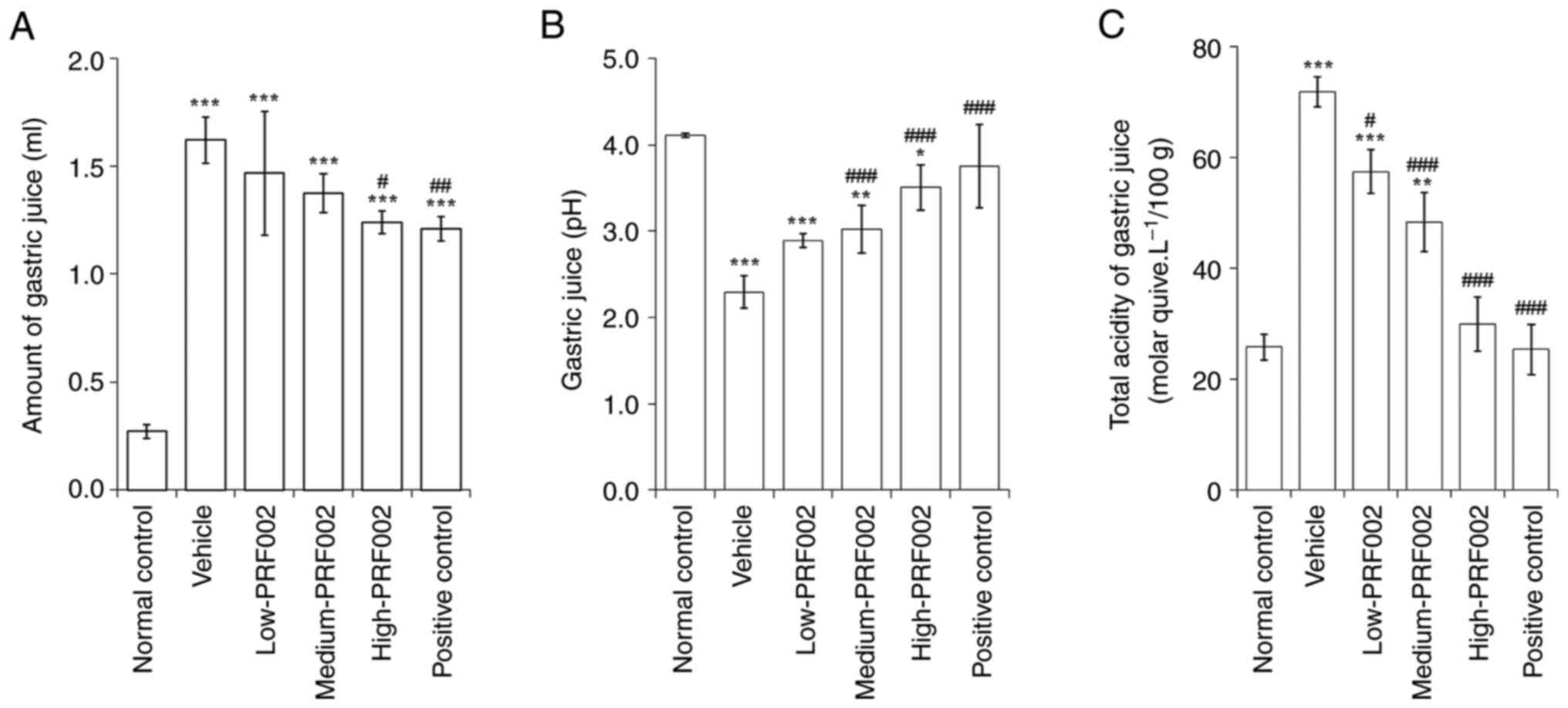
Inhibition of gastric acid secretion
and recovery of pH and acidity by f-PDRN
To confirm the protective effect of f-PDRN on the
gastric mucosa, changes in gastric juice secretion, total acidity
and decrease in gastric juice pH after oral administration of
f-PDRN were analysed. The results indicated that the amount of
gastric juice collected from the gastric pyloric area of rats was
significantly increased in all gastric mucosal injury-induced
groups, compared with that in the normal control (Fig. 2A). In addition, the pH of the
gastric juice was significantly decreased in all of the
PRF002-administered groups compared with that in the normal control
(Fig. 2B). The total acidity was
significantly higher in the vehicle, low-PRF002 and medium-PRF002
groups than in the normal controls (Fig. 2C). To summarise the changes in
gastric juice secretion, total acidity and pH after f-PDRN
administration, it was indicated that the amount of gastric juice
secreted was decreased in each administration group compared with
that in the vehicle group. In particular, the volumes in the
high-PRF002 and positive control groups significantly decreased.
The total acidity of gastric juice was significantly decreased in
the medium-PRF002, high-PRF002 and positive control groups compared
to that in the vehicle group. The total acidity of gastric juice in
the high-PRF002 and positive control groups was restored to almost
the level of the normal control. In addition, the pH of gastric
juice was significantly higher in the medium-PRF002, high-PRF002
and positive control groups than in the vehicle group.
f-PDRN reduces the mRNA expression of
gastric acid secretion-related factors
The expression levels of the receptors related to
gastric acid secretion and inflammation were compared using RT-qPCR
(Fig. 3). The results revealed that
the mRNA expression of H2R, CHRM3, H+/K+
ATPase, MMP-3 and MMP-9 was markedly increased in the vehicle group
compared with that in the normal control, and significantly reduced
in the high-dose PRF002 and positive control groups compared to
that in the vehicle group with gastric mucosal damage, and was
almost restored to the expression level in the normal control
(Fig. 3A, B, D, F and G). The mRNA
expression of MUC5AC increased significantly in the medium-PRF002,
high-PRF002 and positive control groups compared with that in the
vehicle group (Fig. 3E). In
addition, the mRNA expression of CCK2R decreased significantly in
the low-PRF002, medium-PRF002, high-PRF002 and positive control
groups compared with that in the vehicle group (Fig. 3C). These results suggested that
f-PDRN decreased the mRNA expression of pre-stage gastric acid
secretion factors, such as H2R, CHRM3 and CCK2R (9–13),
which led to a decrease in the H+/K+ ATPase
protein transcription factor.
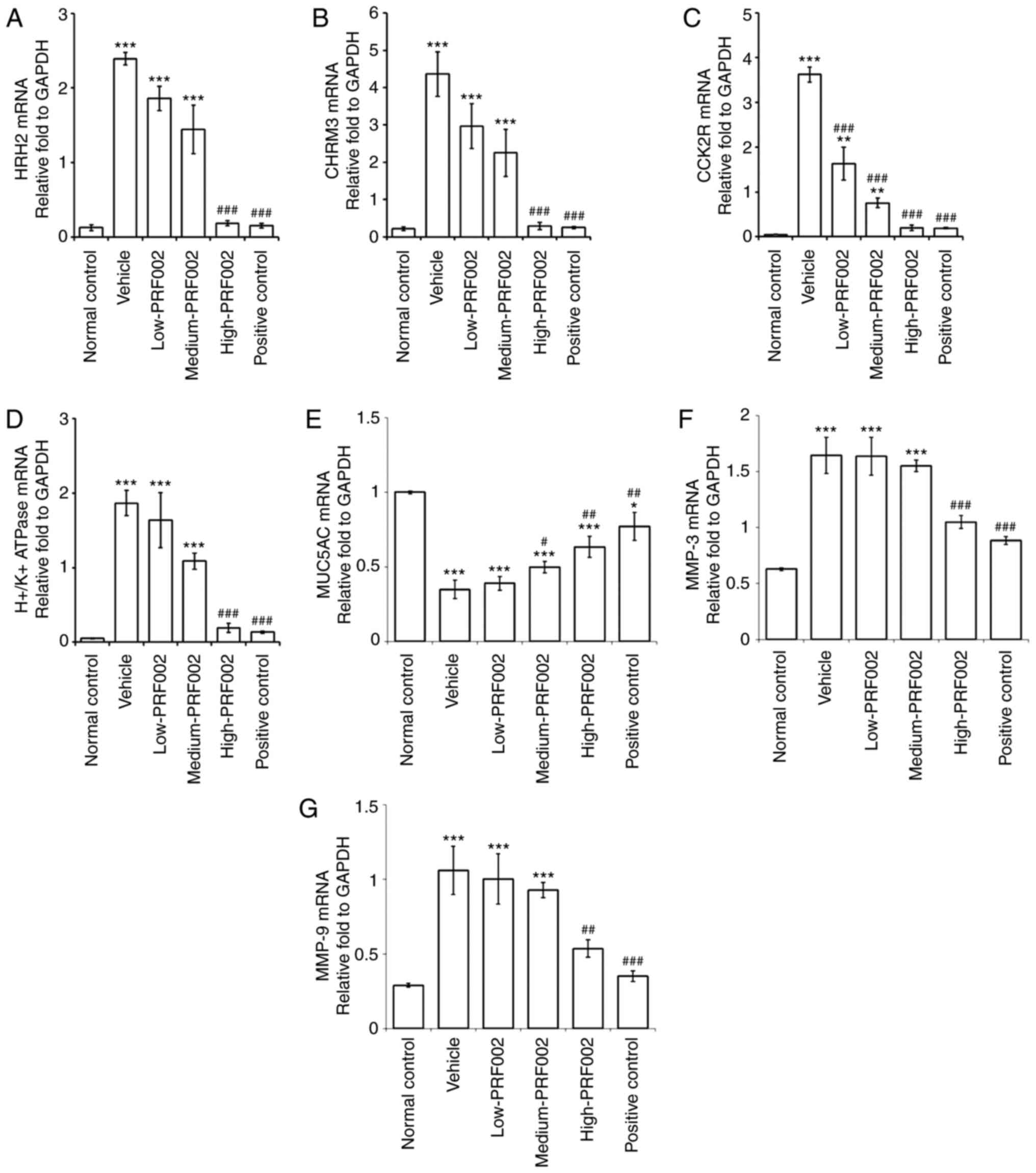 | Figure 3.mRNA expression of (A) H2R, (B)
CHRM3, (C) CCK2R, (D) H+/K+ ATPase, (E) MUC5AC; (F) MMP-3 and (G)
MMP-9 in cells isolated from the gastric wall and gastric mucosa.
The groups were the normal control, vehicle, low-PRF002,
medium-PRF002, high-PRF002 and positive control. *P<0.05,
**P<0.01, ***P<0.001 compared to the normal control;
#P<0.05, ##P<0.01,
###P<0.001 compared to vehicle. H2R, histamine
receptor H2; M3R, muscarinic acetylcholine receptor 3; CCK2R,
cholecystokinin 2 receptor; MUC, mucin. |
Regulatory effect of f-PDRN on
histamine, MPO, cAMP and PGE2
To determine the changes in factors related to
gastric juice secretion induced by f-PDRN administration, the
levels of histamine, MPO, cAMP and PGE2 were compared among the
groups of rats with gastrointestinal mucosal damage. The results
indicated that the inflammation-related factors histamine and MPO
were markedly upregulated in the vehicle group compared with that
in the normal control, and significantly downregulated in the low-,
medium- and high-dose PRF002 groups compared with those in the
vehicle groups (Fig. 4A and B).
Furthermore, cAMP, a precursor of proton pump activation, was also
significantly downregulated in the f-PDRN-treated groups
low-PRF002, medium-PRF002 and high-PRF002 compared with that in the
vehicle group (Fig. 4C). In
addition, PGE2, a factor that mediates gastrointestinal protective
effects, was upregulated in the low-PRF002, medium-PRF002 and
high-PRF002 groups compared with that in the vehicle group with
gastric mucosal damage (Fig. 4D).
These results suggested that f-PDRN exerted potent gastroprotective
effects against gastric mucosal damage.
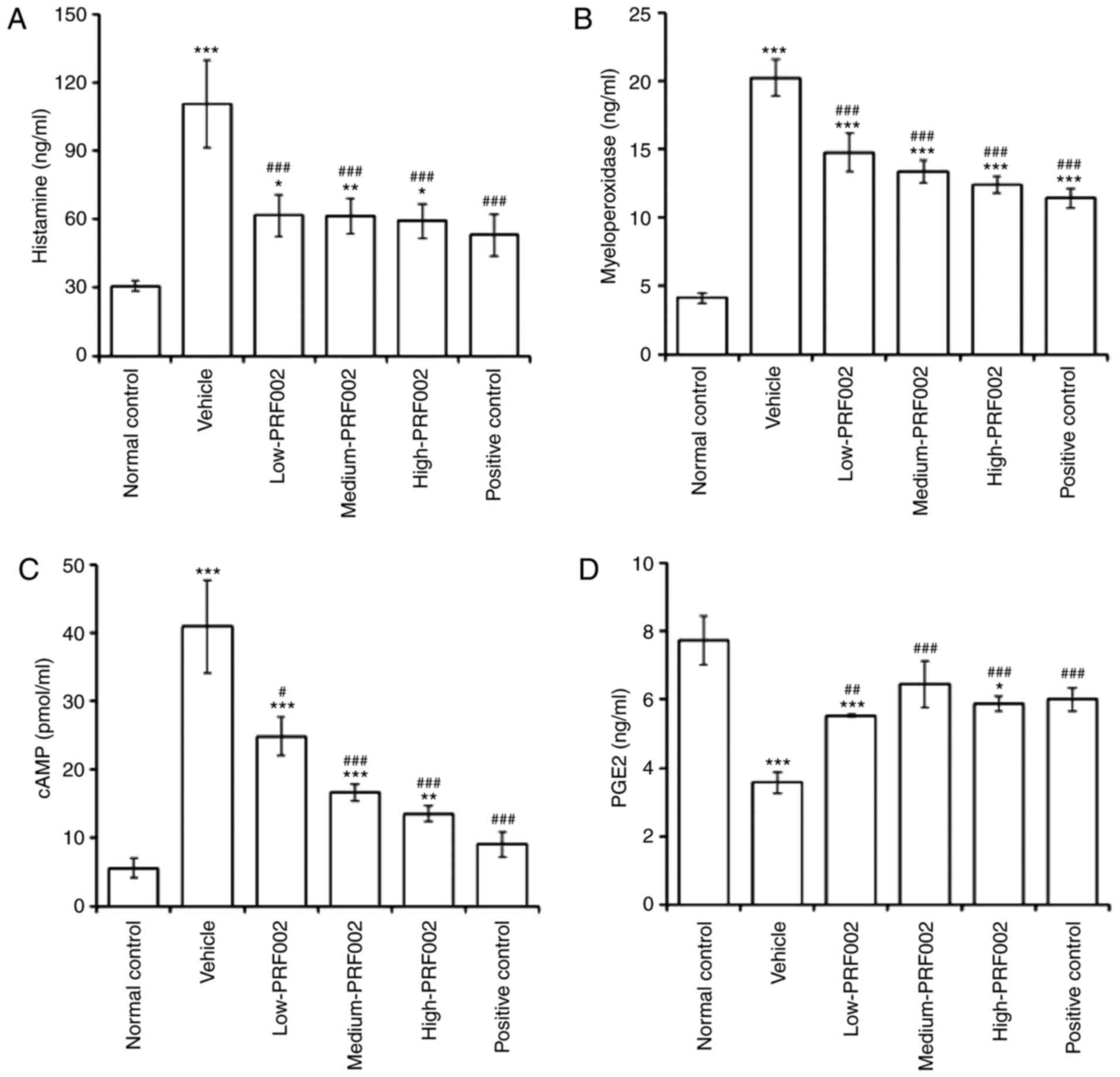 | Figure 4.(A) Concentration of histamine, (B)
MPO activity, (C) level of cAMP and (D) PGE2 in damaged gastric
mucosal tissues. The groups are the normal control, vehicle,
low-PRF002, medium-PRF002, high-PRF002 and positive control.
*P<0.05, **P<0.01, ***P<0.001 compared to the normal
control, #P<0.05, ###P<0.001 compared
to vehicle. MPO, myeloperoxidase; cAMP, cyclic adenosine
monophosphate; PGE2, prostaglandin E2. |
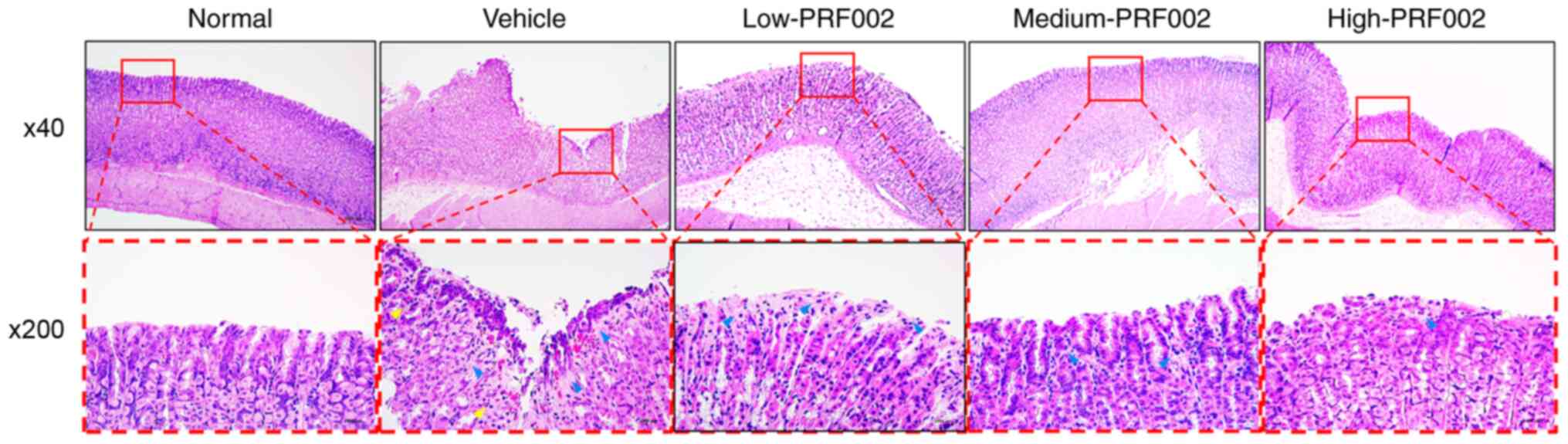
Histological effects of oral
administration of f-PDRN
The present results confirmed the prevention of the
gastric mucosa damage by f-PDRN administration and it was indicated
that the effect of the administration of high concentrations of
PRF002 (high-PRF002) was most similar to that in the positive
control (Fig. 5). The gastric
mucosa in the various groups, including the normal control group
with gastric tissue, the vehicle group with damaged gastric tissue
and the high-PRF002 group with reduced damage of gastric tissue,
was compared using histopathology. The results revealed epithelial
cell loss, mucosal or submucosal distortion (blue arrow) and
invasive inflammatory cells in the vehicle-administered group
(yellow arrow). However, epithelial cell loss and inflammatory cell
infiltration were reduced in the high-PRF002-administered group
compared with those in the vehicle-administered group. Therefore,
these observations confirmed that the gastric mucosa damage was
prevented by administration of high-PRF002.
Discussion
The major objective of the present study was to
investigate whether f-PDRN (PRF002) is helpful in gastric mucosal
maintenance and protects against HCl/EtOH-induced acute gastric
mucosal damage in rats. The present study first investigated the
protective effect of f-PDRN on the macroscopic and subtle changes
caused by HCl/EtOH in the gastric mucosa of rats. It was
demonstrated that administration of f-PDRN significantly prevented
the HCl/EtOH-induced morphological and structural changes in the
gastric mucosa. Previous data from a rat model suggested that acute
ethanol poisoning causes severe lesions and damage through gastric
leukocyte infiltration of the submucosal layer (8,16,34).
In addition, ethanol-induced gastric mucosal damage is associated
with overproduction of gastric juice, resulting from increased
expression of receptors such as H2R, CHRM3 and
H+/K+ ATPase, and increased CCK2R at the mRNA
level, which are associated with gastric acid secretion (13,48,56).
This was further confirmed by changes in histamine, MPO, cAMP and
PGE2, which are factors related to gastric juice secretion.
Therefore, one of the strategies to inhibit gastric acid secretion
is to use receptor antagonists (10,12).
Three major receptors have been identified in the parietal cells
and antagonists have been developed for each of them (10,12).
H2R antagonists and muscarinic receptor antagonists are available
for clinical use (10). In
addition, E2 type prostaglandins block binding to histamine
receptors and cAMP production. Muscarinic receptor antagonists such
as atropine and selective M1-antagonists such as pirenzepine are
effective inhibitors of acid secretion by dietary stimulation but
have certain side effects (57–59).
This is owing to the generalised presence of these receptors in
numerous organs of the body (60).
Furthermore, the degree of inhibition with high doses of these
antagonists is insufficient to reduce acid to low-threshold stable
levels even in duodenal ulcer disease (61,62).
In addition, a close relationship exists between gastric juice
production and gastric inflammation. Therefore, it is endeavoured
to examine the effect of f-PDRN on pro-inflammatory cytokine
production in a future study. Stillen, which was used as a positive
control in the present study, has been widely used in the treatment
of acute and chronic gastritis (63). It provides anti-gastritis effects
following the administration of NSAIDs. More importantly, the
present results demonstrated that f-PDRN significantly attenuated
HCl-EtOH-induced gastric mucosal damage in a dose-dependent manner.
EtOH-induced excessive secretion of gastric juice (associated with
the aetiology of gastric damage) reduced the pH and increased the
total acidity of gastric acid.
The upregulation of histamine, acetylcholine and
gastrin is known to increase gastric acid secretion (64–66).
Gastric acid secretion is also further increased following the
upregulation of histamine, acetylcholine, gastrin-associated
receptors, H2R, M3R and CCK2R (67,68).
The present results confirmed the effect of increased mRNA
expression of HRH2, CHRM3 and CCK2R (factors related to gastric
acid secretion) and their decreased expression following
administration of PRF002 in rat models of HCl-EtOH-induced gastric
mucosa damage. In addition, the administration of PRF002 increased
the expression of MUC5AC and decreased the expression of MMP-3 and
MMP-9 by administration of PRF002. In addition, PRF002 treatment
decreased plasma histamine activity and MPO and cAMP levels
compared with those in the vehicle group and significantly
increased PGE2 production. Therefore, the preventive effect of
PRF002 on EtOH-induced gastric mucosal injury may involve two
mechanistic components: Inhibition of inflammation and protection
of the gastric mucosa.
Reducing neutrophil infiltration into ulcerated
gastric tissues prevents damage to the gastric mucosa in rats
(69,70). Since neutrophils contain the blood
cell protein MPO, the accumulation of neutrophils in gastric
mucosal tissue may be measured by MPO activity. In the present
study, high PRF002 (78 mg/kg) protected the histological structure
of the gastric mucosa and prevented the infiltration of
inflammatory cells (neutrophils). Compared to the ethanol group,
PRF002 administration clearly inhibited the production of gastric
secretory mediators (H2R, CHRM3, CCK2R and proton pumps) during
ethanol-induced gastric mucosal damage. Thus, PRF002 reduces
gastric mucosal damage caused by gastric juice secretion and
protects the stomach.
As observed previously, PDRN interacts with
adenosine A2A receptors, stimulates VEGF expression,
promotes wound healing and contributes to gastric ulcer healing,
probably through the inhibition of inflammation and apoptosis
(71). The f-PDRN used in the
present study is similar to that used in conventional medicine;
however, the extraction, manufacturing method and formulation are
different. The mechanism of f-PDRN in gastric mucosal protection is
related to cell migration, proliferation, re-epithelialization,
gland reconstruction and angiogenesis-bone marrow-derived (stem
cell-derived) blood vessel formation. It is also expected to be a
complex tissue regeneration process involving the formation of new
blood vessels from the newly formed progenitor cells. All of these
processes are regulated by growth factors, cytokines, hormones and
transcription factors (72–76). In addition, it may be identified by
genes encoding early growth factors or growth factors (e.g. EGF,
bone-derived fibroblast growth factor, hepatocyte growth factor,
VEGF and trefoil peptides) derived from platelets, macrophages and
damaged tissues (72,77,78).
The possibility of using f-PDRN for mucosal healing was thus
confirmed.
Overall, the present results indicated that oral
administration of f-PDRN is helpful to gastric mucosal maintenance
and protects against alcohol-induced gastric mucosal injury. In
addition, the results of the present study are consistent with the
confirmation of gastroprotective effects of f-PDRN administration
in HCl/EtOH-induced gastric mucosal injury in a rat model.
Therefore, the administration of f-PDRN represents a potentially
useful treatment option to prevent the progression of
alcohol-induced gastric mucosal damage.
In conclusion, the results of the present in
vivo f-PDRN administration experiments demonstrated that f-PDRN
prevents the progression of alcohol-induced gastric mucosal damage
by modulating factors associated with gastric acid secretion.
f-PDRN reduced the expression of HRH2, CHRM3, CCK2R and
H+/K+ ATPase related to gastric acid
secretion and exerted the protective effects against the gastric
mucosa damage through downregulation of histamine, MPO, cAMP, MMP-3
and MMP-9. Therefore, given the therapeutic potential of f-PDRN,
future studies are required to further explore the detailed
mechanisms of action of f-PDRN.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was supported by the Korea Institute
of Marine Science & Technology Promotion grant funded by the
Korea government of 2019 (MOF) (no. 20190036, Development of the
functional food for gastric health improvement using salmon
DNA).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JoK, SC, SOO and SL performed the experiments and
analysed the data. SaK and JuK extracted f-PDRN from salmon milt
and characterized its properties. JoK, HK and SuK interpreted the
data and drafted the manuscript. JoK and SuK confirm the
authenticity of all the raw data. All authors contributed to
editing and revising the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of KNOTUS IACUC (approval no. 19-KE-262).
Patient consent for publication
Not applicable.
Competing interests
All authors were employees of PharmaResearch Co.,
the supplier of PRF002. The authors have no other competing
interests to declare.
References
|
1
|
Caselli M, LaCorte R, DeCarlo L, Aleotti
A, Trevisani L, Ruina M, Trotta F and Alvisi V: Histological
findings in gastric mucosa in patients treated with non-steroidal
anti-inflammatory drugs. J Clin Pathol. 48:553–555. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheli R, Testino G, Giacosa A and
Cornaggia M: Chronic gastritis: Its clinical and physiopathological
meaning. J Clin Gastroenterol. 21:193–197. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ernst PB, Jin Y, Reyes VE and Crowe SE:
The role of the local immune response in the pathogenesis of peptic
ulcer formation. Scand J Gastroenterol Suppl. 205:22–28. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glickman JN, Wang H, Das KM, Goyal RK,
Spechler SJ, Antonioli D and Odze RD: Phenotype of Barrett's
esophagus and intestinal metaplasia of the distal esophagus and
gastroesophageal junction: An immunohistochemical study of
cytokeratins 7 and 20, Das-1 and 45 MI. Am J Surg Pathol. 25:87–94.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Franke D, Philippi A, Tores F, Hager J,
Ziegler A and König IR: On confidence intervals for genotype
relative risks and attributable risks from case parent trio designs
for candidate-gene studies. Hum Hered. 60:81–88. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Srivastava A and Lauwers GY: Pathology of
non-infective gastritis. Histopathology. 50:15–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bujanda L: The effects of alcohol
consumption upon the gastrointestinal tract. Am J Gastroenterol.
95:3374–3382. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu ES and Cho CH: Relationship between
ethanol-induced gastritis and gastric ulcer formation in rats.
Digestion. 62:232–239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malinowska DH, Sachs G and Cuppoletti J:
Gastric H+ secretion: Histamine (cAMP-mediated) activation of
protein phosphorylation. Biochim Biophys Acta. 972:95–109. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prinz C, Kajimura M, Scott D, Helander H,
Shin J, Besancon M, Bamberg K, Hersey S and Sachs G: Acid secretion
and the H,K ATPase of stomach. Yale J Biol Med. 65:577–596.
1992.PubMed/NCBI
|
|
11
|
Rabben HL, Zhao CM, Hayakawa Y, Wang TC
and Chen D: Vagotomy and gastric tumorigenesis. Curr
Neuropharmacol. 14:967–972. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shamburek RD and Schubert ML: Control of
gastric acid secretion. Histamine H2-receptor antagonists and
H+K(+)-ATPase inhibitors. Gastroenterol Clin North Am. 21:527–550.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheng W, Malagola E, Nienhüser H, Zhang Z,
Kim W, Zamechek L, Sepulveda A, Hata M, Hayakawa Y, Zhao CM, et al:
Hypergastrinemia expands gastric ECL cells through CCK2R+
progenitor cells via ERK activation. Cell Mol Gastroenterol
Hepatol. 10:434–449.e1. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Helander HF and Keeling DJ: Cell biology
of gastric acid secretion. Baillieres Clin Gastroenterol. 7:1–21.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lundgren O, Haglind E and Mårdh S: Failure
to deduce a peptide inhibitor of Na, K-ATPase from the gene coding
for the catalytic alpha-subunit of Na,K-ATPase. Acta Physiol Scand.
136:281–286. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raghavendran HR, Sathivel A and Devaki T:
Efficacy of brown seaweed hot water extract against HCl-ethanol
induced gastric mucosal injury in rats. Arch Pharm Res. 27:449–453.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Whittingham S and Mackay IR: Autoimmune
gastritis: Historical antecedents, outstanding discoveries, and
unresolved problems. Int Rev Immunol. 24:1–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang T, Huo Z, Ma J, He C and Zhong G:
The plasmid-encoded pGP3 promotes chlamydia evasion of acidic
barriers in both stomach and vagina. Infect Immun. 87:e00844–18.
2019. View Article : Google Scholar
|
|
19
|
Homan CS, Singer AJ, Henry MC and Thode HC
Jr: Thermal effects of neutralization therapy and water dilution
for acute alkali exposure in canines. Acad Emerg Med. 4:27–32.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Langkamp-Henken B, Glezer JA and Kudsk KA:
Immunologic structure and function of the gastrointestinal tract.
Nutr Clin Pract. 7:100–108. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szabo S: Mechanisms of gastric mucosal
injury and protection. J Clin Gastroenterol. 13 (Suppl 2):S21–S34.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walker WA: Gastrointestinal host defence:
Importance of gut closure in control of macromolecular transport.
Ciba Found Symp. 16-18:201–219. 1979.PubMed/NCBI
|
|
23
|
Code CF: Defense mechanisms of the gastric
mucosa. Scand J Gastroenterol Suppl. 67:201–204. 1981.PubMed/NCBI
|
|
24
|
Abdel-Salam OM, Czimmer J, Debreceni A,
Szolcsányi J and Mózsik G: Gastric mucosal integrity: Gastric
mucosal blood flow and microcirculation. An overview. J Physiol
Paris. 95:105–127. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Turnberg LA: Gastric mucosal defence
mechanisms. Scand J Gastroenterol Suppl. 110:37–40. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sevak R, Paul A, Goswami S and Santani D:
Gastroprotective effect of beta3 adrenoreceptor agonists ZD 7114
and CGP 12177A in rats. Pharmacol Res. 46:351–356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wallace JL, Boichot E, Sidoti C, Brecx A
and Paubert-Braquet M: Protective effects of somatostatin against
gastric damage induced by hemorrhagic shock, stress and PAF in the
rat. Regul Pept. 47:195–203. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bang BW, Maeng JH, Kim MK, Lee DH and Yang
SG: Hemostatic action of EGF-endospray on mucosectomy-induced ulcer
bleeding animal models. Biomed Mater Eng. 25:101–109.
2015.PubMed/NCBI
|
|
29
|
Toljamo KT, Niemelä SE, Karttunen TJ,
Karvonen AL and Lehtola JK: Clinical significance and outcome of
gastric mucosal erosions: A long-term follow-up study. Dig Dis Sci.
51:543–547. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Farré R: Pathophysiology of
gastro-esophageal reflux disease: A role for mucosa integrity?
Neurogastroenterol Motil. 25:783–799. 2013.PubMed/NCBI
|
|
31
|
Sánchez-Fayos Calabuig P, Martín Relloso
MJ and Porres Cubero JC: Gastric mucosa as a target of persistent
proinflammatory aggression: Pathogenic models of chronic gastritis.
Gastroenterol Hepatol. 32:294–306. 2009.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baron JH: Treatments of peptic ulcer. Mt
Sinai J Med. 67:63–67. 2000.PubMed/NCBI
|
|
33
|
Hammer R and Koss FW: Mechanism of action
of the gastric secretion inhibitor pirenzepin. Fortschr Med.
98:549–554. 1980.(In German). PubMed/NCBI
|
|
34
|
Brzozowski T, Konturek PC, Drozdowicz D,
Konturek SJ, Zayachivska O, Pajdo R, Kwiecien S, Pawlik WW and Hahn
EG: Grapefruit-seed extract attenuates ethanol-and stress-induced
gastric lesions via activation of prostaglandin, nitric oxide and
sensory nerve pathways. World J Gastroenterol. 11:6450–6458. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lima RLS, Oliveira EJSG, Pereira EC, Costa
LDS, Dourado TS, Valadão JA, Lima RC, Campelo GP, Brito RM,
Oliveira CMB, et al: Comparative analysis between patients
undergoing gastric bypass and sleeve gastroplasty in a private
hospital in Sao Luis-MA. Acta Cir Bras. 35:e2020003072020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Medical Advisory Secretariat, . Gastric
electrical stimulation: An evidence-based analysis. Ont Health
Technol Assess Ser. 6:1–79. 2006.
|
|
37
|
Lee JH, Han JW, Byun JH, Lee WM, Kim MH
and Wu WH: Comparison of wound healing effects between
Oncorhynchus keta-derived polydeoxyribonucleotide (PDRN) and
Oncorhynchus mykiss-derived PDRN. Arch Craniofac Surg. 19:20–34.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bertone C and Sgro LC: Clinical data on
topical application in gynaecology of polydeoxiribonucleotide of
human placenta. Int J Tissue React. 4:165–167. 1982.PubMed/NCBI
|
|
39
|
Buffoli B, Favero G, Borsani E, Boninsegna
R, Sancassani G, Labanca M, Rezzani R, Nocini PF, Albanese M and
Rodella LF: Sodium-DNA for bone tissue regeneration: An
experimental study in rat calvaria. Biomed Res Int.
2017:73209532017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hwang KH, Kim JH, Park EY and Cha SK: An
effective range of polydeoxyribonucleotides is critical for wound
healing quality. Mol Med Rep. 18:5166–5172. 2018.PubMed/NCBI
|
|
41
|
Raposio E, Guida C, Coradeghini R,
Scanarotti C, Parodi A, Baldelli I, Fiocca R and Santi PL: In vitro
polydeoxyribonucleotide effects on human pre-adipocytes. Cell
Prolif. 41:739–754. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Veronesi F, Dallari D, Sabbioni G, Carubbi
C, Martini L and Fini M: Polydeoxyribonucleotides (PDRNs) from skin
to musculoskeletal tissue regeneration via adenosine A2A
receptor involvement. J Cell Physiol. 232:2299–2307. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Han JH, Jung J, Hwang L, Ko IG, Nam OH,
Kim MS, Lee JW, Choi BJ and Lee DW: Anti-inflammatory effect of
polydeoxyribonucleotide on zoledronic acid-pretreated and
lipopolysaccharide-stimulated RAW 264.7 cells. Exp Ther Med.
16:400–405. 2018.PubMed/NCBI
|
|
44
|
Minutoli L, Antonuccio P, Squadrito F,
Bitto A, Nicotina PA, Fazzari C, Polito F, Marini H, Bonvissuto G,
Arena S, et al: Effects of polydeoxyribonucleotide on the
histological damage and the altered spermatogenesis induced by
testicular ischaemia and reperfusion in rats. Int J Androl.
35:133–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Irrera N, Arcoraci V, Mannino F, Vermiglio
G, Pallio G, Minutoli L, Bagnato G, Anastasi GP, Mazzon E, Bramanti
P, et al: Activation of A2A receptor by PDRN reduces neuronal
damage and stimulates WNT/β-CATENIN driven neurogenesis in spinal
cord injury. Front Pharmacol. 9:5062018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jeong H, Chung JY, Ko IG, Kim SH, Jin JJ,
Hwang L, Moon EJ, Lee BJ and Yi JW: Effect of
polydeoxyribonucleotide on lipopolysaccharide and
sevoflurane-induced postoperative cognitive dysfunction in human
neuronal SH-SY5Y cells. Int Neurourol J. 23 (Suppl 2):S93–S101.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ko IG, Hwang JJ, Chang BS, Kim SH, Jin JJ,
Hwang L, Kim CJ and Choi CW: Polydeoxyribonucleotide ameliorates
lipopolysaccharide-induced acute lung injury via modulation of the
MAPK/NF-κB signaling pathway in rats. Int Immunopharmacol.
83:1064442020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ko IG, Jin JJ, Hwang L, Kim SH, Kim CJ,
Han JH, Kwak MS, Yoon JY and Jeon JW: Evaluating the mucoprotective
effect of polydeoxyribonucleotide against indomethacin-induced
gastropathy via the MAPK/NF-κB signaling pathway in rats. Eur J
Pharmacol. 874:1729522020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jang J, Eom J, Cheong D and Lee C:
Monitoring of the estuary sand bar related with tidal inlet in
namdaecheon stream using landsat imagery. Korean J Remote Sensing.
33:481–493. 2017.
|
|
50
|
Choi YJ, Lee DH, Choi MG, Lee SJ, Kim SK,
Song GA, Rhee PL, Jung HY, Kang DH, Lee YC, et al: Evaluation of
the efficacy and safety of DA-9601 versus its new formulation,
DA-5204, in patients with gastritis: Phase III, randomized,
double-blind, non-inferiority study. J Korean Med Sci.
32:1807–1813. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cho JH: Effects of aloe-fermented products
on improving gastrointestinal functions in an inflammatory bowel
disease mouse model. J Agric Life Environ Sci. 104–120. 2017.
|
|
52
|
Ra HJ, Oh MY, Kim HJ, Lee SY, Eom DW, Lee
SK, Kim SN, Chung KS and Jang HJ: Effects of salmon DNA fraction in
vitro and in a monosodium iodoacetate-induced osteoarthritis rat
model. Korean J Physiol Pharmacol. 22:163–172. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Barrachina MD, Martinez V, Wang L, Wei JY
and Taché Y: Synergistic interaction between leptin and
cholecystokinin to reduce short-term food intake in lean mice. Proc
Natl Acad Sci USA. 94:10455–10460. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2ˆ(−delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
55
|
Yamashina M, Takami T, Kanemura T, Orii T
and Ojima A: Immunohistochemical demonstration of complement
components in formalin-fixed and paraffin-embedded renal tissues.
Lab Invest. 60:311–316. 1989.PubMed/NCBI
|
|
56
|
Wilson DE: Therapeutic aspects of
prostaglandins in the treatment of peptic ulcer disease. Dig Dis
Sci. 31 (Suppl 2):42S–46S. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Blum AL: Therapeutic approach to ulcer
healing. Am J Med. 79:8–14. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Howden CW, Burget DW, Silletti C and Hunt
RH: Single nocturnal doses of pirenzepine effectively inhibit
overnight gastric secretion. Hepatogastroenterology. 32:240–242.
1985.PubMed/NCBI
|
|
59
|
Simon B, Müller P and Dammann HG:
Antisecretory and mucosa-protecting drugs in the acute care of
peptic ulcer. Review of action principles, healing rates and side
effects of approved ulcer drugs and drugs under clinical trial.
Fortschr Med. 102:683–687. 1984.(In German). PubMed/NCBI
|
|
60
|
Kolasińska-Ćwikła A, Łowczak A,
Maciejkiewicz KM and Ćwikła JB: Peptide receptor radionuclide
therapy for advanced gastroenteropancreatic neuroendocrine
tumors-from oncology perspective. Nucl Med Rev Cent East Eur.
21:2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Smout AJ: Is the sensitivity to gastric
acid inhibition Helicobacter pylori status-dependent? Scand J
Gastroenterol Suppl. 225:32–35. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yamane T, Uchiyama K, Ishii T, Omura M,
Fujise K and Tajiri H: Two cases of refractory post-bulbar duodenal
ulcer. Intern Med. 46:1413–1417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jeong HJ, Kim JH, Kim NR, Yoou MS, Nam SY,
Kim KY, Choi Y, Jang JB, Kang IC, Baek NI and Kim HM:
Antidepressant effect of stillen. Arch Pharm Res. 38:1223–1231.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dimaline R and Struthers J: Expression and
regulation of a vesicular monoamine transporter in rat stomach: A
putative histamine transporter. J Physiol. 490:249–256. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Inui H, Yasuno R, Takenoshita M, Ohnishi
Y, Sakamoto M, Matsuzaki J, Yamaji R, Miyatake K, Yamatodani A and
Nakano Y: Increases in gastric histidine decarboxylase activity and
plasma gastrin level in streptozotocin-induced type 1 diabetic
rats. J Nutr Sci Vitaminol (Tokyo). 46:144–148. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sandvik AK, Brenna E and Waldum HL: Review
article: The pharmacological inhibition of gastric acid
secretion-tolerance and rebound. Aliment Pharmacol Ther.
11:1013–1018. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kim JY, Park SD, Nam W, Nam B, Bae CH, Kim
HJ, Kim J, Lee JL and Sim JH: Gastroprotective effects of cudrania
tricuspidata leaf extracts by suppressing gastric cAMP and
increasing gastric mucins. Prev Nutr Food Sci. 25:158–165. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Schwarz P, Kübler JA, Strnad P, Müller K,
Barth TF, Gerloff A, Feick P, Peyssonnaux C, Vaulont S, Adler G and
Kulaksiz H: Hepcidin is localised in gastric parietal cells,
regulates acid secretion and is induced by Helicobacter pylori
infection. Gut. 61:193–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kim CD and Hong KW: Preventive effect of
rebamipide on gastric lesions induced by ischemia-reperfusion in
the rat. J Pharmacol Exp Ther. 275:340–344. 1995.PubMed/NCBI
|
|
70
|
Melarange R, Gentry C, Toseland CD, Smith
PH and Fuller J: Neutropenia does not prevent etodolac- or
indomethacin-induced gastrointestinal damage in the rat. Dig Dis
Sci. 40:2694–2703. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Minutoli L, Arena S, Bonvissuto G, Bitto
A, Polito F, Irrera N, Arena F, Fragalà E, Romeo C, Nicotina PA, et
al: Activation of adenosine A2A receptors by
polydeoxyribonucleotide increases vascular endothelial growth
factor and protects against testicular damage induced by
experimental varicocele in rats. Fertil Steril. 95:1510–1513. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Konturek JW, Brzozowski T and Konturek SJ:
Epidermal growth factor in protection, repair, and healing of
gastroduodenal mucosa. J Clin Gastroenterol. 13 (Suppl 1):S88–S97.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mustoe TA, O'Shaughnessy K and Kloeters O:
Chronic wound pathogenesis and current treatment strategies: A
unifying hypothesis. Plast Reconstr Surg. 117 (Suppl 7):35S–41S.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Syam AF, Sadikin M, Wanandi SI and Rani
AA: Molecular mechanism on healing process of peptic ulcer. Acta
Med Indones. 41:95–98. 2009.PubMed/NCBI
|
|
75
|
Tarnawski A, Tanoue K, Santos AM and
Sarfeh IJ: Cellular and molecular mechanisms of gastric ulcer
healing. Is the quality of mucosal scar affected by treatment?
Scand J Gastroenterol Suppl. 210:9–14. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tarnawski AS: Cellular and molecular
mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 50
(Suppl 1):S24–S33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Brzozowski T, Konturek PC, Konturek SJ,
Schuppan D, Drozdowicz D, Kwiecień S, Majka J, Nakamura T and Hahn
E: Effect of local application of growth factors on gastric ulcer
healing and mucosal expression of cyclooxygenase-1 and −2.
Digestion. 64:15–29. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wong WM, Playford RJ and Wright NA:
Peptide gene expression in gastrointestinal mucosal ulceration:
Ordered sequence or redundancy? Gut. 46:286–292. 2000. View Article : Google Scholar : PubMed/NCBI
|