Introduction
Colorectal cancer (CRC) is one of the main causes of
cancer-related mortality worldwide (1). In the past decades, treatment
strategies for CRC have significantly improved (2,3).
However, the fatality and survival rates of patients with CRC
remain unsatisfactory (4). In 2018,
the incidence of CRC reached 1.8 million new cases and ~861,000
CRC-related mortalities globally (5). Various factors complicate the
pathogenesis of CRC, especially gene mutagenesis events induced by
inherited and environmental factors, which contribute to the
proliferation, apoptosis and differentiation of CRC (6). Thus, numerous studies have focused on
molecular therapy in the treatment of CRC (7,8).
Collectively, identifying the potential molecular mechanisms
involved in the progression of CRC is of great importance.
Long non-coding RNAs (lncRNAs) are non-coding RNAs
with a length of ~200 nucleotides (9). lncRNAs, which lack an open reading
frame and therefore are unable to encode proteins, are involved in
biological processes, including proliferation and apoptosis, by
regulating epigenetic changes and gene transcription (10). Accumulating evidence has shown that
lncRNAs serve a crucial role in the development of numerous
diseases, particularly in the occurrence and progression of cancer
(11–14). lncRNA plasmacytoma variant
translocation 1 (PVT1) is located near Myc at human chromosome 8q24
(15). Yu et al (16) reported that PVT1 functions as an
oncogene in human CRC via the microRNA (miRNA/miR)-30d-5p/RUNX
family transcription factor 2 axis. Moreover, PVT1 facilitates CRC
progression by targeting miR-26b (17). It has also been shown that PVT1
could affect the prognosis of CRC by regulating cyclooxygenase-2
expression (18). The upregulation
of PVT1 in cancer predicts poor prognosis (19–22).
PVT1 acts as an oncogene in various cancer types, including CRC,
and regulates the biological progression of cancer (23,24).
In CRC, the upregulation of PVT1 predicts advance stage and poor
prognosis, as well as induces chemoresistance (15,25).
However, the possible molecular mechanisms of PVT1 involved in the
progression of CRC are yet to be fully elucidated.
miRNAs are one-stranded short endogenous RNAs
(26) that are involved in the
modulation of gene transcription by binding to the 3′ untranslated
region (3′UTR) of target genes (27). Increasing evidence has indicated
that miRNAs are abnormally expressed in CRC, which suggests that
miRNAs are involved in the progression and development of CRC
(28–31). Therefore, verifying the association
between lncRNAs and miRNAs in CRC may be beneficial for the
prevention, diagnosis and therapy of CRC.
The present study aimed to investigate the
functional roles and underlying mechanisms of PVT1 in CRC, which
may provide a novel molecular mechanism that is associated with the
pathology of CRC and offer a new direction for treating CRC.
Materials and methods
Tissue samples
In total, 30 pairs of CRC tissue samples and
adjacent normal tissues (13 female patients and 17 male patients;
age: 46±8 years) were collected from patients with CRC at The
Second Affiliated Hospital of Soochow University (Suzhou, China)
between January 2017 and August 2018. Adjacent normal tissues were
collected from >2 cm from the tumor margin. The major inclusion
criteria were as follows: i) All patients were reviewed by a
pathologist and histologically confirmed as CRC, based on
histopathological evaluation and without other malignancies; ii)
patients who had not received any preoperative chemotherapy or
radiotherapy treatment; and iii) patients with complete clinical
data, including age, sex, ethnicity, tumor size and local invasion.
The exclusion criteria were as follows: i) Patients with other
types of malignant tumors; and ii) patients who were diagnosed with
malignant lymphoma, and poorly differentiated and anaplastic
carcinomas. This study was approved by the Ethics Committee of The
Second Affiliated Hospital of Soochow University (approval no. JS
sz-358). All patients provided written informed consent.
Cell treatment
The CRC cell lines, DLD-1, HT-29, SW480 and SW620,
and the normal colonic mucosa cell line FHC were purchased from the
American Type Culture Collection. Cells were incubated in RPMI-1640
medium (Thermo Fisher Scientific, Inc.) containing 10% FBS (Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Sigma-Aldrich; Merck KGaA) at 37°C with 5% CO2.
Cell transfection
For transfection, SW480 cells (1×104
cells/well) were plated in a 6-well plate. After adhering for 24 h,
miR-negative control (NC) mimics (50 nM;
5′-UUCUCCGAACGUGUCACGUTT-3′), miR-761 mimics (50 nM;
5′-UUAAUGCUAAUUGUGAUAGGGGU-3′), miR-NC inhibitor (50 nM;
5′-CAGUACUUUUGUGUAGUACAA-3′), miR-761 inhibitor (50 nM;
5′-ACCCCUAUCACGAUUAGCAUUAA-3′), MAPK1 overexpression plasmids
(pcDNA3.1 MAPK1; 50 nM) and its NC (empty vector; 50 nM), which
were provided by Shanghai GenePharma Co., Ltd., were added to the
medium for 6 h at 37°C in a CO2 incubator. The small
interfering RNA (siRNA; 50 nM; 5′-GAGCUGCGAGCAAAGAUGU-3′) targeting
PVT1 was provided by Guangzhou RiboBio Co., Ltd. Non-target
scramble controls (50 nM; 5′-UUCUCCGAACGUGUCACGU-3′) (Guangzhou
RiboBio Co., Ltd.) served as the NC. Cell transfection was
performed for 48 h using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. At 24 h post-transfection, subsequent
experiments were performed.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA were collected from tissues or cell lines
(DLD-1, HT-29, SW480, SW620 and FHC) using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. RNA was reverse transcribed into cDNA
using a RevertAid First Strand cDNA Synthesis Kit (Fermentas;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. qPCR was conducted with a SYBR Premix Ex Taq II kit
(Takara Bio, Inc.) under the following conditions: Initial
denaturation at 95°C for 6 min, followed by 40 cycles at 94°C for
30 sec, 60°C for 30 sec and 73°C for 90 sec. U6 and GAPDH were
utilized as the loading controls for miRNA and mRNA, respectively.
The relative mRNA expression levels were calculated using the
2−ΔΔCq method (32). The
primer sequences were as follows: PVT1 forward,
5′-TTGGCACATACAGCCATCAT-3′ and reverse, 5′-GCAGTAAAAGGGGAACACCA-3′;
miR-761 forward, 5′-GCAGCAGGGTGAAACTGAA-3′ and reverse,
5′-GAACATGTCTGCGTATCTC-3′; MAPK1 forward,
5′-CAGTTCTTGACCCCTGGTCC-3′ and reverse, 5′-TACATACTGCCGCAGGTCAC-3′;
GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′; and U6 forward,
5′-CTCGCTTCGGCAGCAGCACATATA-3′ and reverse,
5′-AAATATGGAACGCTTCACGA-3′.
Western blot analysis
A total of 30 µg proteins were isolated from tissues
and SW480 cells using RIPA lysis buffer containing protease
inhibitors (Beyotime Institute of Biotechnology), and the
concentration was determined using a BCA kit. Then, equal amounts
of protein (20 µg) were isolated via SDS-PAGE on a 10% gel, and the
protein was subsequently transferred onto PVDF membranes.
Subsequently, the membranes were blocked with TBS with 0.05%
Tween-20 buffer containing 5% skimmed milk for 1 h at room
temperature. The membranes were incubated with primary antibodies
overnight at 4°C and then incubated with HRP-conjugated secondary
antibodies (cat. no. ab7090; 1:1,000; Abcam) at 37°C for 2 h. The
protein expression levels were determined with an ECL kit (Thermo
Fisher Scientific, Inc.) and semi-quantified with ImageJ version
1.6 software (National Institutes of Health). The specific primary
antibodies used were as follows: Anti-BAX (cat. no. ab182734;
1:1,000; Abcam), anti-Bcl-2 (cat. no. ab32124; 1:1,000; Abcam),
anti-Cleaved caspase-3 (cat. no. ab49822; 1:1,000; Abcam),
anti-Caspase-3 (cat. no. ab13847; 1:1,000; Abcam), anti-MAPK1 (cat.
no. 4695; 1:1,000; Cell Signaling Technology, Inc.) and
anti-β-actin (cat. no. ab8227; 1:1,000; Abcam).
5-Ethynyl-2′-deoxyuridine (EdU)
assay
SW480 cells (1×104) were fixed with 4%
paraformaldehyde for 30 min at room temperature and permeabilized,
and then cell proliferation was determined with an EdU assay kit
(cat. no. C10310; Guangzhou RiboBio Co., Ltd.). After EdU staining,
nuclei were stained with DAPI for 20 min at 37°C (Beyotime
Institute of Biotechnology). EdU-positive cells were examined by
fluorescence microscopy (BX51 microscope; Olympus Corporation) in
five randomly chosen fields.
Cell counting kit-8 (CCK-8)
analysis
SW480 cells (1×104 cells/well) were
seeded in 96-well plates and incubated with 10 µl CCK-8 reagent
(cat. no. CA1210; Beijing Solarbio Science & Technology Co.,
Ltd.), and then incubated in the dark for 2 h at 37°C. Absorbance
at 450 nm was measured at 0, 24, 48 and 72 h using a microplate
reader. All experiments were performed in triplicate.
Flow cytometry analysis
SW480 cells were collected and centrifuged at 1,000
× g for 6 min at 4°C. The apoptosis (early + late) was detected by
an Annexin V-FITC/PI apoptosis detection kit (Nanjing KeyGen
Biotech Co., Ltd.). Next, cells were stained with 5 µl Annexin
V-FITC and 10 µl PI for 20 min in the dark. The apoptotic rates
were calculated using a a FACScan flow cytometer (BD Biosciences)
and Flow cytometry data were analyzed using FlowJo version 10.0
software (FlowJo LLC).
Bioinformatic analyses
Bioinformatics database (http://starbase.sysu.edu.cn/; Starbase version 2.0)
was used to predict the target of PVT1.
Dual-luciferase reporter assay
The sequences containing the MAPK1 3′untranslated
region (UTR) or PVT1 3′UTR wild-type (WT) and mutant (MUT), which
was generated by site-directed mutagenesis, binding sites on
miR-761 were synthesized and inserted into pmiR-RB-REPORT™ plasmids
(Guangzhou RiboBio Co., Ltd.). Then, luciferase reporter plasmids
were co-transfected with miR-761 mimics and miR-NC mimics using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h. After 24 h, the relative luciferase
activity was calculated using a Dual-Luciferase Reporter Assay
System (Promega Corporation). Luciferase activities were normalized
to Renilla luciferase activities.
Statistical analysis
The experiments were performed in triplicate. The
data were evaluated using GraphPad version 7.0 software (GraphPad
Software, Inc.) and presented as the mean ± SD. A paired Student's
t-test was carried out to evaluate significant differences between
two groups. The differences among multiple groups were analyzed
using a one-way ANOVA, followed by a Tukey's post hoc test. The
Pearson's method was used for correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Downregulated expression levels of
PVT1 suppress the progression of CRC
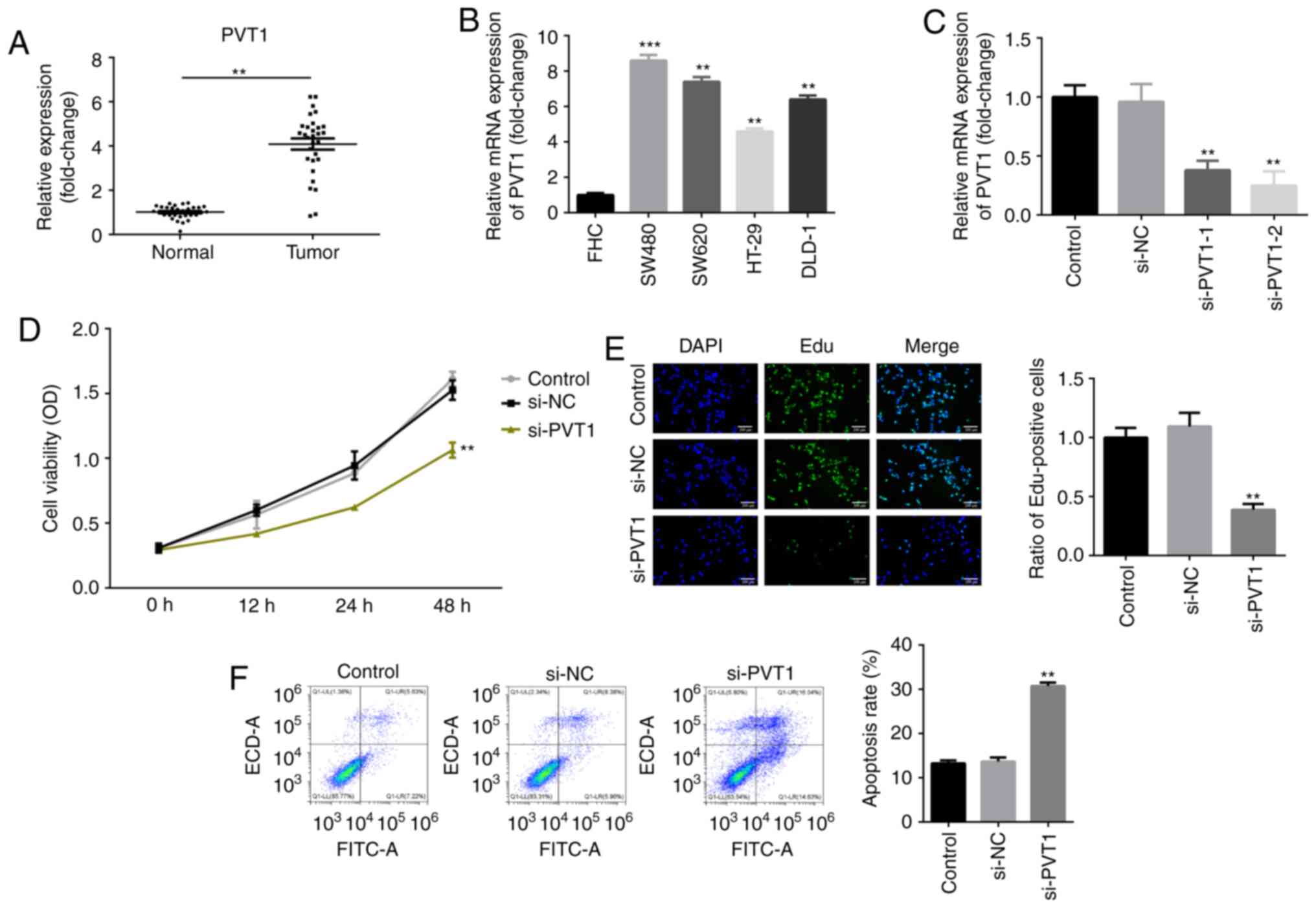
RT-qPCR analysis was performed to examine the
expression levels of PVT1 in CRC tissues. As presented in Fig. 1A, the mRNA expression of PVT1 was
significantly upregulated in CRC tissues. RT-qPCR analysis was also
performed to examine the expression levels of PVT1 in various CRC
cell lines. Similarly, it was found that PVT1 expression was
upregulated in CRC cell lines compared with the normal colonic
mucosa cell line FHC (Fig. 1B).
Moreover, the expression of PVT1 in SW480 cells was higher compared
with that in DLD-1, HT-29 and SW620. Therefore, SW480 cells were
used in the subsequent experiments (Fig. 1B).
It was identified that the expression of PVT1 was
significantly decreased after knockdown of PVT1, which was more
efficient in the si-PVT1-2 group (Fig.
1C). Thus, si-PVT1-2 was used in the following experiments.
CCK-8 assay results indicated that cell viability was significantly
decreased in si-PVT1-transfected cells compared with the controls
(Fig. 1D). Similarly, the results
of the EdU assay demonstrated that the number of EdU-positive cells
was significantly decreased following inhibition of PVT1 in SW480
cells (Fig. 1E). Additionally,
knockdown of PVT1 significantly increased the apoptotic rate of
SW480 CRC cells (Fig. 1F) in
comparison with the control group.
PVT1 acts as a competing endogenous
(ce)RNA by binding to miR-761
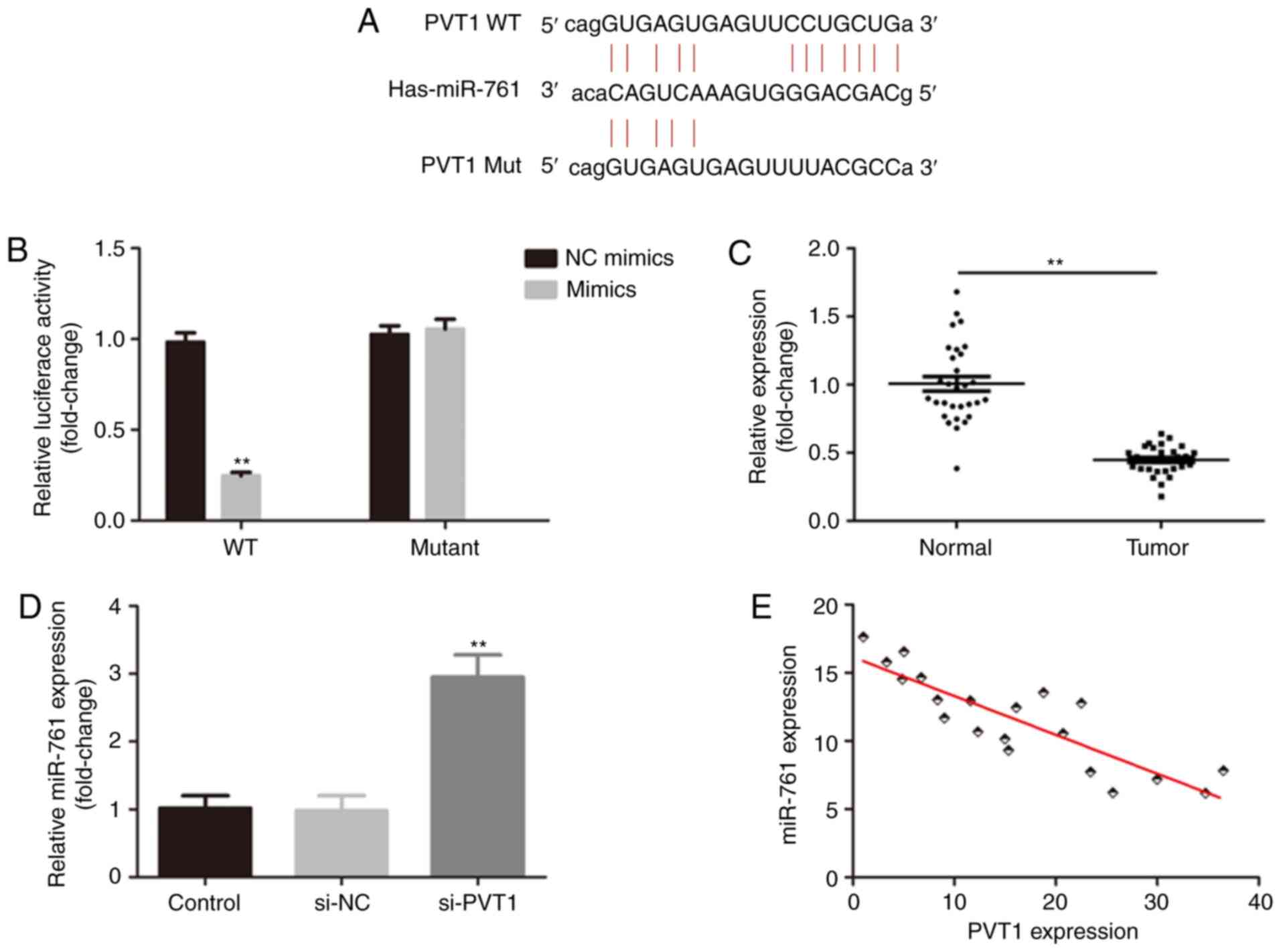
Accumulating evidence has revealed that lncRNAs
function as ceRNAs to regulate biological processes by sponging
miRNAs (33,34). The online bioinformatics database
(Starbase version 2.0) predicted that miR-761 was a target of PVT1
(Fig. 2A). The relative luciferase
activity of CRC cells transfected with PVT1 3′UTR WT and miR-761
mimics was significantly decreased (Fig. 2B). Moreover, miR-761 expression was
decreased in CRC tissues (Fig. 2C).
It was found that knockdown of PVT1 increased the expression of
miR-761 (Fig. 2D), and PVT1 was
negatively correlated with miR-761 (r=0.75; Fig. 2E).
PVT1 regulates the progression of CRC
by binding to miR-761
The significant increase of PVT1 expression in CRC
prompted the investigation into the possible biological
significance of PVT1 in CRC tumorigenesis. As presented in Fig. 3A, the expression of miR-761 was
significantly increased by miR-761 mimics and decreased by miR-761
inhibitor. Furthermore, the CCK-8 results demonstrated that
knockdown of PVT1 suppressed the viability of CRC cells, which was
more potent in the si-PVT1 + miR-761 mimic group (Fig. 3B). An EdU assay was conducted to
further confirm the CCK-8 results, and it was found that the
proliferative activity was inhibited by knockdown of PVT1 in the
SW480 cells, while miR-761 overexpression partially enhanced the
action of PVT1 knockdown (Fig. 3C).
By contrast, knockdown of PVT1 increased the apoptosis of SW480
cells, which was more obvious in the si-PVT1 + miR-761 mimic group
(Fig. 3D). Additionally, si-PVT1 +
miR-761 mimics transfection was more efficient in upregulating BAX
and cleaved caspase-3 expression levels and downregulating Bcl-2
expression compared with the si-PVT1 group (Fig. 3E).
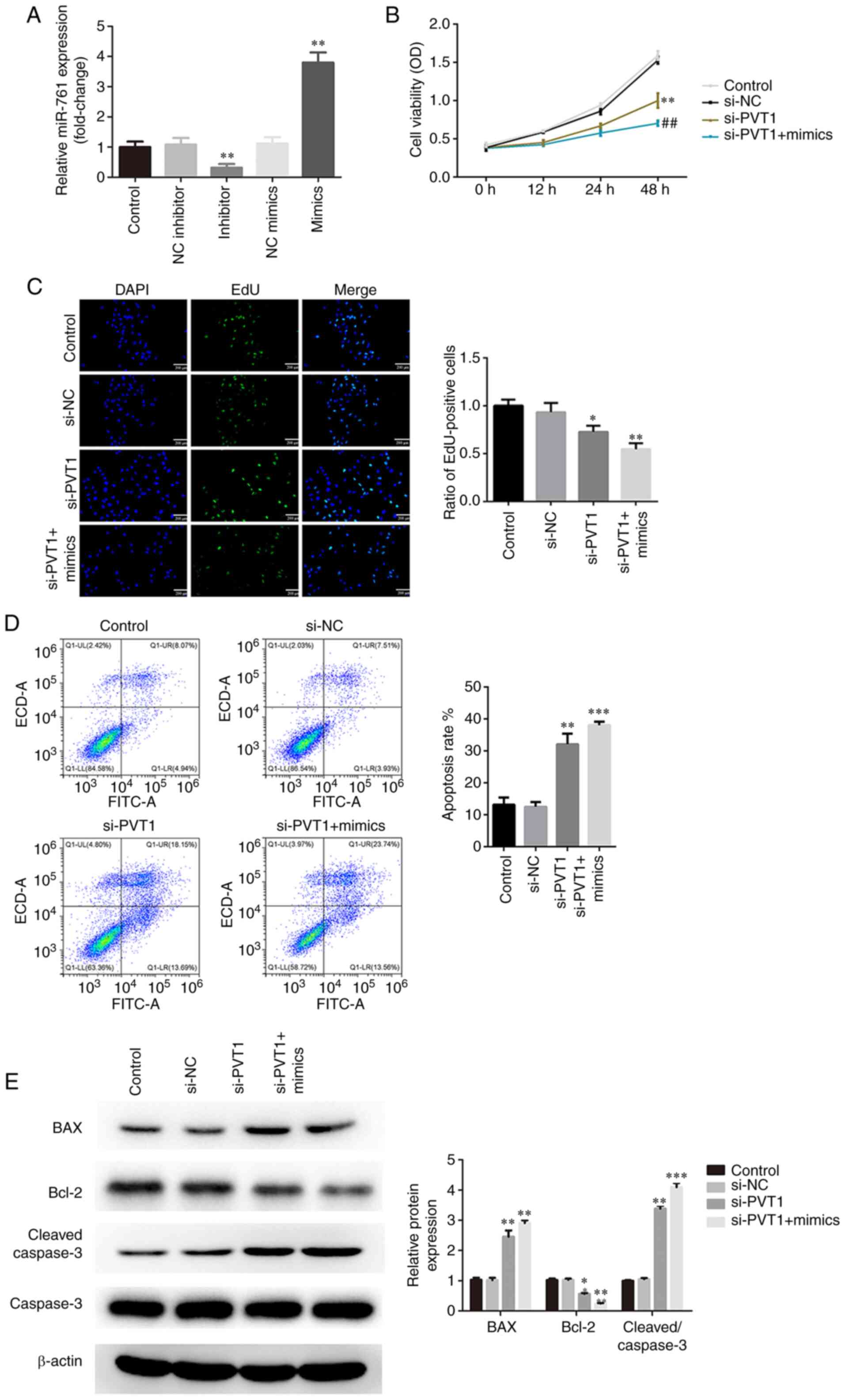 | Figure 3.PVT1 regulates the proliferation and
apoptosis of CRC cells by binding to miR-761. (A) Expression levels
of miR-761 were evaluated after cells were transfected with
inhibitor or mimics. si-PVT1 + miR-761 mimics had a greater effect
on inhibiting the viability and proliferation of CRC cells, as
determined via (B) Cell Counting Kit-8 and (C) EdU assays
(magnification ×200). **P<0.01 vs. NC inhibitor or NC mimics.
(D) si-PVT1 + miR-761 mimics had a greater effect in promoting the
apoptosis of CRC cells, as measured via flow cytometry. (E) si-PVT1
+ miR-761 mimics were more efficient in regulating the expression
levels of Bcl-2/BAX and caspase-3, as detected via western
blotting. *P<0.05, **P<0.01, ***P<0.001 vs. si-NC;
##P<0.01 vs. si-PVT1. PVT1, plasmacytoma variant
translocation 1; miR, microRNA; CRC, colorectal cancer; si-, small
interfering RNA; EdU, 5-Ethynyl-2′-deoxyuridine; NC, negative
control. |
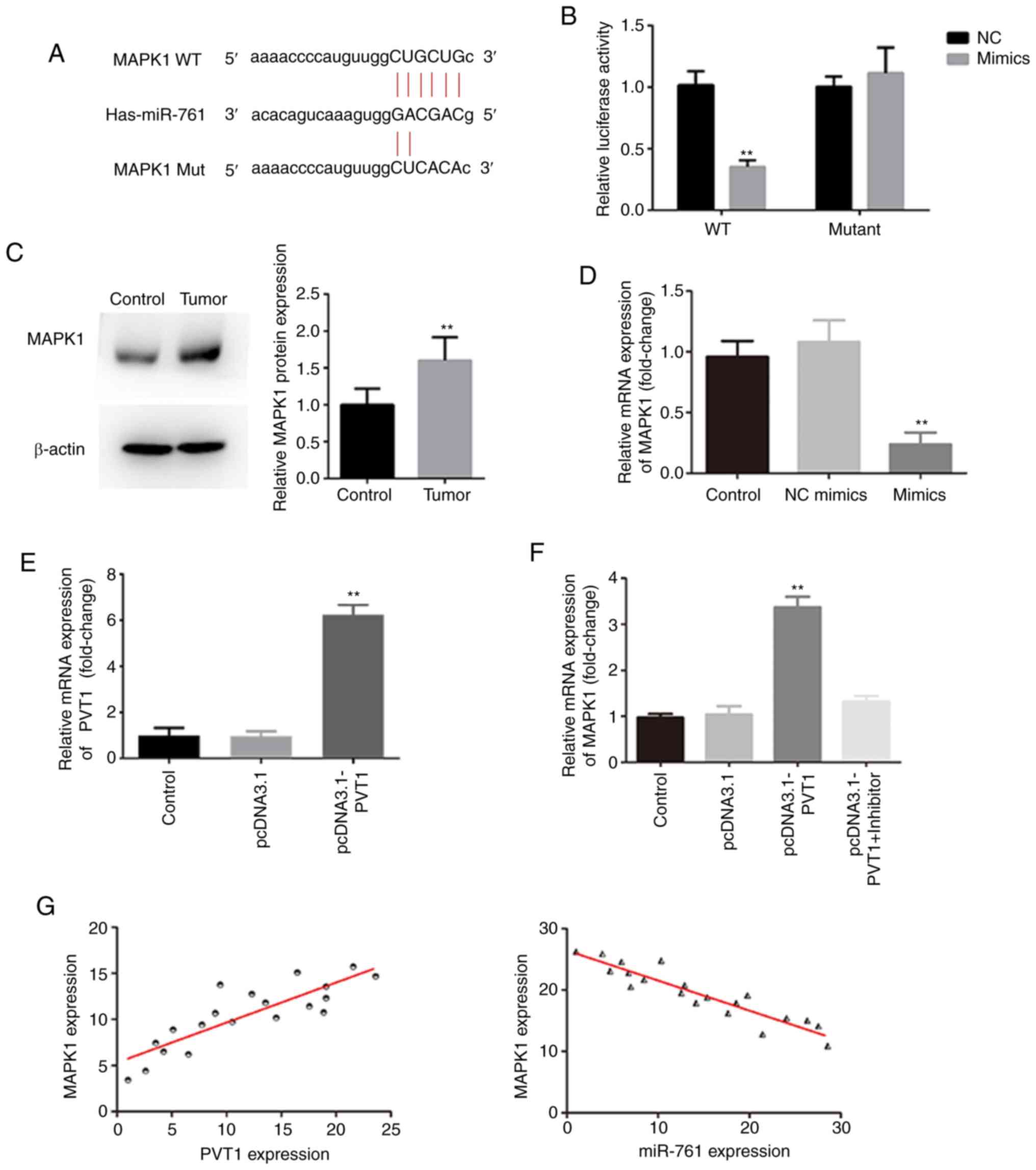
PVT1 regulates MAPK1 by sponging
miR-761
The binding site of MAPK1 on miR-761 is shown in
Fig. 4A. A dual-luciferase assay
demonstrated that co-transfection of miR-761 mimics and MAPK1 3′UTR
WT significantly decreased cell luciferase activity (Fig. 4B). MAPK1 was highly expressed in CRC
tissues (Fig. 4C), and MAPK1
expression was significantly decreased by miR-761 mimics (Fig. 4D). Overexpression of PVT1 following
transfection with the pcDNA3.1 PVT1 was confirmed, as presented in
Fig. 4E. Furthermore, the
expression of MAPK1 was upregulated by PVT1 overexpression, and was
restored to normal levels by miR-761 mimics, suggesting that PVT1
regulated the expression of MAPK1 via miR-761 (Fig. 4F). It was also identified that the
expression of MAPK1 was negatively correlated with miR-761 (r=0.86)
and positively correlated with PVT1 (r=0.72) (Fig. 4G).
Knockdown of PVT1 suppress the
progression of CRC via the miR-761/MAPK1 axis
To further identify whether PVT1 exerted its role in
CRC via the miR-761/MAPK1 axis, additional experiments were
performed. Overexpression of MAPK1 following transfection with the
pcDNA3.1 MAPK1 was confirmed, as presented in Fig. 5A. First, CCK-8 and EdU assays
revealed that cell viability and proliferation were significantly
inhibited by knockdown of PVT1, and was partially restored
following the overexpression of MAPK1 (Fig. 5B and C). Moreover, flow cytometric
analysis results demonstrated that knockdown of PVT1 significantly
increased the apoptotic rate of CRC cells, which was partially
alleviated by MAPK1 overexpression (Fig. 5D). It was also found that the
increase of Bax and cleaved caspase-3 expression levels and the
decrease of Bcl-2 expression induced by si-PVT1 were partially
alleviated after transfection with pcDNA3.1-MAPK1 (Fig. 5E).
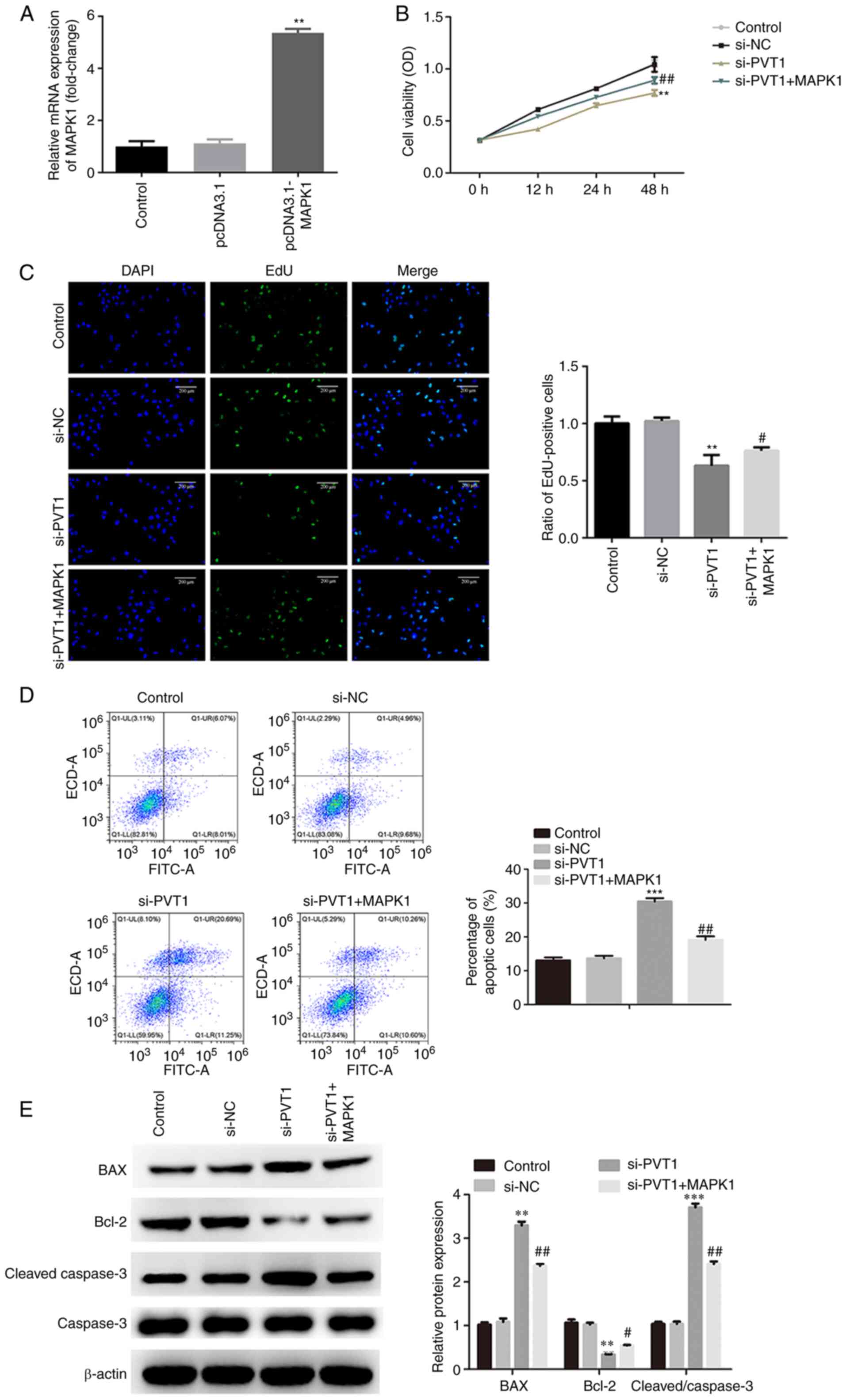 | Figure 5.PVT1 regulates the proliferation and
apoptosis of CRC cells via the miR-761/MAPK1 axis. (A) mRNA
expression levels of MAPK1 following transfection with
pcDNA3.1-MAPK1 were determined via reverse
transcription-quantitative PCR. MAPK1 overexpression reversed the
inhibitory effects of cell viability and proliferation induced by
si-PVT1, as determined via (B) Cell Counting Kit-8 and (C) EdU
assays (magnification, ×200). (D) MAPK1 overexpression alleviated
the apoptosis of CRC cells induced by si-PVT1, as measured via flow
cytometry. (E) MAPK1 overexpression reversed the effects of si-PVT1
on the expression levels of Bcl-2/BAX and caspase-3, as detected
via western blotting. **P<0.01, ***P<0.001 vs. control;
#P<0.05, ##P<0.01 vs. si-PVT1. PVT1,
plasmacytoma variant translocation 1; miR, microRNA; si-, small
interfering RNA; CRC, colorectal cancer; EdU,
5-Ethynyl-2′-deoxyuridine; NC, negative control. |
Discussion
Accumulating evidence has revealed the presence of
dysregulated lncRNAs in CRC, including SLC25A25 antisense RNA 1,
AFAP1 antisense RNA 1, colorectal neoplasia differentially
expressed and growth arrest specific 5 (19–22).
The aberrant expression of lncRNAs participates in the
tumorigenesis and progression of CRC by regulating autophagy,
proliferation, migration, invasion, metastasis and other biological
processes (9–12,35).
Therefore, investigating the possible roles of lncRNAs in CRC is
crucial to identify a therapeutic strategy for CRC. Previous
studies have reported that abnormally expressed PVT1 contributes to
the initiation and development of CRC, and predicts poor prognosis
(15). For example, Wu et al
(36) found that PVT1 promotes CRC
tumorigenesis via a miR-16-5p/VEGFA/VEGFR1/AKT axis. In addition,
knockdown of PVT1 could suppress CRC progression by regulating the
miR-106b-5p/four-jointed box kinase 1 axis (37). In the present study, PVT1 was
upregulated in CRC tissues and cells. Furthermore, PVT1 knockdown
suppressed the progression of CRC. These results suggested that
PVT1 may be an oncogene in CRC, which was consistent with a study
by Ping et al (15).
However, the underlying mechanisms via which PVT1 modulates the
tumorigenic processes in CRC have not been fully elucidated.
lncRNAs function as ceRNAs by sponging miRNAs to
participate in carcinogenesis, including in CRC (1–4,38).
PVT1, located in a cancer-associated region-8q24, acts as an
oncogene by promoting the proliferation and invasion of cervical
cancer via binding to miR-140-5p, and knockdown of PVT1 combined
with the overexpression of miR-214 inhibits hepatocarcinogenesis
(39,40). A previous study also revealed that
overexpression of PVT1 induced the proliferation of CRC by
regulating miR-216a-5p (41).
Moreover, bioinformatics analyses have identified that PVT1 binds
to various miRNAs, including miR-186-5p, miR-16-5p and miR-761.
Therefore, PVT1 may regulate the initiation and development of CRC
by binding to other miRNAs.
Abnormally expressed miRNAs act as oncogenes or
antitumor genes in cancer and regulate cell proliferation,
differentiation, apoptosis and the epithelial-mesenchymal phenotype
(42). miR-761 is downregulated in
CRC, while its overexpression suppresses the progression of CRC and
enhances chemosensitivity (43).
These results suggested that miR-761 may function as an antitumor
gene in CRC (44). In the present
study, the expression of miR-761 was negatively correlated with
PVT1. Moreover, the overexpression of miR-761 facilitated the
effects of PVT1 knockdown on the progression of CRC. The regulatory
role of si-PVT1 in regulating Bcl-2/BAX and caspase-3 was promoted
by the overexpression of miR-761. These results indicated that PVT1
regulated the progression of CRC via miR-761. Therefore, the
PVT1/miR-761 axis may be a promising biomarker for CRC. However,
the underlying mechanisms remain unknown.
The MAPK signaling cascade is prominently conserved
in evolution (45). MAPK1, located
in the cytoplasm, is often activated or upregulated in tumors
(43,44). Accumulating evidence has verified
that the upregulation of MAPK1 contributes to tumorigenesis
(46–48). In CRC, the activation of MAPK1
induces the proliferation and inhibits the apoptosis of CRC cells
(49). The present study identified
a novel upstream mechanism of MAPK1. PVT1 functioned as a ceRNA to
regulate MAPK1 by sponging miR-761. MAPK1 was predicted and shown
to be a target of miR-761. Furthermore, the expression of MAPK1 was
negatively correlated with miR-761, while it was positively
correlated with PVT1. Overexpression of MAPK1 has been demonstrated
to alleviate the effects of PVT1 knockdown on the proliferation and
apoptosis of CRC cells, as well as the regulatory role of
apoptosis-related genes, such as Bcl-2/BAX and caspase-3 (45,46).
Thus, the PVT1/miR-761/MAPK1 axis may serve a crucial role in the
progression of CRC.
The main limitation of the present study was the
small sample size of the patients and lack of an animal model,
which could make the results more convincing. Therefore, an
increased number of patients and animal model will be included in a
future study.
In conclusion, the present study demonstrated that
PVT1 was upregulated in CRC tissues. Knockdown of PVT1 inhibited
the proliferation and triggered the apoptosis of CRC cells by
functioning as a ceRNA towards miR-761 and subsequently
downregulating MAPK1 expression. The present findings may provide a
promising diagnostic biomarker and therapeutic strategy for
CRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW designed the experiments. YL and WD were the
major contributors in writing the manuscript and performed the
experiments. YL and WD confirm the authenticity of all the raw
data. ZZ and JG performed the experiments and analyzed data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Second Affiliated Hospital of Soochow University (Suzhou,
China; approval no. JS sz-358). All patients provided informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lian J, Zhang H, Wei F, Li Q, Lu Y, Yu B,
Yu L, Liang X, Wen Y, Jin K, et al: Long non-coding RNA DANCR
promotes colorectal tumor growth by binding to lysine
acetyltransferase 6A. Cell Signal. 67:1095022020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang Y, Sun H, Ma X, Zeng Y, Pan Y, Yu D,
Liu Z and Xiang Y: HLA-F-AS1/miR-330-3p/PFN1 axis promotes
colorectal cancer progression. Life Sci. 254:1171802020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Cho KB, Li Y, Tao G, Xie Z and Guo
B: Long noncoding RNA (lncRNA)-mediated competing endogenous RNA
networks provide novel potential biomarkers and therapeutic targets
for colorectal cancer. Int J Mol Sci. 20:57582019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang Y, Liu G, Ye W, Xie J, Shao C, Wang
X and Li X: ZEB2-AS1 accelerates epithelial/mesenchymal transition
through miR-1205/CRKL pathway in colorectal cancer. Cancer Biother
Radiopharm. 35:153–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng Y, Nie P and Xu S: Long noncoding
RNA CASC21 exerts an oncogenic role in colorectal cancer through
regulating miR-7-5p/YAP1 axis. Biomed Pharmacother. 121:1096282020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu F, Jia J, Sun L, Yu Q, Duan H, Jiao D,
Gong Z, Zhu S, Jiang K, He Y, et al: lncRNA DSCAM-AS1 downregulates
miR-216b to promote the migration and invasion of colorectal
adenocarcinoma cells. Onco Targets Ther. 12:6789–6795. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Ma S, Lin T, Li Y, Yang S, Zhang W,
Zhang R and Wang Y: Comprehensive analysis of therapy-related
messenger RNAs and long noncoding RNAs as novel biomarkers for
advanced colorectal cancer. Front Genet. 10:8032019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun W, Ren S, Li R, Zhang Q and Song H:
LncRNA, a novel target biomolecule, is involved in the progression
of colorectal cancer. Am J Cancer Res. 9:2515–2530. 2019.PubMed/NCBI
|
|
9
|
Bermúdez M, Aguilar-Medina M,
Lizárraga-Verdugo E, Avendaño-Félix M, Silva-Benítez E,
López-Camarillo C and Ramos-Payán R: LncRNAs as regulators of
autophagy and drug resistance in colorectal cancer. Front Oncol.
9:10082019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amirkhah R, Naderi-Meshkin H, Shah JS,
Dunne PD and Schmitz U: The intricate interplay between epigenetic
events, alternative splicing and noncoding RNA deregulation in
colorectal cancer. Cells. 8:9292019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang C, Zhao T, Li H, He F, Zhao X, Zhang
Y, Chu X, Hua C, Qu Y, Duan Y, et al: Long non-coding RNA ITIH4-AS1
accelerates the proliferation and metastasis of colorectal cancer
by activating JAK/STAT3 signaling. Mol Ther Nucleic Acids.
18:183–193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan Y, Sheng W, Meng Y, Cao Y and Li R:
LncRNA PTENP1 inhibits cervical cancer progression by suppressing
miR-106b. Artif Cells Nanomed Biotechnol. 48:393–407. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yue X, Wu WY, Dong M and Guo M: LncRNA
MALAT1 promotes breast cancer progression and doxorubicin
resistance via regulating miR-570-3p. Biomed J.
43:4982020.PubMed/NCBI
|
|
14
|
Ouyang J, Liu Z, Yuan X, Long C, Chen X,
Wang Y, Liu L, Liu S and Liang H: LncRNA PRNCR1 promotes breast
cancer proliferation and inhibits apoptosis by modulating
microRNA-377/CCND2/MEK/MAPK axis. Arch Med Res. 52:471–482. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ping G, Xiong W, Zhang L, Li Y, Zhang Y
and Zhao Y: Silencing long noncoding RNA PVT1 inhibits
tumorigenesis and cisplatin resistance of colorectal cancer. Am J
Transl Res. 10:138–149. 2018.PubMed/NCBI
|
|
16
|
Yu X, Zhao J and He Y: Long non-coding RNA
PVT1 functions as an oncogene in human colon cancer through
miR-30d-5p/RUNX2 axis. J BUON. 23:48–54. 2018.PubMed/NCBI
|
|
17
|
Zhang R, Li J, Yan X, Jin K, Li W, Liu X,
Zhao J, Shang W and Liu Y: Long noncoding RNA plasmacytoma variant
translocation 1 (PVT1) promotes colon cancer progression via
endogenous sponging miR-26b. Med Sci Monit. 24:8685–8692. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang W, Xiao J, Lu X, Liu T, Jin X, Xiao
Y and He X: PVT1 (rs13281615) and miR-146a (rs2910164)
polymorphisms affect the prognosis of colon cancer by regulating
COX2 expression and cell apoptosis. J Cell Physiol.
234:17538–17548. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Huang S, Li Y, Zhang W, He K, Zhao
M, Lin H, Li D, Zhang H, Zheng Z and Huang C: Decreased expression
of LncRNA SLC25A25-AS1 promotes proliferation, chemoresistance, and
EMT in colorectal cancer cells. Tumour Biol. 37:14205–14215. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang F, Ni H, Sun F, Li M and Chen L:
Overexpression of lncRNA AFAP1-AS1 correlates with poor prognosis
and promotes tumorigenesis in colorectal cancer. Biomed
Pharmacother. 81:152–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han P, Li JW, Zhang BM, Lv JC, Li YM, Gu
XY, Yu ZW, Jia YH, Bai XF, Li L, et al: The lncRNA CRNDE promotes
colorectal cancer cell proliferation and chemoresistance via
miR-181a-5p-mediated regulation of Wnt/β-catenin signaling. Mol
Cancer. 16:92017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei Y, Jing-Jing L, Ke-Nan Z, Qing-Zhong T
and Jin L: A tumor suppressive role of lncRNA GAS5 in human
colorectal cancer. Open Life Sci. 11:105–109. 2016. View Article : Google Scholar
|
|
23
|
Wang Y, Chen W, Lian J, Zhang H, Yu B,
Zhang M, Wei F, Wu J, Jiang J, Jia Y, et al: The lncRNA PVT1
regulates nasopharyngeal carcinoma cell proliferation via
activating the KAT2A acetyltransferase and stabilizing HIF-1α. Cell
Death Differ. 27:695–710. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan H, Zhu JH and Yao XQ: Knockdown of
long non-coding RNA PVT1 reverses multidrug resistance in
colorectal cancer cells. Mol Med Rep. 17:8309–8315. 2018.PubMed/NCBI
|
|
25
|
Gharib E, Anaraki F, Baghdar K, Ghavidel
P, Sadeghi H, Nasrabadi PN, Peyravian N, Aghdaei HA, Zali MR and
Mojarad EN: Investigating the diagnostic performance of HOTTIP,
PVT1, and UCA1 long noncoding RNAs as a predictive panel for the
screening of colorectal cancer patients with lymph node metastasis.
J Cell Biochem. 120:14780–14790. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Zhang S, Yin J and Xu R: MiR-566
mediates cell migration and invasion in colon cancer cells by
direct targeting of PSKH1. Cancer Cell Int. 19:3332019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan L, Zhang Y, Li K, Wang M, Li J, Qi Z,
Wu J, Wang Z, Ling L, Liu H, et al: miR-593-5p inhibit cell
proliferation by targeting PLK1 in non small cell lung cancer
cells. Pathol Res Pract. 216:1527862020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mou TY, Zhang RR and Wang YN: MiRNA-212
acts as a tumor-suppressor in colorectal carcinoma through
targeting SOX4. Eur Rev Med Pharmacol Sci. 23:10751–10760.
2019.PubMed/NCBI
|
|
29
|
Zhang D, Li Y and Sun P: miR-770-5p
modulates resistance to methotrexate in human colorectal
adenocarcinoma cells by downregulating HIPK1. Exp Ther Med.
19:339–346. 2020.PubMed/NCBI
|
|
30
|
Zhao Q, Liu C, Cui Q, Luan X, Wang Q and
Zhou C: miR-190b promotes colorectal cancer progression through
targeting forkhead box protein P2. Exp Ther Med. 19:79–84.
2020.PubMed/NCBI
|
|
31
|
Li H, Zhu G, Xing Y, Zhu Y and Piao D:
miR-4324 functions as a tumor suppressor in colorectal cancer by
targeting HOXB2. J Int Med Res. Dec 18–2019.(Epub ahead of print).
doi: 10.1177/0300060519883731.
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang M, Zheng S, Li X, Ding Y, Zhang M,
Lin L, Xu H, Cheng Y, Zhang X, Xu H and Li S: Integrated analysis
of lncRNA-miRNA-mRNA ceRNA network identified lncRNA EPB41L4A-AS1
as a potential biomarker in non-small cell lung cancer. Front
Genet. 11:5116762020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xue C, Zhang X, Gao P, Cui X, Zhu C and
Qin X: LncRNA loc339803 acts as CeRNA of miR-30a-5p to promote the
migration and invasion of hepatocellular carcinoma cells. J Cancer.
12:1061–1072. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ni X, Xie JK, Wang H and Song HR:
Knockdown of long non-coding RNA LINC00324 inhibits proliferation,
migration and invasion of colorectal cancer cell via targeting
miR-214-3p. Eur Rev Med Pharmacol Sci. 23:10740–10750.
2019.PubMed/NCBI
|
|
36
|
Wu H, Wei M, Jiang X, Tan J, Xu W, Fan X,
Zhang R, Ding C, Zhao F, Shao X, et al: lncRNA PVT1 promotes
tumorigenesis of colorectal cancer by stabilizing miR-16-5p and
interacting with the VEGFA/VEGFR1/AKT axis. Mol Ther Nucleic Acids.
20:438–450. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu F, Wu R, Guan L and Tang X: Knockdown
of PVT1 suppresses colorectal cancer progression by regulating
MiR-106b-5p/FJX1 axis. Cancer Manag Res. 12:8773–8785. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li S, Wu T, Zhang D, Sun X and Zhang X:
The long non-coding RNA HCG18 promotes the growth and invasion of
colorectal cancer cells through sponging miR-1271 and upregulating
MTDH/Wnt/β-catenin. Clin Exp Pharmacol Physiol. 47:703–712. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chang QQ, Chen CY, Chen Z and Chang S:
LncRNA PVT1 promotes proliferation and invasion through enhancing
Smad3 expression by sponging miR-140-5p in cervical cancer. Radiol
Oncol. 53:443–452. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiong X, Yuan J, Zhang N, Zheng Y, Liu J
and Yang M: Silencing of lncRNA PVT1 by miR-214 inhibits the
oncogenic GDF15 signaling and suppresses hepatocarcinogenesis.
Biochem Biophys Res Commun. 521:478–484. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zeng X, Liu Y, Zhu H, Chen D and Hu W:
Downregulation of miR-216a-5p by long noncoding RNA PVT1 suppresses
colorectal cancer progression via modulation of YBX1 expression.
Cancer Manag Res. 11:6981–6993. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aravindan N, Subramanian K, Somasundaram
DB, Herman TS and Aravindan S: MicroRNAs in neuroblastoma
tumorigenesis, therapy resistance, and disease evolution. Cancer
Drug Resist. 2:1086–1105. 2019.PubMed/NCBI
|
|
43
|
Ren Y, Shi G, Jiang P and Meng Q:
MicroRNA-761 is downregulated in colorectal cancer and regulates
tumor progression by targeting Rab3D. Exp Ther Med. 17:1841–1846.
2019.PubMed/NCBI
|
|
44
|
Cao S, Lin L, Xia X and Wu H: MicroRNA-761
promotes the sensitivity of colorectal cancer cells to
5-fluorouracil through targeting FOXM1. Oncotarget. 9:321–331.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang J, Xiao T and Zhao M: MicroRNA-675
directly targets MAPK1 to suppress the oncogenicity of papillary
thyroid cancer and is sponged by long non-coding RNA RMRP. Onco
Targets Ther. 12:7307–7321. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu L, Yang S and Wang J: miR-217 inhibits
the migration and invasion of HeLa cells through modulating MAPK1.
Int J Mol Med. 44:1824–1832. 2019.PubMed/NCBI
|
|
47
|
Zabihula B, Yiliyasi M, Lu Y and Salai A:
MicroRNA-490-3p inhibits proliferation and stimulates apoptosis of
ESCC cells via MAPK1 downregulation. Oncol Lett. 18:3170–3176.
2019.PubMed/NCBI
|
|
48
|
Anand V, Khandelwal M, Appunni S, Gupta N,
Seth A, Singh P, Mathur S and Sharma A: CD44 splice variant
(CD44v3) promotes progression of urothelial carcinoma of bladder
through Akt/ERK/STAT3 pathways: Novel therapeutic approach. J
Cancer Res Clin Oncol. 145:2649–2661. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wei WT, Nian XX, Wang SY, Jiao HL, Wang
YX, Xiao ZY, Yang RW, Ding YQ, Ye YP and Liao WT: miR-422a inhibits
cell proliferation in colorectal cancer by targeting AKT1 and
MAPK1. Cancer Cell Int. 17:912017. View Article : Google Scholar : PubMed/NCBI
|