Introduction
Renal cell carcinoma (RCC) is one of the most common
types of malignant cancer of the urinary system, accounting for
~2–3% of adult malignant tumors (1). The early diagnosis of RCC is difficult
and the risk of cancer metastasis is high due to the undetermined
mechanism of onset of the disease and non-specific clinical
manifestations (2). Metastatic RCC
has a poor prognosis with high recurrent rate and mortality and
accounts for 20–30% of newly diagnosed RCC cases (3). It is not sensitive to radiotherapy or
chemotherapy, and traditional cytokine therapy has limited efficacy
in patients with metastatic RCC (4,5). In
recent years, following the emergence of targeted drugs and immune
checkpoint inhibitors, the overall survival and progression-free
survival of patients with metastatic RCC have greatly improved
(6).
Tumor development is a complex multifactorial
process. Matrix metalloproteinases (MMPs) are key enzymes involved
in the process of cancer invasion and metastasis (7). MMPs have been reported to degrade the
extracellular matrix and basement membrane, facilitate the
formation of vessels and enhance cell invasion and metastasis in
RCC (8,9). Epithelial-mesenchymal transition (EMT)
is a process in which epithelial cells transform into mesenchymal
cells (10). Previous studies have
demonstrated that EMT could regulate numerous genes (such as Twist,
Slug, Snail and zinc finger E-box-binding homeobox 1) and signaling
pathways (such as Wnt/β-catenin, Notch/Jagged and Hedgehog
signaling pathway) to influence the invasion and metastasis of
tumors (11,12). Thus, regulating the EMT process and
the expression levels of MMPs may represent a key axis for RCC
prevention and therapy (13).
EGFR is a member of the tyrosine kinase type I
receptor family and a well-established target for cancer therapy
(14). EGFR inhibitors have been
approved for the treatment of multiple types of cancer, such as
non-small cell lung cancer, colon cancer and glioblastoma (15). AKT is an important member of the
PI3K signaling pathway (16). Thus,
determining the effect of AKT inhibitors may represent a novel
targeted drug treatment for cancer as >50% of tumors are
overactivated by AKT (17,18). A previous study reported that
inhibiting the activity of the EGFR/AKT signaling pathway regulated
RCC growth, metastasis and EMT (19). Thus, it is of urgent significance to
identify effective methods to suppress the EGFR/AKT signaling
pathway.
Rhomboid domain-containing protein 1 (RHBDD1) is a
serine protease that has a role in regulated intramembrane
proteolysis (20). RHBDD1 has been
identified to serve a role in cancer. For example, RHBDD1
expression levels were upregulated in breast cancer (21). In vitro, knockdown of RHBDD1
could inhibit cell proliferation, migration, invasion and EMT in
breast cancer (22). Moreover, Song
et al (23) discovered that
RHBDD1 could promote colorectal cancer growth by activating the
EGFR signaling pathway.
The present study aimed to investigate the
functional role of RHBDD1 in RCC, in addition to its underlying
molecular mechanism. In more detail, the study investigated the
influence of RHBDD1 on RCC cell proliferation, migration, invasion
and EMT in vitro and sought to determine whether RHBDD1
exerted its functions through modulating the EGFR/AKT signaling
pathway.
Materials and methods
Cell culture
Normal renal tubule epithelium cell lines, HKC 5 and
HK-2, and human RCC cell lines, 786O and Caki-1, were purchased
from The Cell Bank of Type Culture Collection of The Chinese
Academy of Sciences. The human RCC cell lines, LoMet-ccRCC and
A498, were obtained from the China Infrastructure of Cell Line
Resources, Institute of Basic Medical Sciences, Chinese Academy of
Medical Sciences. Cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.), and maintained in an
atmosphere containing 5% CO2 and 95% air at 37°C.
Cell transfection
The pGPU6/GFP/Neo vector (Shanghai GenePharma Co.,
Ltd.) was used to construct a vector containing short hairpin RNA
(shRNA) targeting RHBDD1. A RHBDD1 genomic fragment was cloned into
a pcDNA3.1 vector (Shanghai GenePharma Co., Ltd.) to overexpress
RHBDD1. The empty vectors served as their negative controls (NCs),
shRNA-NC or OV-NC, respectively. For transfection, cells were
plated into six-well plates and cultured to 90% confluence.
Subsequently, 50 nM individual vectors [shRNA-NC, shRNA-RHBDD1-1,
shRNA-RHBDD1-2, overexpression (Ov)-NC or Ov-RHBDD1] and 5 µl
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) were separately incubated in serum-free Opti-MEM
(Gibco; Thermo Fisher Scientific, Inc.) for 5 min at room
temperature and then mixed for another 20 min incubation at room
temperature. Subsequently, the transfection mixture was added into
cells and cultured for 6–8 h at 37°C. Following the incubation, the
medium was replaced with complete DMEM and cultured at 37°C for 48
h for use in subsequent experiments.
Cell treatment
Cells transfected with Ov-RHBDD1 were treated with 1
µM gefitinib (Selleck Chemicals) in the presence of 10% FBS at 37°C
for 24 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using RNA-Trip
reagent (Applygen Technologies, Inc.). Total RNA was reverse
transcribed into cDNA using a PrimeScript RT reagent kit with gDNA
Eraser (Takara Bio, Inc.) according to the manufacturer's
instructions. qPCR was subsequently performed using SYBR Green PCR
reagent kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) on
an ABI 7000 Real-Time PCR Detection system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95°C for
5 min; followed by 40 cycles of denaturation at 95°C for 10 sec,
annealing at 60°C for 30 sec and extension at 72°C for 35 sec, and
a final extension at 72°C for 5 min. The following primer pairs
were used for the qPCR: RHBDD1 forward, 5′-ATCTGGCTGGGATTCTTGTTG-3′
and reverse, 5′-GGCTGGCTTGTAATGCTCTC-3′; and GAPDH forward,
5′-TCAACGACCACTTTGTCAAGCTCA-3′ and reverse,
5′-GCTGGTGGTCCAGGGGTCTTACT-3′. The relative expression levels were
quantified using the 2−ΔΔCq method (24) and RHBDD1 expression levels were
normalized to GAPDH.
Nuclear/cytoplasmic separation
A Nuclear Protein Extraction kit (Beijing Solarbio
Science & Technology Co., Ltd.) was used to separate the
cytoplasmic and nuclear proteins, according to the manufacturer's
protocol. The expression levels of Ki67 and proliferating cell
nuclear antigen (PCNA) in nuclear proteins were detected by western
blotting.
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology) supplemented
with proteinase inhibitors. Following centrifugation at 12,000 × g
for 10 min at 4°C, protein quantification was performed using a BCA
Protein Assay kit (Beyotime Institute of Biotechnology) and equal
amounts of protein (30 µg/lane) were separated via SDS-PAGE on
8–10% gels. The proteins were subsequently transferred onto PVDF
membranes (Beyotime Institute of Biotechnology) and blocked in 5%
BSA (Beijing Solarbio Science & Technology Co., Ltd.) for 1 h
at room temperature. The membranes were then incubated with the
following primary antibodies at 4°C overnight: Anti-RHBDD1 (Abcam;
cat. no. ab254805; 1:1,000; 34 kDa), anti-Ki67 (Abcam; cat. no.
ab16667; 1:1,000; 358 kDa), anti-PCNA (Abcam; cat. no. ab18197;
1:1,000; 29 kDa), anti-MMP2 (Abcam; cat. no. ab97779; 1:3,000; 74
kDa.), anti-MMP9 (Abcam; cat. no. ab228402; 1:1,000; 81 kDa),
anti-MMP13 (Abcam; cat. no. ab51072; 1:1,000; 54 kDa),
anti-E-cadherin (Abcam; cat. no. ab40772; 1:10,000; 97 kDa),
anti-N-cadherin (Abcam; cat. no. ab18203; 1:1,000; 100 kDa),
anti-vimentin (Abcam; cat. no. ab137321; 1:3,000; 54 kDa),
anti-Slug (Abcam; cat. no. ab27568; 1:1,000; 30 kDa), anti-EGFR
(Abcam; cat. no. ab52894; 1:10,000; 134 kDa), anti-phosphorylated
(p)-AKT (Abcam; cat. no. ab38449; 1:1,000; 56 kDa), anti-AKT
(Abcam; cat. no. ab8805; 1:500; 56 kDa), anti-Lamin B1 (Abcam; cat.
no. ab133741; 1:10,000; 66 kDa) and anti-GAPDH (Abcam; cat. no.
ab8245; 1:10,000; 40.2 kDa). Following the primary antibody
incubation, the membranes were incubated with a goat anti-rabbit
HRP-conjugated secondary antibody (Abcam; cat. no. ab205718;
1:50,000) or a goat anti-mouse HRP-conjugated secondary antibody
(Abcam; cat. no. ab205719; 1:20,000) at room temperature for 1 h.
Protein bands were visualized using an ImageQuant LAS 4000
apparatus (Cytiva) and analyzed using ImageJ software (version
1.47; National Institutes of Health).
Cell Counting Kit-8 (CCK-8) assay
Cell viability was analyzed using a CCK-8 assay
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. Briefly, cells were seeded at a density of
5×104 cells/ml in an incubator with standard culture
conditions (37°C, 5% CO2) for 24 h following the
aforementioned transfections and gefitinib treatment. Following the
incubation, 10 µl/well CCK-8 solution was added and incubated at
37°C for another 4 h. Finally, the absorbance was measured at a
wavelength of 450 nm used a microplate reader (Thermo Fisher
Scientific, Inc.) to obtain the optical density value.
Wound healing assay
Following the aforementioned transfections and
gefitinib treatment, cells were cultured in DMEM supplemented with
10% FBS for use in the wound healing assay. Upon reaching 90%
confluence, the cell monolayer was scratched with a 200-µl pipette
tip and the detached cells were washed with PBS. Subsequently, the
cells were cultured at 37°C in serum-free DMEM. Cells migrating
into the wound sites were analyzed over a 24 h period, with plates
being imaged at 0 and 24 h under an inverted light microscope
(magnification, ×100; Leica Microsystems GmbH). Cell migration was
quantified by determining the extent (%) of wound healing as
follows: (0 h scratch area - 24 h scratch area) / scratch area at 0
h ×100%.
Transwell invasion assay
The invasive ability of cells was analyzed using
Transwell chambers precoated with Matrigel (Beijing Solarbio
Science & Technology Co., Ltd.) for 8 h at 37°C. Briefly,
2×105 cells/ml suspended in serum-free DMEM were plated
into the upper chambers of Transwell plates, while DMEM
supplemented with 10% FBS as a chemoattractant was plated into the
lower chambers. Following incubation for 24 h at 37°C, non-invasive
cells were removed and the invasive cells in the lower chambers of
the Transwell plates were fixed with 4% paraformaldehyde for 30 min
and stained with 0.4% crystal violet (Sigma-Aldrich; Merck KGaA)
for 5 min at room temperature. Invasive cells were counted in five
randomly selected fields of view using an inverted light microscope
(magnification, ×100; Leica Microsystems GmbH).
Statistical analysis
Experiments were performed in triplicate and data
are presented as mean ± standard deviation. Statistical differences
among groups were determined using one-way ANOVA followed by a
Tukey's post hoc test. Statistical analysis was performed using
GraphPad Prism 6.01 software (GraphPad Software, Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
RHBDD1 expression levels are
upregulated in RCC cell lines
To determine whether RHBDD1 was involved in RCC,
RHBDD1 expression levels were analyzed in the normal renal tubule
epithelium cell lines, HKC-5 and HK-2, and the human RCC lines,
786O, LoMet-ccRCC, A498 and Caki-1, using western blotting and
RT-qPCR. Compared with normal renal tubule epithelium cell lines,
the protein (Fig. 1A) and mRNA
(Fig. 1B) expression levels of
RHBDD1 were significantly upregulated in RCC cell lines. These
findings suggested that RHBDD1 may play an important role in RCC.
The expression levels of RHBDD1 were the highest in the RCC cell
line, 786O. Therefore, 786O cells were selected for use in
subsequent experiments.
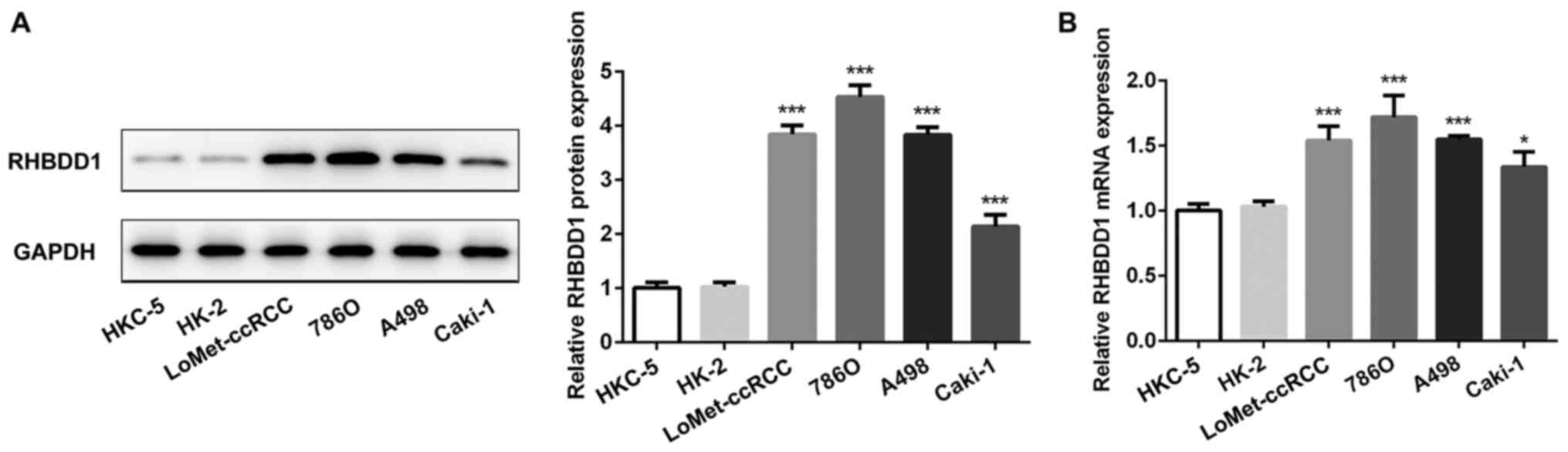 | Figure 1.RHBDD1 expression levels are
significantly upregulated in RCC cell lines. (A) Protein and (B)
mRNA expression levels of RHBDD1 in normal renal tubule epithelium
cell lines, HKC-5 and HK-2, and human RCC cell lines, 786O,
LoMet-ccRCC, A498 and Caki-1, were determined using western
blotting and reverse transcription-quantitative PCR, respectively.
*P<0.05, ***P<0.001 vs. HKC-5 and HK-2 cell lines. RHBDD1,
rhomboid domain-containing protein 1; RCC, renal cell
carcinoma. |
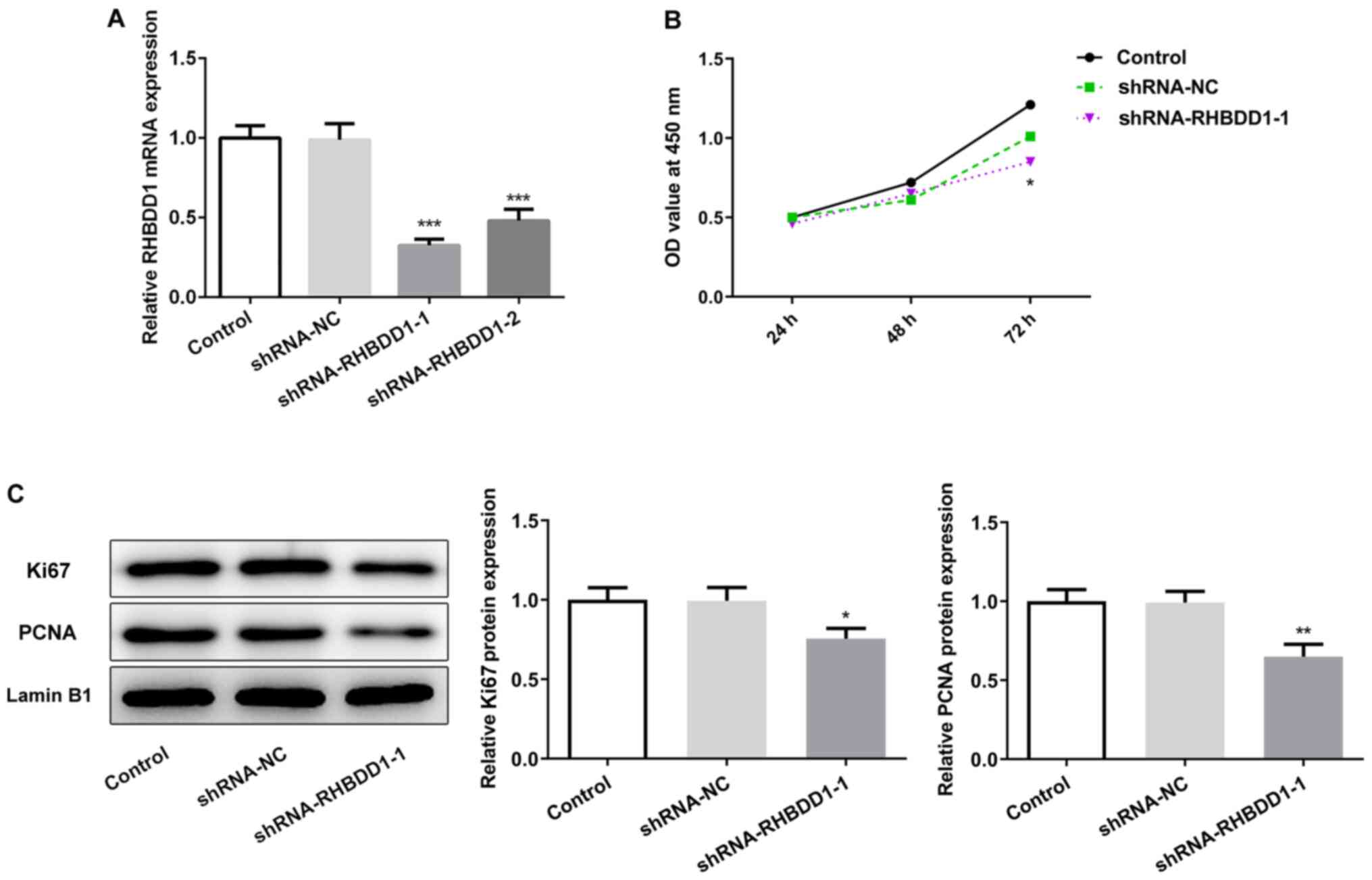
Knockdown of RHBDD1 inhibits cell
proliferation in RCC
As RHBDD1 expression may be associated with the
progression of RCC, shRNA-RHBDD1-1 and shRNA-RHBDD1-2 were
transfected into the 786O cell line to determine the biological
functions of RHBDD1 in RCC. Compared with the shRNA-NC group,
transfection with shRNA-RHBDD1 significantly downregulated the
expression levels of RHBDD1. Due to the optimized transfection
efficiency, shRNA-RHBDD1-1 was chosen for the functional
experiments (Fig. 2A). Data
obtained from CCK-8 assay revealed that cell proliferation was
significantly inhibited following RHBDD1 knockdown in comparison
with the shRNA-NC group (Fig. 2B).
Moreover, the expression levels of the proliferation-associated
proteins, Ki67 and PCNA, were analyzed using western blotting to
further verify the effects of RHBDD1 on cell proliferation in
vitro. As expected, the protein expression levels of Ki67 and
PCNA were significantly downregulated in the 786O cell lines
following the transfection with shRNA-RHBDD1-1 compared with the
shRNA-NC group (Fig. 2C). These
results suggested that the knockdown of RHBDD1 expression may
suppress RCC cell proliferation.
Knockdown of RHBDD1 suppresses cell
migration, invasion and EMT in RCC
The present study subsequently aimed to determine
the function of RHBDD1 in cell migration, invasion and EMT of RCC.
The results of the wound healing assay revealed that the migration
of 786O cells was significantly reduced following the transfection
with shRNA-RHBDD1-1 compared with the shRNA-NC group (Fig. 3A). Similarly, the knockdown of
RHBDD1 significantly decreased RCC cell invasion compared with the
shRNA-NC group (Fig. 3B). In
addition, western blotting was performed to analyze the expression
levels of key proteins related to cell migration and invasion.
MMP2, MMP9 and MMP13 expression levels were significantly
downregulated following the knockdown of RHBDD1 expression compared
with the shRNA-NC group, which indicated that the knockdown of
RHBDD1 may suppress cell migration and invasion in RCC (Fig. 3C). The expression levels of several
EMT markers, including E-cadherin, N-cadherin, vimentin and Slug,
were also analyzed to determine the underlying mechanism of RHBDD1
in tumor progression. The expression levels of E-cadherin were
upregulated, while the expression levels of N-cadherin, vimentin
and Slug were downregulated following the knockdown of RHBDD1
compared with the shRNA-NC group, which suggested that RHBDD1 may
suppress EMT in RCC (Fig. 3D).
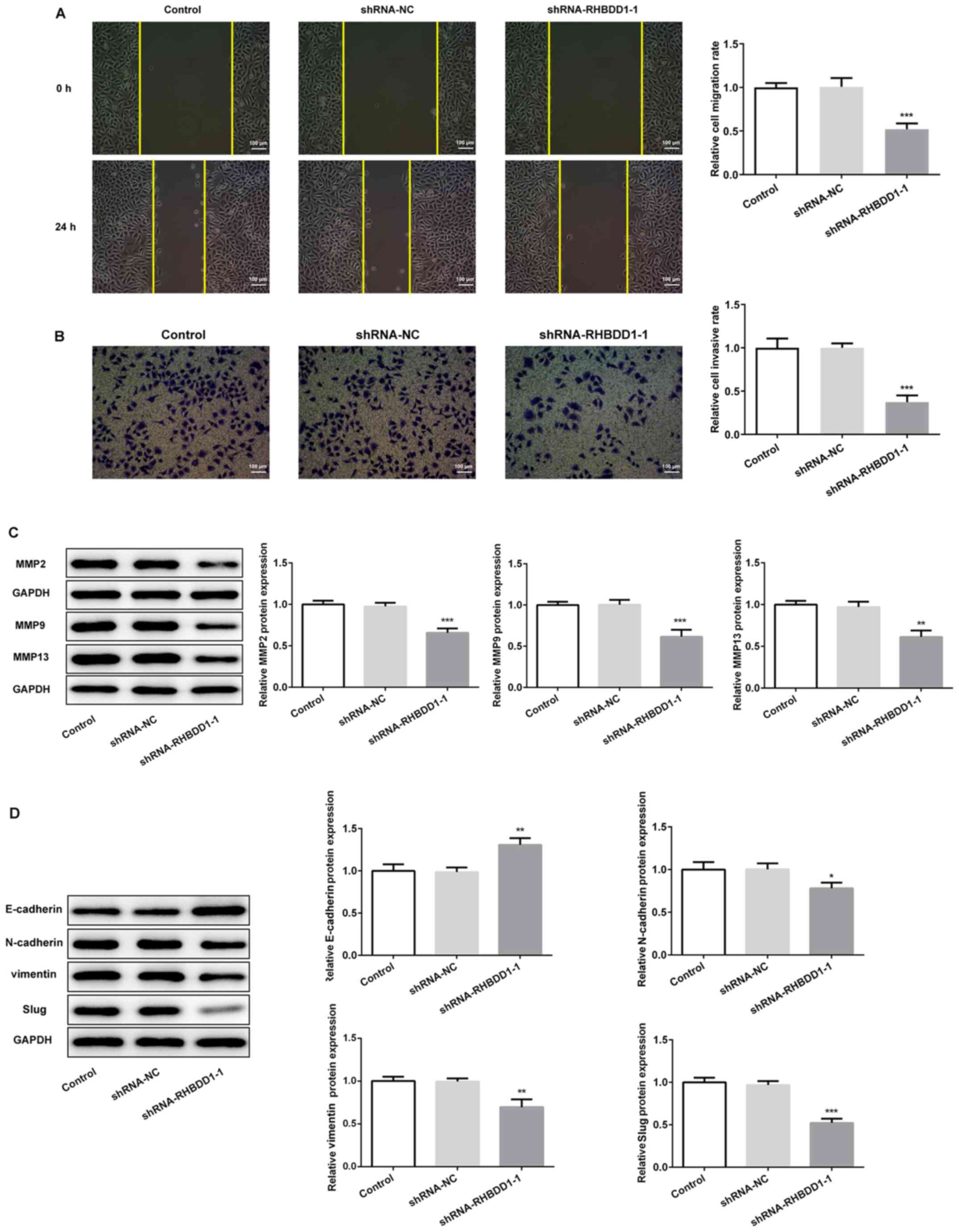 | Figure 3.Knockdown of RHBDD1 suppresses cell
migration, invasion and EMT in renal cell carcinoma. (A) Wound
healing assay was performed to determine cell migration
(magnification, ×100; scale bar, 100 µm). (B) Transwell assay was
performed to determine cell invasion (magnification, ×100; scale
bar, 100 µm). Western blotting was performed to analyze the
expression levels of proteins involved in (C) cell migration and
invasion, including MMP2, MMP9 and MMP13 and (D) EMT, including
E-cadherin, N-cadherin, vimentin and Slug. *P<0.05, **P<0.01,
***P<0.001 vs. shRNA-NC. RHBDD1, rhomboid domain-containing
protein 1; EMT, epithelial-mesenchymal transition; MMP, matrix
metalloproteinase; shRNA, short hairpin RNA; NC, negative
control. |
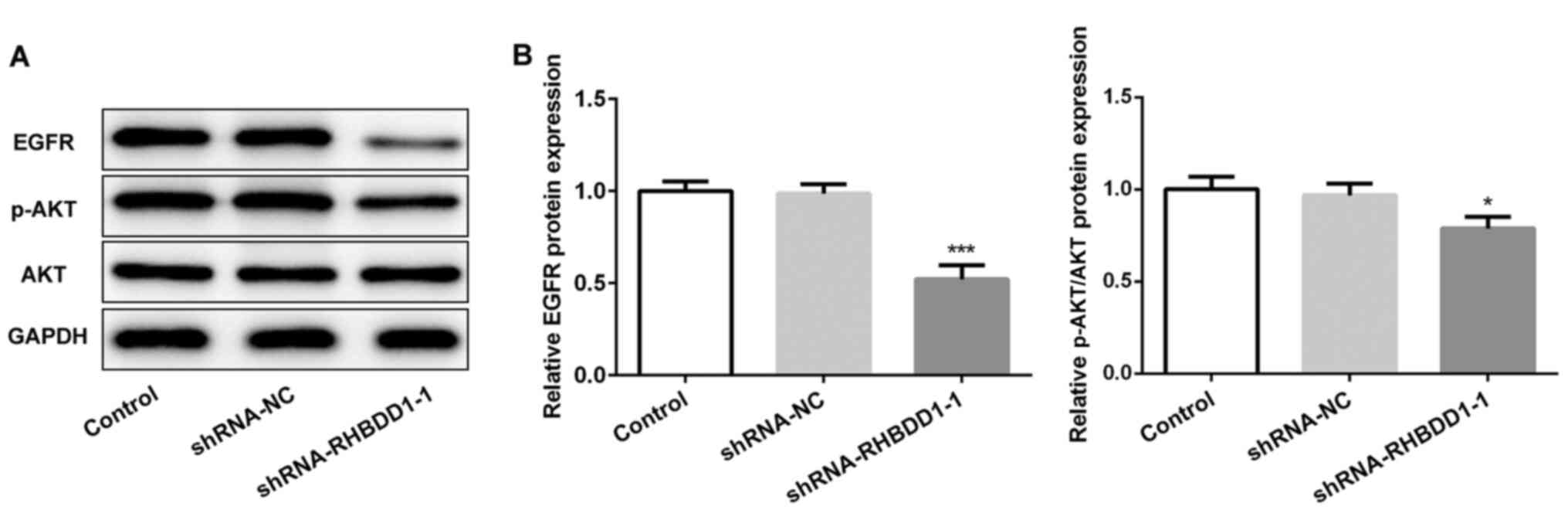
Knockdown of RHBDD1 inhibits EGFR/AKT
signaling pathway in RCC
To determine the molecular mechanism through which
RHBDD1 may regulate RCC, the expression levels of EGFR/AKT
signaling pathway-related proteins were analyzed using western
blotting. EGFR and p-AKT expression levels were significantly
downregulated following the transfection with shRNA-RHBDD1-1
compared with the shRNA-NC group, indicating that RHBDD1 silencing
may suppress EGFR/AKT signaling pathway (Fig. 4A and B).
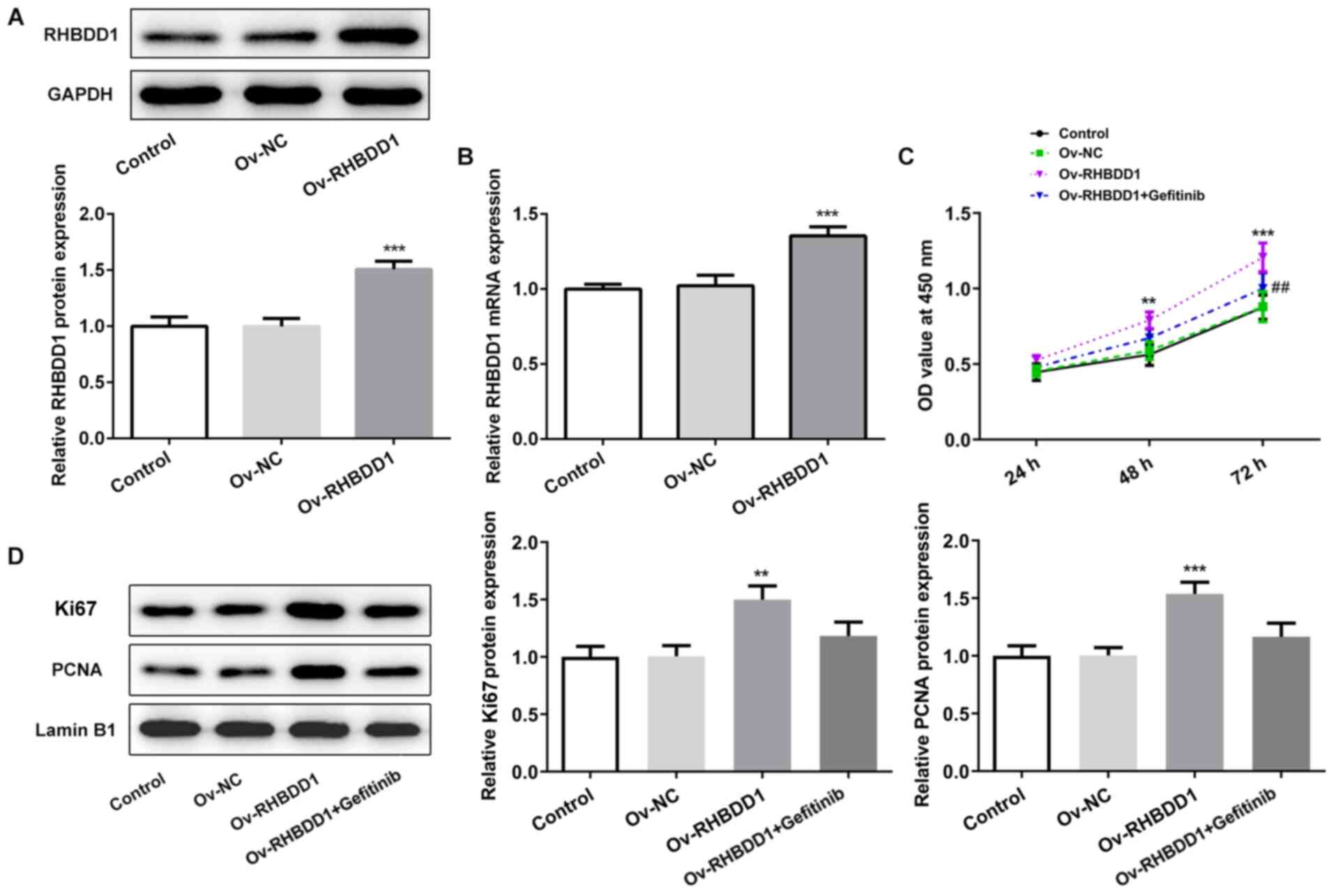
RHBDD1 promotes cell proliferation via
EGFR/AKT signaling pathway in RCC
To further investigate the underlying molecular
mechanism through which RHBDD1 regulated cell proliferation in RCC,
RHBDD1 overexpression vectors were transfected into the 786O cell
line. Data from western blotting (Fig.
5A) and RT-qPCR (Fig. 5B)
analyses both demonstrated that transfection with Ov-RHBDD1
significantly upregulated RHBDD1 expression levels compared with
the Ov-NC group. The results of CCK-8 assay revealed that the
promoting effect of RHBDD1 on cell proliferation was markedly
reversed following treatment with gefitinib, an EGFR inhibitor
(Fig. 5C). Similarly, the
upregulation of proliferation-associated proteins, including Ki67
and PCNA was partly reversed by gefitinib treatment (Fig. 5D). These results suggested that
RHBDD1 may promote cell proliferation via EGFR/AKT signaling
pathway in RCC.
RHBDD1 promotes cell migration,
invasion and EMT via EGFR/AKT signaling pathway in RCC
The underlying mechanism through which RHBDD1 may
promote RCC cell invasion and migration was also identified.
Transfection with Ov-RHBDD1 significantly promoted cell migration
compared with the Ov-NC group, while this effect was significantly
abolished by gefitinib treatment (Fig.
6A). Similarly, RHBDD1 overexpression significantly promoted
cell invasion compared with the Ov-NC group and this effect was
also rescued by gefitinib treatment (Fig. 6B). In addition, the expression
levels of key proteins related to cell migration and invasion
(MMP2, MMP9 and MMP13) were significantly upregulated following
transfection with Ov-RHBDD compared with the Ov-NC group, while
gefitinib treatment downregulated the protein expression levels of
MMP2, MMP9 and MMP13 (Fig. 6C).
Simultaneously, the expression levels of proteins involved in EMT
were also evaluated. Gefitinib treatment reversed the
downregulation in E-cadherin expression levels and upregulation in
N-cadherin, vimentin and Slug expression levels induced by RHBDD1
overexpression (Fig. 6D). These
results suggested that RHBDD1 may promote cell migration, invasion
and EMT via EGFR/AKT signaling pathway in RCC.
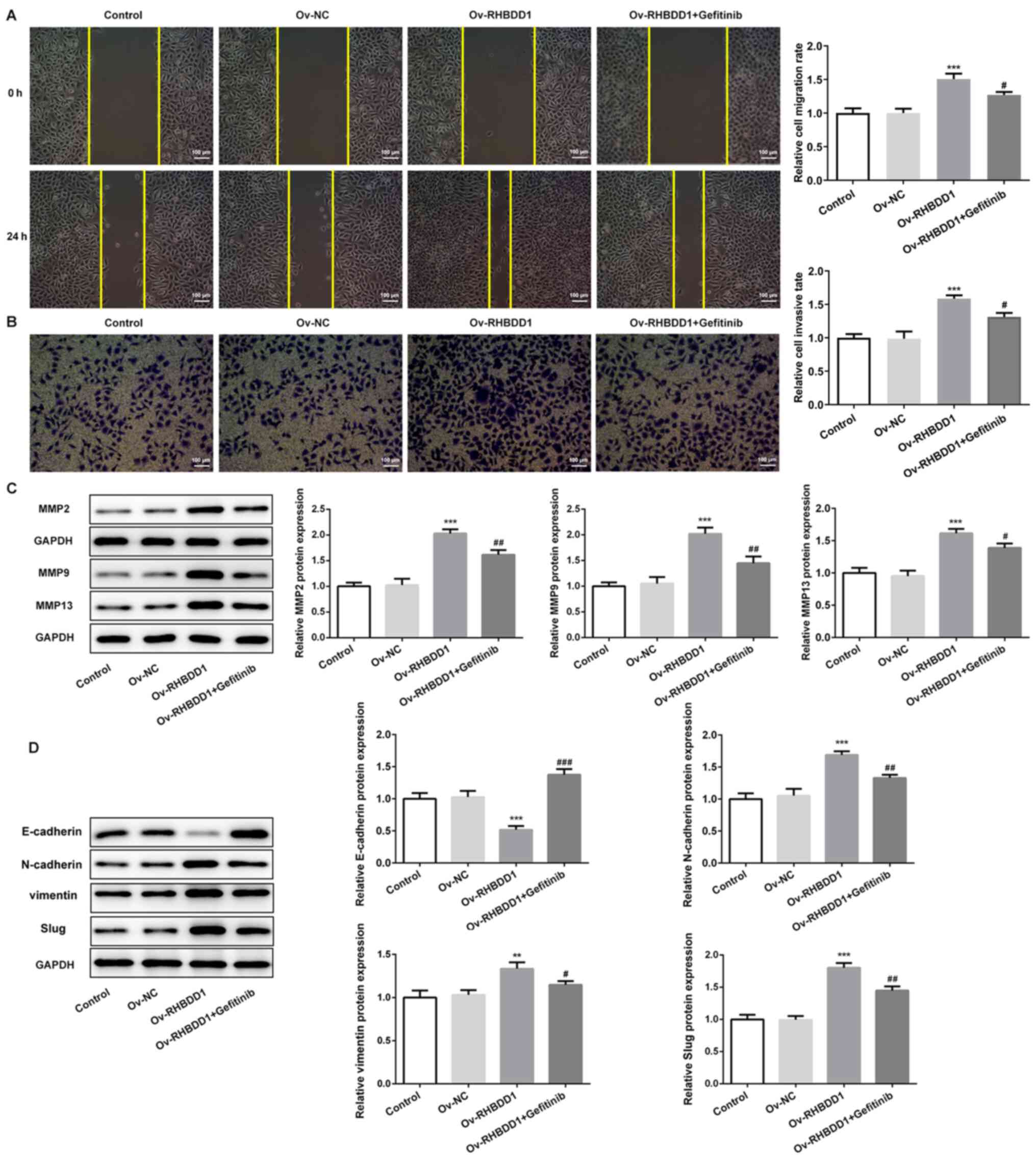 | Figure 6.RHBDD1 promotes cell migration,
invasion and EMT via EGFR/AKT signaling pathway in renal cell
carcinoma. (A) Wound healing assay was performed to determine cell
migration (magnification, ×100; scale bar, 100 µm). (B) Transwell
assay was performed to determine cell invasion (magnification,
×100; scale bar, 100 µm). Western blotting was performed to analyze
the expression levels of key proteins involved in (C) cell
migration and invasion, such as MMP2, MMP9 and MMP13 and (D) EMT,
such as E-cadherin, N-cadherin, vimentin and Slug. **P<0.01,
***P<0.001 vs. Ov-NC; #P<0.05,
##P<0.01, ###P<0.001 vs. Ov-RHBDD1.
RHBDD1, rhomboid domain-containing protein 1; EMT,
epithelial-mesenchymal transition; MMP, matrix metalloproteinase;
Ov, overexpression; NC, negative control. |
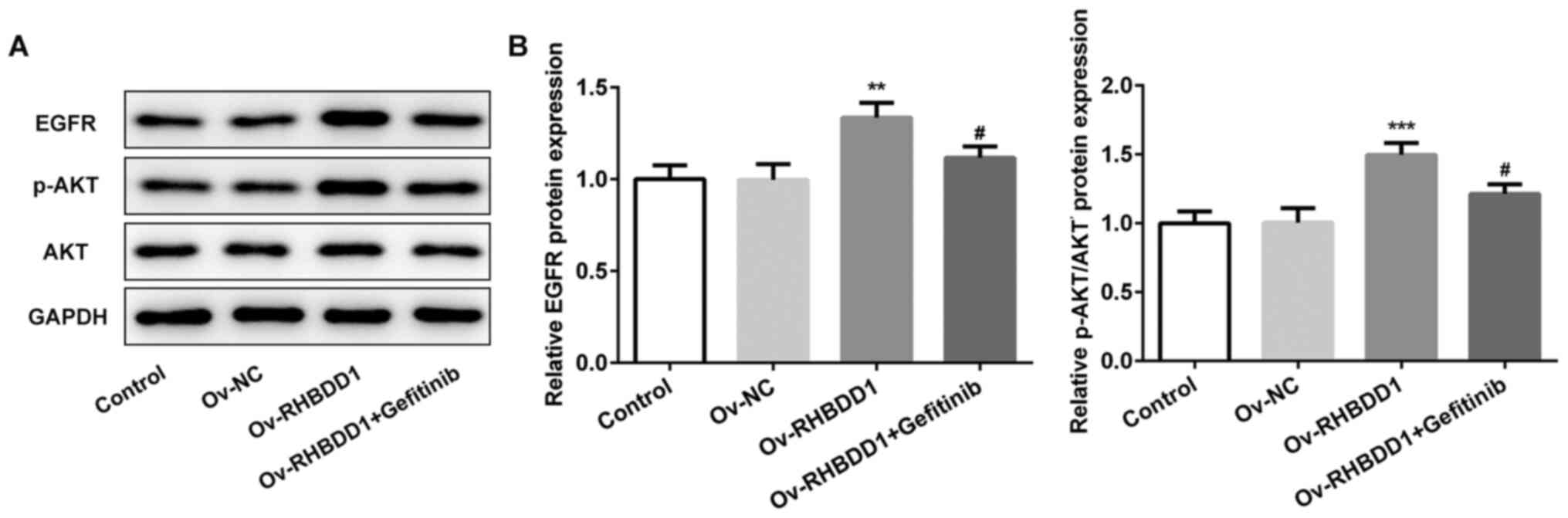
Gefitinib effectively inhibits
EGFR/AKT signaling pathway in RCC
The expression levels of proteins involved in
EGFR/AKT signaling pathway (EGFR and p-AKT) were significantly
upregulated following RHBDD1 overexpression compared with the Ov-NC
group, while the expression levels of EGFR and p-AKT were
downregulated following gefitinib treatment (Fig. 7A and B). Collectively, these results
suggested that gefitinib may effectively suppress EGFR/AKT
signaling pathway in RCC.
Discussion
RCC is highly resistant to radiotherapy and
chemotherapy, and immunotherapy is currently less effective in
treating the disease (4,5). In recent years, with the continuous
development of medical technology and increase in research on the
pathogenesis of RCC, molecular targeted drugs (sunitinib,
pazopanib, axitinib and everolimus) have provided a novel method
for the treatment of advanced RCC (25,26).
The present study aimed to determine the biological characteristics
of RHBDD1 and analyzed the potential value of RHBDD1 as a
therapeutic target for RCC.
Targeted anticancer therapy has been widely applied
for the treatment of malignant cancers following surgical
resection, radiotherapy and chemotherapy (25). A previous study reported that
silencing of RHBDD1 can significantly suppress cell proliferation
and promote the apoptosis of HepG2 cells (27). Accumulating studies have begun to
pay attention to the role of MMPs and EMT in cancer metastasis
(7,10). Zhao et al (21) verified that downregulation of RHBDD1
can inhibit the migratory and invasive abilities and EMT of breast
cancer cells. Moreover, Huang et al (22) demonstrated that RHBDD1 knockdown can
inhibit breast cancer cell migration and invasion by repressing
MMP2/9 protein levels and EMT. In addition, it has been confirmed
that RHBDD1 expression in colorectal tumor tissues is positively
associated with distal metastasis and lymphatic metastasis
(28). The results of the present
study revealed that RHBDD1 expression levels were markedly
upregulated in human RCC cell lines. The overexpression of RHBDD1
could significantly promote cell proliferation, migration, invasion
and EMT in RCC, while the application of EGFR inhibitor (gefitinib)
reversed the effects of RHBDD1 overexpression.
EGFR, a member of the family of protein tyrosine
kinase receptors, plays a crucial role in the proliferation,
apoptosis, invasive ability and metastasis of tumor cells (29,30).
EGFR inhibitors are vital antitumor agents used to prevent the
progression of multiple types of cancer (14,15). A
previous study demonstrated that promoting EGFR degradation and
suppressing EGF-induced AKT signaling can inhibit the growth and
metastasis of kidney clear cell carcinoma (31). Moreover, RHBDD1 can activate EGFR
signaling pathway in colorectal cancer (23). As expected, the present results
revealed that knockdown of RHBDD1 suppressed EGFR/AKT signaling
pathway activity in RCC.
In conclusion, the findings of the present study
demonstrated that RHBDD1 promoted cell proliferation, migration,
invasion and EMT through modulating EGFR/AKT signaling pathway in
RCC. The current data highlighted a pivotal role of RHBDD1 in the
tumorigenesis of RCC, which may provide an improved understanding
of the pathogenesis of RCC and a novel therapeutic target or
biomarker for RCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML, LC, XW, CL and CZ designed the present study.
ML, LC, XW, YY, WJ, GB, ZG, JG and JZ performed the experiments.
ML, LC, XW, YY, WJ and GB analyzed and interpreted the data. ML,
LC, XW, YY, WJ, GB, CL and CZ drafted the manuscript. CL and CZ
revised the manuscript. All authors have read and approved the
final manuscript. CZ and CL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen S, Wang Y, Xiong Y, Peng T, Lu M,
Zhang L and Guo Z: Wild-type IDH1 inhibits the tumor growth through
degrading HIF-α in renal cell carcinoma. Int J Biol Sci.
17:1250–1262. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang T, Wang X, Yang X, Ji J, Wang Q, Yue
X and Dong Z: long non-coding RNA DUXAP8 enhances renal cell
carcinoma progression via downregulating miR-126. Med Sci Monit.
24:7340–7347. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen HT and McGovern FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu H, Wang Z, Xu Q, Zhang Y, Zhai Y, Bai
J, Liu M, Hui Z and Xu N: Inhibition of STAT1 sensitizes renal cell
carcinoma cells to radiotherapy and chemotherapy. Cancer Biol Ther.
13:401–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teng ZY, Cheng XL, Cai XT, Yang Y, Sun XY,
Xu JD, Lu WG, Chen J, Hu CP, Zhou Q, et al: Ancient Chinese Formula
Qiong-Yu-Gao Protects Against Cisplatin-Induced Nephrotoxicity
Without Reducing Anti-tumor Activity. Sci Rep. 5:155922015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flippot R, Escudier B and Albiges L:
Immune Checkpoint Inhibitors: Toward New Paradigms in Renal Cell
Carcinoma. Drugs. 78:1443–1457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong Y, Lu YT, Sun Y, Shi ZH, Li NG, Tang
YP and Duan JA: Recent opportunities in matrix metalloproteinase
inhibitor drug design for cancer. Expert Opin Drug Discov.
13:75–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu QH, Wang Y, Yong HM, Hou PF, Pan J,
Bai J and Zheng JN: XRCC1 serves as a potential prognostic
indicator for clear cell renal cell carcinoma and inhibits its
invasion and metastasis through suppressing MMP-2 and MMP-9.
Oncotarget. 8:109382–109392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang F, Wang YG and Wang C: Metformin
inhibited growth, invasion and metastasis of esophageal squamous
Cell Carcinoma in vitro and in vivo. Cell Physiol Biochem.
51:1276–1286. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsai JH, Donaher JL, Murphy DA, Chau S and
Yang J: Spatiotemporal regulation of epithelial-mesenchymal
transition is essential for squamous cell carcinoma metastasis.
Cancer Cell. 22:725–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang P, Sun Y and Ma L: ZEB1: At the
crossroads of epithelial-mesenchymal transition, metastasis and
therapy resistance. Cell Cycle. 14:481–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan G, Liu Y, Shang L, Zhou F and Yang S:
EMT-associated microRNAs and their roles in cancer stemness and
drug resistance. Cancer Commun (Lond). 41:199–217. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chuang MJ, Sun KH, Tang SJ, Deng MW, Wu
YH, Sung JS, Cha TL and Sun GH: Tumor-derived tumor necrosis
factor-alpha promotes progression and epithelial-mesenchymal
transition in renal cell carcinoma cells. Cancer Sci. 99:905–913.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao BC, Lin CC and Yang JC: Second and
third-generation epidermal growth factor receptor tyrosine kinase
inhibitors in advanced nonsmall cell lung cancer. Curr Opin Oncol.
27:94–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh D, Attri BK, Gill RK and Bariwal J:
Review on EGFR Inhibitors: Critical Updates. Mini Rev Med Chem.
16:1134–1166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Xu H, Cheng X, Yang J, Yan Z, Ma
H, Zhao Y, Ommati MM, Manthari RK and Wang J: Calcium relieves
fluoride-induced bone damage through the PI3K/AKT pathway. Food
Funct. 11:1155–1164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Manning BD and Toker A: AKT/PKB Signaling:
Navigating the Network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brown JS and Banerji U: Maximising the
potential of AKT inhibitors as anti-cancer treatments. Pharmacol
Ther. 172:101–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L, Miao L, Liu Y, Qi A, Xie P, Chen J
and Zhu H: S100A11 regulates renal carcinoma cell proliferation,
invasion, and migration via the EGFR/Akt signaling pathway and
E-cadherin. Tumour Biol. 39:10104283177053372017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Guan X, Fok KL, Li S, Zhang X,
Miao S, Zong S, Koide SS, Chan HC and Wang L: A novel member of the
Rhomboid family, RHBDD1, regulates BIK-mediated apoptosis. Cell Mol
Life Sci. 65:3822–3829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao C, Ling X, Li X, Hou X and Zhao D:
MicroRNA-138-5p inhibits cell migration, invasion and EMT in breast
cancer by directly targeting RHBDD1. Breast Cancer. 26:817–825.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang C, Ji X, Peng Y, Wu M, Wu W, Luo Y,
Cheng G and Zhu Y: Silencing of rhomboid domain containing 1 to
inhibit the metastasis of human breast cancer cells in vitro. Iran
J Basic Med Sci. 21:1161–1166. 2018.PubMed/NCBI
|
|
23
|
Song W, Liu W, Zhao H, Li S, Guan X, Ying
J, Zhang Y, Miao F, Zhang M, Ren X, et al: Rhomboid domain
containing 1 promotes colorectal cancer growth through activation
of the EGFR signalling pathway. Nat Commun. 6:80222015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kitano H, Kitadai Y, Teishima J, Yuge R,
Shinmei S, Goto K, Inoue S, Hayashi T, Sentani K, Yasui W, et al:
Combination therapy using molecular-targeted drugs modulates tumor
microenvironment and impairs tumor growth in renal cell carcinoma.
Cancer Med. 6:2308–2320. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hawkins R, Fife K, Hurst M, Wang M,
Naicker N, Nolasco S, Eisen T, Matakidou A and Gordon J: Treatment
patterns and health outcomes in metastatic renal cell carcinoma
patients treated with targeted systemic therapies in the UK. BMC
Cancer. 20:6702020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu XN, Tang ZH, Zhang Y, Pan QC, Chen XH,
Yu YS and Zang GQ: Lentivirus-mediated silencing of rhomboid domain
containing 1 suppresses tumor growth and induces apoptosis in
hepatoma HepG2 cells. Asian Pac J Cancer Prev. 14:5–9. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang M, Miao F, Huang R, Liu W, Zhao Y,
Jiao T, Lu Y, Wu F, Wang X, Wang H, et al: RHBDD1 promotes
colorectal cancer metastasis through the Wnt signaling pathway and
its downstream target ZEB1. J Exp Clin Cancer Res. 37:222018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Souza JL, Martins-Cardoso K, Guimarães IS,
de Melo AC, Lopes AH, Monteiro RQ and Almeida VH: Interplay Between
EGFR and the Platelet-Activating Factor/PAF Receptor Signaling Axis
Mediates Aggressive Behavior of Cervical Cancer. Front Oncol.
10:5572802020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Q, Huang T, Jiang Z, Ge C, Chen X,
Zhang L, Zhao F, Zhu M, Chen T, Cui Y, et al: Upregulation of SNX5
predicts poor prognosis and promotes hepatocellular carcinoma
progression by modulating the EGFR-ERK1/2 signaling pathway.
Oncogene. 39:2140–2155. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin L, Gao S, Shi H, Wang K, Yang H and
Peng B: TIP-B1 promotes kidney clear cell carcinoma growth and
metastasis via EGFR/AKT signaling. Aging (Albany NY). 11:7914–7937.
2019. View Article : Google Scholar : PubMed/NCBI
|